Abstract
Due to the unique hydrogeological environment of karst areas, pollutants are more likely to enter the soil and water, showing a special migration and transformation behavior. In this work, the binding behaviors between strontium (Sr2+) and dissolved organic matter (DOM) extracted from soil under the influence of pH and Ca2+ in a typical karst area were investigated by applying three-dimensional fluorescence spectroscopy combined with parallel factor analysis (EEM–PARAFAC) and two-dimensional correlation analysis (2D-COS) of synchronous fluorescence spectra (SF). The results show that DOM extracted from soil was dominated by tryptophan-like and tyrosine-like materials (77% in total). Two-dimensional COS of SF showed that the tryptophan-like substance in DOM extracted from soil preferentially bound to Sr2+. When the pH was 7, the binding coefficient (logKa) of the four DOM components ranged from 2.69 to 4.04, which was more conducive to the binding of DOM extracted from soil and Sr2+ than under acidic and alkaline conditions. Ca2+ in soil weakened the binding of DOM extracted from soil to Sr2+ by competing for binding sites and changing the molecular surface potential. This research is helpful for acknowledging the migration and transformation of Sr2+ and offers a reference for groundwater protection in karst areas.
1. Introduction
In the karstic dynamic system formed by the carbon cycle of “carbon dioxide, organic carbon and carbonate” coupled with the unbalanced open system of CO2-H2O-CO32−, matter and energy move in different directions, ways and intensities, producing various surface and underground karstic forms. Water, soil and rock are closely related in karst areas [1]. Due to the unique geological environment of karst areas, the soil is thin and rocky, and rocks such as carbonates are corrosive and permeable. Under strong karstification (dissolution, erosion), most atmospheric precipitation and surface water enter the groundwater [2]. Thus, karst groundwater usually contains a certain number of mineral salts, trace elements or carbon dioxide gas and is characterized by the physicochemical properties of being calcium-rich and partially alkaline [3]. According to statistics, one-quarter of the world’s population relies on karst groundwater for their living and production [4]. Strontium (Sr), as an important trace metal element, is generally enriched in rocks, soils, and sediments and accumulates in surface water and groundwater in the dissolved state through the process of weathering and dissolution [5,6]. As one of the trace essential elements for human bone growth, a proper level of strontium in the groundwater (a content less than 5 mg/L) also has certain curative effects on cardiovascular disease [7,8]. However, the long-term drinking of strontium-rich groundwater can cause kidney damage and abnormal bone development and osteomalacia in humans, especially in karst areas. Therefore, considering the higher Sr content in soil and rocks in karst areas, there is an urgent need for their remediation for the protection of groundwater resources.
The remediation process of different types of soils depends on their physical and chemical properties and environmental media, such as pH, oxidation-reduction potential (Eh), cation content, and organic matter content [9,10]. Hereinto, dissolved organic matter (DOM), as a kind of organic mixture with intricate functional groups and environmental behavior, exists widely in the soil and can impact the transplantation, conversion, and toxicity of contaminants [11]. Although DOM accounts for a small portion of soil organic matter, it is the most positive constituent directly involved in the chemical process of the soil ecological environment [12]. Generally, approximately 25% to 50% of DOM components comprise humic and fulvic acids, while the rest include proteins, polysaccharides, and hydrophilic organic acids [13,14]. The effects of DOM are as follows: on the one hand, the main functional groups of soil DOM, including hydroxyl, carboxyl, and amidogen, possess a high chemical activity and can promote the oxido reduction of inorganic pollutants such as chromium (Cr (VI)), arsenic (As (V)), and mercury (Hg (II)) [15]. On the other hand, it can not only bind to heavy metals through complexation, such as nickel (Ni), copper (Cu), and zinc (Zn), to inhibit the precipitation and adsorption of heavy metals, but also interact with soil minerals, which enhances the migration capacity of heavy metals [16]. Thus, DOM is a considerable element for the migration and transformation of Sr in karst soil.
Relevant studies have shown that the binding behavior of DOM and pollutants is affected by DOM attributes (e.g., components and functional groups), pollutant types, and the external hydrochemical environment (e.g., pH, temperature, and cations) [16,17,18]. Previous studies reported that the binding behavior effect of protein-like groups on Cu2+ is stronger than that of humic-like groups, and different humic-like groups have different affinities for Cu2+ and Hg2+ [19]. Due to the unique calcium-rich and alkali-inclined soil environment in karst areas, pH and Ca2+ become essential factors affecting the complex of DOM and Sr2+ [20,21]. Some studies have shown that under acidic and alkaline conditions, the content of fulvic-like and humic-like matter in soil DOM decreases, and protein-like substances and some functional groups disappear. The number of functional groups decreased to a greater extent under alkaline conditions than under acidic conditions. The optimal condition for the binding of Pb2+ or Sb(V) and DOM was pH 7.0 [22]. Protons and Ca2+ may reduce the effective functional groups of DOM binding to other metal ions through competition and inhibit the binding behavior [20]. Information on how pH and Ca2+ affect soil DOM and Sr complex behavior in karst areas is still insufficient and needs to be explored. This is of practical significance for karst soil pollution remediation and groundwater protection.
Fluorescence quenching, as a susceptible, nondestructive, and convenient method, has been widely employed to analyze the complexation of DOM with metal ions, such as copper (Cu), mercury (Hg), iron (Fe), and zinc (Zn) [22,23,24]. The principle of this method is that when the quenching agent is present, the emission energy of the fluorophore is suppressed, resulting in a decrease in the fluorescence peak intensity. Three-dimensional fluorescence spectroscopy combined with parallel factor analysis (EEM-PARAFAC) can extract the independent fluorescence components with minimum residuals from multiple EEM datasets, which is helpful to study the response of different fluorescence components to quenchers [17]. In addition, synchronous fluorescence spectroscopy combined with two-dimensional correlation spectroscopy (2D-COS) analysis can also afford more detailed information about the quenching effect [25,26].
In this study, Sr2+ was selected as the potential contaminant. The sampling area, Xintian County, Hunan Province, China, is a long-term field observation site of the Institute of Karst Geology, Chinese Academy of Geological Sciences, which has accumulated more than 10 years of field observation data. In previous studies, a large amount of surface water and groundwater was collected, and a preliminary understanding of the occurrence form, migration and transformation rule of DOM and Sr2+ in the sampling area was obtained [4,8,27]. On this basis, we selected and collected one soil sample from key stations to extract DOM and conducted a fluorescence quenching experiment. EEM–PARAFAC analysis and 2D-COS analysis of synchronous fluorescence spectra (SF) were employed to reveal the binding ability between DOM precipitated by soil leaching and Sr2+ and the influences of pH and Ca2+ on DOM–Sr interactions. This study is beneficial for deepening the understanding of the influence of DOM from soil leaching on Sr2+ migration and transformation in Sr-rich karst groundwater systems and provides a reference value for the remediation of heavy metal pollution in karst soil and the protection of groundwater.
2. Materials and Methods
2.1. Study Area and DOM Preparation
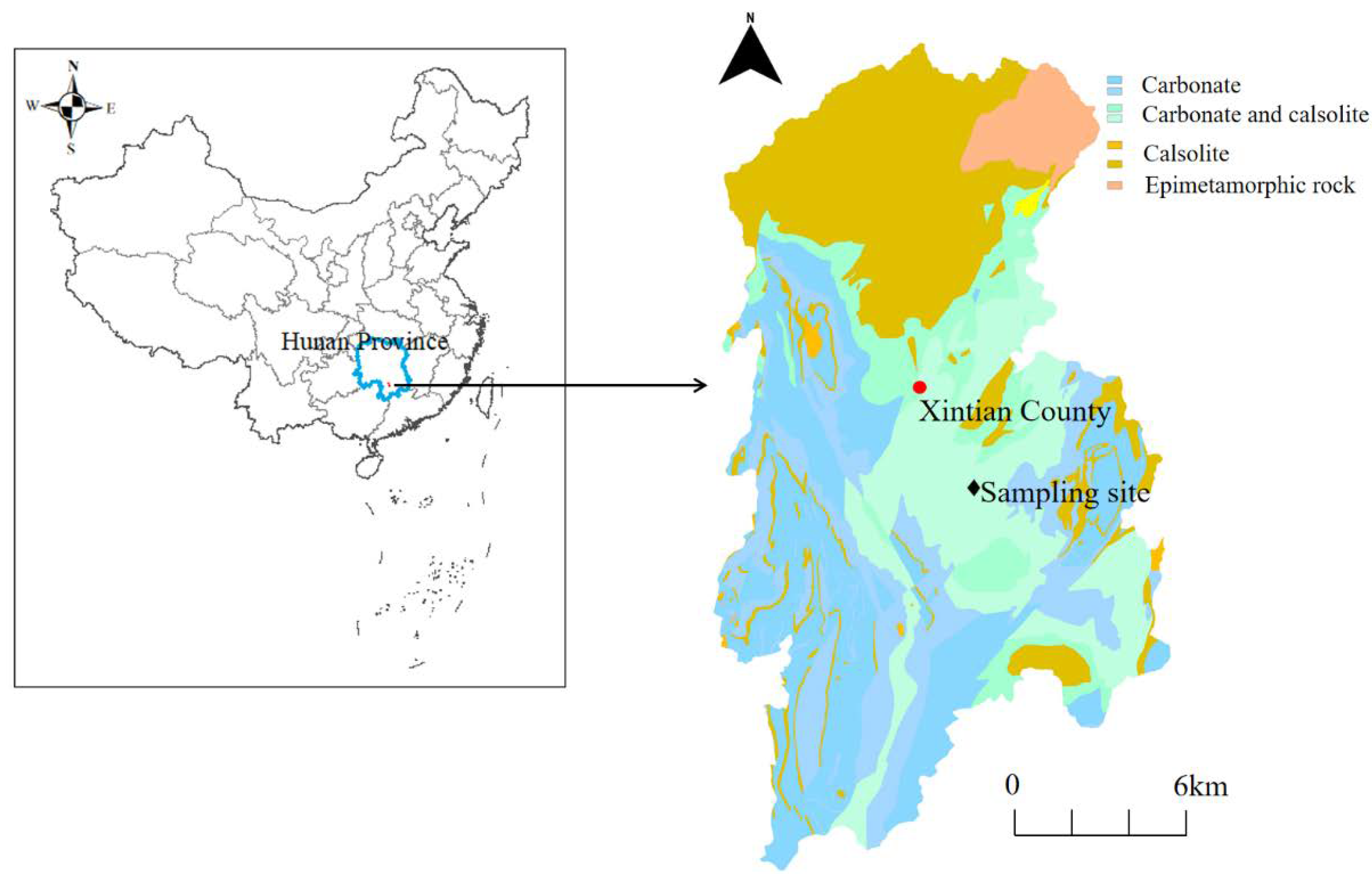
Xintian County is located in southern Hunan Province, China (112°04′–112°23′ E, 25°36′–26°06′ N) (Figure 1). This region is dominated by karst hills and karst dissolved hills with carbonate outcrops, distributing a large area of red loam, black lime soil and paddy soil. The strontium (Sr) content range of the soil was 67.3–115.72 mg/kg in this area, and the maximum content of Sr in the water-bearing rock group was 838 mg/kg, which has the potential risk of groundwater pollution [4,8]. The ranges of pH values and calcium ions (Ca2+) in karst groundwater were 6.91–8.11 and 4.7–160.7 mg/kg, respectively, characterizing a slightly alkaline property and Ca2+ enrichment of karst water [4].
