Abstract
Herbivory is a common forest regeneration challenge across ecosystems. While fencing effectively reduces animal browse damage, it can be impractical. Tree shelters are an alternative forest restoration tool to protect seedlings from herbivory that may also provide a favorable microclimate. Yet, regeneration responses to tree shelters often vary among tree species, environmental conditions, and shelter specifications. To restore the once dominant Quercus virginiana (live oak) and its associated conservation values to subtropical U.S. maritime forests, control of animal browsing is critical. We evaluated the effects of tube and mesh tree shelters to exclude animal browse, combined with the use of controlled-release fertilizer to promote Q. virginiana seedling growth. After two growing seasons, mean seedling survival was 83% for protected seedlings, either from tube or mesh shelters, compared to 68% with non-sheltered seedlings. Seedlings in solid-wall tube shelters had significantly less browse incidence compared to both seedlings in mesh shelters and non-sheltered seedlings. Seedlings in tube shelters had greater height and diameter, followed by mesh shelters, and lastly, the no shelter treatment. Fertilizer resulted in higher browsing incidence and greater seedling height and diameter after the first growing season only, with no shelter treatment interactions. Our findings illustrate the efficacy of tree shelters to improve early regeneration success of Q. virginiana and may have application to the restoration of other forest ecosystems.
1. Introduction
Herbivory by a diverse range of animals is a common forest regeneration challenge across ecosystems [1,2,3,4,5,6,7]. Many factors such as landscape changes, predator reduction, and shifts in food sources have contributed to increased animal browsing pressure [8,9]. This browsing pressure on trees due to both herbivore behavior and herbivore population sizes is a great challenge to forest systems [9]. Herbivory is particularly damaging to newly planted seedlings due to the accessibility of terminal buds and increased mortality potential during the regeneration phase [10,11,12]. While various tools can be used to mitigate herbivory, it is important to consider the material cost, worker hours, maintenance, accessibility, site-specific factors, and many other factors that may influence the decision-making process.
Fencing is an effective tool for excluding animal browse and improving regeneration success; however, fencing requires expensive material and labor, and its use on certain sites can be restrictive based on landowner policies, legalities, size of the reforestation footprint (i.e., large burn scars), and physical access [13,14,15]. There are many alternatives, including individual tree shelters, nurse trees, dense natural regeneration, genetically select seed sources, repellents, fertilizer, and lethal population control, as well as the interactions of these alternatives; success is variable and depends on several factors [7,16,17,18,19,20]. From a meta-analysis of research literature on deer herbivory, Redick and Jacobs [19] concluded that fences and other physical protection such as tree shelters provided the most effective protection to seedlings from herbivory.
The height, color, and material of tree shelters can affect tree responses. Shelter height should be above the herbivore browse line to ensure that emerging seedlings are not browsed [21,22]. If the shelter height is too short and below the browse line, then shelter effectiveness decreases [19,23]. Shelter color and material can influence light, temperature, and airflow, thereby affecting the microenvironment within the shelter [18,19,24,25]. In comparison to solid tube shelters, fabric and mesh shelters typically have less of a greenhouse effect on the microenvironment, radiation is direct, and air movement occurs through the tree shelter, improving evaporative demand; these microenvironmental differences between both types of shelters have intensive effects on survival and growth [18,19,26,27]. In some cases, these microenvironments can be beneficial, but are both species- and location-dependent [18,27,28]. There is still a need to improve knowledge about the specific effects of the type of shelter in a wide array of forest ecosystems affected by herbivores.
In addition to controlling for animal browse, minimizing transplant stress and optimizing the growing environment for planted seedlings are important considerations. When seedlings are planted into clearcut or heavily modified overstory areas, seedlings can be quickly suppressed by competing vegetation [29,30]. Competition may impair basic plant physiological processes, thereby affecting seedling survival and growth due to a lack of soil nutrients, water, and light [31,32]. Additionally, seedlings must survive a period of transplant shock in which moisture and nutrients may be limiting [33]. Field fertilization at the time of planting can improve seedling survival and growth by reducing nutrient stress during establishment and promoting faster growth to outcompete vegetation and reach “free-to-grow” status above animal browse height [34]. Fertilization can also enhance general plant growth by supplementing nutrient-poor soils. However, fertilization may also increase animal browse [19]. Therefore, combining tree shelters with fertilization at planting may provide an effective silvicultural strategy to cope with high browsing pressure.
Two key limiting factors, animal browse and nutrient availability, both of which can inhibit the early establishment of newly planted seedlings, are pertinent to maritime forest regeneration. Maritime forests of the southern U.S. Atlantic coast occur along barrier islands and adjacent mainland from North Carolina to Florida. Quercus virginiana Mill. (live oak) is a semi-evergreen species and is a defining species of maritime forests. Maritime forests and Q. virginiana are well adapted to withstand and survive coastal stressors (e.g., saltwater spray and inundation, prevailing winds), thereby stabilizing soil, facilitating groundwater recharge, and providing critical habitat for threatened and endangered plant and animal species, especially migratory birds [35,36]. However, much of the maritime forest type has been heavily degraded along the southern Atlantic coast, necessitating increased restoration efforts [37,38]. Additionally, landscape changes and the elimination of predators across the eastern U.S. have increased deer populations and decreased oak regeneration [39,40]. Following this trend, Q. virginiana seedlings planted in fenced plots to exclude white-tailed deer (Odocoileus virginianus Zimm.) had nearly double the height and stem caliper of non-fenced seedlings [30]. This negative effect of animal browse is likely enhanced on barrier islands because of coastal ecosystems’ inherent abiotic stressors, including nutrient-poor soils [41]. Reduced light and nutrients from overstory and herbaceous competition decreased Q. virginiana seedling growth, particularly in comparison to clearcut or high light environments [42], suggesting that field fertilization (specifically with controlled released fertilizers) could be beneficial. In comparison to conventional fertilizers, controlled released fertilizers slowly release nutrients more directly to the root zone, limiting undesired benefits to competing vegetation and nutrient losses [33,43,44].
Although Q. virginiana is a dominant, charismatic species in this region, the foundational ecological and biological knowledge is still limited, especially as it relates to restoration efforts. Despite animal browse, nutrient limitation, and tree regeneration being a well-studied topic, overcoming this herbivory challenge and other site limiting factors to increase forest regeneration requires creative approaches specific to ecosystems and species. Given coastal Georgia’s subtropical climate, it is unclear how microenvironments created by tree shelters may affect seedling survival and growth. There are known species-specific effects of shelter type based upon species ecophysiological characteristics, so it is important to specifically consider species and ecosystems [18]. Therefore, our objective was to evaluate the effects of two types of tree shelters (solid-wall and mesh) in a maritime forest restoration context to exclude animal browse combined with the use of controlled-release fertilizer to facilitate Q. virginiana seedling establishment. We hypothesized that (i) Q. virginiana seedling survival and growth would be greatest for sheltered seedlings due to browsing control and this effect would be augmented further with field fertilization; (ii) between the two shelter types, seedlings in the mesh shelters would have better survival and growth due to better air flow and temperature modulation relative to the solid-wall tube shelters; and (iii) an interaction would occur in which non-sheltered seedlings with fertilizer would have reduced survival and growth due to increased palatability.
2. Materials and Methods
2.1. Experimental Sites
This experiment was conducted at two sites, Cannon’s Point Preserve (N 31°15′29″ W 81°20′45″) and Harris Neck National Wildlife Refuge (N 31°37′35″ W 81°16′04″), along the U.S. southern Atlantic coast in Georgia. The region is considered subtropical, with average summer high/low temperatures of 29.3/21.1 °C and winter average high/low temperatures of 19.6/10.1 °C [45]. The region receives an average annual precipitation of 114 cm [45].
Cannon’s Point Preserve is a 246-ha wilderness tract on the north end of St. Simon’s Island, with a mixture of fine sandy soils dominated by Mandarin and Cainhoy fine sands, and with 0–5% slopes. Pottsburg sand and Rutlege fine sand are also present [46]. Designated in 1962, Harris Neck National Wildlife Refuge is adjacent to St. Catherines Island and consists of approximately 1,117-ha. Soils at Harris Neck are also a mixture of fine sandy soils but are dominated by Palm Beach fine sand dark with Rutlege, Ona, and Galestown fine sands also present [46].
