Effective Treatments for the Successful Establishment of Milkweed (Calotropis procera L.) under Water Deficit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Seeds Coat
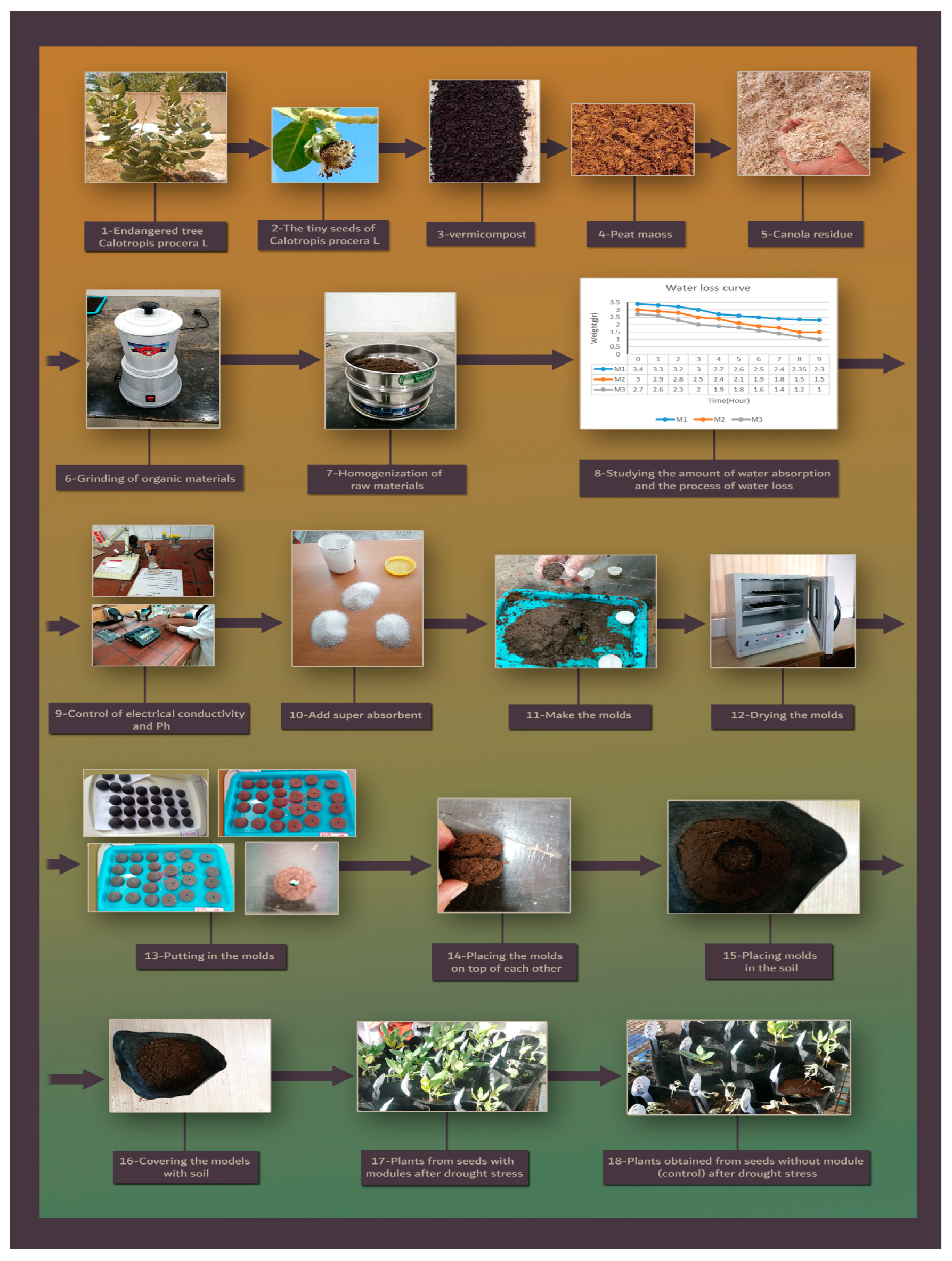
2.2. Experimental Detail
2.3. Measurements
2.3.1. Plant Morphology
2.3.2. Leaf Free Proline Content
2.3.3. Leaf Chlorophyll Content
2.3.4. Activity of Enzymatic Antioxidant
2.4. Statistical Analysis
3. Results and Discussion
3.1. Emergence Percentage, Leaf Area, Specific Leaf Weight, Shoot and Root Dry Weights, and Root/Shoot Dry Weights
3.2. Free Leaf Proline Content
3.3. Leaf Chlorophyll Content
3.4. Activity of Enzymatic Antioxidant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland Management and Ecological Adaptation Analysis Model for Astragalus curvirostris Boiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Mirdavoudi, H.; Ghorbanian, D.; Zarekia, S.; Soleiman, J.M.; Ghonchepur, M.; Sweeney, E.M.; Mastinu, A. Ecological Niche Modelling and Potential Distribution of Artemisia sieberi in the Iranian Steppe Vegetation. Land 2022, 11, 2315. [Google Scholar] [CrossRef]
- Moradi, P.; Aghajanloo, F.; Moosavi, A.; Monfared, H.H.; Khalafi, J.; Taghiloo, M.; Khoshzaman, T.; Shojaee, M.; Mastinu, A. Anthropic Effects on the Biodiversity of the Habitats of Ferula gummosa. Sustainability 2021, 13, 7874. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Ahmadikhah, A.; Fotovat, R.; Rohani, L.; Soheily, F.; Uberti, D.L.; Mastinu, A. Molecular Characterization of a New Ecotype of Holoparasitic Plant Orobanche L. on Host Weed Xanthium spinosum L. Plants 2022, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.D.; Kostka, S.J.; Inouye, A.L.; Zvirzdin, D.L. Postfire Restoration of Soil Hydrology and Wildland Vegetation Using Surfactant Seed Coating Technology. Rangel. Ecol. Manag. 2012, 65, 253–259. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K.; Page-Dumroese, D.S.; Hardegree, S.P. Can biochar be used as a seed coating to improve native plant germination and growth in arid conditions? J. Arid. Environ. 2016, 125, 8–15. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophysics 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Ravi, S.; Breshears, D.D.; Huxman, T.E.; D’Odorico, P. Land degradation in drylands: Interactions among hydrologic–aeolian erosion and vegetation dynamics. Geomorphology 2010, 116, 236–245. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Tribulato, A.; Patanè, C. Effects of drought stress on seed germination of ornamental sunflowers. Acta Physiol. Plant 2017, 39, 184. [Google Scholar] [CrossRef]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae 2022, 8, 193. [Google Scholar] [CrossRef]
- Chaichi, M.; Nemati, A.; Dadrasi, A.; Heydari, M.; Hassanisaadi, M.; Yousefi, A.R.; Baldwin, T.C.; Mastinu, A. Germination of Triticum aestivum L.: Effects of Soil-Seed Interaction on the Growth of Seedlings. Soil Syst. 2022, 6, 37. [Google Scholar] [CrossRef]
- Jam, B.J.; Shekari, F.; Andalibi, B.; Fotovat, R.; Jafarian, V.; Najafi, J.; Uberti, D.; Mastinu, A. Impact of Silicon Foliar Application on the Growth and Physiological Traits of Carthamus tinctorius L. Exposed to Salt Stress. Silicon 2022, 15, 1235–1245. [Google Scholar] [CrossRef]
