Effect of Gated Weir Opening on the Topography and Zooplankton Community of Geum River, South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Topographic Structure Analysis
2.3. Monitoring Strategy
2.4. Data Analysis
3. Results
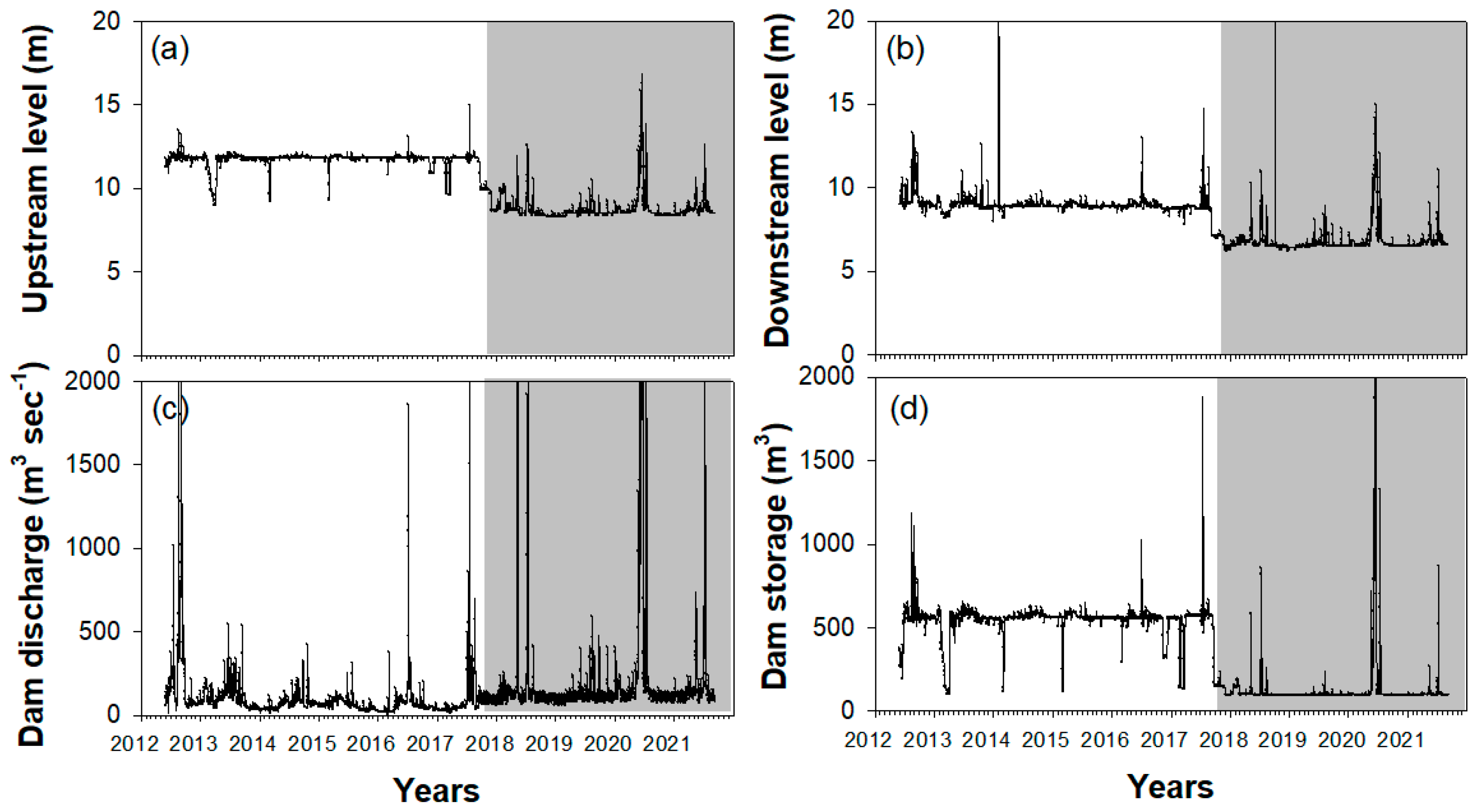
3.1. Hydrological Factors and Environmental Variables
3.2. Topographic Structure
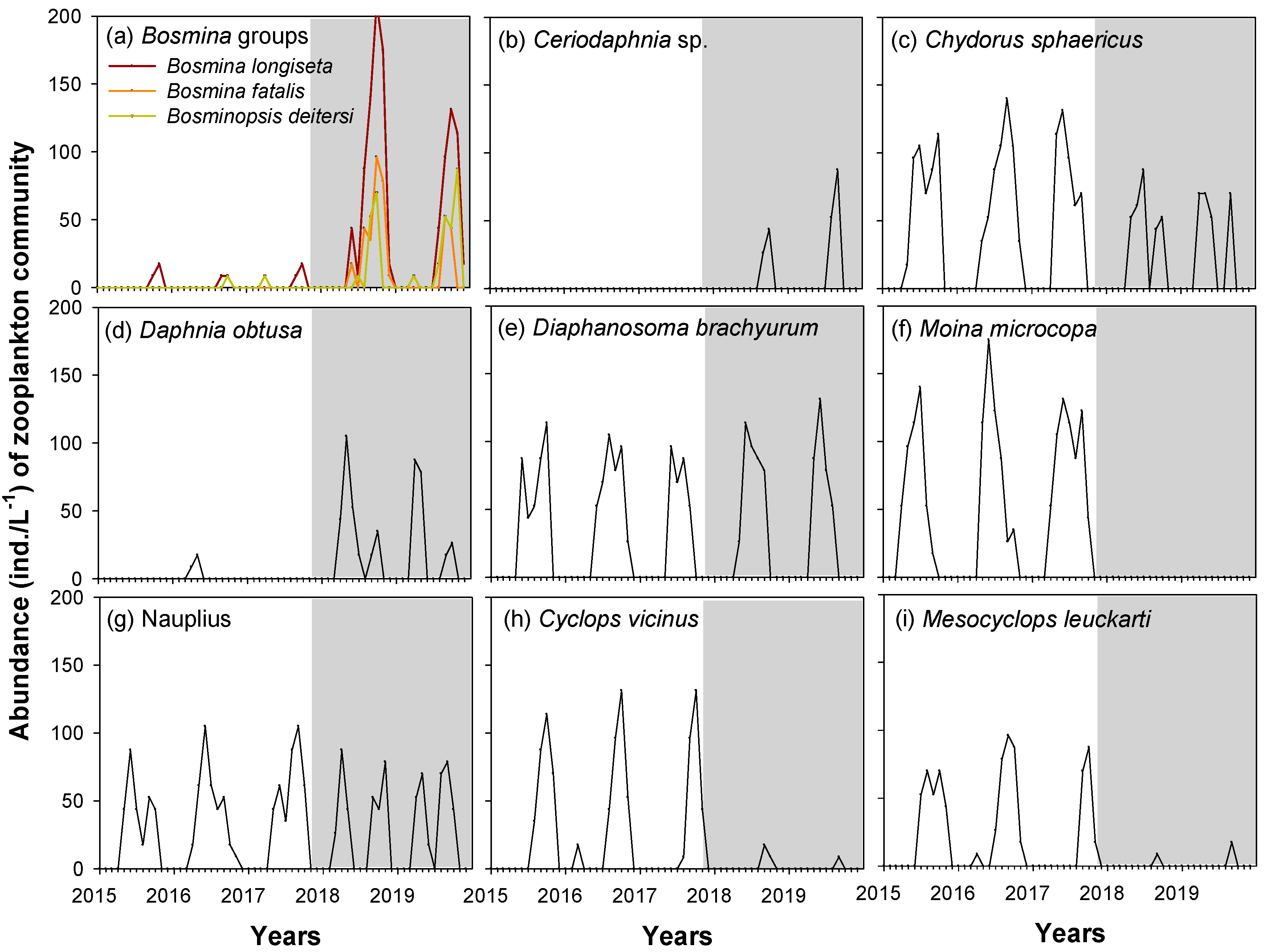
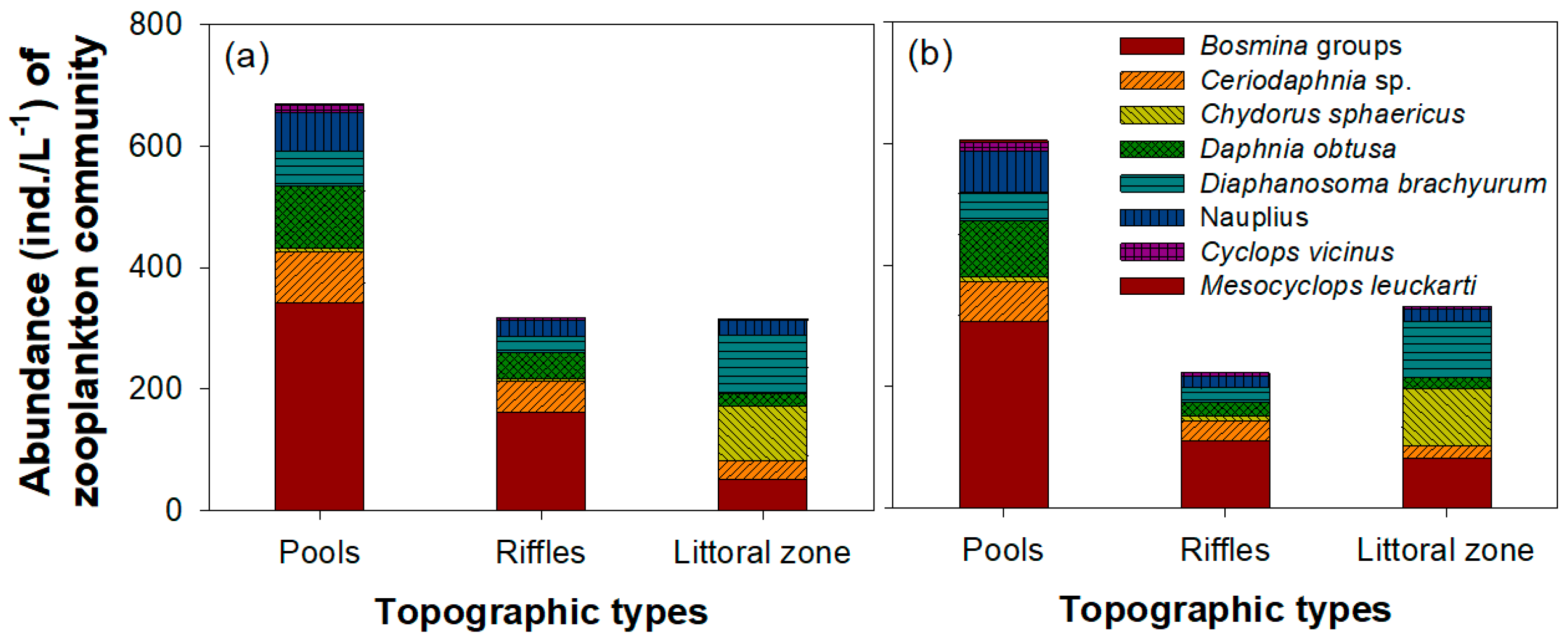
3.3. Zooplankton Community Composition
4. Discussion
4.1. Environmental Variables and Topographic Changes after Weir Opening
4.2. Change of Zooplankton Community Composition after Weir Opening
4.3. Weir Management for River Ecosystem Conservation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, N.; Qu, Y.; Guse, B.; Makarevičiūtė, K.; To, S.; Riis, T.; Fohrer, N. Hydrological and environmental variables outperform spatial factors in structuring species, trait composition, and beta diversity of pelagic algae. Ecol. Evol. 2018, 8, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dong, X.; Chen, Y.; Yang, X.; Xu, M.; Davidson, T.A.; Jeppesen, E. Hydrological alterations as the major driver on environmental change in a floodplain Lake Poyang (China): Evidence from monitoring and sediment records. J. Great Lakes Res. 2018, 44, 377–387. [Google Scholar] [CrossRef]
- Harvey, J.; Gomez-Velez, J.; Schmadel, N.; Scott, D.; Boyer, E.; Alexander, R.; Eng, K.; Golden, H.; Kettner, A.; Konrad, C.; et al. How hydrologic connectivity regulates water quality in river corridors. J. Am. Water Res. Assoc. 2019, 55, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Knösche, R. Organic sediment nutrient concentrations and their relationship with the hydrological connectivity of floodplain waters (River Havel, NE Germany). Hydrobiologia 2006, 560, 63–76. [Google Scholar] [CrossRef]
- Bowling, L.C.; Merrick, C.; Swann, J.; Green, D.; Smith, G.; Neilan, B.A. Effects of hydrology and river management on the distribution, abundance and persistence of cyanobacterial blooms in the Murray River, Australia. Harmful Algae 2013, 30, 27–36. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Saito, Y.; Liu, J.P.; Sun, X. Interannual and seasonal variation of the Huanghe (Yellow River) water discharge over the past 50 years: Connections to impacts from ENSO events and dams. Glob. Planet. Chang 2006, 50, 212–225. [Google Scholar] [CrossRef]
- Jeong, K.S.; Kim, D.K.; Joo, G.J. Delayed influence of dam storage and discharge on the determination of seasonal proliferations of Microcystis aeruginosa and Stephanodiscus hantzschii in a regulated river system of the lower Nakdong River (South Korea). Water Res. 2007, 41, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.M.; Lee, S.; Kang, T. Evaluation of dams and weirs opening for water resource management of the Geum River. Sci. Total Environ. 2014, 478, 103–115. [Google Scholar] [CrossRef]
- Woo, H. Trends in ecological river engineering in Korea. J. Hydro-Environ. Res. 2010, 4, 269–278. [Google Scholar] [CrossRef]
