Mapping Agricultural Lands: From Conventional to Regenerative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selecting a Functional Form for a Regenerative–Conventional Agricultural Index
2.2. Exploring, Choosing, and Preparaing Regenerative–Conventional Variables
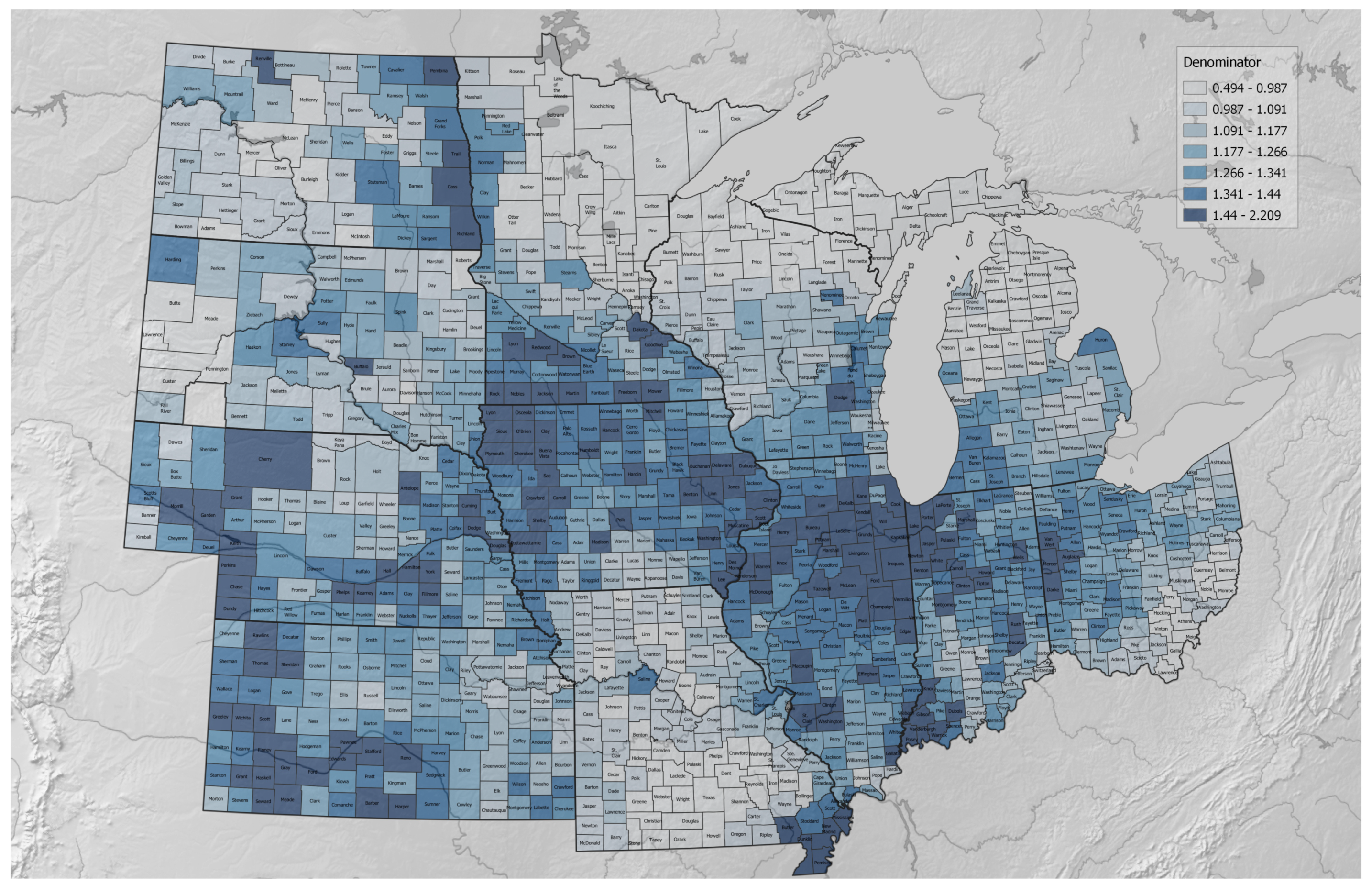
2.3. Formulating the Regenerative–Conventional Agricultural Index Expression
2.4. Clustering Regenerative–Conventional Variables
