Reclaiming Wetlands after Oil Sands Mining in Alberta, Canada: The Changing Vegetation Regime at an Experimental Wetland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.1.1. Weather
2.1.2. Site Characteristics
2.1.3. Water Management
2.1.4. Early Vegetation Structure: Years 1–3
2.2. Methods
2.2.1. Vegetation and Plant Communities
2.2.2. Water Chemistry
2.2.3. Water Tables
2.2.4. Spatial Analyses
3. Results
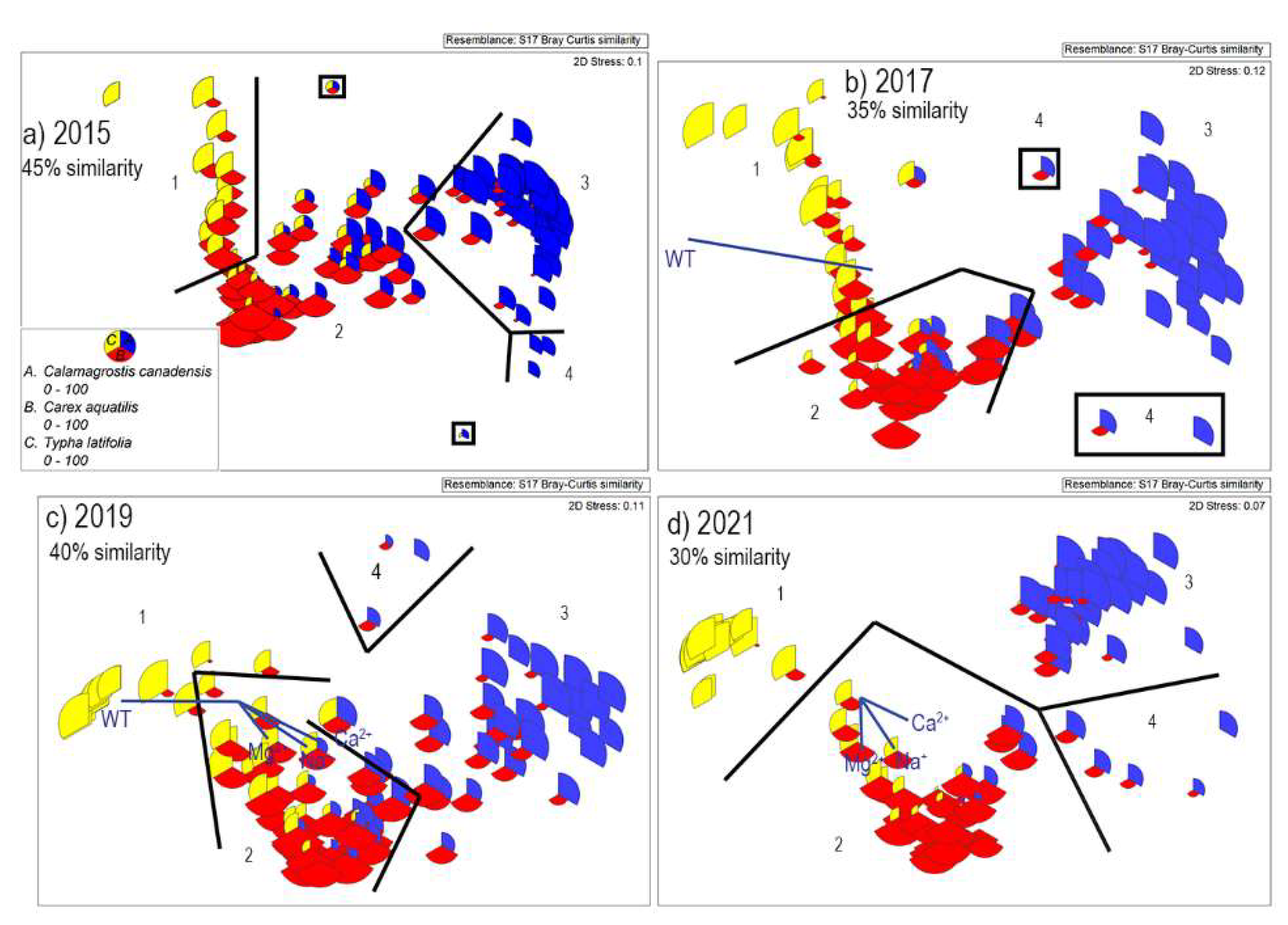
3.1. Plant Communities on Sandhill Wetland
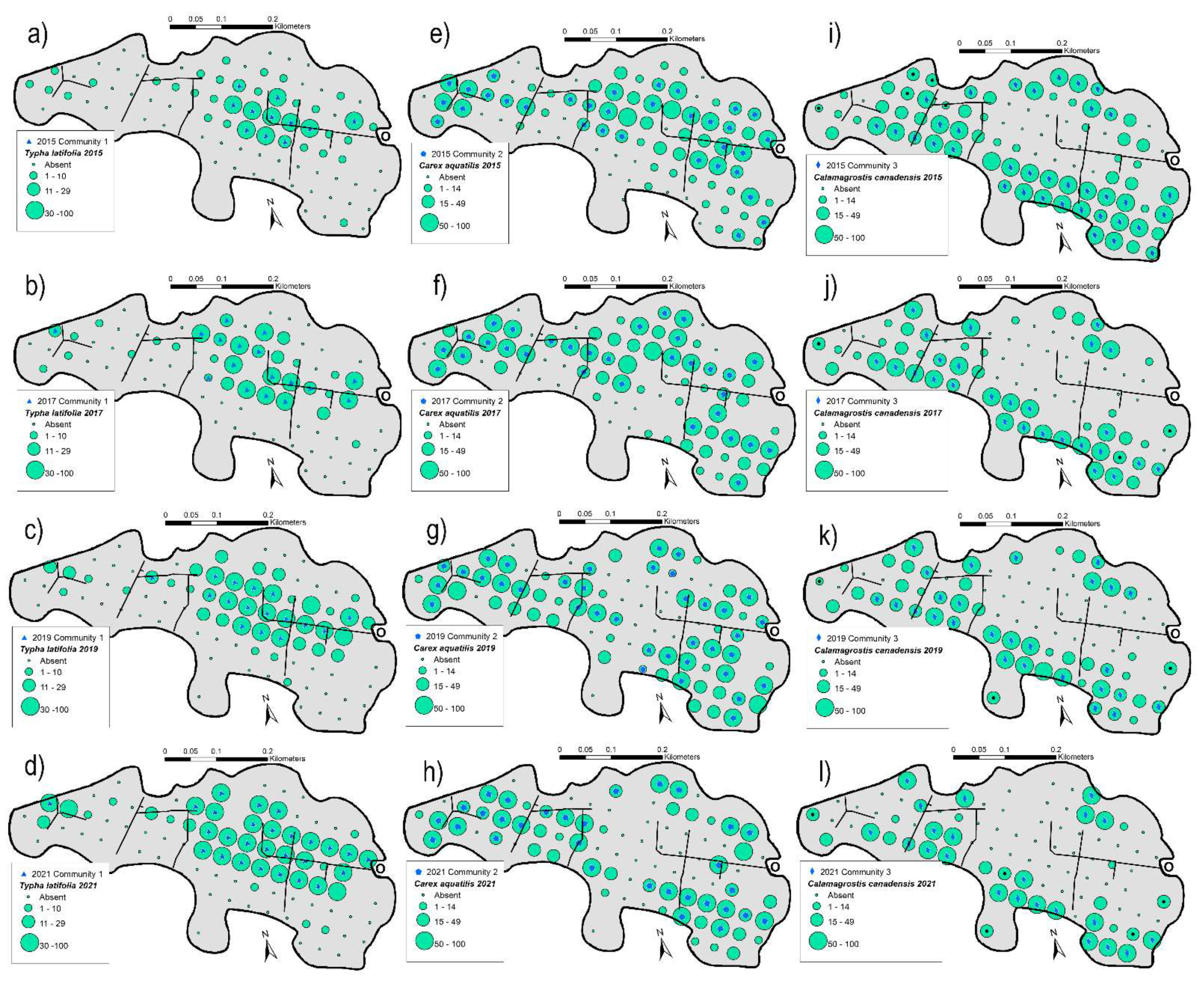
3.2. Spatial Changes of Plant Communities
3.3. Bryophyte Species Richness
