Variations in Soil Biological and Biochemical Indicators under Different Grazing Intensities and Seasonal Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Soil Sampling
2.4. Laboratory Measurements
2.5. Physical Properties
2.6. Chemical Properties
2.7. Biological Properties
2.7.1. Fungal Biomass
2.7.2. Basal Soil Respiration
2.7.3. Microbial Biomass Carbon
2.7.4. Microbial Biomass Carbon to Microbial Biomass Nitrogen Ratio (MBC/MBN)
2.7.5. Microbial Metabolic Quotient
2.7.6. Metabolic Coefficient (qCO2)
2.7.7. Arbuscular Mycorrhizal Spore Number (AMSN)
2.8. Substrate-Induced Respiration (SIR)
2.9. Data Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Fungal Biomass
3.3. Microbial Biomass Carbon (MBC)
3.4. Basal Soil Respiration (BSR)
3.5. Microbial Biomass Carbon to Microbial Biomass Nitrogen Ratio (MBC/MBN)
3.6. Microbial Metabolic Quotient (qCO2)
3.7. Arbuscular Mycorrhizal Spore Number (AMSN)
3.8. Total N and MBN/N
3.9. Substrate-Induced Respiration (SIR)
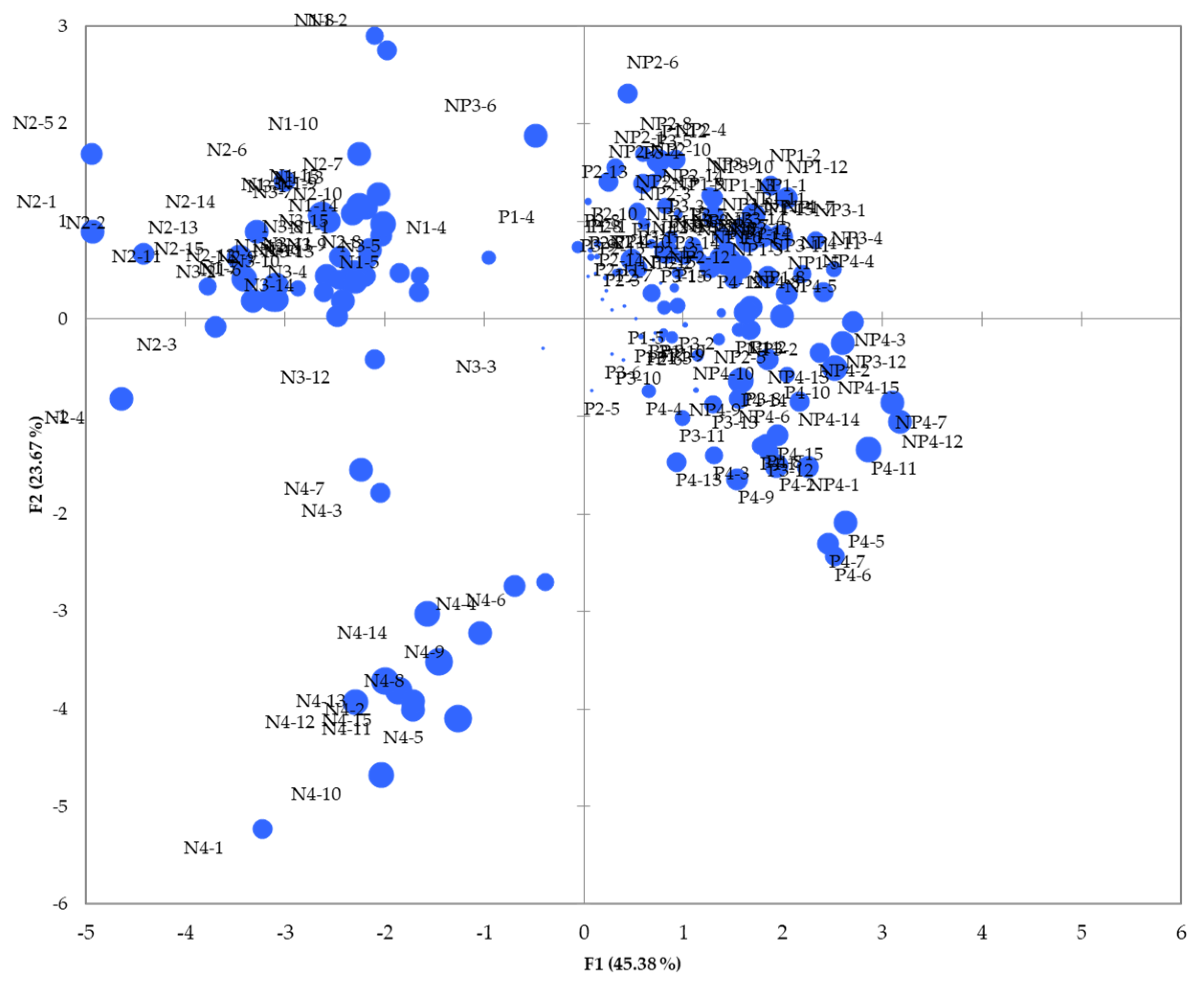
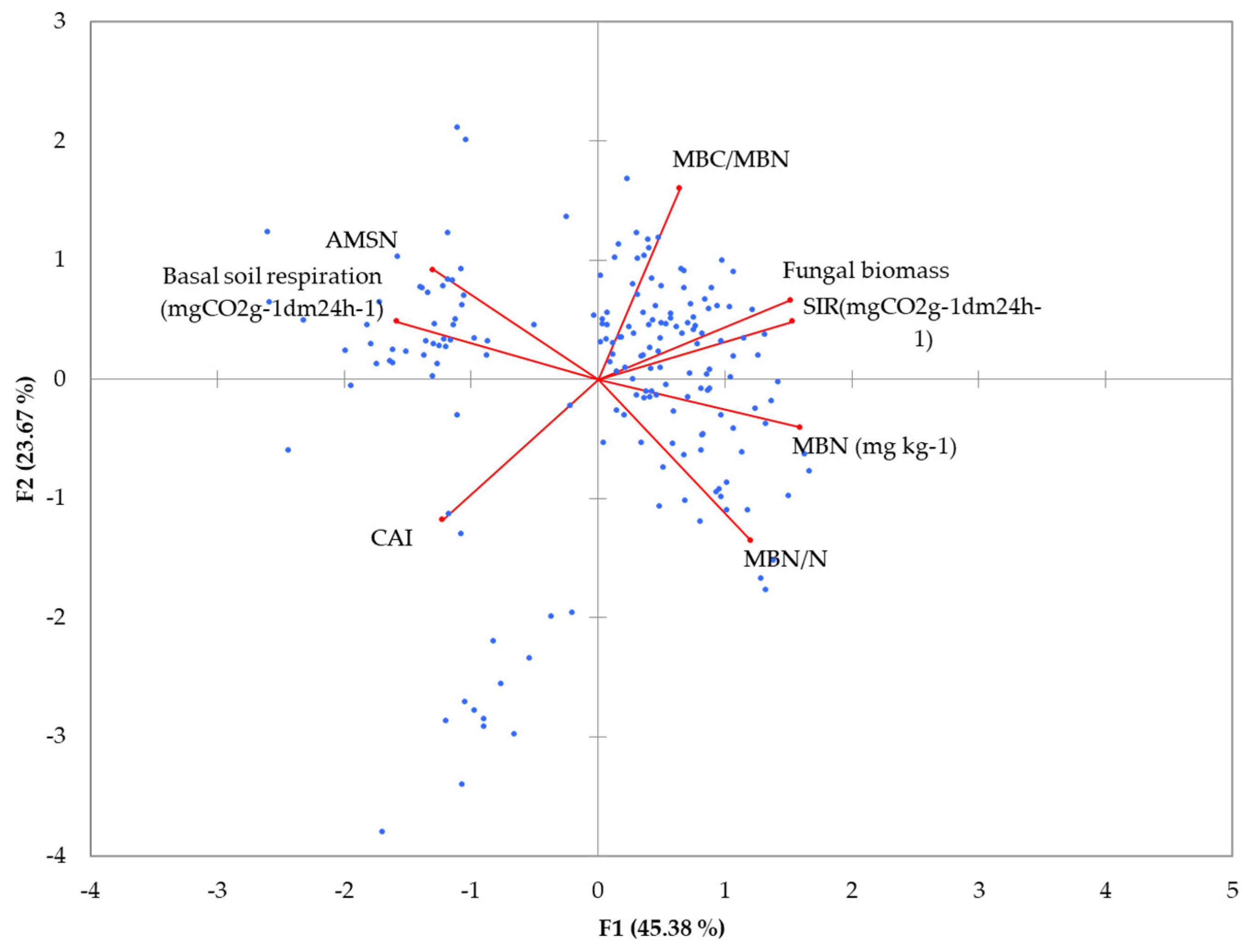
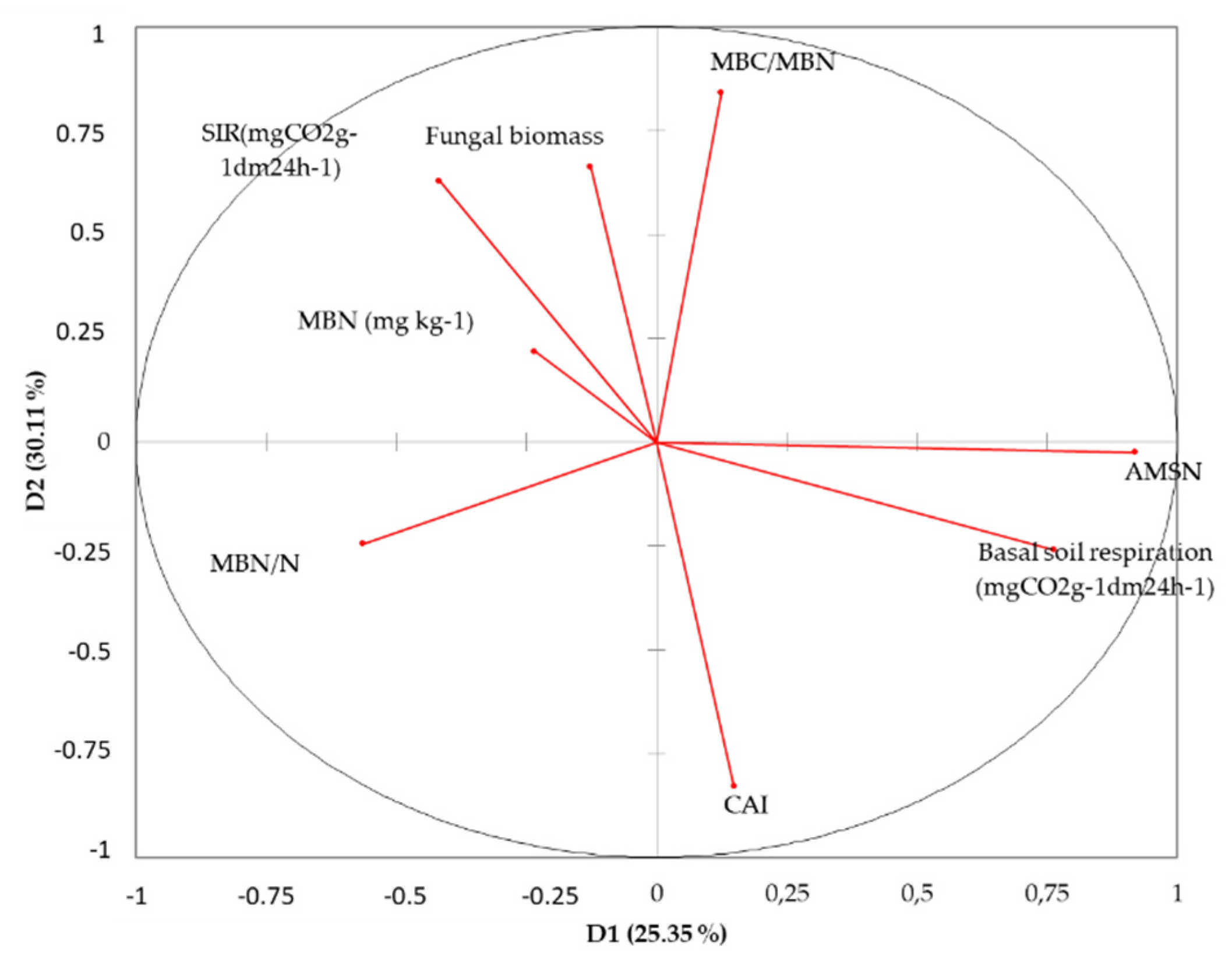
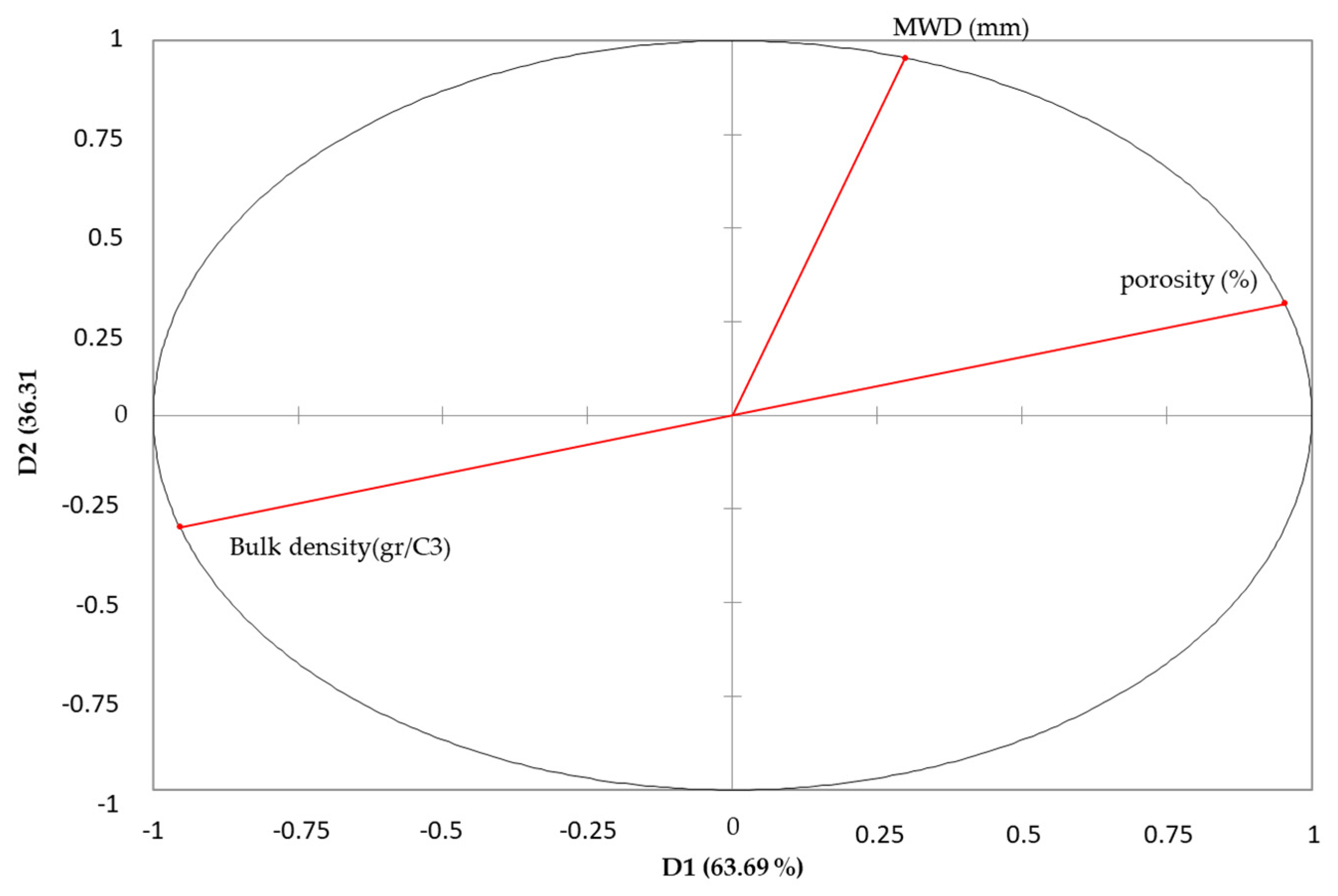
3.10. Principal Component Analysis (PCA) Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alemu, M.M. Environmental Role of National Parks. J. Sustain. Dev. 2015, 9, 1. [Google Scholar] [CrossRef]
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland Management and Ecological Adaptation Analysis Model for Astragalus curvirostris Boiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Moradi, P.; Aghajanloo, F.; Moosavi, A.; Monfared, H.H.; Khalafi, J.; Taghiloo, M.; Khoshzaman, T.; Shojaee, M.; Mastinu, A. Anthropic Effects on the Biodiversity of the Habitats of Ferula gummosa. Sustainability 2021, 13, 7874. [Google Scholar] [CrossRef]
- Narouei, M.; Javadi, S.A.; Khodagholi, M.; Jafari, M.; Azizinejad, R. Modeling the effects of climate change on the potential distribution of the rangeland species Gymnocarpus decander Forssk (case study: Arid region of southeastern Iran). Env. Monit Assess 2022, 194, 1–15. [Google Scholar] [CrossRef]
- Villamil, M.B.; Amiotti, N.M.; Peinemann, N. Soil degradation related to overgrazing in the semi-arid southern Caldenal area of Argentina. Soil. Sci. 2001, 166, 441–452. [Google Scholar] [CrossRef]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae 2022, 8, 193. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrao, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Chaichi, M.; Nemati, A.; Dadrasi, A.; Heydari, M.; Hassanisaadi, M.; Yousefi, A.R.; Baldwin, T.C.; Mastinu, A. Germination of Triticum aestivum L.: Effects of Soil-Seed Interaction on the Growth of Seedlings. Soil Syst. 2022, 6, 37. [Google Scholar] [CrossRef]
- Fawad, M.; Khan, M.A.; Wahid, F.; Khan, H.; Gul, B.; Khattak, A.M.; Jamal, A.; Mastinu, A. Irrigation Scheduling and Weed Management: A Sustainable Approach for Managing Broomrape and Other Weeds in Tomato Crop. Horticulturae 2022, 8, 676. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Mac Sweeney, E.; Mastinu, A. Competitive Ability Effects of Datura stramonium L. and Xanthium strumarium L. on the Development of Maize (Zea mays) Seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Mac Sweeney, E.; Mastinu, A. Biological Response of Lallemantia iberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.R.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Yousefvand, P.; Sohrabi, Y.; Heidari, G.; Weisany, W.; Mastinu, A. Salicylic Acid Stimulates Defense Systems in Allium hirtifolium Grown under Water Deficit Stress. Molecules 2022, 27, 1083. [Google Scholar] [CrossRef] [PubMed]