- Kamali, N.; Sadeghipour, A.; Souri, M.; Mastinu, A. Variations in Soil Biological and Biochemical Indicators under Different Grazing Intensities and Seasonal Changes. Land 2022, 11, 1537. [Google Scholar] [CrossRef]
- Taghvaei, M.; Nasrolahizadehi, A.; Mastinu, A. Effect of Light, Temperature, Salinity, and Halopriming on Seed Germination and Seedling Growth of Hibiscus sabdariffa under Salinity Stress. Agronomy 2022, 12, 2491. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Anastasi, U.; Santonoceto, C.; Maggio, A. Effect of PEG-induced drought stress on seed germination of four lentil genotypes. J. Plant Interact. 2013, 9, 354–363. [Google Scholar] [CrossRef]
- Lotfi, N.; Soleimani, A.; Vahdati, K.; Çakmakçı, R. Comprehensive biochemical insights into the seed germination of walnut under drought stress. Sci. Hortic. 2019, 250, 329–343. [Google Scholar] [CrossRef]
- Partheeban, C.; Chandrasekhar, C.N.; Jeyakumar, P.; Ravikesavan, R.; Gnanam, R. Effect of PEG Induced Drought Stress on Seed Germination and Seedling Characters of Maize (Zea mays L.) Genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1095–1104. [Google Scholar] [CrossRef]
- Amirkhani, M.; Mayton, H.; Loos, M.; Taylor, A. Development of Superabsorbent Polymer (SAP) Seed Coating Technology to Enhance Germination and Stand Establishment in Red Clover Cover Crop. Agronomy 2023, 13, 438. [Google Scholar] [CrossRef]
- Beigi, S.; Azizi, M.; Iriti, M. Application of Super Absorbent Polymer and Plant Mucilage Improved Essential Oil Quantity and Quality of Ocimum basilicum var. Keshkeni Luvelou. Molecules 2020, 25, 2503. [Google Scholar] [CrossRef]
- Johnson, E.N.; Miller, P.R.; Blackshaw, R.E.; Gan, Y.; Harker, K.N.; Clayton, G.W.; Kephart, K.D.; Wichman, D.M.; Topinka, K.; Kirkland, K.J. Seeding date and polymer seed coating effects on plant establishment and yield of fall-seeded canola in the Northern Great Plains. Can. J. Plant Sci. 2004, 84, 955–963. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M. Zinc seed coating improves the growth, grain yield and grain biofortification of bread wheat. Acta Physiol. Plant 2016, 38, 238. [Google Scholar] [CrossRef]
- Gorim, L.; Asch, F. Seed coating reduces respiration losses and affects sugar metabolism during germination and early seedling growth in cereals. Funct. Plant Biol. 2015, 42, 209. [Google Scholar] [CrossRef] [PubMed]
- Cerasola, V.A.; Perlotti, L.; Pennisi, G.; Orsini, F.; Gianquinto, G. Potential Use of Superabsorbent Polymer on Drought-Stressed Processing Tomato (Solanum lycopersicum L.) in a Mediterranean Climate. Horticulturae 2022, 8, 718. [Google Scholar] [CrossRef]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Motamedi, M.; Zahedi, M.; Karimmojeni, H.; Motamedi, H.; Mastinu, A. Effect of rhizosphere bacteria on antioxidant enzymes and some biochemical characteristics of Medicago sativa L. subjected to herbicide stress. Acta Physiol. Plant 2022, 44, 84. [Google Scholar] [CrossRef]
- AbdAllah, A.M.; Mashaheet, A.M.; Burkey, K.O. Super absorbent polymers mitigate drought stress in corn (Zea mays L.) grown under rainfed conditions. Agric. Water Manag. 2021, 254, 106946. [Google Scholar] [CrossRef]
- Bagherifard, A.; Hamidoghli, Y.; Biglouei, M.H.; Ghaedi, M. Effects of drought stress and superabsorbent polymer on morpho-physiological and biochemical traits of Caper (Capparis spinosa L.). Aust. J. Crop Sci. 2020, 14, 13–20. [Google Scholar] [CrossRef]
