Bioremediation of Copper- and Chromium-Contaminated Soils Using Agrostis capillaris L., Festuca pratensis Huds., and Poa pratensis L. Mixture of Lawn Grasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theory/Calculation
2.2. Soil Physico-Chemical Properties
2.3. Preparations of Cu2+ and CrO42− Solutions
2.4. Plant Sowing and Cultivation in the Presence of Cu(II) and Cr(VI)
2.5. Determination of the Effect of Cu(II) and Cr(VI) on the Amount of Microorganisms in the Soil Microbiome
2.6. X-ray Fluorescence Analysis of the Samples
2.7. Statistical Analysis
3. Results
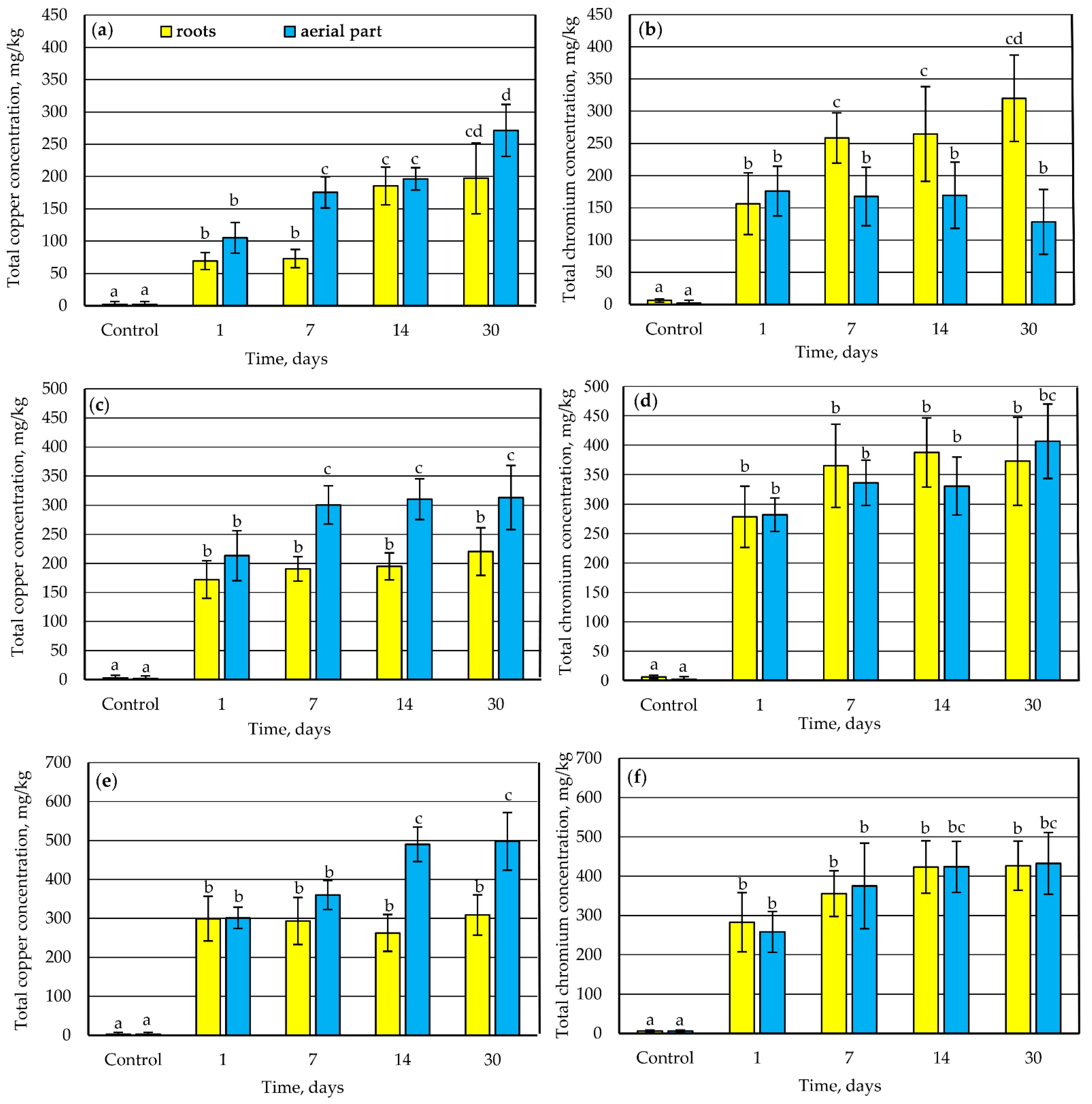
3.1. Accumulation of Copper and Chromium Compounds by Lawn Grass
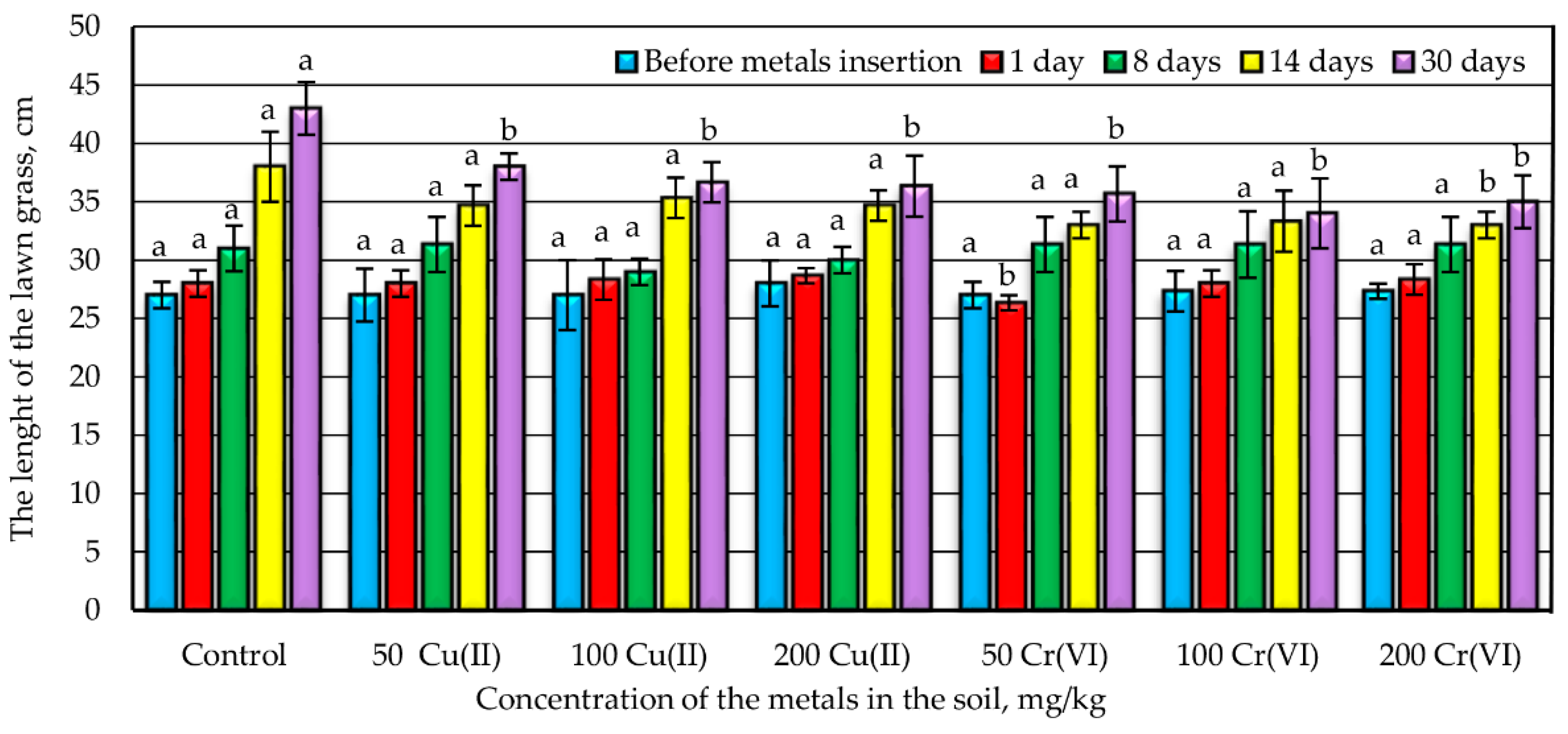
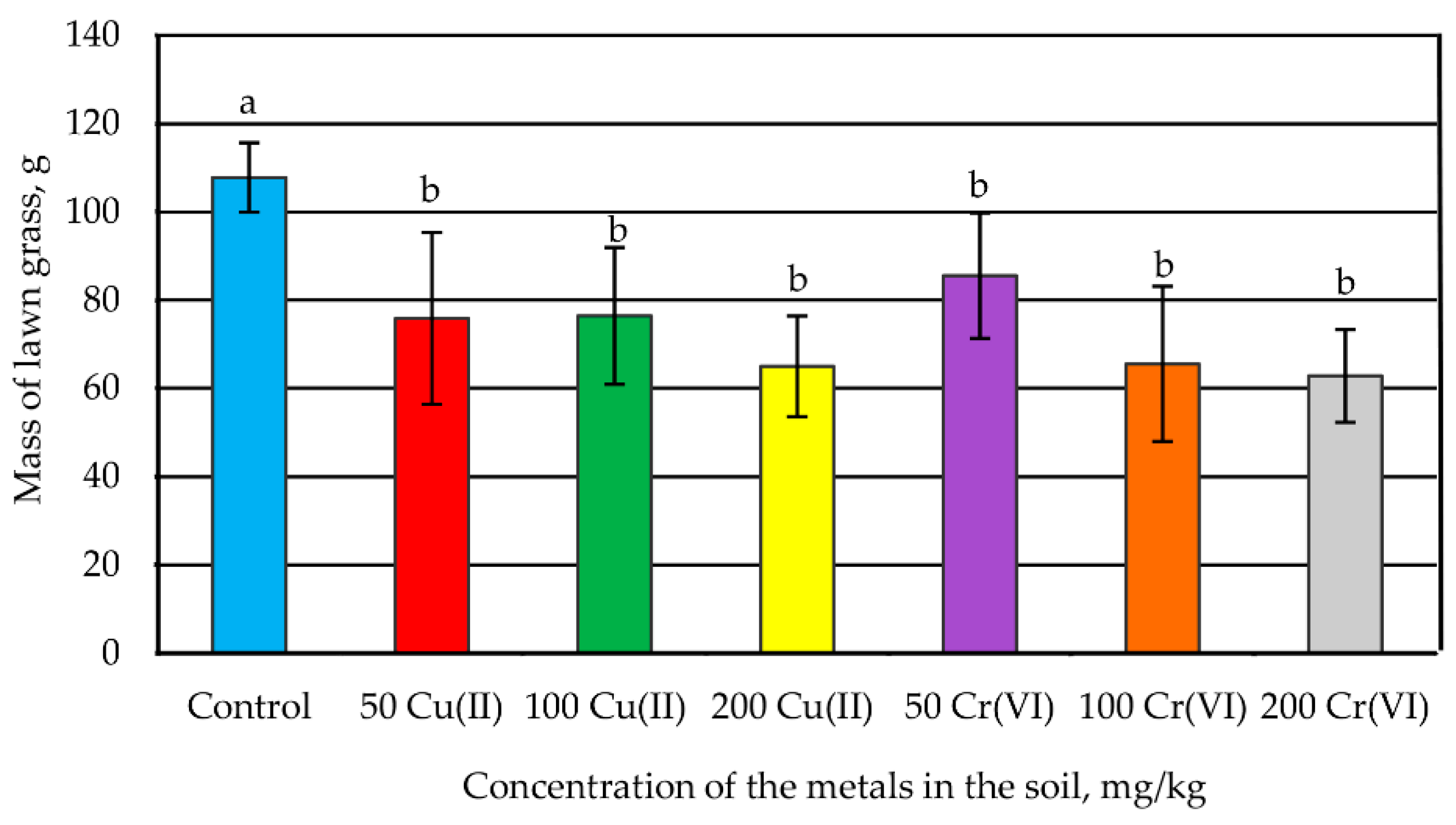
3.2. The Influence of Metals on the Growth of Lawn Grass
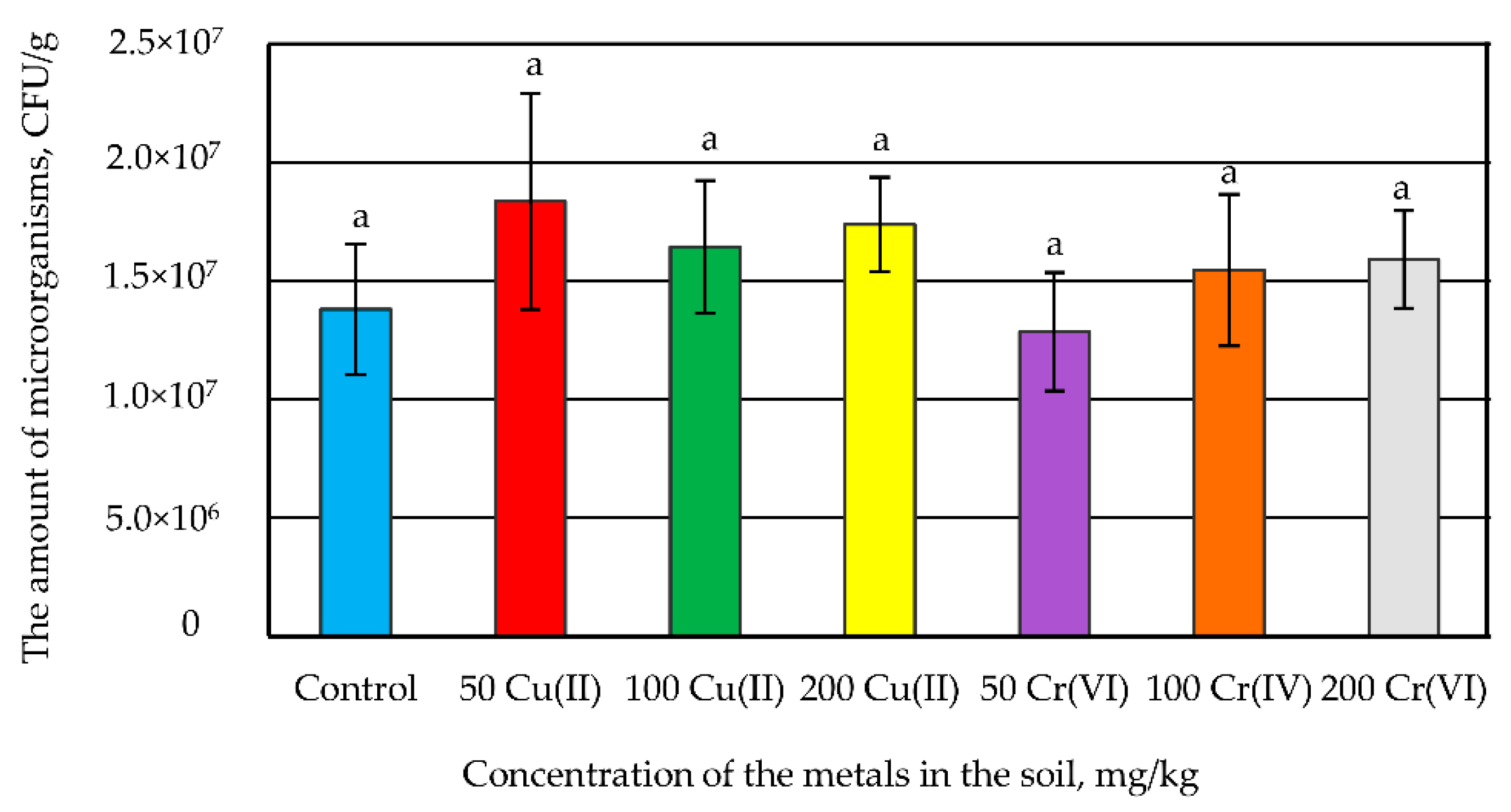
3.3. The Influence of Metals on the Amount of Microorganisms in the Soil Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals, 1st ed.; Saleh, H., Aglan, R.F., Eds.; IntechOpen: London, UK, 2018; Volume 7, pp. 115–133. [Google Scholar] [CrossRef] [Green Version]
- Aleksandra, S.-N. Phytotechnologies: Importance in Remediation of Heavy Metal–Contaminated Soils. In Biomanagement of Metal-Contaminated Soils. Environmental Pollution, 1st ed.; Khan, M., Zaidi, A., Goel, R., Musarrat, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 20, pp. 277–295. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, X.; Wang, P.; Hu, Y.; Cheng, H. Heavy Metal Pollution Caused by Small-Scale Metal Ore Mining Activities: A Case Study from a Polymetallic Mine in South China. Sci. Total Environ. 2018, 639, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy Metal Pollution from Gold Mines: Environmental Effects and Bacterial Strategies for Resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Galán, M.; Baena-Moreno, F.M.; Vázquez, S.; Arroyo-Torralvo, F.; Vilches, L.F.; Zhang, Z. Remediation of Acid Mine Drainage. Environ. Chem. Lett. 2019, 17, 1529–1538. [Google Scholar] [CrossRef]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of Heavy Metals in Wastewater and Soil Samples from Open Drainage Channels in Nairobi, Kenya: Community Health Implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef] [PubMed]