3.4. Vascular Plant Species Richness
3.5. Environmental Gradients at Sandhill Wetland
3.5.1. Stoichiometric Comparisons of Base Cations at Sandhill Wetland to Natural Sites
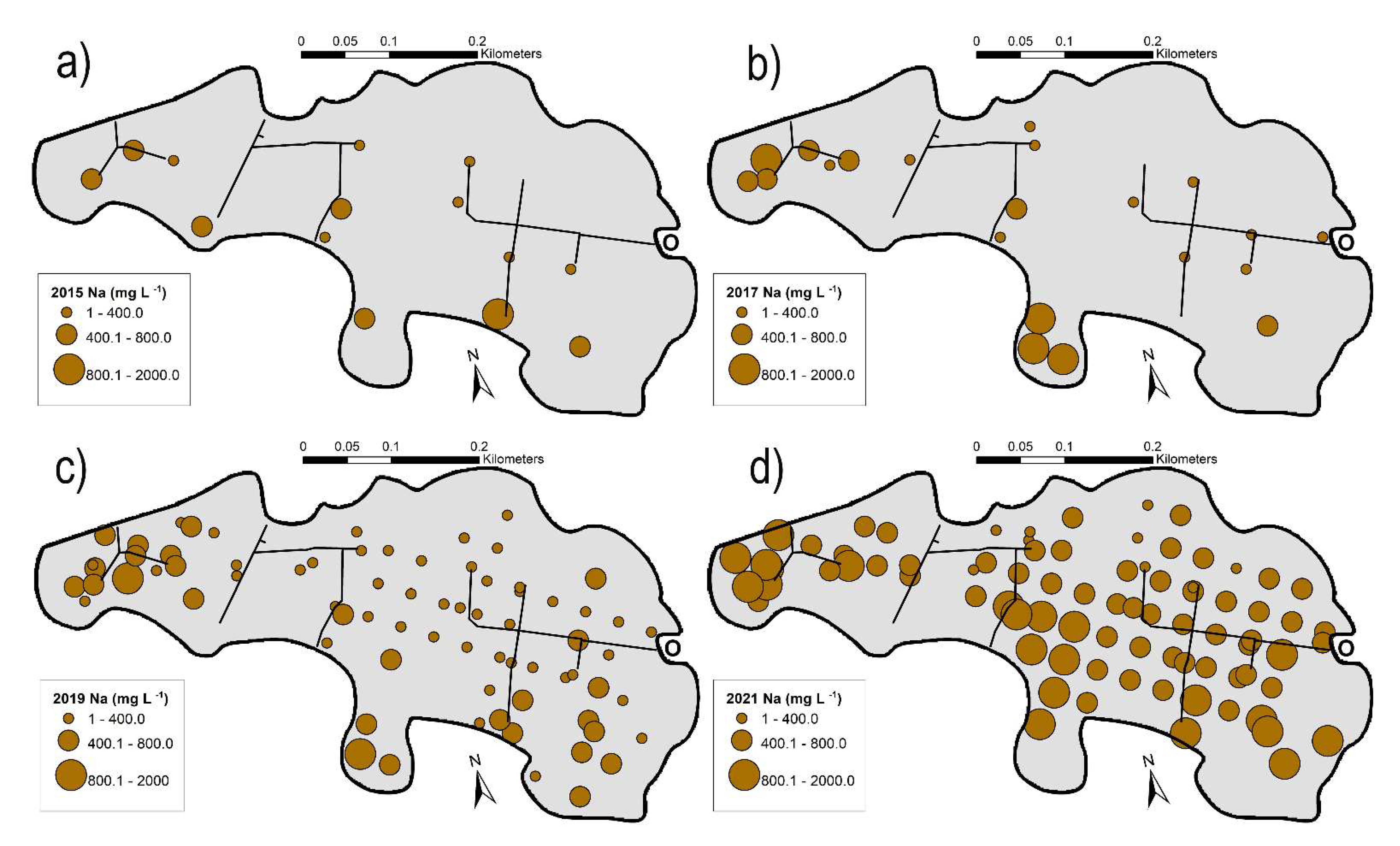
3.5.2. Variation of Base Cations and pH of Surficial Waters
3.5.3. Variation in Na+/Ca2+ + Mg2+ Ratios
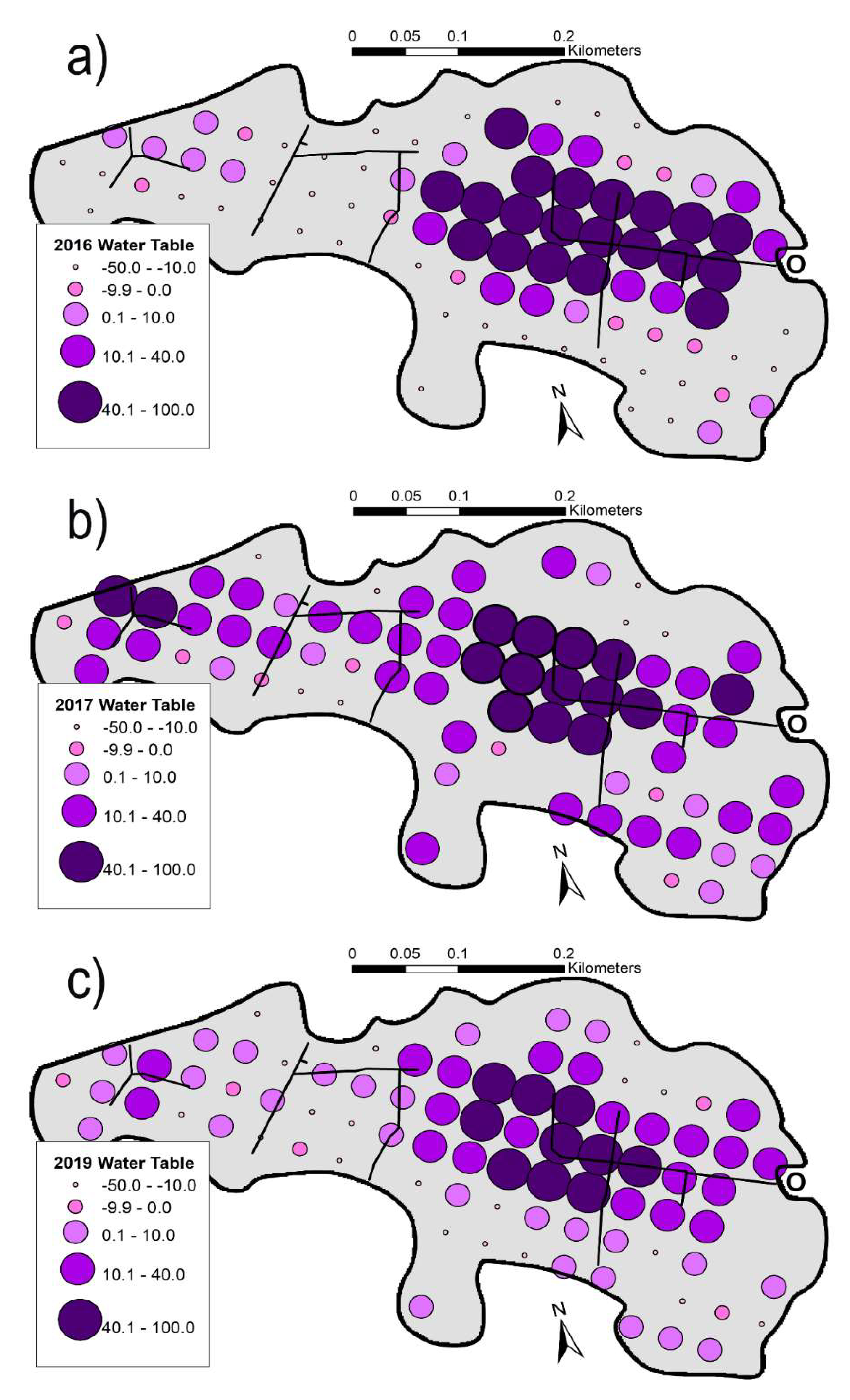
3.5.4. Variation in Water Tables
4. Discussion
4.1. Changing Plant Communities
4.2. Changing Water Chemistry
4.3. Changing Water Tables
4.4. Linkages between Environmental Factors and Plant Community Change
5. Conclusions and Implications
5.1. Conclusions
