Ecovoltaics: Maintaining Native Plants and Wash Connectivity inside a Mojave Desert Solar Facility Leads to Favorable Growing Conditions
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Abiotic Factors
3.1.1. Precipitation
3.1.2. Soil Water in Storage
3.1.3. Infiltration
3.1.4. Photosynthetically Active Radiation
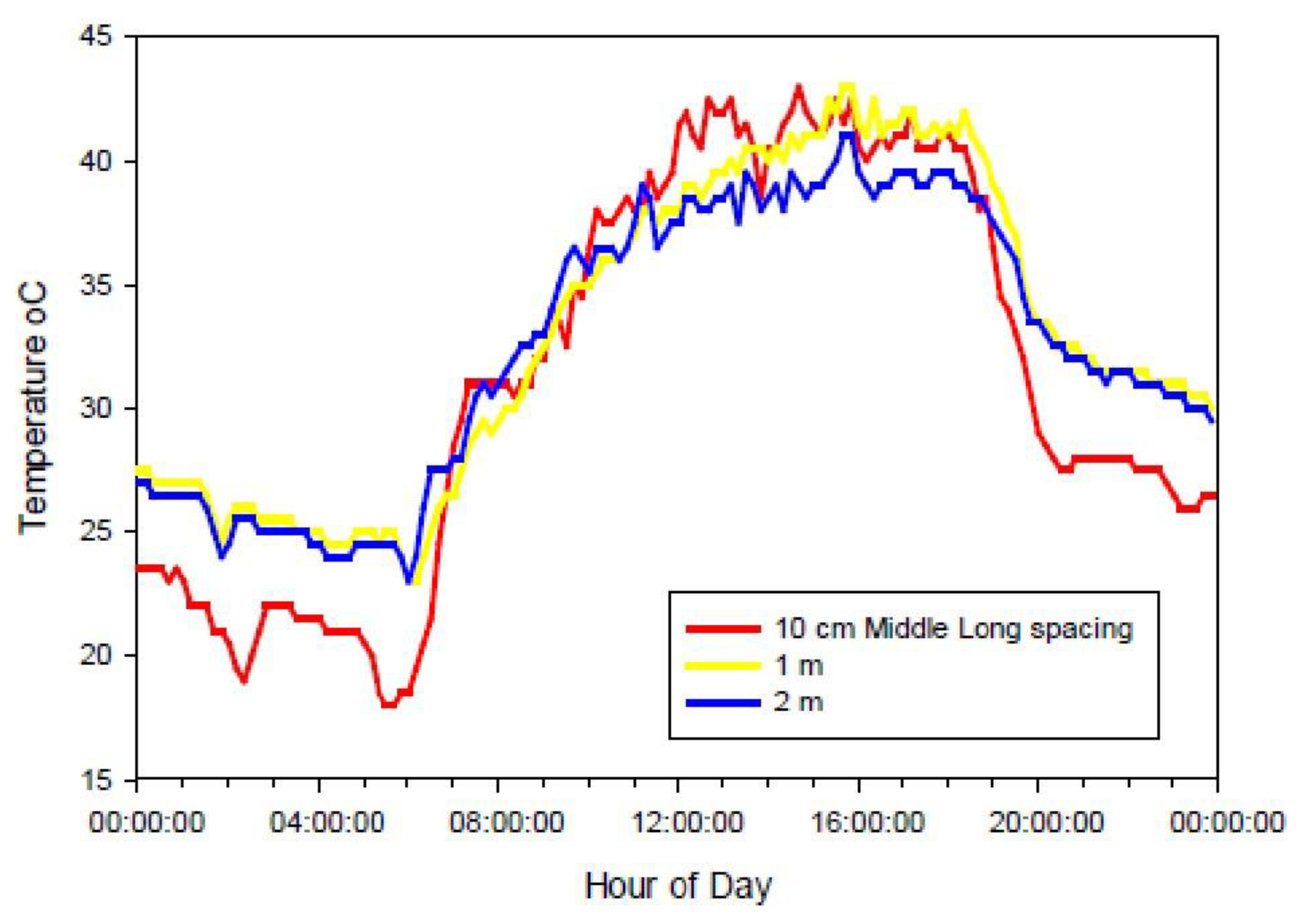
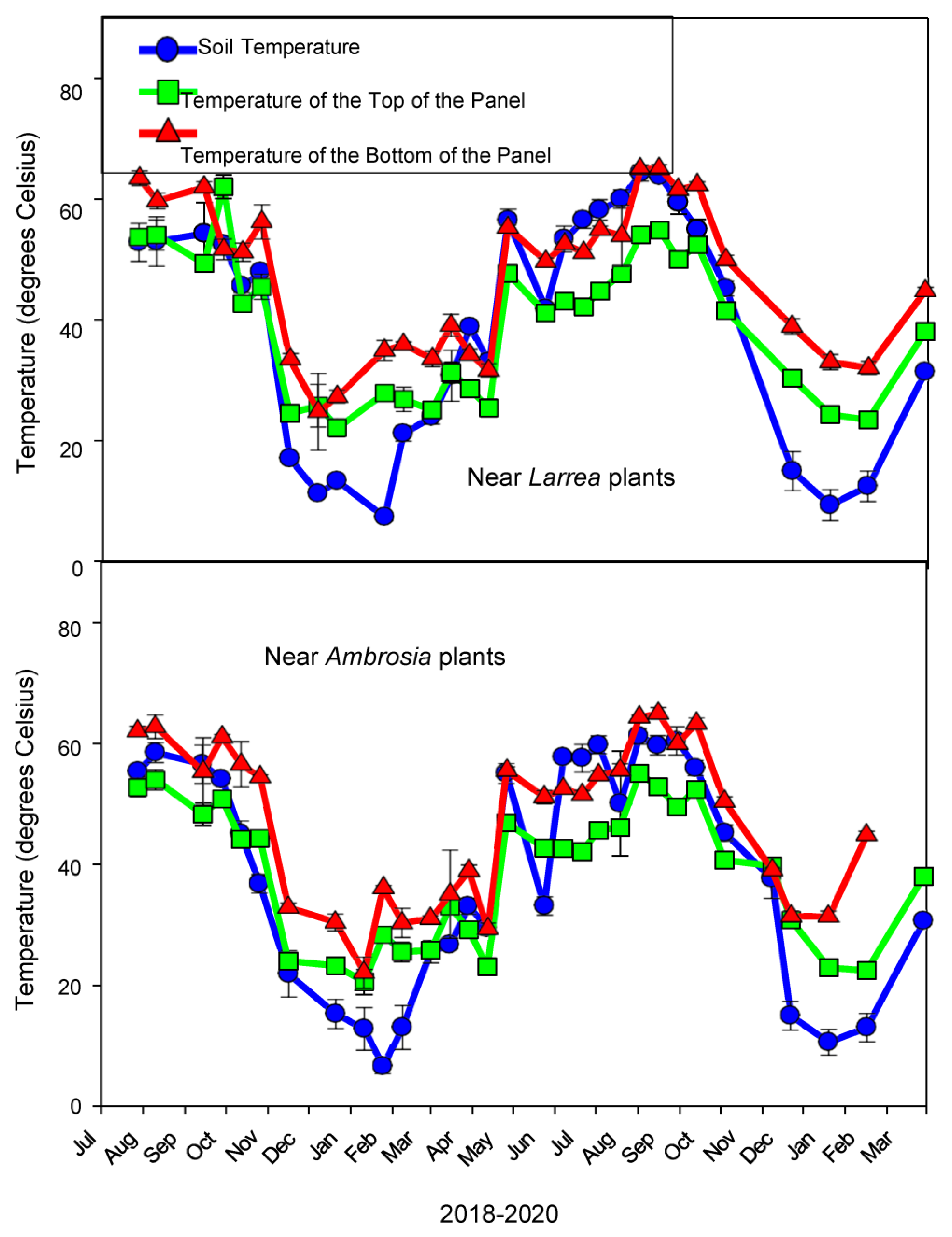
3.1.5. Temperature
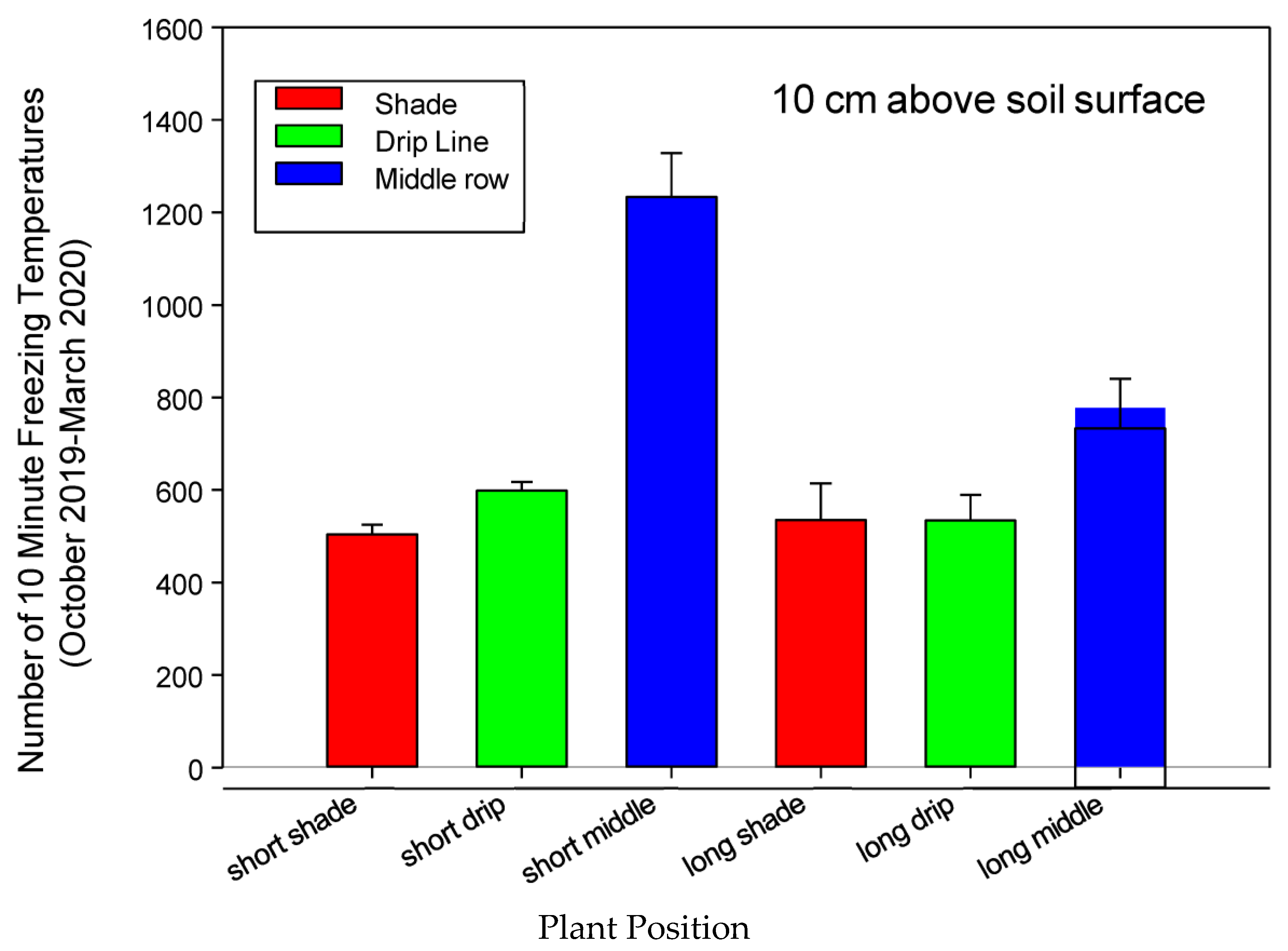
3.1.6. Heat Index
3.2. Biotic Responses
3.2.1. Leaf Xylem Water Potential
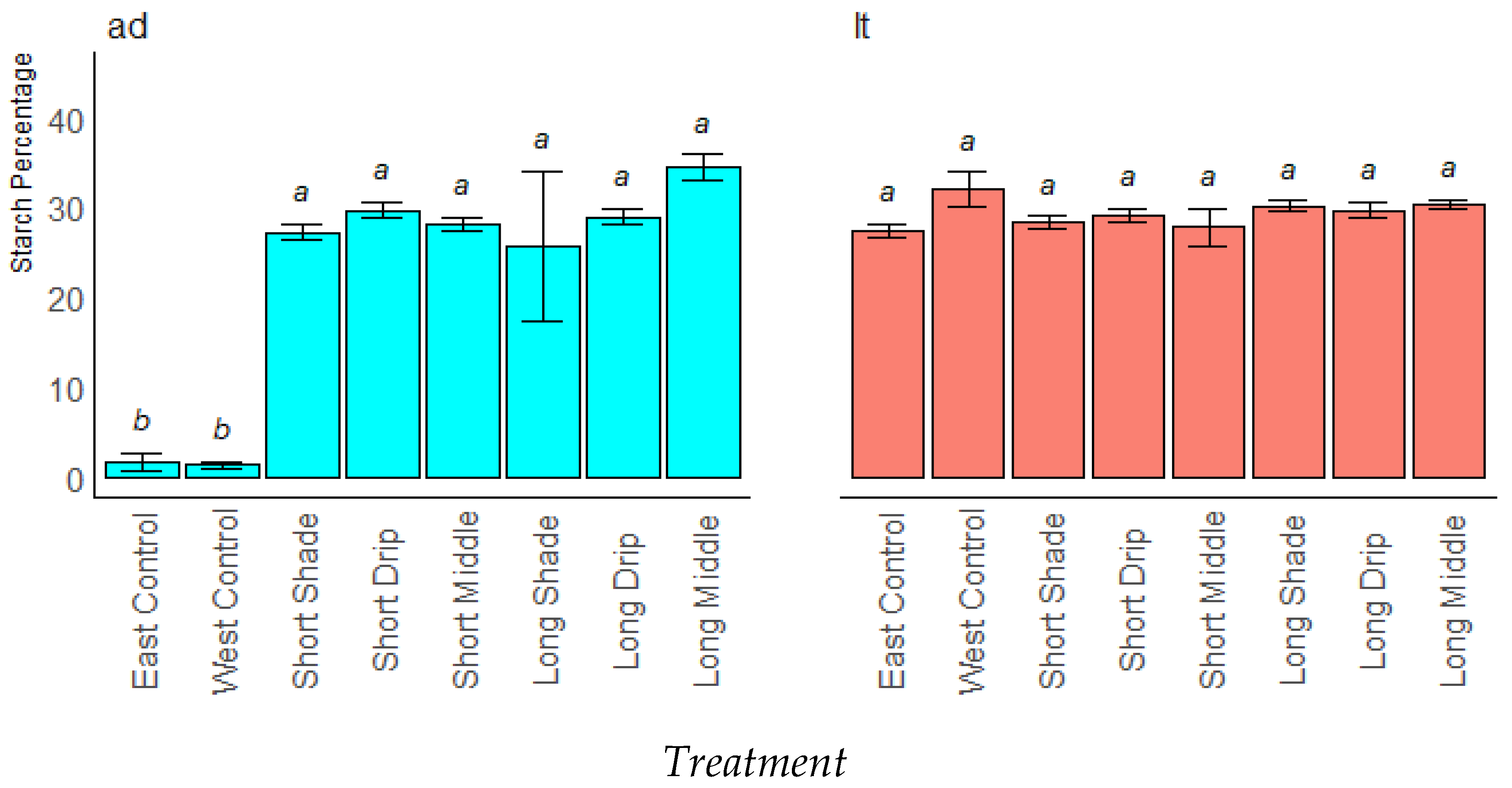
3.2.2. Stored Sugars and Starch
3.2.3. δ13 Carbon
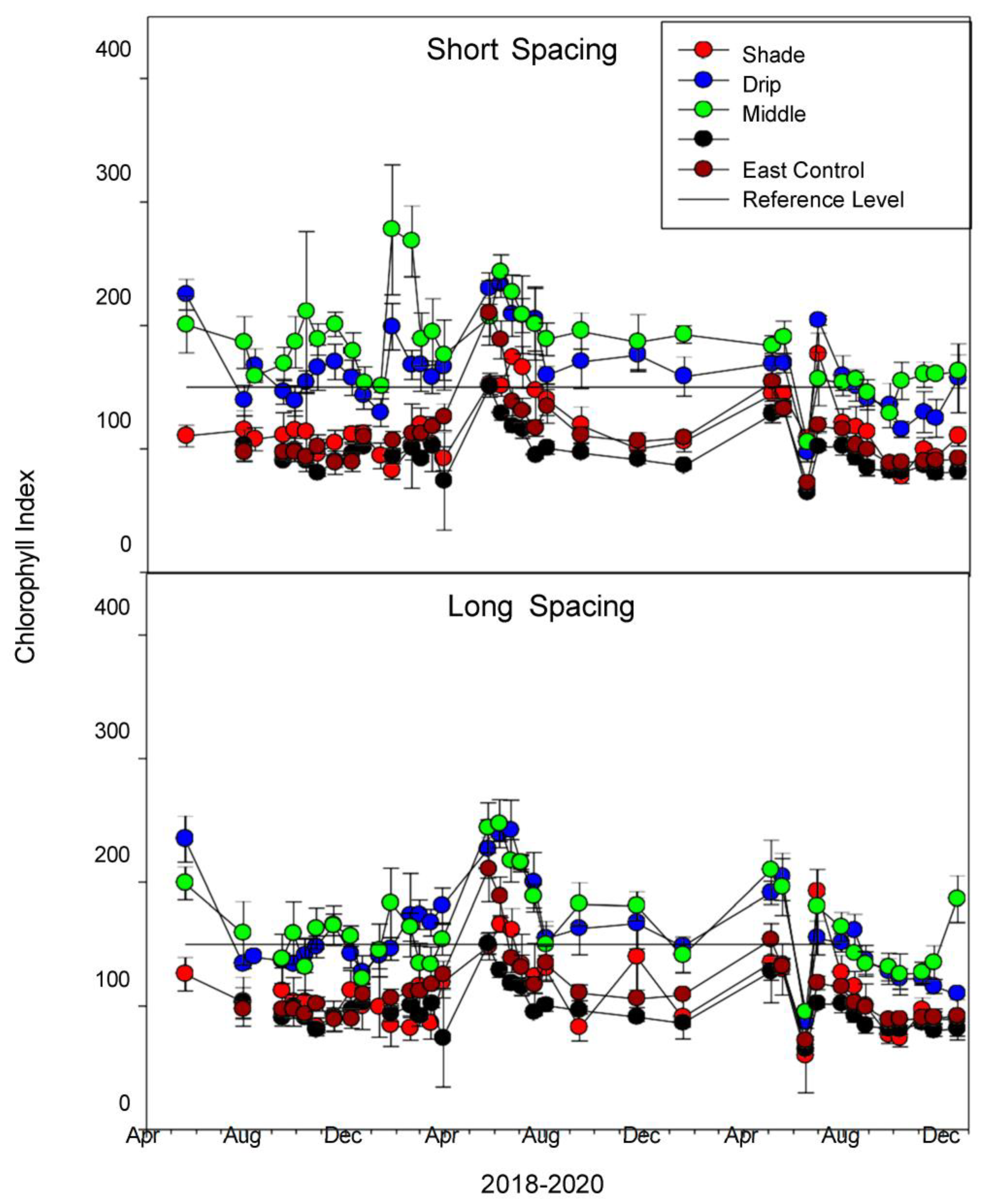
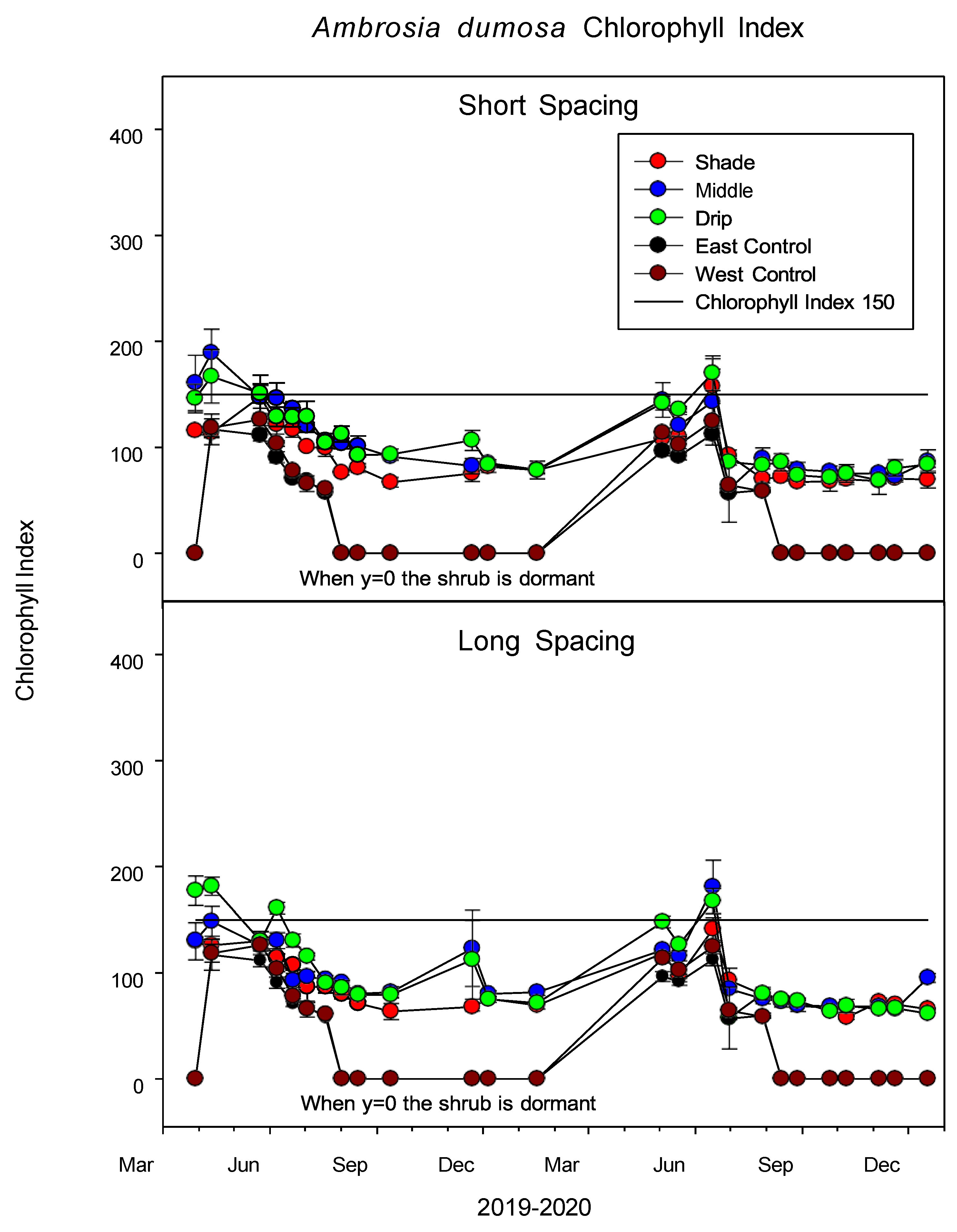
3.2.4. Chlorophyll Index
Canopy Temperature Minus Ambient Temperature
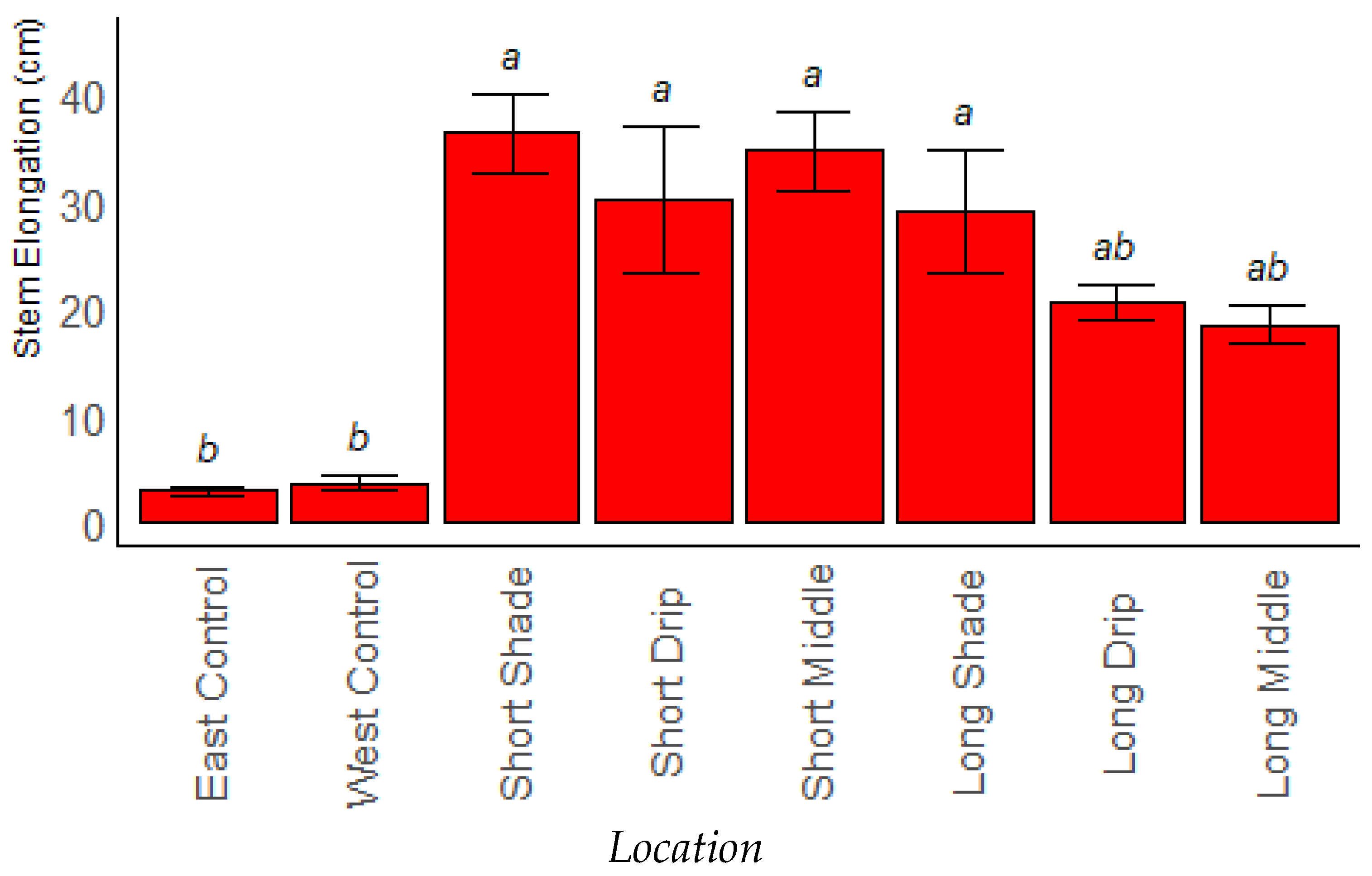
Stem Elongation
Stomatal Density
Seed Yield
Tissue Ion Concentrations
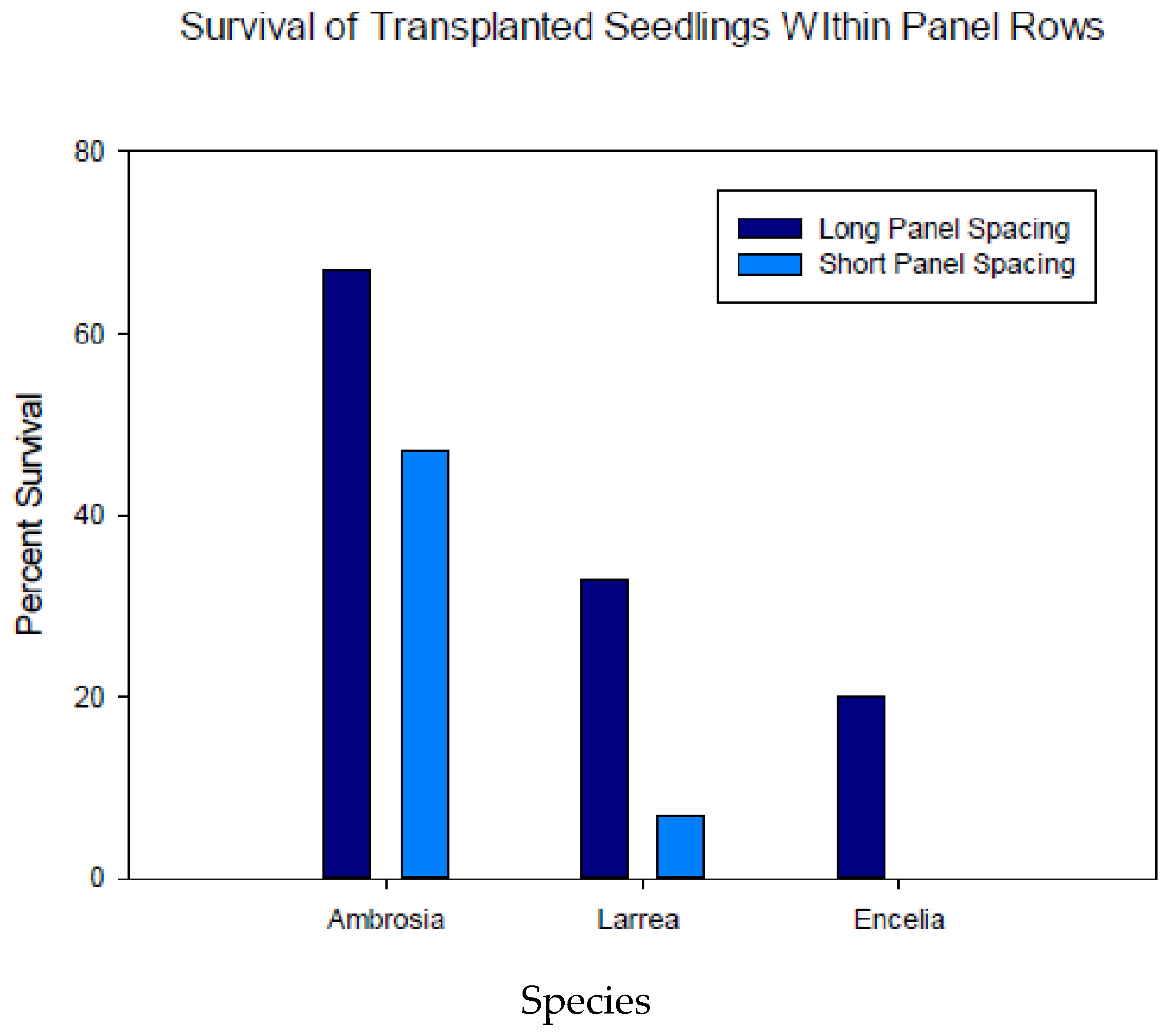
Survival of Transplants
4. Discussion
5. Concluding Comments
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef]
- Andrews, A. Fragmentation of Habitat by Roads and Utility Corridors: A Review. Aust. Zool. 1990, 26, 130–141. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Lovich, J.E.; Ennen, J.R. Wildlife Conservation and Solar Energy Development in the Desert Southwest. USA Biosci. 2011, 61, 982–991. [Google Scholar] [CrossRef]
- Devitt, D.A.; Young MHPierre, J.P. Assessing the potential for greater solar development in West Texas, USA. Energy Strategy Reviews 2020, 29, 100490. [Google Scholar] [CrossRef]
- Bolling, J.D.; Walker, L.R. Plant and Soil Recovery along a Series of Abandoned Desert Roads. J. Arid. Environ. 2000, 46, 1–24. [Google Scholar] [CrossRef]
- Vasek, F.C.; Johnson, H.B.; Brum, C.D. Effects of power transmission lines on vegetation of the Mojave Desert. Madrone 1975, 23, 114–130. [Google Scholar]
