Corn and Wheat Residue Management Effects on Greenhouse Gas Emissions in the Mid-Atlantic USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.2. Experiment 2 and 3
2.3. GHG Measurements
2.4. GHG Flux Calculation
2.5. Weather Data
2.6. Data Analysis
3. Results and Discussion
3.1. Data Analysis
3.2. Effect of Corn Stover Retained Rates on GHG Emissions
3.3. Effect of Wheat Straw Retained Rates on GHG Emissions
3.4. Combined Effect of Corn Stover and Wheat Straw Retained Rates on GHG Emissions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.A.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; et al. Agriculture, Forestry and Other Land Use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- IPCC. Implications of Different Definitions and Generic Issues. Land Use, Land-Use Change and Forestry; Cambridge University Press: Cambridge, UK, 2000; p. 375. [Google Scholar]
- Wilhelm, W.W.; Johnson, J.M.; Hatfield, J.L.; Voorhees, W.B.; Linden, D.R. Crop and soil productivity response to corn residue removal: A literature review. Agron. J. 2004, 96, 1–17. [Google Scholar] [CrossRef]
- Macedo, I.C.; Nassar, A.M.; Cowie, A.L.; Seabra, J.E.A.; Marelli, L.; Otto, M.; Wang, M.Q.; Tyner, W.E. Greenhouse Gas Emissions from Bioenergy; Souza, G.M., Victoria, R.L., Joly, C.A., Verdade, L.M., Eds.; Bioenergy Sustainability: Paris, France, 2015; pp. 582–617. [Google Scholar]
- Souza, G.M.; Ballester, M.V.R.; de Brito Cruz, C.H.; Chum, H.; Dale, B.; Dale, V.H.; Fernandes, E.C.; Foust, T.; Karp, A.; Lynd, L.; et al. The role of bioenergy in a climate-changing world. Environ. Dev. 2017, 23, 57–64. [Google Scholar] [CrossRef]
- Battaglia, M.L.; Thomason, W.E.; Fike, J.H.; Evanylo, G.; von Cossel, M.; Babur, E.; Iqbal, Y.; Diatta, A. The broad impacts of corn stover and wheat straw removal on crop productivity, soil health and greenhouse gases emissions: A review. GCB Bioenergy 2021, 13, 45–57. [Google Scholar] [CrossRef]
- Battaglia, M.; Fike, J.; Fike, W.; Sadeghpour, A.; Diatta, A. Miscanthus × giganteus biomass yield and quality in the Virginia Piedmont. Grassl. Sci. 2019, 65, 1–10. [Google Scholar] [CrossRef]
- Kumar, P.; Lai, L.; Battaglia, M.L.; Kumar, S.; Owens, V.; Fike, J.; Galbraith, J.; Hong, C.O.; Farris, R.; Crawford, R.; et al. Impacts of nitrogen fertilization rate and landscape position on select soil properties in switchgrass field at four sites in the USA. CATENA 2019, 180, 183–193. [Google Scholar] [CrossRef]
- Kumar, S.; Lai, L.; Kumar, P.; Feliciano, Y.M.V.; Battaglia, M.L.; Hong, C.O.; Owens, V.N.; Fike, J.; Farris, R.; Galbraith, J. Impacts of nitrogen rate and landscape position on soils and switchgrass root growth parameters. Agron. J. 2019, 111, 1046–1059. [Google Scholar] [CrossRef]
- Golden, J.S.; Handfield, R.B.; Daystar, J.; McConnell, T.E. An Economic Impact Analysis of the US Biobased Products Industry: A Report to the Congress of the United States of America. A Joint Publication of the Duke Center for Sustainability Commerce and the Supply Chain Resource Cooperative at North Carolina State University. 2015. Available online: https://biopreferred.gov/BPResources/files/EconomicReport_6_12_2015.pdf (accessed on 10 February 2022).