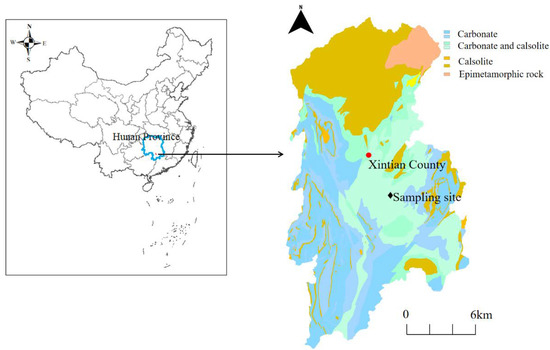
Figure 1.
Map of Xintian County showing the locations of the sampling sites.
The soil sample was collected in July 2017 from Xintian County, Hunan Province, China, and was freeze-dried, ground and passed through a 2 mm sieve (Figure 1). The soil sample was loaded into the plexiglass column and leached at velocities of 0.2, 0.4, 0.7, 1.5, 1.8, and 2.5 mL/min. The initial indexes (pH, Sr2+, and Ca2+ concentration) were measured by taking the exudate. The values of pH, Sr2+, and Ca2+ were 8.26 ± 0.16, 0.002 ± 0.01 mM, and 0.412 ± 0.08 mM in the soil sample, respectively. Forty grams of soil was added to an inorganic glass bottle containing 400 mL of Milli-Q water (solid–liquid ratio 1:10). Then, all liquid after mixing was shaken at 25 °C (rotate speed: 200 rpm) under dark conditions for 48 h. The hybrid liquid was decentered at 5000 rpm for 10 min, and the suspension fluid was filtered with 0.7 µm glass fiber filters (Waterman GF/F) to obtain the DOM extracted from the soil. The filtrate was dialyzed with dialysis bags with a pore size of 3500 Da for 24 h at 4 °C to remove inorganic salts and small organic compounds. The DOC concentration of the DOM stock solution was 57 mg/L. To eliminate the influence of the DOM concentration and internal filtration effect, the extracted DOM stock solution was diluted 5 times with Milli-Q water to a final DOC concentration of 10 mg/L for the following experiments.
2.2. Fluorescence Quenching Titration
2.2.1. DOM Interaction with Sr2+ and pH Condition Setting
Sr2+ and Ca2+ (Sino-Pharm Chemical Reagent Co, Shanghai, China) stock solutions were prepared. Various concentrations of Sr2+ stock solution were dropped into a series of brown sealed bottles containing 10 mL of DOM extracted from the soil. Then, 100 μL of a potassium chloride (KCl, pH 7.0) solution was used to maintain the ion strength and ensure an effective binding reaction. The final volume was 20 mL, and the concentrations of DOC, KCl, and Sr2+ were 10 mg/L, 10 mM, and 0–1.6 mM, respectively. In addition, the concentration of Sr2+ in this experiment was generally higher than that in the actual environment, meaning it could better characterize the complexation reaction. Finally, all bottles containing DOM and Sr2+ were shaken for 24 h in the dark. The UV-vis absorption, EEM, and synchronous fluorescence (SF) spectra of the solutions were measured.
To research the influence of pH on the interaction of DOM extracted from soil with Sr2+, a succession of brown sealed bottles containing 10 mL of DOM and various concentrations of Sr2+ stock solution were adjusted to adjust the pH of DOM with NaOH and HCl. The pH values of the mixed solution were 3.0–11.0. The above processing steps were repeated, and relevant indicators were measured.
2.2.2. Effect of Ca2+ on the Interaction between DOM and Sr2+
Various concentrations of Ca2+ stock solution were dropped into a series of brown sealed bottles containing 10 mL of DOM extracted from the soil. Then, 100 μL of a potassium chloride (KCl, pH 7.0) solution was used to maintain the ion strength and assure a better binding effect. The final volume was 20 mL, and the concentrations of DOC, KCl, and Ca2+ were 10, 10, and 0–1.6 mM, respectively. After shaking in the dark for 24 h at 25 °C, all solutions were measured by using EEM, UV-vis absorption, and synchronous fluorescence (SF) spectra.
To investigate the influence of Ca2+ on the interaction of DOM extracted from the soil with Sr2+, various concentrations of Ca2+ stock solution were dropped into several brown sealed bottles containing 10 mL of DOM and various concentrations of Sr2+ stock solution. A certain amount of potassium chloride (KCl, pH of 7.0) solution was dropped into the bottles to maintain a volume of 20 mL, DOC at 10 mg/L, Sr2+ at 0–1.6 mM, KCl at 10 mM, Ca2+ at 0.9 mM and 1.6 mM. After that, the UV-vis spectra and EEM spectra of the mixed solution were detected.
2.3. Fluorescence and Absorbance Measurement
The dissolved organic carbon (DOC) of the soil sample was gauged by a TOC-Vcph analyzer (Shimadzu, Kyoto, Japan) within a week after the sample arrived at the laboratory. UV-vis absorption spectra were determined by a UV-vis spectrophotometer (Shimadzu, UV-2550). The wavelength ranges were from 200 to 800 nm with intervals of 1 nm. A Hitachi F-7000 fluorescence spectrometer (Hitachi High Technologies, Tokyo, Japan) was applied to measured fluorescence spectra (EEMs and SF spectra). The scanning excitation (Ex) and emission (Em) of EEMs were 250–550 nm with 5 nm intervals, and the Ex wavelength region of SF spectra was 200–450 nm with a constant offset (60 nm).
2.4. Data Analysis
2.4.1. Two-Dimensional COS Analysis
“2Dshige” is a practical software used to reveal the binding sequence of DOM extracted from soil and pollutants. The 2D-COS of SF spectroscopy was analyzed using “2Dshige” software to exhibit the complexation behaviors of DOM–Sr. Detailed information is shown through synchronous and asynchronous diagrams. In the synchronous figures, the autopeak on the diagonal describes the overall degree of variation in spectral intensity in the external variable interval. When the crosspeak at λ1/λ2 is positive, it means possible coupling or correlation at λ1 or λ2. The negative peak at λ1/λ2 shows an opposite change at λ1 and λ2. In the asynchronous figure, the positive crosspeak at λ1/λ2 indicates that the variation at λ1 occurs preferentially at λ2. The negative cross-peak at λ1/λ2 suggests that the variation at λ1 occurs after that at λ2.
2.4.2. PARAFAC Modeling
The interactions of DOM components with Sr2+ were analyzed by PARAFAC. PARAFAC analysis involves the separation of a set of EEM data into mathematically and chemically individual fluorescent components. Fluorescent species are identified by the emission (Em) and excitation (Ex) wavelengths of different components. The PARAFAC modeling was operated by the drEEM toolbox of MATLAB (R2018b) [28]. MATLAB is a mathematical software produced by MathWorks Inc., which contains a large number of computational algorithms. The model was verified by split-half analysis, where six different half-datasets were collected and three validity tests “S4C6T3” (Splits-4, Combinations-6, Tests-3) were generated. The number of components was successively increased, and the residual was compared to determine the optimal number of components. More information on the PARAFAC model can be found elsewhere [29].
2.4.3. Complexation Modeling
The modified Stern–Volmer equation was utilized to evaluate the effectiveness of the fluorescence quenching for components. The modified Stern–Volmer equation is exhibited as Equation (1):
where F0 and F are the fluorescence intensities when the concentration of Sr2+/Ca2+ (mol·L−1) was 0 and Cx. Ka represents the quenching constant, and f is the proportion of quenchable fluorescence groups.
To further explore the reaction of DOM components with Sr2+/Ca2+, the site-binding equation was applied to explore the stability constant and the binding points of components as shown in Equation (2):
where Kb and n indicate the binding constant and the number of binding points, respectively.
2.5. Nomenclature
DOM: dissolved organic matter; EEM–PARAFAC: three-dimensional fluorescence spectroscopy combined with parallel factor analysis; 2D-COS: two-dimensional correlation analysis; SF: synchronous fluorescence spectra; logKa: the binding coefficient; DOC: dissolved organic carbon; Four components extracted from EEM–PARAFAC: C1-C4.