- Webb, R.H.; Wilshire, H.G. Environmental Effects of Off-Road Vehicles: Impacts and Management in arid Regions; Springer Series on Environmental management; Springer: New York, NY, USA, 1983. [Google Scholar]
- Lovich, J.E.; Bainbridge, D. Anthropogenic Degradation of the Southern California Desert Ecosystem and Prospects for Natural Recovery and Restoration. Environ. Manag. 1999, 24, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, R.Q.; Huang, Z.; Cheng, Z.; López-Vicente, M.; Ma, X.R.; Wu, G.L. Solar photovoltaic panels significantly promote vegetation recovery by modifying the soil surface micro habitats in an arid sandy ecosystem. Land Degrad. Dev. 2019, 30, 2177–2186. [Google Scholar] [CrossRef]
- Moriarty, P.; Honnery, D. Ecosystem maintenance energy and the need for a green EROI. Energy Policy 2019, 131, 229–234. [Google Scholar] [CrossRef]
- Northrup, J.M.; Wittemyer, G. Characterising the impacts of emerging energy development on wildlife, with an eye towards mitigation. Ecol. Lett. 2012, 16, 112–125. [Google Scholar] [CrossRef]
- Devitt, D.A.; Apodaca, L.; Bird, B.; Dawyot, J.P.; Fenstermaker, L.; Petrie, M.D. Assessing the Impact of a Utility Scale Solar Photovoltaic Facility on a Down Gradient Mojave Desert Ecosystem. Land 2022, 11, 1315. [Google Scholar] [CrossRef]
- Tsoutsos, T.; Frantzekaki, N.; Gekas, V. Environmental Impacts from the Solar Energy Technologies. Energy Policy 2005, 33, 289–296. [Google Scholar] [CrossRef]
- Cameron, D.R.; Cohen, B.S.; Morrison, S.A. An Approach to Enhance the Conservation-Compatibility of Solar Energy Development. PLoS ONE 2012, 7, 38437. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.R.; Hoffacker, M.K.; Field, C.B. Efficient use of land to meet sustainable energy needs. Nat. Clim. Change 2015, 5, 353–358. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Hoffacker, M.K.; Murphy-Mariscal, M.L.; Wu, G.C.; Allen, M.F. Solar energy development impacts on land cover change and protected areas. PNAS 2015, 112, 13579–13584. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, K. Land Use and Electricity generation: A life-cycle analysis. Renew. Sustain. Energy Rev. 2009, 13, 1465–1474. [Google Scholar] [CrossRef]