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sust. Energ. Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Singh, A.; Pant, D.; Korres, N.E.; Nizami, A.; Prasad, S.; Murphy, J.D. Key issues in life cycle assessment of ethanol production from lignocellulosic biomass: Challenges and perspectives. Bioresour. Technol. 2010, 101, 5003–5012. [Google Scholar] [CrossRef]
- Tenenbaum, D.J. Food vs. Fuel: Diversion of Crops Could Cause More Hunger. Environ. Health Persperct. 2008, 116, A254–A257. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.B. The Agricultural Ethics of Biofuels: The Food vs. Fuel Debate. Agric. J. 2012, 2, 339–358. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, M.L.; Groover, G.; Thomason, W.E. Harvesting and Nutrient Replacement Costs Associated with Corn Stover Removal in Virginia. Virginia Cooperative Extension Publication CSES-229NP. Blacksburg, VA. 2018. Available online: https://pubs.ext.vt.edu/content/dam/pubs_ext_vt_edu/CSES/cses-229/CSES-229.pdf (accessed on 10 February 2022).
- Babur, E.; Uslu, Ö.S.; Battaglia, M.L.; Diatta, A.; Fahad, S.; Datta, R.; Zafar-ul-Hye, M.; Danish, S. Studying soil erosion by evaluating changes in Physico-chemical properties of soils under different land-use types. J. Saudi. Soc. Agric. Sci. 2021, 20, 190–197. [Google Scholar] [CrossRef]
- Chum, H.; Faaij, A.; Moreira, J.; Berndes, G.; Dhamija, P.; Dong, H.; Gabrielle, B.; Eng, A.G.; Lucht, W.; Mapako, M. Bioenergy. In IPCC Special Report on Renewable Energy Sources and Climate Change Mitigation; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Seyboth, K., Matschoss, P., Kadner, S., Zwickel, T., Eickemeier, P., Hansen, G., Schlömer, S., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2011; pp. 209–332. [Google Scholar]
- Hassan, M.U.; Aamer, M.; Mahmood, A.; Awan, M.I.; Barbanti, L.; Seleiman, M.F.; Bakhsh, G.; Alkharabsheh, H.M.; Babur, E.; Shao, J.; et al. Management strategies to mitigate N2O emissions in agriculture. Life 2022, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Fassbinder, J.; Lamb, J.A. The impact of corn stover removal on N2O emission and soil respiration: An investigation with automated chambers. BioEnergy Res. 2014, 7, 503–508. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Khera, T.S.; Doran, J.W.; Bronson, K.F. Denitrification, N2O and CO2 fluxes in rice-wheat cropping system as affected by crop residues, fertilizer N and legume green manure. Biol. Fertil. Soils 2001, 34, 375–389. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Ball, B.C.; Scott, A.; Parker, J.P. Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality. Soil Till. Res. 1999, 53, 29–39. [Google Scholar] [CrossRef]
- Fontaine, S.; Bardoux, G.; Benest, D.; Verdier, B.; Mariotti, A.; Abbadie, L. Mechanisms of the priming effect in a savannah soil amended with cellulose. Soil Sci. Soc. Am. J. 2004, 68, 125. [Google Scholar] [CrossRef]
- Derrien, D.; Plain, C.; Courty, P.E.; Gelhaye, L.; Moerdijk-Poortvliet, T.C.W.; Thomas, F.; Versini, A.; Zeller, B.; Koutika, L.S.; Boschker, H.T.S.; et al. Does the addition of labile substrate destabilise old soil organic matter? Soil Biol. Biochem. 2014, 76, 149–160. [Google Scholar] [CrossRef]
- Badagliacca, G.; Ruisi, P.; Rees, R.M.; Saia, S. An assessment of factors controlling N2O and CO2 emissions from crop residues using different measurement approaches. Biol. Fertil. Soils 2017, 53, 547–561. [Google Scholar] [CrossRef] [Green Version]
- Dendooven, L.; Patiño-Zúñiga, L.; Verhulst, N.; Luna-Guido, M.; Marsch, R.; Govaerts, B. Global warming potential of agricultural systems with contrasting tillage and residue management in the central highlands of Mexico. Agric. Ecosyst. Environ. 2012, 152, 50–58. [Google Scholar] [CrossRef]