2.5. Regional Geographies of Conventional and Regenerative Agricultural Lands
3. Results
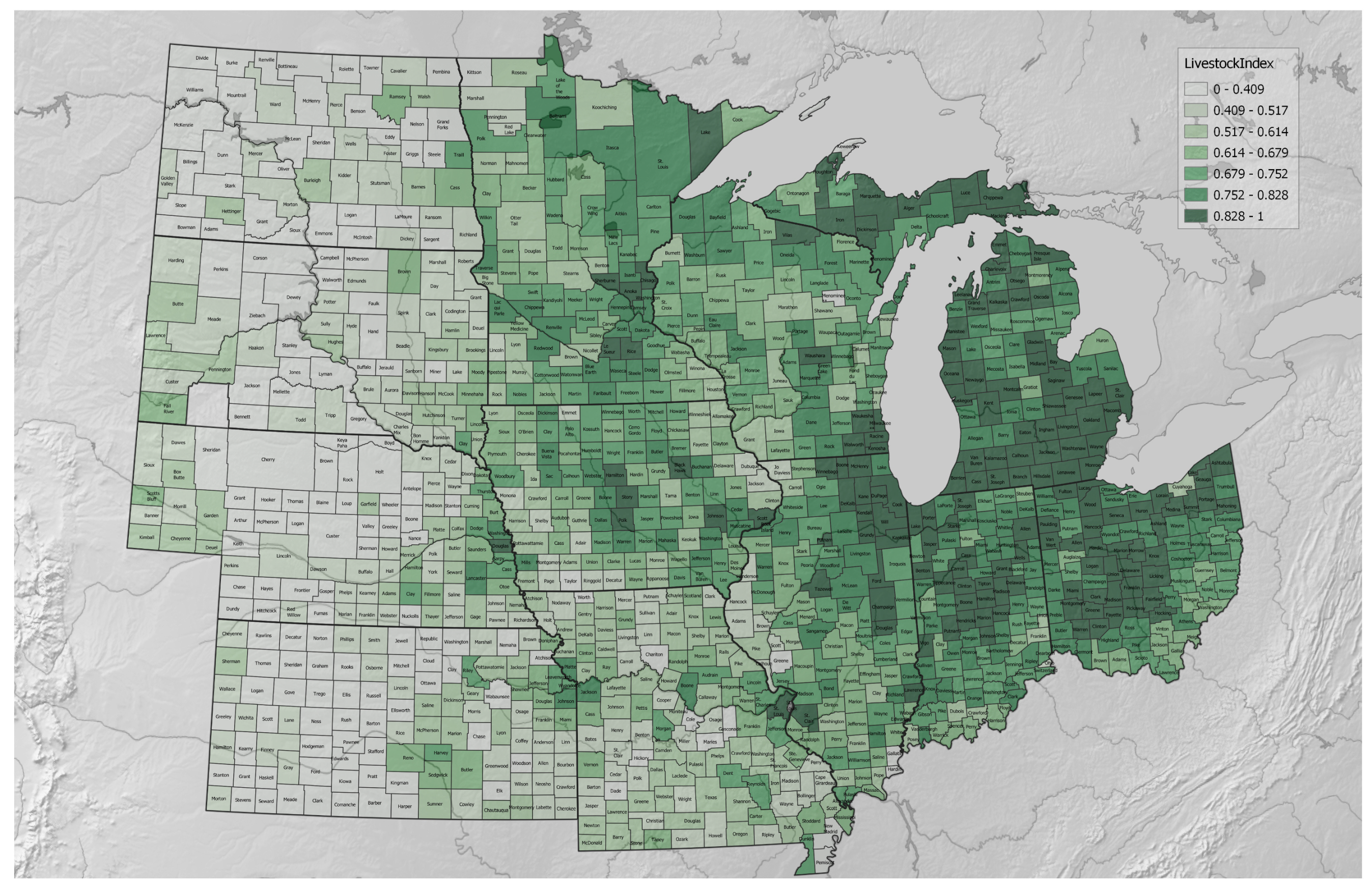
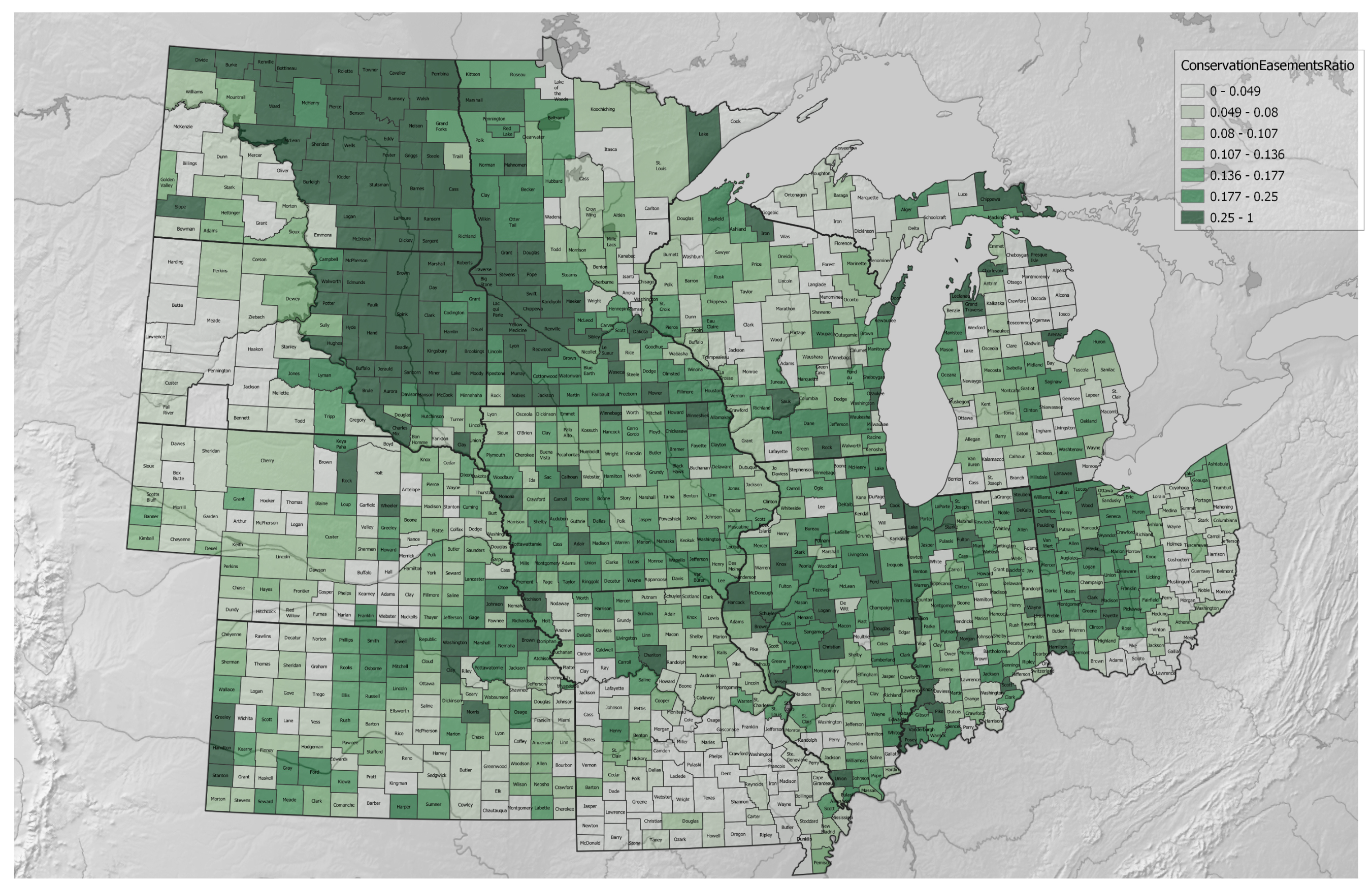
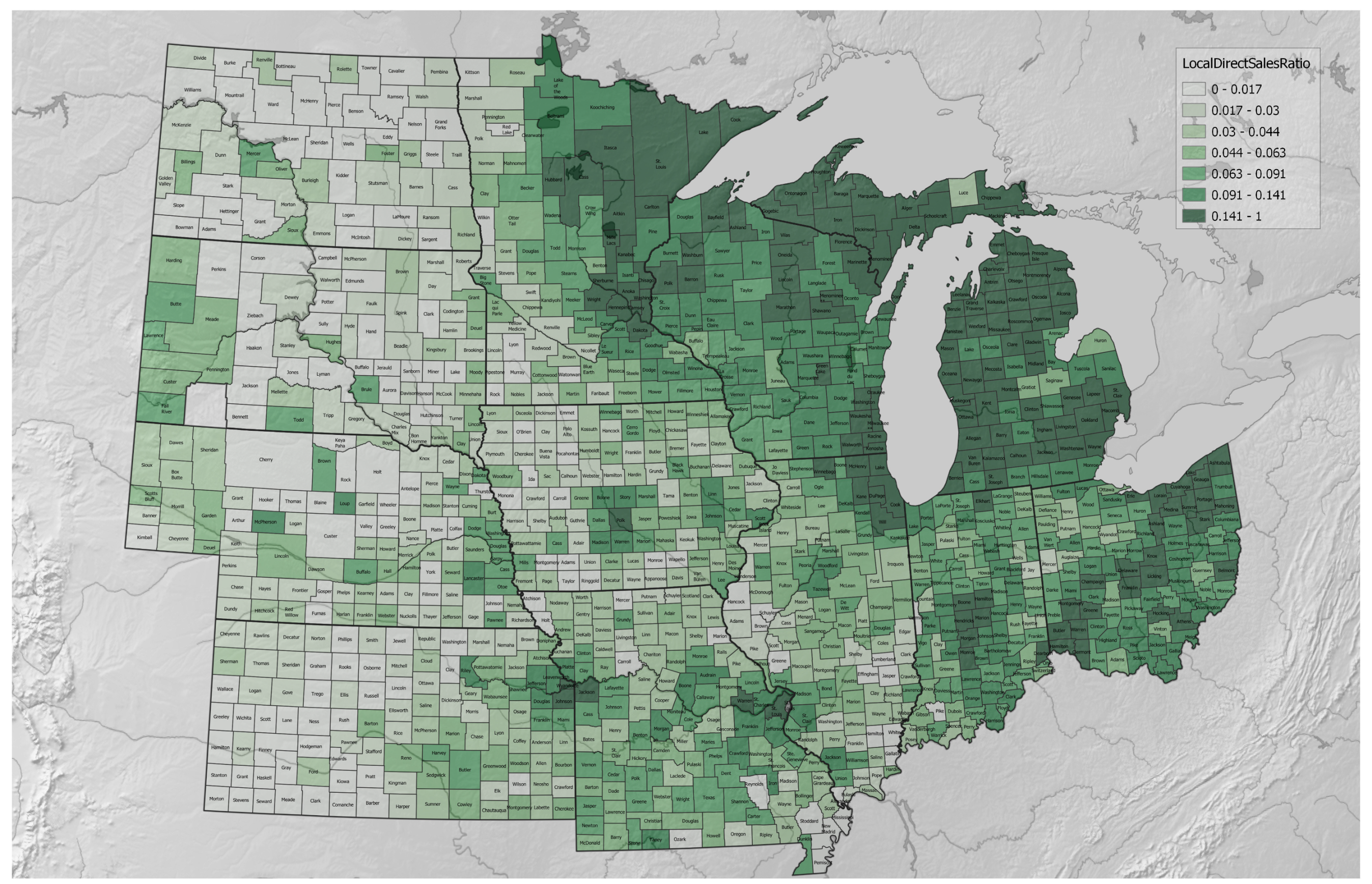
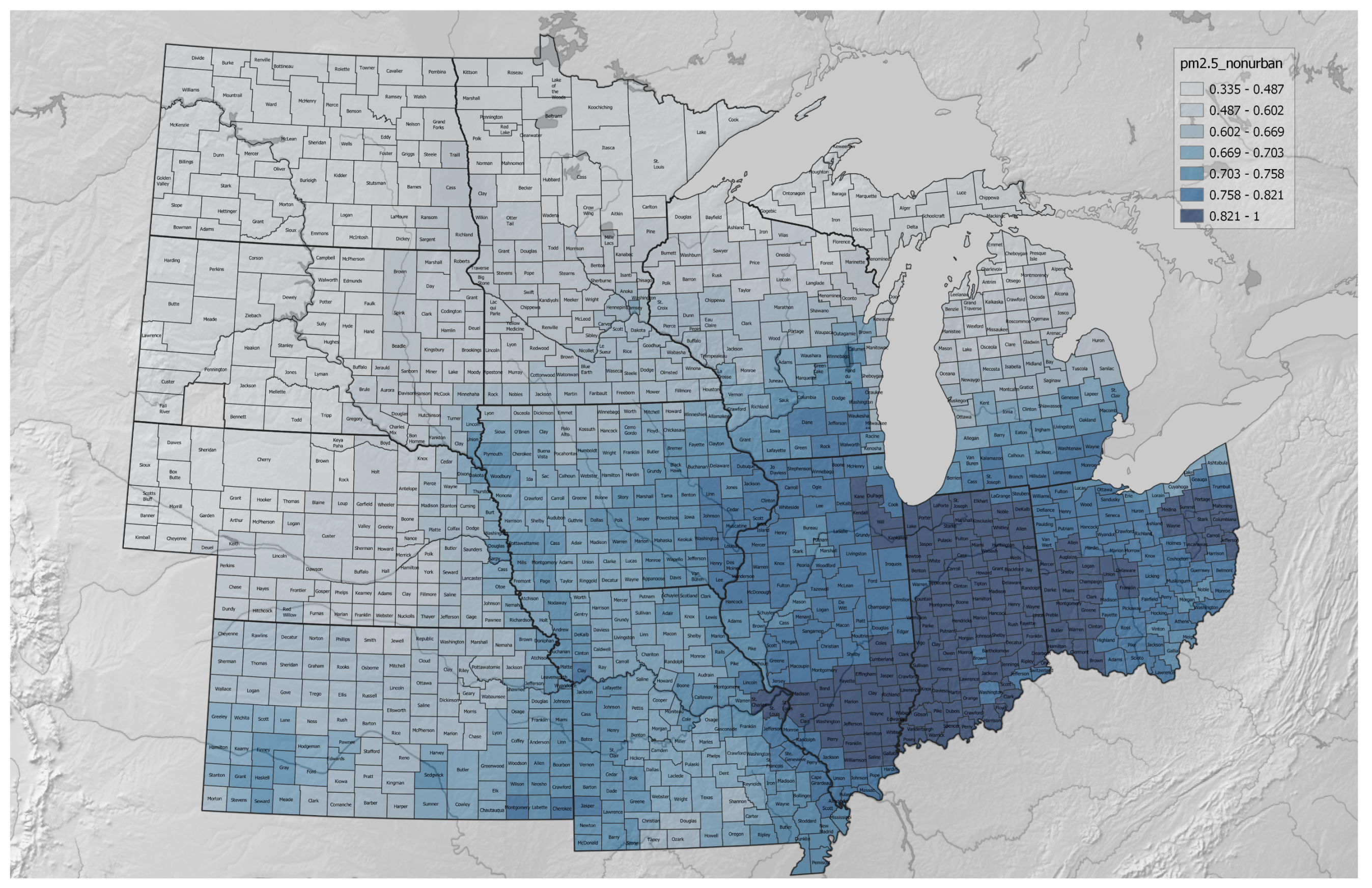
3.1. Agricultural Regenerative–Conventional Variables
3.2. Agricultural Regenerative–Conventional Index
3.2.1. Lands of Conventional Agriculture
3.2.2. Lands of Regenerative Agriculture
