Comparative Habitat Divergence and Fragmentation Analysis of Two Sympatric Pheasants in the Qilian Mountains, China
Abstract
:1. Introduction
2. Materials and Methods
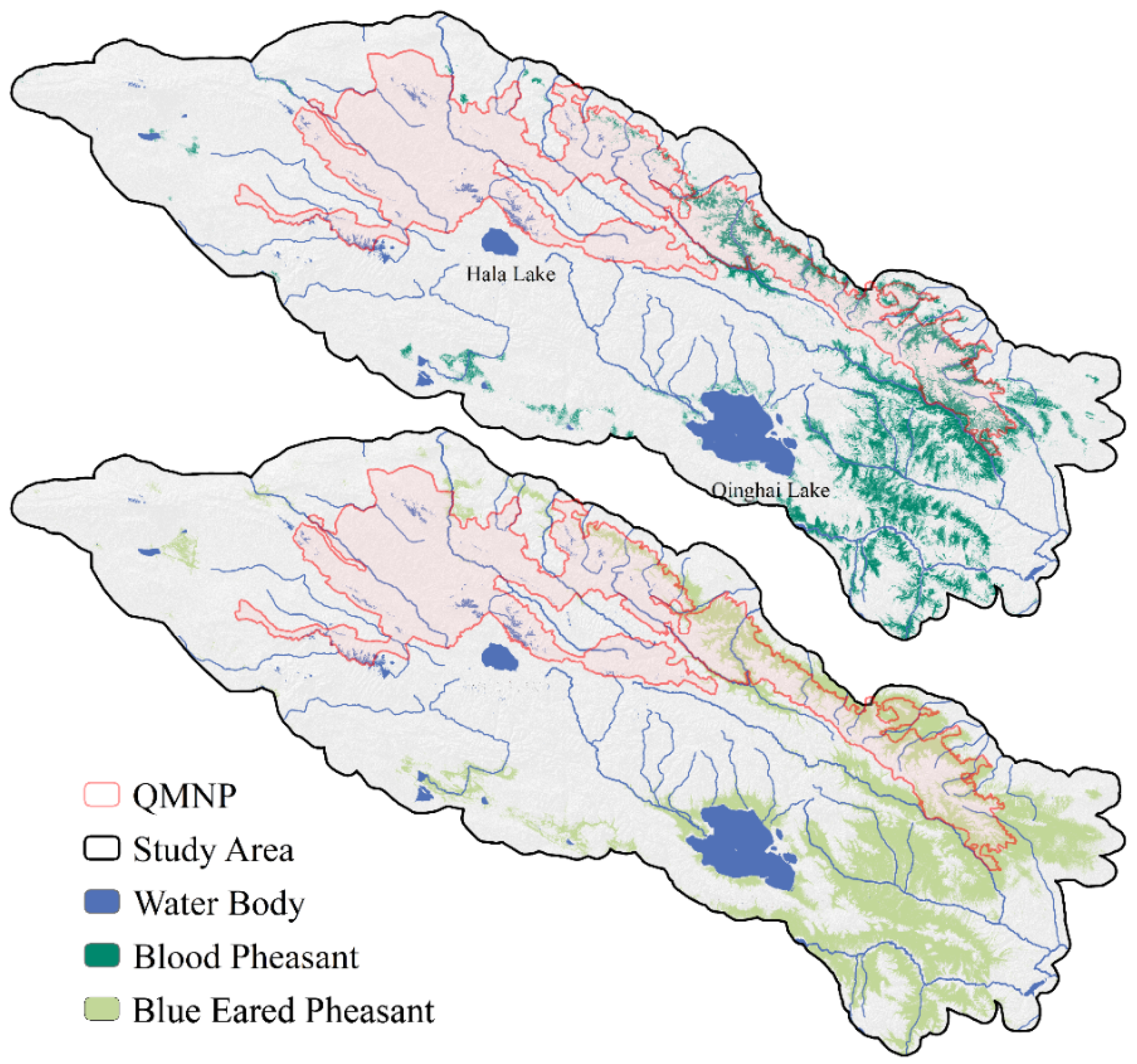
2.1. Study Area
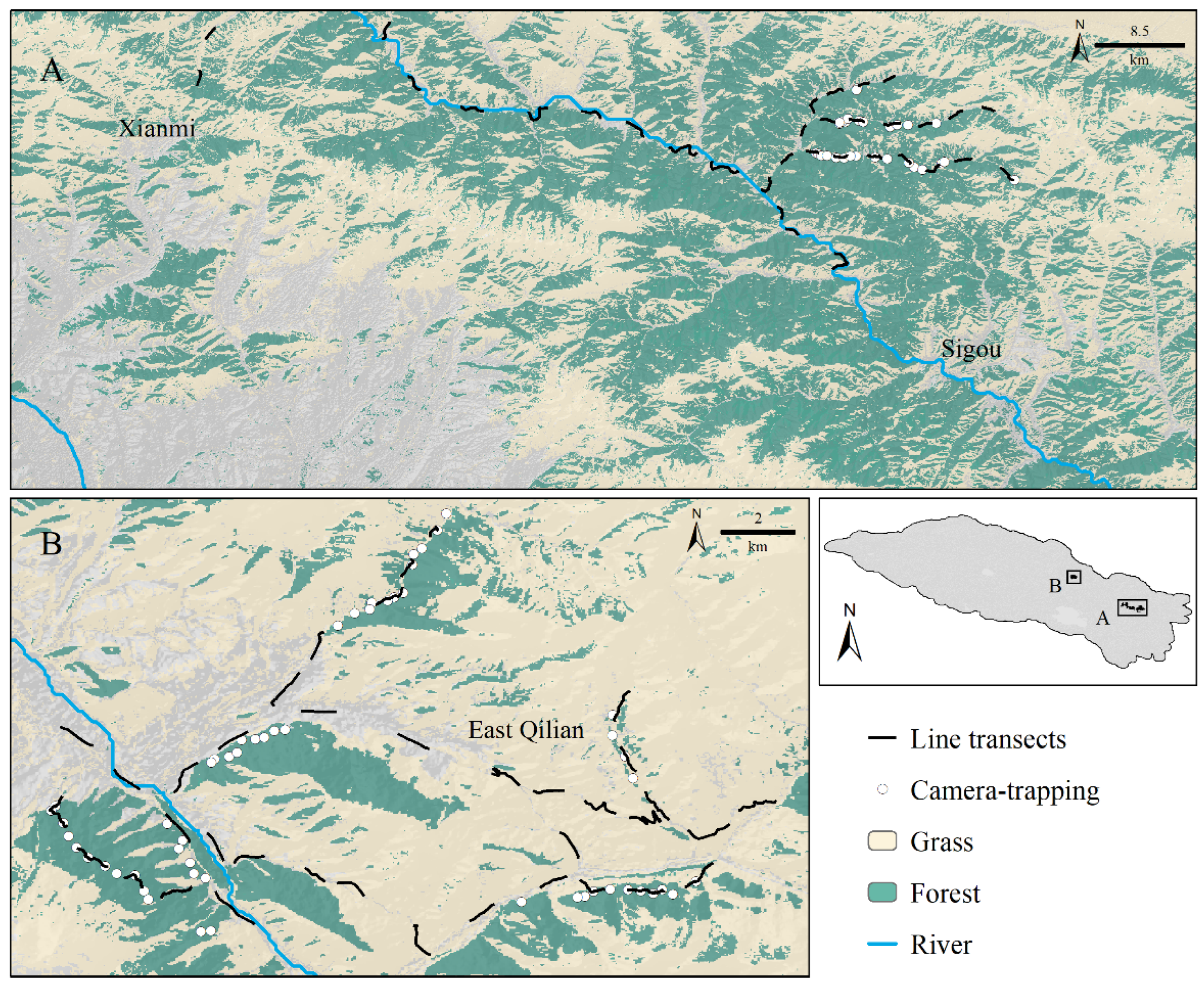
2.2. Field Study
2.3. Occurrence Data
2.4. Environmental Variables
2.5. Model Building and Evaluation
2.6. Data Analysis
3. Results
3.1. Model Validation
3.2. Potential Distribution and Niche Differentiation
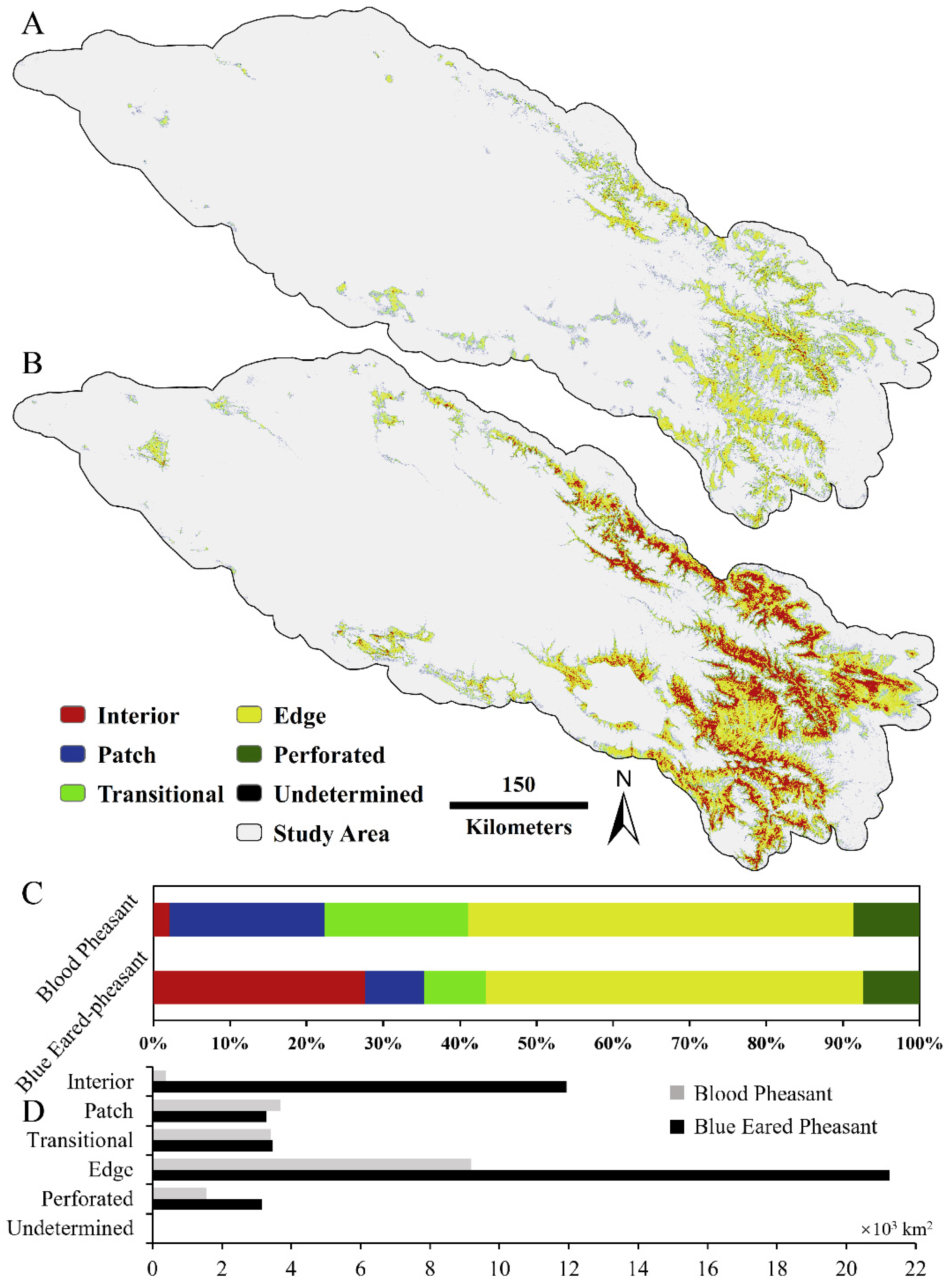
3.3. Habitat Fragmentation and Protection GAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A. Land Cover and Land Use

Appendix B. Result of Field Study
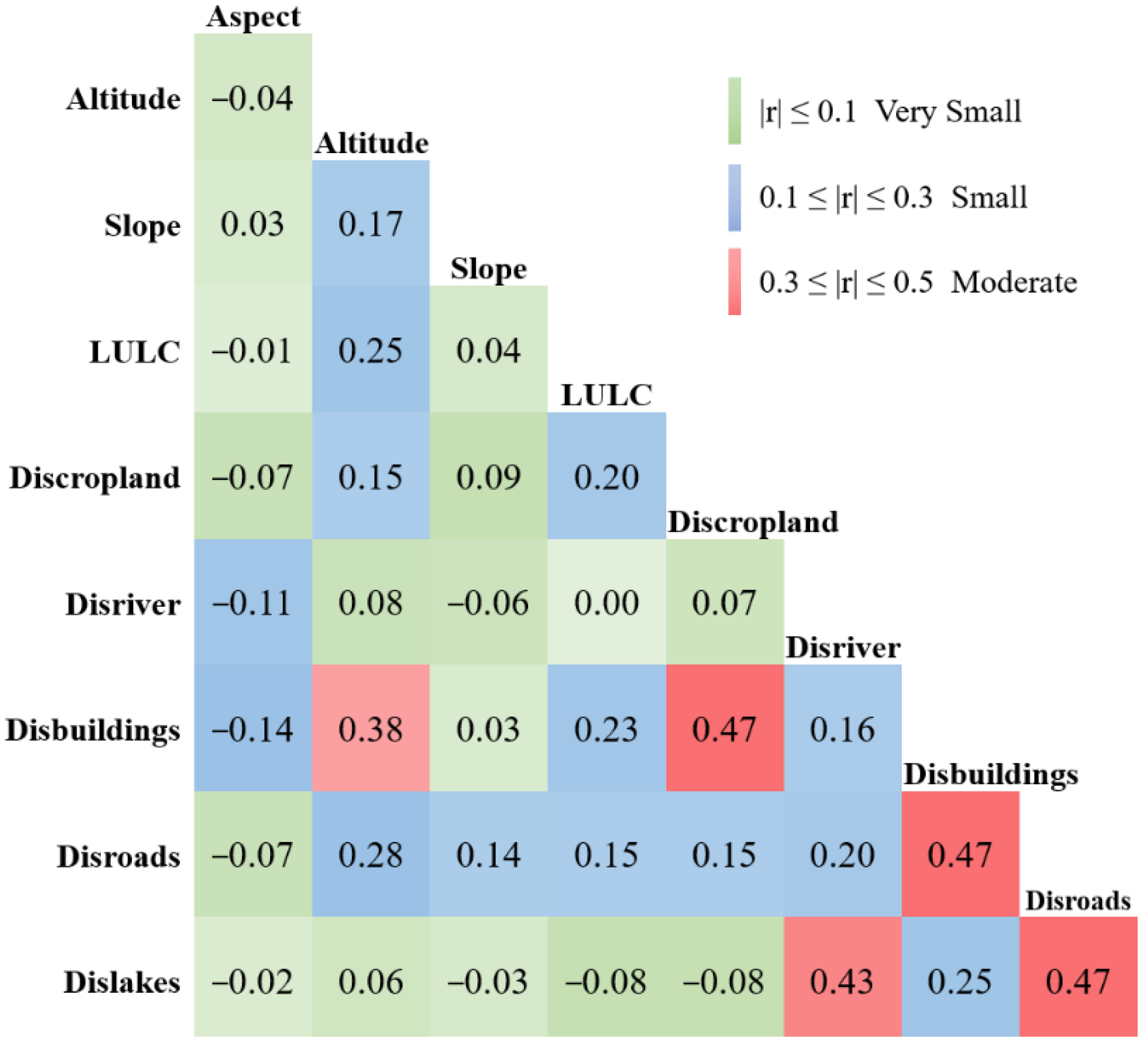
Appendix C. Correlation of Environmental Variable
Appendix D. Occurrence Data Source
| Species | Total | Selected | GBIF | Field Survey | Previous Studies |
|---|---|---|---|---|---|
| Blood Pheasant | 196 | 172 | 30 | 105 | 37 |
| Blue Eared Pheasant | 263 | 258 | 55 | 85 | 118 |
Appendix E. Field Survey Result
| Survey Site | Survey Period | Total Cameras | No. of Blood Pheasant Obs. | No. of Blue Eared Pheasant Obs. |
|---|---|---|---|---|
| Xian-mi | 26–27 April | 16 | 14 | 16 |
| Si-gou | 28–29 April | 27 | 9 | 13 |
| East of Qilian country | 30 April–2 May | 57 | 87 | 56 |
Appendix F. Regularized Training Gain

Appendix G. Fragmentation Analysis
| Species | Dispersal Distance (km) | NP | LPI | PLAND | CL |
|---|---|---|---|---|---|
| Blood Pheasant | 0.5 | 1699 | 5.08 | 7.82 | 62,591.64 |
| 1 | 518 | 8.01 | 9.85 | 98,775.56 | |
| 1.5 | 266 | 9.22 | 11.27 | 103,526.01 | |
| 2 | 183 | 10.07 | 12.41 | 108,159.23 | |