- Islam, M.R.; Xue, X.; Mao, S.; Ren, C.; Eneji, A.E.; Hu, Y. Effects of water-saving superabsorbent polymer on antioxidant enzyme activities and lipid peroxidation in oat (Avena sativa L.) under drought stress. J. Sci. Food Agric. 2011, 91, 680–686. [Google Scholar] [CrossRef]
- Taghvaei, M.; Sadeghi, H.; Khaef, N. Cardinal Temperatures for Germination of the Medicinal Anddesert Plant, Calotropis procera. Planta Daninha 2015, 33, 671–678. [Google Scholar] [CrossRef]
- Khanzada, A.K.; Shaikh, W.; Kazi, T.G.; Kabir, S.; Soofia, S. Antifungal activity, elemental analysis and determination of total protein of seaweed, Solieria robusta (Greville) Kylin from the coast of Karachi. Pak. J. Bot. 2007, 39, 931–937. [Google Scholar]
- Nouman, W.; Aziz, U. Seed priming improves salinity tolerance in Calotropis procera (Aiton) by increasing photosynthetic pigments, antioxidant activities, and phenolic acids. Biologia 2022, 77, 609–626. [Google Scholar] [CrossRef]
- Taghvaei, M.; Kordestani, M.D.; Saleh, M.; Mastinu, A. The Reinforcement of Early Growth, Extract, and Oil of Silybum marianum L. by Polymer Organic Cover and Bacteria Inoculation under Water Deficit. Soil Syst. 2023, 7, 61. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gong, Y.P.; Toivonen, P.M.A.; Lau, O.L.; Wiersma, P.A. Antioxidant system level in ‘Braeburn’ apple is related to its browning disorder. Bot. Bull. Acad. Sin. 2001, 42, 259–264. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen-Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach-Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase. J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and Physiological Plant Responses to Drought Stress in Thymus citriodorus. Int. J. Agron. 2016, 2016, 4165750. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Chaves, M.M.; Costa, J.M.; Saibo, N.J.M. Recent Advances in Photosynthesis Under Drought and Salinity. Adv. Bot. Res. 2011, 57, 49–104. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Sivritepe, N.; Erturk, U.; Yerlikaya, C.; Turkan, I.; Bor, M.; Ozdemir, F. Response of the cherry rootstock to water stress induced in vitro. Biol. Plant. 2008, 52, 573–576. [Google Scholar] [CrossRef]
- Nemeskeri, E.; Kovacs-Nagy, E.; Nyeki, J.; Sardi, E. Responses of apple tree cultivars to drought: Carbohydrate composition in the leaves. Turk. J. Agric. For. 2015, 39, 949–957. [Google Scholar] [CrossRef]
- Nge, T.T.; Hori, N.; Takemura, A.; Ono, H. Swelling behavior of chitosan/poly(acrylic acid) complex. J. Appl. Polym. Sci. 2004, 92, 2930–2940. [Google Scholar] [CrossRef]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. Int. J. Polym. Sci. 2022, 2022, 4914836. [Google Scholar] [CrossRef]
- Yazdani, F.; Allahdadi, I.; Akbari, G.A. Impact of Superabsorbent Polymer on Yield and Growth Analysis of Soybean (Glycine max L.) Under Drought Stress Condition. Pak. J. Biol. Sci. 2007, 10, 4190–4196. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mardeh, A.S.-S.; Poustini, K.; Jahromi, M.E. Influence of Osmo and Hydropriming on Seed Germination and Seedling Growth in Wheat (Triticum aestivum L.) Cultivars under Different Moisture and Temperature Conditions. Pak. J. Biol. Sci. 2007, 10, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, L.