- Gubiani, E.A.; Gomes, L.C.; Agostinho, A.A.; Okada, E.K. Persistence of fish populations in the upper Paraná River: Effects of water regulation by dams. Ecol. Freshw. Fish 2007, 16, 191–197. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.S.; Kim, G. Nutrient input from submarine groundwater discharge versus intermittent river-water discharge through an artificial dam in the Yeongsan River estuary, Korea. Ocean Sci. J. 2010, 45, 179–186. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Rajwar, G.S. Physico-chemical and microbiological study of Tehri dam reservoir, Garhwal Himalaya, India. J. Am. Sci. 2010, 6, 65–71. [Google Scholar]
- Grabowska, M. The role of a eutrophic lowland reservoir in shaping the composition of river phytoplankton. Ecohydrol. Hydrobiol. 2012, 12, 231–242. [Google Scholar] [CrossRef]
- Shamloo, H.; Rajaratnam, N.; Katopodis, C. Hydraulics of simple habitat structures. J. Hydraul. Res. 2001, 39, 351–366. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Zhu, L.; Liu, W.; Han, J.; Yang, Y. Influence of large reservoir operation on water-levels and flows in reaches below dam: Case study of the Three Gorges Reservoir. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Feurer, D.; Bailly, J.S.; Puech, C.; Le Coarer, Y.; Viau, A.A. Very-high-resolution mapping of river-immersed topography by remote sensing. Prog. Phys. Geogr. 2008, 32, 403–419. [Google Scholar] [CrossRef]
- Mermillod-Blondin, F.; Creuzé des Châtelliers, M.; Marmonier, P.; Dole-Olivier, M.J. Distribution of solutes, microbes and invertebrates in river sediments along a riffle-pool-riffle sequence. Freshw. Biol. 2000, 44, 255–269. [Google Scholar] [CrossRef]
- Walker, K.F. A review of the ecological effects of river regulation in Australia. In Perspectives in Southern Hemisphere Limnology; Davies, B.R., Walmsley, R.D., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1985; pp. 111–129. [Google Scholar]
- Mulani, S.K.; Mule, M.B.; Patil, S.U. Studies on water quality and zooplankton community of the Panchganga river in Kolhapur city. J. Environ. Biol. 2009, 30, 455–459. [Google Scholar]
- Choi, J.Y.; Jeong, K.S.; Kim, H.W.; Chang, K.H.; Joo, G.J. Inter-annual variability of a zooplankton community: The importance of summer concentrated rainfall in a regulated river ecosystem. J. Ecol. Environ. 2011, 34, 49–58. [Google Scholar] [CrossRef][Green Version]
- Choi, J.Y.; Kim, J.C.; Kim, S.K. Changing distributions of zooplankton communities in a coastal lagoon in response to rainfall seasonality. J. Coast. Res. 2020, 102, 69–74. [Google Scholar] [CrossRef]
- Neschuk, N.; Claps, M.; Gabellone, N. Planktonic rotifers of a saline-lowland river: The Salado River (Argentina). Ann. Limnol.-Int. J. Limnol. 2002, 38, 191–198. [Google Scholar] [CrossRef]
- Maia-Barbosa, P.M.; Peixoto, R.S.; Guimarães, A.S. Zooplankton in littoral waters of a tropical lake: A revisited biodiversity. Braz. J. Biol. 2008, 68, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, S.K. Responses of rotifer community to microhabitat changes caused by summer-concentrated rainfall in a shallow reservoir, South Korea. Diversity 2020, 12, 113. [Google Scholar] [CrossRef]
- Tekile, A.; Kim, I.; Kim, J. Mini-review on river eutrophication and bottom improvement techniques, with special emphasis on the Nakdong River. J. Environ. Sci. 2015, 30, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Manca, M.; De Bernardi, R. Eutrophication-like response to climate warming: An analysis of Lago Maggiore (N. Italy) zooplankton in contrasting years. J. Limnol. 2008, 67, 87. [Google Scholar] [CrossRef]