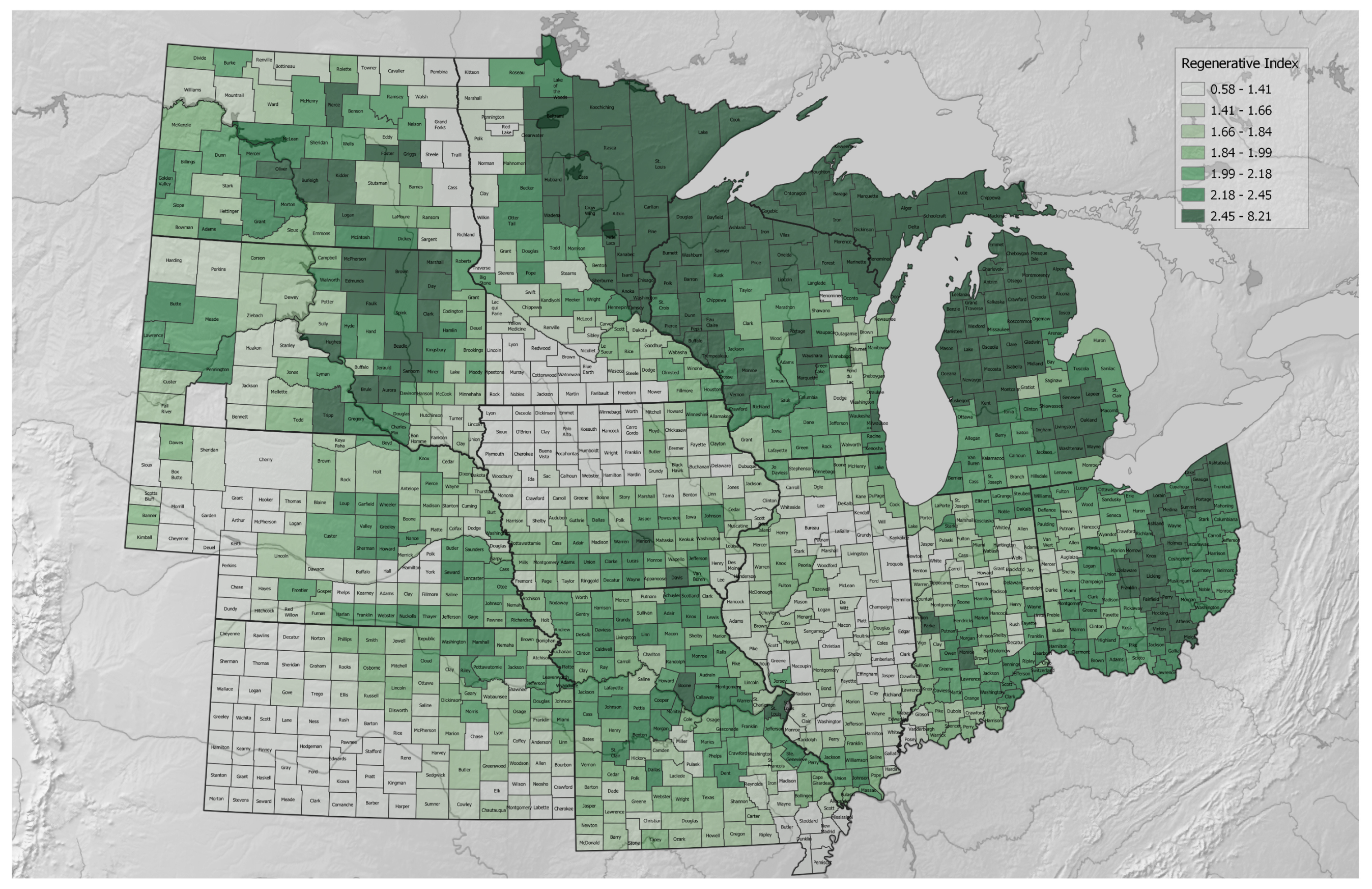
3.2.3. Lands of the Regenerative–Conventional Agricultural Index
3.3. Agricultural Regenerative–Conventional Variable Clustering
4. Discussion
4.1. Co-producing Measurements and Mappings
4.2. Reading Multiple Mappings and Regionalizations
4.3. Revitalizing Regional Agricultural Geographies
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A











References
- Wender, M.J. Goodbye family farms and hello agribusiness: The story of how agricultural policy is destroying the family farm and the environment. Environ. Law J. 2011, 22, 141. [Google Scholar]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, L.F. The Dynamics of Latifundia Formation. PLoS ONE 2013, 8, e82863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, P.H. Concentration and Power in the Food System: Who Controls What We Eat? Bloomsbury Publishing: London, UK, 2021. [Google Scholar]
- Hill, J.; Goodkind, A.; Tessum, C.; Thakrar, S.; Tilman, D.; Polasky, S.; Smith, T.; Hunt, N.; Mullins, K.; Clark, M.; et al. Air-quality-related health damages of maize. Nat. Sustain. 2019, 2, 397–403. [Google Scholar] [CrossRef]
- General Mills. Regenerative Agriculture 2020. Available online: https://www.generalmills.com/en/Responsibility/Sustainability/Regenerative-agriculture (accessed on 14 February 2022).