- Kamali, N.; Siroosi, H.; Sadeghipour, A. Impacts of wind erosion and seasonal changes on soil carbon dioxide emission in southwestern Iran. J. Arid Land 2020, 12, 690–700. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Poore, A.G.B.; Ruiz-Colmenero, M.; Letnic, M.; Soliveres, S. Ecosystem structure, function, and composition in rangelands are negatively affected by livestock grazing. Ecol. Appl. 2016, 26, 1273–1283. [Google Scholar] [CrossRef]
- Zamin, T.J.; Grogan, P. Caribou exclusion during a population low increases deciduous and evergreen shrub species biomass and nitrogen pools in low Arctic tundra. J. Ecol. 2013, 101, 671–683. [Google Scholar] [CrossRef]
- Stark, S.; Mannisto, M.K.; Ganzert, L.; Tiirola, M.; Haggblom, M.M. Grazing intensity in subarctic tundra affects the temperature adaptation of soil microbial communities. Soil. Biol. Biochem. 2015, 84, 147–157. [Google Scholar] [CrossRef]
- Stark, S.; Julkunen-Tiitto, R.; Kumpula, J. Ecological role of reindeer summer browsing in the mountain birch (Betula pubescens ssp. czerepanovii) forests: Effects on plant defense, litter decomposition, and soil nutrient cycling. Oecologia 2007, 151, 486–498. [Google Scholar] [CrossRef]
- Olofsson, J.; Stark, S.; Oksanen, L. Reindeer influence on ecosystem processes in the tundra. Oikos 2004, 105, 386–396. [Google Scholar] [CrossRef]
- Liang, C.; MacDonald, J.D.; Desjardins, R.L.; McConkey, B.G.; Beauchemin, K.A.; Flemming, C.; Cerkowniak, D.; Blondel, A. Beef cattle production impacts soil organic carbon storage. Sci. Total Environ. 2020, 718, 137273. [Google Scholar] [CrossRef]
- Steffens, M.; Kölbl, A.; Totsche, K.U.; Kögel-Knabner, I. Grazing effects on soil chemical and physical properties in a semiarid steppe of Inner Mongolia (P.R. China). Geoderma 2008, 143, 63–72. [Google Scholar] [CrossRef]
- Becker, A.E.; Horowitz, L.S.; Ruark, M.D.; Jackson, R.D. Surface-soil carbon stocks greater under well-managed grazed pasture than row crops. Soil Sci. Soc. Am. J. 2022, 86, 758–768. [Google Scholar] [CrossRef]
- Bertol, F.D.Z.; Martins, A.P.; Denardin, L.G.D.; Kunrath, T.R.; de Souza, W.; Goulart, M.W.; Carvalho, P.C.D.; Anghinoni, I. Liming and grazing intensities effects on soil mineral nitrogen throughout the pasture cycle in a subtropical integrated crop-livestock system. Rev. Bras. Cienc Solo 2022, 46, e0210042. [Google Scholar] [CrossRef]
- Capstaff, N.M.; Domoney, C.; Miller, A.J. Real-time monitoring of rhizosphere nitrate fluctuations under crops following defoliation. Plant Methods 2021, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Mahanta, S.K.; Manna, M.C.; Singh, S.; Bhattacharyya, R.; Tyagi, V.C.; Singh, J.B.; Ram, S.N.; Srinivasan, R.; Singh, A.K.; et al. Long-Term Grazing Mediates Soil Organic Carbon Dynamics by Reorienting Enzyme Activities and Elemental Stoichiometry in Semi-arid Tropical Inceptisol. J. Soil Sci. Plant Nut. 2022, 22, 1422–1433. [Google Scholar] [CrossRef]
- Li, J.W.; Shangguan, Z.P.; Deng, L. Free particulate organic carbon plays critical roles in carbon accumulations during grassland succession since grazing exclusion. Soil Till. Res. 2022, 220, 105380. [Google Scholar] [CrossRef]