- Yoshida, T.; Urabe, J.; Elser, J.J. Assessment of ‘top-down’and ‘bottom-up’forces as determinants of rotifer distribution among lakes in Ontario, Canada. Ecol. Res. 2003, 18, 639–650. [Google Scholar] [CrossRef]
- Du, X.; García-Berthou, E.; Wang, Q.; Liu, J.; Zhang, T.; Li, Z. Analyzing the importance of top-down and bottom-up controls in food webs of Chinese lakes through structural equation modeling. Aquat. Ecol. 2015, 49, 199–210. [Google Scholar] [CrossRef]
- Sommer, U.; Sommer, F. Cladocerans versus copepods: The cause of contrasting top–down controls on freshwater and marine phytoplankton. Oecologia 2006, 147, 183–194. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeong, K.S.; Kim, S.K.; La, G.H.; Chang, K.H.; Joo, G.J. Role of macrophytes as microhabitats for zooplankton community in lentic freshwater ecosystems of South Korea. Ecol. Inform. 2014, 24, 177–185. [Google Scholar] [CrossRef]
- Warfe, D.M.; Barmuta, L.A. Habitat structural complexity mediates food web dynamics in a freshwater macrophyte community. Oecologia 2006, 150, 141–154. [Google Scholar] [CrossRef]
- Carmignani, J.R.; Roy, A.H. Annual winter water-level drawdowns influence physical habitat structure and macrophytes in Massachusetts, USA, lakes. Ecosphere 2021, 12, e03442. [Google Scholar] [CrossRef]
- Musil, J.; Horký, P.; Slavík, O.; Zbořil, A.; Horká, P. The response of the young of the year fish to river obstacles: Functional and numerical linkages between dams, weirs, fish habitat guilds and biotic integrity across large spatial scale. Ecol. Indic. 2012, 23, 634–640. [Google Scholar] [CrossRef]
- Mbaka, J.G.; Wanjiru Mwaniki, M. A global review of the downstream effects of small impoundments on stream habitat conditions and macroinvertebrates. Environ. Rev. 2015, 23, 257–262. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K.; Kim, J.C.; Yun, J.H. Effect of microhabitat structure on the distribution of an endangered fish, Coreoperca kawamebari (Temminck & Schlegel, 1843) in the Geum River, South Korea. Water 2020, 12, 1690. [Google Scholar]
- Kobayashi, S.; Nakanishi, S.; Akamatsu, F.; Yajima, Y.; Amano, K. Differences in amounts of pools and riffles between upper and lower reaches of a fully sedimented dam in a mountain gravel-bed river. Landsc. Ecol. Eng. 2012, 8, 145–155. [Google Scholar] [CrossRef]
- Johansen, K.; Phinn, S.; Witte, C. Mapping of riparian zone attributes using discrete return LiDAR, QuickBird and SPOT-5 imagery: Assessing accuracy and costs. Remote Sens. Environ. 2010, 114, 2679–2691. [Google Scholar] [CrossRef]
- Holmes, K.L.; Goebel, P.C. A functional approach to riparian area delineation using geospatial methods. J. For. 2011, 109, 233–241. [Google Scholar]
- Wetzel, R.G.; Likens, G.E. Limnological Analyses; Springer: New York, NY, USA, 2000; p. 429. [Google Scholar]
- Haney, J.F.; Hall, D.J. Sugar-coated Daphnia: A preservation technique for Cladocera 1. Limnol. Oceanogr. 1973, 18, 331–333. [Google Scholar] [CrossRef]
- Mizuno, T.; Takahashi, E. An Illustrated Guide to Freshwater Zooplankton in Japan; Tokai University Press: Tokyo, Japan, 1999; p. 532. [Google Scholar]
