Abstract
The European Union’s ‘Green Deal’ proposes an ambitious roadmap towards climate neutrality by 2050 and the adoption of a circular economy. Functional AgroBiodiversity (FAB) measures, which balance food production with minimised impacts on nature, are a promising way to achieve this on farmland. Here, we undertake a rapid evidence assessment to highlight Functional Agro-Biodiversity (FAB) management measures which help to realise biodiversity, climate neutrality, efficiency in use of natural resources and the circular economy. We report evidence on the effectiveness of 10 common FAB measures employed in Europe following a resurgence of interest and increased availability of data on their impact. The review found that the outcomes of implementing FAB measures were largely positive, with a number of mixed effects. There are evidence gaps, e.g., the impact of FAB measures on yield, the magnitude and timescale of impacts, the effect of landscape context. We signpost the most relevant and well-documented FAB measures, providing a reference for land managers and practitioners to select FAB measures to achieve specific ecological and agricultural outcomes. It is also important to note that a combination of measures implemented in a strategic way can enhance the output success.
1. Introduction
Over the past 50 years, unprecedented increases in agricultural productivity, driven by economic motivations and technological advances, have led to widespread loss of biodiversity, soil degradation and global environmental change [1,2,3]. Increasing agricultural productivity in a sustainable way without further destroying biodiversity and degrading soils and water bodies, or jeopardising earth’s life support systems, will be critical as we move towards the projected peak in human population of 10.4 billion people during the 2080s [4]. The UN’s sustainable development goals (SDGs) articulate this challenge (https://unstats.un.org/sdgs (accessed on 1 January 2021). The EU’s green deal1 will contribute towards the SDGs, with one vehicle being the new EU missions within Horizon Europe—one of which is in the area of ‘soil health and food’. The ongoing effort through the EU green deal recognises the need for ‘joint action by stakeholders, researchers, policy-makers, industry and citizens working together to co-design, co-create and implement solutions’ (European Union, 2015), which is synonymous with a Functional Agro-Biodiversity (FAB) approach.
Within the European Union, the concept of Functional Agro-Biodiversity emerged alongside the ecosystem services concepts embodied in the Millennium Ecosystem Assessment (MEA) [5]. FAB is defined by the European Learning Network (ELN) as ‘those elements of biodiversity on the scale of agricultural fields or landscapes, which provide ecosystem services that support sustainable agricultural production and can also deliver benefits to the regional and global environment and the public at large’ [6]. It recognises the importance of a range of measures that support both above and below ground biodiversity. The functional component indicates the importance of biodiversity that can enhance ecosystem services, and specifically those ecosystem services that support agriculture [7]. These include the prioritisation of biodiversity that supports biomass production or pest and disease regulation. The FAB approach is a pragmatic one that recognises the need to achieve food production in a way that works with nature, exploiting synergies as far as possible. It seeks to reconcile the often deep rift between conservation and intensive agriculture goals while generating more resilient agricultural production systems [6,8] that use nature where possible over synthetic products.
Whilst Pillar 1 of the EU Common Agricultural Policy (created in 1999) concentrates on direct income support payments to farmers, Pillar 2 has been focused, at least partly, on ensuring the sustainable management of natural resources and climate. This has resulted in the development of agri-environment schemes across the EU, but the quality of natural resources on farmland continues to decline despite these schemes [1]. Evidence is limited on how agri-environment schemes have moderated this decline; the evidence that is available suggests that conservation actions for biodiversity have had mixed effects [9]. Agriculture was responsible for 51% of the total EU water use in 2014, and in 2012 more than 90% of the assessed ‘River Basin Management Plans’ indicated that agriculture was a significant pressure on water bodies2. Since farming covers 48% of the land surface area of the EU, agriculture also has an enormous influence over soil resources in arable areas or intensively managed grassland (i.e., through the decline of soil organic matter, soil erosion, soil compaction), while also being heavily dependent on them (i.e., for soil fertility and productivity). Linear intensive practices have resulted in costly degradation [10], indicating an urgent need for alternative land management approaches. Such approaches will need to include increased resource efficiency as part of the transition to a more circular economy; circular agro-ecosystems, which maintain production capacity, circulate products and material, depend less on external inputs, regenerate nature and conserve natural resources, but can also generate a sustainable income for farmers. FAB practises aim to achieve this.
Knowledge regarding the implementation, impact and outcomes of FAB is still highly fragmented and insufficiently embedded in agricultural practice, policy and society [7]. Furthermore, well-optimised FAB ecosystems could be quite different from existing ones, in which key functions are largely underpinned by fossil fuel inputs [11]. It is preferable not to view FAB measures (or agri-environment options) as stand-alone measures, but rather as part of whole-farm or landscape systems approaches delivering at a range of scales, from field to landscape and beyond.
The aim of this evidence assessment is to evaluate ten popular FAB measures which are the focus of the EU Interreg FABulous Farmers project [12], a transdisciplinary living laboratory project. This paper summarises from a longer report [13]. The measures were determined through consultation between partners and user communities, including professional growers’ organisations and farmer’s unions, in north-west Europe. We summarise the current understanding of the impacts and outcomes of each of the ten FAB measures, highlighting where key knowledge is still missing that may limit farm uptake and implementation.
2. Materials and Methods
We focused on ten FAB measures: conservation tillage techniques; mixed crops and crop rotations, including sward diversity (herbal leys); cover and catch crops, including legumes; organic matter input; modified manure quality and diversity; agroforestry; hedgerow management; field margin management; and reduction in the use of plant protection products. We also considered the cross-cutting effects of semi-natural landscape elements and landscape context. A definition of each measure is included in Appendix A. Measures were chosen through consultation and discussion with stakeholders and are considered to offer the most potential for achieving FAB through implementation across EU farming systems.
To address our research objectives, we conducted a widespread literature search to estimate the impacts of FAB measures. A literature survey was conducted using keyword searches in the ISI Web of Science database (to September 2019) to extract potential articles, which were subsequently filtered by title and keywords, abstract and finally full publication content [14,15]. Combinations of the following keywords were used for literature searches: reduced/conservation tillage, agroforestry, hedgerow management, mixed crops/crop rotation, manure, sward diversity, field margins, pesticides, reduced pesticide protection, organic, carbon (C) storage. Studies from Europe were targeted, but for some options evidence is more geographically specialised, so other continents were included. We also utilised recent reviews of agri-environment options [16,17] and searched the reference lists of previous syntheses on related topics. We have included material from the academic literature and the grey literature (government reports—often peer-reviewed). We have also added elements from more recent research during manuscript preparation and review. Using the reviewed literature, each of the ten FAB measures was defined and assessed in terms of impacts on yield, biodiversity (here, biodiversity refers to biodiversity that supports ecosystem services of agriculture (FAB)—also covered by pollination and pests—as well as wider biodiversity at species or ecosystem level; this varies by individual study), pollination, pests and diseases, soil quality, carbon sequestration and greenhouse gas (GHG) emissions, water quality and conservation. The strength of evidence was recorded for each FAB measure using the following categories: weak (evidence from only a few studies or contextually limited, e.g., geographically), intermediate (some evidence but not fully conclusive, contested, some contextual limitations), strong (large number of studies across range of contexts). Subsequent assessments of the co-benefits and trade-offs, magnitude, timescale, spatial issues, displacement, longevity, climate interactions, and social and economic barriers were made for each of the identified impacts of measures.
3. Results
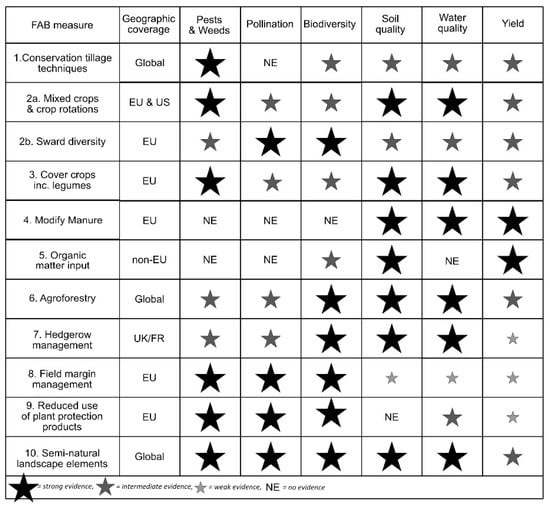
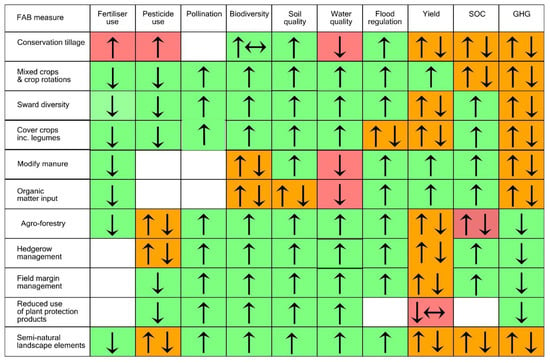
The assessment evidenced the potential contribution of FAB measures to ecosystem service provision, yet highlighted the lack of evidence in key areas for some measures (Figure 1). Figure 2 gives an overview of the main impacts found; it is an abbreviated version from the full table in the Supplementary Information (Table S1). Evidence of the nature of interactions between measures was also collected where possible. This was included in the text where appropriate and is summarised in the Supplementary Material (Table S2).
