Coexistence of Native and Invasive Freshwater Turtles: The Llobregat Delta (NE Iberian Peninsula) as a Case Study
Abstract
1. Introduction
2. Materials and Methods
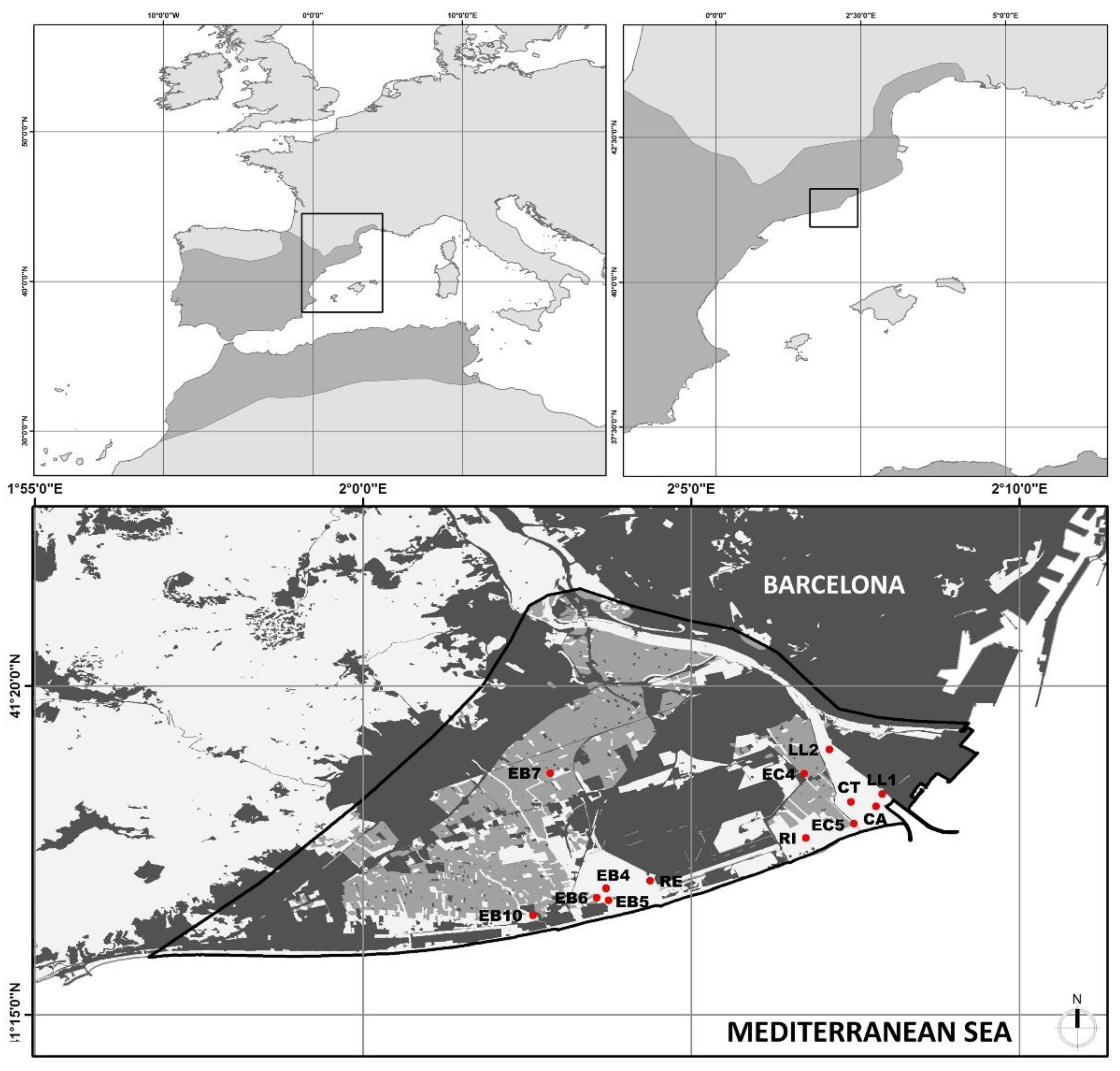
2.1. Study Site and Species Description
2.2. Sampling Methodology
2.3. Landscape and Environmental Variables
2.4. Data Analysis
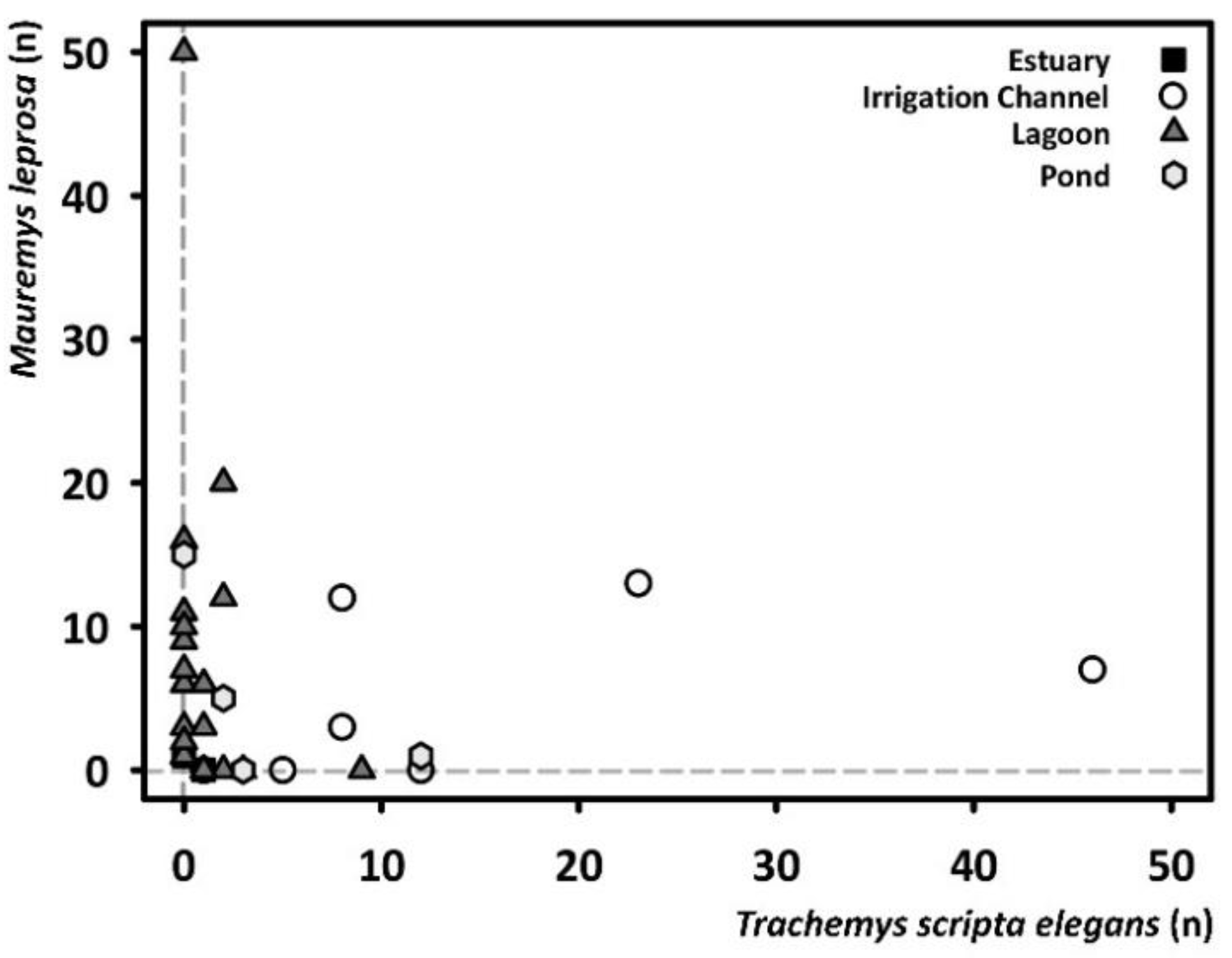
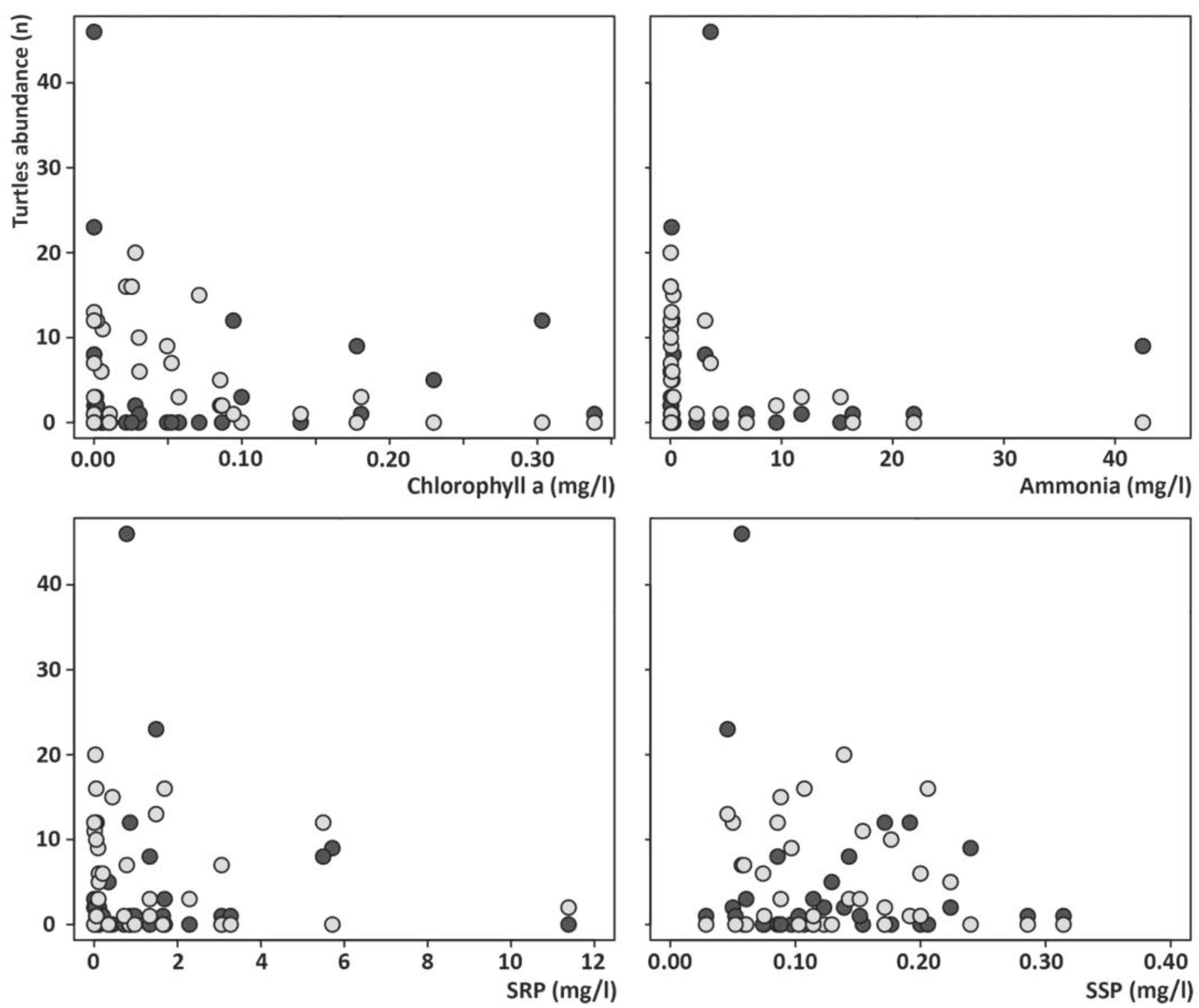
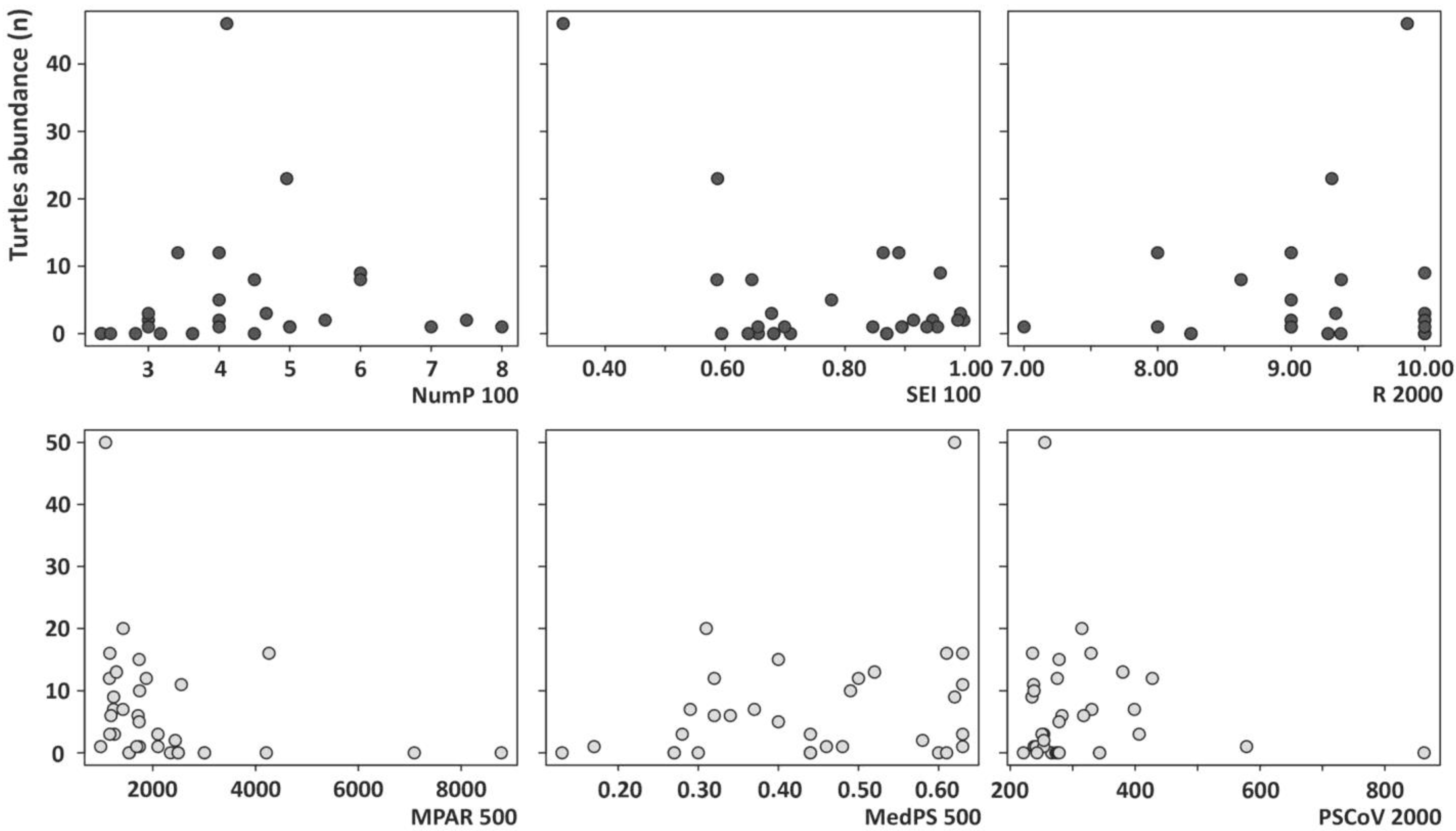
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonada, N.; Cañedo-Argüelles, M.; Obrador, B.; Rodríguez-Lozano, P.; Verkaik, I. In Memoriam: Maria Rieradevall (1960–2015). Limnetica 2015, 34, 1–6. [Google Scholar]
- Clavero, M.; García-Berthou, E. Invasive Species Are a Leading Cause of Animal Extinctions. Trends Ecol. Evol. 2005, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Gurevitch, J.; Padilla, D. Are Invasive Species a Major Cause of Extinctions? Trends Ecol. Evol. 2004, 19, 470–474. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, M.A.; Butchart, S.H.M.; Spear, D.; Marais, E.; Kleynhans, E.J.; Symes, A.; Chanson, J.; Hoffmann, M. Global Indicators of Biological Invasion: Species Numbers, Biodiversity Impact and Policy Responses. Divers. Distrib. 2010, 16, 95–108. [Google Scholar] [CrossRef]
- O’Connor, R.J.; Usher, M.B.; Gibbs, A.; Brown, K.C. Biological Characteristics of Invaders among Bird Species in Britain [and Discussion]. Philos. Trans. R. Soc. B Biol. Sci. 1986, 314, 583–598. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Harpole, W.S.; Reichman, O.J.; Tilman, D. Invasion, Competitive Dominance, and Resource Use by Exotic and Native California Grassland Species. Proc. Natl. Acad. Sci. USA 2003, 100, 13384–13389. [Google Scholar] [CrossRef]
- Griffen, B.D.; Altman, I.; Bess, B.M.; Hurley, J.; Penfield, A. The Role of Foraging in the Success of Invasive Asian Shore Crabs in New England. Biol. Invasions 2012, 14, 2545–2558. [Google Scholar] [CrossRef]
- Tonella, L.H.; Fugi, R.; Vitorino, O.B.; Suzuki, H.I.; Gomes, L.C.; Agostinho, A.A. Importance of Feeding Strategies on the Long-Term Success of Fish Invasions. Hydrobiologia 2018, 817, 239–252. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Most Invasive Species Largely Conserve Their Climatic Niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cleland, E.E. The Evolutionary Impact of Invasive Species. Proc. Natl. Acad. Sci. USA 2001, 98, 5446–5451. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Gemmell, N.J.; Rand, T.A.; Ewers, R.M. Interactive Effects of Habitat Modification and Species Invasion on Native Species Decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological Impacts of Invasive Alien Plants: A Meta-Analysis of Their Effects on Species, Communities and Ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Deane, D.C.; Li, S.; Wu, Y.; Sui, X.; Xu, H.; Chu, C.; He, F.; Fang, S. Invasion Success and Impacts Depend on Different Characteristics in Non-native Plants. Divers. Distrib. 2021, 27, 1194–1207. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding Remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Chase, J.M.; Leibold, M.A. Ecological Niches: Linking Classical and Contemporary Approaches; University of Chicago Press: Chicago, IL, USA, 2009. [Google Scholar]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Carscadden, K.A.; Emery, N.C.; Arnillas, C.A.; Cadotte, M.W.; Afkhami, M.E.; Gravel, D.; Livingstone, S.W.; Wiens, J.J. Niche Breadth: Causes and Consequences for Ecology, Evolution, and Conservation. Q. Rev. Biol. 2020, 95, 179–214. [Google Scholar] [CrossRef]
- Vázquez, D.P. Exploring the Relationship between Nichie Breadth and Invasion Success. In Conceptual Ecology and Invasion Biology: Reciprocal Approaches to Nature SE—14; Cadotte, M., Mcmahon, S., Fukami, T., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 1, pp. 307–322. [Google Scholar] [CrossRef]
- Ricciardi, A. Invasive Species. In Ecological Systems; Leemans, R., Ed.; Springer: New York, NY, USA, 2013; pp. 161–178. [Google Scholar] [CrossRef]
- Sol, D. Do Successful Invaders Exist? Pre-Adaptations to Novel Environments in Terrestrial Vertebrates. In Biological Invasions; Nentwig, W., Ed.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2007; Volume 193, pp. 127–141. [Google Scholar] [CrossRef]