- Province, J.; Pan, H.; Cheng, Z.; Zhou, G.; Yang, R.; Sun, B. Geochemical and Mineralogical Characterization of Tailings of the Dexing Geochemical and Mineralogical Characterization of Tailings of the Dexing Copper Mine, Jiangxi Province, China. Geochem. Explor. Environ. Anal. 2017, 17, 334–344. [Google Scholar] [CrossRef]
- Abraham, M.R.; Susan, T.B. Water Contamination with Heavy Metals and Trace Elements from Kilembe Copper Mine and Tailing Sites in Western Uganda; Implications for Domestic Water Quality. Chemosphere 2017, 169, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Solgi, E.; Parmah, J. Analysis and Assessment of Nickel and Chromium Pollution in Soils around Baghejar Chromite Mine of Sabzevar Ophiolite Belt, Northeastern Iran. Trans. Nonferrous Met. Soc. China 2015, 25, 2380–2387. [Google Scholar] [CrossRef]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic Life and Human Health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef] [Green Version]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative Stress and Heavy Metals in Plants. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2018; Volume 245, pp. 129–156. ISBN 978-3-319-75037-8. [Google Scholar]
- David, E.; Cosio, C. New Insights into Impacts of Toxic Metals in Aquatic Environments. Environments 2021, 8, 1. [Google Scholar] [CrossRef]
- Roa Rivera, C.A.; Garavito Aguilar, Z.V.; Delgadillo Méndez, D.A. Effects of Environmental Pollutants in Oxidative Stress of Reptiles and Amphibians: A Comparative Analysis. Bachelor’s Thesis, Universidad de Los Andes, Bogotá, Colombia, 2020. [Google Scholar]