Both sites have some of the last intact maritime forests in Georgia; however, there are many areas dominated by abandoned land use (e.g., pine plantations, agriculture, airfield). At both sites, areas of abandoned pine plantations (mostly Pinus taeda L. with some P. elliotti Englem.) were clearcut to salvage timber in response to southern pine beetle (Dendroctonus frontalis Zimm.) outbreaks. White-tailed deer populations are present at both sites, creating high levels of browsing pressure on planting efforts. Additionally, Harris Neck Wildlife Refuge has a larger presence of wild pigs (Sus spp.) that are also destructive to planting efforts.
2.2. Experimental Design and Treatments
The experiment was a completely randomized factorial design at each site with two fixed independent factors, browse protection and fertilizer, and site as a random factor. With three levels of animal browse protection and two levels of fertilizer, there were six treatment combinations. Each treatment combination had a sample size of 50 (25 seedlings per site).
The browse protection levels were based on seedlings assigned to either a mesh tree shelter, a solid-wall tube tree shelter, or no tree shelter (non-sheltered) (Figure 1). The mesh shelter from Ben Meadows (Janesville, Wisconsin) was a Rigid Seedling Protector Tube® constructed of ultraviolet-inhibited polyethylene and polypropylene material. The mesh is designed to slowly photodegrade, ideally once seedlings have reached free-to-grow status. The mesh tree shelter diameter varied from 8.26 cm to 10.16 cm due to the nested shipping design. The solid-wall tube tree shelter from Tree Pro (West Lafayette, IN, USA) was a flat sheet, slit tube design. There were approximately 40 ventilation holes, 8 to 10 mm in diameter, and the ventilation holes extended from the top to the bottom of the shelter. The flat sheet was formed into a 10.5 cm diameter tube with releasable zip ties. A preliminary study we conducted found that the Tree Pro tree shelters in this experiment reduced photosynthetically active radiation (PAR) by an average of 44.1% (range of 26.1% to 58.1%) compared to full sunlight conditions across three measurement times (08:00, 11:00, and 14:00).
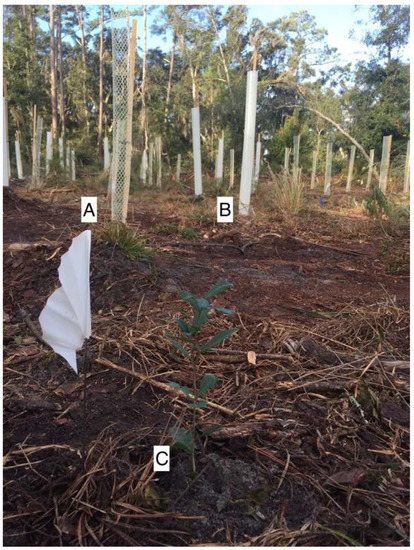
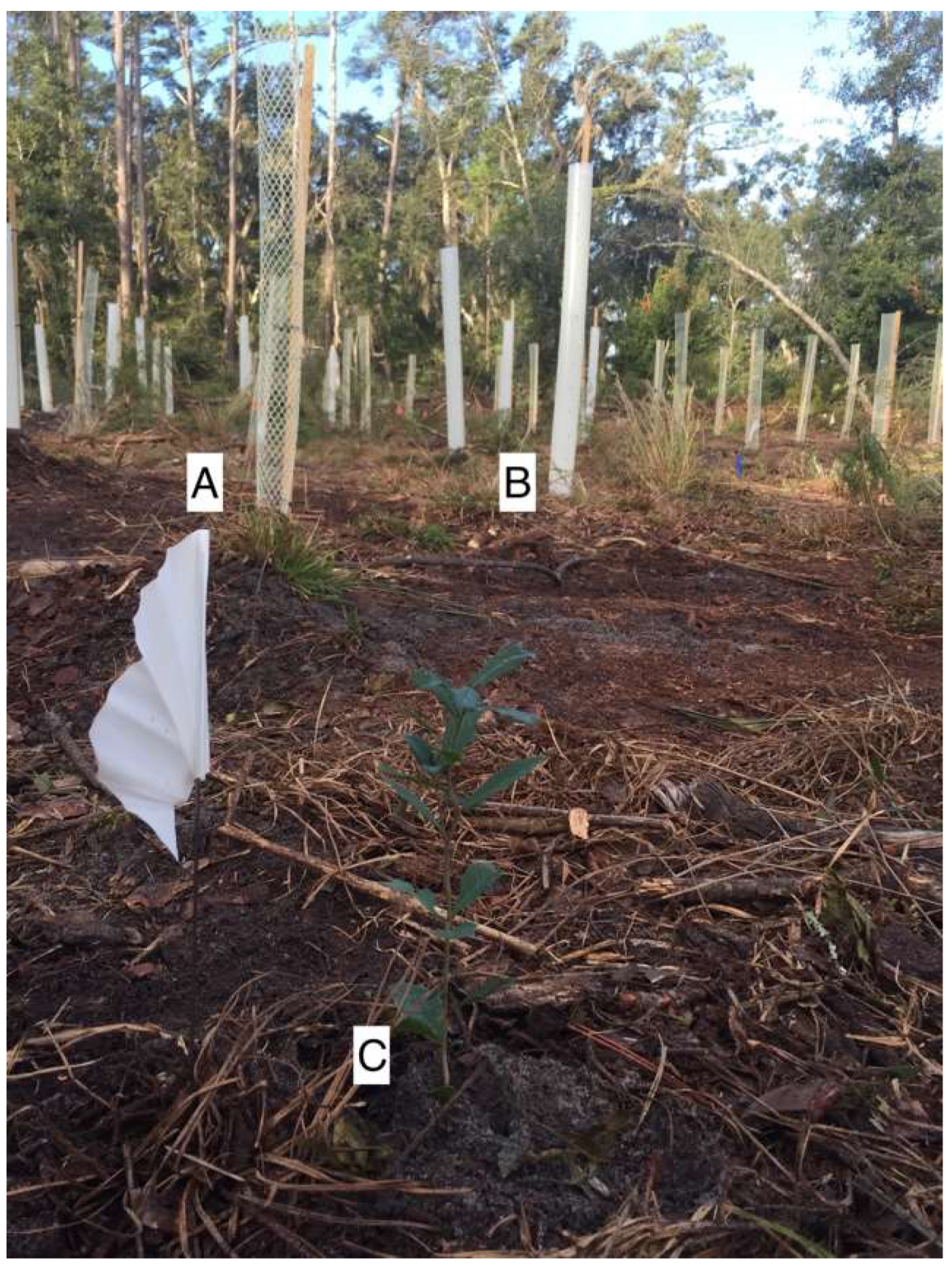
Figure 1.
(A) mesh tree shelter, (B) solid-wall tube tree shelter with ventilation holes, and (C) seedling with no shelter, all at the time of planting (photo credit: E. Thyroff).
Both tree shelters (mesh or solid-wall tube) were approximately 1.5 m tall, which is a sufficient height for protection from white-tailed deer in the eastern U.S. [16,19]. White oak wooden stakes were used to keep all tree shelters in place and remain protecting the seedlings until they reach free-to-grow status above the deer browse line.
The fertilizer treatment levels were defined by fertilizer applications of either 20 g or 0 g of controlled-release fertilizer (15N-9P-12K, Osmocote® Exact Lo-Start, Scotts Co., Marysville, OH, USA). The fertilizer was applied with a dibble bar adjacent to the seedling root plug at the time of planting. The controlled-release fertilizer had a labeled release period of 8 to 9 months.