- Minocha, R.; Contosta, A.R.; Lawrence, G.B.; Kohli, R.K.; Minocha, S.C.; Long, S. Changes in Soil Chemistry and Foliar Metabolism of Himalayan Cedar (Cedrus deodara) and Himalayan Spruce (Picea smithiana) along an Elevational Gradient at Kufri, HP, India: The Potential Roles of Regional Pollution and Localized Grazing. Forests 2021, 12, 400. [Google Scholar] [CrossRef]
- Strand, L.T.; Fjellstad, W.; Jackson-Blake, L.; De Wit, H.A. Afforestation of a pasture in Norway did not result in higher soil carbon, 50 years after planting. Landsc. Urban. Plan. 2021, 207, 104007. [Google Scholar] [CrossRef]
- Khan, A.N.; Ali, A. Desertification Risk Reduction Approaches in Pakistan. In Disaster Risk Reduction Approaches in Pakistan; Springer: Tokyo, Japan, 2015; pp. 161–173. [Google Scholar] [CrossRef]
- Shaheb, M.R.; Venkatesh, R.; Shearer, S.A. A Review on the Effect of Soil Compaction and its Management for Sustainable Crop Production. J. Biosyst. Eng. 2021, 46, 417–439. [Google Scholar] [CrossRef]
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef]
- Yin, R.; Kardol, P.; Thakur, M.P.; Gruss, I.; Wu, G.-L.; Eisenhauer, N.; Schädler, M. Soil functional biodiversity and biological quality under threat: Intensive land use outweighs climate change. Soil Biol. Biochem. 2020, 147, 107847. [Google Scholar] [CrossRef] [PubMed]
- Sajedi, T.; Prescott, C.E.; Seely, B.; Lavkulich, L.M. Relationships among soil moisture, aeration and plant communities in natural and harvested coniferous forests in coastal British Columbia, Canada. J. Ecol. 2012, 100, 605–618. [Google Scholar] [CrossRef]
- Aislabie, J.; Deslippe, J.R. Soil Microbes and Their Contribution to Soil Services. In Ecosystem Services in New Zealand: Conditions and Trends; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 143–161. [Google Scholar]
- Sacca, M.L.; Caracciolo, A.B.; Di Lenola, M.; Grenni, P. Ecosystem Services Provided By Soil Microorganisms. In Soil Biological Communities and Ecosystem Resilience; Springer: Cham, Switzerland, 2017; pp. 9–14. [Google Scholar] [CrossRef]
- Motamedi, M.; Zahedi, M.; Karimmojeni, H.; Motamedi, H.; Mastinu, A. Effect of rhizosphere bacteria on antioxidant enzymes and some biochemical characteristics of Medicago sativa L. subjected to herbicide stress. Acta Physiol. Plant 2022, 44, 1–12. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.J.; Chang, S.J.; Kan, H.M.; Lin, L.J. Impact of Grazing on Soil Carbon and Microbial Biomass in Typical Steppe and Desert Steppe of Inner Mongolia. PLoS ONE 2012, 7, e36434. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Anderson, I.C.; Singh, B.K. Response of Soil Properties and Microbial Communities to Agriculture: Implications for Primary Productivity and Soil Health Indicators. Front. Plant Sci. 2016, 7, 990. [Google Scholar] [CrossRef]
- Schimel, J. Soil Microbiology, Ecology, and Biochemistry for the 21st Century. In Soil Microbiology, Ecology and Biochemistry; Academic Press: Cambridge, MA, USA, 2007; pp. 503–514. [Google Scholar] [CrossRef]
- Piao, H.C.; Hong, Y.T.; Yuan, Z.Y. Seasonal changes of microbial biomass carbon related to climatic factors in soils from karst areas of southwest China. Biol. Fert. Soils 2000, 30, 294–297. [Google Scholar] [CrossRef]