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of Climatic Niche Shift during Biological Invasion. Ecol. Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef]
- Bates, O.K.; Bertelsmeier, C. Climatic Niche Shifts in Introduced Species. Curr. Biol. 2021, 31, R1252–R1266. [Google Scholar] [CrossRef]
- Wilbur, H.M. Experimental Ecology of Food Webs: Complex Systems in Temporary Ponds. Ecology 1997, 78, 2279–2302. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of Biological Invasions: What’s What and the Way Forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Chen, T.H.; Lue, K.Y. Ecological Notes on Feral Populations of Trachemys scripta elegans in Northern Taiwan. Chelonian Conserv. Biol. 1998, 3, 87–90. [Google Scholar]
- Romero, D.; Báez, J.C.; Ferri-Yáñez, F.; Bellido, J.J.; Real, R. Modelling Favourability for Invasive Species Encroachment to Identify Areas of Native Species Vulnerability. Sci. World J. 2014, 2014, 519710. [Google Scholar] [CrossRef] [PubMed]
- González-Lagos, C.; Cardador, L.; Sol, D. Invasion Success and Tolerance to Urbanization in Birds. Ecography 2021, 44, 1642–1652. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The Population Biology of Invasive Species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Williams, J.L.; Kendall, B.E.; Levine, J.M. Rapid Evolution Accelerates Plant Population Spread in Fragmented Experimental Landscapes. Science 2016, 353, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Borden, J.B.; Flory, S.L. Urban Evolution of Invasive Species. Front. Ecol. Environ. 2021, 19, 184–191. [Google Scholar] [CrossRef]
- Blair, R.B. Land Use and Avian Species Diversity Along an Urban Gradient. Ecol. Appl. 1996, 6, 506–519. [Google Scholar] [CrossRef]
- Fine, P.V.A. The Invasibility of Tropical Forests by Exotic Plants. J. Trop. Ecol. 2002, 18, 687–705. [Google Scholar] [CrossRef]
- Marvier, M.; Kareiva, P.; Neubert, M.G. Habitat Destruction, Fragmentation, and Disturbance Promote Invasion by Habitat Generalists in a Multispecies Metapopulation. Risk Anal. 2004, 24, 869–878. [Google Scholar] [CrossRef]
- Pouteau, R.; Hulme, P.E.; Duncan, R.P. Widespread Native and Alien Plant Species Occupy Different Habitats. Ecography 2015, 38, 462–471. [Google Scholar] [CrossRef]
- Finlayson, M.C. Forty Years of Wetland Conservation and Wise Use. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 139–143. [Google Scholar] [CrossRef]
- Maltby, E. Wetland Management Goals: Wise Use and Conservation. Landsc. Urban Plan. 1991, 20, 9–18. [Google Scholar] [CrossRef]
- Sánchez-Carrillo, S.; Angeler, D.G. Ecology of Threatened Semi-Arid Wetlands: Long-Term Research in Las Tablas de Daimiel; Wetlands: Ecology, Conservation and Management; Springer Science & Business Media: Dordrecht, The Netherlands, 2012; Volume 2. [Google Scholar]
- Lázaro-Lobo, A.; Ervin, G.N. Wetland Invasion: A Multi-Faceted Challenge during a Time of Rapid Global Change. Wetlands 2021, 41, 64. [Google Scholar] [CrossRef]
- Tockner, K. Freshwaters: Global Distribution, Biodiversity, Ecosystem Services, and Human Pressures. In Handbook of Water Resources Management: Discourses, Concepts and Examples; Springer International Publishing: Cham, Switzerland, 2021; pp. 489–501. [Google Scholar] [CrossRef]
- Burke, V.J.; Gibbons, J.W. Terrestrial Buffer Zones and Wetland Conservation: A Case Study of Freshwater Turtles in a Carolina Bay. Conserv. Biol. 1995, 9, 1365–1369. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Biological Delineation of Terrestrial Buffer Zones for Pond-Breeding Salamanders. Conserv. Biol. 1998, 12, 1113–1119. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Jensen, J.B. Core Habitat, Not Buffer Zone. Natl. Wetl. Newsl. 2001, 23, 4–6. [Google Scholar]
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Lovich, J.E. Turtles. In Our living Resources: A Report to the Nation on the Distribution, Abundance, and Health of U.S. Plants, Animals, and Ecosystems; LaRoe, E.T., Farris, G.S., Puckett, C.E., Doran, P.D., Mac, M.J., Eds.; U.S. Dept. of the Interior, National Biological Service: Washington, DC, USA, 1995; p. 548. [Google Scholar] [CrossRef]
- Gibbs, J.P.; Shriver, W.G. Estimating the Effects of Road Mortality on Turtle Populations. Conserv. Biol. 2002, 16, 1647–1652. [Google Scholar] [CrossRef]
- Marchand, M.N.; Litvaitis, J.A. Effects of Habitat Features and Landscape Composition on the Population Structure of a Common Aquatic Turtle in a Region Undergoing Rapid Development. Conserv. Biol. 2004, 18, 758–767. [Google Scholar] [CrossRef]