Figure 2.
Selected FAB measures and their contribution to ecosystem service provision and farm management. GHG = GHG emissions, SOC = soil organic carbon, ↓ = decrease; ↔ = no significant effect, ↑ = increase. The cells have been shaded green (positive effect on ES), red (negative effect on ES), orange (mixed). Presence of multiple arrows indicates good evidence for different effects, often depending on specific context.
3.1. Conservation Tillage and No-Tillage
Conservation tillage and no-tillage cover a continuum of practices to decrease soil disturbance and increase crop residues, with no-tillage representing the extreme case [18]. Practices along this continuum are often reported to promote soil quality, improve soil structure, reduce soil erosion and reduce costs and GHG emissions associated with fuel consumption [19,20]. Outcomes for biodiversity include maintaining high populations of soil-ameliorating fauna and insect pest predators and small mammals [21,22], although undesirable species (e.g., voles) may also increase [23]. There is some evidence for the effects of reduced tillage on birds, but it is inconclusive [24].
However, there is mixed evidence regarding the impact of conservation tillage and no-tillage on soil organic carbon (SOC) [25,26]. Tillage of the soil is a major driver of reduction in SOC on agricultural land, so reduced tillage has been recommended for C sequestration [27]. Recently, a meta-analysis [28] reported a boost to SOC (~5%) under conservation tillage. Others have shown that stock remains the same and that the distribution of C in the soil profile alters, removing the potential for slower C degradation in deeper soil horizons under reduced tillage or no-tillage [29,30,31,32].
Furthermore, reduced tillage may lead to reduced yields, with a reported −12% without nitrogen (N) fertiliser addition and −4% with inorganic N addition based on a global meta-analysis, resulting in a −4.5% in Europe, where soils are generally fertilised [21]. This loss can be partly offset with the combination of other measures such as cover crops [33,34] and crop rotation. Zikeli et al. [35] found that stubble tillage combined with reduced primary tillage can sustain yield levels without compromising the beneficial effects from reduced tillage on soil C and available nutrient content. Water management improvements can be made with restored water infiltration resulting from better soil structure and moisture conservation, especially important in drier clay and sandy soils [21]. Yet, water quality in some locations has reduced due to the higher herbicide use to tackle weed infestations, commonly due to a lack of soil inversion [36,37]. However, this effect can again be offset by improving weed control using diverse crop rotations and cover crops [36,38], together with non-chemical cover crop destruction (see Supplementary Table S2). Research has also suggested that reduced tillage may be associated with increased N2O emissions in poorly aerated soils (often found in NW Europe); this is concerning, due to the increased global warming potential of N2O [39,40], but it is likely to be limited in extent. Hence, the picture is mixed, with greater understanding needed on where gains can and cannot be made to inform intervention policy.
3.2. Mixed Crops and Crop Rotations including Sward Diversity
Mixed cropping (spatial diversity) and crop rotations (temporal diversity) are popular measures in the creation of a circular agricultural system. Synergistic interactions between crops can effectively reduce the requirement for fertiliser [22,41], especially when clover or other legumes are part of the rotation/mixture. They increase yield, improve yield stability and potentially increase the diversity and longevity of nectar resources for pollinators and other beneficial insects (depending on what is planted). More diverse crop rotations can increase the soil’s microbial richness and diversity [42]. Crop rotations can disrupt pest and disease cycles, potentially reducing the need for pesticide application. Mixing species with different properties and within a crop rotation can maintain a higher density of crop cover with better weed control and lower weed densities [22]. This helps to reduce reliance on fertiliser inputs and increases below ground [42] and above ground biodiversity, including invertebrates and invertebrate pest predators [43,44].
In pastures, there is some evidence that plant species richness is associated with increased grassland productivity and fodder quality and a reduced need for supplementary feed [45,46]. Beyond benefits to plant diversity, the successful diversification of grassland swards can benefit other above-ground and below-ground species including moths, spiders and beetles [47]. However, below-ground diversity has been found to respond less strongly compared with above-ground taxa [46].
Crop rotations and improved sward diversity can promote soil health by improving the diversity of the root architecture and soil structure, reducing soil compaction [48,49], improving water conservation and reducing risk of flooding [46]. Some increases in SOC content with plant species richness have been found [46,50,51]. However, Luo et al. [31] reported that when more crop species were used in rotation, it reduced C accumulation in the topsoil and led to a greater loss of C in deeper soil layers. Potentially, there may be a reduction in nitrate leaching from the soil, resulting from the varying ability of crops to remove nitrate from different places in the soil profile and reductions in the use of N fertiliser if legumes are introduced. However, if legumes are added to swards that did not previously receive manufactured N fertiliser, the additional fixed N can result in a net emission of N2O [52,53]. The design of the rotation is a key factor in trying to match crop uptake with available N [49].
3.3. Cover and Catch Crops, including Legumes
Cover and catch crops have been utilised in the agricultural system by some for many years and have received revived interest recently. Planting cover crops during fallow periods reduces N-leaching and soil erosion by avoiding periods of bare soil, often over winter, and limiting surface run-off [54,55,56]. Cover crops can increase soil quality by improving the soil structure through rooting systems [57], preventing erosion, increasing soil organic carbon stocks [58] and improving nutrient cycling. Increased crop phosphorus (P) nutrition [59] and increased soil mineral N availability, with the use of legumes or the incorporation of the cover crop, allows a reduction in the use of chemical N fertilisers and also lowers the risk of nutrient leaching [60]. The increased vegetative mulch can be very effective at suppressing weeds when retained on the soil surface [61,62], and soil biological activity and diversity are higher in systems with a surface mulch or cover crop.
Cover crops can have a positive effect on the biodiversity of many taxa, especially invertebrates and invertebrate pest predators [63,64], and may be beneficial to pollinators, although there may be trade-off between generalist and specialists [65]. However, some cover crops may increase pests, which then causes a decrease in yield, e.g., grassy cover crops give shelter to (stages of) wireworms when followed by maize.
The trade-offs for the use of cover crops mostly revolve around the increased expense to the farmer for planting a crop that is not harvested. It is unclear if cover crops provide an increase in the yield of the following crop, with no such increase found in a review by Scopel et al. [57]. Increased yields have been found with N-fixing cover crops in low-N soils, but decreased yields in intermediate- or high-N soils [55].
Environmental variables (e.g., soil type, climate), crop type and management practices (e.g., sowing date of cover crop) will all be important. Cover crops, particularly perennial and/or leguminous crops, may increase SOC sequestration [28,66]. Although N2O emissions may decrease in the short term, they may increase in the longer-term due to interactions with the C cycle [67]. Different factors such as the incorporation of residues within the soil, the species of cover crop and timing throughout the year all affect (N2O) emissions [68].
3.4. Modified Manure Management Quality and Diversity
The use of manure, possibly amended with small amounts of mineral N, can provide an effective fertiliser for cropping (Table S1). Manure amendments can rebuild a depleted soil microbial community [69], SOC [70], and improve soil structure and water retention capacity [39]. Replacing mineral fertilisers with organic fertilisers will not necessarily benefit biodiversity above ground, because the negative effects of fertilisation will occur regardless of the specific source of crop nutrients [24]; for instance, the encouragement of dense, fast-growing competitive plants.
Manure use will result in a reduced need for inorganic fertiliser, thereby reducing emissions from their manufacture [71]. The risk of increased N2O emission with the application of organic fertilisers [70] can be reduced by avoiding surface broadcasting [72], but may increase costs for appropriate machinery. If applied in the long-term, manure applications can reduce water quality via nitrate leaching, especially if over-applied [73].
It is important to note that different types of organic input are associated with different benefits and risks to ecosystem services. For example, a large experiment compared the effects of compost, manure, digestates and slurry on various soil properties and crop yields across seven UK sites [74]. Overall, the experiment found that composts and farmyard manures increased soil organic matter as compared with digestates or slurry (given roughly equivalent N in dosage). However, many recent studies suggest that labile organic inputs (such as those from slurry) can form SOM that is stable in the long-term, especially in clay soils [75]. Furthermore, slurry can supply more crop-available N than farmyard manure, allowing reduced inorganic N fertiliser use. However, GHG savings from reducing inorganic fertiliser use are negated by considerably higher N2O emissions associated with slurry than farmyard manure [76]. Similarly, poultry manure and sludge pellets may be associated with high N2O emissions [70]. It is important to ensure the quality of organic inputs to avoid contaminants such as heavy metals.