- Abalos, D.; Sanz-Cobena, A.; Garcia-Torres, L.; van Groenigen, J.W.; Vallejo, A. Role of maize stover incorporation on nitrogen oxide emissions in a non-irrigated Mediterranean barley field. Plant Soil 2013, 364, 357–371. [Google Scholar] [CrossRef] [Green Version]
- Lenka, N.K.; Lal, R. Soil aggregation and greenhouse gas flux after 15 years of wheat straw and fertilizer management in a no-till system. Soil Tillage Res. 2013, 126, 78–89. [Google Scholar] [CrossRef]
- Jin, V.L.; Baker, J.M.; Johnson, J.M.F.; Karlen, D.L.; Lehman, R.M.; Osborne, S.L.; Sauer, T.J.; Stott, D.E.; Varvel, G.E.; Venterea, R.T.; et al. Soil greenhouse gas emissions in response to corn stover removal and tillage management across the US Corn Belt. BioEnergy Res. 2014, 7, 517–527. [Google Scholar] [CrossRef] [Green Version]
- Parton, W.J.; Stewart, J.W.B.; Cole, C.V. Dynamics of C, N, P and S in grassland soils: A model. Biogeochemistry 1988, 5, 109–131. [Google Scholar] [CrossRef]
- Hartman, M.D.; Parton, W.J.; Del Grosso, S.F.; Easter, M. The Daily Century Ecosystem, Soil Organic Matter, Nutrient Cycling, Nitrogen Trace Gas, and Methane Model. NREL-Colorado State University, 2016. Available online: http://warnercnr.colostate.edu/lam/shared/25hFJQJu/DayCent_Manual_07.29.201/6.pdf (accessed on 10 February 2022).
- Kim, S.; Dale, B.E. Life cycle assessment of various cropping systems utilized for producing biofuels: Bioethanol and biodiesel. Biomass Bioenergy 2005, 29, 426–439. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E.; Jenkins, R. Life cycle assessment of corn grain and corn stover in the US. Int. J. Life Cycle Assess. 2009, 14, 160–174. [Google Scholar] [CrossRef]
- Argonne National Laboratory, GREET Model. 2017. Available online: https://greet.es.anl.gov/ (accessed on 10 February 2022).
- Wang, M.Q.; Han, J.; Haq, Z.; Tyner, W.E.; Wu, M.; Elgowainy, A. Energy and greenhouse gas emission effects of corn and cellulosic ethanol with technology improvements and land use changes. Biomass Bioenergy 2011, 35, 1885–1896. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Han, J.; Dunn, J.B.; Cai, H.; Elgowainy, A. Well-to-wheels energy use and greenhouse gas emissions of ethanol from corn, sugarcane and cellulosic biomass for US use. Environ. Res. Lett. 2012, 7, 045905. [Google Scholar] [CrossRef] [Green Version]
- Gentile, R.; Vanlauwe, B.; Chivenge, P.; Six, J. Interactive effects from combining fertilizer and organic residue inputs on nitrogen transformations. Soil Biol. Biochem. 2008, 40, 2375–2384. [Google Scholar] [CrossRef]
- Lin, S.; Iqbal, J.; Hu, R.; Shaaban, M.; Cai, J.; Chen, X. Nitrous oxide emissions from yellow brown soil as affected by incorporation of crop residues with different carbon-to-nitrogen ratios: A case study in central China. Arch. Environ. Contam. Toxicol. 2013, 65, 183–192. [Google Scholar] [CrossRef]
- Shan, J.; Yan, X. Effects of crop residue returning on nitrous oxide emissions in agricultural soils. Atmos. Environ. 2013, 71, 170–175. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Mosier, A.R.; Smith, K.A.; Winiwater, W. N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos. Chem. Phys. 2008, 8, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, M.L.; Groover, G.; Thomason, W.E. Value and Implications of Corn Stover Removal from Virginia Fields. Virginia Cooperative Extension Publication CSES-180. Blacksburg, VA. 2017. Available online: http://pubs.ext.vt.edu/CSES/CSES-180/CSES-180.html (accessed on 10 February 2022).
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NRCS, Natural Resource Conservation Service. Web Soil Survey. 2018. Available online: http://websoilsurvey.nrcs.usda.gov/app/HomePage.htm (accessed on 10 February 2022).