3. Results and Discussion
3.1. Characteristics of DOM Extracted from Soil
3.1.1. UV-Vis Analysis
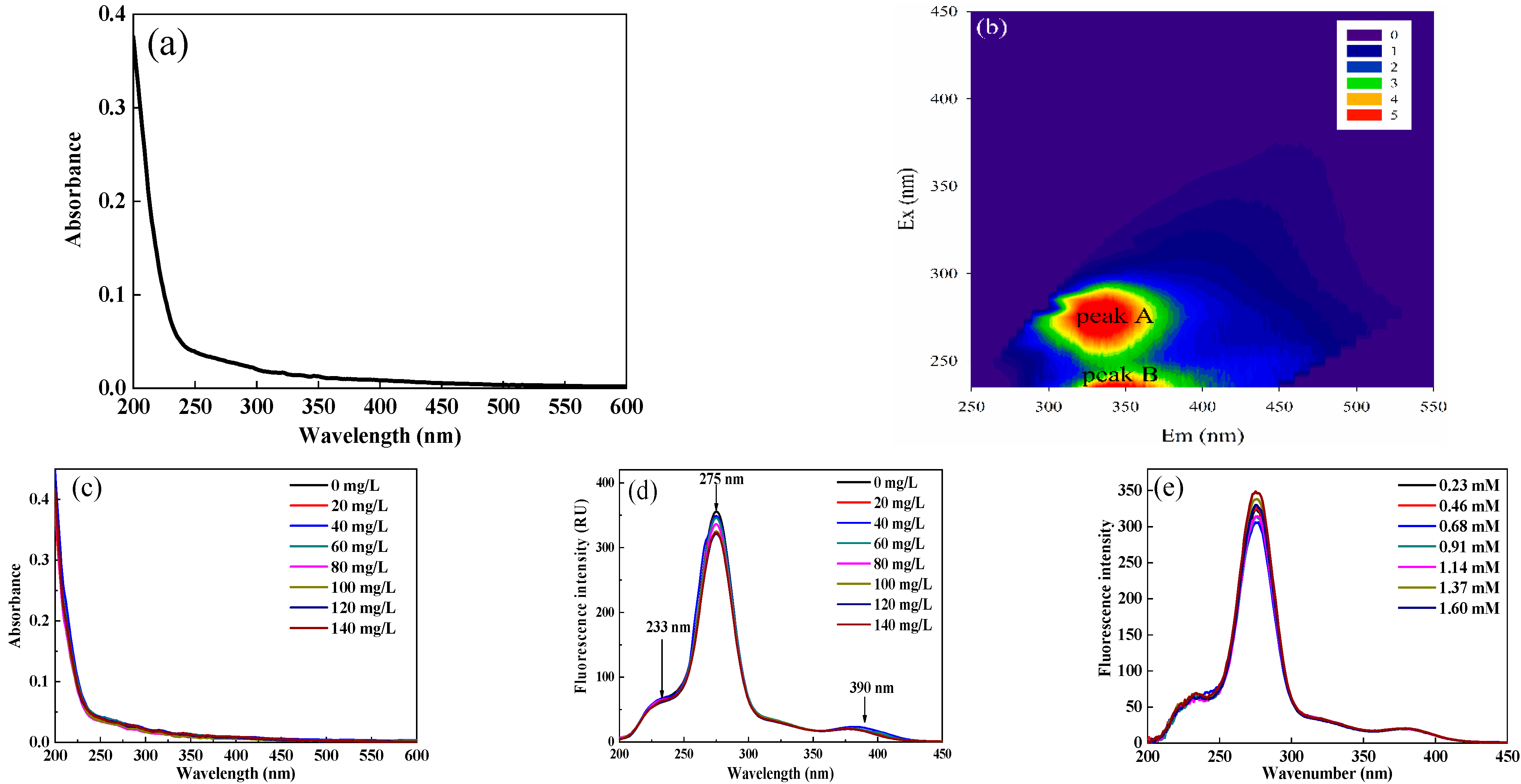
As shown in Figure 2d, the UV-vis spectra suggested that the absorbance of DOM extracted from soil increased logarithmically when the wavelength was lower than 260 nm and decreased gradually with increasing wavelength. Generally, the degree of humification is proportional to the absorbance of UV-vis spectra, which indicated that DOM extracted from soil humification was at a lower level in karst areas. In addition, when the value of SUVA254 is greater than 4, it indicates that there is a considerable amount of aromatic hydrophobic substances in DOM [30]. In this study, the SUVA254 value of DOM extracted from soil was 0.85 L/mg C m–1, which indicated that DOM was mainly composed of hydrophilic small molecular aliphatic substances and hydrophilic compounds.
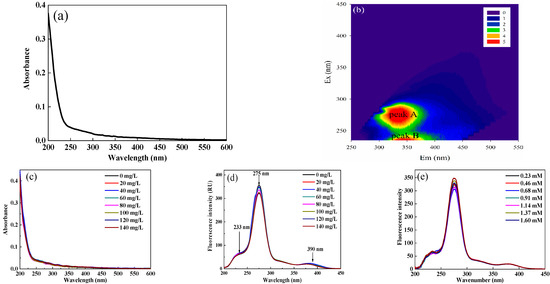
Figure 2.
UV-vis (a) and EEM spectra (b) of DOM extracted from soil; UV-vis spectra of DOM extracted from soil after adding Sr2+ (c); Change in synchronous fluorescence (SF) spectra of DOM extracted from soil after adding Sr2+ or Ca2+ (d,e).
3.1.2. EEM-PARAFAC Analysis
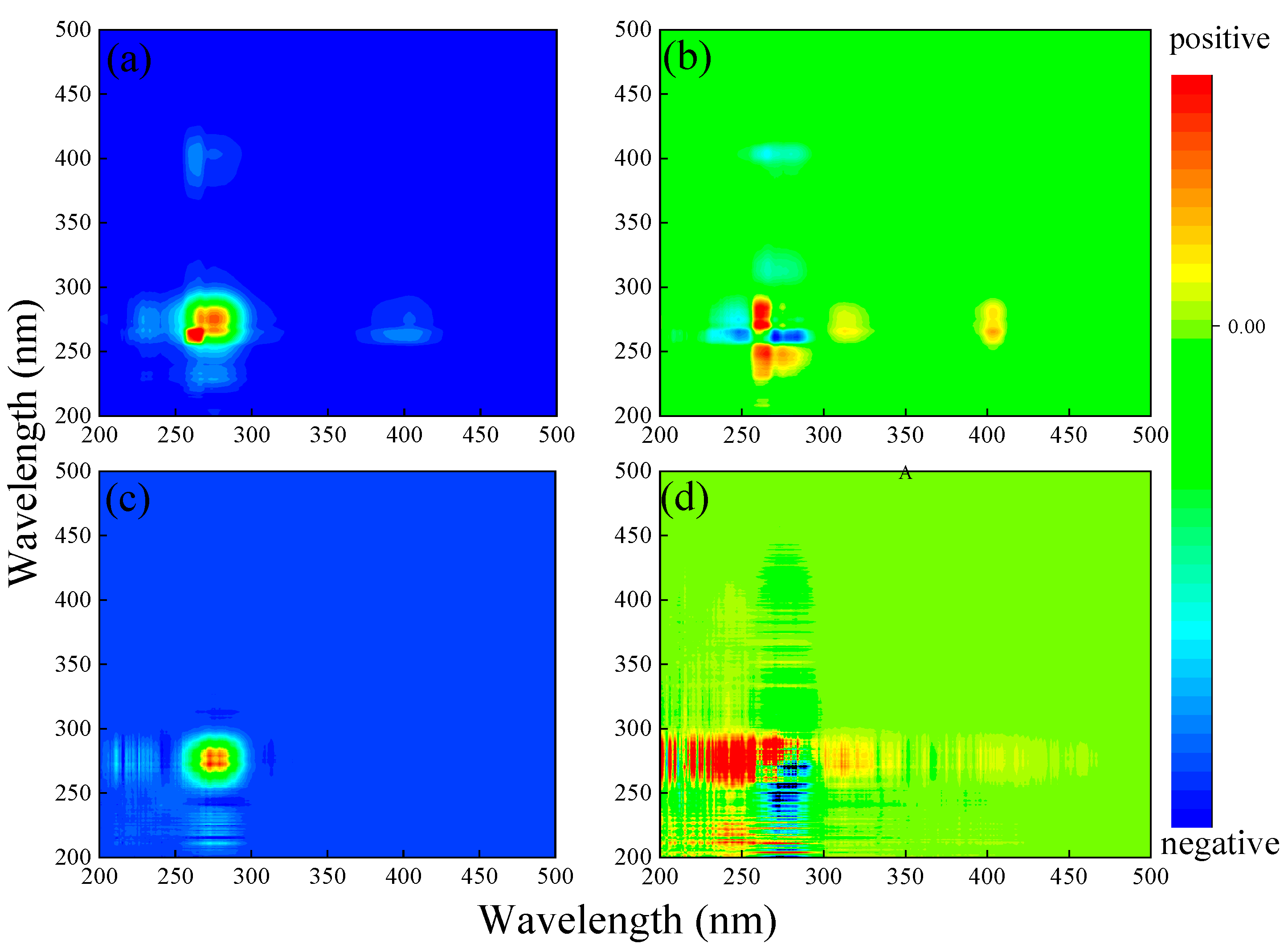
There were generally two visible fluorescence peaks, including peak A (Ex/Em 275/330 nm) and peak B (Ex/Em 235/350 nm) (Figure 2e). It is deemed that the fluorescence peak with an emission wavelength less than 380 nm in the EEM spectra contains benzene rings and electron donors such as hydroxyl and amino groups, while the fluorescence peak with an emission wavelength greater than 380 nm is related to the polycyclic aromatic structure. In other words, the fluorescence groups with excitation wavelengths greater than 380 nm are classified as humic-like matter, while those with excitation wavelengths less than 380 nm are classified as protein-like materials. In this study, the two fluorescence peaks could be classified as protein-like substances, which characterized a relatively simple molecular structure and reflected the microbial activity and bioavailability of DOM [16,31]. There was no significant fluorescence peak in the humic-like fluorescence region, indicating that DOM extracted from soil was mainly composed of protein-like materials and that humic-like substances had a low content.