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol. Biochem. 2020, 155, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, C.; Magdich, S.; Ben Rouina, B.; Boukhris, M.; Ben Abdullah, F. Saline water irrigation effects on soil salinity distribution and some physiological responses of field grown Chemlali olive. J. Environ. Manag. 2012, 113, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Towhidi, A.; Saberifar, T.; Dirandeh, E. Nutritive value of some herbage for dromedary camels in the central arid zone of Iran. Trop. Anim. Health Prod. 2011, 43, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, M.J.; Niknejad-Kazempour, H. Effect of dormancy breaking treatments and salinity on seed germination of two desert shrubs. Arid. Land. Res. Manag. 2007, 21, 107–118. [Google Scholar] [CrossRef]
- Su, L.-Q.; Li, J.-G.; Xue, H.; Wang, X.-F. Super absorbent polymer seed coatings promote seed germination and seedling growth of Caragana korshinskii in drought. J. Zhejiang Univ.-Sci. B 2017, 18, 696–706. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Sankar, B.; Murali, P.V.; Gomathinayagam, M.; Lakshmanan, G.M.A.; Panneerselvam, R. Water deficit stress effects on reactive oxygen metabolism in Catharanthus roseus; impacts on ajmalicine accumulation. Colloids Surf. B Biointerfaces 2008, 62, 105–111. [Google Scholar] [CrossRef]
- Poormohammad Kiani, S.; Maury, P.; Sarrafi, A.; Grieu, P. QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water-stressed conditions. Plant Sci. 2008, 175, 565–573. [Google Scholar] [CrossRef]
- Tahkokorpi, M.; Taulavuori, K.; Laine, K.; Taulavuori, E. After-effects of drought-related winter stress in previous and current year stems of Vaccinium myrtillus L. Environ. Exp. Bot. 2007, 61, 85–93. [Google Scholar] [CrossRef]
- Tongo, A.; Jalilvand, H.; Hosseininasr, M.; Naji, H.R. Leaf morphological and physiological variations in response to canopy dieback of Persian Oak (Quercus brantii Lindl.). Forest Pathol. 2021, 51, e12671. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef]
- Zhang, H.-Q.; Zou, Y.-B.; Xiao, G.-C.; Xiong, Y.-F. Effect and Mechanism of Cold Tolerant Seed-Coating Agents on the Cold Tolerance of Early Indica Rice Seedlings. Agric. Sci. China 2007, 6, 792–801. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon Regulates Antioxidant Activities of Crop Plants under Abiotic-Induced Oxidative Stress: A Review. Front. Plant Sci. 2017, 8, 00510. [Google Scholar] [CrossRef]
- Terzi, R.; Kadioglu, A. Drought stress tolerance and the antioxidant enzyme system in Ctenanthe setosa. Acta Biol. Cracov. Bot. 2006, 48, 89–96. [Google Scholar]
- Sales, C.R.G.; Ribeiro, R.V.; Silveira, J.A.G.; Machado, E.C.; Martins, M.O.; Lagôa, A.M.M.A. Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol. Biochem. 2013, 73, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef]
- Azarabadi, S.; Abdollahi, H.; Torabi, M.; Salehi, Z.; Nasiri, J. ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur. J. Plant Pathol. 2016, 147, 279–294. [Google Scholar] [CrossRef]
- Lai, K.M.; Ye, D.Y.; Wong, J.W.C. Enzyme activities in a sandy soil amended with sewage sludge and coal fly ash. Water Air Soil Poll. 1999, 113, 261–272. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, S.; Hu, X.; Cai, J.; Tang, Z.; Xu, Q. Whole Process Inhibition of a Composite Superabsorbent Polymer-Based Antioxidant on Coal Spontaneous Combustion. Arab. J. Sci. Eng. 2018, 43, 5999–6009. [Google Scholar] [CrossRef]