- Delille, D.; Fiala, M.; Kuparinen, J.; Kuosa, H.; Plessis, C. Seasonal changes in microbial biomass in the first-year ice of the Terre Adélie area (Antarctica). Aquat. Microb. Ecol. 2002, 28, 257–265. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Seasonal changes in soil phosphorus and associated microbial properties under adjacent grassland and forest in New Zealand. For. Ecol. Manag 2003, 177, 539–557. [Google Scholar] [CrossRef]
- Prihar, S.S.; Hundal, S.S. Determination of bulk density of soil clod by saturation. Geoderma 1971, 5, 283–286. [Google Scholar] [CrossRef]
- Danielson, R.E.; Sutherland, P.L. Porosity. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods, 5.1, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 2018; pp. 443–461. [Google Scholar] [CrossRef]
- Chen, H.Q.; Hou, R.X.; Gong, Y.S.; Li, H.W.; Fan, M.S.; Kuzyakov, Y. Effects of 11 years of conservation tillage on soil organic matter fractions in wheat monoculture in Loess Plateau of China. Soil Till. Res. 2009, 106, 85–94. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Simple modification of the Walkley-Black method for simultaneous determination of organic carbon and potentially mineralizable nitrogen in tropical rice soils. Plant Soil 1982, 69, 73–77. [Google Scholar] [CrossRef]
- Alvarado, J.; Marquez, M.; Leon, L.E. Determination of Organic Nitrogen by the Kjeldahl Method Using Microwave Acid Digestion. Anal. Lett. 1988, 21, 357–365. [Google Scholar] [CrossRef]
- Huluka, G.; Evans, C.E. Correlation of Potassium Extracted by Different Methods with Vegetative Growth of Teff. Commun. Soil Sci. Plan. 1992, 23, 1427–1437. [Google Scholar] [CrossRef]
- Kelepertzis, E.; Paraskevopoulou, V.; Argyraki, A.; Fligos, G.; Chalkiadaki, O. Evaluation of single extraction procedures for the assessment of heavy metal extractability in citrus agricultural soil of a typical Mediterranean environment (Argolida, Greece). J. Soil Sediment. 2015, 15, 2265–2275. [Google Scholar] [CrossRef]
- Fardeau, J.C.; Morel, C.; Boniface, R. Why the Olsen Method Should Be Used to Estimate Available Soil-Phosphorus. Agronomie 1988, 8, 577–584. [Google Scholar] [CrossRef]
- Beni, A.; Lajtha, K.; Kozma, J.; Fekete, I. Application of a Stir Bar Sorptive Extraction sample preparation method with HPLC for soil fungal biomass determination in soils from a detrital manipulation study. J. Microbiol. Meth. 2017, 136, 1–5. [Google Scholar] [CrossRef]
- Anderson, J.P.E. Soil Respiration. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 2015; pp. 831–871. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil—V. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Soon, Y.K.; Abboud, S. A comparison of some methods for soil organic carbon determination. Commun. Soil Sci Plan. 2008, 22, 943–954. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Turkovskaya, O.V.; Golubev, S.N. The Collection of Rhizosphere Microorganisms: Its importance for the study of associative plant-bacterium interactions. Vavilovskii J. Genet. 2020, 24, 315–324. [Google Scholar] [CrossRef]
- Drewry, J.J.; Lowe, J.A.H.; Paton, R.J. Effect of sheep stocking intensity on soil physical properties and dry matter production on a Pallic Soil in Southland. N. Z. J. Agr. Res. 1999, 42, 493–499. [Google Scholar] [CrossRef]