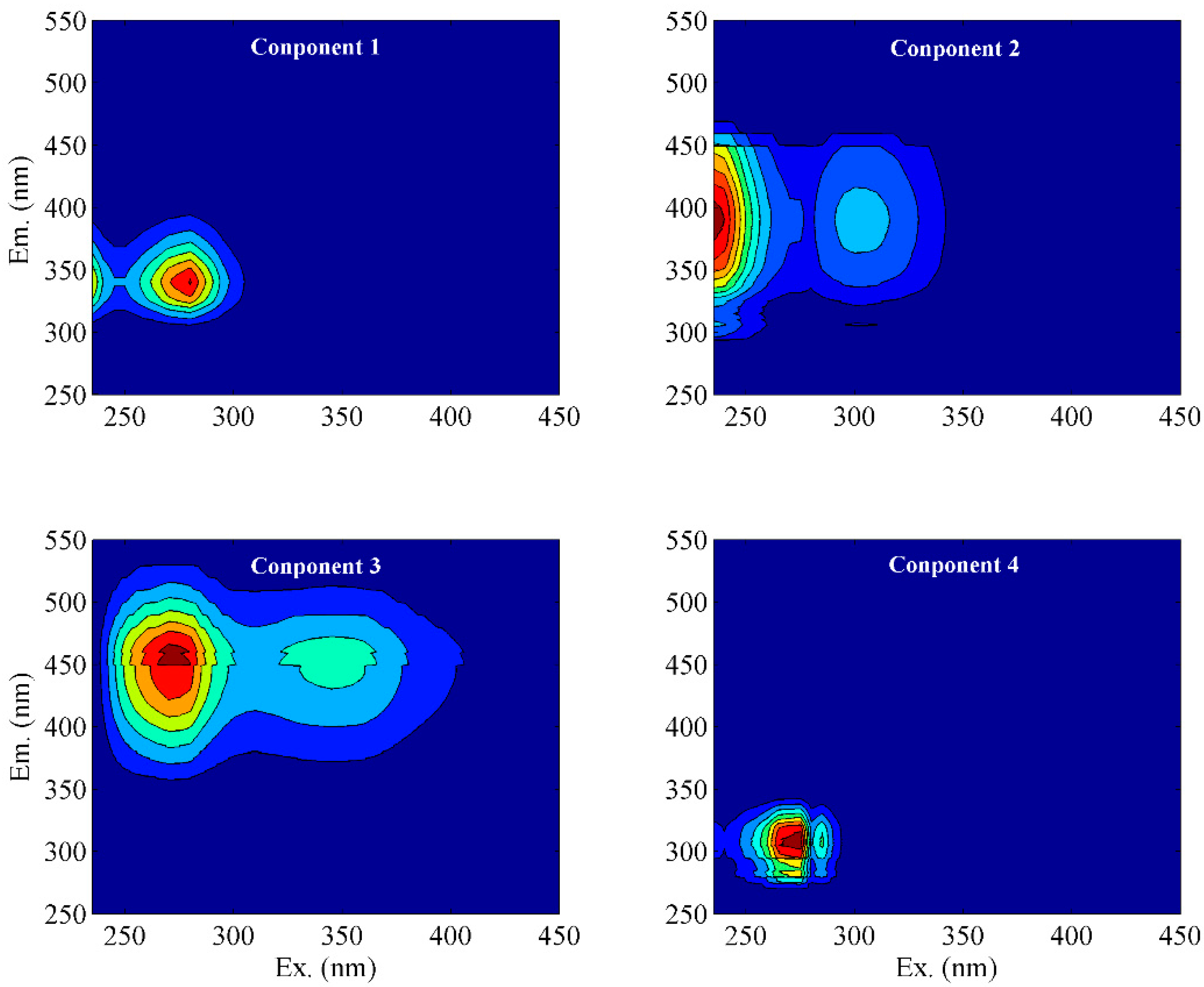
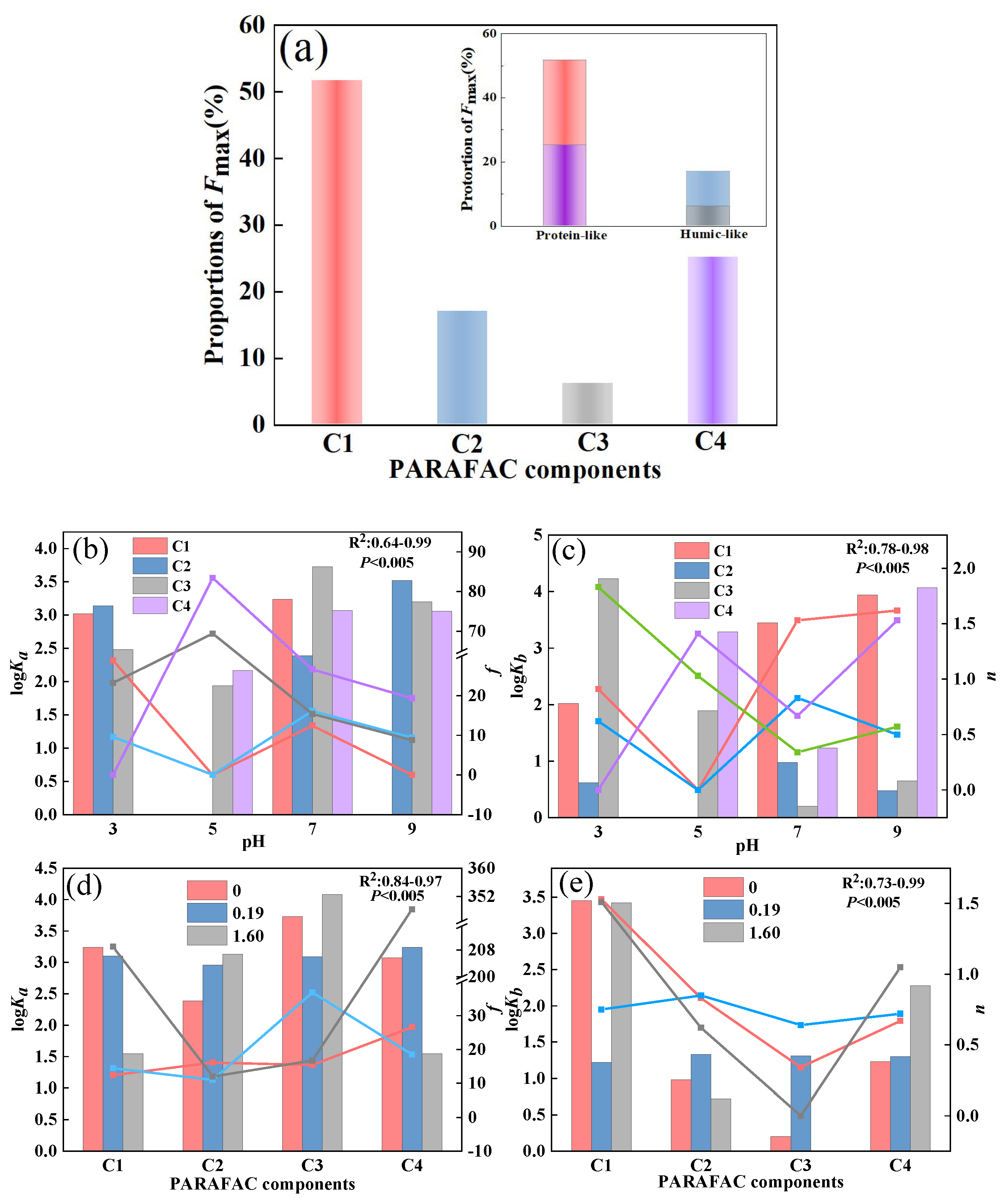
To further quantify the properties of DOM, PARAFAC analysis was applied, and we obtained a total of four components (C1–C4) (Figure 3). The locations of components C1 (Ex/Em = 260 (320)/400 nm) and C4 (Ex/Em = 230/430 nm) corresponded to tryptophan-like substances and tyrosine-like substances [31,32,33]. Components C2 (Ex/Em = 235 (302)/390 nm) and C3 (Ex/Em = 270 (345)/460 nm) could be classified as fulvic-like and humic-like materials, respectively [31,34]. Comparatively, component C4 has a longer emission wavelength than C3, indicating a tighter structure and higher molecular weight. According to the Fmax value of each component, C1, C2, C3, and C4 accounted for 52%, 17%, 6%, and 25% of the total fluorescence intensity of DOM extracted from the soil, respectively (Figure 4a). This result suggests that DOM extracted from the soil in karst areas was mainly composed of tryptophan-like and tyrosine-like materials, while the contents of fulvic-like and humic-like matter were insignificant. According to relevant studies, protein-like substances are mainly precursors of humic polymers and energy sources of microorganisms, which are preferentially utilized to degrade and promote the increase in soil humic substances [31,35]. Meanwhile, a high proportion of protein-like matter could improve soil fertility and the continuous nitrogen supply [36]. This fluorescent feature may be related to the climatic conditions in karst regions. High summer temperatures lead to an increased abundance of microorganisms in the form of bacteria [37]. Active microbial activity is advantageous to DOM mineralization and the formation of biodegradable intermediates such as amino acids [35]. In addition, surface pollution caused by agricultural activities also contributes to the increase in protein-like substances [38]. This fluorescence characteristic of low humus was also found in the well water in the study area, which may be related to the higher cation concentration in the well water than that in the descending spring and surface water [3].
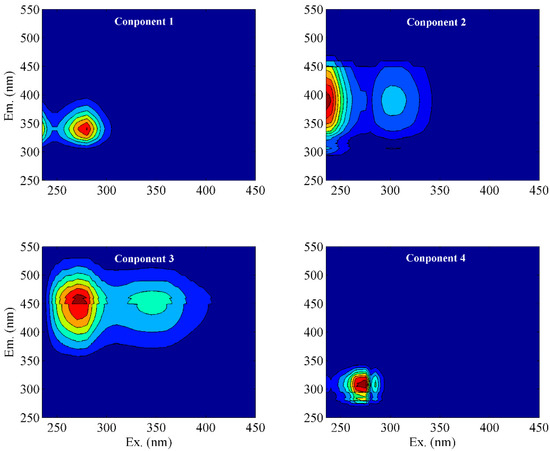
Figure 3.
EEM spectra of components isolated from PARAFAC models.
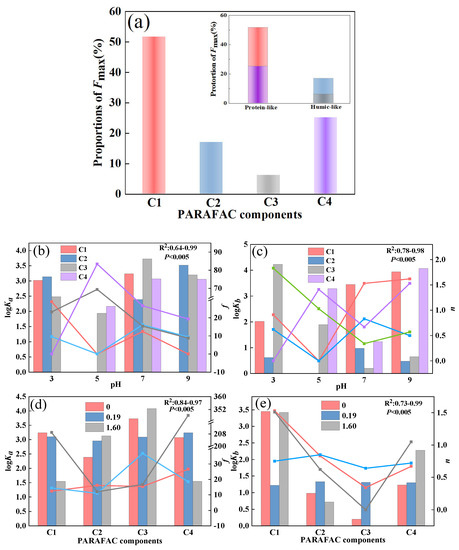
Figure 4.
The ratio of the four PARAFAC components to the total fluorescence intensity (a); the binding constants (logKa), initial spectral intensities (f), binding constant (logKb), and binding site number (n) between PARAFAC components and Sr2+ at different pH values (b,c); the binding constants (logKa), initial spectral intensities (f), binding constant (logKb), and binding site number (n) between PARAFAC components and Sr2+ when Ca2+ was added at different pH values (d,e).