| 2.5 | 135 | 10.78 | 13.39 | 109,810.40 | |
| Blue Eared Pheasant | 3 | 43 | 16.10 | 20.03 | 127,128.70 |
| 4 | 31 | 17.73 | 21.50 | 142,703.49 | |
| 5 | 23 | 20.41 | 22.78 | 163,100.33 | |
| 5.5 | 20 | 20.88 | 23.38 | 164,575.24 | |
| 6 | 19 | 21.28 | 23.96 | 165,054.78 |
References
- Bełcik, M.; Lenda, M.; Amano, T.; Skórka, P. Different response of the taxonomic, phylogenetic and functional diversity of birds to forest fragmentation. Sci. Rep. 2020, 10, 20320. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Coomes, D.A.; Gibson, L.; Hu, G.; Liu, J.; Luo, Y.; Wu, C.; Yu, M. Forest fragmentation in China and its effect on biodiversity. Biol. Rev. 2019, 94, 1636–1657. [Google Scholar] [CrossRef]
- Wang, P.; Xia, W.; Zhou, E.; Li, Y.; Hu, J. Suitable Habitats of Chrysolophus spp. Need Urgent Protection from Habitat Fragmentation in China: Especially Suitable Habitats in Non-Nature Reserve Areas. Animals 2022, 12, 2047. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.M.; Whittaker, R.J.; Freudenberger, D. Bird community responses to habitat fragmentation: How consistent are they across landscapes? J. Biogeogr. 2005, 32, 1353–1370. [Google Scholar] [CrossRef]
- Lu, N.; Jia, C.-X.; Lloyd, H.; Sun, Y.-H. Species-specific habitat fragmentation assessment, considering the ecological niche requirements and dispersal capability. Biol. Conserv. 2012, 152, 102–109. [Google Scholar] [CrossRef]