3.5. Organic Inputs: Biochar and Organic Matter Inputs, including Biosolids
The application of biochar, organic or inorganic fertilisers and biosolids has been reported to increase nutrient retention and use efficiency, reducing fertiliser needs [77,78,79]. Biochar has been reported to increase crop yields by ~20% (at an application rate of about 10 t ha−1) [80], and by 10% with substantial variability [81], with coarse low pH soils benefitting yields most.
The plant residual return results in higher SOC stocks [30,82], with biochar application shown to increase SOC by ~39% [28]. However, organic inputs could cause a priming effect on microbial activity, which may result in SOC mineralisation and CO2 efflux [83]. The application of biochar reduced the soil GHG emissions [79], yet consideration of the transport emission of inputs must be considered as these may offset the positive effects of GHG mitigation. Biochar and biosolids have the capacity to bind toxic components (bioremediation), which may negatively affect or change the soil microbial community [8], possibly also building up heavy metals and organic pollutants in the soil [39]. There is also some evidence that biochar could have positive effects on the composition of the soil microbial community, increasing species associated with increasing N fixation, N cycling, C cycling, P availability and decreasing potential plant pathogens [84,85].
As with manure, N2O emissions and N leaching are possible trade-offs of organic matter addition and increased SOC [39].
3.6. Agroforestry
Agroforestry is the practice of deliberately integrating woody vegetation (trees or shrubs) with crop and/or animal production systems to benefit from the resulting ecological and economic interactions [86]. It can include silvoarable systems, silvopastoral systems and agrosilvopastoral systems. Here, we consider all systems. Agroforestry has been shown to have a significant positive effect on biodiversity at landscape scales compared to solely agricultural or forestry systems [87], creating habitats for plants, birds, small mammals, bats, pollinators and other invertebrates [88,89]. Agroforestry can result in a higher abundance of natural pest predators and a lower abundance of weeds [90], although the agroforestry component can also act as a source of weeds or pests. Agroforestry can reduce soil erosion [15], enhance soil fertility, nutrient retention and nutrient cycling [91] and improve air quality through pollutant capture. Agroforestry systems require fewer fertiliser inputs, which in turn reduces emissions from the manufacture and application of fertilisers. If agroforestry is used for buffer strips in riparian zones, water quality is improved by reducing non-point source pollution [88] and by promoting stream bank stability, and controlling erosion and retaining water can also help prevent small floods downstream [92,93].
Agroforestry is a potential way to store C in biomass and soil, which may help to mitigate climate change. The potential for the trees to sequester C depends upon the stage of the woodland cycle [60]. Initially, C sequestration may decline, but agroforestry is planned over longer cycles and in the longer-term C sequestration should increase. Nair [87] states that “assuming that one hectare of agroforestry could save five hectares from deforestation”, C emission from deforestation can be reduced significantly by implementing agroforestry systems.
SOC generally increases when woody species are introduced, and land is converted from less complex systems to agroforestry. For example, a recent meta-analysis revealed SOC stock increases of 26–40% at various depths on conversion from agriculture to agroforestry [94]. In reviews, agroforestry (shelterbelts, hedgerows, alley cropping and silvopastoral systems) was found to sequester significant amounts of SOC in topsoils and subsoils [95,96]. Other studies found that the afforestation of grassland (not necessarily as agroforestry) can have mixed effects on SOC [47,97]. SOC can be higher in grasslands, and afforestation may release aggregated C from the subsoil at 30–80 cm, [98], but eventually the system should have a net gain from increased organic matter inputs. Research from France and the Mediterranean has shown an accumulation of SOC under alley-cropping systems [99,100], although there were more increases in coarse labile fractions that could affect stability. Research on an agroforestry system in Canada showed that SOC stocks to 30 cm were significantly greater in the forested areas than in adjacent herblands [101]. Cardinael et al. [102] present a set of C stock change factors and SOC accumulation/loss rates for three main land use changes (LUCs), cropland to agroforestry; forest to agroforestry; and grassland to agroforestry, showing positive relationships.
In addition to C sequestration, agroforestry has potential climate mitigation and adaptation benefits; it can influence micro-climatic conditions by providing a windbreak, retaining soil moisture and reducing the extreme changes in soil temperature during periods of drought or extreme cold weather conditions [103]. There can be costs at the establishment of agroforestry, for instance, in pastoral systems, where there is a need for protective fencing, and more widely by taking land out of production.
There is mixed evidence for the impact of agroforestry on yield. The combination of tree and crop systems could lead to a more efficient capture of resources than separate tree or crop systems [10,104]. However, whilst some studies have found an increase in yield of up to 40%, in alleys between trees compared with conventional fields [10,103], other studies have shown declines in yields, possibly due to competition for solar radiation and water [105,106]. Kanzler [107] found a decrease in sugar beet and winter wheat yields measured close to the tree hedgerows (3 m); however, at distances greater than 9 m away from the hedgerows, yields were higher.
A meta-analysis by Torralba et al. [15] suggested that often studies only calculated partial production, for the trees or crops alone. The whole system should be assessed (crop, wood, berries, nuts, feed), and this may be more likely to be positive for total biomass production.
3.7. Hedgerow Management
Hedgerows need to be kept in a management cycle to ensure that they continue to form effective hedges and produce associated ecosystem services. The structure and form of a hedgerow and its management, in terms of species present, width, height, presence of buffer strips, frequency and severity of cutting, presence of mature standard trees, etc., as well as the management of the adjacent field, all have a big effect on biodiversity [24] and function [108]. Larger, denser, more structurally and floristically complex hedgerows have been found to benefit many species by providing a greater habitat area and more niche space [109,110,111].
Hedgerows can provide a wide range of microhabitats (i.e., soil, bank, ground vegetation underneath and adjacent, shrub layer, tree canopy) to enhance populations of the natural enemies of crop pests [109] and a healthy diverse invertebrate and pollinator population, potentially increasing yield [108]. However, pest species themselves may utilise the additional resources managed or created to benefit their natural enemies. Hedgerow extent is positively associated with the richness and abundance of flora, invertebrates and birds [112,113].
Hedgerows also reduce soil loss, water course pollution from fields and the volume of water reaching a river or stream through increased rates of infiltration and interception of soil, nutrients, pollutants and sediment, particularly if placed along contours or beside water bodies [108,114]. A study by Borin et al. [115] showed that N losses and run-off in sites with hedgerows were reduced substantially (N by 44–100% and P 50–100% depending on tree age), and Caubel-Forget et al. [116] found nitrate levels in groundwater to be three times lower where a hedgerow was present, compared to a site without a hedge. Hedgerows could also improve biosecurity against some livestock diseases by reducing transmission between stocks in adjacent fields. As with agroforestry, hedgerows provide climate change mitigation through micro-climatic influences and the storage and accumulation of C above and below ground [87,108]. Positive effects of hedgerows on SOC have been found [117].
Hedgerows play an important role in preventing soil erosion through helping to reduce wind speeds and have been shown to decrease average wind speed by up to 60% [118].
However, also similar to agroforestry, there is mixed (and insufficient long term) evidence for impacts on the yield of adjacent crops, associated with their role in the provision of habitats for crop pests, pest predators and pollinators, impacts on hydrology, climate and wind, etc., with increases reported in some studies [103,107] and reductions found in others [105,106,107].
3.8. Field Margin Management
Although land may be taken out of production to create field margins, land at the edges of fields tends to be less productive and benefits can outweigh costs [119,120]. There is good evidence that field margins can significantly increase the biodiversity of invertebrates, plants, reptiles and amphibians and birds [119,121,122,123]. Field margins can, depending on the species chosen, provide resources that benefit pest predators, thus reducing pesticide use [124,125]. The margin can also act as a buffer strip for pollution from pesticides and fertilisers, which can protect adjacent semi-natural habitat and improve water quality and water flow (depending upon location and topography) [119].
Different types of margin management deliver different resources or benefits for different taxa, so there would be benefits from including multiple types of margin [126]; there will also be interactions between margins and hedgerows [127]. Field margins, if planted with wildflowers, have the ability to increase wild bee and pollinator abundance [121,123], which can lead to enhanced crop pollination and positive impacts on yield. The complexity and therefore the stability of invertebrate food webs has been found to be higher in non-cropped margins than in cropped, particularly those sown with wildflowers [128]. In some cases, field margins may favour pest species [129].
Responses to field margin management have been strongest in more intensively farmed landscapes [130,131], although for specialised species, responses may be strongest near to semi-natural habitats [132]. Grass margins benefit invertebrates (including beetles, spiders, earthworms) [133], hedgehogs, small mammals and some birds, although tall, dense swards could have negative impacts on ground nesting birds [122]. They also act as a buffer strip, protecting boundary features from spray drift and nutrient run-off, reducing soil erosion and increasing water quality [24]. A study suggested that the creation of grass margins on the edges of arable fields may not adversely impact yield at the field scale [127]. Beetle banks are designed to provide a source of predatory invertebrates to suppress pests in the adjacent crop [109], and so reduce plant protective product (PPP) application. Winter bird food/cover strips are planted to enhance bird populations, although there have been some negative effects such as increased predation or disease transmission [134].