- USDA-NASS, Quick stats Lite (Beta). 2018. Available online: http://www.nass.usda.gov/Quick_Stats/Lite/index.php (accessed on 10 February 2022).
- Brann, D.E.; Holshouser, D.L.; Mullins, G.L. Agronomy Handbook; Virginia Tech Cooperative Extension: Blacksburg, VA, USA, 2000; Volume 424–100, p. 134. [Google Scholar]
- Abendroth, L.J.; Elmore, R.W.; Boyer, M.J.; Marlay, S.K. Corn Growth and Development. PMR 1009; Iowa State University Extension: Ames, IA, USA, 2011. [Google Scholar]
- SAS Institute. SAS 9.4 User’s Guide; SAS Inst.: Cary, NC, USA, 2014. [Google Scholar]
- Ball, B.C.; Crichton, I.; Horgan, G.W. Dynamics of upward and downward N2O and CO2 fluxes in ploughed or no-tilled soils in relation to water-filled pore space, compaction and crop presence. Soil Till. Res. 2008, 101, 20–30. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Change Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Borken, W.; Davidson, E.A.; Savage, K.; Gaudinski, J.; Trumbore, S.E. Drying and Wetting Effects on Carbon Dioxide Release from Organic Horizons. Soil Sci. Soc. Am. J. 2003, 67, 1888–1896. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Zhang, X.; McLaughlin, N.B.; Liang, A.; Jia, S.; Chen, X.; Chen, X. Effect of soil temperature and soil moisture on CO2 flux from eroded landscape positions on black soil in Northeast China. Soil Tillage Res. 2014, 144, 119–125. [Google Scholar] [CrossRef]
- Dalal, R.C.; Allen, D.E. Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot. 2008, 56, 369–407. [Google Scholar] [CrossRef]
- Gritsch, C.; Zimmermann, M.; Zechmeister-Boltenstern, S. Interdependencies between temperature and moisture sensitivities of CO2 emissions in European land ecosystems. Biogeosciences 2015, 12, 5981–5993. [Google Scholar] [CrossRef] [Green Version]
- Mosier, A.; Wassmann, R.; Verchot, L.; King, J.; Palm, C. Methane and nitrogen oxide fluxes in tropical agricultural soils, sources, sinks and mechanisms. Environ. Dev. Sustain. 2004, 6, 11–49. [Google Scholar] [CrossRef]
- Guzman, J.; Al-Kaisi, M.; Parkin, T. Greenhouse Gas Emissions Dynamics as Influenced by Corn Residue Removal in Continuous Corn System. Soil Sci. Soc. Am. J. 2015, 79, 612–625. [Google Scholar] [CrossRef]
- Behnke, G.D.; Zuber, S.M.; Pittelkow, C.M.; Nafziger, E.D.; Villamil, M.B. Long-term crop rotation and tillage effects on soil greenhouse gas emissions and crop production in Illinois, USA. Agric. Ecosyst. Environ. 2018, 261, 62–70. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilised fields: Summary of available measurement data. Glob. Biogeochem. Cycles 2002, 16, 1058. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Modeling global annual N2O and NO emissions from fertilised fields. Glob. Biogeochem. Cycles 2002, 16, 1080. [Google Scholar] [CrossRef]
- Novemberoa, R.; Tejeda, H.R. Evaluation of the N2O emissions from N in plant residues as affected by environmental and management factors. Nutr. Cycl. Agroecosyst. 2006, 75, 29–46. [Google Scholar] [CrossRef]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosyst. 2006, 74, 207–228. [Google Scholar] [CrossRef]
- De Klein, C.; Novemberoa, R.S.A.; Ogle, S.; Smith, K.A.; Rochette, P.; Wirth, T.C.; McConkey, B.G.; Mosier, A.; Rypdal, K. N2O Emissions from Managed Soils, and CO2 Emissions from Lime and Urea Application. In IPCC Guidelines for National Greenhouse Gas Inventories, Agriculture, Forestry and Other Land Use; IPCC: Geneva, Switzerland, 2006; Volume 4, pp. 1–54. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_11_Ch11_N2O&CO2.pdf (accessed on 1 June 2022).