- Song, K.; Gao, B.; Halvarsson, P.; Fang, Y.; Klaus, S.; Jiang, Y.-X.; Swenson, J.E.; Sun, Y.-H.; Höglund, J. Demographic history and divergence of sibling grouse species inferred from whole genome sequencing reveal past effects of climate change. BMC Ecol. Evol. 2021, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wen, C.; Gu, Y.; Wang, H.; Gu, L.; Shi, X.; Zhong, J.; Wei, M.; He, F.; Lu, Z. A bird’s view of new conservation hotspots in China. Biol. Conserv. 2017, 211, 47–55. [Google Scholar] [CrossRef]
- Lei, F.-M.; Qu, Y.-H.; Tang, Q.-Q.; An, S.-C. Priorities for the conservation of avian biodiversity in China based on the distribution patterns of endemic bird genera. Biodivers. Conserv. 2003, 12, 2487–2501. [Google Scholar] [CrossRef]
- Zhang, D.; An, B.; Chen, L.; Sun, Z.; Mao, R.; Zhao, C.; Zhang, L. Camera Trapping Reveals Spatiotemporal Partitioning Patterns and Conservation Implications for Two Sympatric Pheasant Species in the Qilian Mountains, Northwestern China. Animals 2022, 12, 1657. [Google Scholar] [CrossRef]
- Li, D.-H.; Li, C.-Q. Blue-eared Phessant and Blood Pheasant in the Forestry of Qi-Lian Xian, Qinghai. Zool. Res. 1981, 1, 77–82. [Google Scholar]
- Song, K.; Gao, B.; Halvarsson, P.; Fang, Y.; Klaus, S.; Jiang, Y.-X.; Swenson, J.E.; Han, Z.-M.; Sun, Y.-H.; Höglund, J. Conservation genomics of sibling grouse in boreal forests reveals introgression and adaptive population differentiation in genes controlling epigenetic variation. Zool. Res. 2022, 43, 184. [Google Scholar] [CrossRef] [PubMed]
- Johnsgard, P.A. The Pheasants of the World: Biology and Natural History. Hist. Philos. Life Sci. 2002, 23, 547. [Google Scholar]
- Jia, C.-X.; Sun, Y.-H.; Swenson, J.E. Unusual Incubation Behavior and Embryonic Tolerance of Hypothermia by the Blood Pheasant (Ithaginis cruentus). Auk 2010, 127, 926–931. [Google Scholar] [CrossRef]