5.2. Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wieder, R.K.; Vitt, D.H.; Benscoter, B. Peatlands and the boreal forest. In Boreal Peatland Ecosystems; Wieder, R.K., Vitt, D.H., Eds.; Springer: Berlin/Heidelburg, Germany; New York, NY, USA, 2006; pp. 1–8. [Google Scholar]
- Yu, Z. Northern peatland carbon stocks and dynamics: A review. Biogeosciences 2012, 9, 4071–4085. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Morris, P.J.; Liu, J.; Holden, J. PEATMAP: Refining estimates of global peatland distribution based on a meta-analysis. Catena 2018, 160, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Loisel, J.; Yu, Z.; Beilman, D.W.; Camill, P.; Alm, J.; Amesbury, M.J.; Anderson, D.; Andersson, S.; Bochicchio, C.; Barber, K.; et al. A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation. Holocene 2014, 24, 1028–1042. [Google Scholar] [CrossRef]
- Vitt, D.H.; Halsey, L.A.; Bauer, I.E.; Campbell, C. Spatial and temporal trends of carbon sequestration in peatlands of continental western Canada through the Holocene. Can. J. Earth Sci. 2000, 37, 683–693. [Google Scholar] [CrossRef]
- Government of Alberta. Oil Sands Facts and Statistics [www Document] 2022. Available online: https://www.alberta.ca/oil-sands-facts-and-statistics.aspx (accessed on 3 March 2022).