- Duiker, S.W.; Lal, R. Crop residue and tillage effects on carbon sequestration in a Luvisol in central Ohio. Soil Till. Res. 1999, 52, 73–81. [Google Scholar] [CrossRef]
- Jacinthe, P.A.; Lal, R.; Kimble, J.M. Carbon budget and seasonal carbon dioxide emission from a central Ohio Luvisol as influenced by wheat residue amendment. Soil Till. Res. 2002, 67, 147–157. [Google Scholar] [CrossRef]
- McSwiney, C.P.; Robertson, G.P. Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Glob. Change Biol. 2005, 11, 1712–1719. [Google Scholar] [CrossRef]




| Location | Treatment | Corn Stover | Wheat Straw | Total Residue † |
|---|---|---|---|---|
| Mg ha−1 | ||||
| Blacksburg | 1 | 0.00 | - | - |
| 2 | 3.33 | - | - | |
| 3 | 6.66 | - | - | |
| 4 | 10.00 | - | - | |
| 5 | 20.00 | - | - | |
| New Kent (NK1 and NK2) | 1 | 0.00 | 0.00 | 0.00 |
| 2 | 0.00 | 1.00 | 1.00 | |
| 3 | 0.00 | 2.00 | 2.00 | |
| 4 | 0.00 | 3.00 | 3.00 | |
| 5 | 3.33 | 0.00 | 3.33 | |
| 6 | 3.33 | 1.00 | 4.33 | |
| 7 | 3.33 | 2.00 | 5.33 | |
| 8 | 3.33 | 3.00 | 6.33 | |
| 9 | 6.66 | 0.00 | 6.66 | |
| 10 | 6.66 | 1.00 | 7.66 | |
| 11 | 6.66 | 2.00 | 8.66 | |
| 12 | 6.66 | 3.00 | 9.66 | |
| 13 | 10.00 | 0.00 | 10.00 | |
| 14 | 10.00 | 1.00 | 11.00 | |
| 15 | 10.00 | 2.00 | 12.00 | |
| 16 | 10.00 | 3.00 | 13.00 | |
| 2015 | 2016 | 2017 | |
|---|---|---|---|
| Fertilizer application date | 4 May | 4 July | 19 June |
| N–P2O5–K2O, kg ha−1 | 43-43-43 | 56-0-0 | 56-0-0 |
| Seeding date | 3 May | 9 May | 11 May |
| Tillage | No-till | No-till | No-till |
| Genotype | P1498HR | P1498HR | P1498HR |
| Seed rate, seeds ha−1 | 69,189 | 69,189 | 69,189 |
| Seed rate, kg ha−1 | - | - | - |
| Row width, cm | 76 | 76 | 76 |
| Herbicides date | 4 May (pre-emerg); 10 June (post-emerg) | 9 May (all); 24 May (barley silage) | 2 May |
| Pre-emergent | |||
| 2,4 D (Dimethylamine salt) | 0.498 kg a.i. ha−1 | 0.498 kg a.i. ha−1 | |
| Bicep II Magnum (Atrazine + S-Metolachlor) ƛ | 0.573 kg a.i. ha−1 + 0.351 kg a.i. ha−1 | 0.717 kg a.i. ha−1 + 0.439 kg a.i. ha−1 | 0.717 kg a.i. ha−1 + 0.439 kg a.i. ha−1 |