- Zeng, D.; Zhao, H. Activity test and mechanism study of an environmentally friendly wheat seed coating agent. Agric. Sci. 2013, 4, 334–339. [Google Scholar] [CrossRef]
| Permanent Wilting Point (%) | Field Capacity (%) | Silt (%) | Clay (%) | Sand (%) | EC (d/Sm) | pH | Organic Matter (%) |
|---|---|---|---|---|---|---|---|
| 11 | 28 | 53 | 33 | 14 | 2.1 | 7.1 | 0.268 |
| Vermicompost Properties | Peat Moss Properties | Canola Residue Properties (Above-Ground Plant at Flowering) | |
|---|---|---|---|
| pH | 7.75 | 4.4 | 6.14 |
| Electrical conductivity(EC) | 3.8 (ds/m) | 0.76 (ds/m) | 3.27 (ds/m) |
| Organic matter | 44.2 (%) | 45.68 (%) | 43.80 (%) |
| Organic carbon | 113.48 (mg/kg) | 192.19 (mg/kg) | 46.46 (mg/kg) |
| Total nitrogen | 21.7 (mg/kg) | 12.21 (mg/kg) | 3.7 (mg/kg) |
| Phosphorus | 14,194 (mg/kg) | 0.09 (mg/kg) | 0.25 (%) |
| Potassium | 10,000 (mg/kg) | 0.03 (mg/kg) | 1.4 (%) |
| Iron | 3274 (mg/kg) | 563 (mg/kg) | 19 (mg/kg) |
| Zinc | 112.3 (mg/kg) | 104 (mg/kg) | 15 (mg/kg) |
| Manganese | 248.8 (mg/kg) | 25 (mg/kg) | 14 (mg/kg) |
| Copper | 28.7 (mg/kg) | 340 (mg/kg) | 2.7 (mg/kg) |
| Variables | Kolmogorov–Smirnov | Shapiro–Wilk | ||||
|---|---|---|---|---|---|---|
| Statistic | df | Sig. | Statistic | df | Sig. | |
| EP | 0.155 | 192 | 0.142 * | 0.935 | 192 | 0.095 * |
| LA | 0.197 | 192 | 0.151 * | 0.912 | 192 | 0.123 * |
| LDW | 0.135 | 192 | 0.091 * | 0.937 | 192 | 0.071 * |
| SLA | 0.090 | 192 | 0.161 * | 0.957 | 192 | 0.008 * |
| SDW | 0.067 | 192 | 0.200 * | 0.987 | 192 | 0.568 * |
| RDW | 0.095 | 192 | 0.066 * | 0.965 | 192 | 0.066 * |
| R/S | 0.078 | 192 | 0.200 * | 0.969 | 192 | 0.044 * |
| Source of Variability | df | EP | LA | SLA | SDW | RDW | R/S | CHO | PRO | SOD | CAT | APX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 3 | 124.82 ** | 13.92 ** | 0.73 ** | 17.91 ** | 12.39 ** | 0.005 ns | 0.007 ** | 1.89 ** | 1230.18 ** | 987.05 ** | 2613.01 ** |
| M | 3 | 1470 ** | 242.42 ** | 0.017 ns | 256.71 ** | 127.30 ** | 0.003 ns | 0.06 ** | 73.07 ** | 35,186.41 ** | 4277.51 ** | 12,697.13 ** |
| I | 2 | 7376 ** | 8360.86 ** | 0.19 ** | 22,391.5 ** | 5204.56 ** | 0.26 ** | 18.25 ** | 2994.02 ** | 1,386,722.81 ** | 38,578.42 ** | 4,327,279.04 ** |
| C × M | 9 | 4.6 ** | 0.047 ns | 0.010 ns | 0.17 ns | 0.07 ns | 0.009 ns | 0.0 ns | 0.02 ns | 8.27 ns | 3.33 ns | 3.10 ns |
| C × I | 6 | 236.12 ** | 1.307 ** | 0.021 * | 3.05 ** | 2.56 ** | 0.006 ns | 0.002 ** | 0.63 ** | 318.65 ** | 247.45 ** | 42.22 ** |
| M× I | 6 | 2.98 * | 53.76 ** | 0.054 ** | 74.09 ** | 30.25 ** | 0.043 ns | 0.015 ** | 21.02 ** | 11,088.60 ** | 1066.64 ** | 246.09 ** |
| C × M × I | 18 | 4.50 ** | 0.063 ns | 0.21 ns | 0.14 ns | 0.13 ns | 0.023 ns | 0.0 ns | 0.04 ns | 8.58 ns | 1.42 ns | 16.49 ns |
| Error | 144 | 1.29 | 0.23 | 0.16 | 0.58 | 0.41 | 0.12 | 0 | 0.03 | 6.67 | 2.06 | 14.06 |
| CV (%) | - | 1.68S | 1.1 | 12.2 | 3.02 | 4.21 | 20.12 | 2.12 | 1.65 | 4.36 | 3.02 | 6.12 |
| 100% FC | 50% FC | 25% FC | ||
|---|---|---|---|---|
| Emergence Percentage | ||||
| Control | 87.11 ± 1.5 a | 68 ± 0.71 b | 62.5 ± 0.81 b | 77.54 ± 10.55 A |