- Lee, P.; Cheng, R. Bitumen and Biocarbon: Land Use Changes and Loss of Biological Carbon Due to Bitumen Operations in the Boreal Forests of Alberta, Canada; Global Forest Watch Canada: Edmonton, AB, Canada, 2009; p. 40. [Google Scholar]
- Bauer, I.E.; Vitt, D.H. Autogenic succession and its importance for the peatlands of Canada’s western boreal forest. In Peatlands, Archaeological Sites—Archives Of Nature—Nature Conservation—Wise Use. In Proceedings of the Peatland Conference, Hannover, Germany, 26–30 October 2002; Bauerochse, A., Haßmann, H., Eds.; Verlag Leidorf GmbH (Rahden/Westf): Hannover, Germany, 2003; pp. 179–187. [Google Scholar]
- Sjörs, H. On the relation between vegetation and electrolytes in North Swedish mire waters. Oikos 1950, 2, 241–258. [Google Scholar] [CrossRef]
- Vitt, D.H. An overview of factors that influence the development of Canadian peatlands. Mem. Ent. 1994, 169, 7–20. [Google Scholar] [CrossRef]
- Wieder, R.K.; Vitt, D.H.; Vile, M.A.; Graham, J.A.; Hartsock, J.A.; Fillingim, H.; House, M.; Quinn, J.C.; Scott, K.D.; Petix, M.; et al. Experimental nitrogen addition alters structure and function of a boreal bog: Critical loads and thresholds revealed. Ecol. Monogr. 2019, 89, e01371. [Google Scholar] [CrossRef]
- Wieder, R.K.; Vitt, D.H.; Vile, M.A.; Graham, J.A.; Hartsock, J.A.; House, M.; Popma, J.M.; Fillingim, H.; Quinn, J.C.; Scott, K.D.; et al. Experimental nitrogen addition alters structure and function of a boreal poor fen: Implications for critical loads. Sci. Total Environ. 2020, 733, 138619. [Google Scholar] [CrossRef]
- Couwenberg, J.; Joosten, H.C.A. Weber and Raised Bog of Augstumal—With a Translation of the 1902 Monograph by Weber on the “Vegetation and Development of the Raised Bog of Augstumal in the Memel Delta”; International Mire Conservation Group/PPE “Grif & K”: Tula, Russia, 2002; 278p. [Google Scholar]
- Trites, M.; Bayley, S.E. Vegetation communities in continental boreal wetlands along a salinity gradient: Implications for oil sands mining reclamation. Aquat. Bot. 2009, 91, 27–39. [Google Scholar] [CrossRef]
- Harris, M.L. Guideline for Wetland Establishment on Reclaimed Oil Sands Leases, 2nd ed.; Reclamation Working Group, Cumulative Environmental Management Association: Fort McMurray, AB, Canada, 2007. [Google Scholar]
- Foote, L. Threshold considerations and wetland reclamation in Alberta’s mineable oil sands. Ecol. Soc. 2012, 17, 35. [Google Scholar] [CrossRef] [Green Version]
- Ketcheson, S.J.; Price, J.S.; Carey, S.K.; Petrone, R.M.; Mendoza, C.A.; Devito, K.J. Constructing fen peatlands in post-mining oil sands landscapes: Challenges and opportunities from a hydrological perspective. Earth-Sci. Rev. 2016, 161, 130–139. [Google Scholar] [CrossRef]
- Biagi, K.M.; Oswald, C.J.; Nicholls, E.M.; Carey, S.K. Increases in salinity following a shift in hydrologic regime in a constructed wetland watershed in a post-mining oil sands landscape. Sci. Total Environ. 2019, 653, 1445–1457. [Google Scholar] [CrossRef]
- Wytrykush, C.; Vitt, D.H.; McKenna, G.; Vassov, R. Designing landscapes to support peatland development on soft tailings deposits. In Restoration and Reclamation of Boreal Ecosystems; Vitt, D.H., Bhatti, J., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 161–178. [Google Scholar]
- Kessler, S.S.; Barbour, L.; Van Rees, K.C.J.; Dobchuk, B.S. Salinization of soil over saline-sodic overburden from the oil sands in Alberta. Can. J. Soil Sci. 2010, 90, 637–647. [Google Scholar] [CrossRef]
- Vessey, C.J.; Lindsay, M.B.J.; Barbour, S.L. Sodium transport and attenuation in soil cover materials for oil sands mine reclamation. Appl. Geochem. 2019, 100, 42–54. [Google Scholar] [CrossRef]