- Marchand, M.N.; Litvaitis, J.A. Effects of Landscape Composition, Habitat Features, and Nest Distribution on Predation Rates of Simulated Turtle Nests. Biol. Conserv. 2004, 117, 243–251. [Google Scholar] [CrossRef]
- Bodie, J.R.; Semlitsch, R.D. Spatial and Temporal Use of Floodplain Habitats by Lentic and Lotic Species of Aquatic Turtles. Oecologia 2000, 122, 138–146. [Google Scholar] [CrossRef]
- Bury, R. Population Ecology of Freshwater Turtles. In Turtles: Perspectives and Research; Harless, M., Morlock, H., Eds.; Wiley: New York, NY, USA, 1979; pp. 571–602. [Google Scholar]
- Vogt, R.C. Food Partitioning in Three Sympatric Species of Map Turtle, Genus Graptemys (Testudinata, Emydidae). Am. Midl. Nat. 1981, 105, 102. [Google Scholar] [CrossRef]
- Luiselli, L.; Akani, G.C.; Ajong, S.N.; George, A.; Di Vittorio, M.; Eniang, E.A.; Dendi, D.; Hema, E.M.; Petrozzi, F.; Fa, J.E. Predicting the Structure of Turtle Assemblages along a Megatransect in West Africa. Biol. J. Linn. Soc. 2020, 130, 296–309. [Google Scholar] [CrossRef]
- Aresco, M.J. Competitive Interactions of Two Species of Freshwater Turtles, a Generalist Omnivore and an Herbivore, under Low Resource Conditions. Herpetologica 2010, 66, 259–268. [Google Scholar] [CrossRef]
- Nori, J.; Tessarolo, G.; Ficetola, G.F.; Loyola, R.; Di Cola, V.; Leynaud, G. Buying Environmental Problems: The Invasive Potential of Imported Freshwater Turtles in Argentina. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 685–691. [Google Scholar] [CrossRef]
- Espindola, S.; Vázquez-Domínguez, E.; Nakamura, M.; Osorio-Olvera, L.; Martínez-Meyer, E.; Myers, E.A.; Overcast, I.; Reid, B.N.; Burbrink, F.T. Complex Genetic Patterns and Distribution Limits Mediated by Native Congeners of the Worldwide Invasive Red-eared Slider Turtle. Mol. Ecol. 2022, 31, 1766–1782. [Google Scholar] [CrossRef]
- Balzani, P.; Vizzini, S.; Santini, G.; Masoni, A.; Ciofi, C.; Ricevuto, E.; Chelazzi, G. Stable Isotope Analysis of Trophic Niche in Two Co-Occurring Native and Invasive Terrapins, Emys orbicularis and Trachemys scripta elegans. Biol. Invasions 2016, 18, 3611–3621. [Google Scholar] [CrossRef]
- Franch, M.; Llorente, G.A.; Montori, A.; Albornà, P.X.; Richter-Boix, À. Estudi i Seguiment de l’estat de Les Poblacions Dels Rèptils Al Delta Del Llobregat. In Seguiment de Paràmetres Biològics i Detecció de Bioindicadors de L’estat del Sistema al Llarg del Període de Creació de noves Infraestructures al Delta del Llobregat. Memòria 2005; Llorente, G.A., Ed.; Universitat de Barcelona: Barcelona, Spain, 2005; pp. 314–398. [Google Scholar]
- Ballesteros, T.; Degollada, A. Distribució Dels Amfibis i Rèptils Al Delta Del Llobregat. SPARTINA Butlletí Nat. Llobregat 1996, 2, 85–96. [Google Scholar]
- de Roa, E. Projecte de Reintroducció i Estudi de la Tortuga d’aigua Ibèrica (Mauremys leprosa) al delta del Llobregat. Primers Resultats. SPARTINA Butlletí Nat. Llobregat 1994, 1, 21–27. [Google Scholar]
- Arribas, Ó. Primera Cita de Trachemys emolli (Legler, 1990) Asilvestrada en la Península Ibérica. Bol. Asoc. Herpetol. Esp. 2008, 19, 115–117. [Google Scholar]
- Bertolero, A.; Busack, S.D. Mauremys leprosa (Schoepff in Schweigger 1812)–Mediterranean Pond Turtle, Spanish Terrapin, Mediterranean Stripe-Necked Terrapin. Chelonian Res. Monogr. 2017, 5, 102. [Google Scholar] [CrossRef]
- da Silva, E. Distribución de Los Emídidos Mauremys leprosa Schw.(1812) y Emys orbicularis L.(1758) de la Provincia de Badajoz: Factores Que Pudieran Influir en Sus Áreas de Ocupación. Donana Acta Vertebr. 1993, 20, 260–266. [Google Scholar]
- Segurado, P.; Araujo, A.P.R.; Paula, A.; Araújo, R. Coexistence of Emys orbicularis and Mauremys leprosa in Portugal at Two Spatial Scales: Is There Evidence of Spatial Segregation? Biologia 2004, 59, 61–72. [Google Scholar]
- Cox, N.A.; Temple, H.J. European Red List of Reptiles; Office for Official Publications of of the European Communities: Luxembourg, 2009. [Google Scholar]
- Díaz-paniagua, C.; Andreu, A.C.; Keller, C. Galápago Leproso—Mauremys leprosa (Schweigger, 1812). In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Marco, A., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2015; pp. 1–49. [Google Scholar]