- Diaconu, M.; Pavel, L.V.; Hlihor, R.-M.; Rosca, M.; Fertu, D.I.; Lenz, M.; Corvini, P.X.; Gavrilescu, M. Characterization of Heavy Metal Toxicity in Some Plants and Microorganisms—A Preliminary Approach for Environmental Bioremediation. New Biotechnol. 2020, 56, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, P.; Yang, F.; Sun, D.; Zhang, D.-X.; Zhou, Y.-K. Assessment of Heavy Metal Pollution and Human Health Risks in Urban Soils around an Electronics Manufacturing Facility. Sci. Total Environ. 2018, 630, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Abedi Sarvestani, R.; Aghasi, M. Health Risk Assessment of Heavy Metals Exposure (Lead, Cadmium, and Copper) through Drinking Water Consumption in Kerman City, Iran. Environ. Earth Sci. 2019, 78, 714. [Google Scholar] [CrossRef]
- Bourgeois, C.; Alfaro, A.C.; Dencer-Brown, A.; Duprey, J.L.; Desnues, A.; Marchand, C. Stocks and Soil-Plant Transfer of Macro-Nutrients and Trace Metals in Temperate New Zealand Estuarine Mangroves. Plant Soil 2019, 436, 565–586. [Google Scholar] [CrossRef]
- Lyu, Z.; Chai, J.; Xu, Z.; Qin, Y. Environmental Impact Assessment of Mining Activities on Groundwater: Case Study of Copper Mine in Jiangxi Province, China. J. Hydro. Eng. 2019, 24, 05018027. [Google Scholar] [CrossRef]
- Bhakta, J.N. Metal Toxicity in Microorganism. Handb. Res. Inven. Bioremediat. Tech. 2017, 1, 1–23. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, A.; Srivastava, P.; Choudhary, K.K.; Dikshit, A. 1–Plant Growth-Promoting Microorganisms in Sustainable Agriculture. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Kumar, A., Singh, A.K., Choudhary, K.K., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–19. ISBN 978-0-12-817004-5. [Google Scholar]
- Xu, Y.; Seshadri, B.; Bolan, N.; Sarkar, B.; Ok, Y.S.; Zhang, W.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; et al. Microbial Functional Diversity and Carbon Use Feedback in Soils as Affected by Heavy Metals. Environ. Int. 2019, 125, 478–488. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Kidd, P.S.; Álvarez-López, V.; Becerra-Castro, C.; Cabello-Conejo, M.; Prieto-Fernández, Á. Chapter Three–Potential Role of Plant-Associated Bacteria in Plant Metal Uptake and Implications in Phytotechnologies. In Advances in Botanical Research; Cuypers, A., Vangronsveld, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 83, pp. 87–126. ISBN 0065-2296. [Google Scholar]