Sown margins may even enhance topsoil C [128]; one study estimates that 0.1% to 2.4% of 1990 UK CO2-C emissions could be sequestered using grass margins on arable fields [135].
3.9. Reduction in Use of Plant Protection Products
Most evidence on the impacts of reducing plant protection products comes from organic farming where inputs are prohibited, with less evidence available on more gradual reductions, particularly linking the size of the reduction to the magnitude of the response [136]. The reduction in the use of pesticides increases the potential for biological pest control [137]. Long-term organic farming and the application of farmyard manure has been found to promote soil quality and microbial biomass [138].
Pesticides negatively impact pollinators, species diversity of plants, carabids and ground-nesting farmland birds [123,139,140] and can end up in water [141,142]. Therefore, the reduction of these PPPs is important. However, without PPPs, yields may be lower [136] and high populations of problem weeds could build up in the seedbank [143]. Although, where pesticides are used intensively, the likelihood of ‘weed’ species (e.g., blackgrass, Lolium sp.) becoming resistant to herbicides increases and alternative management solutions to their management are being sought. These include ecological solutions, manipulating the diversity and abundance of weeds and reducing resource availability, like FAB measures, such as better crop rotations, cover crops, inclusions of leys and increasing semi-natural habitats in the wider landscape [144]. The extent to which some reduction in PPPs can have no significant effect on yield is still under debate, with studies suggesting that reductions of 30–50% would not result in a loss of yield [145].
Reducing yields through the removal of all PPPs could displace the problem, as the intensity of food production will just move elsewhere if there is no change in consumer food preferences and nothing is done to minimise food waste. If PPPs’ application is currently high, there may be potential for reductions with little or no impact on yield [146], particularly if combined with other FAB options (e.g., field margins, increased semi-natural habitat, cover crops, crop rotations) (Supplementary Table S2).
3.10. Semi-Natural Landscape Elements and Landscape Context
In addition to the implementation of the individual FAB measures outlined above, it is important to consider the wider impact of semi-natural habitats across the landscape [1]. Increasing the amount of semi-natural habitats may involve taking land out of production. Although this may reduce direct yield, costs may be offset by the additional services provided. Semi-natural habitats can increase yield by providing habitats for pest predators and pollinators, suppressing pest species and improving soil quality and nutrient cycling [120,147]. Semi-natural habitats can also reduce soil loss from fields through intercepting water-borne sediment and reducing surface flow rate, acting as buffer strips that significantly decrease pollution run-off [91,103,122]. There is a lot of evidence demonstrating positive relationships between the distance from semi-natural habitats and the abundance of pollinators and crops [148,149,150,151].
The extent and quality of semi-natural habitats influence landscape complexity and habitat heterogeneity; they are generally believed to be correlated with increased species diversity and larger species pools (as providers of source material for re-colonisation) through the provision of more niche space, increasing the variability of microclimatic conditions, providing habitats for shelter, food, predator escape and breeding [152,153,154,155,156] and increasing the ease with which species can move through the landscape and achieve viable meta-populations [157].
A number of studies have looked at whether simple, intermediate or complex landscapes will be the most effective at improving biodiversity-related measures. Some believe that species richness is greatest at intermediate landscape complexity [125,158], whilst others think that measures will be more effective in simple landscapes [14]; however, there is not yet a consensus and evidence suggests that impacts are context- and taxa-specific [14,157,158]. Comparative studies of biodiversity on organic and non-organic farms showed taxa-specific responses (highly positive for plants and more variable but generally positive for invertebrates, bats and birds) to organic practices which were in part related to greater field- and farm-level complexity on organic farms [159]. It has also been hypothesised that no relationship between organic farming and increased bird diversity could be related to the pre-existing large spatial scale of pesticide application across the landscape [140]. Such studies indicate the importance of considering the context, extent and configuration of semi-natural habitats when assessing the effects of introducing agri-environment measures.
4. Discussion
4.1. Impact of FAB on Nature, Resource Use and Yield
There is an urgent need for increased resource efficiency in farming systems to make the transition to more circular agro-ecosystems, which depend less on external inputs and conserve natural resources (soil, water, biodiversity), especially in the context of climate change. FAB refers to the application of farm management practices that enhance and exploit elements of biodiversity for their role in providing ecosystem services (e.g., pollination, biological pest control, soil erosion control, water retention) and ecosystem functions, and in supporting sustainable agricultural production and human well-being [160,161]. In this review, we found that the outcomes of implementing FAB measures were largely positive, with a number of mixed effects. Positive outcomes include improvements in above- and below-ground biodiversity (e.g., [43,47,58,63,64,88,89,127]), improvements in soil structure [57], and the diversity of root architecture that can reduce soil compaction [48,49], improve water conservation and reduce risks of flooding [46]. Many of the measures lead to reductions in fertiliser application [60], which has direct impacts, e.g., on water quality, and indirect impacts, e.g., reductions in GHG from their production. Many of the measures that improve soil erosion also positively benefit water quality. Mixed effects include impacts on GHG emissions, with reductions in some GHGs offset by increased N2O emissions [39,40,52,53,77] or mixed impacts on soil organic carbon (SOC) [25,26]. There are also large uncertainties in some areas (Figure 1), particularly surrounding the impact of FAB measures on yield, with a range of both positive and negative results being reported. This is predominantly due to the metrics, location and timeframe reviewed in each study and complex interactions between management and context. Some effects on yield could be short-term, and the longer-term benefits of a more sustainable system may exceed yield loss with time [35,103,107,119,120,136]. There may be issues with the methods used to calculate yield, e.g., not using a systems approach to calculate productivity in agroforestry [15]. Evidence is also currently limited in other areas (Figure 1); for example, organic matter input and reduced tillage are often implemented to enhance soil quality [35], yet effects on biodiversity are less well studied. There may be negative effects on biodiversity from fertilisation [24], regardless of the source (i.e., organic vs. non-organic), and mixed effects on soil microbial diversity from biochar and biosolids [83].
The review highlighted that there are some important trade-offs to be considered when implementing FAB measures [162]. For example, potential increases in pesticide use with conservation tillage could lead to reduced water quality [36,37]. Another example is the decline in water quality with the addition of organic matter [73]. These trade-offs need to be considered in each region for their broader implications, or offset with the use of other FAB measures; in the first example, above, the integration of conservation tillage with mixed and cover crops could offset the requirement for increased pesticide use. There are multiple potential synergies between measures (Supplementary Table S2) when implementing several different interventions as a package at farm or field level—the effect of the whole may be greater than the sum of the parts. Spatial configuration and landscape context are important considerations when locating these measures [14,87,125,144,158].
4.2. Knowledge Gaps, Future Possibilities and Limitations of FAB
Although the possibilities of FAB measures are extensive and many of the core evidence gaps are closing, the impact size, timescale and socio-economic barriers that exist before broad-scale uptake could occur are still significant and need further investigation and testing [7].
The evidence of the magnitude of impact that many of the measures can have upon yield, soil health and water quality over a broad spectrum of space and time is often still lacking (Figure 1). Quantitative evidence for how much of a given intervention is needed to deliver a given benefit is lacking and is also likely to be context-specific [24]. There are knowledge gaps on biodiversity, and studies often focus on selected taxa (e.g., [63,64,119]), perhaps those more easily studied. Often, when an intervention has been implemented for its effect on a different Ecosystem Service, biodiversity impacts may not be as well documented. Cases of success, in particular in farming environments, provide hope for the circular system applicability, yet the evidence in some instances produces conflicting results, possibly due to variance in FAB intervention suitability to an environment (e.g., soil type) or even differences in FAB application or impact monitoring and assessment. It could be that the agricultural matrix is already so degraded (e.g., species pools, soil quality) that it does not have the capacity to recover in the short term or without remedial action [163]. Furthermore, the potential for publication bias in the literature, which tends to report mostly successful trials rather than the negligible or negative results that often never make it to publication, must be noted [15,21,28,95]. This may have an impact when meta-analysis is used to develop the evidence base.
A key step in the successful implementation of FAB measures is the understanding that one intervention alone will not solve all challenges faced, yet a combination of measures implemented in a strategic way can enhance the output success, especially when adopted on a whole farm basis (Table S2). When implementing several different interventions as a package at farm or field level, the effect of the whole is greater than the sum of the parts [164].
Understanding past land management practices and having access to expertise for measuring impacts is vital to the successful application and effective management of the co-benefits and trade-offs at farm and regional scales (e.g., [22,24,35,41,74,108,119]).
However, beyond the closing of evidence gaps, FAB intervention uptake will continue to be limited unless social and economic barriers are removed [7,49]. For many of the FAB measures investigated here, the financial implications of uptake limit the viability of such options, with the business case to invest in long-term nature-based measures currently not adequately supported. Farmers are often proud of their yields, but it is important to convince farmers to look at profit as the difference between income and expenses. Not only yield is taken in consideration in the economic balance, but also the reduction of costs, like expenses on PPPs, chemical fertilisers and fuel.