2.3. Plant Material and Planting Operation
One-year-old Quercus virginiana container seedlings were planted in November 2018. Seedlings were obtained from Urban Forestry Services (Mike Campbell, Micanopy, FL, USA) with a north Florida seed source. Seedlings were grown in 98 cm3 conical containers with a top diameter of 4.8 cm and a depth of 8.9 cm (Groove Tube 51D, Growing Systems, Milwaukee, WI, USA). A total of 300 seedlings were hand planted 1.25 m apart (150 seedlings per site) in a rectangular plot arrangement (15 × 10 rows). Seedlings were well sorted and randomized resulting in an initial mean height of 23 ± 0.4 cm and an initial mean diameter of 2.3 ± 0.1 mm. An additional 54 seedlings were planted around the plot perimeter to maintain density and interspecific seedling competition at each site. Competing vegetation was mechanically controlled within the research plot prior to planting as the only site preparation and vegetation control applied.
2.4. Measurements
At the time of planting (November 2018), ground line diameter (mm) and height (cm) to the last live bud were measured. At the beginning of the following two growing seasons (March 2020, February 2021), ground line diameter and height to the last live bud were re-measured. Additionally, survival, browse evidence, and shelter damage were recorded as binary responses. Browse evidence was marked as present if there was any apparent damage caused by deer, rabbits, or pigs. Shelter damage was marked as present if the shelter had fallen and/or required re-alignment over the seedling. In addition, mildew presence was recorded as a binary response after the second growing season (February 2021), due to widespread mildew on seedlings in shelters.
At both sites and across the three shelter levels, 24 dataloggers (METER Group, Inc., product number MX2301A, Pullman, WA, USA) were installed at the base of randomly selected tree shelters representative of the treatments to collect air temperature (°C) and relative humidity (%). Data were registered every 15 min from April 2019 to January 2021. Datalogger data from the non-shelter treatments was not included because radiation shields were not installed around the dataloggers. This likely resulted in inaccurate data collection due to direct sun on the equipment. Since the mesh shelter openings allowed for typical air flow, it was assumed that the air temperature and relative humidity within the mesh shelter was comparable to the no shelter seedlings [47].
2.5. Statistical Analysis
A logistic regression model was used to analyze survival, browse evidence, and the presence of mildew. A general linear model was used to analyze air temperature. Diameter, height, crown width, and foliar N were analyzed with repeated measures general linear mixed models with shelter type, fertilizer, and time as fixed factors; site and individual tree as random factors. Residuals from all response variables were tested to ensure normality and homogeneity of variance. For all analyses across all measured response variables, interaction terms were found to be non-significant at p ≤ 0.05, and so the interaction terms were dropped from the final models and figures. When significant treatment effects were detected (p ≤ 0.05), Tukey’s HSD test was used to test for pairwise comparisons (α = 0.05). All data were analyzed with R software version 4.0.2 [48] using the lme4 package [49] for general linear models and logistic regression, the nlme package [50] for repeated measures models, and the multcomp package [51] for pairwise comparisons.
3. Results
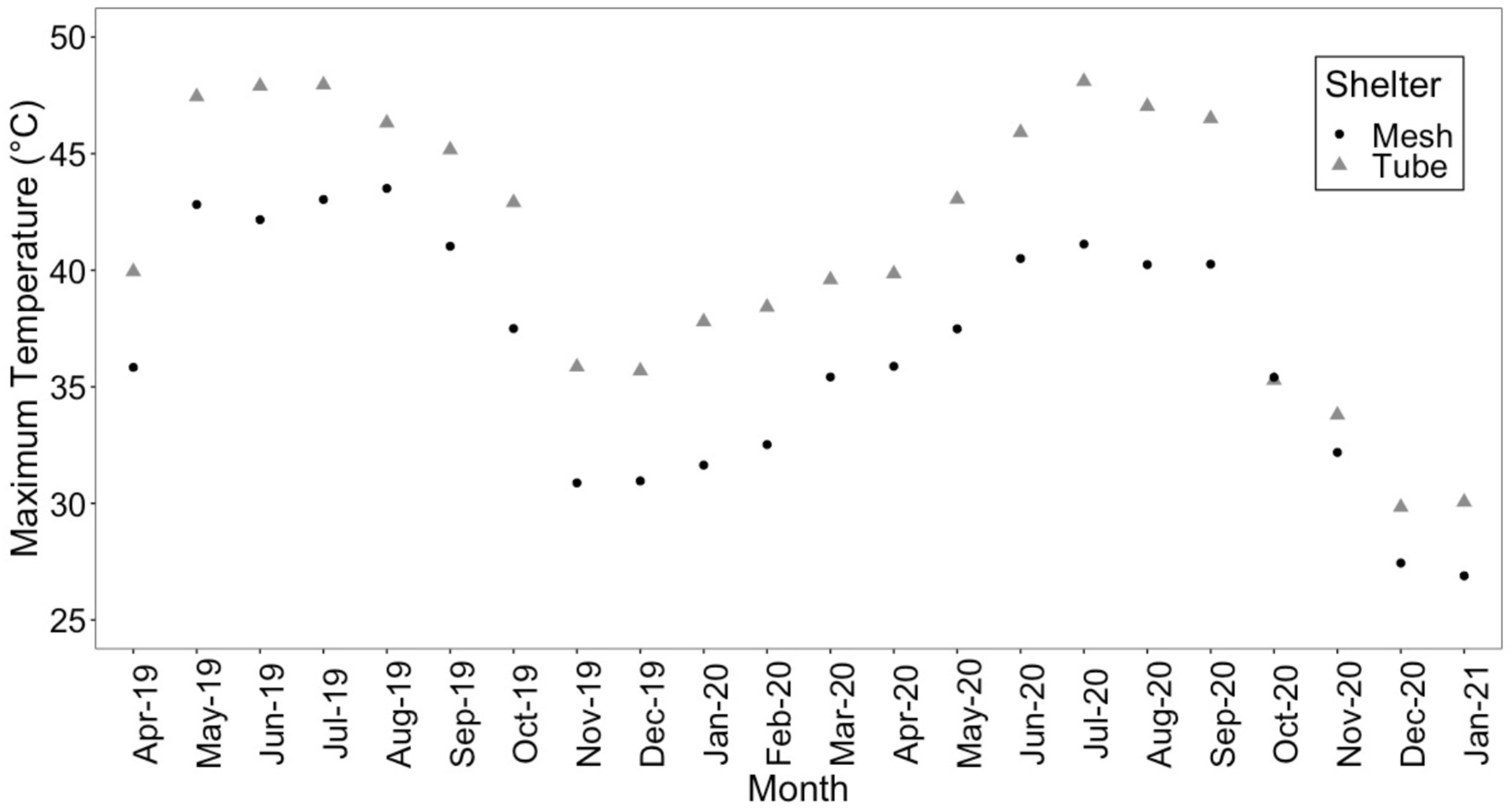
An examination of air temperature across the three shelter treatments found that the maximum and mean air temperature was higher in the solid-wall tube shelters (Figure 2 and Figure S1). Relative humidity followed the opposite pattern, with greater relative humidity more often in the mesh shelters (Figure S2). Shelter type had a significant effect on the presence of mildew on seedlings (X22,300 = 12.96, p = 0.002). Non-sheltered seedlings did not have mildew, whereas seedlings in solid-wall tube shelters had more mildew (27%) than seedlings in mesh shelters (8%). Repairs to tree shelters were required after the first growing season, with 11% of mesh shelters and 4% of tube shelters requiring maintenance. After the second growing season, 46% of mesh shelters needed to be repaired compared to 13% of tube shelters.
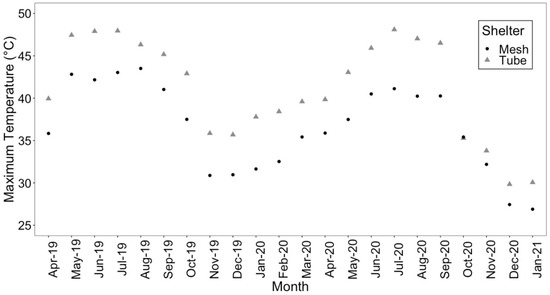
Figure 2.
Maximum air temperatures (°C) averaged by shelter treatment (mesh and tube shelters included) from April 2019 to January 2021. Air temperature was recorded every 15 min.