3.2. DOM–Sr Binding Behavior
3.2.1. UV-Vis and SF Spectra
The changes in UV-vis spectra after adding Sr2+ are shown in Figure 2c. Generally, the peptide skeleton structure had a high absorbance value located near 200 nm. The absorption shoulders at 230 nm were mainly caused by the amino acid side chain groups in tryptophan and tyrosine [39]. With the gradual addition of Sr2+, the absorbance and shape did not change significantly, indicating that Sr2+ molecules did not cause changes in the DOM molecular microenvironment.
SF spectra can not only reveal the effect of the quenching agent on DOM conformation, but also reveal the characteristics of the microenvironment near fluorescence functional groups. With the increase in the Sr2+ concentration, the fluorescence peak at 275 nm in the SF spectra of DOM extracted from soil was quenched (Figure 2g). Since the emission wavelength of tryptophane-like substances is longer than that of tyrosine-like materials, the fluorescence quenching at 275 nm was due to the interaction between tryptophane-like fluorophores and Sr2+. The relatively strong fluorescence quenching effect of tyrosine-like substances indicated a change in the electron structure in protein-like substances [29]. However, the changes in fluorescence intensities at 233 nm and 390 nm were insignificant, indicating that tyrosine-like and humic-like matter contributed little to the binding of DOM to Sr2+. Therefore, the binding sites interacting with Sr2+ in different components of DOM extracted from soil were not evenly distributed.
3.2.2. Two-Dimensional COS of SF Spectra
To study the binding sequence of Sr2+ and its components, the SF spectra of DOM extracted from soil were analyzed by 2D-COS (Figure 5a,b). In the synchronous map, there was a positive autopeak at 275 nm along the diagonal, indicating that the corresponding region had the strongest spectral variation, which agreed with the change in the SF spectra (Figure 5a). In addition, the two positive autopeaks at 275/230 nm and 390/275 nm suggested the same variation trend of the three fluorophore intensities at 230, 275, and 390 nm (decrease with increasing Sr2+ concentration).
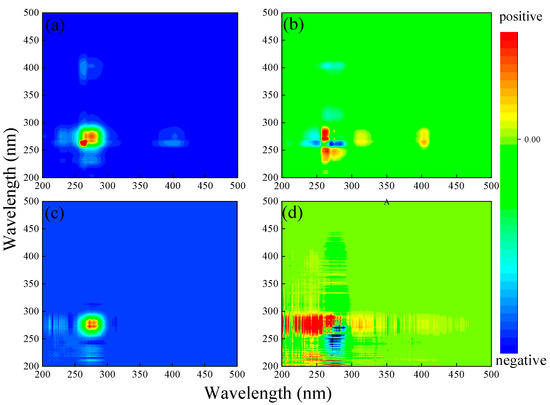
Figure 5.
Two-dimensional DOS analysis of DOM extracted from soil after adding Sr2+ (a,b) or Ca2+ (c,d). Left is the synchronous graph, and right is the asynchronous graph; red and blue represent positive and negative correlation, respectively. Darker and lighter colors represent stronger and weaker correlations, respectively.
In the asynchronous map, one positive crosspeak exists at 260 (275)/235 nm below the diagonal, while one negative crosspeak exists at 275/260 nm, which indicates that the fluorescence quenching of shortwave strength occurred preferentially in a long wave strength (Figure 5b). Specifically, the sequence of fluorescence quenching at different wavelengths is 260 > 275 > 235 nm [40]. Therefore, tryptophan-like substances were preferred to tyrosine-like substances for DOM–Sr binding, while humic-like substances had no significant strength change due to their low content, and 2D-COS analysis could not reveal the order of DOM–Sr binding.
3.2.3. Sr-Binding Abilities of PARAFAC Components
Figure 5d depicts the variations in the fluorescent intensity of the components after adding Sr2+ to the DOM extracted from the soil. The fluorescence quenching was most obvious when the added amount of Sr2+ was 0–60 mg/L. With the increase in Sr2+ addition, the fluorescence quenching gradually stabilized. It is noteworthy that the response behavior of the fluorescence intensity of the four fluorescence components to Sr2+ was different. Low concentrations of Sr2+ (<40 mg/L) induced an obvious fluorescence quenching effect on C3 and C4, while there was no fluorescence quenching phenomenon on C1 and C2. With increasing Sr2+ concentration, the fluorescence intensity of C3 and C4 continued to weaken and stabilize, while the fluorescence intensity of C1 also began to decrease. When the added amount of Sr2+ was 70 mg/L, the fluorescence quenching ratios of C1, C2, C3, and C4 were 9%, 4%, 17%, and 19%, respectively. The dynamic collision between the fluorescent substance and the quencher or the formation of a nonfluorescent complex can lead to fluorescence quenching. However, some studies have found that the fluorescence property was enhanced after DOM interacts with some metals, which was mainly caused by the variation of the molecular environment of the fluorescent component with the increased metal concentration [29]. The results of EEM-PARAFAC and SF spectra seem to be inconsistent, mainly because SF spectra are a two-dimensional spectrum, and DOM components at different wavelengths have cross coverage, which may cover part of the fluorescence quenching information. Various DOM components have different intensities of fluorescence quenching effects. Some studies indicate that the quenching degree of the tryptophan standard was higher than that of the humic standard under the same temperature conditions [41]. It was also reported that the fluorescence quenching effect of the L-tyrosine solution (52%) was more potent than that of the L-tryptophane solution [42].
To explore the interaction of Sr2+ with the components, the modified Stern–Volmer equation was employed to calculate the effective quenching constant (logKa). The excellent linear relationships between F0/(F0 − F) and 1/CSr for all components suggested that the equation depicted the fluorescence quenching well (R2 > 0.73). The logKa values of the four DOM components ranged from 2.69 to 4.04, and the order was C3 > C1 > C4 > C2, indicating that the interaction between humic acid and Sr2+ was the strongest, followed by tryptophane-like and tyrosine-like substances (Table 1). The fulvic acid component had the weakest binding effect with Sr2+. Humic-like substances are one of the most important metal chelating agents in the natural world. Due to thier abundant functional groups and large molecular weight, they have a strong affinity for Sr2+ [43]. Therefore, the strong complexation ability of humic-like matter reduces its abundance and retains the increased accumulation of protein-like substances in karst soil.

Table 1.
The binding parameters of different PARAFAC components with Sr2+ and Ca2+. “-” suggests the modified Stern–Volmer equation could not be modeled due to the weak fluorescence quenching effects.
The linear relation between log[(F0 − F)/F] and Log [Sr2+] was good (R2 > 0.89), indicating that the point-position combination mode can also better fit the interaction between DOM and Sr2+ (Table 1). Among the four fluorescence components, the binding constant of C3 and Sr2+ was the smallest, which was due to the low content of humic-like matter in soil DOM and the few effective binding points (n = 0.34). In contrast, the content of tryptophan-like substances was higher than that of other substances, and the effective binding sites of Sr2+ were more abundant than those of other substances, meaning that the effect was more stable. The binding sites for Sr2+ in the tyrosine-like and humic-like fractions were all less than 1. In general, protein-like component C3 had strong binding abilities and high stabilities to Sr2+, which were the leading fraction in the interaction between Sr2+ and DOM extracted from soil. It was proven that the complex of DOM extracted from soil and Sr2+ contributed to the migration and transformation of Sr2+.
3.3. Influence of pH Conditions on the Interaction between DOM Extracted from Soil and Sr2+
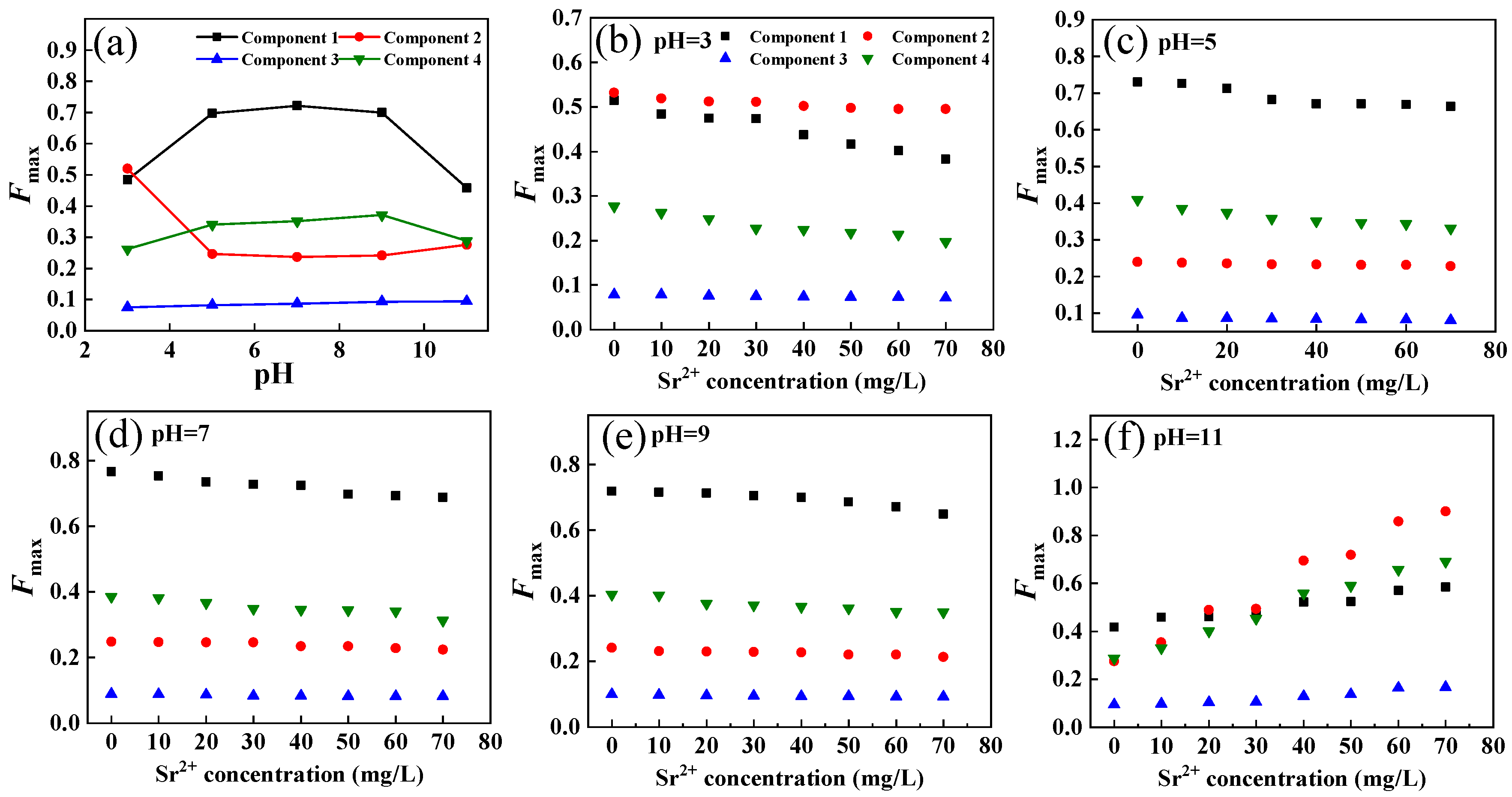
The fluorescence intensity of DOM extracted from soil components under different pH conditions is shown in Figure 6a. Under neutral conditions, the fluorescence intensity of C1, C3, and C4 was higher than that of C1 but decreased with decreasing and increasing pH. The change in pH might cause the ionization of fluorescence components or functional groups in DOM molecules, thus changing the molecular orbital of excited electrons and affecting their fluorescence properties [44]. The fluorescence intensity of compounds was affected by electron functional groups such as amino and hydroxyl groups [45,46]. Carboxyl chromophores make a major contribution to the change in fluorescence between pH 3.0 and 5.0, while phenolic chromophores have a key function in the shift at pH 9.0 and 11.0 [47]. It has been reported that the effect of pH on fluorescence is related to changes in DOM macromolecular structure [48]. Generally, the tighter the structure is, the stronger the fluorescence. When the pH increased, the molecular structure of the humic-like substance tended to be linear, and some fluorescent molecules were wrapped in acidic conditions. They expanded and emitted fluorescence under alkaline conditions, so the fluorescence intensity increased [45]. However, the fluorescence of protein-like matter mainly comes from aromatic amino acid residues (tryptophane tyrosine and phenylalanine). Some molecules in components C1 and C4, which were covered under acidic and alkaline conditions, spread out under neutral conditions, showing the strongest fluorescence. For the fulvic acid components, the fluorescence was the strongest under acidic conditions, and the fluorescence decreased with increasing pH, implying that low pH was conducive to the development of fluorescent molecules.