Furthermore, education and an improved understanding of the benefits changes in the agricultural system could have at a farm and regional scale is lacking in most cases, limited to small networks rather than incentivised at regional or national governmental level. These and other barriers to implementing FAB measures must be addressed by future policy and transdisciplinary research programs, for which FABulous Farmers is an example [12], and those that will be developed in the future under the European Green Deal.
5. Conclusions
This review has drawn together evidence on ten popular FAB measures. The review highlights current information and knowledge gaps towards effecting an increased uptake and implementation of FAB measures. The results indicate that the implementation of FAB measures as part of a shift from linear systems focused on inputs and intensification towards more circular systems which work with natural processes to enhance the farming system for future environmental and business sustainability is likely to be beneficial. There is evidence that FAB interventions can decrease fertiliser use (by improving soil quality and reducing soil erosion), decrease pesticide use (increasing beneficial pest predators and disrupting pest and disease cycles), improve above- and below-ground biodiversity, improve soil quality (improving soil structure, preventing erosion) and improve water quality and conservation. However, evidence is also conflicting for some of the measures, for instance on yield and on impacts on soil organic carbon and GHGs, perhaps in part due to a lack of evidence at the requisite spatial and temporal scale. The review also demonstrated that there is a lot of complexity and detail to be considered, there are synergistic interactions and trade-offs in implementing FAB measures, and success may be influenced by landscape context. Evaluations of the FAB measures are generally limited to approaches which evaluate the measures from singular perspectives, and therefore a need for more holistic approaches to evaluating the measures (both individually and in combination) from social, economic and environmental perspectives and in the different contexts to which they may be applied are required to evaluate their potential contribution towards the development of more sustainable agricultural systems. The FABulous Farmers project, of which this review is a part, seeks to carry out such an evaluation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/land12112078/s1, Table S1: Selected FAB solutions and their contribution to ecosystem service provision and farm management (text to accompany arrows shown in Figure 2). Table S2: Interactions between FAB measures. References cited [165,166,167,168,169,170,171,172,173].
Author Contributions
L.C.M.: Investigation, Writing—original draft, Writing—review and editing. A.R.: Visualisation, Writing—original draft, Writing—review and editing. D.A.R.: Project administration, Supervision, Writing—original draft, Writing—review and editing. S.R.: Investigation, Writing—original draft. J.A.: Investigation, Writing—review and editing. L.R.N.: Writing—review and editing. L.B.: Writing—review and editing. K.G.: Project administration, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the European Union’s Interreg North-West Europe programme, part of the European Territorial Cooperation Programme, and by ERDF funding. The work was supported by grant agreement No. NWE 810, project FABulous Farmers (Functional Agro-Biodiversity in farming). In addition, UKCEH staff were supported by the Natural Environment Research Council award number NE/R016429/1 as part of the UK-SCaPE programme delivering National Capability.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
We would like to thank the Welsh Government and the Environment and Rural Affairs Monitoring and Modelling Programme (ERAMMP) (Welsh Government Contract C210/2016/2017) and the ERAMMP team. Whilst not directly funding this work, we did benefit from complementary activities reviewing evidence for agri-environment measures (https://erammp.wales/en/r-sfs-evidence-pack).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Table A1.
Definitions of FAB measures.
| FAB Measure | Description |
| Conservation tillage and no-tillage | Conventional tillage uses multiple trips across a field to invert the soil to quite a deep depth. Reduced tillage (RT), a key component of conservation tillage, replaces heavy and deep ploughing with a lighter tillage implement that disturbs less of the surface crop residue, often in a single pass. There are different reduced tillage options, e.g., reduction of tillage in rotation, reduction in tillage depth whilst maintaining inversion tillage and non-inversion tillage at shallow soil [35]. In no-till (NT), a self-contained planting unit is used to plant the crop in a single pass with no seedbed preparation [174] and may be referred to as direct drilling or seeding [175]. Conservation tillage and no-tillage are targeted at improvements in soil quality. |
| Mixed crops or crop rotations, including sward diversity | Rather than agricultural monoculture systems, crop rotation includes the use of multiple crop species and varieties in a rotation, including the integration of short-term grass or other non-woody perennial leys into previously arable-only rotations, and alternating spring and winter crop use to manage weeds. For crop rotation, the variation in crops happens in time. For mixed crops, the variation in crops happens spatially. Diverse species are in the field at the same time. This can be as a very close clustering of the species (like in herbal leys, but also in the mixture of crops such as maize and legumes) or by the creation of crop mosaics. Improving sward diversity can be conducted through the addition of grass, forb and legume species, normally carried out through reseeding, over-sowing or slot seeding, but may also include the introduction of plug plants or feeding animals with high-quality hay containing seeds (from nearby sites). |
| Cover or catch-crops | Cover crops are fast-maturing crops grown within a system to maintain soil cover during fallow periods, and are typically ploughed under as green manure, or killed with herbicides under no-till systems. Cover crops sequester C below ground through increased primary productivity and maintenance of organic input throughout the rotation. This option also includes the use of legumes (i.e., peas, beans or clover) in arable rotations. The legumes could be introduced to break a long arable run, or grown with the arable crop (intercropped or inter-sown). |
| Modified manure management, quality and diversity | Organic fertiliser (manure) is mainly derived from cattle, pig and poultry farming, with liquid manure (slurry) having a lower dry matter content than solid manure. In general, slurry has lower concentrations (g kg−1 fresh weight) of C and N than solid manure, but pH stays largely unaffected. Poultry manure (solid or liquid) generally has higher C and N concentrations than cattle and pig manure. By combining a diversity of manure types, manure can be better adjusted to the needs of the crop. This measure is targeted at improvements in soil quality, SOC. |
| Organic matter input | Organic inputs, including compost, woodchips, farmyard manure, biosolids (recycled from sewage) and incorporation of crop residues. Biochar is another form of organic material that can be added to soil. Biochars are obtained through the thermal treatment of organic material in low oxygen conditions [79] and can be a side-product of liquid biofuel production. This measure is targeted at improvements in soil quality, SOC. |
| Agroforestry | Agroforestry is the practice of deliberately integrating woody vegetation (trees or shrubs) into crop and/or animal production systems to benefit from the resulting ecological and economic interactions. The diversity of practices behind the term agroforestry is vast, ranging from the installation of shelter belts [86] to alley cropping forest farming and many variations in between. |
| Hedgerow management | Hedgerows are a component of agroforestry. They are linear features over 20 m long and <5 m wide within farmed landscapes that incorporate a shrub component, hedgerow trees (where present) and associated ground flora. A hedgerow may also encompass not just the lines of trees or shrubs, but the base of the hedge, which may be an earth bank, an associated ditch and permanent herbaceous margins where the management is influenced by the presence of shrubs or trees [108]. Hedgerows need to be kept in a management cycle to maintain condition and provide ecosystem services. Management options could include “gapping up”, rejuvenation through hedge laying or coppicing and improvement of the ground flora. |
| Field margin management | Field margins can be managed to help protect hedgerow flora and fauna and to benefit biodiversity, pollination and pest control, by taking the often less-productive field edges out of arable crop production [119]. There are different types of field margin management that could be practiced, including conservation headlands, beetle banks, uncultivated margins, perennial grass strips or floral strips [125,126,128]. |
| Reduction in the use of plant protection products (PPPs) | Plant protection products (PPPs) are products that protect plants or plant products from harmful organisms. They include herbicides, fungicides and insecticides. Organic agriculture is the ultimate end point of reduced PPPs, being defined as farming systems where the use of pesticides, herbicides and chemical fertilisers is prohibited [137], but reduction should be considered along a gradient. |
| Semi-natural landscape elements | Many of the above sections include the use of selected semi-natural habitats in an agricultural landscape (e.g., agroforestry, hedgerows and field margins). However, there are other semi-natural features that have not been considered, such as ponds, ditches or fallow land, and semi-natural habitats should also be considered in their entirety as contributors to habitat heterogeneity and landscape complexity (potentially increasing species richness) [1,158], connectivity (the characteristics of the landscape that affect the movement of organisms), ecosystem functioning and resilience and species pools to enable the successful implementation of options. |
Notes
| 1 | https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 1 September 2020). |
| 2 | https://www.eca.europa.eu/Lists/ECADocuments/SR14_04/SR14_04_EN.pdf (accessed on 1 September 2020). |
References
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Firbank, L.B.; McCracken, D.; Stoate, C.; Goulding, K.; Harmer, R.; Hess, T.; Jenkins, A.; Pilgrim, E.; Potts, S.; Smoith, P.; et al. (Eds.) Chapter 7: Enclosed Farmland; UNEP-WCMC: Cambridge, UK, 2011. [Google Scholar]
- Strohbach, M.W.; Kohler, M.L.; Dauber, J.; Klimek, S. High Nature Value farming: From indication to conservation. Ecol. Indic. 2015, 57, 557–563. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Ten Key Messages. 2022. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd_2022_wpp_key-messages.pdf (accessed on 1 September 2020).