- He, M.Z.; Zheng, J.G.; Li, X.R.; Qian, Y.L. Environmental factors affecting vegetation composition in the Alxa Plateau, China. J. Arid Env. 2007, 69, 473–489. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Wittig, R. The impact of grazing intensity on soil characteristics of Stipa grandis and Stipa bungeana steppe in northern China (autonomous region of Ningxia). Acta Oecol. 2004, 25, 197–204. [Google Scholar] [CrossRef]
- Liebig, M.A.; Gross, J.; Kronberg, S.L.; Hanson, J.D.; Frank, A.B.; Phillips, R.L. Soil response to long-term grazing in the northern Great Plains of North America. Agr. Ecosyst. Env. 2006, 115, 270–276. [Google Scholar] [CrossRef]
- Teague, R.; Kreuter, U. Managing Grazing to Restore Soil Health, Ecosystem Function, and Ecosystem Services. Front. Sustain. Food Syst. 2020, 4, 534187. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Are, K.S.; Huang, Z.; Yu, H.; Zhang, Q. Livestock grazing significantly accelerates soil erosion more than climate change in Qinghai-Tibet Plateau: Evidenced from 137Cs and 210Pbex measurements. Agric. Ecosyst. Environ. 2019, 285, 106643. [Google Scholar] [CrossRef]
- Minoshima, H.; Jackson, L.E.; Cavagnaro, T.R.; Sanchez-Moreno, S.; Ferris, H.; Temple, S.R.; Goyal, S.; Mitchell, J.P. Soil food webs and carbon dynamics in response to conservation tillage in California. Soil. Sci. Soc. Am. J. 2007, 71, 952–963. [Google Scholar] [CrossRef]
- Soderstrom, B.E. Seasonal Fluctuations of Active Fungal Biomass in Horizons of a Podzolized Pine-Forest Soil in Central Sweden. Soil Biol. Biochem. 1979, 11, 149–154. [Google Scholar] [CrossRef]
- Alberton, O.; Kuyper, T.W.; Gorissen, A. Taking mycocentrism seriously: Mycorrhizal fungal and plant responses to elevated CO2. New Phytol. 2005, 167, 859–868. [Google Scholar] [CrossRef]
- Hartley, I.P.; Armstrong, A.F.; Murthyw, R.; Barron-Gafford, G.; Ineson, P.; Atkin, O.K. The dependence of respiration on photosynthetic substrate supply and temperature: Integrating leaf, soil and ecosystem measurements. Glob. Change Biol. 2006, 12, 1954–1968. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Hartley, I.P.; Ineson, P.; Fitter, A.H. Soil temperature affects carbon allocation within arbuscular mycorrhizal networks and carbon transport from plant to fungus. Glob. Change Biol. 2008, 14, 1181–1190. [Google Scholar] [CrossRef]
- Castano, C.; Lindahl, B.D.; Alday, J.G.; Hagenbo, A.; de Aragon, J.M.; Parlade, J.; Pera, J.; Bonet, J.A. Soil microclimate changes affect soil fungal communities in a Mediterranean pine forest. New Phytol. 2018, 220, 1211–1221. [Google Scholar] [CrossRef]
- Xu, X.K.; Inubushi, K.; Sakamoto, K. Effect of vegetations and temperature on microbial biomass carbon and metabolic quotients of temperate volcanic forest soils. Geoderma 2006, 136, 310–319. [Google Scholar] [CrossRef]
- Zarafshar, M.; Bazot, S.; Matinizadeh, M.; Bordbar, S.S.K.; Rousta, M.J.; Kooch, Y.; Enayati, K.; Abbasi, A.; Negahdarsaber, M. Do tree plantations or cultivated fields have the same ability to maintain soil quality as natural forests? Appl. Soil Ecol. 2020, 151, 103536. [Google Scholar] [CrossRef]
- Strebel, D.; Elberling, B.; Morgner, E.; Knicker, H.E.; Cooper, E.J. Cold-season soil respiration in response to grazing and warming in High-Arctic Svalbard. Polar Res. 2010, 29, 46–57. [Google Scholar] [CrossRef]
- Abbott, L.K.; Robson, A.D. Factors Influencing the Occurrence of Vesicular Arbuscular Mycorrhizas. Agr. Ecosyst. Env. 1991, 35, 121–150. [Google Scholar] [CrossRef]