- Andrejkovičová, S.; Sudagar, A.; Rocha, J.; Patinha, C.; Hajjaji, W.; da Silva, E.F.; Velosa, A.; Rocha, F. The Effect of Natural Zeolite on Microstructure, Mechanical and Heavy Metals Adsorption Properties of Metakaolin Based Geopolymers. Appl. Clay Sci. 2016, 126, 141–152. [Google Scholar] [CrossRef]
- Urbina, L.; Guaresti, O.; Requies, J.; Gabilondo, N.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Design of Reusable Novel Membranes Based on Bacterial Cellulose and Chitosan for the Filtration of Copper in Wastewaters. Carbohydr. Polym. 2018, 193, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Nassef, E.; El-taweel, Y.A. Removal of Copper from Wastewater By Cementation from Simulated Leach Removal of Copper from Wastewater by Cementation from Simulated Leach Liquors. J. Chem. Eng. Process Technol. 2015, 6, 214. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Liu, J.; Sui, M.; Qu, Y.; Ambuchi, J.J.; Wang, H.; Feng, Y. A Combined Microbial Desalination Cell and Electrodialysis System for Copper-Containing Wastewater Treatment and High-Salinity-Water Desalination. J. Hazard Mater. 2017, 321, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Husain, T.; Hejazi, R. An Overview and Analysis of Site Remediation Technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Sims, J.L.; Sims, R.C.; Matthews, J.E. Approach to Bioremediation of Contaminated Soil. Hazard Waste Hazard Mater. 1990, 7, 117–149. [Google Scholar] [CrossRef]
- Romantschuk, M.; Sarand, I.; Petänen, T.; Peltola, R.; Jonsson-Vihanne, M.; Koivula, T.; Yrjälä, K.; Haahtela, K. Means to Improve the Effect of in Situ Bioremediation Ofcontaminated Soil. Environ. Pollut. 2000, 107, 179–185. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological Advances in Bioremediation of Heavy Metals Contaminated Ecosystems: An Overview with Special Reference to Phytoremediation. Int. J. Environ. Sci. Technol. 2014, 11, 843–872. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of Heavy Metal Contaminated Soils: Phytoremediation as a Potentially Promising Clean-up Technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Cornu, J.Y.; Huguenot, D.; Jézéquel, K.; Lollier, M.; Lebeau, T. Bioremediation of Copper-Contaminated Soils by Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 26. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, H.B.; Prabha, R. Plant-Microbe Interactions in Agro-Ecological Perspectives. Plant-Microbe Interact. Agro-Ecol. Perspect. 2017, 2, 1–763. [Google Scholar] [CrossRef]
- Sun, L.; Yang, J.; Fang, H.; Xu, C.; Peng, C.; Huang, H.; Lu, L.; Duan, D.; Zhang, X.; Shi, J. Mechanism Study of Sulfur Fertilization Mediating Copper Translocation and Biotransformation in Rice (Oryza sativa L.) Plants. Environ. Pollut. 2017, 226, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Zupfer, K.R.; Vasiluk, L.; Dutton, M.D.; Bellantino-Perco, M.; Hale, B.A. Modeling Phytoremediation of Aged Soil Ni from Anthropogenic Deposition Using Alyssum murale. Chemosphere 2021, 267, 128861. [Google Scholar] [CrossRef] [PubMed]
- Azab, E.; Hegazy, A.K. Monitoring the Efficiency of Rhazya stricta L. Plants in Phytoremediation of Heavy Metal-Contaminated Soil. Plants 2020, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C.; Rai, A.; Korstad, J. Aromatic Crops in Phytoremediation: From Contaminated to Waste Dumpsites. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 255–275. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, D. Phytoremediation Potential of Perennial Grasses; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 0-12-817733-0. [Google Scholar]
- De Conti, L.; Ceretta, C.A.; Melo, G.W.; Tiecher, T.L.; Silva, L.O.; Garlet, L.P.; Mimmo, T.; Cesco, S.; Brunetto, G. Intercropping of Young Grapevines with Native Grasses for Phytoremediation of Cu-Contaminated Soils. Chemosphere 2019, 216, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Gajić, G.; Mitrović, M.; Pavlović, P. Feasibility of Festuca rubra L. Native Grass in Phytoremediation. Phytoremediat. Potential Perenn. Grasses 2020, 115–164. [Google Scholar] [CrossRef]