- MEA. Millenium Ecosystem Assessment Ecosystems and Human Well-Being World Resources Institute, Washington, DC. 2005. Available online: https://www.millenniumassessment.org/en/index.html (accessed on 1 September 2020).
- ELN-FAB 2012; Functional Agrobiodiversity: Nature Serving Europe’s Farmers. ECNC-European Centre for Nature Conservation: Tilburg, The Netherlands, 2012.
- Bianchi, F.; Mikos, V.; Brussaard, L.; Delbaere, B.; Pulleman, M. Opportunities and limitations for functional agrobiodiversity in the European context. Environ. Sci. Policy 2013, 27, 223–231. [Google Scholar] [CrossRef]
- Berkes, F. Rethinking Community-Based Conservation. Conserv. Biol. 2004, 18, 621–630. [Google Scholar] [CrossRef]
- Kleijn, D.; Rundlöf, M.; Scheper, J.; Smith, H.G.; Tscharntke, T. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 2011, 26, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Graves, A.; Burgess, P.; Palma, J.; Herzog, F.; Moreno, G.; Bertomeu, M.; Dupraz, C.; Liagre, F.; Keesman, K.; van der Werf, W.; et al. Development and application of bio-economic modelling to compare silvoarable, arable, and forestry systems in three European countries. Ecol. Eng. 2007, 29, 434–449. [Google Scholar] [CrossRef]
- Swift, M.; Izac, A.-M.; van Noordwijk, M. Biodiversity and ecosystem services in agricultural landscapes—Are we asking the right questions? Agric. Ecosyst. Environ. 2004, 104, 113–134. [Google Scholar] [CrossRef]
- INTERREG. FABulous Farmers. 2020. Available online: https://www.nweurope.eu/projects/project-search/fabulous-farmers/ (accessed on 17 September 2020).
- Maskell, L.; Norton, L.; Alison, J.; Reinsch, S.; Robinson, D. Review of Current Methods and Approaches for Simple on Farm Environmental Monitoring of FAB Solutions Report for EU INTERREG FABulous Farmers NEC06872. 2020. Available online: https://www.nweurope.eu/media/12309/wpt1-2-fabfarmers-intervention-review.pdf (accessed on 1 January 2020).
- Batáry, P.; Báldi, A.; Kleijn, D.; Tscharntke, T. Landscape-moderated biodiversity effects of agri-environmental management: A meta-analysis. Proc. Biol. Sci. 2011, 278, 1894–1902. [Google Scholar] [CrossRef]
- Torralba, M.; Fagerholm, N.; Burgess, P.J.; Moreno, G.; Plieninger, T. Do European agroforestry systems enhance biodiversity and ecosystem services? A meta-analysis. Agric. Ecosyst. Environ. 2016, 230, 150–161. [Google Scholar] [CrossRef]
- ERAMMP Evidence Pack. Available online: https://erammp.wales/en/r-sfs-evidence-pack (accessed on 1 September 2019).
- AGFORWARD Project. Available online: https://www.agforward.eu/ (accessed on 1 October 2022).
- Reicosky, D.C. Conservation tillage is not conservation agriculture. J. Soil Water Conserv. 2015, 70, 103A–108A. [Google Scholar] [CrossRef]
- Govaerts, B.; Verhulst, N.; Castellanos-Navarrete, A.; Sayre, K.D.; Dixon, J.; Dendooven, L. Conservation Agriculture and Soil Carbon Sequestration: Between Myth and Farmer Reality. Crit. Rev. Plant Sci. 2009, 28, 97–122. [Google Scholar] [CrossRef]
- Buckingham, S.; Rees, R.M.; Watson, C.A. Issues and pressures facing the future of soil carbon stocks with particular emphasis on Scottish soils. J. Agric. Sci. 2014, 152, 699–715. [Google Scholar] [CrossRef]
- Van den Putte, A.; Govers, G.; Diels, J.; Gillijns, K.; Demuzere, M. Assessing the effect of soil tillage on crop growth: A meta-regression analysis on European crop yields under conservation agriculture. Eur. J. Agron. 2010, 33, 231–241. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Barbercheck, M.E.; Murrell, E.G.; Harper, J.; Finney, D.M.; Kaye, J.P.; Mortensen, D.A.; Smith, R.G. Balancing multiple objectives in organic feed and forage cropping systems. Agric. Ecosyst. Environ. 2017, 239, 219–227. [Google Scholar] [CrossRef]
- Roos, D.; Saldaña, C.C.; Arroyo, B.; Mougeot, F.; Luque-Larena, J.J.; Lambin, X. Unintentional effects of environmentally-friendly farming practices: Arising conflicts between zero-tillage and a crop pest, the common vole (Microtus arvalis). Agric. Ecosyst. Environ. 2019, 272, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Keenleyside, C.B.; Beaufoy, G.; Alison, J.; Gunn, I.D.M.; Healey, J.; Jenkins, T.; Pagella, T.; Siriwardena, G.M. Technical Annex 4: Building Ecosystem Resilience. In Environment and Rural Affairs Monitoring & Modelling Programme (ERAMMP): Sustainable Farming Scheme Evidence Review; Report to Welsh Government (Contract C210/2016/2017): UK Centre for Ecology & Hydrology Project NEC06297; UK Centre for Ecology & Hydrology: Bailrigg, UK, 2019; Available online: https://erammp.wales/sites/default/files/2023-09/04-ERAMMP-SFS-Evidence-Review-4-Ecosystem-resilience-v1.1.pdf (accessed on 1 January 2020).
- Manley, J.; van Kooten, G.C.; Moeltner, K.; Johnson, D.W. Creating Carbon Offsets in Agriculture through No-Till Cultivation: A Meta-Analysis of Costs and Carbon Benefits. Clim. Chang. 2005, 68, 41–65. [Google Scholar] [CrossRef]
- Powlson, D.S.; Stirling, C.M.; Jat, M.L.; Gerard, B.G.; Palm, C.A.; Sanchez, P.A.; Cassman, K.G. Limited potential of no-till agriculture for climate change mitigation. Nat. Clim. Chang. 2014, 4, 678–683. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Y.; Ren, W.; Coyne, M.; Jacinthe, P.-A.; Tao, B.; Hui, D.; Yang, J.; Matocha, C. Responses of soil carbon sequestration to climate-smart agriculture practices: A meta-analysis. Glob. Chang. Biol. 2019, 25, 2591–2606. [Google Scholar] [CrossRef]
- Angers, D.A.; Eriksen-Hamel, N.S. Full-Inversion Tillage and Organic Carbon Distribution in Soil Profiles: A Meta-Analysis. Soil Sci. Soc. Am. J. 2008, 72, 1370–1374. [Google Scholar] [CrossRef]
- Dimassi, B.; Mary, B.; Wylleman, R.; Labreuche, J.; Couture, D.; Piraux, F.; Cohan, J.-P. Long-term effect of contrasted tillage and crop management on soil carbon dynamics during 41 years. Agric. Ecosyst. Environ. 2014, 188, 134–146. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Sun, O.J. Can no-tillage stimulate carbon sequestration in agricultural soils? A meta-analysis of paired experiments. Agric. Ecosyst. Environ. 2010, 139, 224–231. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Hedlund, K.; Jackson, L.E.; Kätterer, T.; Lugato, E.; Thomsen, I.K.; Jørgensen, H.B.; Isberg, P.-E. How does tillage intensity affect soil organic carbon? A systematic review. Environ. Evid. 2017, 6, 30. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; van Groenigen, K.J.; Lee, J.; Lundy, M.E.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. Productivity limits and potentials of the principles of conservation agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef]
- Zikeli, S.; Gruber, S.; Teufel, C.-F.; Hartung, K.; Claupein, W. Effects of Reduced Tillage on Crop Yield, Plant Available Nutrients and Soil Organic Matter in a 12-Year Long-Term Trial under Organic Management. Sustainability 2013, 5, 3876–3894. [Google Scholar] [CrossRef]
- Carmona, I.; Griffith, D.M.; Soriano, M.-A.; Murillo, J.M.; Madejón, E.; Gómez-Macpherson, H. What do farmers mean when they say they practice conservation agriculture? A comprehensive case study from southern Spain. Agric. Ecosyst. Environ. 2015, 213, 164–177. [Google Scholar] [CrossRef]
- Armengot, L.; Blanco-Moreno, J.; Bàrberi, P.; Bocci, G.; Carlesi, S.; Aendekerk, R.; Berner, A.; Celette, F.; Grosse, M.; Huiting, H.; et al. Tillage as a driver of change in weed communities: A functional perspective. Agric. Ecosyst. Environ. 2016, 222, 276–285. [Google Scholar] [CrossRef]
- Peigné, J.; Ball, B.C.; Roger-Estrade, J.; David, C. Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 2007, 23, 129–144. [Google Scholar] [CrossRef]
- Freibauer, A.; Rounsevell, M.D.; Smith, P.; Verhagen, J. Carbon sequestration in the agricultural soils of Europe. Geoderma 2004, 122, 1–23. [Google Scholar] [CrossRef]
- Rochette, P. No-till only increases N2O emissions in poorly-aerated soils. Soil Tillage Res. 2008, 101, 97–100. [Google Scholar] [CrossRef]
- Weigelt, A.; Weisser, W.W.; Buchmann, N.; Scherer-Lorenzen, M. Biodiversity for multifunctional grasslands: Equal productivity in high-diversity low-input and low-diversity high-input systems. Biogeosciences 2009, 6, 1695–1706. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.-J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Scherber, C.; Eisenhauer, N.; Weisser, W.W.; Schmid, B.; Voigt, W.; Fischer, M.; Schulze, E.-D.; Roscher, C.; Weigelt, A.; Allan, E.; et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 2010, 468, 553–556. [Google Scholar] [CrossRef]
- Newell Price, J.P.; Siriwardena, G.M.; Williams, A.P.; Alison, J.; Williams, J.R. Technical Annex 2: Sward Management. Environment and Rural Affairs Monitoring & Modelling Programme (ERAMMP): Sustainable Farming Scheme Evidence Review; Report to Welsh Government (Contract C210/2016/2017). UK Centre for Ecology & Hydrology Project NEC06297; UK Centre for Ecology & Hydrology: Bailrigg, UK, 2019; Available online: https://erammp.wales/sites/default/files/2023-08/02-ERAMMP-SFS-Evidence-Review-2-Sward-v1.1.pdf (accessed on 1 January 2020).