| Canola residue | 90.48 ± 1.12 a | 69.5 ± 1.12 b | 66.75 ± 1.08 c | 75.57 ± 10.60 B |
| Peat moss | 78.64 ± 1.45 a | 72.75 ± 1.92 b | 67 ± 0.71 c | 72.791 ± 4.75 AB |
| Vermicompost | 97.45 ± 1.545 a | 75 ± 1.41 b | 70.75 ± 0.82 c | 81.06 ± 11.71 A |
| Mean | 88.42 ± 6.76 A | 71.31 ± 2.74 B | 66.75 ± 2.92 C | |
| S1 | 86.94 ± 7.03 a | 69.5 ± 2.29 b | 65.75 ± 2.86 b | 67.66 ± 2.05 B |
| S2 | 87.25 ± 6.45 a | 71 ± 2.73 b | 66.5 ± 3.57 b | 61 ± 0.82 C |
| S3 | 89.5 ± 6.8 a | 71.75 ± 2.86 b | 66.75 ± 2.48 c | 69.66 ± 1.24 B |
| S4 | 90 ± 6.81 | 73 ± 3.16 | 68 ± 2.82 | 77.66 ± 2.05 A |
| Mean | 88.42 ± 1.35 A | 71.31 ± 1.26 B | 66.75 ± 0.82 C | |
| Leaf area (cm2) | ||||
| Control | 35.05 ± 0.11 a | 21.77 ± 2.62 bc | 11.86 ± 2.94 cd | 22.89 ± 9.49 C |
| Canola residue | 35.17 ± 0.11 a | 22.22 ± 2.79 b | 12.17 ± 3.02 dc | 23.19 ± 9.41 B |
| Peat moss | 35.44 ± 0.11 a | 22.75 ± 2.76 b | 12.63 ± 2.93 c | 23.61 ± 9.32 B |
| Vermicompost | 35.54 ± 0.13 a | 23.55 ± 2.85 b | 13.31 ± 2.96 c | 24.13 ± 9.08 A |
| Mean | 35.3 ± 0.197 A | 22.58 ± 0.65 B | 12.49 ± 0.54 C | |
| S1 | 35.29 ± 0.19 a | 22.99 ± 0.78 b | 12.82 ± 0.44 c | 23.70 ± 9.17 B |
| S2 | 35.14 ± 0.22 a | 18.03 ± 0.52 bc | 7.61 ± 0.57 d | 20.26 ± 11.3 C |
| S3 | 35.34 ± 0.19 a | 23.88 ± 0.62 b | 13.13 ± 0.65 c | 24.10 ± 9.08 A |
| S4 | 35.45 ± 0.21 a | 25.37 ± 0.72 b | 15.41 ± 0.51 c | 25.41 ± 8.18 A |
| Mean | 35.30 ± 0.11 A | 22.56 ± 2.7 B | 12.24 ± 2.8 C | |
| Specific leaf area | ||||
| Control | 0.54 ± 0.001 a | 0.488 ± 0.020 b | 0.34 ± 0.154 c | 0.45 ± 0.081 AB |
| Canola residue | 0.53 ± 0.001 a | 0.49 ± 0.017 b | 0.43 ± 0.082 b | 0.48 ± 0.035 AB |
| Peat moss | 0.50 ± 0.001 a | 0.49 ± 0.024 b | 0.43 ± 0.072 b | 0.49 ± 0.041 A |
| Vermicompost | 0.53 ± 0.002 a | 0.503 ± 0.012 a | 0.451 ± 0.092 b | 0.49 ± 0.035 A |
| Mean | 0.53 ± 0.018 a | 0.49 ± 0.004 a | 0.45 ± 0.017 b | |
| S1 | 0.53 ± 0.002 a | 0.45 ± 0.13 b | 0.48 ± 0.15 ab | 0.48 ± 0.036 B |
| S2 | 0.5 ± 0.002 a | 0.49 ± 0.005 b | 0.32 ± 0.011 c | 0.44 ± 0.092 AB |
| S3 | 0.45 ± 0.002 b | 0.50 ± 0.003 a | 0.46 ± 0.009 b | 0.47 ± 0.022 B |
| S4 | 0.55 ± 0.002 a | 0.53 ± 0.008 a | 0.50 ± 0.025 ab | 0.52 ± 0.021 A |
| Mean | 0.51 ± 0.039 A | 0.49 ± 0.028 A | 0.44 ± 0.070 B | |
| Shoot dry weight (g) | ||||
| Control | 66.55 ± 0.04 a | 45.43 ± 3.7 b | 28.45 ± 2.01 c | 46.79 ± 15.5 AB |
| Canola residue | 66.67 ± 0.04 a | 45.84 ± 3.8 b | 28.48 ± 3.00 c | 46.99 ± 15.6 AB |
| Peat moss | 66.75 ± 0.04 a | 46.39 ± 3.5 b | 29.41 ± 2.32 c | 47.51 ± 15.2 A |
| Vermicompost | 66.8 ± 0.51 a | 47.30 ± 3.8 b | 30.59 ± 2.40 c | 48.24 ± 14.7 A |
| Mean | 66.68 ± 0.12 A | 47.25 ± 0.72 B | 29.23 ± 0.87 C | |
| S1 | 66.69 ± 0.09 a | 47.30 ± 0.55 b | 27.22 ± 4.07 cd | 47.07 ± 16.1 B |
| S2 | 66.63 ± 0.10 a | 39.90 ± 0.71 bc | 25.66 ± 0.56 cd | 44.06 ± 16.9 C |
| S3 | 66.72 ± 0.10 a | 48.56 ± 0.72 b | 30.78 ± 0.82 c | 48.68 ± 14.6 A |
| S4 | 66.75 ± 0.10 a | 49.23 ± 0.83 b | 31.47 ± 0.92 c | 49.15 ± 14.4 A |
| Mean | 66.69 ± 0.044 A | 46.27 ± 3.72 B | 28.78 ± 2.41 C | |
| Root dry weight (g) | ||||
| Control | 35.90 ± 0.04 a | 27.75 ± 2.1 b | 17.26 ± 1.96 c | 26.97 ± 7.62 C |
| Canola residue | 35.92 ± 0.04 a | 28.17 ± 2.1 b | 17.67 ± 1.97 c | 27.25 ± 7.47 AB |
| Peat moss | 35.95 ± 0.04 a | 28.72 ± 2.1 b | 18.25 ± 1.97 c | 27.64 ± 7.26 AB |
| Vermicompost | 36.03 ± 0.14 a | 29.56 ± 2.2 b | 18.84 ± 2.12 c | 28.14 ± 7.08 A |