- Ernst, C.H.; Barbour, R.W. Turtles of the World; Press, S.I., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1989. [Google Scholar]
- Seidel, M.E. Taxonomic Observations on Extant Species and Subspecies of Slider Turtles, Genus Trachemys. J. Herpetol. 2002, 36, 285–292. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Avery, H.W. Life History and Ecology of the Slider Turtle, 2nd ed.; Smithsonian Institution Press: Washington, DC, USA, 2000. [Google Scholar]
- Ernst, C.H.; Lovich, J.E. Turtles of the United States and Canada; Ernst, C.H., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2009. [Google Scholar]
- Buhlmann, K.; Tuberville, T.; Gibbons, J.W. Turtles of the Southeast. A Wormsloe Foundation Nature Book; University of Georgia Press: Athens, GA, USA, 2008. [Google Scholar]
- Parmenter, R.R.; Avery, H.W. The Feeding Ecology of the Slider Turtle. In Life History and Ecology of the Slider Turtle; Gibbons, J.W., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1990; pp. 257–266. [Google Scholar]
- Luiselli, L.; Capula, M.; Capizzi, D.; Filippi, E.; Trujillo Jesus, V.; Anibaldi, C. Problems for Conservation of Pond Turtles (Emys orbicularis) in Central Italy: Is the Introduced Red-Eared Turtle (Trachemys scripta) a Serious Threat? Chelonian Conserv. Biol. 1997, 2, 417–419. [Google Scholar]
- García-Díaz, P.; Ross, J.V.; Ayres, C.; Cassey, P.; García-Díaz, P.; Ross, J.V.; Ayres, C.; Cassey, P. Understanding the Biological Invasion Risk Posed by the Global Wildlife Trade: Propagule Pressure Drives the Introduction and Establishment of Nearctic Turtles. Glob. Chang. Biol. 2015, 21, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Rödder, D.; Padoa-Schioppa, E. Trachemys scripta (Slider Terrapin). In Handbook of Global Freshwater Invasive Species. Earthscan, Taylor & Francis Group, Abingdon; Francis, R.A., Ed.; Earthscan from Routledge: Abingdon, UK, 2012; pp. 331–339. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; ISSG, SSC, IUCN, Eds.; Invasive Species Specialist Group: Auckland, New Zealand, 2000. [Google Scholar]
- Rayner, M.J.; Hauber, M.E.; Imber, M.J.; Stamp, R.K.; Clout, M.N. Spatial Heterogeneity of Mesopredator Release within an Oceanic Island System. Proc. Natl. Acad. Sci. USA 2007, 104, 20862–20865. [Google Scholar] [CrossRef]
- Sanders, H.; Mills, D.N. Predation Preference of Signal Crayfish (Pacifastacus leniusculus) on Native and Invasive Bivalve Species. River Res. Appl. 2022. [Google Scholar] [CrossRef]
- Simberloff, D. Invasive Species. In Conservation Biology for All; Sodhi, N.S., Ehrlich, P.R., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 131–152. [Google Scholar] [CrossRef]
- Dominguez Almela, V.; South, J.; Britton, J.R. Predicting the Competitive Interactions and Trophic Niche Consequences of a Globally Invasive Fish with Threatened Native Species. J. Anim. Ecol. 2021, 90, 2651–2662. [Google Scholar] [CrossRef]
- Falaschi, M.; Melotto, A.; Manenti, R.; Ficetola, G.F. Invasive Species and Amphibian Conservation. Herpetologica 2020, 76, 216. [Google Scholar] [CrossRef]
- LaForgia, M.L.; Harrison, S.P.; Latimer, A.M. Invasive Species Interact with Climatic Variability to Reduce Success of Natives. Ecology 2020, 101, e03022. [Google Scholar] [CrossRef]
- Parham, J.F.; Papenfuss, T.J.; Sellas, A.B.; Stuart, B.L.; Simison, W.B. Genetic Variation and Admixture of Red-Eared Sliders (Trachemys scripta elegans) in the USA. Mol. Phylogenet. Evol. 2020, 145, 106722. [Google Scholar] [CrossRef] [PubMed]
- Kraus, F. Impacts from Invasive Reptiles and Amphibians. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 75–97. [Google Scholar] [CrossRef]
- Sancho, V.; Lacomba, I.; Bataller, J.V.; Veríssimo, J.; Velo-Antón, G. First Report of Hybridization between Mauremys leprosa and Mauremys sinensis Highlights the Risk of Exotic Mauremys spp. Pet Trade. Basic Appl. Herpetol. 2020, 34, 75–81. [Google Scholar] [CrossRef]
- Demkowska-Kutrzepa, M.; Studzińska, M.; Roczeń-Karczmarz, M.; Tomczuk, K.; Abbas, Z.; Różański, P. A Review of the Helminths Co-Introduced with Trachemys scripta elegans—A Threat to European Native Turtle Health. Amphibia-Reptilia 2018, 39, 177–189. [Google Scholar] [CrossRef]
- Weitzman, C.L.; Kaestli, M.; Gibb, K.; Brown, G.P.; Shine, R.; Christian, K. Disease Exposure and Antifungal Bacteria on Skin of Invasive Cane Toads, Australia. Emerg. Infect. Dis. 2019, 25, 1770–1771. [Google Scholar] [CrossRef]