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture—A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Altieri, M.A. Agroecology: The Science of Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I.; Montalba, R. Technological approaches to sustainable agriculture at a crossroads: An agroecological perspective. Sustainability 2017, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.J. The imperative for regenerative agriculture. Sci. Prog. 2017, 100, 80–129. [Google Scholar] [CrossRef]
- Urrutia, A.L.; González-Gónzalez, C.; Van Cauwelaert, E.M.; Rosell, J.A.; García Barrios, L.; Benítez, M. Landscape heterogeneity of peasant-managed agricultural matrices. Agric. Ecosyst. Environ. 2020, 292, 106797. [Google Scholar] [CrossRef]
- Gebru, H. A review on the comparative advantages of intercropping to mono-cropping system. J. Biol. Agric. Healthc. 2015, 5, 1–13. [Google Scholar]
- Kantola, I.; Masters, M.; DeLucia, E. Soil particulate organic matter increases under perennial bioenergy crop agriculture. Soil Biol. Biochem. 2017, 113, 184–191. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, M. Using cover crops to mitigate and adapt to climate change—A review. Agron. Sustain. Dev. 2017, 37, 4. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Huang, D.; Liu, N.; Zhang, Y.; Badgery, W.; Wang, X.; Shen, Y. Improved grazing management may increase soil carbon sequestration in temperate steppe. Sci. Rep. 2015, 5, 10892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulc, R.M.; Tracy, B.F. Integrated Crop–Livestock Systems in the U.S. Corn Belt. Agron. J. 2007, 99, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Griffon, D.; Hernandez, M.-J. Some theoretical notes on agrobiodiversity: Spatial heterogeneity and population interactions. Agroecol. Sustain. Food Syst. 2020, 44, 795–823. [Google Scholar] [CrossRef]
- Levins, R.; Wilson, M. Ecological Theory and Pest-Management. Annu. Rev. Entomol. 1980, 25, 287–308. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Ecologically based pest management: A key pathway to achieving agroecosystem health. In Managing for Healthy Ecosystems; CRC Press: Boca Raton, FL, USA, 2002; pp. 999–1010. [Google Scholar]
- Borin, M.; Passoni, M.; Thiene, M.; Tempesta, T. Multiple functions of buffer strips in farming areas. Eur. J. Agron. 2010, 32, 103–111. [Google Scholar] [CrossRef]
- Scherr, S.J.; Shames, S.; Friedman, R. From climate-smart agriculture to climate-smart landscapes. Agric. Food Secur. 2012, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Riparian buffer strips: Their role in the conservation of insect pollinators in intensive grassland systems. Agric. Ecosyst. Environ. 2015, 211, 207–220. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodale Institute. Regenerative Organic Agriculture and Climate Change: A Down-to-Earth Solution to Global Warming. 2015. Available online: https://rodaleinstitute.org/wp-content/uploads/rodale-white-paper.pdf (accessed on 14 February 2022).
- Rui, Y.; Jackson, R.D.; Cotrufo, M.F.; Sanford, G.R.; Spiesman, B.J.; Deiss, L.; Culman, S.W.; Liang, C.; Ruark, M.D. Persistent soil carbon enhanced in Mollisols by well-managed grasslands but not annual grain or dairy forage cropping systems. Proc. Natl. Acad. Sci. USA 2022, 119, e2118931119. [Google Scholar] [CrossRef] [PubMed]
- Toensmeier, E. The Carbon Farming Solution; A global toolkit of perennial crops and regenerative agriculture practices for climate change mitigation and food security; Chelsea Green Publishing: Hartford, VT, USA, 2016. [Google Scholar]
- Montgomery, D.R.; Biklé, A.; Archuleta, R.; Brown, P.; Jordan, J. Soil health and nutrient density: Preliminary comparison of regenerative and conventional farming. PeerJ 2022, 10, e12848. [Google Scholar] [CrossRef] [PubMed]
- Perfecto, I.; Vandermeer, J.; Wright, A. Nature’s Matrix: Linking Agriculture, Biodiversity Conservation and Food Sovereignty; Routledge: London, UK, 2019. [Google Scholar]
- Giraldo, O.F. Political Ecology of Agriculture; Springer: Cham, Switzerland, 2019; 150p. [Google Scholar]
- Duncan, J.; Carolan, M.S.; Wiskerke, J.S. Routledge Handbook of Sustainable and Regenerative Food Systems; Routledge: London, UK, 2021. [Google Scholar]
- Longo, P. Food justice and sustainability: A new revolution. Agric. Agric. Sci. Procedia 2016, 8, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Penniman, L. Farming While Black: Soul Fire Farm’s Practical Guide to Liberation on the Land; Chelsea Green Publishing: Hartford, VT, USA, 2018. [Google Scholar]
- Arias, P.F.; Jonas, T.; Munksgaard, K. Farming Democracy: Radically Transforming the Food System from the Ground Up; Australian Food Sovereignty Alliance: Melbourne, VIC, Australia, 2019. [Google Scholar]
- Slocum, R.; Cadieux, K.V. Notes on the practice of food justice in the US: Understanding and confronting trauma and inequity. J. Political Ecol. 2015, 22, 27. [Google Scholar]
- Rotz, S.; Fraser, E.D. Resilience and the industrial food system: Analyzing the impacts of agricultural industrialization on food system vulnerability. J. Environ. Stud. Sci. 2015, 5, 459–473. [Google Scholar] [CrossRef]
- Frison, E.A.; IPES-Food. From Uniformity to Diversity: A Paradigm Shift from Industrial Agriculture to Diversified Agroecological Systems; IPES: Louvain-la-Neuve, Belgium, 2016; 96p. [Google Scholar]
- Burchi, F.; Fanzo, J.; Frison, E. The role of food and nutrition system approaches in tackling hidden hunger. Int. J. Environ. Res. Public Health 2011, 8, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Zenk, S.N.; Schulz, A.J.; Israel, B.A.; James, S.A.; Bao, S.; Wilson, M.L. Fruit and vegetable access differs by community racial composition and socioeconomic position in Detroit, Michigan. Ethn. Dis. 2006, 16, 275–280. [Google Scholar] [PubMed]