- Nicholls, E.M.; Carey, S.K.; Humphreys, E.R.; Clark, M.G.; Drewitt, G.B. Multi-year water balance assessment of a newly constructed wetland, Fort McMurray, Alberta. Hydrol. Process. 2016, 30, 2739–2753. [Google Scholar] [CrossRef]
- Spennato, H.M.; Ketcheson, S.J.; Mendoza, C.A.; Carey, S.K. Water table dynamics in a constructed wetland, Fort McMurray, Alberta. Hydrol. Process. 2018, 32, 3824–3836. [Google Scholar] [CrossRef]
- Biagi, K.M.; Carey, S.K. The hydrochemical evolution of a constructed peatland in a post-mining landscape six years after construction. J. Hydrol. Reg. 2021, 39, 1445–1457. [Google Scholar] [CrossRef]
- Koropchak, S.; Vitt, D.H. Survivorship and growth of Typha latifolia L. across a NaCl gradient: A greenhouse study. Int. J. Min. Reclam. Environ. 2012, 26, 143–150. [Google Scholar] [CrossRef]
- Vitt, D.H.; Glaeser, L.C.; House, M.; Kitchen, S. Structural and functional responses of Carex aquatilis to increasing sodium concentrations. Wetl. Ecol. Manag. 2020, 28, 753–763. [Google Scholar] [CrossRef]
- Glaeser, L.C.; House, M.; Vitt, D.H. Reclaiming to brackish wetlands in the Alberta oil sands: Comparison of responses to sodium concentrations for Carex atherodes and C. aquatilis. Plants 2021, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, L.C.; Vitt, D.H.; Ebbs, S. Responses of the wetland grass, Beckmannia syzigachne, to salinity and soil wetness: Consequences for wetland reclamation in the oil sands area of Alberta, Canada. Ecol. Eng. 2016, 86, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Koropchak, S.; Vitt, D.H.; Bloise, R.; Wieder, R.K. Fundamental paradigms, foundation species selection, and early plant responses to peatland initiation on mineral soils. In Restoration and Reclamation of Boreal Ecosystems; Vitt, D.H., Bhatti, J., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 76–100. [Google Scholar]
- Vitt, D.H.; House, M. Establishment of bryophytes from indigenous sources after disturbance from oil sands mining. Bryologist 2015, 118, 123–129. [Google Scholar] [CrossRef]
- Vitt, D.H.; House, M.; Hartsock, J.A. Sandhill Fen, an initial trial for wetland species assembly on in-pit substrates: Lessons after three years. Botany 2016, 94, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Hartsock, J.A.; Piercey, J.; House, M.; Vitt, D.H. An evaluation of water quality at Sandhill Wetland: Implications for reclaiming wetlands above soft tailings deposits in northern Alberta, Canada. Wetl. Ecol. Manag. 2021, 29, 1–17. [Google Scholar] [CrossRef]
- Hartsock, J.; House, M.; Clark, M.G.; Vitt, D.H. A comparison of plant communities and water chemistry at Sandhill Wetland to natural Albertan peatlands and marshes. Ecol. Eng. 2021, 169, 106313. [Google Scholar] [CrossRef]
- Biagi, K.M.; Clark, M.G.; Carey, S.K. Hydrological functioning of a constructed peatland watershed in the Athabasca oil sands region: Potential trajectories and lessons learned. Ecol. Eng. 2021, 166, 106236. [Google Scholar] [CrossRef]
- Syncrude, 2008. Syncrude Land Reclamation. Available online: https://www.syncrude.ca/environment/land-reclamation/ (accessed on 18 November 2019).
- Syncrude Canada Ltd. Composite Tailings Capping Knowledge Synthesis, Oil Sands Research and Information Network (OSRIN); University of Alberta: Edmonton, AB, Canada, 2020. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef] [Green Version]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- SigmaPlot, Version 11; Systat Software, Inc.: San Jose, CA, USA, 2008.
- Vitt, D.H.; Li, Y.; Belland, R.J. Patterns of bryophyte diversity in peatlands of continental western Canada. Bryologist 1995, 98, 218–227. [Google Scholar] [CrossRef]