- Xin, L.; Guangmei, Z.; Binyuan, G. A preliminary investigation on taxonomy, distribution and evolutionary relationship of the eared pheasants, Crossoptilon. Dong Wu Xue Bao 1998, 44, 131–137. [Google Scholar]
- Li, X.; Huang, Y.; Lei, F. Comparative mitochondrial genomics and phylogenetic relationships of the Crossoptilon species (Phasianidae, Galliformes). BMC Genom. 2015, 16, 42. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Xu, J.; Li, J.; Zhang, M.; Wang, Y. Response of Reeves’s Pheasants Distribution to Human Infrastructure in the Dabie Mountains over the Last 20 Years. Animals 2021, 11, 2037. [Google Scholar] [CrossRef]
- Yang, G.-J.; Xiao, D.-N.; Zhao, C.-Z. GIS-based Analysis on the Forest Landscape Patterns in the Qilian Mountain. Arid Zone Res. 2004, 1, 27–32. [Google Scholar]
- Yang, G.; Xiao, D. Forest landscape pattern and fragmentation: A case study on Xishui Natural Reserve in Qilian Mountain. Chin. J. Ecol. 2003, 22, 56–61. [Google Scholar]
- Tang, C.; Zhang, H.; Chen, Y.; Yao, X.; Wang, L.; Xiao, D. Vegetation landscape pattern and its fragmentation on southern slope of Qilian Mountain. Chin. J. Ecol. 2009, 28, 2305–2310. [Google Scholar]
- Fuller, R.A.; Garson, P.J. (Eds.) Pheasants: Status Survey and Conservation Action Plan 2000–2004; IUCN: Gland, Switzerland; Cambridge, UK; The World Pheasant Association: Reading, UK, 2000. [Google Scholar]
- Li, S.; Wang, D.; Xiao, Z.; Li, X.; Wang, T.; Feng, L.; Wang, Y. Camera-trapping in wildlife research and conservation in China: Review and outlook. Biodivers. Sci. 2014, 22, 685–695. [Google Scholar]