- Wiangkham, N.; Prapagdee, B. Potential of Napier Grass with Cadmium-Resistant Bacterial Inoculation on Cadmium Phytoremediation and Its Possibility to Use as Biomass Fuel. Chemosphere 2018, 201, 511–518. [Google Scholar] [CrossRef]
- Tashyrev, O. Theoretical Aspects of Interaction of Microorganisms with Metals. Microbial Accumulation of Metals Due to Their Stereochemical Analogy with Macronutrients. Microbiol. J. 1994, 56, 89–100. [Google Scholar]
- Sumner, M. Measurement of Soil pH: Problems and Solutions. Commun. Soil. Sci. Plant Anal. 1994, 25, 859–879. [Google Scholar] [CrossRef]
- Suslova, O.; Govorukha, V.; Brovarskaya, O.; Matveeva, N.; Tashyreva, H.; Tashyrev, O. Method for Determining Organic Compound Concentration in Biological Systems by Permanganate R.edox Titration. Int. J. Bioautomation 2014, 18, 45–52. [Google Scholar]
- Beckhoff, B.; Kanngießer, B.; Langhoff, N.; Wedell, R.; Wolff, H. Handbook of Practical X-Ray Fluorescence Analysis; Springer Science & Business Media: New York, NY, USA, 2007; ISBN 3-540-36722-5. [Google Scholar]
- Taylor, J. The Estimation of Numbers of Bacteria by Tenfold Dilution Series. J. Appl. Bacteriol. 1962, 25, 54–61. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef] [PubMed]
- Wolski, K.; Czarnecki, J.; Brennensthul, M.; Ptak, W. Color Assessment of Selected Lawn Grass Mixtures. Grassl. Sci. 2021, 67, 198–206. [Google Scholar] [CrossRef]
- Gladkov, E.A.; Tashlieva, I.I.; Gladkova, O.V. Ornamental Plants Adapted to Urban Ecosystem Pollution: Lawn Grasses and Painted Daisy Tolerating Copper. Environ. Sci. Pollut. Res. 2021, 28, 14115–14120. [Google Scholar] [CrossRef] [PubMed]
- Gladkov, E.; Tashlieva, I.; Dolgikh, Y.; Gladkova, O. Increasing Tolerance Agrostis stolonifera, Festuca rubra, Brachycome iberidifolia, Chrysanthemum carinatum to Copper: Modeling Natural Environments. In Proceedings of the 2nd International Conference on BioGeoSciences, Bulgaria, 19 December 2018; pp. 167–174, ISBN 978-3-030-04232-5. [Google Scholar]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Srivastava, S.; Prakash, S.; Srivastava, M.M. Fate of Trivalent Chromium in Presence of Organic Acids: A Hydroponic Study on the Tomato Plant. Chem. Speciat. Bioavailab. 1998, 10, 147–150. [Google Scholar] [CrossRef]