| Mean | 35.95 ± 0.05 A | 28.55 ± 0.67 A | 18 ± 0.59 A | |
| S1 | 35.93 ± 0.02 a | 29.022 ± 0.80 b | 17.73 ± 0.31 cd | 27.56 ± 7.50 ABb |
| S2 | 35.86 ± 0.02 a | 24.86 ± 0.58 bc | 14.84 ± 0.62 c | 25.18 ± 8.58 C |
| S3 | 35.96 ± 0.02 a | 29.9 ± 0.74 b | 14.07 ± 6.85 c | 26.64 ± 9.22 AB |
| S4 | 36.05 ± 0.12 a | 30.42 ± 0.61 b | 19.87 ± 0.70 c | 28.78 ± 6.70 A |
| Mean | 35.95 ± 0.068 A | 28.55 ± 2.18 B | 16.62 ± 2.32 C | |
| Root/shoot dry weight | ||||
| Control | 0.70 ± 0.0394 b | 0.83 ± 0.006 a | 0.835 ± 0.027 a | 0.7.88 ± 0.062 B |
| Canola residue | 0.72 ± 0.003 ab | 0.82 ± 0.056 a | 0.841 ± 0.02 b | 0.793 ± 0.052 A |
| Peat moss | 0.729 ± 0.001 b | 0.82 ± 0.003 a | 0.854 ± 0.02 a | 0.801 ± 0.055 A |
| Vermicompost | 0.729 ± 0.002 b | 0.94 ± 0.01 a | 0.854 ± 0.06 a | 0.841 ± 0.052 A |
| Mean | 0.722 ± 0.018 B | 0.872 ± 0.01 A | 0.846 ± 0.02 A | |
| S1 | 0.546 ± 0.0001 b | 0.667 ± 0.06 a | 0.623 ± 0.009 a | 0.584 ± 0.046 B |
| S2 | 0.523 ± 0.03 b | 0.637 ± 0.004 a | 0.590 ± 0.017 ab | 0.621 ± 0.052 A |
| S3 | 0.547 ± 0.001 b | 0.659 ± 0.05 a | 0.657 ± 0.008 a | 0.622 ± 0.053 A |
| S4 | 0.548 ± 0.001 b | 0.650 ± 0.04 a | 0.669 ± 0.032 a | 0.623 ± 0.053 A |
| Mean | 0.542 ± 0.01 A | 0.653 ± 0.011 A | 0.634 ± 0.031 A | |
| Leaf Chlorophyll Content (mg Chl g−1 FW) | ||||
|---|---|---|---|---|
| 100% FC | 50% FC | 25% FC | ||
| Control | 1.62 ± 0.002 a | 0.93 ± 0.039 bc | 0.607 ± 0.055 cd | 1.071 ± 0.44 AB |
| Canola residue | 1.67 ± 0.002 a | 0.94 ± 0.037 b | 0.6205 ± 0.05 c | 1.078 ± 0.43 AB |
| Peat moss | 1.672 ± 0.002 a | 0.95 ± 0.037 b | 0.64075 ± 0.049 c | 1.088 ± 0.43 A |
| Vermicompost | 1.673 ± 0.003 a | 0.96 ± 0.037 b | 0.65425 ± 0.048 c | 1.098 ± 0.42 A |
| Mean | 1.67 ± 0.00 A | 0.95 ± 0.012 B | 0.63 ± 0.018 C | |
| S1 | 1.66 ± 0.0005 a | 0.95 ± 0.01 b | 0.63 ± 0.012 c | 1.086 ± 0.43 AB |
| S2 | 1.67 ± 0.0004 a | 0.87 ± 0.05 bc | 0.56 ± 0.025 cd | 1.024 ± 0.47 AB |
| S3 | 1.67 ± 0.0004 a | 0.96 ± 0.01 b | 0.68 ± 0.021 c | 1.104 ± 0.42 A |
| S4 | 1.67 ± 0.0012 a | 0.97 ± 0.01 b | 0.68 ± 0.013 c | 1.112 ± 0.41 A |
| Mean | 1.67 ± 0.00 A | 0.97 ± 0.04 B | 0.63 ± 0.05 C | |
| Free leaf proline content (μmol g−1 FW) | ||||
| Control | 4.04 ± 0.012 c | 9.54 ± 1.34 b | 18.04 ± 1.81 a | 10.55 ± 5.7 A |
| Canola residue | 4.03 ± 0.007 c | 9.4175 ± 1.34 b | 17.7825 ± 1.89 a | 10.44 ± 5.6 A |
| Peat moss | 4.02 ± 0.011 c | 9.2625 ± 1.32 b | 17.4925 ± 1.94 a | 10.22 ± 5.5 A |
| Vermicompost | 3.92 ± 0.15 c | 9.145 ± 1.31 b | 17.0875 ± 1.97 a | 10.01 ± 5.4 A |
| Mean | 4 ± 0.04 C | 9.34 ± 0.15 B | 17.08 ± 0.35 A | |
| S1 | 4.03 ± 0.007 d | 8.91 ± 0.19 d | 17.95 ± 0.16 b | 10.29 ± 5.76 B |
| S2 | 4.04 ± 0.010 d | 11.6 ± 0.16 b | 20.45 ± 0.34 a | 12.04 ± 6.72 A |
| S3 | 4.02 ± 0.007 d | 8.62 ± 0.12 b | 16.65 ± 0.42 b | 9.755 ± 5.20 C |
| S4 | 4.01 ± 0.021 d | 8.22 ± 0.12 b | 15.35 ± 0.47 bc | 9.186 ± 4.66 C |
| Mean | 4.02 ± 0.011 C | 9.34 ± 1.33 B | 17.59 ± 1.91 A | |
| Superoxide Dismutase Activity (Ug−1 FW) | ||||
|---|---|---|---|---|
| 100% FC | 50% FC | 25% FC | Mean | |
| Control | 80.44 ± 0.040 c | 156.32 ± 25.36 bc | 369.125 ± 42.45 a | 201.98 ± 122.19 B |
| Canola residue | 80.45 ± 0.041 c | 160.42 ± 25.21 b | 361.9375 ± 43.88 a | 200.98 ± 118.43 B |
| Peat moss | 80.45 ± 0.047 c | 166.32 ± 26.04 b | 367.125 ± 43.28 a | 204.68 ± 120.12 A |