- Riitters, K.; Wickham, J.; O’Neill, R.; Jones, B.; Smith, E. Global-scale patterns of forest fragmentation. Conserv. Ecol. 2000, 4, 3. [Google Scholar] [CrossRef]
- Deng, S.-F.; Yang, T.-B.; Zeng, B.; Zhu, X.-F.; Xu, H.-J. Vegetation cover variation in the Qilian Mountains and its response to climate change in 2000–2011. J. Mt. Sci. 2013, 10, 1050–1062. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, C.; Feng, Z. Species distribution models to estimate the deforested area of Picea crassifolia in arid region recently protected: Qilian Mts. National Natural Reserve (China). Pol. J. Ecol. 2012, 60, 515–524. [Google Scholar]
- Rong, Z.; Zhao, C.; Liu, J.; Gao, Y.; Zang, F.; Guo, Z.; Mao, Y.; Wang, L. Modeling the Effect of Climate Change on the Potential Distribution of Qinghai Spruce (Picea crassifolia Kom.) in Qilian Mountains. Forests 2019, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-C.; Peng, M.; Huang, R.-F.; Lu, X.-F. Vegetation Characteristics and Its Distribution of Qilian Mountain Region. Acta Bot. Sin. 1994, 36, 10. [Google Scholar]
- Song, K.; Mi, C.-R.; Zhao, Y.-Z.; Yang, N.; Sun, Y.-H.; Xu, J.-L. Zonation of nature reserve according to the habitat requirement of conservation target: A case study on the endangered Brown Eared-Pheasant at Baihuashan Nature Reserve. Glob. Ecol. Conserv. 2021, 32, e01941. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Chen, X.; Gao, Y.; Xie, S.; Mi, J. GLC_FCS30: Global land-cover product with fine classification system at 30 m using time-series Landsat imagery. Earth Syst. Sci. Data 2021, 13, 2753–2776. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; pp. 655–662. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Coudrat, C.N.; Nekaris, K.A. Modelling Niche Differentiation of Co-Existing, Elusive and Morphologically Similar Species: A Case Study of Four Macaque Species in Nakai-Nam Theun National Protected Area, Laos. Animals 2013, 3, 45–62. [Google Scholar] [CrossRef]
- Meza Mori, G.; Barboza Castillo, E.; Torres Guzman, C.; Cotrina Sanchez, D.A.; Guzman Valqui, B.K.; Oliva, M.; Bandopadhyay, S.; Salas Lopez, R.; Rojas Briceno, N.B. Predictive Modelling of Current and Future Potential Distribution of the Spectacled Bear (Tremarctos ornatus) in Amazonas, Northeast Peru. Animals 2020, 10, 1816. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Carvalheiro, L.G.; Polce, C.; van Loon, E.E.; Raes, N.; Reemer, M.; Biesmeijer, J.C. Fit-for-Purpose: Species Distribution Model Performance Depends on Evaluation Criteria—Dutch Hoverflies as a Case Study. PLoS ONE 2013, 8, e63708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Tang, Y.; Winkler, J.A.; Viña, A.; Liu, J.; Zhang, Y.; Zhang, X.; Li, X.; Wang, F.; Zhang, J.; Zhao, Z.J.P.O. Uncertainty of future projections of species distributions in mountainous regions. PLoS ONE 2018, 13, e0189496. [Google Scholar] [CrossRef] [Green Version]
- Song, K. Habitat Suitability Assessment of the Eastern Population of Brown Eared Pheasant (Crossoptilon mantchuricum) and Its Implication in Conservation. Master’s Thesis, Beijing Forestry University, Beijing, China, 2015. [Google Scholar]
- McGarigal, K. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 1995; Volume 351.