| Atrazine 4L (Atrazine) ‡ | 0.235 kg a.i. ha−1 | ||
| Glystar plus (Glyphosate) § | 0.919 kg a.i. ha−1 | 0.316 kg a.i. ha−1 | 0.316 kg a.i. ha−1 |
| Post-emergent | |||
| Glystar plus (Glyphosate) § | 0.460 kg a.i. ha−1 | 0.316 kg a.i. ha−1 | |
| Insecticide date | None | None | None |
| Fungicide date | None | None | None |
| Irrigation management | None | None | None |
| Harvest date € | 8-October | Not harvested | 20-October |
| 2014 | 2015 | 2016 | 2017 | |||
|---|---|---|---|---|---|---|
| Wheat | Wheat (Cont.) | Soybean | Corn | Wheat | Wheat (Cont.) | |
| Fertilizer application date | 5 November | 19 March | - | 2 November | 15 March | |
| 2-April | 5-April | |||||
| N–P2O5–K2O, kg ha−1 | 40-60-80 | 67-0-0 | - | 40-60-80 | 67-0-0 | |
| 67-0-0 | - | 67-0-0 | ||||
| Seeding date | 28 October | - | 23 June | 15 April | 25 October | - |
| Tillage | No-till | No-till | No-till | No-till | No-till | No-till |
| Genotype | USG 3404 | - | Asgrow 5332 | Channel 197-31 | USG 3404 | USG 3404 |
| Seed rate, seeds ha−1 | - | - | 346,000 | 59,300 | ||
| Seed rate, kg ha−1 | 0 | - | - | - | 135 | - |
| Row width, cm | 19 | 19 | 19 | 76 | 19 | 19 |
| Herbicides | - | |||||
| Pre-emergent | 15 April | |||||
| Atrazine 4L (Atrazine) ₰ | - | - | - | 0.235 kg a.i. ha−1 | - | - |
| Glystar plus (Glyphosate) ∑ | - | - | - | 0.919 kg a.i. ha−1 | - | - |
| Post-emergent | 23 November | 1 April | - | 15 May | 21 November | 3 April |
| Metribuzin 75 (Metribuzin) ¥ | 0.039 kg a.i. ha−1 | - | - | - | 0.039 kg a.i. ha−1 | - |
| Prowl H2O (Pendimethalin) ₤ | 0.309 kg a.i. ha−1 | - | - | - | 0.309 kg a.i. ha−1 | - |
| Glystar plus (Glyphosate) ∑ | - | - | - | 0.460 kg a.i. ha−1 | - | - |
| Harmony SG (Thifensulfuron-methyl) | - | 0.092 kg a.i. ha−1 | - | - | - | 0.092 kg a.i. ha−1 |
| Insecticide date | 23-November | 1 April | - | - | 23-November | 1 April |
| Baythroid XL (ß-cyfluthrin) Ω | 0.002 kg a.i. ha−1 | - | - | - | 0.002 kg a.i. ha−1 | - |