- Harden, N.M.; Ashwood, L.L.; Bland, W.L.; Bell, M.M. For the public good: Weaving a multifunctional landscape in the Corn Belt. Agric. Hum. Values 2013, 30, 525–537. [Google Scholar] [CrossRef]
- Low, S.A.; Adalja, A.; Beaulieu, E.; Key, N.; Martinez, S.; Melton, A.; Perez, A.; Ralston, K.; Stewart, H.; Suttles, S.; et al. Trends in US Local and Regional Food Systems: A Report to Congress; Cornell SC Johnson College of Business: Ithaca, NY, USA, 2015. [Google Scholar]
- Rotz, S.; Fraser, E.D.; Martin, R.C. Situating tenure, capital and finance in farmland relations: Implications for stewardship and agroecological health in Ontario, Canada. J. Peasant Stud. 2019, 46, 142–164. [Google Scholar] [CrossRef]
- Chappell, M.J. Beginning to End Hunger: Food and the Environment in Belo Horizonte, Brazil, and Beyond; University of California Press: Berkeley, CA, USA, 2018. [Google Scholar]
- Fenster, T.L.; LaCanne, C.E.; Pecenka, J.R.; Schmid, R.B.; Bredeson, M.M.; Busenitz, K.M.; Michels, A.M.; Welch, K.D.; Lundgren, J.G. Defining and validating regenerative farm systems using a composite of ranked agricultural practices. F1000Research 2021, 10, 115. [Google Scholar] [CrossRef]
- Ludden, M.T.; Welsh, R.; Weissman, E.; Hilchey, D.; Gillespie, G.W.; Guptill, A. The Progressive Agriculture Index: Assessing the Advancement of Agri-food Systems. J. Agric. Food Syst. Community Dev. 2018, 8, 159–185. [Google Scholar] [CrossRef]
- Kuo, H.-J.; Peters, D.J. The socioeconomic geography of organic agriculture in the United States. Agroecol. Sustain. Food Syst. 2017, 41, 1162–1184. [Google Scholar] [CrossRef] [Green Version]
- Jonasson, O. Agricultural Regions of Europe. Econ. Geogr. 1925, 1, 277–314. [Google Scholar] [CrossRef]
- Baker, O.E. Agricultural Regions of North America. Part I. The Basis of Classification. Econ. Geogr. 1926, 2, 459–493. [Google Scholar] [CrossRef]
- Whittlesey, D. Major Agricultural Regions of the Earth. Ann. Assoc. Am. Geogr. 1936, 26, 199–240. [Google Scholar] [CrossRef]
- Sommer, J.E. Diversity in US Agriculture: A New Delineation by Farming Characteristics; US Department of Agriculture, Economic Research Service: Washington, DC, USA, 1991.
- U.S. Department of Agriculture, Economic Research Service. Farm Resource Regions; AIB-760; US Department of Agriculture, Economic Research Service: Washington, DC, USA, 2000. Available online: www.ers.usda.gov/publications/aib760/ (accessed on 11 March 2022).
- United States Department of Agriculture, National Agricultural Statistics Service. 2017 Census of Agriculture Washington DC: United States Department of Agriculture. 2019. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/index.php (accessed on 14 February 2022).
- Sulc, R.M.; Franzluebbers, A.J. Exploring integrated crop–livestock systems in different ecoregions of the United States. Eur. J. Agron. 2014, 57, 21–30. [Google Scholar] [CrossRef]
- Scherr, S.J.; McNeely, J.A. Biodiversity conservation and agricultural sustainability: Towards a new paradigm of ‘ecoagriculture’ landscapes. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 477–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Wardropper, C.B.; Rissman, A.R.; Turner, M.G. Spatial fit between water quality policies and hydrologic ecosystem services in an urbanizing agricultural landscape. Landsc. Ecol. 2017, 32, 59–75. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Archer, D.; Hendrickson, J.; Kronberg, S.; Liebig, M.; Nichols, K.; Schmer, M.; Tanaka, D.; Aguilar, J. Diversification and ecosystem services for conservation agriculture: Outcomes from pastures and integrated crop–livestock systems. Renew. Agric. Food Syst. 2013, 28, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Willis, G.H.; McDowell, L.L. Pesticides in agricultural runoff and their effects on downstream water quality. Environ. Toxicol. Chem. 1982, 1, 267–279. [Google Scholar] [CrossRef]
- Rasmussen, J.J.; Wiberg-Larsen, P.; Baattrup-Pedersen, A.; Monberg, R.J.; Kronvang, B. Impacts of pesticides and natural stressors on leaf litter decomposition in agricultural streams. Sci. Total Environ. 2012, 416, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, A.K. Researches in water pollution: A review. Int. Res. J. Nat. Appl. Sci. 2017, 4, 118–142. [Google Scholar]
- Chaudhry, F.N.; Malik, M. Factors affecting water pollution: A review. J. Ecosyst. Ecography 2017, 7, 1–3. [Google Scholar]
- Shaddick, G.; Thomas, M.; Green, A.; Brauer, M.; Van Donkelaar, A.; Burnett, R.; Chang, H.H.; Cohen, A.; Van Dingenen, R.; Dora, C.; et al. Data integration model for air quality: A hierarchical approach to the global estimation of exposures to ambient air pollution. J. R. Stat. Soc. Ser. C (Appl. Stat.) 2017, 67, 231–253. [Google Scholar] [CrossRef]
- Dewitz, J. National Land Cover Database (NLCD) 2016 Products. In U.S. Geological Survey Data Release; editor. ver. 2.0, July 2020 ed2019; U.S. Geological Survey: Sioux Falls, SD, USA, 2019. [Google Scholar] [CrossRef]
- Domingo, N.G.G.; Balasubramanian, S.; Thakrar, S.K.; Clark, M.A.; Adams, P.J.; Marshall, J.D.; Muller, N.Z.; Pandis, S.N.; Polasky, S.; Robinson, A.L.; et al. Air quality–related health damages of food. Proc. Natl. Acad. Sci. USA 2021, 118, e2013637118. [Google Scholar] [CrossRef]
- Food, Conservation, and Energy Act of 2008, Pub. L. 110-246, 122 Stat. 1929. Available online: https://www.govinfo.gov/content/pkg/PLAW-110publ246/html/PLAW-110publ246.htm (accessed on 5 March 2022).