- Slack, N.G.; Vitt, D.H.; Horton, D.G. Vegetation gradients of minerotrophically rich fens in western Alberta. Can. J. Bot. 1980, 58, 330–350. [Google Scholar] [CrossRef]
- Chee, W.L.; Vitt, D.H. The vegetation, surface water chemistry, and peat chemistry of moderate-rich fens in central Alberta, Canada. Wetlands 1989, 9, 227–262. [Google Scholar] [CrossRef]
- Vitt, D.H.; Wieder, R.K. The structure and function of bryophyte-dominated peatlands. In Bryophyte Biology: Second Edition; Goffinet, B., Shaw, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 357–392. [Google Scholar]
- Campbell, C.; Vitt, D.H.; Halsey, L.A.; Campbell, I.D.; Thormann, M.N.; Bayley, S.E. Net primary production and standing biomass in the northern continental wetlands. In Northern Forestry Centre Information Report NOR-X-369; Canadian forestry Service: Ottawa, ON, Canada, 2000; 57p. [Google Scholar]
- Vitt, D.H.; House, M. Bryophytes as key indicators of ecosystem function and structure of northern peatlands. Bryophyt. Divers. Evol. 2021, 43, 253–264. [Google Scholar] [CrossRef]
- Vitt, D.H.; Wieder, R.W.; Scott, K.D.; Faller, S. Decomposition and peat accumulation in rich fens of boreal Alberta, Canada. Ecosystems 2009, 12, 360–373. [Google Scholar] [CrossRef]
- Tahvanainen, T. Water chemistry of mires in relation to the poor-rich vegetation gradient and contrasting geochemical zones of the north-eastern Fennoscandian Shield. Folia Geobot. 2004, 39, 353–369. [Google Scholar] [CrossRef]
- Vitt, D.H.; Chee, W.L. The relationships of vegetation to surface water chemistry and peat chemistry in fens of Alberta, Canada. Vegetatio 1990, 89, 87–106. [Google Scholar] [CrossRef]
- Mollard, F.P.O.; Roy, M.C.; Frederick, K.; Foote, L. Growth of the dominant macrophyte Carex aquatilis is inhibited in oil sands affected wetlands in northern Alberta, Canada. Ecol. Eng. 2012, 38, 11–19. [Google Scholar] [CrossRef]
- Mollard, F.P.O.; Roy, M.C.; Foote, A.L. Typha latifolia plant performance and stand biomass in wetlands affected by surface oil sands mining. Ecol. Eng. 2013, 58, 26–34. [Google Scholar] [CrossRef]
- Sabovljevic, M.; Sabovljevic, A. Contribution to the coastal bryophytes of the northern Mediterranean: Are there halophytes among bryophytes. Phytol. Balc. 2007, 13, 131–135. [Google Scholar]
- Pouliot, R.; Rochefort, L.; Graf, M.D. Fen mosses can tolerate some saline conditions found in oil sands process water. Environ. Exp. Bot. 2013, 89, 44–50. [Google Scholar] [CrossRef]
- Stotler, R.E.; Crandall-Stotler, B. A synopsis of the liverwort flora of North America, north of Mexico. Ann. Missouri Bot. Gard. 2017, 102, 574–709. [Google Scholar] [CrossRef]
- BFNA. Flora of North America North of Mexico, Volume 27, Bryophyta, Part 1; Oxford University Press: Oxford, NY, USA, 2007. [Google Scholar]
- BFNA. Flora of North America North of Mexico, Volume 28, Bryophyta, Part 2; Oxford University Press: Oxford, NY, USA, 2014. [Google Scholar]
- Johnson, D.; Kershaw, L.; MacKinnon, A.; Pojar, J. Plants of the Western Forest Alaska to Minnesota Boreal and Aspen Parkland; Lone Pine Publishing: Edmonton, AB, Canada, 1995; 392p. [Google Scholar]


| Plant Community 1 | 2015 | 2017 | 2019 | 2021 |
|---|---|---|---|---|
| Alpha diversity (vasc) | 3.3 | 3.6 | 2.1 | 1.7 |
| Alpha diversity (bryo) | 0.0 | 0.1 | 0.0 | 0.0 |
| Alpha diversity (total) | 3.3 | 3.6 | 2.1 | 1.7 |
| Gamma diversity (vasc) | 9 | 14 | 7 | 5 |
| Gamma diversity (bryo) | 0 | 1 | 0 | 0 |
| Gamma diversity (total) | 9 | 15 | 7 | 5 |
| Beta diversity (vasc) | 2.7 | 3.9 | 3.3 | 2.9 |
| Beta diversity (bryo) | - | - | - | - |
| Beta diversity (total) | 2.7 | |||
| Evenness | 0.46 | 0.38 | 0.34 | 0.08 |
| No. of plots | 11 | 17 | 16 | 26 |