- Wang, W.; Ren, G.; He, Y.; Zhu, J. Habitat Degradation and Conservation Status Assessment of Gallinaceous Birds in the Trans-Himalayas, China. J. Wildl. Manag. 2008, 72, 1335–1341. [Google Scholar] [CrossRef]
- Clark, W.R.; Schmitz, R.A.; Bogenschutz, T.R. Site selection and nest success of ring-necked pheasants as a function of location in Iowa landscapes. J. Wildl. Manag. 1999, 63, 976–989. [Google Scholar] [CrossRef]
- Johnson, M.; Reich, P.; Mac Nally, R. Bird assemblages of a fragmented agricultural landscape and the relative importance of vegetation structure and landscape pattern. Wildl. Res. 2007, 34, 185–193. [Google Scholar] [CrossRef]
- Das, P.; Behera, M.D.; Murthy, M.S.R. Forest fragmentation and human population varies logarithmically along elevation gradient in Hindu Kush Himalaya-utility of geospatial tools and free data set. J. Mt. Sci. 2017, 14, 2432–2447. [Google Scholar] [CrossRef]
- Mohammadi, A.; Almasieh, K.; Nayeri, D.; Ataei, F.; Khani, A.; Lopez-Bao, J.V.; Penteriani, V.; Cushman, S.A. Identifying priority core habitats and corridors for effective conservation of brown bears in Iran. Sci. Rep. 2021, 11, 1044. [Google Scholar] [CrossRef]
- Cushman, S.A.; Landguth, E.L. Ecological Associations, Dispersal Ability, and Landscape Connectivity in the Northern Rocky Mountains; Research Paper RMRS-RP-90; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2012; 21p.
- Elliot, N.B.; Cushman, S.A.; Macdonald, D.W.; Loveridge, A.J. The devil is in the dispersers: Predictions of landscape connectivity change with demography. J. Appl. Ecol. 2014, 51, 1169–1178. [Google Scholar] [CrossRef]
- O’Neill, R.V.; Hunsaker, C.T.; Timmins, S.P.; Jackson, B.L.; Jones, K.B.; Riitters, K.H.; Wickham, J.D. Scale problems in reporting landscape pattern at the regional scale. Landsc. Ecol. 1996, 11, 169–180. [Google Scholar] [CrossRef]
- Li, C.Q.; Li, D.H. Blood Pheasant (Ithaginis cruentus) and Blue Eared Pheasant (Crossoptilon auritum) in Qilian forests district, Qinghai province. Zool. Res. 1981, 2, 80–85. [Google Scholar]
- Jin, X.; Zhang, Y.; Schaepman, M.E.; Clevers, J.; Su, Z.; Cheng, J.; Jiang, J.; van Genderen, J. Impact of Elevation and Aspect on the Spatial Distribution of Vegetation in the Qilian Mountain Area with Remote Sensing Data; International Society for Photogrammetry and Remote Sensing: Nice, France, 2008. [Google Scholar]
- Sun, J.; Liang, E.; Barrio, I.C.; Chen, J.; Wang, J.; Fu, B. Fences undermine biodiversity targets. Science 2021, 374, 269. [Google Scholar] [CrossRef]
- Song, K.; Mi, C.R.; Zhao, Y.Z.; Yang, N.; Sun, Y.H.; Xu, J.L. Modeling habitat factors and suitability for the Brown Eared Pheasant (Crossoption mantchuricum) in Baihuashan National Nature Reserve, Beijing. Chin. J. Zool. 2016, 51, 363–372. [Google Scholar]