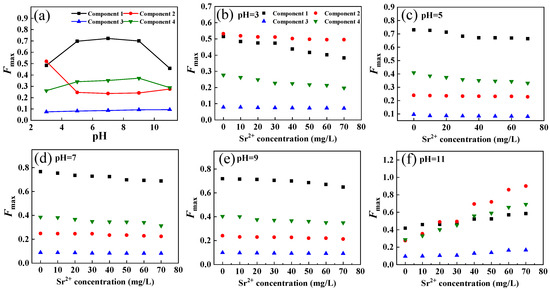
Figure 6.
Intensity changes of fluorescent components in DOM extracted from soil under different pH conditions (a); intensity changes of fluorescent components in DOM extracted from soil with the increase in Sr2+ concentration under different pH conditions (b–f).
The interaction between the four DOM components and Sr2+ under different pH conditions is shown in Figure 6b–f. When the pH was 3, 5, 7, and 9, the fluorescence intensity of DOM components decreased with increasing Sr2+ concentration, indicating the formation of the nonfluorescent complex. However, when the pH value was 11, the fluorescence intensity of DOM components increased significantly with increasing Sr2+ concentration, indicating that the interaction between DOM components and Sr2+ under alkaline conditions changed the molecular microenvironment of DOM extracted from soil and formed a stronger fluorescence complex or precipitates [49].
Furthermore, the modified Stern–Volmer equation and the point-position binding equation were selected to fit the binding parameters of the four components with Sr2+ at pH 3, 5, 7, and 9 (Figure 4b,c and Table S1). For components C1, C3, and C4, logKa was the maximum when the pH value was 7, indicating that the affinity of the three components to Sr2+ is the strongest under neutral conditions. However, when the pH increased or decreased, the fluorescence molecular structure was compacted, and its binding ability to Sr2+ decreased. At the same time, protons might compete with Sr2+ for DOM extracted from soil binding sites under acidic conditions, weakening the interaction between DOM and Sr2+. This phenomenon has also been observed in the complexation of cadmium (Cd) or copper (Cu) with DOM [49,50].
The interaction of component C2 with Sr2+ was stronger under acidic and alkaline conditions than under neutral conditions, indicating enhanced protonation, negative charge and electrostatic action [50,51]. Therefore, pH changes had different effects on the binding points of DOM extracted from soil and Sr2+. For C1 and C2, the decrease in pH led to the protonation of the fluorophore (the increase in H+), reducing the effective binding sites [52]. For C3, the reduction in pH increased the effective binding point of DOM extracted from soil to Sr2+, which might be due to the higher H+ concentration leading to the precipitation and desorption of Sr2+ in the form of sulfate and hydroxide, and the stability of the binding effect was enhanced [52]. The interaction between C4 and Sr2+ was more stable under acidic and alkaline conditions. In general, neutral conditions were more conducive to the binding of DOM extracted from soil and Sr2+. Alkaline environments in karst areas are not conducive to Sr2+ migration and transformation.
3.4. Effect of Ca2+ on the Interaction between DOM Extracted from Soil and Sr2+
3.4.1. Interaction of Ca2+ with DOM Extracted from Soil
As shown in Figure 2e, with increasing Ca2+ concentration, the fluorescence peak intensity at 275 nm in the SF spectra changed remarkably, indicating that the tryptophan-like fluorophore was the main component in the interaction between DOM extracted from soil and Ca2+, while tyrosine-like and humic-like substances contributed negligibly. This was similar to the interaction characteristics of DOM extracted from soil and Sr2+. Two-dimensional COS was further used to study the binding sequence of Ca2+ and different components (Figure 5c,d). In the synchronous map, a positive autopeak at 275 nm along the diagonal indicated that the corresponding region had the strongest spectral change, which agreed with the characteristic variation in the SF spectra (Figure 5c). In addition, the positive cross-peak at 275/230 nm indicated that the intensity of the two fluorophore groups at 230 and 275 nm had the same trend. In the asynchronous graph, there was a negative cross peak at 275/235 nm below the diagonal, indicating that tyrosine-like substances reacted with Ca2+ before tryptophane-like substances, which was inconsistent with the results of Sr2+ (Figure 5d). This meant that DOM extracted from the soil had different and similar binding mechanisms for different elements. Due to the low content of humic-like matter, the strength of humic-like materials did not change significantly, and 2D-COS analysis could not reveal the action sequence of humic-like substances with Ca2+.
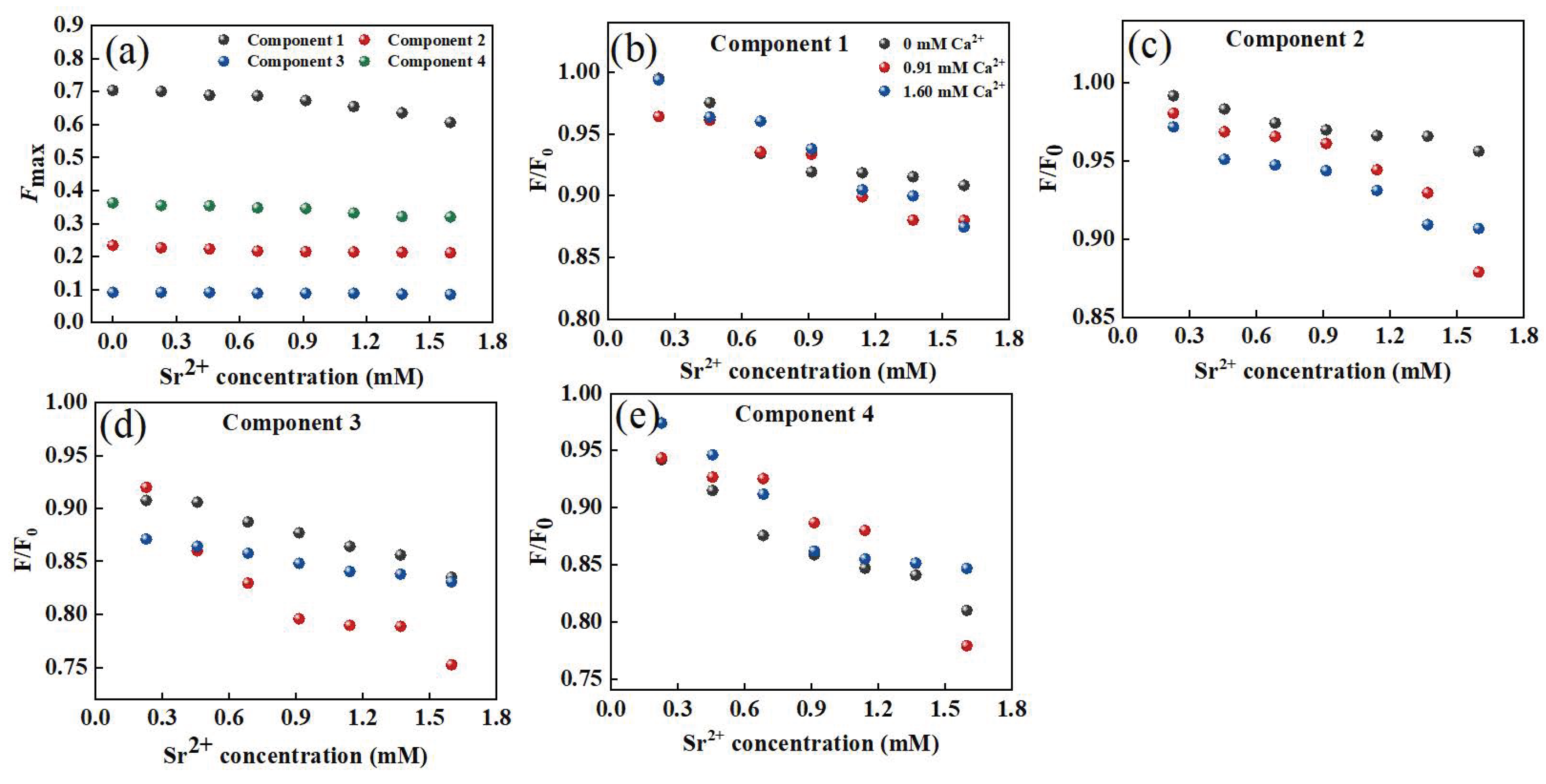
Furthermore, Ca2+ could quench the fluorescence intensity of the four components to different degrees (Figure 7a). When the added amount of Ca2+ increased to 1.6 mM, the fluorescence quenching ratios of C1, C2, C3, and C4 were 14%, 10%, 7%, and 12%, respectively, indicating that most of the tryptophan-like substances could bind to Ca2+. However, most components of humic acid could not be quenched by Ca2+. By using the modified Stern–Volmer equation and the point-position binding equation, it was found that the order of the binding constants logKa was C2 > C4 > C3, confirming the weakest interaction between the humic acid component and Ca2+, which differed significantly from the DOM–Sr2+ results (Table 1). However, the high logKb value of humic-like components indicated that although there were fewer fluorescence components that could bind to Ca2+ in humic acid substances, they had the strongest mutual stabilities and the most effective adsorption sites. While components C2 and C4 had strong affinities for Ca2+, their utilization was unstable. In general, tryptophan-like, tyrosine-like, and fulvic-like components were the main functional groups of DOM extracted from soil interacting with Ca2+. Compared with Sr2+, the lower logKa value of DOM extracted from soil interacting with Ca2+ suggested that the main mechanism of DOM extracted from soil interacting with Ca2+ was electrostatic force. The mechanism of DOM extracted from soil and Sr2+ was the formation of a complex, so the logKa value was higher than that of DOM–Ca.