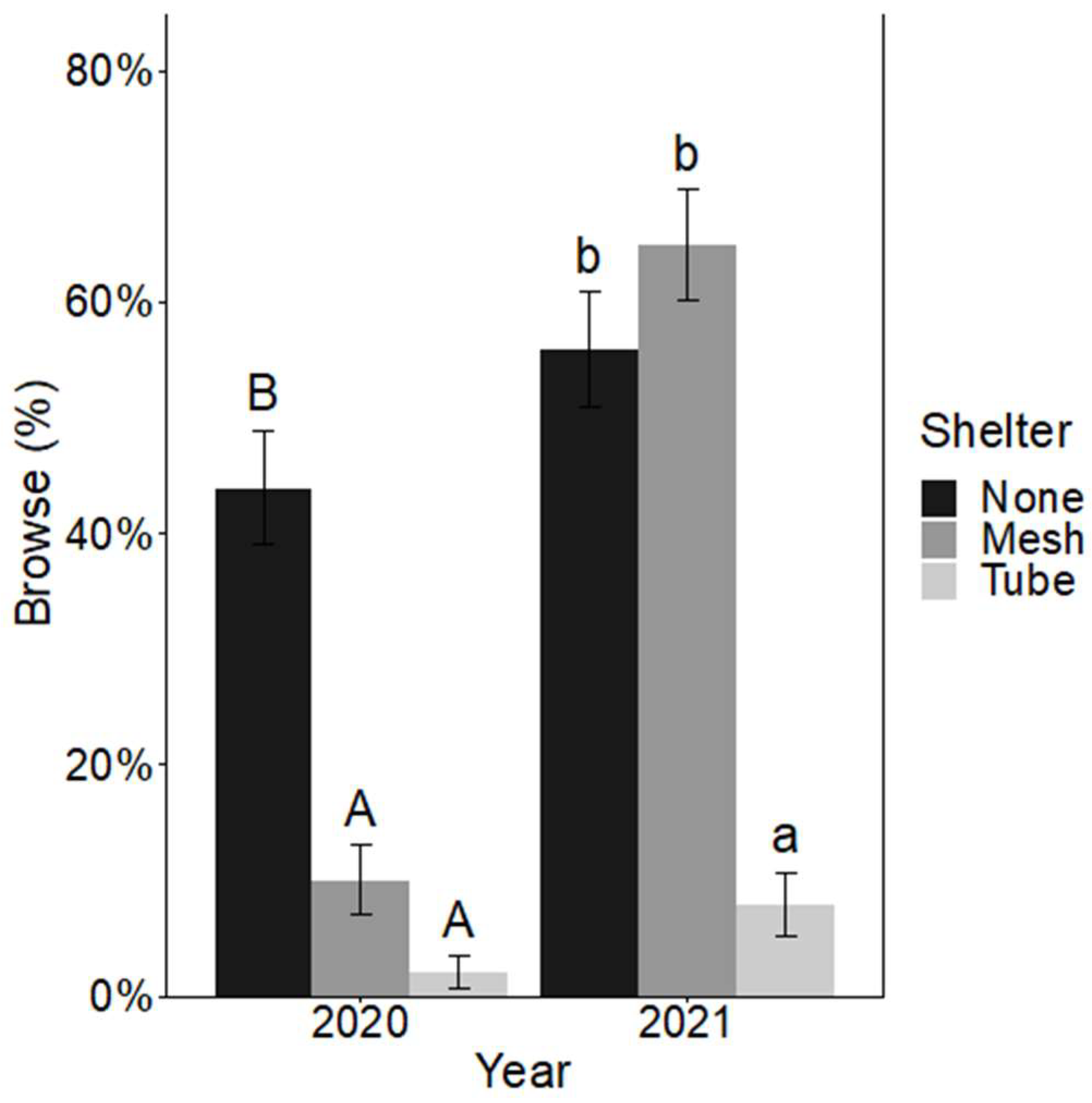
There was no significant interaction between shelter and fertilizer for browse incidence after either growing season. After the first growing season, only the effect of shelter on browse incidence was significant, with sheltered seedlings having significantly less browse incidence (2% browse for solid-wall tube shelters and 10% browse for mesh shelters) than non-sheltered seedlings at 44% (X22,300 = 47.79, p < 0.001) (Figure 3). After the second growing season, mesh shelters no longer had a significant effect on overall browse incidence compared to the non-sheltered seedlings. Seedlings in solid-wall tube shelters had significantly less browse incidence at 8% compared to both non-sheltered seedlings at 55% and seedlings in mesh shelters at 66% browse incidence (Figure 3) (X22,300= 55.36, p < 0.001).
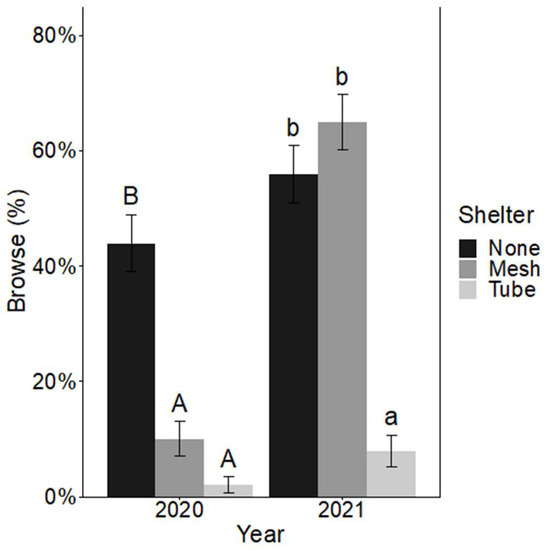
Figure 3.
Mean ± SE percent browse incidence (%) on Quercus virginiana seedlings after the first growing season (2020) and the second growing season (2021). Seedlings either had no tree shelter, a mesh tree shelter, or solid-wall tube tree shelter. Different letters (uppercase letters for 2020 comparisons and lowercase letters for 2021 comparisons) indicate significant differences among the shelter treatment levels (α = 0.05).
Average survival across all treatments after two growing seasons was 81 ± 6.8%, with no interactions between factors. Of the two main effects, only shelter was significant, with protected seedlings having significantly greater survival (90% solid-wall tube and 86% mesh shelters) than non-sheltered seedlings (68%, X22,295 = 32.18, p < 0.001).
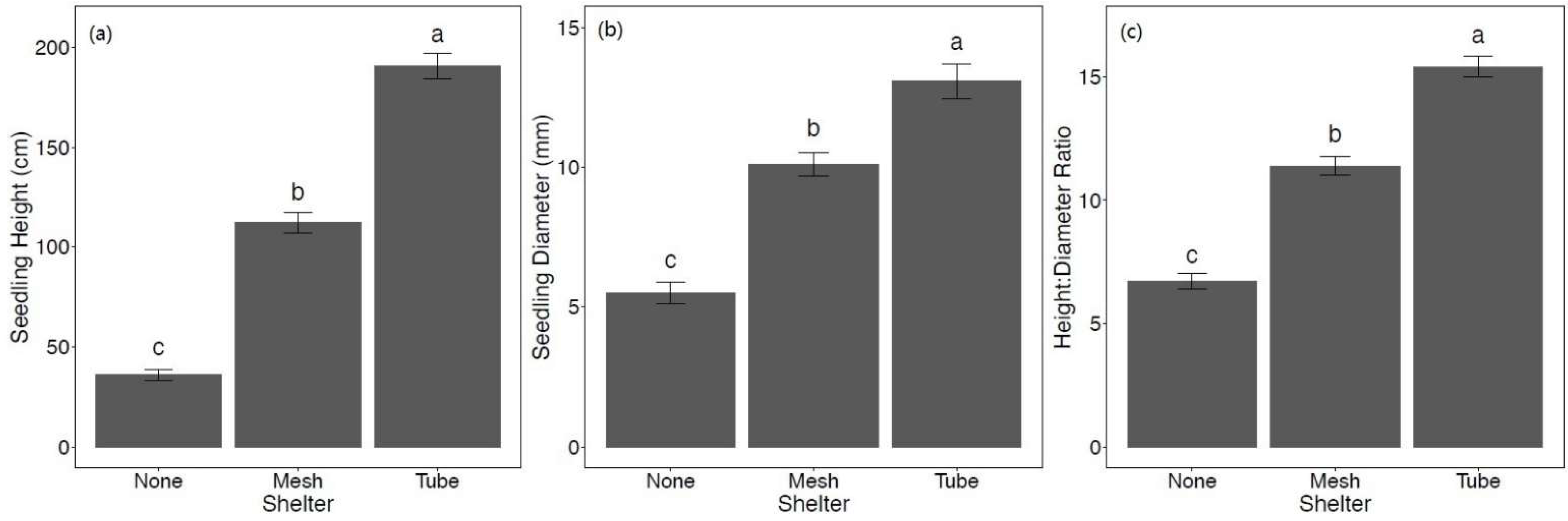
For both mean height and mean diameter, there was no significant interaction between shelter and fertilizer. After two growing seasons, the effect of shelters was significant for height (F2,241 = 174.43, p < 0.001), diameter (F2,241 = 49.14, p < 0.001), and height:diameter ratio (F2,241 = 122.00, p < 0.001). Overall, seedlings protected by solid-wall tube shelters had the greatest growth, as indicated by height and diameter followed by intermediate growth for mesh shelter seedlings, and the least growth for non-sheltered seedlings (Figure 4a,b). This pattern also held for height:diameter ratios (Figure 4c).