- Zhou, C.; Xu, J.; Zhang, Z. Dramatic decline of the Vulnerable Reeves’s pheasant Syrmaticus reevesii, endemic to central China. Oryx 2015, 49, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Mi, C.-R.; Yang, N.; Sun, L.; Sun, Y.-H.; Xu, J.-L. Improve the roles of nature reserves in conservation of endangered pheasant in a highly urbanized region. Sci. Rep. 2020, 10, 17673. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga-Palacios, J.; Zuria, I.; Castellanos, I.; Lara, C.; Sánchez-Rojas, G. What do we know (and need to know) about the role of urban habitats as ecological traps? Systematic review and meta-analysis. Sci. Total Environ. 2021, 780, 146559. [Google Scholar] [CrossRef] [PubMed]


| Type | Variable | Description | Unit | Source |
|---|---|---|---|---|
| Topographic variables | Altitude | m | ASTER_GDEM_30M, From: https://earthexplorer.usgs.gov/ (Accessed on 23 June 2021) | |
| Aspect | ° | |||
| Slope | ° | |||
| Land cover variables | LULC | Land Use and Land Cover | - | GLC_FCS30-2020, From: http://data.casearth.cn/sdo/detail/5d904b7a0887164a5c7fbfa0 (Accessed on 10 July 2021) |
| Euclidean distance factor | Cropland | Distance to croplands | m | |
| Lake | Distance to lakes | m | ||
| River | Distance to rivers | m | River data from https://hydrosheds.org/page/hydrorivers (Accessed on 11 July 2021) | |
| Road | Distance to roads | m | Gaode Map | |
| Building | Distance to buildings | m | GHSL, From: https://ghslsys.jrc.ec.europa.eu/download.php (Accessed on 10 July 2021) |
| Blood Pheasant | Blue Eared Pheasant | |||
|---|---|---|---|---|
| Variable | Rank | Contribution (%) | Rank | Contribution (%) |
| LULC *** | 1 | 63.6 | 1 | 41.3 |
| Discropland | 2 | 9.3 | 2 | 28.1 |
| Disbuildings *** | 3 | 9.1 | 4 | 2.7 |
| Altitude ** | 4 | 7.6 | 3 | 21.6 |
| Disroads *** | 5 | 4.5 | 8 | 0.7 |
| Disriver *** | 6 | 3 | 5 | 2 |
| Slope | 7 | 1.2 | 9 | 0.7 |
| Dislakes *** | 8 | 1.2 | 6 | 1.7 |
| Aspect | 9 | 0.5 | 7 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.-D.; Jia, J.; Song, K.; Bu, C.-L.; Ma, L.-M.; Wang-Jie, G.-S.; Li, Q.-L.; Yin, H.-Q.; Xu, F.-Y.; Ma, D.-F.; et al. Comparative Habitat Divergence and Fragmentation Analysis of Two Sympatric Pheasants in the Qilian Mountains, China. Land 2022, 11, 2104. https://doi.org/10.3390/land11122104
Xie W-D, Jia J, Song K, Bu C-L, Ma L-M, Wang-Jie G-S, Li Q-L, Yin H-Q, Xu F-Y, Ma D-F, et al. Comparative Habitat Divergence and Fragmentation Analysis of Two Sympatric Pheasants in the Qilian Mountains, China. Land. 2022; 11(12):2104. https://doi.org/10.3390/land11122104
Chicago/Turabian StyleXie, Wen-Dong, Jia Jia, Kai Song, Chang-Li Bu, Li-Ming Ma, Ge-Sang Wang-Jie, Quan-Liang Li, Heng-Qing Yin, Feng-Yi Xu, Dui-Fang Ma, and et al. 2022. "Comparative Habitat Divergence and Fragmentation Analysis of Two Sympatric Pheasants in the Qilian Mountains, China" Land 11, no. 12: 2104. https://doi.org/10.3390/land11122104