- McClung, M.R.; Moran, M.D. Minimizing impacts of future renewable energy development on the worlds desert ecosystems. Front. Sustain. 2022, 3, 900468. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Cagle, A.E.; Grodsky, S.M.; Exley, G.; Jordan, S.M. Comments on: Land use for United States power generation: A critical review of existing metrics with suggestions for going forward. Renew. Sustain. Energy Rev. 2021, 143, 110911. [Google Scholar]
- Hernandez, R.R.; Armstrong, A.; Burney, J.; Ryan, G.; Moore-O’leary, K.; Diédhiou, I.; Grodsky, S.M.; Saul-Gershenz, L.; Davis, R.; Macknick, J.; et al. Techno–ecological synergies of solar energy for global sustainability. Nat. Sustain. 2019, 2, 560–568. [Google Scholar] [CrossRef]
- Parker, S.S.; Cohen, B.S.; Moore, J. Impact of solar and wind development on conservation values in the Mojave Desert. PLoS ONE 2018, 13, e0207678. [Google Scholar] [CrossRef] [PubMed]
- Popcewicz, J.A.; Copeland, H. Potential impacts of energy development on shrublands in western North America. Nat. Resour. Environ. 2011, 17, 93–97. [Google Scholar]
- Grodsky, S.M.; Hernandez, R.R. Reduced ecosystem services of desert plants form ground-mounted solar energy development. Nat. Sustain. 2020, 3, 1036–1043. [Google Scholar] [CrossRef]
- Rao, L.E.; Allen, E.B. Combined effects of precipitation and nitrogen depositon. Oecologia 2010, 162, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Yavari R., D.; Zaliwciw, D.; Cibin, R.; McPhillips, L. Minnimizing environmental impacts of solar farms: A review of current science on landscape hydrology and guidance on stormwater management. Environ. Res. Infrastruct. Sustain. 2022, 2, 032002. [Google Scholar] [CrossRef]