- Janos, D.P.; Sahley, C.T. Rodent Dispersal of Vesicular-Arbuscular Mycorrhizal Fungi in Amazonian Peru. Ecology 1995, 76, 1852–1858. [Google Scholar] [CrossRef]
- Ramírez-Gerardo, M.; Álvarez-Sánchez, J.; Guadarrama Chávez, P.; Sánchez-Gallén, I. Estudio de hongos micorrizógenos arbusculares bajo árboles remanentes en un pastizal tropical. Bot. Sci. 2017, 61, 15–20. [Google Scholar] [CrossRef]
- Guadarrama, P.; Alvarez-Sanchez, F.J. Abundance of arbuscular mycorrhizal fungi spores in different environments in a tropical rain forest, Veracruz, Mexico. Mycorrhiza 1999, 8, 267–270. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Patra, A.K.; Abbadie, L.; Clays-Josserand, A.; Degrange, V.; Grayston, S.J.; Loiseau, P.; Louault, F.; Mahmood, S.; Nazaret, S.; Philippot, L.; et al. Effects of grazing on microbial functional groups involved in soil N dynamics. Ecol. Monogr. 2005, 75, 65–80. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wu, L.W.; Lin, Q.Y.; Yuan, M.T.; Xu, D.P.; Yu, H.; Hu, Y.G.; Duan, J.C.; Li, X.Z.; He, Z.L.; et al. Responses of the functional structure of soil microbial community to livestock grazing in the Tibetan alpine grassland. Glob. Change Biol. 2013, 19, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Tscherko, D.; Kandeler, E.; Jones, T.H. Effect of temperature on below-ground N-dynamics in a weedy model ecosystem at ambient and elevated atmospheric CO2 levels. Soil Biol. Biochem. 2001, 33, 491–501. [Google Scholar] [CrossRef]
- Beare, M.H.; Neely, C.L.; Coleman, D.C.; Hargrove, W.L. A Substrate-Induced Respiration (Sir) Method for Measurement of Fungal and Bacterial Biomass on Plant Residues. Soil Biol. Biochem. 1990, 22, 585–594. [Google Scholar] [CrossRef]
- Cochran, V.L.; Horton, K.A.; Cole, C.V. An Estimation of Microbial Death Rate and Limitations of N or C during Wheat Straw Decomposition. Soil Biol. Biochem. 1988, 20, 293–298. [Google Scholar] [CrossRef]
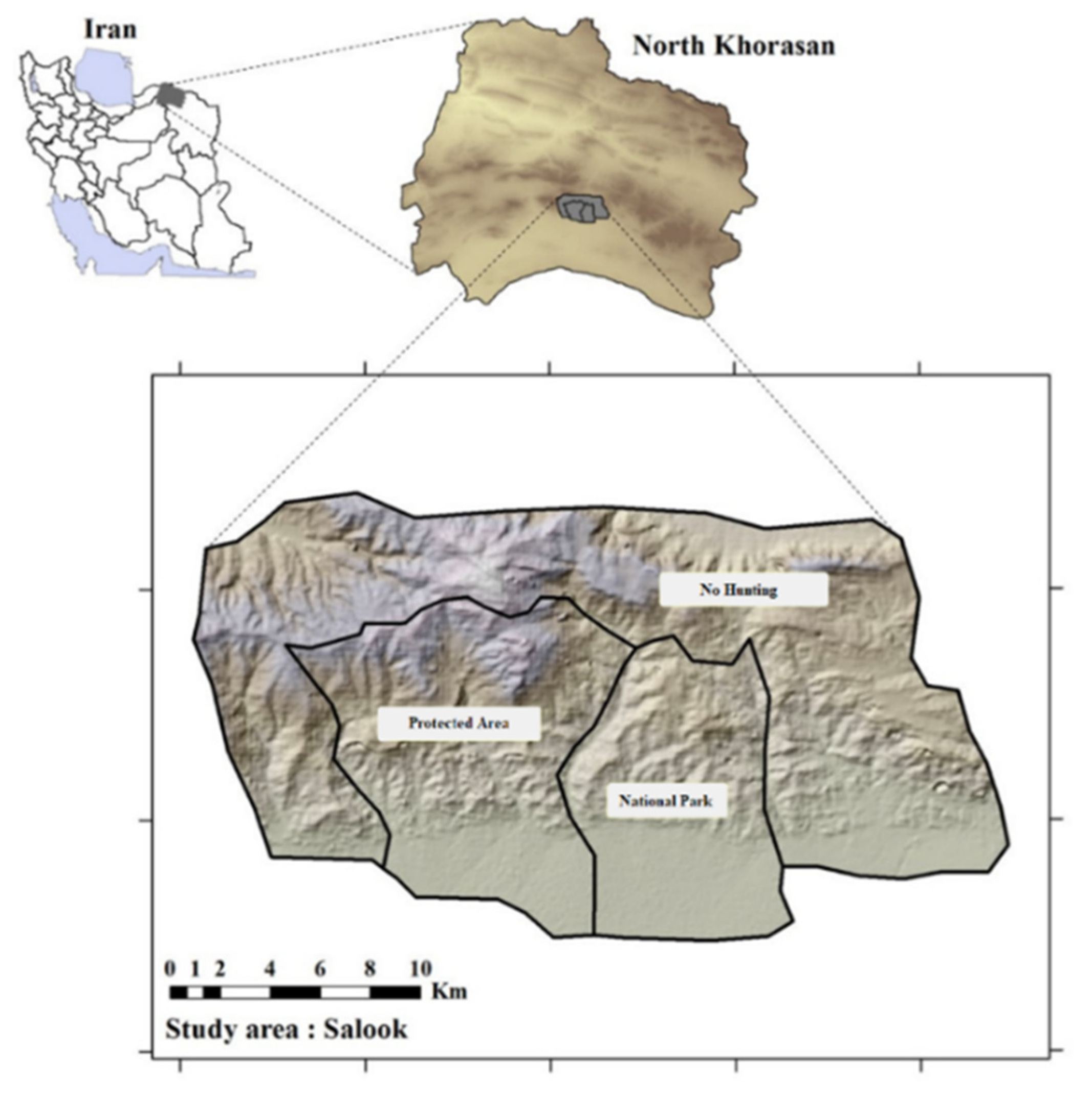

| Conservative Management Level | Site Description | Grazing Time | Sheep ha−1 | Dominant Rangeland Species (Canopy Cover, %) | Associated Plant Species Biomass, (kg ha−1) | Associated Plant Species (Canopy Cover, %) | Soil Texture (0–30 cm Depth) |
|---|---|---|---|---|---|---|---|
| National Park | Only grazed by wild animals for 19 years, dominated by rangeland species (cover: ~51%) | 0 days | - | Artemisia aucheri Boiss. (8.96), Cousinia umbrosa Bunge. (5.40) | Artemisia aucheri Boiss. (98.3), Cousinia umbrosa Bunge. (12.3) | Stipa barbata Desf. (2.8), annual grasses (2.67) | Sandy loam (sand: 58.30%, silt: 22.18%, clay: 18.9%) |
| Protected Area | Grazed by nomadic livestock for 60 days of the year for 48 years, dominated by rangeland species (cover: ~43%) | 60 days of the year (about 22 May to end of July) | 18–20 | Artemisia aucheri Boiss. (6.66), Cousinia umbrosa Bunge (4.55) | Artemisia aucheri Boiss. (73.42), Cousinia umbrosa Bunge. (9.8) | Lactuca orientalis (Boiss.) Boiss. (3.2), Rosa persica Michx. ex Juss. (3.28) | Sandy loam (sand: 58.77%, silt: 21.74%, clay: 19.48%) |
| No Hunting | Just free grazing of nomadic livestock takes place in all seasons, dominated by woody species (cover: ~24%) | Free grazing in all seasons | 35–50 | Artemisia aucheri Boiss. (5.23), Rosa persica Michx. ex Juss. (4.90) | Artemisia aucheri Boiss. (57.46), Rosa persica Michx. ex Juss. (10.5) | Hulthemia persica (Michx. ex Juss.) Bornm. (3.22), Lactuca orientalis (Boiss.) Boiss. (2.75) | Sandy loam (sand: 59.12%, silt: 22.093%, clay: 18.78%) |