- Lin, X.; Ruess, P.J.; Marston, L.; Konar, M. Food flows between counties in the United States. Environ. Res. Lett. 2019, 14, 084011. [Google Scholar] [CrossRef]
- Miller, M. Identifying Critical Thresholds for Resilient Regional Food Flows: A Case Study from the U.S. Upper Midwest. Front. Sustain. Food Syst. 2021, 5, 371. [Google Scholar] [CrossRef]
- Maasakkers, J.D.; Jacob, D.J.; Sulprizio, M.P.; Turner, A.; Weitz, M.; Wirth, T.; Hight, C.; Defigueiredo, M.; Desai, M.; Schmeltz, R.; et al. Gridded National Inventory of U.S. Methane Emissions. Environ. Sci. Technol. 2016, 50, 13123–13133. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Harper, M.; Meinen, R.; Day, R.; Lopes, J.; Ott, T.; Venkatesh, A.; Randles, C.A. Discrepancies and Uncertainties in Bottom-up Gridded Inventories of Livestock Methane Emissions for the Contiguous United States. Environ. Sci. Technol. 2017, 51, 13668–13677. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K. Data clustering: 50 years beyond K-means. Pattern Recognit. Lett. 2010, 31, 651–666. [Google Scholar] [CrossRef]
- Kazakov, E. Attribute Based Clustering 2019. Available online: https://github.com/eduard-kazakov/attributeBasedClustering (accessed on 5 March 2022).
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunge, W. Locations are not unique. Ann. Assoc. Am. Geogr. 1966, 56, 375–376. [Google Scholar] [CrossRef]
- Hartshorne, R. Perspective on the Nature of Geography; Association of American Geographers: Philadelphia, PA, USA, 1959; 201p. [Google Scholar]
- United States Department of Agriculture, National Agricultural Statistics Service. Corn: Production Acreage by County—2019; United States Department of Agriculture National Agricultural Statistics Service: Washington, DC, USA, 2020. Available online: https://www.nass.usda.gov/Charts_and_Maps/Crops_County/cr-pr.php (accessed on 5 March 2022).
- Anand, S.; Sen, A. Human Development Index: Methodology and Measurement; Human Development Report Office: New York, NY, USA, 1994; 19p. [Google Scholar]
- Malczewski, J.; Rinner, C. Multicriteria Decision Analysis in Geographic Information Science; Springer: New York, NY, USA, 2015; 331p. [Google Scholar]
- Lobell, D.B. Remote Sensing of Soil Degradation: Introduction. J. Environ. Qual. 2010, 39, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cécillon, L.; Barthès, B.G.; Gomez, C.; Ertlen, D.; Genot, V.; Hedde, M.; Stevens, A.; Brun, J.J. Assessment and monitoring of soil quality using near-infrared reflectance spectroscopy (NIRS). Eur. J. Soil Sci. 2009, 60, 770–784. [Google Scholar] [CrossRef] [Green Version]
- Paz-Kagan, T.; Zaady, E.; Salbach, C.; Schmidt, A.; Lausch, A.; Zacharias, S.; Notesco, G.; Ben-Dor, E.; Karnieli, A. Mapping the Spectral Soil Quality Index (SSQI) Using Airborne Imaging Spectroscopy. Remote Sens. 2015, 7, 15748–15781. [Google Scholar] [CrossRef] [Green Version]
- De Paul Obade, V.; Lal, R. Assessing land cover and soil quality by remote sensing and geographical information systems (GIS). Catena 2013, 104, 77–92. [Google Scholar] [CrossRef]
- Roth, J. County Distance Database: National Bureau of Economic Research. 2014. Available online: https://data.nber.org/data/county-distance-database.html (accessed on 15 February 2022).
- United States Census Bureau. 2017 TIGER/Line Shapefiles. Available online: https://www.census.gov/geographies/mapping-files/time-series/geo/tiger-line-file.html (accessed on 15 February 2022).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergmann, L.; Chaves, L.F.; Betz, C.R.; Stein, S.; Wiedenfeld, B.; Wolf, A.; Wallace, R.G. Mapping Agricultural Lands: From Conventional to Regenerative. Land 2022, 11, 437. https://doi.org/10.3390/land11030437
Bergmann L, Chaves LF, Betz CR, Stein S, Wiedenfeld B, Wolf A, Wallace RG. Mapping Agricultural Lands: From Conventional to Regenerative. Land. 2022; 11(3):437. https://doi.org/10.3390/land11030437
Chicago/Turabian StyleBergmann, Luke, Luis Fernando Chaves, Carolyn R. Betz, Serena Stein, Brian Wiedenfeld, Ann Wolf, and Robert G. Wallace. 2022. "Mapping Agricultural Lands: From Conventional to Regenerative" Land 11, no. 3: 437. https://doi.org/10.3390/land11030437
APA StyleBergmann, L., Chaves, L. F., Betz, C. R., Stein, S., Wiedenfeld, B., Wolf, A., & Wallace, R. G. (2022). Mapping Agricultural Lands: From Conventional to Regenerative. Land, 11(3), 437. https://doi.org/10.3390/land11030437