- Dueñas, M.-A.; Hemming, D.J.; Roberts, A.; Diaz-Soltero, H. The Threat of Invasive Species to IUCN-Listed Critically Endangered Species: A Systematic Review. Glob. Ecol. Conserv. 2021, 26, e01476. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ Warning on Invasive Alien Species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Bellard, C.; Ricciardi, A. Alien versus Native Species as Drivers of Recent Extinctions. Front. Ecol. Environ. 2019, 17, 203–207. [Google Scholar] [CrossRef]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine Invasive Alien Species: A Threat to Global Biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Linders, T.E.W.; Schaffner, U.; Eschen, R.; Abebe, A.; Choge, S.K.; Nigatu, L.; Mbaabu, P.R.; Shiferaw, H.; Allan, E. Direct and Indirect Effects of Invasive Species: Biodiversity Loss Is a Major Mechanism by Which an Invasive Tree Affects Ecosystem Functioning. J. Ecol. 2019, 107, 2660–2672. [Google Scholar] [CrossRef]
- Polo-Cavia, N. Factores Que Afectan a la Competencia Entre el Galápago Leproso (Mauremys leprosa) y el Introducido Galápago de Florida (Trachemys scripta). Ph.D. Thesis, Consejo Superior de Investigaciones Científicas (CSIC), Madrid, Spain, 2009. [Google Scholar]
- Polo-Cavia, N.; López, P.; Martín, J. Interference Competition between Native Iberian Turtles and the Exotic Trachemys scripta. Basic Appl. Herpetol. 2015, 28, 5–20. [Google Scholar] [CrossRef]
- Polo-Cavia, N.; López, P.; Martín, J. Interspecific Differences in Chemosensory Responses of Freshwater Turtles: Consequences for Competition between Native and Invasive Species. Biol. Invasions 2009, 11, 431–440. [Google Scholar] [CrossRef]
- Polo-Cavia, N.; López, P.; Martín, J. Competitive Interactions during Basking between Native and Invasive Freshwater Turtle Species. Biol. Invasions 2010, 12, 2141–2152. [Google Scholar] [CrossRef]
- Polo-Cavia, N.; López, P.; Martín, J. Aggressive Interactions during Feeding between Native and Invasive Freshwater Turtles. Biol. Invasions 2011, 13, 1387–1396. [Google Scholar] [CrossRef]
- Polo-Cavia, N.; López, P.; Martín, J. Feeding Status and Basking Requirements of Freshwater Turtles in an Invasion Context. Physiol. Behav. 2012, 105, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Paniagua, C.; Pérez-Santigosa, N.; Hidalgo-Vila, J.; Florencio, M. Does the Exotic Invader Turtle, Trachemys scripta elegans, Compete for Food with Coexisting Native Turtles? Amphibia-Reptilia 2011, 32, 167–175. [Google Scholar] [CrossRef]
- Hidalgo-Vila, J.; Martínez-Silvestre, A.; Pérez-Santigosa, N.; León-Vizcaíno, L.; Díaz-Paniagua, C. High Prevalence of Diseases in Two Invasive Populations of Red-Eared Sliders (Trachemys scripta elegans) in Southwestern Spain. Amphibia-Reptilia 2020, 41, 509–518. [Google Scholar] [CrossRef]
- Meyer, O.L.; Du Preez, L.; Bonneau, E.; Héritier, L.; Franch, M.; Valdeón, A.; Sadaoui, A.; Kechemir-Issad, N.; Palacios, C.; Verneau, O. Parasite Host-Switching from the Invasive American Red-Eared Slider, Trachemys scripta elegans, to the Native Mediterranean Pond Turtle, Mauremys leprosa, in Natural Environments. Aquat. Invasions 2015, 10, 79–91. [Google Scholar] [CrossRef]
- Verneau, O.; Palacios, C.; Platt, T.; Alday, M.; Billard, E.; Allienne, J.-F.; Basso, C.; du Preez, L.H. Invasive Species Threat: Parasite Phylogenetics Reveals Patterns and Processes of Host-Switching between Non-Native and Native Captive Freshwater Turtles. Parasitology 2011, 138, 1778–1792. [Google Scholar] [CrossRef]
- Keller, C. Ecología de Poblaciones de Mauremys Leprosa y Emys Orbicularis en el Parque Nacional de Doñana; Universidad de Sevilla: Sevilla, Spain, 1997. [Google Scholar]
- Franch, M.; Llorente, G.A.; Montori, A. Primeros Datos Sobre la Biología de Trachemys scripta elegans en Sintopía Con Mauremys leprosa en el delta del Llobregat (NE Ibérico). In Invasiones Biológicas: Un Factor del Cambio Global, Proceedings of the EEI 2006 Actualización de Conocimientos. 2º Congreso Nacional de Especies Exóticas Invasoras. EEI 2006, León, Spain, 19–22 September 2016; Series Técnica N.o 3; Grupo Especialista en Invasiones Biológicas (GEIB): León, Spain, 2007; pp. 85–101. [Google Scholar]
- ESRI. ArcGIS 10.2 Released. Support Services Blog; ESRI: Redlands, CA, USA, 2013; Available online: https://blogs.esri.com/esri/supportcenter/2013/07/31/arcgis-10-2-released/ (accessed on 16 August 2022).