- Von Seckendorff Hoff, K.; Marlow, R.W. Impacts of Vehicle Road Traffic on Desert Tortoise Populations with Consideration of Conservation of Tortoise Habitat in Southern Nevada. Chelonium. Conserv. Biol. 2002, 4, 449–456. [Google Scholar]
- Barron-Gafford, G.A.; Minor, R.L.; Allen, N.A.; Cronin, A.D.; Brooks, A.E.; Pavao-Zuckerman, M.A. The Photovoltaic Heat Island Effect: Larger solar power plants increase local temperatures. Sci. Rep. 2016, 6, 35070. [Google Scholar] [CrossRef] [PubMed]
- Hulin, V.; Delmas, V.; Girondot, M.; Godfrey, M.H.; Guillon, J.M. Temperature-dependent sex determination and global change: Are some species at greater risk? Oecologia 2009, 160, 493–506. [Google Scholar] [CrossRef]
- Graham, M.; Ates, S.; Melathopoulos, A.P.; Moldenke, A.R.; Debano, S.J.S.; Bes, L.R.; Higgins, C.W. Floral abundance during the late=season pollinators in a dryland, agrivoltaic ecosystem. Sci. Rep. 2021, 11, 7452. [Google Scholar] [CrossRef]
- Chock, R.Y.; Clucas, B.; Peterson, E.K.; Blackwell, B.F.; Blumstein, D.T.; Church, K.; Fernández-Juricic, E.; Francescoli, G.; Greggor, A.L.; Kemp, P.; et al. Evaluating potential effects of solar power facilities on wildlife from an animal behavior perspective. Conserv. Sci. Pract. 2021, 3, e319. [Google Scholar] [CrossRef]
- Armstrong, A.; Ostle, N.J.; Whitaker, J. Solar park microclimate and vegetation management effects on grassland carbon cycling. Environ. Res. Lett. 2016, 11, 074016. [Google Scholar] [CrossRef]
- Tanner, K.E.; Moore-O’Leary, K.A.; Parker, I.M.; Pavlik, B.M.; Hernarndez, R.R. Simulated solar panels create altered microhabitats in desert landforms. Ecosphere 2020, 11, e03089. [Google Scholar] [CrossRef]
- Dupraz, C.; Marrou, H.; Talbot, G.; Dufour, L.; Nogier, A.; Ferard, Y. Combining solar photovoltaic panels and food crops for optimizing land use: Towards new agrivoltaic schemes. Renew. Energy 2011, 36, 2725–2732. [Google Scholar] [CrossRef]