| Warrior T (Lambda-cyhalothrin) † | - | 0.001 kg a.i. ha−1 | - | - | - | 0.001 kg a.i. ha−1 |
| Fungicide date | - | 1 April; 14 May | - | - | 2 April; 11 May | |
| QuiltXcel (Azoxystrobin + Propiconazole) ƛ | - | 0.022 + 0.017 kg a.i. ha−1 | - | - | 0.022 + 0.017 kg a.i. ha−1 | |
| Prosaro (Prothioconazole + Tebuconazole) § | - | 0.020 + 0.020 kg a.i. ha−1 | - | - | 0.020 + 0.020 kg a.i. ha−1 | |
| Growth regulator date | None | 1 April | - | - | - | 3 April |
| Palisade EC ‡ | - | 0.002 kg a.i. ha−1 | - | - | - | 0.002 kg a.i. ha−1 |
| Irrigation management | None | None | None | None | ||
| Harvest date | - | 22 June | 6 September | - | 22 June | |
| Blacksburg (BB) | Parameter | R2 | Pr > t | Range | Mean | |||
| Timing † | a | b | c | |||||
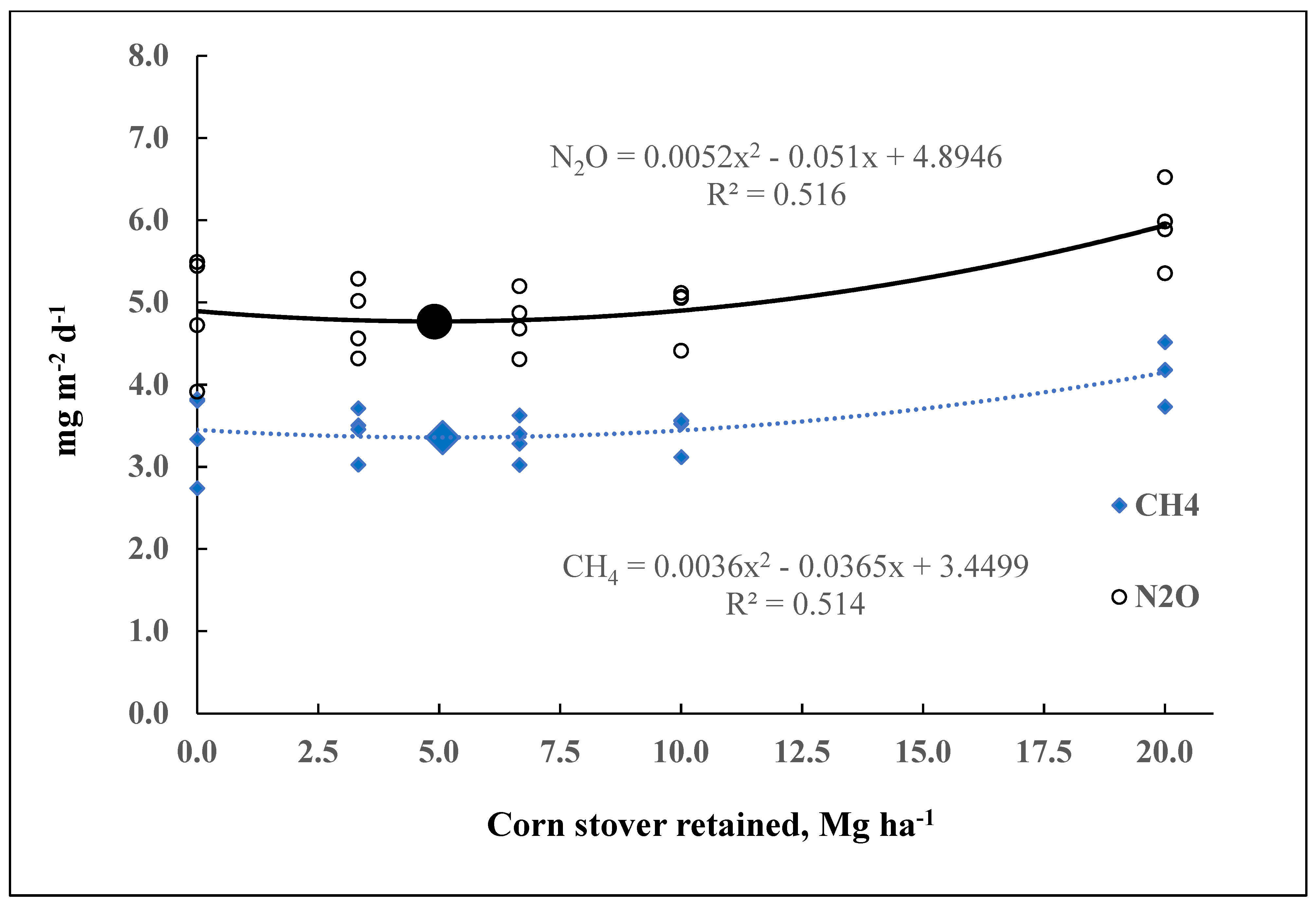
| June 2016 (CS) | CH4, mg m−2 day−1 | 0.0036 | −0.0365 | 3.45 | 0.514 | 0.048 | 2.74–4.52 | 3.55 |
| N2O, mg m−2 day−1 | 0.0052 | −0.0510 | 4.89 | 0.516 | 0.051 | 3.91–6.52 | 5.06 | |
| CO2, g m−2 day−1 | 0.0048 | −0.0556 | 4.69 | 0.258 | 0.195 | 3.83–6.40 | 4.78 | |
| June 2017 (CS) | CH4, mg m−2 day−1 | −0.0011 | 0.0413 | 4.18 | 0.121 | 0.363 | 3.42–5.01 | 4.38 |
| N2O, mg m−2 day−1 | −0.0025 | 0.0612 | 6.19 | 0.042 | 0.467 | 5.10–7.51 | 6.39 | |
| CO2, g m−2 day−1 | −0.0015 | 0.0523 | 6.60 | 0.067 | 0.494 | 5.59–8.11 | 6.86 | |
| New Kent, experiment 1 (NK1) | Parameter | R2 | Pr > t | Range | Mean | |||
| Timing ‡ | a | b | c | |||||
| October, 2016 (WS) | CH4, mg m−2 day−1 | −0.2239 | 0.5448 | 1.34 | 0.110 | 0.304 | 0.04–3.93 | 1.71 |
| N2O, mg m−2 day−1 | 0.0600 | −0.1371 | 0.96 | 0.020 | 0.669 | 0.02–0.92 | 0.41 | |
| CO2, g m−2 day−1 | 0.0095 | −0.5457 | 3.62 | 0.020 | 0.718 | 0.22–2.14 | 0.99 | |
| New Kent, experiment 2 (NK2) | Parameter | R2 | Pr > t | Range | Mean | |||
| Timing | a | b | c | |||||
| July 2016 (CS) | CH4, mg m−2 day−1 | 0.0152 | −0.1506 | 4.02 | 0.132 | 0.223 | 3.17–5.06 | 4.07 |