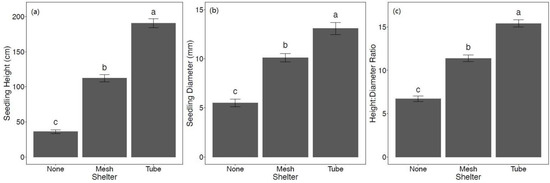
Figure 4.
Mean ± SE (a) height (cm), (b) diameter (mm), and (c) height:diameter ratio of Quercus virginiana seedlings after the second growing season (February 2021). Seedlings either had no tree shelter, a mesh tree shelter, or solid-wall tube tree shelter. Different letters indicate significant differences among the shelter treatment levels (α = 0.05).
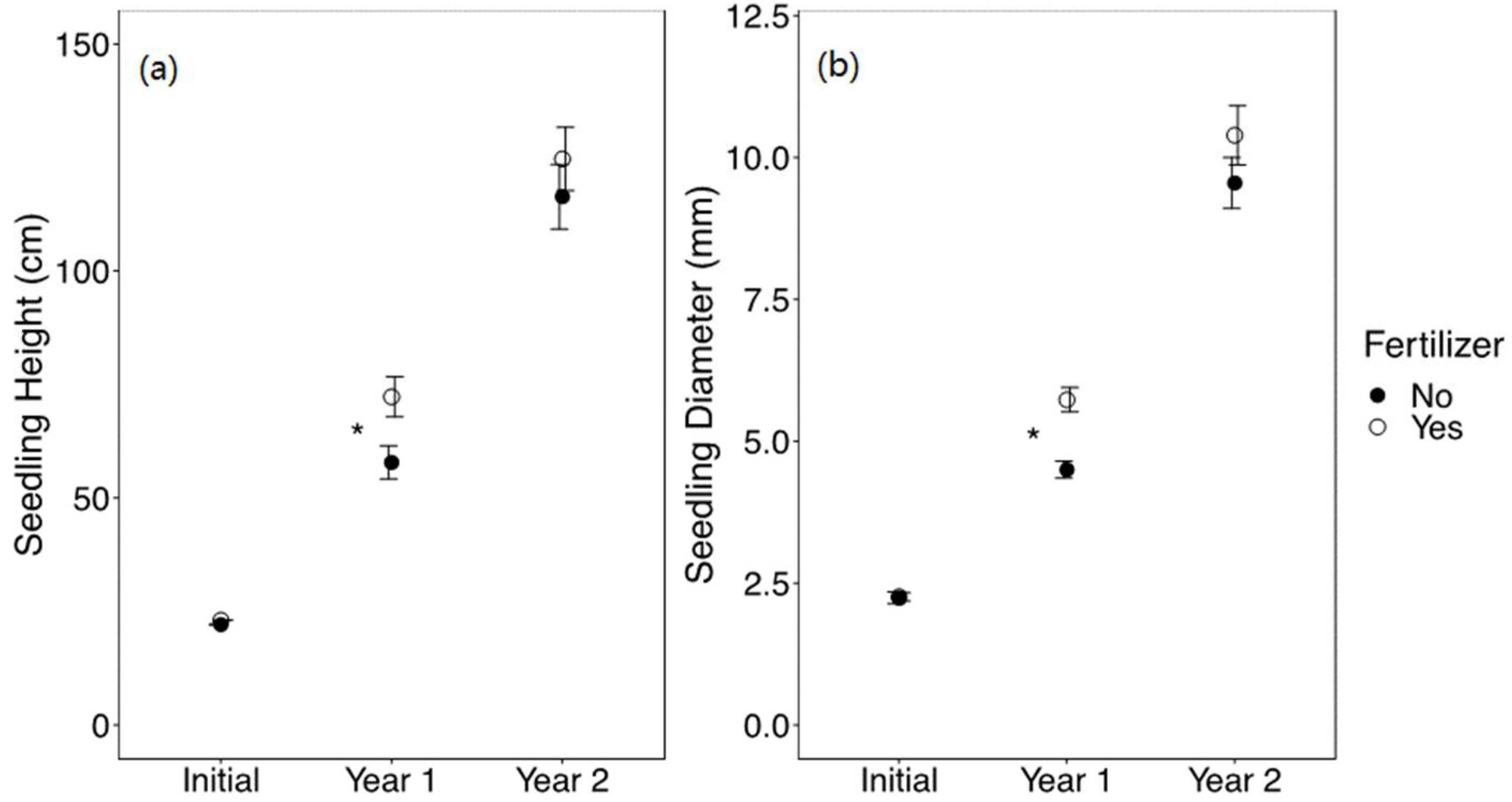
The effect of fertilizer on seedling growth was significant after the first growing season, with fertilized seedlings having greater height (F1,254 = 7.03, p = 0.009) and diameter (F1,254 = 10.22, p = 0.002); however, this effect was not significant after the second growing season (Figure 5). After the first growing season, fertilized seedlings had higher browse incidence at 21% than non-fertilized seedlings at 16% (X21,300 = 4.25, p = 0.039), whereas after the second growing season, there was no significant difference in browse incidence associated with fertilizer treatment (Figure S3).
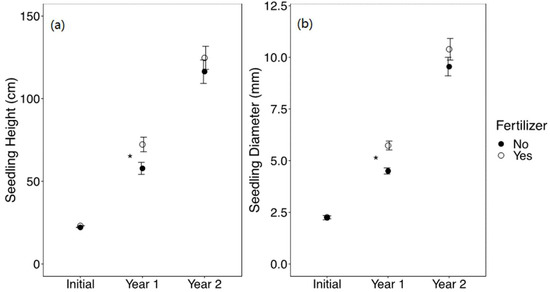
Figure 5.
Mean ± SE (a) height (cm) and (b) diameter (mm) of Quercus virginiana seedlings at the time of planting (initial), after the first growing season (Year 1), and after the second growing season (Year 2). Seedlings either did not receive fertilizer or received 20 g of fertilizer. Asterisks indicate significant differences among the fertilizer treatment levels (α = 0.05).
Throughout this experiment, we observed differences between the tube and mesh shelters in branch architecture and maintenance requirements (Figure 6). Trees in mesh shelters frequently had branches growing through the mesh holes, which sometimes restricted their lateral growth and caused branch deformities. Both shelter types required periodic maintenance to minimize damage to seedlings from fallen shelters (Figure 6).

Figure 6.
Clockwise from top-left: successful tube shelter after one growing season (photo credit: D. Jacobs), successful mesh shelter after one growing season (photo credit: D. Jacobs), an unsheltered seedling after one growing season (photo credit: D. Jacobs), a fallen tube after two growing seasons (photo credit: C. Redick), a mesh shelter with some branches growing through it after one growing season (photo credit: D. Jacobs).