| df | Sand | Silt | Clay | Potassium | Bulk Density | pH | |
|---|---|---|---|---|---|---|---|
| Between Groups | 2 | 0.480 | 0.788 | 2.086 | 1371.212 | 0.348 ** | 0.002 |
| Within Groups | 42 | 15.045 | 12.280 | 15.628 | 2044.678 | 0.018 | 0.068 |
| Total | 44 | ||||||
| df | Phosphorus | EC | Ca | T.N.V | MDW | Porosity | |
| Between Groups | 2 | 350.688 ** | 0.046 | 0.550 | 16.613 | 0.919 ** | 411.764 ** |
| Within Groups | 42 | 19.914 | 0.145 | 0.534 | 69.477 | 0.026 | 22.555 |
| Total | 44 |
| Treatments | Bulk Density | Porosity | Phosphorus | MWD |
|---|---|---|---|---|
| No hunting | 1.483 a | 46.33 b | 26.866 c | 0.781 b |
| National park | 1.202 b | 56.326 a | 36.533 a | 1.246 a |
| Protected area | 1.232 b | 54.06 a | 31.933 b | 1.171 a |
| Source | df | Fungal Biomass | Basal Soil Respiration | Microbial Biomass Carbon | MBN | MBC/MBN | Microbial Quotient |
|---|---|---|---|---|---|---|---|
| Rep | 14 | 0.220 | 0.020 | 118.574 | 0.798 | 7.986 | 0.613 |
| Site | 2 | 82.148 ** | 2.549 ** | 105,690.617 ** | 181.103 ** | 579.191 ** | 21.207 ** |
| Season | 3 | 4.040 ** | 0.869 ** | 5002.696 ** | 14.694 ** | 370.855 ** | 4.310 ** |
| Site × Season | 6 | 0.894 ** | 0.300 ** | 164.624 | 1.832 * | 60.539 ** | 1.389 |
| Error | 154 | 0.199 | 0.026 | 179.429 | 0.740 | 12.194 | 0.813 |
| CV% | 29.81 | 41.59 | 14.73 | 14.78 | 22.42 | 37.5 | |
| Source | df | QCo2 | Mycorrhiza | Corg | MBN/N | SIR | CAI |
| Rep | 14 | 0.002 | 99.81 | 0.38 | 0.06 | 0.3 | 0.31 |
| Site | 2 | 0.3 ** | 4788.74 ** | 352.69 ** | 4.28 ** | 29.36 ** | 31.68 ** |
| Season | 3 | 0.01 ** | 8897.14 ** | 10.4 ** | 6.75 ** | 4.23 ** | 5.69 ** |
| Site × Season | 6 | 0.004 | 363.12 ** | 0.82 | 0.34 ** | 3.75 ** | 6.7 ** |
| Error | 154 | 0.0004 | 71.85 | 0.59 | 0.07 | 0.18 | 0.06 |
| CV% | 32.79 | 18.18 | 17.62 | 6.25 | 28.47 | 42.67 |
| Treatments | Fungal Biomass | Basal Soil Respiration | MBN | MBC/MBN | AMSN | N Total | MBN/N | SIR | CAI |
|---|---|---|---|---|---|---|---|---|---|
| N × season 1 | 0.253 d | 0.544 b | 2.589 f | 17.404 ab | 59.53 b | 0.444 fg | 0.6 e | 1.083 de | 0.567 c |
| N × season 2 | 0.293 d | 0.965 a | 4.335 d | 14.057 cd | 77.73 a | 0.728 a | 0.6 e | 0.673 f | 1.552 b |
| N × season 3 | 0.256 d | 0.579 b | 3.613 e | 11.7 d | 57.07 b | 0.739 a | 0.48 e | 1.071 e | 0.578 c |
| N × season 4 | 0.231 d | 0.235 cde | 4.76 d | 5.352 e | 33.27 def | 0.306 h | 1.612 b | 0.137 g | 2.926 a |
| P × season1 | 1.849 b | 0.266 cd | 6.7 bc | 17.329 ab | 40.8 c | 0.627 bc | 1.08 cd | 1.661 c | 0.175 cd |
| P × season2 | 1.883 b | 0.308 c | 7.213 ab | 17.232 ab | 60.87 b | 0.582 cd | 1.268 c | 1.117 de | 0.282 cd |
| P × season3 | 1.945 b | 0.231 d | 6.767 abc | 16.995 ab | 37.4 cd | 0.617 bc | 1.112 cd | 1.411 cd | 0.172 cd |
| P × season4 | 1.025 c | 0.119 e | 7.4 a | 14.102 cd | 29.93 f | 0.4 g | 1.921 a | 2.165 b | 0.066 d |
| NP × season 1 | 2.761 a | 0.228 cde | 6.105 c | 19.542 a | 38 cd | 0.536 de | 1.145 cd | 2.219 b | 0.108 cd |
| NP × season 2 | 2.796 a | 0.265 cd | 6.678 bc | 19.795 a | 57.4 b | 0.641 bc | 1.055 d | 1.333 de | 0.202 cd |
| NP × season 3 | 2.847 a | 0.237 cde | 6.441 c | 18.164 a | 36.73 cde | 0.657 b | 1.016 d | 2.113 b | 0.116 cd |