- Marrou, H.; Wery, J.; Dufour, L.; Dupraz, C. Productivity and radiation use efficiency of lettuces grown in the partial shade of photovoltaic panels. Eur. J. Agron. 2013, 44, 54–66. [Google Scholar] [CrossRef]
- Hassanpour, A.E.; Selker, J.S.; Higgins, C.W. Remarkable agrivoltaic influence on soil moisture, micrometeorology and water use efficiency. PLoS ONE 2018, 13, e0203256. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.S.; Cagle, A.E.; Macknick, J.; Bloom, D.E.; Caplan, J.S.; Ravi, S. Effects of Revegetation on Soil Physical and Chemical Properties in Solar Photovoltaic Infrastructure. Front. Environ. Sci. 2020, 8, 140. [Google Scholar] [CrossRef]
- Wright, J.L. New Evapotranspiration crop coefficients. J. Irrig. Drain. Eng. Div. 1982, 108, 57–74. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cooper, T.A. Correlations between carbon isotope ratio and microhabitat in desert plants. Oecologia 1988, 76, 562–566. [Google Scholar] [CrossRef]
- Ehleringer, J.R. Variation in leaf carbon isotope discrimination in Encelia farinosa: Implications for growth, competition, and drought survival. Oecologia 1993, 95, 340–346. [Google Scholar] [CrossRef]
- Devitt, D.A.; Smith, S.D.; Neuman, D.S. Carbon isotope discrimination in three Landscape species growing in an arid environment. J. Arid. Environ. 1997, 36, 249–257. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Chow, P.S.; Dickman, L.T.; E Furze, M.; Kuhlman, I.; Schmid, S.; Wiesenbauer, J.; Wild, B.; Gleixner, G.; Hartmann, H.; et al. Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol. 2018, 38, 1764–1778. [Google Scholar] [CrossRef]
- Hickman, J.C.; Jepson, W.L. The Jepson Manual: Higher Plants of California; University of California Press: Berkeley, CA, USA, 1993. [Google Scholar]
- R Core Team 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 10 October 2020).