| Vermicompost | 80.46 ± 0.053 c | 171.82 ± 25.53 b | 375.88 ± 41.31 a | 209.48 ± 123.48 A |
| Mean | 80.45 ± 0.00 C | 163.73 ± 5.89 B | 368.51 ± 4.99 A | |
| S1 | 80.46 ± 0.014 e | 179.19 ± 5.29 c | 377.44 ± 5.72 ab | 212.36 ± 123.48 AB |
| S2 | 80.43 ± 0.11 e | 199.03 ± 5.70 bc | 429.14 ± 5.37 a | 236.23 ± 144.78 A |
| S3 | 80.42 ± 0.008 e | 149.125 ± 5.61 d | 341.64 ± 9.75 b | 190.39 ± 110.55 B |
| S4 | 80.41 ± 0.007 e | 130.63 ± 6.01 d | 313.34 ± 7.63 b | 174.78 ± 100.07 C |
| Mean | 80.43 ± 0.021 C | 164.50 ± 26.43 B | 365.39 ± 43.27 A | |
| Catalase activity (U g−1 FW) | ||||
| Control | 24.20 ± 0.014 d | 48.44 ± 12.59 c | 65.12 ± 12.26 ab | 45.92 ± 16.79 B |
| Canola residue | 24.20 ± 0.014 d | 52.25 ± 12.06 bc | 69.75 ± 12.05 ab | 49.11 ± 18.83 AB |
| Peat moss | 24.21 ± 0.015 d | 58.44 ± 12.62 bc | 74.68 ± 12.41 a | 52.50 ± 21.05 A |
| Vermicompost | 24.22 ± 0.012 d | 65.25 ± 12.10 bc | 80.31 ± 12.04 a | 56.40 ± 23.63 A |
| Mean | 24.21 ± 0.00 C | 56.28 ± 60.04 B | 72.49 ± 5.65 A | |
| S1 | 23.45 ± 1.293 d | 54.95 ± 5.86 bc | 71.93 ± 5.37 ab | 50.11 ± 20.08 AB |
| S2 | 24.235 ± 0.005 d | 76.31 ± 5.63 ab | 92.31 ± 5.57 a | 64.28 ± 29.06 A |
| S3 | 24.205 ± 0.005 d | 49.12 ± 6.67 c | 65.37 ± 6.57 b | 46.23 ± 16.93 B |
| S4 | 24.21 ± 0.007 d | 43.81 ± 5.45 c | 60.25 ± 5.17 b | 42.75 ± 14.73 BC |
| Mean | 24.025 ± 0.33 C | 56.048 ± 12.34 B | 72.46 ± 72.49 A | |
| Ascorbate peroxidase activity (U g−1 FW) | ||||
| Control | 897.37 ± 0.96 d | 1184.93 ± 15.29 c | 1403.44 ± 27.34 ab | 1161.917 ± 207.23 A |
| Canola residue | 897 ± 0.82 d | 1190.43 ± 16.09 c | 1411.44 ± 26.34 a | 1166.207 ± 210.61 A |
| Peat moss | 898.18 ± 0.73 d | 1198.5 ± 17.1 c | 1420.44 ± 24.34 a | 1172.227 ± 213.83 A |
| Vermicompost | 898 ± 0.83 d | 1209.5 ± 17.93 c | 1435.44 ± 18.34 a | 1181 ± 220.35 A |
| Mean | 897.64 ± 0.47 C | 1195.84 ± 9.24 B | 1417.53 ± 11.91 A | |
| S1 | 898.93 ± 0.48 c | 1198.12 ± 8.7 b | 1413.06 ± 9.8 a | 1170.04 ± 210.82 A |
| S2 | 872.93 ± 43.4 c | 1221.56 ± 11.13 b | 1456.62 ± 6.8 a | 1183.70 ± 239.78 A |
| S3 | 896.93 ± 0.48 c | 1188.75 ± 6.7 b | 1405.93 ± 10 a | 1163.87 ± 208.54 A |
| S4 | 896.81 ± 0.56 c | 1177.43 ± 6.2 b | 1387.31 ± 11 a | 1153.85 ± 200.93 A |
| Mean | 891.41 ± 10.69 B | 1196.49 ± 16.23 AB | 1415.73 ± 25.41 A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolatkordestani, M.; Taghvaei, M.; Mastinu, A. Effective Treatments for the Successful Establishment of Milkweed (Calotropis procera L.) under Water Deficit. Land 2023, 12, 1987. https://doi.org/10.3390/land12111987
Dolatkordestani M, Taghvaei M, Mastinu A. Effective Treatments for the Successful Establishment of Milkweed (Calotropis procera L.) under Water Deficit. Land. 2023; 12(11):1987. https://doi.org/10.3390/land12111987
Chicago/Turabian StyleDolatkordestani, Mojtaba, Mansour Taghvaei, and Andrea Mastinu. 2023. "Effective Treatments for the Successful Establishment of Milkweed (Calotropis procera L.) under Water Deficit" Land 12, no. 11: 1987. https://doi.org/10.3390/land12111987
APA StyleDolatkordestani, M., Taghvaei, M., & Mastinu, A. (2023). Effective Treatments for the Successful Establishment of Milkweed (Calotropis procera L.) under Water Deficit. Land, 12(11), 1987. https://doi.org/10.3390/land12111987



_Kazoglou.png)