| NP × season 4 | 1.914 b | 0.163 de | 7.181 ab | 15.168 bc | 30.73 ef | 0.474 ef | 1.568 b | 2.849 a | 0.062 d |
| Treatment | Microbial Biomass Carbon | Microbial Quotient | qCO2 |
|---|---|---|---|
| Site | |||
| No hunting | 42.55 b | 3.076 a | 0.142 a |
| National park | 112.72 a | 2.01 b | 0.02 b |
| Protected area | 117.53 a | 2.085 b | 0.018 b |
| Season | |||
| 1 | 92.11 b | 2.695 a | 0.062 a |
| 2 | 104.16 a | 2554 a | 0.07 a |
| 3 | 88.91 b | 1.965 b | 0.068 a |
| 4 | 78.56 c | 2.38 a | 0.043 b |
| Variables | Fungal Biomass | Basal Soil Respiration | MBN | MBC/MBN | AMSN | MBN/N | SIR | CAI |
|---|---|---|---|---|---|---|---|---|
| Fungal biomass | 1 | |||||||
| Basal soil respiration | −0.473 * | 1 | ||||||
| MBN | 0.603 * | −0.510 * | 1 | |||||
| MBC/MBN | 0.479 * | −0.100 | 0.043 | 1 | ||||
| AMSN | −0.285 * | 0.637 * | −0.378 * | 0.075 | 1 | |||
| MBN/N | 0.189 * | −0.556 * | 0.648 * | −0.251 * | −0.557 * | 1 | ||
| SIR | 0.517 * | −0.413 * | 0.480 * | 0.333 * | −0.405 * | 0.286 * | 1 | |
| CAI | −0.481 * | 0.366 * | −0.344 * | −0.513 * | 0.108 | 0.026 | −0.604 * | 1 |
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | |
|---|---|---|---|---|---|---|---|---|
| Eigenvalue | 3.630 | 1.894 | 0.713 | 0.590 | 0.441 | 0.364 | 0.233 | 0.135 |
| Variability (%) | 45.381 | 23.672 | 8.912 | 7.375 | 5.510 | 4.544 | 2.917 | 1.689 |
| Cumulative % | 45.381 | 69.053 | 77.966 | 85.340 | 90.850 | 95.394 | 98.311 | 100.000 |
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | |
|---|---|---|---|---|---|---|---|---|
| Fungal biomass | 0.754 | 0.330 | 0.261 | −0.347 | 0.137 | −0.291 | 0.067 | −0.161 |
| Basal soil respiration | −0.787 | 0.241 | 0.274 | 0.219 | 0.370 | 0.019 | −0.232 | −0.098 |
| MBN (mg kg−1) | 0.789 | −0.200 | 0.517 | 0.049 | −0.023 | −0.014 | −0.153 | 0.210 |
| MBC/MBN | 0.319 | 0.793 | −0.092 | −0.314 | 0.110 | 0.379 | −0.053 | 0.060 |
| AMSN | −0.645 | 0.455 | 0.491 | 0.106 | −0.222 | 0.100 | 0.255 | −0.011 |
| MBN/N | 0.597 | −0.671 | 0.173 | 0.042 | −0.004 | 0.351 | 0.023 | −0.194 |
| SIR | 0.760 | 0.242 | −0.118 | 0.445 | 0.318 | 0.004 | 0.221 | 0.046 |
| CAI | −0.608 | −0.585 | 0.093 | −0.331 | 0.349 | 0.038 | 0.186 | 0.111 |
| Variables | Bulk Density (gr/C3) | MWD (mm) | Porosity (%) |
|---|---|---|---|
| Bulk density (gr/C3) | 1 | ||
| MWD (mm) | −0.570 * | 1 | |
| porosity (%) | −1.000 * | 0.570 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamali, N.; Sadeghipour, A.; Souri, M.; Mastinu, A. Variations in Soil Biological and Biochemical Indicators under Different Grazing Intensities and Seasonal Changes. Land 2022, 11, 1537. https://doi.org/10.3390/land11091537
Kamali N, Sadeghipour A, Souri M, Mastinu A. Variations in Soil Biological and Biochemical Indicators under Different Grazing Intensities and Seasonal Changes. Land. 2022; 11(9):1537. https://doi.org/10.3390/land11091537
Chicago/Turabian StyleKamali, Nadia, Ahmad Sadeghipour, Mahshid Souri, and Andrea Mastinu. 2022. "Variations in Soil Biological and Biochemical Indicators under Different Grazing Intensities and Seasonal Changes" Land 11, no. 9: 1537. https://doi.org/10.3390/land11091537
APA StyleKamali, N., Sadeghipour, A., Souri, M., & Mastinu, A. (2022). Variations in Soil Biological and Biochemical Indicators under Different Grazing Intensities and Seasonal Changes. Land, 11(9), 1537. https://doi.org/10.3390/land11091537