| Plant Community 2 | 2015 | 2017 | 2019 | 2021 |
| Alpha diversity (vasc) | 11.3 | 4.5 | 4.1 | 3.0 |
| Alpha diversity (bryo) | 3.3 | 0.3 | 0.4 | 0.7 |
| Alpha diversity (total) | 14.6 | 4.8 | 4.5 | 3.6 |
| Gamma diversity (vasc) | 68 | 32 | 30 | 17 |
| Gamma diversity (bryo) | 18 | 5 | 7 | 9 |
| Gamma diversity (total) | 86 | 37 | 37 | 26 |
| Beta diversity (vasc) | 6.0 | 7.7 | 7.3 | 5.7 |
| Beta diversity (bryo) | 5.5 | - | - | - |
| Beta diversity (total) | 5.9 | |||
| Evenness | 0.45 | 0.34 | 0.38 | 0.37 |
| No. of plots | 34 | 31 | 37 | 31 |
| Plant Community 3 | 2015 | 2017 | 2019 | 2021 |
| Alpha diversity (vasc) | 15.5 | 11.1 | 11.5 | 8.8 |
| Alpha diversity (bryo) | 4.9 | 5.8 | 3.5 | 2.0 |
| Alpha diversity (total) | 20.4 | 16.9 | 15.0 | 10.8 |
| Gamma diversity (vasc) | 73 | 50 | 48 | 38 |
| Gamma diversity (bryo) | 19 | 23 | 15 | 13 |
| Gamma diversity(total) | 92 | 73 | 63 | 51 |
| Beta diversity (vasc) | 4.7 | 4.5 | 4.2 | 4.3 |
| Beta diversity (bryo) | 3.9 | 4.0 | 4.3 | 6.5 |
| Beta diversity (total) | 4.5 | 4.3 | 4.2 | 4.7 |
| Evenness | 0.52 | 0.56 | 0.65 | 0.50 |
| No. of plots | 37 | 27 | 28 | 22 |
| Group 4 | 2015 | 2017 | 2019 | 2021 |
| Alpha diversity (vasc) | 17.8 | 14.0 | 8.7 | 4.6 |
| Alpha diversity (bryo) | 4.2 | 4.7 | 0.0 | 1.8 |
| Alpha diversity (total) | 22.0 | 18.7 | 8.7 | 6.4 |
| Gamma diversity(vasc) | 48 | 30 | 17 | 12 |
| Gamma diversity (bryo) | 7 | 10 | 0 | 7 |
| Gamma diversity(total) | 55 | 40 | 17 | 19 |
| Beta diversity (vasc) | 2.7 | 2.1 | 2.0 | 2.6 |
| Beta diversity (bryo) | 1.7 | 2.1 | - | 3.9 |
| Beta diversity (total) | 2.5 | 2.1 | 2.0 | 3.0 |
| Evenness | 0.78 | 0.63 | 0.56 | 0.57 |
| No. of plots | 5 | 3 | 3 | 5 |
| Year | 2015 | 2017 | 2019 | 2021 |
|---|---|---|---|---|
| Gamma diversity | 24 | 25 | 16 | 13 |
| No. of occurrences | 316 | 182 | 112 | 74 |
| No. of plots with >6 species | 17 | 19 | 6 | 3 |
| No. of plots with no bryophytes | 23 | 58 | 64 | 71 |
| Alpha diversity | 4.5 | 5.7 | 3.9 | 3.3 |
| No. of species occurring in >20% of plots | 5 | 2 | 1 | 0 |
| 2015 | 2017 | 2019 | 2021 | |||||
|---|---|---|---|---|---|---|---|---|
| Na (mg L−1) | 379 ± 71 | 89–918 (n = 14) | 476 ± 83 | 139–1232 (n = 22) | 364 ± 17 | 56–962 (n = 89) | 705 ± 35 | 64–1950 (n = 83) |
| Mg (mg L−1) | 60 ± 7.4 | 20–102 (n = 14) | 116 ± 15 | 41–360 (n = 21) | 76 ± 3 | 17–262 (n = 90) | 93 ± 3.6 | 27–209 (n = 82) |
| Ca (mg L−1) | 153 ± 19 | 79–302 (n = 14) | 275 ± 15 | 174–489 (n = 23) | 236 ± 7.5 | 101–477 (n = 88) | 169 ± 7.3 | 80–420 (n = 82) |
| pH | 8.10 ± 0.04 | 7.8–8.32 (n = 14) | 8.08 ± 0.03 | 7.8–8.31 (n = 21) | 7.47 ± 0.03 | 6.66–8.01 (n = 89) | 7.86 ± 0.02 | 7.43–8.24 (n = 84) |
| EC (mScm−1) | 2.37 ± 0.34 | 0.91–5.36 (n = 14) | 3.05 ± 0.18 | 1.83–5.89 (n = 29) | 2.75 ± 0.87 | 0.65–5.89 (n = 89) | 3.65 ± 0.10 | 1.26–6.41 (n = 84) |
| Na:Ca + Mg (mmol) | 2.56505403 | 1.73894056 | 1.72217267 | 3.71185117 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
House, M.; Vitt, D.H.; Glaeser, L.C.; Hartsock, J.A. Reclaiming Wetlands after Oil Sands Mining in Alberta, Canada: The Changing Vegetation Regime at an Experimental Wetland. Land 2022, 11, 844. https://doi.org/10.3390/land11060844
House M, Vitt DH, Glaeser LC, Hartsock JA. Reclaiming Wetlands after Oil Sands Mining in Alberta, Canada: The Changing Vegetation Regime at an Experimental Wetland. Land. 2022; 11(6):844. https://doi.org/10.3390/land11060844
Chicago/Turabian StyleHouse, Melissa, Dale H. Vitt, Lilyan C. Glaeser, and Jeremy A. Hartsock. 2022. "Reclaiming Wetlands after Oil Sands Mining in Alberta, Canada: The Changing Vegetation Regime at an Experimental Wetland" Land 11, no. 6: 844. https://doi.org/10.3390/land11060844