- QGIS Development Team. QGis 2.4.0 Chugiak. Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project; QGIS Development Team: Berne, Switzerland, 2014; Available online: https://www.qgis.org/en/site/index.html (accessed on 16 August 2022).
- Schubauer, J.P.; Gibbons, J.W.; Spotila, J.R. Home Range and Movement Patterns of Slider Turtles Inhabiting Par Pond. In Life History and Ecology of the Slider Turtle; Gibbons, J.W., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1990; pp. 223–232. [Google Scholar]
- Gibbons, J.W. The Slider Turtle. In Life History and Ecology of the Slider Turtle; Gibbons, J.W., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1990; pp. 3–18. [Google Scholar]
- Moll, D.; Moll, E.O. The Ecology, Exploitation and Conservation of River Turtles; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Pérez-Santigosa, N.; Hidalgo-Vila, J.; Díaz-Paniagua, C. Comparing Activity Patterns and Aquatic Home Range Areas among Exotic and Native Turtles in Southern Spain. Chelonian Conserv. Biol. 2013, 12, 313–319. [Google Scholar] [CrossRef]
- Ibàñez, J.J.; Burriel, J.Á. Mapa de Cubiertas del Suelo de Cataluña: Características de la Tercera Edición y Relación Con SIOSE. Tecnol. Inf. Geográfi. Inf. Geográfi. Serv. Ciudad. 2010, 3, 179–198. [Google Scholar]
- Rempel, R.; Kaukinen, D.; Carr, A.P. Patch Analyst; Ontario Ministry of Natural Resources; Center for Northern Forest Ecosystem Research: Thunder Bay, ON, Canada, 2012; pp. 1–15. [Google Scholar]
- Paudel, S.; Yuan, F. Assessing Landscape Changes and Dynamics Using Patch Analysis and GIS Modeling. Int. J. Appl. Earth Obs. Geoinf. 2012, 16, 66–76. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L. Standard Methods for the Examination of Water & Wastewater, 22nd ed.; Rice, E.W., Bridgewater, L., Health, A.A.P., Works, A.A.W., Federation, W.E., Eds.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Schoener, T.W. Nonsynchronous Spatial Overlap of Lizards in Patchy Habitats. Ecology 1970, 51, 408. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.J. Package “Spaa”: Species Association Analysis. 2013. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.204.8570&rep=rep1&type=pdf (accessed on 16 August 2022).
- Gotelli, N.J. Null model analysis of species co-occurrence patterns. Ecology 2000, 81, 2606–2621. [Google Scholar] [CrossRef]
- Gomes, L.; Grilo, C.; Silva, C.; Mira, A. Identification Methods and Deterministic Factors of Owl Roadkill Hotspot Locations in Mediterranean Landscapes. Ecol. Res. 2009, 24, 355–370. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: London, UK, 2012. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The Vegan Package: Community Ecology Package. 2015. Available online: https://www.researchgate.net/profile/Gavin-Simpson-2/publication/228339454_The_vegan_Package/links/0912f50be86bc29a7f000000/The-vegan-Package.pdf (accessed on 16 August 2022).
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation Partitioning of Species Data Matrices: Estimation and Comparison of Fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef]
- Skrondal, A.; Rabe-Hesketh, S. Generalized Latent Variable Modeling: Multilevel, Longitudinal, and Structural Equation Models; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bartholomew, D.J.; Knott, M.; Moustaki, I. Latent Class Models and Factor Analysis: A Unified Approach, 3rd ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Letten, A.D.; Keith, D.A.; Tozer, M.G.; Hui, F.K.C. Fine-Scale Hydrological Niche Differentiation through the Lens of Multi-Species Co-Occurrence Models. J. Ecol. 2015, 103, 1264–1275. [Google Scholar] [CrossRef]
- Harris, D.J. Generating Realistic Assemblages with a Joint Species Distribution Model. Methods Ecol. Evol. 2015, 6, 465–473. [Google Scholar] [CrossRef]
- Plummer, M. JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003), Vienna, Austria, 20–22 March 2003; Technische Universit at Wien: Vienna, Austria, 2003; pp. 20–22. [Google Scholar]
- Su, Y.-S.; Yajima, M. R2jags: A Package for Running Jags from R. 2012. Available online: https://cran.r-project.org/web/packages/R2jags/R2jags.pdf (accessed on 16 August 2022).