4. Discussion
Tree shelters have been used in a range of ecosystems to prevent seedling herbivory [28] and our results of increased survival and growth suggest that tree shelters are highly effective at protecting Quercus virginiana seedlings in maritime forests of the U.S. southern Atlantic coast (Figure 6). Furthermore, our experimental results suggest that individual tree shelters can serve as an effective alternative to fencing for browse protection. Although we did not have a fencing treatment in this experiment, we can draw comparisons to results from Thyroff et al. [30] that had a fencing treatment two years earlier at the same site. Thyroff et al. [30] reported that fencing significantly increased Q. virginiana seedling height, diameter, and crown width. Specifically, after two growing seasons, height and diameter increased on average by 200% in fenced plots compared to non-fenced plots. Compared to this study, after two growing seasons, diameter increased on average by 150% and 100% for solid-wall tube and mesh shelters, respectively, compared to non-sheltered seedlings. Diameter gains in this study were not nearly as large as gains observed with the fenced plots reported by Thyroff et al. [30]. However, height after two growing seasons increased on average by 400% and 200% for solid-wall tube and mesh shelters, respectively, compared to non-sheltered seedlings, suggesting equal or better height performance compared to the use of fencing.
Such increased growth, though not universal, has been observed in many other studies with tree shelters for various species and climates [27,28,52]. Protection from deer browse as supported by reduced deer browse incidence (Figure 3) yielded increased growth of sheltered Q. virginiana seedlings. However, contrary to our hypothesis (ii), the solid-wall tube shelter had greater height and diameter growth than mesh shelters (Figure 4). This could be due to the increase in browse incidence for seedlings protected by mesh in the second growing season (Figure 3) associated with more deer browse incidence on lateral branches for mesh sheltered seedlings as the seedlings grew out from mesh openings. While mesh shelters protect seedlings from most browse, lateral branches (and sometimes terminal leaders) that grew through the mesh were browsed by deer. Browsed branches and buds represent lost photosynthetic capacity that could have otherwise been utilized. Previous studies have noted similar results [27]. However, because the buds and leaves inside the mesh shelters were protected, this treatment still yielded greater seedling growth compared to non-sheltered seedlings (Figure 4). Other studies have found similar differences in height growth between mesh and solid-wall shelters, with mesh having an intermediate effect [26,27] or even no effect [52]. Yet, a meta-analysis by Abe [28] found a lack of differences in height growth between trees in mesh versus solid-wall tube shelters. For diameter growth, some studies have found that mesh shelters have a greater positive effect on diameter growth [28] and others, such as Sharew and Hairston-Strang [27] also found an intermediate effect of mesh shelters on diameter compared to solid-wall shelters and controls, similar to our results. Despite other studies that have shown tube shelters increased height growth at the expense of diameter growth [53], our study showed that Q. virginiana responded by increasing height and diameter simultaneously. Height growth was most rapid for solid-wall tube shelters, resulting in higher height:diameter ratios (Figure 4), which may be due to reduced PAR levels and because seedlings had to grow vertically as there were no mesh gaps for lateral growth. Diameter and height growth reflect allocation patterns of young trees in response to complex interacting factors such as light intensity, abiotic stress, and shade and stress tolerance of the species. In general, seedlings tend to reduce diameter growth and increase height growth in response to shading [54]. However, tree shelters vary greatly in light transmission values, which could explain the differences in allocation responses among studies [25]. In our experiment, light transmission of the solid-wall tube shelter was relatively high (average PAR reduction of 44.1% as compared to other shelters that have transmissivity as low as 20% [25]), reducing the allocation reaction that inhibits diameter growth at the expense of height.
The improved greenhouse conditions generated in the solid-wall tube shelters was another reason that these shelters may have yielded greater growth than mesh shelters. Solid-wall shelters are known to create a greenhouse environment favorable to the growth of seedlings [26,27,52]. Warmer air temperatures were observed in the solid-wall tube resulting in a greenhouse effect year-round, with the highest maximum temperatures during summer months (Figure 2). Surprisingly, these high temperatures did not appear to damage the seedlings. Q. virginiana, which is adapted to warm, humid coastal maritime climates, may be able to withstand heat stress well, or perhaps are able to persist and repair; similar to seedlings withstanding high light and temperature stressors in clearcut stands [42]. In this study, maximum temperature occurrence was during a season that typically has high water availability from regular thunderstorms. Perhaps if multiple stressors occurred simultaneously (e.g., drought) with the high temperatures, then that may result in a negative impact on seedling performance. Apart from a greenhouse effect that explains growth increments in tubes, improvements in survival suggest that tube shelters exert a protective effect against other abiotic effects. For instance, Oliet et al. [18] suggested that tube shelters not only protected seedlings from browse compared to mesh, but also reduced air movement that increased transpiration demand and water stress. Additionally, the greenhouse effect during the winter months may have been more beneficial than any potential negative effect of high temperatures during the summer months.
The influence of fertilization on seedling growth and browse incidence was evident only during the first growing season (Figure 3 and Figure S3). Again, contrary to our hypotheses (i & iii), by the second growing season, the effect of fertilizer was not detected (Figure 5). After the first year, fertilized seedlings were still short enough (<1 m) for deer to access their terminal buds. It appears that the release rate of the fertilizer applied (8 to 9 months) made nutrients available for increased growth during the first growing season. Thus, the diminished fertilizer treatment effect was likely the result of the rapid release rate, Q. virginiana nutrient requirements, and general acclimation to the specific soils at each site. While fertilizer can help seedlings to grow above the browse line, in some studies, fertilizer has had adverse effects on seedlings by increasing the likelihood of browse, as it did for our study after the first growing season. This depends on how fertilizers affect the palatability of the seedlings to deer [10,55,56]. However, the increase in browse for fertilized seedlings during the first year was not reflected in the level of mortality or height observed at the same time in this study.
From a practical application, maintenance, site goals, feasibility, and cost must be considered when using tree shelters as an alternative to fencing. In addition to fixing fallen shelters (Figure 6), deciding when to remove browse protection is an important consideration. Removal must be carefully timed by waiting until seedlings become free-to-grow with a height above the browse line [57] and before shelters begin to restrict larger saplings [58]. While fencing also requires maintenance and removal decisions, abandoned fencing will not harm the developing trees. Deciding between tree shelters and fencing also depends on the goals of the planting site. Tree shelters only protect individual seedlings, whereas fencing protects all plants within an area. Protecting an entire area could be desirable for other plants and support a more holistic understory restoration; however, undesired (e.g., invasive) plants are also protected and could become competitors. Lastly, the feasibility of acquiring materials (whether tree shelters or fencing) and the associated costs, as well as transportation and installation of materials, should be considered. Shelters may be less expensive and easier to install for smaller plantings, but fences can become increasingly more cost-effective with increasing area, depending on planting density [59].
5. Conclusions
Tree shelters of the appropriate height and type for the target species have proven successful in the restoration of many tree species and forest systems, and we have shown that this also applies to the Quercus virginiana of maritime forests. Solid-wall shelters were more effective than mesh shelters at reducing browse and may have positively altered the seedling microclimate, resulting in the greatest growth response. Thus, restoration land managers and foresters hoping to successfully regenerate Q. virginiana should consider using solid-wall tree shelters on plantings, as well as in cases where fences are not desirable or feasible. We found that the benefit of fertilizers was minimal and short-lived for Q. virginiana under these experimental conditions. While our study was specific to Q. virginiana in the southern U.S. Atlantic coast, this research improves the scientific knowledge base of tree shelters for forest restoration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/land11070966/s1, Figure S1. Mean air temperatures (°C) averaged by shelter treatment (mesh and tube shelters included) from April 2019 to January 2021. Air temperature was recorded every 15 min; Figure S2. Mean percent relative humidity (%) averaged by shelter treatment (mesh and tube shelters included) from April 2019 to January 2021. Relative humidity was recorded every 15 min; Figure S3. Percent browse (%) of fertilized and non-fertilized seedlings after the first growing season (2020) and second growing season (2021). Different letters (uppercase letters for 2020 comparisons and lowercase letters for 2021 comparisons) indicate significant differences among the shelter treatment levels (α = 0.05).