- Jordan, S.M.; Lee, J.; McClung, M.; Moran, M. Quantifying the ecosystem services values of electricity generation in eh US Chihuahuan Desert: Life cycle perspective. J. Indus. Ecol. 2021, 25, 1089–1101. [Google Scholar] [CrossRef]
- Yue, S.; Guo, M.; Zou, P.; Wu, W.; Zhou, X. Effects of photovoltaic panels on soil temperature and moisture in desert areas. Environ. Sci. Pollut. Res. 2021, 28, 17506–17518. [Google Scholar] [CrossRef]
- Schwinning, S.; Hooten, M.M. Mojave Desert Root Systems. The Mojave Desert: Ecosystems Processes and Sustainability; University of Nevada Press: Reno, NV, USA, 2009; pp. 278–311. [Google Scholar]
- Wright, J.I.; Reich, P.B.; Westoby, M. Strategy Shifts in Leaf Physiology, Structure and Nutrient Content between Species of High-and Low-Rainfall and Higher- and Low-Nutrient Habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Schulz, E.D.; Turner, N.C.; Nicolle, D.; Schumacher, J. Leaf and wood carbon isotope ratios, specific leaf areas and wood growth of Eucalyptus species across a rainfall gradient in Australia. Tree Physiol. 2006, 26, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, H.; Yu, Y.; Zhao, W.; Liu, J.; Yu, H.; Yetemen, O. Ecohydrological effects of photovoltaic solar farms on soil microclimates and moisture regimes in arid northwest China: A modeling study. Sci. Total Environ. 2022, 802, 149946. [Google Scholar] [CrossRef] [PubMed]
- Lambert, Q.; Bischoff, A.; Cluchier, A.; Cueff, S.; Gros, R. Effects of solar park construction and solar panels on soil quality, microclimate, CO2 effluxes and vegetation under a Mediterranean climate. Land Degrad. Dev. 2021, 32, 5190–5202. [Google Scholar] [CrossRef]
- Levis, S.; Meehl, G.A.; Han, W.; Washington, W.M.; Oleson, K.W.; van Ruijven, B.J.; Strand, W.G. Impact of solar panels on global climate. Nat. Clim. 2016, 6, 290–294. [Google Scholar]