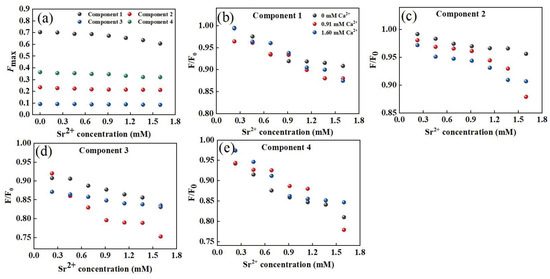
Figure 7.
Intensity changes of fluorescent components in DOM extracted from soil after adding Sr2+ (a); in the presence of Ca2+, the changes in fluorescent intensity for components in DOM extracted from soil with increasing Sr2+ concentration (b–e).
3.4.2. The Interaction between DOM Extracted from Soil and Sr in the Presence of Ca
The interaction between PARAFAC components and Ca2+ in the presence of Ca2+ is shown in Figure 7b,e. When the Ca2+ concentrations were 0.91 mM and 1.60 mM, Ca2+ had no obvious influence on the binding of the tryptophan-like component C1 to Ca2+. The fluorescence quenching of C2 and Ca2+ was sensitive to Ca2+. With increasing Ca2+ concentration, the fluorescence quenching of C2 and Sr2+ was enhanced. The interaction between the humic-like component C3 and Sr2+ showed a similar response trend to Ca2+ to that of C2. The effect of Ca2+ on the tyrosine-like component C4 was similar to that of C1. The presence of Ca2+ enhanced the fluorescence quenching of humic-like substances and Sr2+ to a certain extent, while the interaction between protein-like substances and Sr2+ was weakly affected by Ca2+.
Based on the Stern–Volmer equation and point-position binding equation, the binding abilities of the four components and Sr2+ in the presence of Ca2+ were evaluated (Figure 4d,e and Table S2). For component C1, logKa decreased with increasing Ca2+ concentration, suggesting that Ca2+ reduced the affinity of tryptophan-like substances to Sr2+ by competing with Sr2+ for effective binding sites of tryptophan-like components. The binding constants of components C2 and C3 to Sr2+ increased with increasing Ca2+ and humic acid to Sr2+. However, the logKa values of components C4 and Sr2+ were slightly increased when the Ca2+ concentration was 0.91 mM. The logKa values were significantly decreased when the Ca2+ concentration was 1.60 mM, indicating that a high concentration of Ca2+ inhibited the binding of DOM to Sr2+. It has been reported that the binding effect of DOM and Cu2+ could be affected by 5 mM Ca2+ [53]. Ca2+ can change the zeta potential on the surface of DOM molecules by neutralizing negatively charged functional groups (such as carboxyl and phosphoric groups) in the DOM structure, thus affecting its binding with Sr2+ [49,54]. Different functional groups in different DOM components had different effects on Ca2+. For protein-like substances, Ca2+ could weaken the interaction between protein-like substances and Sr2+ by competing for binding sites and changing surface potentials through cation-π interactions [54]. In addition, Ca2+-(H2O)n formed outer-sphere complexes on the DOM surface, which hindered the binding of protein substances to Sr2+ [55]. However, Ca2+ might promote the binding of humic-like substances to Sr2+ through organic chelation [55]. As the main functional group in DOM was protein-like substances, the existence of Ca2+ inhibited the binding of DOM to Sr2+. According to previous research, the water and soil in the study area were slightly alkaline (pH: 6.91–8.11), implying a relatively strong complexation effect between DOM extracted from soil and Sr2+. The existence of large amounts of Ca2+ in karst areas led to the migration and transformation of Sr being more complicated between surface water and groundwater. The result also verify that Sr might exist in karst water in various forms, mainly including ionic state, complex state, and composite precipitation. The results confirm that DOM extracted from soil can reduce the Sr2+ content and toxicity to help protect groundwater. There are two possible paths: (1) Increasing the organic matter content in karst soil, especially humic-like matter. (2) Regulating the pH of the soil.
4. Conclusions
According to EEM-PARAFAC analysis, DOM extracted from soil is mainly composed of tryptophan-like and tyrosine-like materials (77% in total), while fulvic acid and humic acid were relatively low (23% in total). The tryptophan-like substance in DOM extracted from soil preferentially bound to Sr2+ so that Sr2+ transforms from a free dissolved state to a DOM-bound state. When the pH was 7, the binding coefficients of the components were highest (except C2), following the order of humic-like substance > tryptophane-like substance > tyrosine-like substance > fulvic-like substance. At the same time, tryptophan-like and tyrosine-like substances bound Sr2+ more stably. Acidic conditions were not conducive to the binding of DOM extracted from soil with Sr2+, while new fluorescent complexes appeared during the reaction of DOM extracted from soil with Sr2+ under alkaline conditions. Ca2+ in the soil complexed with DOM extracted from the soil, but it was weaker than that between DOM extracted from soil and Sr2+. Overall, Ca2+ inhibited the binding of protein-like substances to Sr2+ by competing for binding sites and changing the molecular surface potential. Increasing the organic matter content and adjusting the pH in the soil can help to protect groundwater by reducing the Sr2+ content and toxicity. These results compare the ability of DOM extracted from soil to transfer and transform Sr2+ in karst soil under different pH conditions and the role of Ca2+ in this process, providing a new idea for the remediation of soil heavy metal pollution and groundwater protection in karst areas.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/land11091376/s1, Table S1: The binding parameters of PARAFAC components and Sr2+ at different pH conditions; Table S2: The binding parameters between DOM components and Sr2+ in the presence of Ca2+.
Author Contributions
Conceptualization, methodology, and software, T.F. and H.R.; Data curation, writing—original draft preparation, X.Y.; writing—review and editing, C.S. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully thank the Natural Science Foundation of Guangxi Province, China (2020GXNSFAA297025), for their financial support of this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, D.X.; Zhang, C. Karst dynamics theory in China and its Practice. Acta Geosci. Sin. 2008, 29, 355–365. (In Chinese) [Google Scholar]
- Jiang, Y.; Cao, M.; Yuan, D.; Zhang, Y.; He, Q. Hydrogeological characterization and environmental effects of the deteriorating urban karst groundwater in a karst trough valley: Nanshan, SW China. Hydrogeol. J. 2018, 26, 1487–1497. [Google Scholar] [CrossRef]
- Lv, W.; Yao, X.; Su, C.; Ren, H.; Yao, M.; Zhang, B. Characteristics and influencing factors of hydrochemistry and dissolved organic matter in typical karst water system. Environ. Sci. Pollut. Res. 2020, 27, 11174–11183. [Google Scholar]
- Stefan, K.; Gerhard, K.; Miran, V. Decision support systems for groundwater protection: Innovative tools for resource management. Environ. Geol. 2006, 49, 840–848. [Google Scholar]
- Banner, J.L. Radiogenic isotopes: Systematics and applications to earth surface processes and chemical stratigraphy. Earth-Sci. Rev. 2004, 65, 141–191. [Google Scholar] [CrossRef]
- Musgrove, M.L. The occurrence and distribution of strontium in U.S. groundwater. Appl. Geochem. 2021, 126, 104867. [Google Scholar] [CrossRef]
- Nielsen, S.P. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef]
- Su, C.T.; Huang, C.H.; Zou, S.Z.; Xie, D.X.; Zhao, G.S.; Tang, J.S.; Luo, F.; Yang, Y. Enrich environment and sources of strontium of groundwater in Xintian county, Hunan Province. Carsologica Sin. 2017, 36, 678–683. (In Chinese) [Google Scholar]
- Yamaguchi, N.; Nakamura, T.; Dong, D.; Takahashi, Y.; Amachi, S.; Makino, T. Arsenic release from flooded paddy soils is influenced by speciation, Eh, pH, and iron dissolution. Chemosphere 2011, 83, 925–932. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Jho, E.H.; Nam, K. Effect of dissolved humic acid on the Pb bioavailability in soil solution and its consequence on ecological risk. J. Hazard. Mater. 2015, 286, 236–241. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zheng, Y.C.; Wang, X.C.; Wang, Y.K.; Dzakpasu, M. Characterization and biogeochemical implications of dissolved organic matter in aquatic environments. J. Environ. Manag. 2021, 294, 113041. [Google Scholar] [CrossRef] [PubMed]
- Haney, R.L.; Haney, E.B.; Smith, D.R.; Harmel, R.D.; White, M.J. The soil health tool—Theory and initial broad-scale application. Appl. Soil Ecol. 2018, 125, 162–168. [Google Scholar] [CrossRef]
- Araujo, E.; Strawn, D.G.; Morra, M.; Moore, A.; Alleoni, L.R.F. Association between extracted copper and dissolved organic matter in dairy-manure amended soils. Environ. Pollut. 2019, 246, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Polubesova, T.; Chefetz, B. DOM-affected transformation of contaminants on mineral surfaces: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 223–254. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb, and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Hu, X.P.; Qu, C.C.; Han, Y.F.; Chen, W.L.; Huang, Q.Y. Elevated temperature altered the binding sequence of Cd with DOM in arable soils. Chemosphere 2022, 288, 132572. [Google Scholar] [CrossRef]