- Roca-Fernández, A.I.; Peyraud, J.L.; Delaby, L.; Delagarde, R. Pasture intake and milk production of dairy cows rotationally grazing on multi-species swards. Animal 2016, 10, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Weisser, W.W.; Roscher, C.; Meyer, S.T.; Ebeling, A.; Luo, G.; Allan, E.; Beßler, H.; Barnard, R.L.; Buchmann, N.; Buscot, F.; et al. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic Appl. Ecol. 2017, 23, 1–73. [Google Scholar] [CrossRef]
- Alison, J.; Duffield, S.J.; Morecroft, M.D.; Marrs, R.H.; Hodgson, J.A. Successful restoration of moth abundance and species-richness in grassland created under agri-environment schemes. Biol. Conserv. 2017, 213, 51–58. [Google Scholar] [CrossRef]
- Carvell, C.; Meek, W.R.; Pywell, R.F.; Goulson, D.; Nowakowski, M. Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. J. Appl. Ecol. 2006, 44, 29–40. [Google Scholar] [CrossRef]
- DEFRA. Reviewing the Opportunities, Barriers and Constraints for Organic Management Techniques to Improve Sustainability of Conventional Farming—Final Project Report. Prepared as Part of Defra Project OF03111. 2018. Available online: https://agricology.co.uk/research-projects/organic-management-techniques-project/ (accessed on 1 January 2020).
- Fornara, D.A.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Hungate, B.A.; Barbier, E.B.; Ando, A.W.; Marks, S.P.; Reich, P.B.; van Gestel, N.; Tilman, D.; Knops, J.M.H.; Hooper, D.U.; Butterfield, B.J.; et al. The economic value of grassland species for carbon storage. Sci. Adv. 2017, 3, e1601880. [Google Scholar] [CrossRef]
- Henderson, B.B.; Gerber, P.J.; Hilinski, T.E.; Falcucci, A.; Ojima, D.S.; Salvatore, M.; Conant, R.T. Greenhouse gas mitigation potential of the world’s grazing lands: Modeling soil carbon and nitrogen fluxes of mitigation practices. Agric. Ecosyst. Environ. 2015, 207, 91–100. [Google Scholar] [CrossRef]
- Garnett, T.; Godde, C.; Muller, A.; Röös, E.; Smith, P.; de Boer, I.J.M.; zu Ermgassen, E.; Herrero, M.; van Middelaar, C.; Schader, C.; et al. Grazed and Confused? Ruminating on Cattle, Grazing Systems, Methane, Nitrous Oxide, the Soil Carbon Sequestration Question—And What It All Means for Greenhouse Gas Emissions. FCRN, University of Oxford. 2017. Available online: https://edepot.wur.nl/427016 (accessed on 1 January 2020).
- Desjardins, R.L.; Smith, W.; Grant, B.; Campbell, C.; Riznek, R. Management Strategies to Sequester Carbon in Agricultural Soils and to Mitigate Greenhouse Gas Emissions. Clim. Chang. 2005, 70, 283–297. [Google Scholar] [CrossRef]
- Tonitto, C.; David, M.B.; Drinkwater, L.E. Replacing bare fallows with cover crops in fertilizer-intensive cropping systems: A meta-analysis of crop yield and N dynamics. Agric. Ecosyst. Environ. 2006, 112, 58–72. [Google Scholar] [CrossRef]
- Sun, Y.; Zeng, Y.; Shi, Q.; Pan, X.; Huang, S. No-tillage controls on runoff: A meta-analysis. Soil Tillage Res. 2015, 153, 1–6. [Google Scholar] [CrossRef]
- Scopel, E.; Triomphe, B.; Affholder, F.; Da Silva, F.A.M.; Corbeels, M.; Xavier, J.H.V.; Lahmar, R.; Recous, S.; Bernoux, M.; Blanchart, E.; et al. Conservation agriculture cropping systems in temperate and tropical conditions, performances and impacts. A review. Agron. Sustain. Dev. 2013, 33, 113–130. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover Crops and Ecosystem Services: Insights from Studies in Temperate Soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden miners—The roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 2019, 434, 7–45. [Google Scholar] [CrossRef]
- Eory, V.; MacLeod, M.; Topp, C.F.E.; Rees, R.M.; Webb, J.; McVittie, A.; Wall, E.; Borthwick, F.; Watson, C.; Waterhouse, A.; et al. Review and Update the UK Agriculture Marginal Abatement Cost Curve to Assess the Greenhouse Gas Abatement Potential for the 5th Carbon Budget Period and to 2050. Report for the Committee on Climate Change. 2015. Available online: https://www.theccc.org.uk/wp-content/uploads/2015/11/Scotland%E2%80%99s-Rural-Collage-SRUC-Ricardo-Energy-and-Environment-2015-Review-and-update-of-the-UK-agriculture-MACC-to-assess-abatement-potential-for-the-fifth-carbon-budget-period-and-to-2050.pdf (accessed on 1 January 2020).
- Dorn, B.; Jossi, W.; van der Heijden, M.G.A. Weed suppression by cover crops: Comparative on-farm experiments under integrated and organic conservation tillage. Weed Res. 2015, 55, 586–597. [Google Scholar] [CrossRef]
- Carr, P.M. Guest Editorial: Conservation Tillage for Organic Farming. Agriculture 2017, 7, 19. [Google Scholar] [CrossRef]
- Holland, J.; Luff, M. The Effects of Agricultural Practices on Carabidae in Temperate Agroecosystems. Integr. Pest Manag. Rev. 2000, 5, 109–129. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Fergen, J.K. The Effects of a Winter Cover Crop on Diabrotica virgifera (Coleoptera: Chrysome-lidae) Populations and Beneficial Arthropod Communities in No-Till Maize. Environ. Entomol. 2010, 39, 1816–1828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mallinger, R.E.; Franco, J.G.; Prischmann-Voldseth, D.A.; Prasifka, J.R. Annual cover crops for managed and wild bees: Optimal plant mixtures depend on pollinator enhancement goals. Agric. Ecosyst. Environ. 2018, 273, 107–116. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Lugato, E.; Leip, A.; Jones, A. Mitigation potential of soil carbon management overestimated by neglecting N2O emissions. Nat. Clim. Chang. 2018, 8, 219–223. [Google Scholar] [CrossRef]
- Basche, A.D.; Miguez, F.E.; Kaspar, T.C.; Castellano, M.J. Do cover crops increase or decrease nitrous oxide emissions? A meta-analysis. J. Soil Water Conserv. 2014, 69, 471–482. [Google Scholar] [CrossRef]
- Kallenbach, C.; Grandy, A.S. Controls over soil microbial biomass responses to carbon amendments in agri-cultural systems: A meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 241–252. [Google Scholar] [CrossRef]
- Jones, S.K.; Rees, R.M.; Kosmas, D.; Ball, B.C.; Skiba, U.M. Carbon sequestration in a temperate grassland; management and climatic controls. Soil Use Manag. 2006, 22, 132–142. [Google Scholar] [CrossRef]
- Smith, L.P.S.; Pearce, B. Soil Carbon Sequestration and Organic Farming: An Overview of Current Evidence; Organic Centre Wales: Aberystwyth, UK, 2011. [Google Scholar]
- Misselbrook, T.H.; Smith, K.A.; Johnson, R.A.; Pain, B.F. SE—Structures and Environment: Slurry Application Techniques to reduce Ammonia Emissions: Results of some UK Field-scale Experiments. Biosyst. Eng. 2002, 81, 313–321. [Google Scholar] [CrossRef]
- Moxley, J.; Anthony, S.; Begum, K.; Bhogal, A.; Buckingham, S.; Christie, P.; Datta, A.; Dragosits, U.; Fitton, N.; Higgins, A.; et al. Capturing Cropland and Grassland Management Impacts on Soil Carbon in the UK LULUCF Inventory; CEH Project no. C04909, Defra Project no. SP1113; Defra: London, UK, 2014; 90p.