- Thorp, J.H.; Covich, A.P. Ecology and Classification of North American Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Choi, J.Y.; Jeong, K.S.; Joo, G.J. Rainfall as dominant driver of rotifer dynamics in shallow wetlands: Evidence from a long-term data record (Upo Wetlands, South Korea). Int. Rev. Hydrobiol. 2015, 100, 21–33. [Google Scholar] [CrossRef]
- Miskewitz, R.; Uchrin, C. In-stream dissolved oxygen impacts and sediment oxygen demand resulting from combined sewer overflow discharges. J. Environ. Eng. 2013, 139, 1307–1313. [Google Scholar] [CrossRef]
- Null, S.E.; Mouzon, N.R.; Elmore, L.R. Dissolved oxygen, stream temperature, and fish habitat response to environmental water purchases. J. Environ. Manag. 2017, 197, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Connolly, N.M.; Crossland, M.R.; Pearson, R.G. Effect of low dissolved oxygen on survival, emergence, and drift of tropical stream macroinvertebrates. J. N. Am. Benthol. Soc. 2004, 23, 251–270. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Sheng, H.; Dong, F.; Zou, R.; Zhao, L.; Guo, H.; Zhu, X.; He, B. Quantitative evaluation of lake eutrophication responses under alternative water diversion scenarios: A water quality modeling based statistical analysis approach. Sci. Total Environ. 2014, 468, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, H.L.; Read, D.S.; McGowan, S.; Wagner, M.; Roberts, C.; Armstrong, L.K.; Nicholls, D.J.E.; Wickham, H.D.; Hutchins, M.G.; Bowes, M.J. Characterisation of a major phytoplankton bloom in the River Thames (UK) using flow cytometry and high performance liquid chromatography. Sci. Total Environ. 2018, 624, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.T.; Stanley, J.K.; Doyle, R.D.; Forbes, M.G.; Brooks, B.W. River–reservoir transition zones are nitrogen fixation hot spots regardless of ecosystem trophic state. Hydrobiologia 2009, 625, 61–68. [Google Scholar] [CrossRef]
- Sabater, S.; Artigas, J.; Durán, C.; Pardos, M.; Romaní, A.M.; Tornés, E.; Ylla, I. Longitudinal development of chlorophyll and phytoplankton assemblages in a regulated large river (the Ebro River). Sci. Total Environ. 2008, 404, 196–206. [Google Scholar] [CrossRef]
- Buffington, J.M.; Lisle, T.E.; Woodsmith, R.D.; Hilton, S. Controls on the size and occurrence of pools in coarse-grained forest rivers. River Res. Appl. 2002, 18, 507–531. [Google Scholar] [CrossRef]
- Malard, F.; Tockner, K.; Dole-Olivier, M.J.; Ward, J.V. A landscape perspective of surface–subsurface hydrological exchanges in river corridors. Freshw. Biol. 2002, 47, 621–640. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, H.K.; Lee, M.H.; Kim, Y.J.; Lee, H.; Kim, B.H. The effect of monsoon rainfall patterns on epilithic diatom communities in the Hantangang River, Korea. Water 2020, 12, 1471. [Google Scholar] [CrossRef]
- Zahar, Y.; Ghorbel, A.; Albergel, J. Impacts of large dams on downstream flow conditions of rivers: Aggradation and reduction of the Medjerda channel capacity downstream of the Sidi Salem dam (Tunisia). J. Hydrol. 2008, 351, 318–330. [Google Scholar] [CrossRef]