- Havryliuk, О.; Hovorukha, V.; Sachko, A.; Gladka, G.; Bida, I.; Tashyrev, O. Bioremoval of Hazardous Cobalt, Nickel, Chromium, Copper and Cadmium Compounds from Contaminated Soil by Nicotiana tabacum Plants and Associated Microbiome. Biosyst. Divers. 2021, 29, 88–93. [Google Scholar] [CrossRef]
- Choo, T.P.; Lee, C.K.; Low, K.S.; Hishamuddin, O. Accumulation of Chromium (VI) from Aqueous Solutions Using Water Lilies (Nymphaea spontanea). Chemosphere 2006, 62, 961–967. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L. Citric Acid Assisted Phytoextraction of Chromium by Sunflower; Morpho-Physiological and Biochemical Alterations in Plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef]
- Myo, E.M.; Ge, B.; Ma, J.; Cui, H.; Liu, B.; Shi, L.; Jiang, M.; Zhang, K. Indole-3-Acetic Acid Production by Streptomyces Fradiae NKZ-259 and Its Formulation to Enhance Plant Growth. BMC Microbiol. 2019, 19, 155. [Google Scholar] [CrossRef] [Green Version]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of Acetic Acid Bacteria and Their Role in Vinegar and Fermented Beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behera, B.C. Citric Acid from Aspergillus niger: A Comprehensive Overview. Crit. Rev. Microbiol. 2020, 46, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Chu, D. Effects of Heavy Metals on Soil Microbial Community. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012009. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Si, Y.; Chen, H.; Russel, M.; Chen, K.; Qian, Y.; Zaray, G.; Bramanti, E. Short-Time Effect of Heavy Metals upon Microbial Community Activity. J. Hazard. Mater. 2010, 173, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Kucharski, J.; Borowik, A.; Boros, E. Response of Bacteria to Soil Contamination with Heavy Metals. J. Elem. 2008, 13, 28886536. [Google Scholar] [CrossRef]
- Hattori, H. Influence of Heavy Metals on Soil Microbial Activities. Soil Sci. Plant Nutr. 1992, 38, 93–100. [Google Scholar] [CrossRef]