- Bowers, J.E. Effects of drought on shrub survival and longevity in the northern Sonoran Desert. J. Torrey Bot. Soc. 2005, 132, 421–431. [Google Scholar] [CrossRef]
- Curtin, A.J.; Zug, G.R.; Medica, P.A.; Spotila, J.R. Assessing age in the desert tortoise Gopherus agassizii: Testing skeletochronology with individuals of known age. Endanger. Species Res. 2008, 5, 21–27. [Google Scholar] [CrossRef]
- Moore-O’Leary, K.A.; Hernandez, R.R.; Johnston, D.S.; Abella, S.R. Sustainability of utility-scale solar energy- critical ecological concepts. Front. Ecol. Environ. 2017, 15, 385–394. [Google Scholar] [CrossRef]
- Mamun, A.M.A.; Dargusch, P.; Wadley, D.; Zulkarnain, N.A.; Aziz, A.A. A review of research on agrivoltaic systems. Renew. Sustain. Energy Rev. 2022, 161, 112351. [Google Scholar] [CrossRef]
- Elamri, Y.; Cheviron, B.; Lopez, J.-M.; Dejean, C.; Belaud, G. Water budget and crop modelling for agrivoltaic systems: Application to irrigated lettuces. Agric. Water Manag. 2018, 208, 440–453. [Google Scholar] [CrossRef]
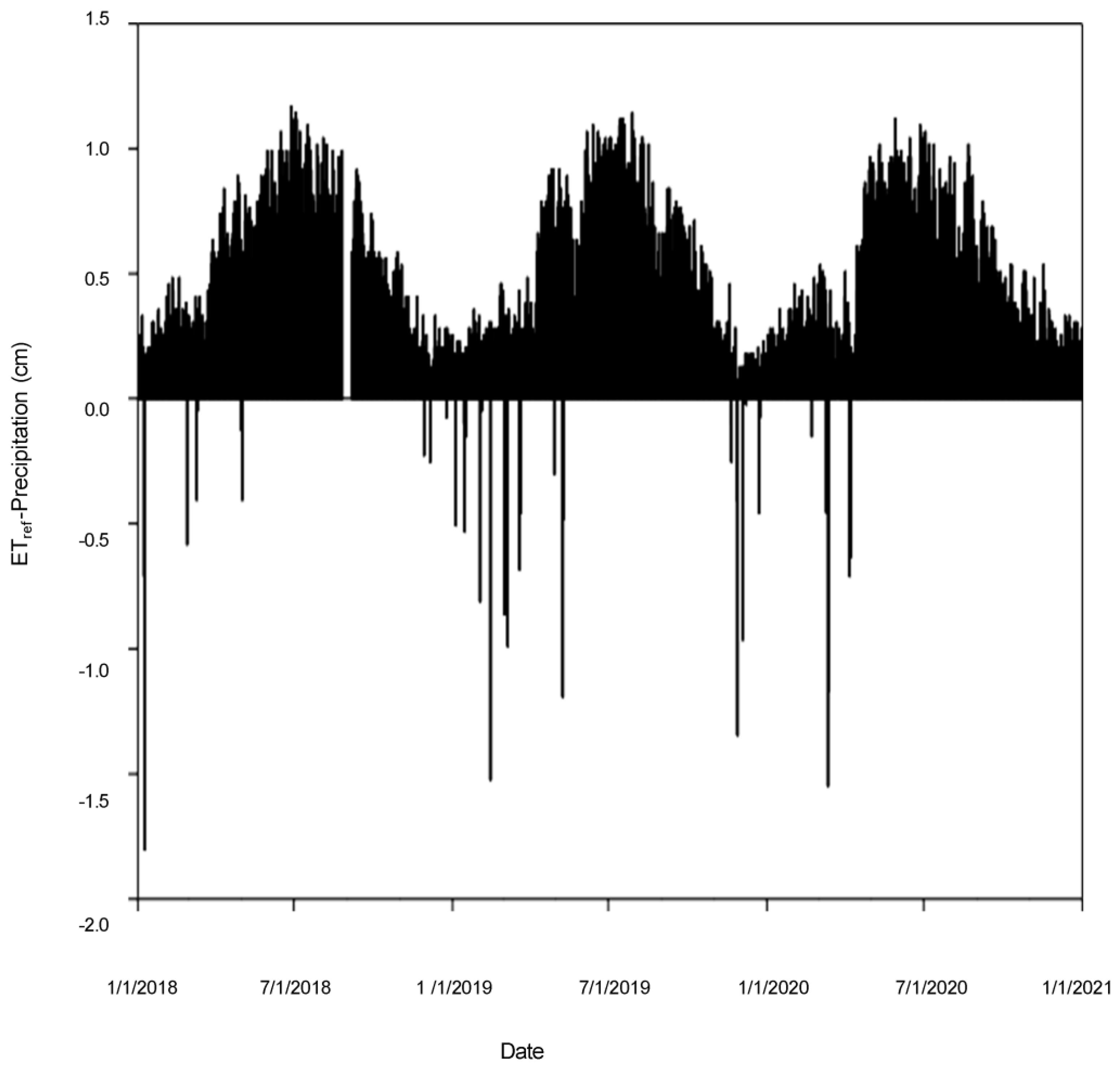
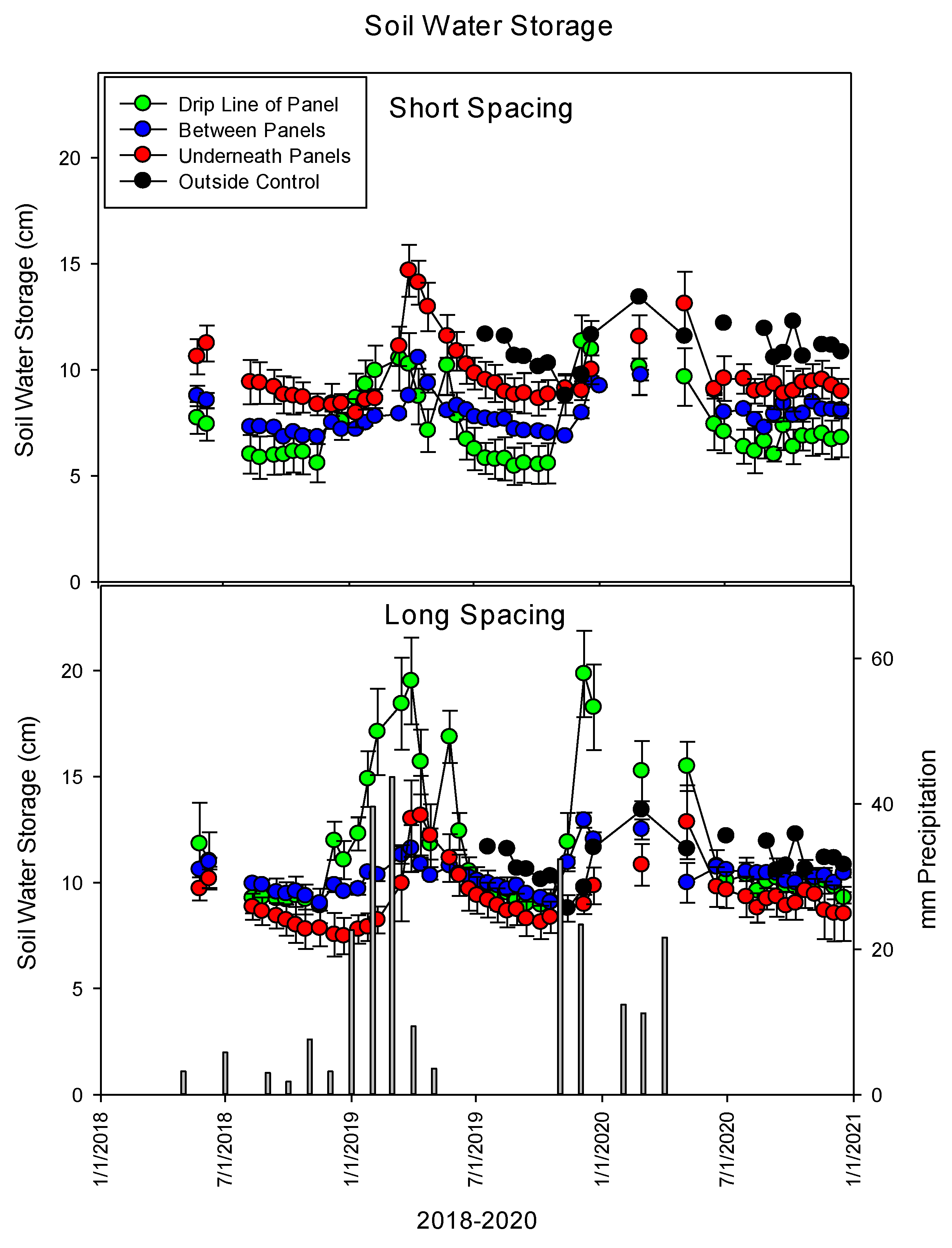
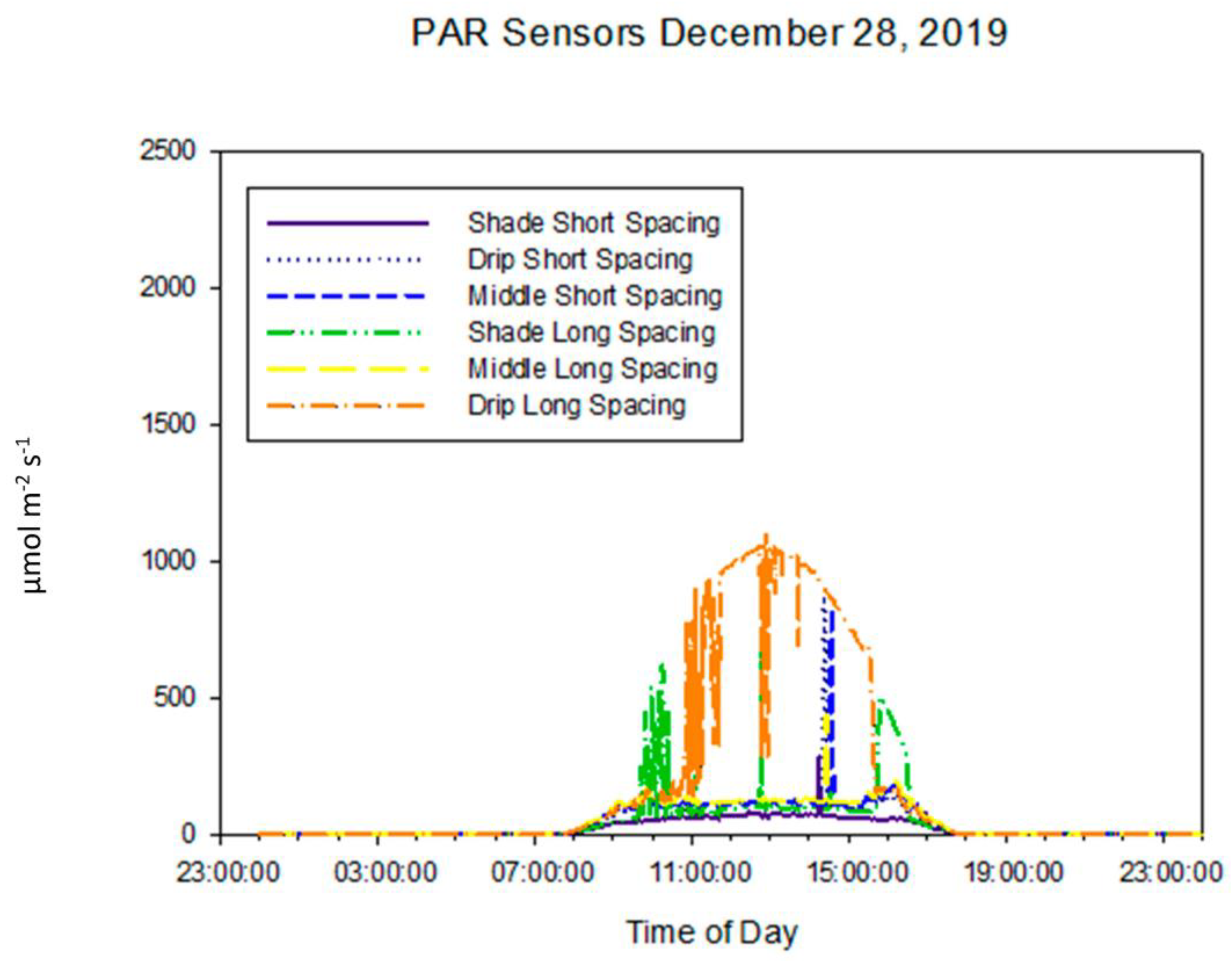

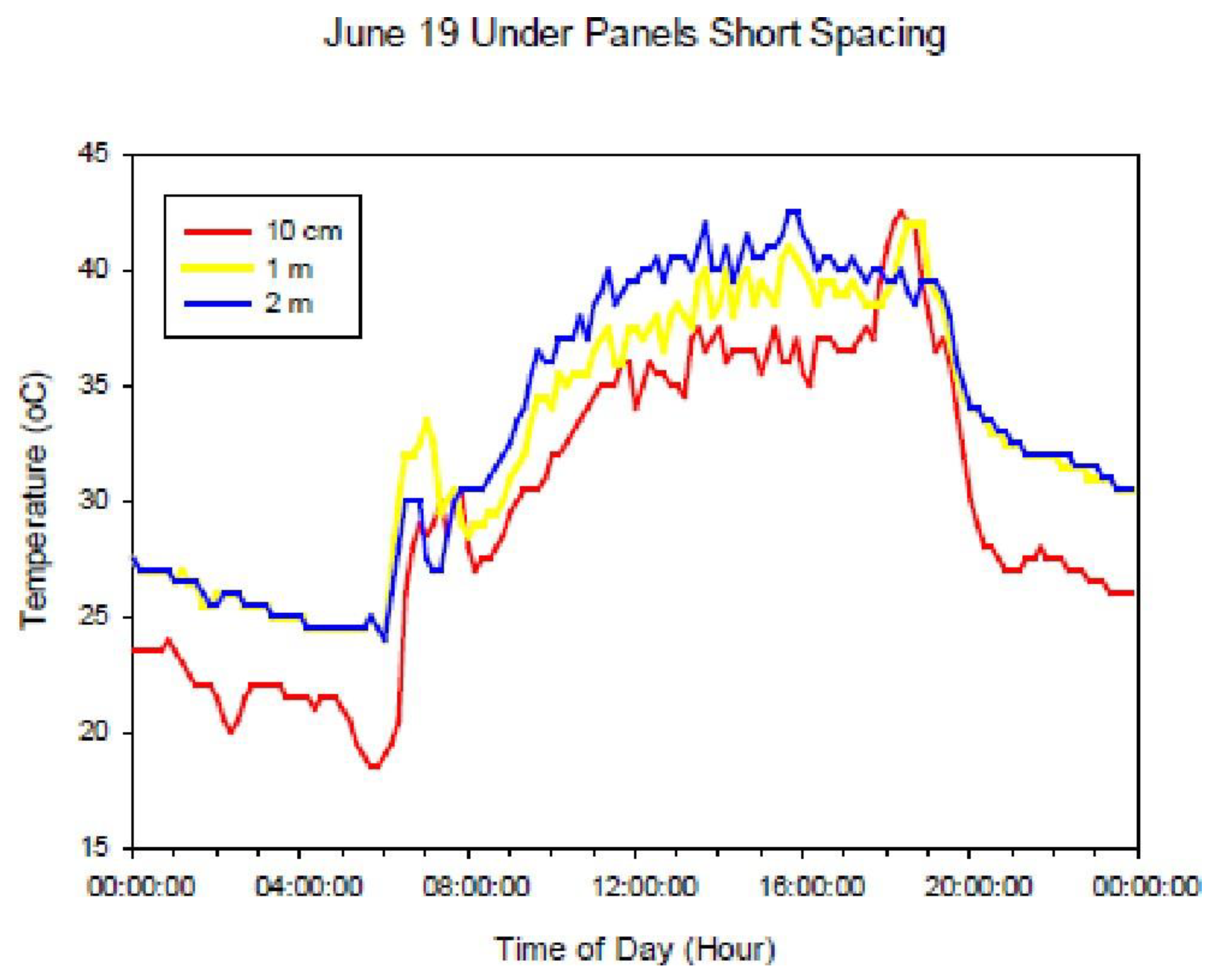



| Year | Precipitation | ETref | ETref−Precipitation |
|---|---|---|---|
| 2018 | 7.3 | 190.8 | 183.5 |
| 2019 | 17.2 | 187.5 | 170.3 |
| 2020 | 7.0 | 187.0 | 180.0 |
| Parameter | Mean+/−SE |
|---|---|
| Panel Height above the Soil | 99.1+/−19.1 cm |
| Panel Slope | 29.4+/−1.3% |
| Soil Slope | 9.0+/−6.1% |
| Position | Long Spacing | Short Spacing |
|---|---|---|
| Drip Line | 0% reduction | 11% |
| Middle of the Row | 16% | 20% |
| Under the Panel | 83% | 85% |
| Variable | Species |
|---|---|
| Seed | L. tridentata Y = 9.76 + 0.027Fe − 1.36 Cu R2 = 0.49 p < 0.001 |
| Weight | A. dumosa Y = 138.2 − 40.4K − 8.03Ca + 151.7Mg + 1.71Mn |
| R2 = 0.56 p = 0.004 | |
| Δ Height | L. tridentata Y = 39.84 − 7.34 position − 127.2S + 76.1Mg R2 = 0.58 p < 0.001 |
| Δ Height | A. dumosa Y = −15.16 + 0.56Zn + 12.58Mo R2 = 0.59 p < 0.001 |