- Leigh, C.; Watkinson, A.; Burford, M.A. Effects of extreme inflows on the water quality and phytoplankton of seven reservoirs in subtropical Australia. Inland Waters 2015, 5, 240–252. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, S. A survey of zooplankton diversity of Challawa River, Kano and evaluation of some of its physico-chemical conditions. Bayero J. Pure Appl. Sci. 2009, 2, 19–26. [Google Scholar] [CrossRef][Green Version]
- Jafari, N.; Nabavi, M.S.; Akhavan, M. Ecological investigation of zooplankton abundance in the river Haraz, northeast Iran: Impact of environmental variables. Arch. Biol. Sci. 2011, 63, 785–798. [Google Scholar] [CrossRef]
- Tavernini, S.; Pierobon, E.; Viaroli, P. Physical factors and dissolved reactive silica affect phytoplankton community structure and dynamics in a lowland eutrophic river (Po river, Italy). Hydrobiologia 2011, 669, 213–225. [Google Scholar] [CrossRef]
- Thorp, J.H.; Black, A.R.; Haag, K.H.; Wehr, J.D. Zooplankton assemblages in the Ohio River: Seasonal, tributary, and navigation dam effects. Can. J. Fish. Aquat. Sci. 1994, 51, 1634–1643. [Google Scholar] [CrossRef]
- Banerjee, A.; Chakrabarty, M.; Rakshit, N.; Bhowmick, A.R.; Ray, S. Environmental factors as indicators of dissolved oxygen concentration and zooplankton abundance: Deep learning versus traditional regression approach. Ecol. Indic. 2019, 100, 99–117. [Google Scholar] [CrossRef]
- Röpke, C.P.; Ferreira, E.; Zuanon, J. Seasonal changes in the use of feeding resources by fish in stands of aquatic macrophytes in an Amazonian floodplain, Brazil. Environ. Biol. Fishes 2014, 97, 401–414. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K.; Jeong, K.S.; Joo, G.J. Distribution pattern of epiphytic microcrustaceans in relation to different macrophyte microhabitats in a shallow wetland (Upo wetlands, South Korea). Oceanol. Hydrobiol. Stud. 2015, 44, 151–163. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeong, K.S.; La, G.H.; Kim, S.K.; Joo, G.J. Sustainment of epiphytic microinvertebrate assemblage in relation with different aquatic plant microhabitats in freshwater wetlands (South Korea). J. Limnol. 2014, 73, 1. [Google Scholar] [CrossRef]
- Cazzanelli, M.; Warming, T.P.; Christoffersen, K.S. Emergent and floating-leaved macrophytes as refuge for zooplankton in a eutrophic temperate lake without submerged vegetation. Hydrobiologia 2008, 605, 113–122. [Google Scholar] [CrossRef]
- Richardson, W.B. Microcrustacea in flowing water: Experimental analysis of washout times and a field test. Freshw. Biol. 1992, 28, 217–230. [Google Scholar] [CrossRef]
- Geraldes, A.M.; Boavida, M.J. Do littoral macrophytes influence crustacean zooplankton distribution? Limnetics 2004, 23, 57–64. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeong, K.S.; Kim, S.K.; Son, S.H.; Joo, G.J. Distribution and attachment characteristics of Sida crystallina (OF Müller, 1776) in lentic freshwater ecosystems of South Korea. J. Ecol. Environ. 2016, 40, 1–10. [Google Scholar] [CrossRef]
- Edwards, W.J.; Rehmann, C.R.; McDonald, E.; Culver, D.A. The impact of a benthic filter feeder: Limitations imposed by physical transport of algae to the benthos. Can. J. Fish. Aquat. Sci. 2005, 62, 205–214. [Google Scholar] [CrossRef]
- Van Donk, E.; van de Bund, W.J. Impact of submerged macrophytes including charophytes on phyto-and zooplankton communities: Allelopathy versus other mechanisms. Aquat. Bot. 2002, 72, 261–274. [Google Scholar] [CrossRef]