- Xiao, L.; Yu, Z.; Liu, H.; Tan, T.; Yao, J.; Zhang, Y.; Wu, J. Effects of Cd and Pb on Diversity of Microbial Community and Enzyme Activity in Soil. Ecotoxicology 2020, 29, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, K.; Movsesyan, H.; Ghazaryan, N.; Watts, B.A. Copper Phytoremediation Potential of Wild Plant Species Growing in the Mine Polluted Areas of Armenia. Environ. Pollut. 2019, 249, 491–501. [Google Scholar] [CrossRef]
- Chandrasekhar, C.; Ray, J.G. Copper Accumulation, Localization and Antioxidant Response in Eclipta alba L. in Relation to Quantitative Variation of the Metal in Soil. Acta Physiol. Plant 2017, 39, 205. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havryliuk, O.; Hovorukha, V.; Bida, I.; Danko, Y.; Gladka, G.; Zakutevsky, O.; Mariychuk, R.; Tashyrev, O. Bioremediation of Copper- and Chromium-Contaminated Soils Using Agrostis capillaris L., Festuca pratensis Huds., and Poa pratensis L. Mixture of Lawn Grasses. Land 2022, 11, 623. https://doi.org/10.3390/land11050623
Havryliuk O, Hovorukha V, Bida I, Danko Y, Gladka G, Zakutevsky O, Mariychuk R, Tashyrev O. Bioremediation of Copper- and Chromium-Contaminated Soils Using Agrostis capillaris L., Festuca pratensis Huds., and Poa pratensis L. Mixture of Lawn Grasses. Land. 2022; 11(5):623. https://doi.org/10.3390/land11050623
Chicago/Turabian StyleHavryliuk, Olesia, Vira Hovorukha, Iryna Bida, Yanina Danko, Galina Gladka, Oleg Zakutevsky, Ruslan Mariychuk, and Oleksandr Tashyrev. 2022. "Bioremediation of Copper- and Chromium-Contaminated Soils Using Agrostis capillaris L., Festuca pratensis Huds., and Poa pratensis L. Mixture of Lawn Grasses" Land 11, no. 5: 623. https://doi.org/10.3390/land11050623
APA StyleHavryliuk, O., Hovorukha, V., Bida, I., Danko, Y., Gladka, G., Zakutevsky, O., Mariychuk, R., & Tashyrev, O. (2022). Bioremediation of Copper- and Chromium-Contaminated Soils Using Agrostis capillaris L., Festuca pratensis Huds., and Poa pratensis L. Mixture of Lawn Grasses. Land, 11(5), 623. https://doi.org/10.3390/land11050623