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike SE—15; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2002. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Lindeman, P.V. Comparative Life History of Painted Turtles (Chrysemys picta) in Two Habitats in the Inland Pacific Northwest. Copeia 1996, 1996, 114–130. [Google Scholar] [CrossRef]
- Moll, D. Dirty River Turtles. Nat. Hist. 1980, 89, 42. [Google Scholar]
- Souza, F.L.; Abe, A.S. Feeding Ecology, Density and Biomass of the Freshwater Turtle, Phrynops geoffroanus, Inhabiting a Polluted Urban River in South-Eastern Brazil. J. Zool. 2000, 252, 437–446. [Google Scholar] [CrossRef]
- da Silva, E. Notes on Clutch Size and Egg Size of Mauremys leprosa from Spain. J. Herpetol. 1995, 29, 484. [Google Scholar] [CrossRef]
- Franch, M. Caracterització de la Tortuga de Rierol Mauremys leprosa (Schweigger, 1812) a l’Alt Empordà: Biometria i Cicle Biològic, Barcelona. Master’s Thesis, Universitat de Barcelona, Barcelona, Spain, 2003. [Google Scholar] [CrossRef]
- Naimi, M.; Znari, M.; Lovich, J.E.; Feddadi, Y.; Baamrane, M.A.A. Clutch and Egg Allometry of the Turtle Mauremys leprosa (Chelonia: Geoemydidae) from a Polluted Peri-Urban River in West-Central Morocco. Herpetol. J. 2012, 22, 43–49. [Google Scholar]
- Gasith, A.; Sidis, I. Polluted Water Bodies, the Main Habitat of the Caspian Terrapin (Mauremys caspica rivulata) in Israel. Copeia 1984, 1984, 216–219. [Google Scholar] [CrossRef]
- Ferronato, B.O.; Marques, T.S.; Guardia, I.; Longo, A.L.B.; Piña, C.I.; Bertoluci, J.; Verdade, L.M.; Pina, C.I.; Bertoluci, J.; Verdade, L.M. The Turtle Trachemys scripta elegans (Testudines, Emydidae) as an Invasive Species in a Polluted Stream of Southeastern Brazil. Herpetol. Bull. 2009, 109, 29–34. [Google Scholar]
- Malkmus, R. Amphibians and Reptiles of Portugal, Madeira and the Azores-Archipelago: Distribution and Natural History Notes; A.R.G. Gantner Verlag K.G.: Königstein, Germany, 2004. [Google Scholar]
- Gibbons, J.W.; Keaton, G.H.; Schuhauer, J.P.; Greene, J.L.; Bennett, D.P.; McAuliffe, J.R.; Sharitz, R.R. Unusual Population Size Structure in Freshwater Turtles on Barrier Islands. Georg. J. Sci. 1979, 37, 155–159. [Google Scholar]
- Meiling, H.; Ke, Z.; Chaohua, S.; Di, X.; Haitao, S. Effect of Salinity on the Survival, Ions and Urea Modulation in Red-Eared Slider(Trachemys scripta elegans). Asian Herpetol. Res. 2014, 5, 128. [Google Scholar] [CrossRef]
- Andrén, H.; Andren, H. Effects of Habitat Fragmentation on Birds and Mammals in Landscapes with Different Proportions of Suitable Habitat: A Review. Oikos 1994, 71, 355. [Google Scholar] [CrossRef]
- Gustafson, E.J. Quantifying Landscape Spatial Pattern: What Is the State of the Art? Ecosystems 1998, 1, 143–156. [Google Scholar] [CrossRef]
- Lloret, F.; Calvo, E.; Pons, X.; Díaz-Delgado, R. Wildfires and Landscape Patterns in the Eastern Iberian Peninsula. Landsc. Ecol. 2002, 17, 745–759. [Google Scholar] [CrossRef]
- Rizkalla, C.E.; Swihart, R.K. Community Structure and Differential Responses of Aquatic Turtles to Agriculturally Induced Habitat Fragmentation. Landsc. Ecol. 2006, 21, 1361–1375. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Marchand, M.N.; Litvaitis, J.A. Terrestrial Habitat Use by Nesting Painted Turtles in Landscapes with Different Levels of Fragmentation. Northeast. Nat. 2004, 11, 41–48. [Google Scholar] [CrossRef]
- Gibbons, J.W.J.W. Terrestrial Habitat: A Vital Component for Herpetofauna of Isolated Wetlands. Wetlands 2003, 23, 630–635. [Google Scholar] [CrossRef]
- Buhlmann, K.A.; Gibbons, J.W. Terrestrial Habitat Use by Aquatic Turtles from a Seasonally Fluctuating Wetland: Implications for Wetland Conservation Boundaries. Chelonian Conserv. Biol. 2001, 4, 115–127. [Google Scholar]
- Semlitsch, R.D.; Bodie, J.R. Biological Criteria for Buffer Zones around Wetlands and Riparian Habitats for Amphibians and Reptiles. Conserv. Biol. 2003, 17, 1219–1228. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M. Ecology of Macroinvertebrate Communities in Transitional Waters: Influence of the Environment, Responde to Disturbance and Successional Processes. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 2009. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Rieradevall, M. Quantification of Environment-Driven Changes in Epiphytic Macroinvertebrate Communities Associated to Phragmites Australis. J. Limnol. 2009, 68, 229. [Google Scholar] [CrossRef][Green Version]
- da Silva, E. Mauremys leprosa (Schweiger, 1812). In Atlas y Libro Rojo de los Anfibios y Reptiles de España; Pleguezuelos, J.M., Márquez, R., Lizana, M., Eds.; Dirección General de Conservación de la Naturaleza—Asociación Herpetológica Española: Madrid, Spain, 2002; pp. 143–146. [Google Scholar]
- Segurado, P.; Figueiredo, D. Coexistence of Two Freshwater Turtle Species along a Mediterranean Stream: The Role of Spatial and Temporal Heterogeneity. Acta Oecol. 2007, 32, 134–144. [Google Scholar] [CrossRef]
- Franch, M.; Montori, A.; Sillero, N.; Llorente, G.A.A. Temporal Analysis of Mauremys leprosa (Testudines, Geoemydidae) Distribution in Northeastern Iberia: Unusual Increase in the Distribution of a Native Species. Hydrobiologia 2015, 757, 129–142. [Google Scholar] [CrossRef]
- Cadi, A.; Joly, P. Impact of the Introduction of the Red-Eared Slider (Trachemys scripta elegans) on Survival Rates of the European Pond Turtle (Emys orbicularis). Biodivers. Conserv. 2004, 13, 2511–2518. [Google Scholar] [CrossRef]
- Díaz-Paniagua, C.; Marco, A.; Andreu, A.C.; Sánchez, C.; Pena, L.; Acosta, M.; Molina, I. Trachemys Scripta en Doñana; Museo Nacional de Ciencias Naturales: Sevilla, Spain, 2002. [Google Scholar]
- Cadi, A.; Joly, P. Competition for Basking Places between the Endangered European Pond Turtle (Emys orbicularis galloitalica) and the Introduced Red-Eared Slider (Trachemys scripta elegans). Can. J. Zool. 2003, 81, 1392–1398. [Google Scholar] [CrossRef]
- Lambert, M.R.; Nielsen, S.N.; Wright, A.N.; Thomson, R.C.; Shaffer, H.B. Habitat Features Determine the Basking Distribution of Introduced Red-Eared Sliders and Native Western Pond Turtles. Chelonian Conserv. Biol. 2013, 12, 192–199. [Google Scholar] [CrossRef]
- Parmenter, R.R. Effects of Food Availability and Water Temperature on the Feeding Ecology of Pond Sliders (Chrysemys s. scripta). Copeia 1980, 1980, 503. [Google Scholar] [CrossRef]
- Prévot-Julliard, A.-C.; Gousset, E.; Archinard, C.; Cadi, A.; Girondot, M. Pets and Invasion Risks: Is the Slider Turtle Strictly Carnivorous? Amphibia-Reptilia 2007, 28, 139–143. [Google Scholar] [CrossRef]