- Keckeis, S.; Baranyi, C.; Hein, T.; Holarek, C.; Riedler, P.; Schiemer, F. The significance of zooplankton grazing in a floodplain system of the River Danube. J. Plankton Res. 2003, 25, 243–253. [Google Scholar] [CrossRef]
- Serafim-Júnior, M.; Lansac-Tôha, F.A.; Lopes, R.M.; Perbiche-Neves, G. Continuity effects on rotifers and microcrustaceans caused by the construction of a downstream reservoir in a cascade series (Iguaçu River, Brazil). Braz. J. Biol. 2016, 76, 279–291. [Google Scholar] [CrossRef]
- Chiu, M.C.; Yeh, C.H.; Sun, Y.H.; Kuo, M.H. Short-term effects of dam removal on macroinvertebrates in a Taiwan stream. Aquat. Ecol. 2013, 47, 245–252. [Google Scholar] [CrossRef]
- Orr, C.H.; Kroiss, S.J.; Rogers, K.L.; Stanley, E.H. Downstream benthic responses to small dam removal in a coldwater stream. River Res. Appl. 2008, 24, 804–822. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, T.; Wu, N.; Fu, X.; Cai, Q. Impacts of a small dam on riverine zooplankton. Int. Rev. Hydrobiol. 2008, 93, 297–311. [Google Scholar] [CrossRef]
- Molisani, M.M.; de Sousa Barroso, H.; Becker, H.; Moreira, M.O.P.; Hijo, C.A.G.; do Monte, T.M.; Vasconcellos, G.H. Trophic state, phytoplankton assemblages and limnological diagnosis of the Castanhão Reservoir, CE, Brazil. Acta Limnol. Bras. 2010, 22, 1–12. [Google Scholar] [CrossRef]
- Xu, D.; Xia, Y.; Li, Z.; Gu, Y.; Lou, C.; Wang, H.; Han, J. The influence of flow rates and water depth gradients on the growth process of submerged macrophytes and the biomass composition of the phytoplankton assemblage in eutrophic water: An analysis based on submerged macrophytes photosynthesis parameters. Environ. Sci. Pollut. Res. 2020, 27, 31477–31488. [Google Scholar] [CrossRef] [PubMed]
- Rennella, A.M.; Geronazzo, M.D.; Romero, M.E.; Boveri, M.; Rosso, J.J. Hydrological variability, zooplankton availability and the shift between planktivore-benthivore feeding behaviour in the visual predator fish, Odontesthes bonariensis. Environ. Biol. Fishes 2019, 102, 713–725. [Google Scholar] [CrossRef]
- Miyazono, S.; Aycock, J.N.; Miranda, L.E.; Tietjen, T.E. Assemblage patterns of fish functional groups relative to habitat connectivity and conditions in floodplain lakes. Ecol. Freshw. Fish 2010, 19, 578–585. [Google Scholar] [CrossRef]
- Nunn, A.D.; Tewson, L.H.; Cowx, I.G. The foraging ecology of larval and juvenile fishes. Rev. Fish Biol. Fish. 2012, 22, 377–408. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K. Effect of the human utilization of northern snakehead (Channa argus Cantor, 1842) on the settlement of exotic fish and cladoceran community structure. Sustainability 2021, 13, 2486. [Google Scholar] [CrossRef]
 ) is 7 km downstream from Sejong Weir. The sampling point for investigation of environmental variable and zooplankton community indicated red-circle (●).
) is 7 km downstream from Sejong Weir. The sampling point for investigation of environmental variable and zooplankton community indicated red-circle (●).
 ) is 7 km downstream from Sejong Weir. The sampling point for investigation of environmental variable and zooplankton community indicated red-circle (●).