| N2O, mg m−2 day−1 | 0.0286 | −0.2667 | 5.44 | 0.202 | 0.151 | 3.15–6.91 | 5.42 | |
| CO2, g m−2 day−1 | 0.0375 | −0.3377 | 5.23 | 0.249 | 0.114 | 2.78–6.86 | 5.27 | |
| May 2017 (CS + WS) | CH4, mg m−2 day−1 | −0.0080 | 0.0911 | 4.04 | 0.107 | 0.020 | 3.57–5.22 | 4.17 |
| N2O, mg m−2 day−1 | −0.0096 | 0.1044 | 5.51 | 0.090 | 0.049 | 4.48–7.30 | 5.65 | |
| CO2, g m−2 day−1 | −0.0081 | 0.0780 | 5.97 | 0.074 | 0.135 | 5.19–7.78 | 6.02 | |
| October 2017 (CS + WS) | CH4, mg m−2 day−1 | −0.0258 | 0.4350 | 2.17 | 0.087 | 0.138 | 0.22–7.48 | 3.51 |
| N2O, mg m−2 day−1 | 0.0028 | −0.0364 | 3.69 | 0.001 | 0.885 | 0.67–7.55 | 3.62 | |
| CO2, g m−2 day−1 | −0.0099 | 0.1942 | 3.28 | 0.012 | 0.682 | 0.43–10.06 | 4.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battaglia, M.L.; Thomason, W.E.; Fike, J.H.; Evanylo, G.K.; Stewart, R.D.; Gross, C.D.; Seleiman, M.F.; Babur, E.; Sadeghpour, A.; Harrison, M.T. Corn and Wheat Residue Management Effects on Greenhouse Gas Emissions in the Mid-Atlantic USA. Land 2022, 11, 846. https://doi.org/10.3390/land11060846
Battaglia ML, Thomason WE, Fike JH, Evanylo GK, Stewart RD, Gross CD, Seleiman MF, Babur E, Sadeghpour A, Harrison MT. Corn and Wheat Residue Management Effects on Greenhouse Gas Emissions in the Mid-Atlantic USA. Land. 2022; 11(6):846. https://doi.org/10.3390/land11060846
Chicago/Turabian StyleBattaglia, Martin L., Wade E. Thomason, John H. Fike, Gregory K. Evanylo, Ryan D. Stewart, Cole D. Gross, Mahmoud F. Seleiman, Emre Babur, Amir Sadeghpour, and Matthew Tom Harrison. 2022. "Corn and Wheat Residue Management Effects on Greenhouse Gas Emissions in the Mid-Atlantic USA" Land 11, no. 6: 846. https://doi.org/10.3390/land11060846
APA StyleBattaglia, M. L., Thomason, W. E., Fike, J. H., Evanylo, G. K., Stewart, R. D., Gross, C. D., Seleiman, M. F., Babur, E., Sadeghpour, A., & Harrison, M. T. (2022). Corn and Wheat Residue Management Effects on Greenhouse Gas Emissions in the Mid-Atlantic USA. Land, 11(6), 846. https://doi.org/10.3390/land11060846