| Stem Elongation | L. tridentata Y = 51.88 − 9.10 position − 13.9K + 0.27Mn R2 = 0.70 p < 0.001 |
| Species Effect | A. dumosa Concentrations of all macro and micronutrients were higher inside facility (p < 0.001) |
| Position Effect | |
| Nutrient concentrations A. dumosa lower in the outside control plants; N, K, S, Mg, and Mo (p < 0.001) | |
| Correlations between nutrient concentrations. Highest three R2 values for each species. | |
| L tridentata %N = 1.02 + 10.13P, R2 = 0.51, p < 0.001 | |
| L. tridentata %P = −0.0004 + 0.0524 N, R2 = 0.51, p < 0.001 | |
| L. tridentata Mo (ppm) = 1.024 + 10.129P R2 = 0.51, p < 0.001 | |
| A. dumosa %K = −0.373 + 0.85N − 0.006B + 0.543Mo, R2 = 0.84, p < 0.001 | |
| A. dumosa %N = 0.71 + 5.48P + 5.03S − 0.005B R2 = 0.78, p < 0.001 | |
| A. dumosa %S = −0.027 + 0.81K + 0.001B R2 = 0.77, p < 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wynne-Sison, T.; Devitt, D.A.; Smith, S.D. Ecovoltaics: Maintaining Native Plants and Wash Connectivity inside a Mojave Desert Solar Facility Leads to Favorable Growing Conditions. Land 2023, 12, 1950. https://doi.org/10.3390/land12101950
Wynne-Sison T, Devitt DA, Smith SD. Ecovoltaics: Maintaining Native Plants and Wash Connectivity inside a Mojave Desert Solar Facility Leads to Favorable Growing Conditions. Land. 2023; 12(10):1950. https://doi.org/10.3390/land12101950
Chicago/Turabian StyleWynne-Sison, Tamara, Dale A. Devitt, and Stanley D. Smith. 2023. "Ecovoltaics: Maintaining Native Plants and Wash Connectivity inside a Mojave Desert Solar Facility Leads to Favorable Growing Conditions" Land 12, no. 10: 1950. https://doi.org/10.3390/land12101950
APA StyleWynne-Sison, T., Devitt, D. A., & Smith, S. D. (2023). Ecovoltaics: Maintaining Native Plants and Wash Connectivity inside a Mojave Desert Solar Facility Leads to Favorable Growing Conditions. Land, 12(10), 1950. https://doi.org/10.3390/land12101950