) is 7 km downstream from Sejong Weir. The sampling point for investigation of environmental variable and zooplankton community indicated red-circle (●).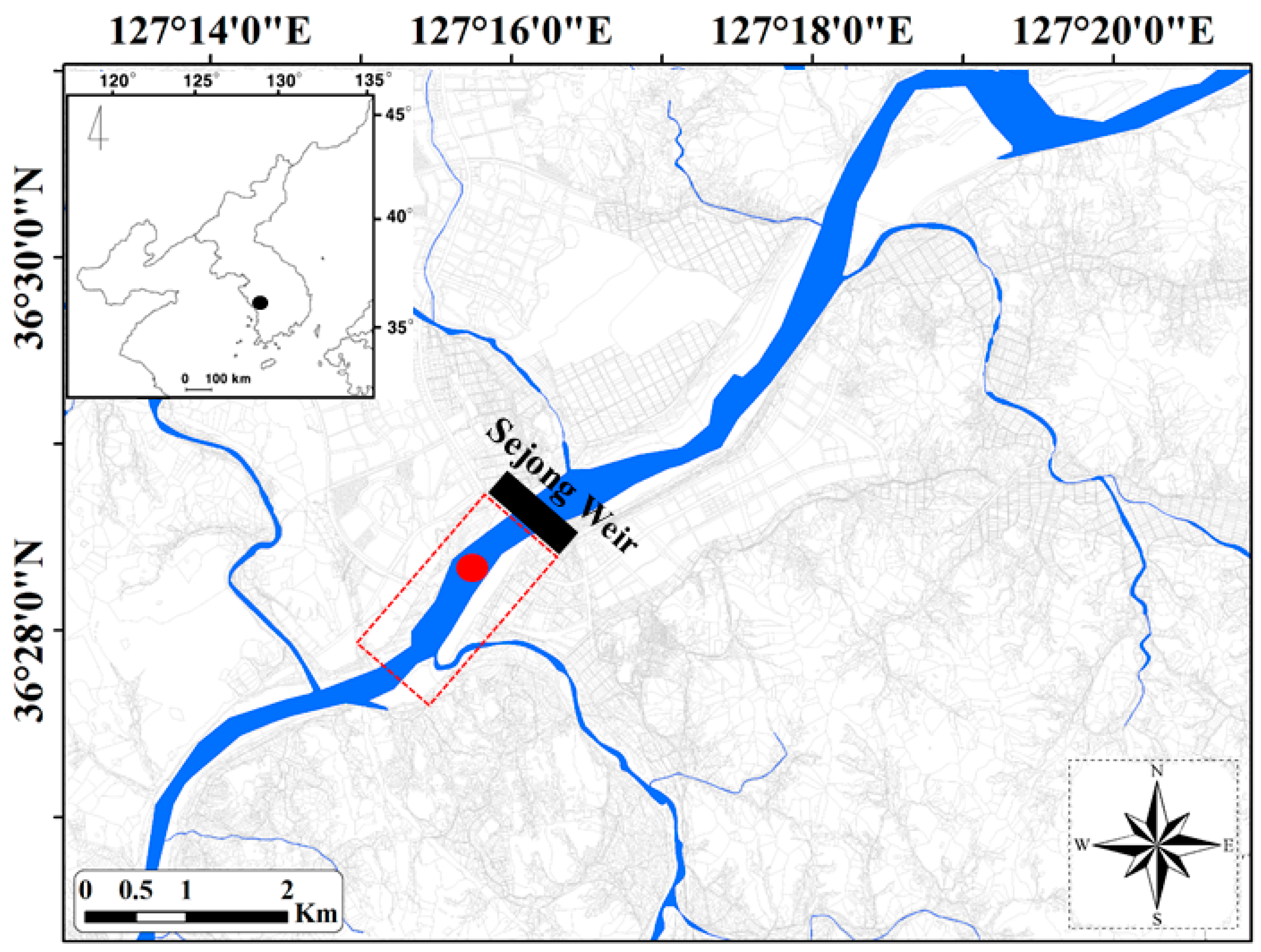
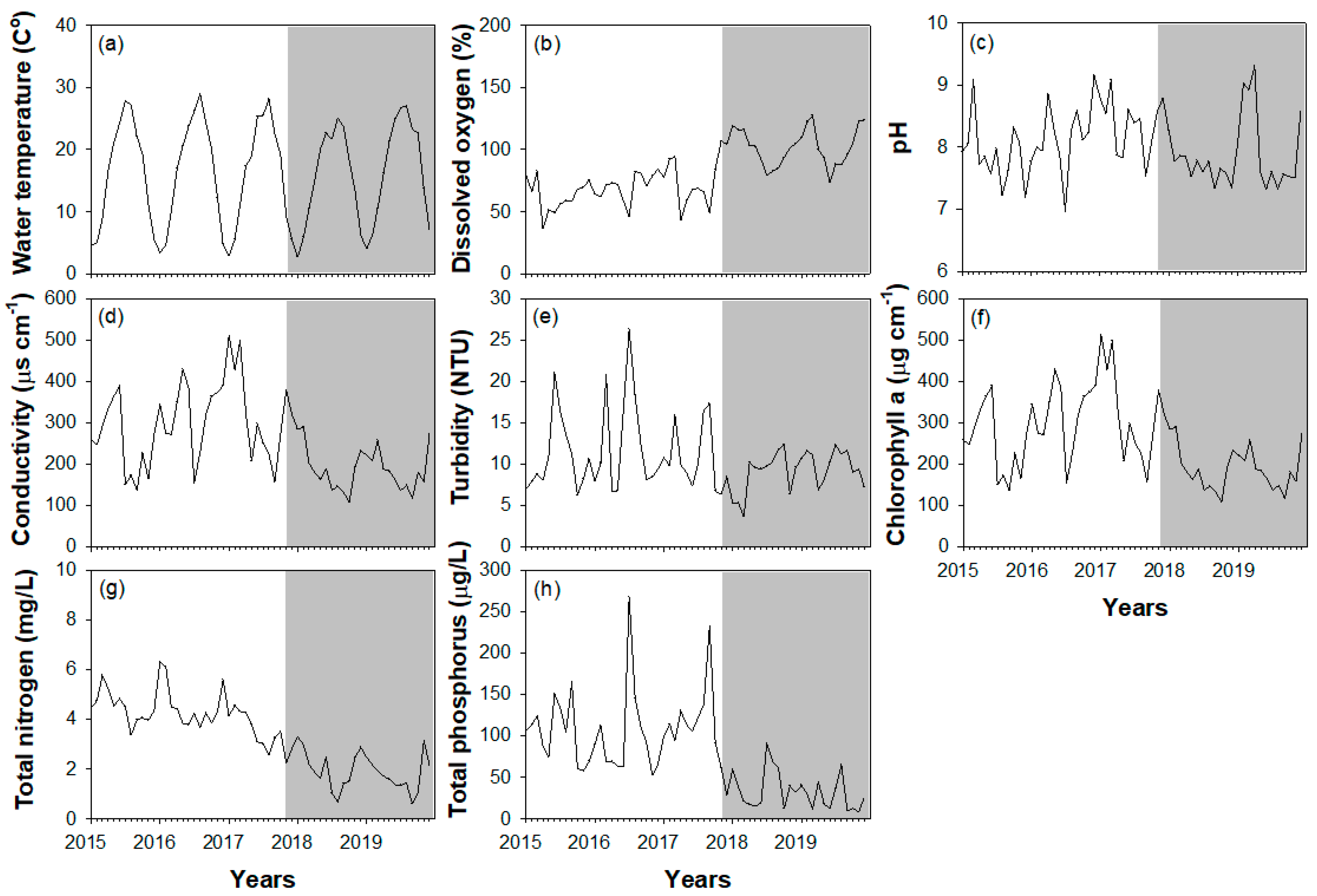

| Environmental Variables | df | F | p |
|---|---|---|---|
| Water temperature (°C) | 46 | 0.477 | 0.493 |
| Dissolved oxygen (%) | 46 | 0.899 | 0.348 |
| pH | 46 | 0.042 | 0.838 |
| Conductivity (µg/L) | 46 | 9.331 | <0.01 |
| Turbidity (NTU) | 46 | 9.085 | <0.01 |
| Chlorophyll a (µg cm−1) | 46 | 21.706 | <0.001 |
| Total nitrogen (mg/L) | 46 | 0.105 | 0.748 |
| Total phosphorus (µg/L) | 46 | 5.469 | <0.05 |
| Structure | 2015 | 2018 | 2020 |
|---|---|---|---|
| Pools | 578,822 m2 (92.7%) | 342,093 m2 (54.8%) | 286,980 m2 (45.9%) |
| Riffles | - | 126,151 m2 (20.2%) | 147,646 m2 (23.6%) |
| Grassland/bare land | 45,680 m2 (7.3%) | 156,258 m2 (25.0%) | 189,876 m2 (30.4%) |
| Total | 624,502 m2 (100%) | 624,502 m2 (100%) | 624,502 m2 (100%) |
| Aquatic Macrophytes | 2015 | 2018 | 2020 |
|---|---|---|---|
| No plants (open water) | 295,411 m2 (51.0%) | 306,124 m2 (89.5%) | 235,141 m2 (81.9%) |
| Paspalum distichum | 28,541 m2 (4.9%) | - | 2684 m2 (0.9%) |
| Phragmites communis | 114,506 m2 (19.8%) | 23,421 m2 (6.8%) | 32,415 m2 (11.3%) |
| Hydrocharis dubia | 3151 m2 (0.5%) | - | 1372 m2 (0.5%) |
| Trapa japonica | 5189 m2 (0.9%) | - | |
| Typha orientalis | 84,541 m2 (14.6%) | 6845 m2 (2.0%) | 11,245 m2 (3.9%) |