Author Contributions
Conceptualization, J.A.O., O.T.B., D.F.J.; methodology, E.C.T., C.H.R., J.A.O., O.T.B., D.F.J.; validation, J.A.O., O.T.B., D.F.J.; formal analysis, E.C.T., C.H.R.; investigation, E.C.T., C.H.R.; resources, O.T.B., D.F.J.; data curation, E.C.T., C.H.R.; writing—original draft preparation, E.C.T., C.H.R., J.A.O., O.T.B., D.F.J.; writing—review and editing, E.C.T., C.H.R., J.A.O., O.T.B., D.F.J.; visualization, E.C.T., C.H.R., D.F.J.; supervision, J.A.O., O.T.B., D.F.J.; project administration, O.T.B., D.F.J.; funding acquisition, O.T.B., D.F.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by USDA National Institute of Food and Agriculture McIntire-Stennis Cooperative Forestry Program (Accession: NM1017308 and IND011535), Hardwood Tree Improvement and Regeneration Center, the Fred M. van Eck Forest Foundation, JTH Forestry Center with NMSU, the St. Simon’s Land Trust, and Harris Neck Wildlife Refuge. In kind support was received from the St. Simon’s Land Trust, The Nature Conservancy, and Georgia Department of Natural Resources.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Acknowledgments
Thank you to Stephanie Knox of St. Simon’s Land Trust and Chuck Hayes of Harris Neck Wildlife Refuge for their support of this project. Thank you to Edward Oehlman, Andrei Toca, Zach Allen, Camila Montoya Gomez, Steve Kipp, and Taylor Senegal for valuable assistance with this project. The tube tree shelters were donated by Tree Pro (West Lafayette, Indiana).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Griscom, H.P.; Griscom, B.W.; Ashton, M.S. Forest Regeneration from Pasture in the Dry Tropics of Panama: Effects of Cattle, Exotic Grass, and Forested Riparia. Restor. Ecol. 2009, 17, 117–126. [Google Scholar] [CrossRef]
- Takatsuki, S. Effects of Sika Deer on Vegetation in Japan: A Review. Biol. Conserv. 2009, 142, 1922–1929. [Google Scholar] [CrossRef]
- Cole, R.J.; Litton, C.M.; Koontz, M.J.; Loh, R.K. Vegetation Recovery 16 Years after Feral Pig Removal from a Wet Hawaiian Forest. Biotropica 2012, 44, 463–471. [Google Scholar] [CrossRef]
- Ashton, M.S.; Goodale, U.M.; Bawa, K.S.; Ashton, P.S.; David Neidel, J. Restoring Working Forests in Human Dominated Landscapes of Tropical South Asia: An Introduction. For. Ecol. Manag. 2014, 329, 335–339. [Google Scholar] [CrossRef]
- Suzuki, M.; Ito, E. Combined Effects of Gap Creation and Deer Exclusion on Restoration of Belowground Systems of Secondary Woodlands: A Field Experiment in Warm-Temperate Monsoon Asia. For. Ecol. Manag. 2014, 329, 227–236. [Google Scholar] [CrossRef]
- Leiva, M.J.; Pérez-Romero, J.A.; Mateos-Naranjo, E. The Effect of Simulated Damage by Weevils on Quercus Ilex Subsp. Ballota Acorns Germination, Seedling Growth and Tolerance to Experimentally Induced Drought. For. Ecol. Manag. 2018, 409, 740–748. [Google Scholar] [CrossRef]
- Maltoni, A.; Mariotti, B.; Tani, A.; Martini, S.; Jacobs, D.F.; Tognetti, R. Natural Regeneration of Pinus Pinaster Facilitates Quercus Ilex Survival and Growth under Severe Deer Browsing Pressure. For. Ecol. Manag. 2019, 432, 356–364. [Google Scholar] [CrossRef]
- Russell, F.L.; Zippin, D.B.; Fowler, N.L. Effects of White-Tailed Deer (Odocoileus Virginianus) on Plants, Plant Populations and Communities: A Review. Am. Midl. Nat. 2001, 146, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Beguin, J.; Tremblay, J.P.; Thiffault, N.; Pothier, D.; Côté, S.D. Management of Forest Regeneration in Boreal and Temperate Deer-Forest Systems: Challenges, Guidelines, and Research Gaps. Ecosphere 2016, 7, e01488. [Google Scholar] [CrossRef]
- Burney, O.T.; Jacobs, D.F. Ungulate Herbivory of Boreal and Temperate Forest Regeneration in Relation to Seedling Mineral Nutrition and Secondary Metabolites. New For. 2013, 44, 753–768. [Google Scholar] [CrossRef]
- Burney, O.T.; Jacobs, D.F. Ungulate Herbivory of Regenerating Conifers in Relation to Foliar Nutrition and Terpenoid Production. For. Ecol. Manag. 2011, 262, 1834–1845. [Google Scholar] [CrossRef]
- Woolery, P.O.; Jacobs, D.F. Planting Stock Type and Seasonality of Simulated Browsing Affect Regeneration Establishment of Quercus rubra. Can. J. For. Res. 2014, 44, 732–739. [Google Scholar] [CrossRef]
- Fargione, J.; Haase, D.L.; Burney, O.T.; Kildisheva, O.A.; Edge, G.; Cook-Patton, S.C.; Chapman, T.; Rempel, A.; Hurteau, M.D.; Davis, K.T.; et al. Challenges to the Reforestation Pipeline in the United States. Front. For. Glob. Chang. 2021, 4, 629198. [Google Scholar] [CrossRef]
- Friday, J.B.; Cordell, S.; Giardina, C.P.; Inman-Narahari, F.; Koch, N.; Leary, J.J.K.; Litton, C.M.; Trauernicht, C. Future Directions for Forest Restoration in Hawai’i. New For. 2015, 46, 733–746. [Google Scholar] [CrossRef]
- Löf, M.; Madsen, P.; Metslaid, M.; Witzell, J.; Jacobs, D.F. Restoring Forests: Regeneration and Ecosystem Function for the Future. New For. 2019, 50, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Stange, E.E.; Shea, K.L. Effects of Deer Browsing, Fabric Mats, and Tree Shelters on Quercus rubra Seedlings. Restor. Ecol. 1998, 6, 29–34. [Google Scholar] [CrossRef]
- Löf, M.; Bolte, A.; Jacobs, D.F.; Jensen, A.M. Nurse Trees as a Forest Restoration Tool for Mixed Plantations: Effects on Competing Vegetation and Performance in Target Tree Species. Restor. Ecol. 2014, 22, 758–765. [Google Scholar] [CrossRef]
- Oliet, J.A.; Blasco, R.; Valenzuela, P.; Melero de Blas, M.; Puértolas, J. Should We Use Meshes or Solid Tube Shelters When Planting in Mediterranean Semiarid Environments? New For. 2018, 50, 267–282. [Google Scholar] [CrossRef]
- Redick, C.H.; Jacobs, D.F. Mitigation of Deer Herbivory in Temperate Hardwood Forest Regeneration: A Meta-Analysis of Research Literature. Forests 2020, 11, 1220. [Google Scholar] [CrossRef]
- Redick, C.H.; McKenna, J.R.; Carlson, D.E.; Jenkins, M.A.; Jacobs, D.F. Silviculture at Establishment of Hardwood Plantations Is Relatively Ineffective in the Presence of Deer Browsing. For. Ecol. Manag. 2020, 474, 118339. [Google Scholar] [CrossRef]
- Taylor, T.S.; Loewenstein, E.F.; Chappelka, A.H. Effect of Animal Browse Protection and Fertilizer Application on the Establishment of Planted Nuttall Oak Seedlings. New For. 2006, 32, 133–143. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Czapka, S.J.; Carol, L.; Petrow, A. How Planting Method, Weed Abatement, and Herbivory Affect Afforestation Success. South. J. Appl. For. 2007, 31, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Keeton, W.S. Evaluation of Tree Seedling Mortality and Protective Strategies in Riparian Forest Restoration. North. J. Appl. For. 2008, 25, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Mariotti, B.; Maltoni, A.; Jacobs, D.F.; Tani, A. Tree Shelters Affect Shoot and Root System Growth and Structure in Quercus Robur during Regeneration Establishment. Eur. J. For. Res. 2015, 134, 641–652. [Google Scholar] [CrossRef]