- Fan, Y.R.; Zheng, C.L.; Huo, A.D.; Wang, Q.R.; Shen, Z.X.; Xue, Z.W.; He, C. Investigating the binding properties between antimony(V) and dissolved organic matter (DOM) under different pH conditions during the soil sorption process using fluorescence and FTIR spectroscopy. Ecotoxicol. Environ. Saf. 2019, 181, 34–42. [Google Scholar] [CrossRef]
- Brigante, M.; Zanini, G.; Avena, M. On the dissolution kinetics of humic acid particles effects of pH, temperature and Ca2+ concentration. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 64–70. [Google Scholar] [CrossRef]
- Chen, W.B.; Smith, D.S.; Gueguen, C. Influence of water chemistry and dissolved organic matter (DOM) molecular size on copper and mercury binding determined by multiresponse fluorescence quenching. Chemosphere 2013, 92, 351–359. [Google Scholar] [CrossRef]
- Habibul, N.; Chen, W. Structural response of humic acid upon binding with lead: A spectroscopic insight. Sci. Total Environ. 2018, 643, 479–485. [Google Scholar] [CrossRef]
- Lu, Y.F.; Allen, H.E. Characterization of copper complexation with natural dissolved organic matter (DOM)—Link to acidic moieties of DOM and competition by Ca and Mg. Water Res. 2002, 36, 5083–5101. [Google Scholar] [CrossRef]
- Xu, H.C.; Yan, Z.S.; Cai, H.Y.; Yu, G.H.; Yang, L.Y.; Jiang, H.L. Heterogeneity in metal binding by individual fluorescent components in a eutrophic algae-rich lake. Ecotoxicol. Environ. Saf. 2013, 98, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Poulin, B.A.; Ryan, J.N.; Aiken, G.R. Effects of iron on optical properties of dissolved organic matter. Environ. Sci. Technol. 2014, 48, 10098–10106. [Google Scholar] [CrossRef]
- Liu, M.X.; Han, X.K.; Liu, C.Q.; Guo, L.D.; Ding, H.; Lang, Y.C. Differences in the spectroscopic characteristics of wetland dissolved organic matter binding with Fe3+, Cu2+, Cd2+, Cr3+ and Zn2+. Sci. Total Environ. 2021, 800, 149476. [Google Scholar] [CrossRef]
- Ren, H.Y.; Fan, T.T.; Yao, X.; Ma, F.Y.; Liu, L.; Ming, J.D.; Wang, S.T.; Zhang, Y.H.; Deng, H.G. Investigation of the variations in dissolved organic matter properties and complexations with two typical heavy metals under the influence of biodegradation: A survey of an entire lake. Sci. Total Environ. 2022, 806, 150485. [Google Scholar] [CrossRef]
- Fan, T.T.; Yao, X.; Ren, H.Y.; Ma, F.Y.; Liu, L.; Huo, X.J.; Lin, T.; Zhu, H.Y.; Zhang, Y.H. Multispectroscopic investigation of the molecular weight distribution and copper binding ability of dissolved organic matter in Dongping Lake, China. Environ. Pollut. 2022, 300, 118931. [Google Scholar] [CrossRef]
- Su, C.T.; Yang, Y.; Ba, J.J.; Luo, F.; Li, X.P.; Zhao, G.S. Dynamic characteristics and genesis of strontium-rich groundwater in Xintian county, Hunan Province. Carsologica Sin. 2020, 39, 24–33. (In Chinese) [Google Scholar]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multiway techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, Y.; Jaffe, R. Characterizing the interactions between trace metals and dissolved organic matter using excitation-emission matrix and parallel factor analysis. Environ. Sci. Technol. 2008, 42, 7374–7379. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Q.; Yang, W.X.; Dinh, Q.T.; Tran, T.A.; Zhao, X.D.; Wu, J.T.; Liu, Y.X.; Liang, D.L. DOM derivations determine the distribution and bioavailability of DOM-Se in selenate applied soil and mechanisms. Environ. Pollut. 2020, 259, 113899. [Google Scholar] [CrossRef]
- Yan, L.L.; Liu, Q.P.; Liu, C.; Liu, Y.; Zhang, M.Y.; Zhang, Y.D.; Zhang, Y.; Gu, W.R. Effect of swine biogas slurry application on soil dissolved organic matter (DOM) content and fluorescence characteristics. Ecotoxicol. Environ. Saf. 2019, 184, 109616. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Zhao, Y.; Chen, Y.N.; Wang, X.Q.; Qian, L.; Jia, L.M.; Wei, Z.M. Assessment of phytotoxicity grade during composting based on EEM/PARAFAC combined with projection pursuit regression. J. Hazard. Mater. 2017, 326, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Chang, C.H.; Lee, D.J.; He, P.J.; Shao, L.M.; Su, A. Dissolved organic matter with multipeak fluorophores in landfill leachate. Chemosphere 2009, 74, 575–582. [Google Scholar] [CrossRef]
- Kowalczuk, P.; Durako, M.J.; Young, H.; Kahn, A.E.; Copper, W.J.; Gonsior, M. Characterization of dissolved organic matter fluorescence in the South Atlantic Bight with use of PARAFAC model: Interannual variability. Mar. Chem. 2009, 113, 182–196. [Google Scholar] [CrossRef]
- Kleber, M. What is recalcitrant soil organic matter? Environ. Chem. 2010, 7, 320. [Google Scholar] [CrossRef]
- McFarland, J.W.; Ruess, R.W.; Kielland, K.; Pregitzer, K.; Hendrick, R. Glycine mineralization in situ closely correlates with soil carbon availability across six North American forest ecosystems. Biogeochemistry 2010, 99, 175–191. [Google Scholar] [CrossRef]
- Liu, H.; Yang, W.; Ai, Z.; Zhang, J.; Zhang, C.; Xue, S.; Liu, G. Effects of the interaction between temperature and revegetation on the microbial degradation of soil dissolved organic matter (DOM)—A DOM incubation experiment. Geoderma 2019, 337, 812–824. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Liu, P.; Qin, X.; Shan, X.; Yao, X. FDOM conversion in Karst watersheds expressed by three-dimensional fluorescence spectroscopy. Water 2018, 10, 1427. [Google Scholar] [CrossRef] [Green Version]
- Noda, I. Techniques useful in two-dimensional correlation and codistribution spectroscopy (2DCOS and 2DCDS) analyses. J. Mol. Struct. 2016, 1124, 29–41. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, R.; Kumar, V.; Sharma, K.; Sharma, V. On the discrimination of soil samples by derivative diffuse reflectance UV-vis-NIR spectroscopy and chemometric methods. Forensic Sci. Int. 2020, 319, 110655. [Google Scholar] [CrossRef]
- Baker, A. Thermal fluorescence quenching properties of dissolved organic matter. Water Res. 2005, 39, 4405–4412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Cao, J.; Meng, F.G. Interactions between protein-like and humic-like components in dissolved organic matter revealed by fluorescence quenching. Water Res. 2015, 68, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.H.; Tian, X.L.; Li, T.T.; Zhang, Z.Y.; He, X.; Xing, B.S. Surface-bound humic acid increased Pb2+ sorption on carbon nanotubes. Environ. Pollut. 2012, 167, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Lanne, R.W.P.M. Influence of pH on the fluorescence of dissolved organic matter. Mar. Chem. 1982, 11, 395–401. [Google Scholar] [CrossRef]
- Yan, M.Q.; Fu, Q.W.; Li, D.C.; Gao, G.F.; Wang, D.S. Study of the pH influence on the optical properties of dissolved organic matter using fluorescence excitation-emission matrix and parallel factor analysis. J. Lumin. 2013, 142, 103–109. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Dryer, D.J.; Korshin, G.V.; Fabbricino, M. In situ examination of the protonation behavior of fulvic acids using differential absorbance spectroscopy. Environ. Sci. Technol. 2008, 42, 6644–6649. [Google Scholar] [CrossRef]
- Patel-Sorrentino, N.; Mounier, S.; Benaim, J.Y. Excitation-emission fluorescence matrix to study pH influence on organic matter fluorescence in the Amazon basin rivers. Water Res. 2002, 36, 2571–2581. [Google Scholar] [CrossRef]
- Yin, K.Y.; Wang, J.Y.; Zhai, S.; Xu, X.; Li, T.T.; Sun, S.C.; Xu, S.; Zhang, X.X.; Wang, C.P.; Hao, Y.S. Adsorption mechanisms for cadmium from aqueous solutions by oxidant-modified biochar derived from Platanus orientalis Linn leaves. J. Hazard. Mater. 2022, 428, 128261. [Google Scholar] [CrossRef]
- Meng, J.; Feng, X.L.; Dai, Z.M.; Liu, X.M.; Wu, J.J.; Xu, J.M. Adsorption characteristics of Cu(II) from aqueous solution onto biochar derived from swine manure. Environ. Sci. Pollut. Res. 2014, 21, 7035–7046. [Google Scholar] [CrossRef]
- Boonamnuayvitaya, V.; Chaiya, C.; Tanthapanichakoon, W.; Jarudilokkul, S. Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay. Sep. Purif. Technol. 2004, 35, 11–22. [Google Scholar] [CrossRef]
- Batool, S.; Idrees, M.; Hussain, Q.; Kong, J. Adsorption of copper (II) by using derived-farmyard and poultry manure biochars: Effificiency and mechanism. Chem. Phys. Lett. 2017, 689, 190–198. [Google Scholar] [CrossRef]
- Cao, Y.; Conklin, M.; Betterton, E. Competitive complexation of trace-metals with dissolved humic-acid. Environ. Health Perspect. 1995, 103, 29–32. [Google Scholar]
- Aggarwal, V.; Hui, L.; Boyd, S.; Teppen, B. Enhanced sorption of trichloroethene by smectite clay exchanged with Cs2+. Environ. Sci. Technol. 2006, 40, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, J.; Yang, X.; Li, A.; Philippe, C. Adsorption of Ni(II) and Cd(II) from water by novel chelating sponge and the effect of alkali-earth metal ions on the adsorption. J. Hazard. Mater. 2014, 264, 332–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).