| Zizania latifolia | 47,483 m2 (8.2%) | 5703 m2 (1.7%) | 4123 m2 (1.4%) |
| Total | 578,822 m2 | 342,093 m2 | 286,980 m2 |
| Zooplankton Species | df | F | p |
|---|---|---|---|
| Bosmina longiseta | 46 | 47.019 | <0.001 |
| Bosmina fatalis | 46 | 35.304 | <0.001 |
| Bosminopsis deiterisi | 46 | 32.829 | <0.001 |
| Ceriodaphnia sp. | 46 | 19.345 | <0.001 |
| Chydrous sphaericus | 46 | 10.176 | <0.01 |
| Daphnia obtusa | 46 | 29.343 | <0.001 |
| Diaphanosoma brachyurum | 46 | 0.175 | 0.677 |
| Moina microcopa | 46 | 68.959 | <0.001 |
| Nauplius | 46 | 0.215 | 0.645 |
| Cyclops vicinus | 46 | 45.518 | <0.001 |
| Mesocyclops leuckarti | 46 | 46.145 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-K.; Joo, G.-J.; Choi, J.-Y. Effect of Gated Weir Opening on the Topography and Zooplankton Community of Geum River, South Korea. Land 2022, 11, 529. https://doi.org/10.3390/land11040529
Kim S-K, Joo G-J, Choi J-Y. Effect of Gated Weir Opening on the Topography and Zooplankton Community of Geum River, South Korea. Land. 2022; 11(4):529. https://doi.org/10.3390/land11040529
Chicago/Turabian StyleKim, Seong-Ki, Gea-Jae Joo, and Jong-Yun Choi. 2022. "Effect of Gated Weir Opening on the Topography and Zooplankton Community of Geum River, South Korea" Land 11, no. 4: 529. https://doi.org/10.3390/land11040529
APA StyleKim, S.-K., Joo, G.-J., & Choi, J.-Y. (2022). Effect of Gated Weir Opening on the Topography and Zooplankton Community of Geum River, South Korea. Land, 11(4), 529. https://doi.org/10.3390/land11040529