- Oliet, J.A.; Puértolas, J.; Valenzuela, P.; Vázquez de Castro, A. Light Transmissivity of Tree Shelters Interacts with Site Environment and Species Ecophysiology to Determine Outplanting Performance in Mediterranean Climates. Land 2021, 10, 753. [Google Scholar] [CrossRef]
- Tuley, G. The Growth of Young Oak Trees in Shelters. Forestry 1985, 58, 181–195. [Google Scholar] [CrossRef]
- Sharew, H.; Hariston-Strang, A. A Comparison of Seedling Growth and Light Transmission among Tree Shelters. North. J. Appl. For. 2005, 22, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Abe, T. Effects of Treeshelter on Seedling Performance: A Meta-Analysis. J. For. Res. 2021, 27, 171–181. [Google Scholar] [CrossRef]
- Dey, D.C.; Jacobs, D.; McNabb, K.; Miller, G.; Baldwin, V.; Foster, G. Artificial Regeneration of Major Oak (Quercus) Species in the Eastern United States--a Review of the Literature. For. Sci. 2008, 54, 77–106. [Google Scholar] [CrossRef]
- Thyroff, E.C.; Burney, O.T.; Jacobs, D.F. Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration. Forests 2019, 10, 950. [Google Scholar] [CrossRef] [Green Version]
- Salifu, K.F.; Jacobs, D.F.; Birge, Z.K.D. Nursery Nitrogen Loading Improves Field Performance of Bareroot Oak Seedlings Planted on Abandoned Mine Lands. Restor. Ecol. 2009, 17, 339–349. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why Seedlings Survive: Influence of Plant Attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Salifu, K.F.; Seifert, J.R. Growth and Nutritional Response of Hardwood Seedlings to Controlled-Release Fertilization at Outplanting. For. Ecol. Manag. 2005, 214, 28–39. [Google Scholar] [CrossRef]
- Rose, K.M.; Friday, J.B.; Oliet, J.A.; Jacobs, D.F. Canopy Openness Affects Microclimate and Performance of Underplanted Trees in Restoration of High-Elevation Tropical Pasturelands. Agric. For. Meteorol. 2020, 292–293, 108105. [Google Scholar] [CrossRef]
- Bellis, V.J. Ecology of Maritime Forests of the Southern Atlantic Coast: A Community Profile; National Biological Service; U.S. Department of the Interior: Lakewood, CO, USA, 1995; pp. 1–95.
- Jones, G.; Snider, A.; Luo, S. Changes in the Extent of North Carolina Barrier Island Maritime Forests 1988-2011: An Assessment of Past Efforts at Protection. J. For. 2013, 111, 186–193. [Google Scholar] [CrossRef]
- Mathews, T.D.; Stapor, F.W.; Richter, C.R.; Miglarese, J.V.; McKenzi, M.D.; Barlcay, L.A.; Joseph, E.B. Ecological Characterization of the Sea Island Coastal Region of South Carolina and Georgia. In Volume I: Physical Features of the Characterization Area; Marine Resources Division: Charleston, SC, USA, 1980. [Google Scholar]
- Lopazanski, M.J.; Evans, J.P.; Shaw, R.E. An Assessment of Maritime Forest Resources on the North Carolina Coast; North Carolina Department of Natural Resources and Community Development: Raleigh, NC, USA, 1988; pp. 1–104.
- Abrams, M.D. Where Has All the White Oak Gone? BioScience 2003, 53, 927–939. [Google Scholar] [CrossRef] [Green Version]
- McEwan, R.W.; Dyer, J.M.; Pederson, N. Multiple Interacting Ecosystem Drivers: Toward an Encompassing Hypothesis of Oak Forest Dynamics across Eastern North America. Ecography 2011, 34, 244–256. [Google Scholar] [CrossRef]
- Taggart, J.; Long, Z. Effects of White-Tailed Deer (Odocoileus virginianus) on the Maritime Forest of Bald Head Island, North Carolina. Am. Midl. Nat. 2015, 173, 283–293. [Google Scholar] [CrossRef]
- Thyroff, E.C.; Burney, O.T.; Mickelbart, M.V.; Jacobs, D.F. Unraveling Shade Tolerance and Plasticity of Semi-Evergreen Oaks: Insights From Maritime Forest Live Oak Restoration. Front. Plant Sci. 2019, 10, 1526. [Google Scholar] [CrossRef] [Green Version]
- van Dyke, F.; Wilderman, S.; Harju, S.; Faulkner, J.; Hindy, K.; Kitzel, P.; Marshall, T.; Redick, C.; Rowley, D.; Tolsma, J.; et al. Effects of Soil Treatments and Tree Species on Reforestation of Well Pads. Restor. Ecol. 2022, e13658. [Google Scholar] [CrossRef]
- Earnshaw, K.M.; Baribault, T.W.; Jacobs, D.F. Alternative Field Fertilization Techniques to Promote Restoration of Leguminous Acacia Koa on Contrasting Tropical Sites. For. Ecol. Manag. 2016, 376, 126–134. [Google Scholar] [CrossRef]
- U.S. Climate Data. Available online: https://www.usclimatedata.com/https://www.usclimatedata.com/ (accessed on 26 August 2021).
- Natural Resources Conservation Service, NRCS. United States Soil Survey. Available online: https://www.usclimatedata.com/ (accessed on 26 August 2021).
- Padilla, F.M.; de Dios Miranda, J.; Ortega, R.; Hervás, M.; Sánchez, J.; Pugnaire, F.I. Does Shelter Enhance Early Seedling Survival in Dry Environments? A 4 Test with Eight Mediterranean Species 5 6. Appl. Veg. Sci. 2011, 14, 31–39. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R. R Core Team R: A Language and Environment for Statistical Computing; R Core Team R: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Karkar, D. R Core Team Nlme: Linear and Nonlinear Mixed Effects Models; R Core Team R: Vienna, Austria, 2018. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, J.S.; Gent, M.P.N.; Stephens, G.R. Effects of Planting Stock Quality and Browse Protection-Type on Height Growth of Northern Red Oak and Eastern White Pine. For. Ecol. Manag. 2000, 127, 205–216. [Google Scholar] [CrossRef]
- West, D.H.; Chappelka, A.H.; Tilt, K.M.; Ponder, H.G.; Williams, J.D. Effect of Tree Shelters on Survival, Growth, and Wood Quality of 11 Tree Species Commonly Planted in the Southern United States. J. Arboric. 1999, 25, 69–74. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The Shade-Avoidance Syndrome: Multiple Signals and Ecological Consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar]
- George, J.F.; Powell, J. Deer Browsing and Browse Production of Fertilized American Elm Sprouts. J. Range Manag. 1977, 30, 357. [Google Scholar] [CrossRef] [Green Version]
- Tripler, C.E.; Canham, C.D.; Inouye, R.S.; Schnurr, J.T. Soil Nitrogen Availability, Plant Luxury Consumption, and Herbivory by White-Tailed Deer. Oecologia 2002, 133, 517–524. [Google Scholar] [CrossRef]
- Johansson, T. Changes in Stem Taper for Birch Plants Growing in Tree Shelters. New For. 2004, 27, 13–24. [Google Scholar] [CrossRef]
- Jacobs, D.F. Reforestation of a Salvage-Logged High-Elevation Clearcut: Engelmann Spruce Seedling Response to Tree Shelters after 11 Growing Seasons. West. J. Appl. For. 2011, 26, 53–56. [Google Scholar] [CrossRef] [Green Version]
- van Lerberghe, P. Protecting Trees from Wildlife Damage-Mesh Tree Guards; CNPF-IDF: Paris, France, 2015.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).