- Lindeman, P.V. A Comparative Spotting-Scope Study of the Distribution and Relative Abundance of River Cooters (Pseudemys concinna) in Western Kentucky and Southern Mississippi. Chelonian Conserv. Biol. 1997, 2, 378–383. [Google Scholar]


| Category | Surface (km2) | Percentage | |
|---|---|---|---|
| Artificial | Unproductive artificial | 51.31 | 78.64 |
| Seminatural | Fields | 9.81 | 15.03 |
| Natural | Dense wooded | 2.31 | 3.54 |
| Shrublands | 0.85 | 1.30 | |
| Unproductive natural | 0.45 | 0.68 | |
| Continental waters | 0.41 | 0.62 | |
| Wetlands | 0.06 | 0.09 | |
| Light wooded | 0.03 | 0.05 | |
| Meadows and grasslands | 0.03 | 0.04 | |
| TOTAL | 65.24 | 100.00 | |
| Landscape Descriptive Metrics | Metric | Description |
|---|---|---|
| Patch richness, diversity and evenness | R | Richness |
| SDI | Shannon’s diversity index | |
| SEI | Shannon’s evenness index | |
| Patch shape and fractal dimension | AWMSI | Area weighted mean shape index |
| MSI | Mean shape index | |
| MPAR | Mean perimeter–area ratio | |
| MPFD | Mean patch fractal dimension | |
| AWMPFD | Area weighted mean patch fractal dimension | |
| Edge density | TE | Total edge (m) |
| ED | Edge density | |
| MPE | Mean Patch Edge (m) | |
| PSCoV | Patch size coefficient of variance | |
| Patch size | MedPS | Median patch size (m2) |
| MPS | Mean patch size (m2) | |
| NumP | Number of patches | |
| PSSD | Patch size standard deviation (m2) | |
| Landscape descriptive variables | CA | Total core area (m2) |
| TLA | Landscape area (m2) | |
| Environmental Variables | Metric | Description |
| General variables | Ox | Dissolved oxygen in water (mg/L) |
| Ox% | Dissolved oxygen saturation in water (%) | |
| pH | pH of water | |
| Secchi | Water transparency (m) | |
| T | Temperature (°C) | |
| Primary production and nutrient concentration | Chl-a | phytoplanktonic chlorophyll-a concentration (μg/L) |
| DIN | Dissolved inorganic nitrogen concentration (mg/L) | |
| NH4+ | Ammonium concentration (mg/L) | |
| NO2− | Nitrite concentration (mg/L) | |
| NO3− | Nitrate concentration (mg/L) | |
| PO43− | Phosphate concentration (mg/L) | |
| SiO42− | Silicate concentration (mg/L) | |
| SRP | Soluble reactive phosphorous concentration (mg/L) | |
| TOC | Total organic carbon concentration (mg/L) | |
| TP | Total phosphorous concentration (mg/L) | |
| Conductivity and ion concentration | Ca2+ | Calcium concentration (mg/L) |
| Cl− | Chloride concentration (mg/L) | |
| Cond | Water conductivity (μS/cm) | |
| Fe2+ | Iron concentration (mg/L) | |
| K+ | Potassium concentration (mg/L) | |
| Mg2+ | Magnesium concentration (mg/L) | |
| Mn2+ | Manganese concentration (mg/L) | |
| Na+ | Sodium concentration (mg/L) | |
| Si2+ | Silicon concentration (mg/L) | |
| SO24− | Sulphates concentration (mg/L) | |
| SSP | Suspended solids concentration (mg/L) |
| Station Name | Code | Longitude (E) | Latitude (N) | Typology | Captures | |
|---|---|---|---|---|---|---|
| M. leprosa | T. s. elegans | |||||
| Braç de la Vidala | EB5 | 2.0605 | 41.2857 | Irrigation channel | 0 | 17 |
| Canal de la Bunyola 1 | EC5 | 2.1243 | 41.2987 | Irrigation channel | 0 | 1 |
| Canal de la Bunyola 2 | EC4 | 2.1150 | 41.3076 | Irrigation channel | 35 | 85 |
| Llera Nova 1 | LL1 | 2.1307 | 41.3061 | Estuary | 1 | 0 |
| Llera Nova 2 | LL2 | 2.1169 | 41.3189 | Estuary | 0 | 2 |
| Cal Tet | CT | 2.1221 | 41.3056 | Lagoon | 68 | 7 |
| La Murtra | EB10 | 2.0396 | 41.2772 | Lagoon | 0 | 9 |
| El Remolar | RE | 2.0723 | 41.2817 | Lagoon | 9 | 2 |
| La Ricarda | RI | 2.1151 | 41.2927 | Lagoon | 49 | 3 |
| Riera de Sant Climent | EB6 | 2.0660 | 41.2771 | Lagoon | 0 | 1 |
| Ca l’Arana | CA | 2.1300 | 41.3037 | Lagoon | 47 | 0 |
| Can Dimoni Gran | EB7 | 2.0480 | 41.3110 | Pond | 1 | 12 |
| Bassa dels Pollancres | EB4 | 2.0655 | 41.2813 | Pond | 20 | 5 |
| Total: | 230 | 144 | ||||
| Environmental | Residual | |
|---|---|---|
| Chl-a | −0.34 | −0.43 |
| C− | −0.04 | −0.45 |
| NH4+ | −0.38 | −0.39 |
| Ox | −0.09 | −0.47 |
| Secchi | −0.12 | −0.53 |
| Na+ | 0.06 | −0.48 |
| SRP | −0.41 | −0.18 |
| SSP | −0.29 | −0.46 |
| T | −0.12 | −0.40 |
| TOC | −0.19 | −0.19 |
| Estimate | Std. Error | t Value | Pr (>|t|) | Sign. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TSE | ML | TSE | ML | TSE | ML | TSE | ML | TSE | ML | ||
| intercept | −1.901 × 102 | 2.015 × 102 | 0.642 × 102 | 1.222 × 102 | −2.963 | 1.649 | 0.006 | 0.112 | ** | - | |
| Ø100 | SEI | −0.456 × 102 | - | 6.557 × 100 | - | −6.957 | - | <0.001 | - | *** | - |
| NumP | −6.051 × 100 | 2.604 × 100 | 1.691 × 100 | 1.449 × 100 | −3.578 | 1.798 | 0.001 | 0.084 | ** | · | |
| PSCoV | 0.263 × 100 | - | 0.118 × 100 | - | 2.221 | - | 0.035 | - | * | - | |
| PSSD | −3.357 × 102 | - | 0.623 × 102 | - | −5.393 | - | <0.001 | - | *** | - | |
| CA | 3.423 × 102 | - | 0.868 × 102 | - | 3.944 | - | <0.001 | - | *** | - | |
| Ø500 | MPAR | - | −2.306 × 10−3 | - | 1.019 × 10−3 | - | −2.263 | - | 0.033 | - | * |
| MPFD | - | −1.357 × 102 | - | 7.889 × 101 | - | −1.720 | - | 0.098 | - | · | |
| MedPS | - | 3.161 × 101 | - | 1.388 × 101 | - | 2.277 | - | 0.032 | - | * | |
| Ø2000 | R | 2.836 × 100 | - | 1.319 × 100 | - | 2.151 | - | 0.041 | - | * | - |
| MedPS | −0.301 × 102 | - | 0.145 × 102 | - | −2.082 | - | 0.047 | - | * | - | |
| SEI | - | 5.854 × 101 | - | 3.176 × 101 | - | 1.843 | - | 0.077 | - | · | |
| MPAR | - | 4.973 × 10−3 | - | 2.863 × 10−3 | - | 1.737 | - | 0.095 | - | · | |
| ED | - | −1.690 × 10−1 | - | 9.427 × 10−2 | - | −1.793 | - | 0.085 | - | · | |
| PSCoV | - | 9.766 × 10−2 | - | 4.147 × 10−2 | - | 2.355 | - | 0.027 | - | * | |
| PSSD | - | −5.398 × 100 | - | 3.894 × 100 | - | −1.386 | - | 0.178 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franch, M.; Llorente, G.A.; Rieradevall, M.; Montori, A.; Cañedo-Argüelles, M. Coexistence of Native and Invasive Freshwater Turtles: The Llobregat Delta (NE Iberian Peninsula) as a Case Study. Land 2022, 11, 1582. https://doi.org/10.3390/land11091582
Franch M, Llorente GA, Rieradevall M, Montori A, Cañedo-Argüelles M. Coexistence of Native and Invasive Freshwater Turtles: The Llobregat Delta (NE Iberian Peninsula) as a Case Study. Land. 2022; 11(9):1582. https://doi.org/10.3390/land11091582
Chicago/Turabian StyleFranch, Marc, Gustavo A. Llorente, Maria Rieradevall, Albert Montori, and Miguel Cañedo-Argüelles. 2022. "Coexistence of Native and Invasive Freshwater Turtles: The Llobregat Delta (NE Iberian Peninsula) as a Case Study" Land 11, no. 9: 1582. https://doi.org/10.3390/land11091582
APA StyleFranch, M., Llorente, G. A., Rieradevall, M., Montori, A., & Cañedo-Argüelles, M. (2022). Coexistence of Native and Invasive Freshwater Turtles: The Llobregat Delta (NE Iberian Peninsula) as a Case Study. Land, 11(9), 1582. https://doi.org/10.3390/land11091582