- Nicholson, F.; Taylor, M.; Bhogal, A.; Rollett, A.; Williams, J.R.; Newell Price, P.; Chambers, B.; Becvar, A.; Wood, M.; Litterick, A.; et al. Field Experiments for Quality Digestate and Compost in Agriculture: Work Package 2 Report—Digestate Nitrogen Supply and Environmental Emissions; Report number: OMK001-001/WR1212; ADAS: Surrey, UK, 2016. [Google Scholar]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Powlson, D.; Bhogal, A.; Chambers, B.; Coleman, K.; Macdonald, A.; Goulding, K.; Whitmore, A. The potential to increase soil carbon stocks through reduced tillage or organic material additions in England and Wales: A case study. Agric. Ecosyst. Environ. 2012, 146, 23–33. [Google Scholar] [CrossRef]
- Powlson, D.S.; Whitmore, A.P.; Goulding, K.W.T. Soil carbon sequestration to mitigate climate change: A critical re-examination to identify the true and the false. Eur. J. Soil Sci. 2011, 62, 42–55. [Google Scholar] [CrossRef]
- Al-Wabel, M.; Hussain, Q.; Usman, A.; Ahmad, M.; Abduljabbar, A.; Sallam, A.; Abdulazeem, S.; Ok, Y.S. 2017. Impact of Biochar Characteristics on Soil Conditions and Agricultural Sustainability: A Review. Land Degrad. Dev. 2017, 29, 2124–2161. [Google Scholar]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Virto, I.; Barré, P.; Burlot, A.; Chenu, C. Carbon input differences as the main factor explaining the variability in soil organic C storage in no-tilled compared to inversion tilled agrosystems. Biogeochemistry 2011, 108, 17–26. [Google Scholar] [CrossRef]
- Pascault, N.; Ranjard, L.; Kaisermann, A.; Bachar, D.; Christen, R.; Terrat, S.; Mathieu, O.; Lévêque, J.; Mougel, C.; Henault, C.; et al. Stimulation of Different Functional Groups of Bacteria by Various Plant Residues as a Driver of Soil Priming Effect. Ecosystems 2013, 16, 810–822. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Burgess, P.J.; Crous-Duran, J.; Den Herder, M.; Dupraz, C.; Fagerholm, N.; Freese, D.; Garnett, K.; Graves, A.R.; Hermansen, J.E.; Liagre, F.; et al. AGFORWARD Project Periodic Report: January to December 2014; AGFORWARD, Ed.; Cranfield University: Silsoe, UK, 2015. [Google Scholar]
- Nair, P.R. The coming of age of agroforestry. J. Sci. Food Agric. 2007, 87, 1613–1619. [Google Scholar] [CrossRef]
- Söderström, B.; Svensson, B.; Vessby, K.; Glimskär, A. Plants, insects and birds in semi-natural pastures in relation to local habitat and landscape factors. Biodivers. Conserv. 2001, 10, 1839–1863. [Google Scholar] [CrossRef]
- Hermansen, J.E.; Novak, S.; Smith, J.; Bondesan, V.; Bestman, M.; Kongsted, A.G.; Mosquera Losada, M.R.; Ferreiro-Domingues, N. Agroforestry for Livestock Farmers: Dissemination of Results and Recommendations; Milestone 25 for EU FP7 Research Project. 2018. Available online: https://www.agforward.eu/documents/MS25%20Dissemination%20for%20livestock%20farmers.pdf (accessed on 1 January 2020).
- Pumariño, L.; Sileshi, G.W.; Gripenberg, S.; Kaartinen, R.; Barrios, E.; Muchane, M.N.; Midega, C.; Jonsson, M. Effects of agroforestry on pest, disease and weed control: A meta-analysis. Basic Appl. Ecol. 2015, 16, 573–582. [Google Scholar] [CrossRef]
- Jäger, M. Lessons Learnt: Agroforestry-Systems with Fruit Trees in Switzerland. 2017. 10p. Available online: http://www.agforward.eu/index.php/en/integrating-trees-with-arable-crops-switzerland.html (accessed on 28 September 2020).
- Wharton, G.; Gilvear, D.J. River restoration in the UK: Meeting the dual needs of the European union water framework directive and flood defence? Int. J. River Basin Manag. 2007, 5, 143–154. [Google Scholar] [CrossRef]
- Stratford, C.; Miller, J.; House, A.; Old, G.; Acreman, M.; Duenas-Lopez, M.A.; Nisbet, T.; Burgess-Gamble, L.; Chappell, N.; Clarke, S.; et al. Do Trees in UK-Relevant River Catchments Influence Fluvial Flood Peaks?: A Systematic Review; CEH: Wallingford, UK, 2017; Available online: https://nora.nerc.ac.uk/id/eprint/517804/7/N517804CR.pdf (accessed on 1 January 2020).
- De Stefano, A.; Jacobson, M.G. Soil carbon sequestration in agroforestry systems: A meta-analysis. Agrofor. Syst. 2018, 92, 285–299. [Google Scholar] [CrossRef]
- Hübner, R.; Kühnel, A.; Lu, J.; Dettmann, H.; Wang, W.; Wiesmeier, M. Soil carbon sequestration by agroforestry systems in China: A meta-analysis. Agric. Ecosyst. Environ. 2021, 315, 107437. [Google Scholar] [CrossRef]
- Mayer, S.; Wiesmeier, M.; Sakamoto, E.; Hübner, R.; Cardinael, R.; Kühnel, A.; Kögel-Knabner, I. Soil organic carbon sequestration in temperate agroforestry systems—A meta-analysis. Agric. Ecosyst. Environ. 2022, 323, 107689. [Google Scholar] [CrossRef]
- Soussana, J.-F.; Loiseau, P.; Vuichard, N.; Ceschia, E.; Balesdent, J.; Chevallier, T.; Arrouays, D. Carbon cycling and sequestration opportunities in temperate grasslands. Soil Use Manag. 2004, 20, 219–230. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Vesterdal, L.; Leifeld, J.; VAN Wesemael, B.; Schumacher, J.; Gensior, A. Temporal dynamics of soil organic carbon after land-use change in the temperate zone—Carbon response functions as a model approach. Glob. Chang. Biol. 2011, 17, 2415–2427. [Google Scholar] [CrossRef]
- Cardinael, R.; Chevallier, T.; Barthès, B.G.; Saby, N.P.; Parent, T.; Dupraz, C.; Bernoux, M.; Chenu, C. Impact of alley cropping agroforestry on stocks, forms and spatial distribution of soil organic carbon—A case study in a Mediterranean context. Geoderma 2015, 259–260, 288–299. [Google Scholar] [CrossRef]
- Cardinael, R.; Chevallier, T.; Cambou, A.; Béral, C.; Barthès, B.G.; Dupraz, C.; Durand, C.; Kouakoua, E.; Chenu, C. Increased soil organic carbon stocks under agroforestry: A survey of six different sites in France. Agric. Ecosyst. Environ. 2017, 236, 243–255. [Google Scholar] [CrossRef]
- Baah-Acheamfour, M.; Chang, S.X.; Carlyle, C.N.; Bork, E.W. Carbon pool size and stability are affected by trees and grassland cover types within agroforestry systems of western Canada. Agric. Ecosyst. Environ. 2015, 213, 105–113. [Google Scholar] [CrossRef]
- Cardinael, R.; Umulisa, V.; Toudert, A.; Olivier, A.; Bockel, L.; Bernoux, M. Revisiting IPCC Tier 1 coefficients for soil organic and biomass carbon storage in agroforestry systems. Environ. Res. Lett. 2018, 13, 124020. [Google Scholar] [CrossRef]
- Vityi, A.; Kiss Szigeti, N.; Schettrer, P.; Marosvölgyi, B. Lessons Learnt: Alley Cropping in Hungary. 2017. 24p. Available online: http://agforward.eu/index.php/en/alley-cropping-systems-in-hungary.html (accessed on 28 September 2017).
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Gosme, M.; Desclaux, D. Lessons Learnt: Screening of Durum Wheat Cultivars for Agroforestry. 2017. Available online: https://www.agforward.eu/documents/LessonsLearnt/WP4_F_Screening_durum_wheat_lessons_learnt.pdf (accessed on 12 October 2017).
- Arenas-Corraliza, M.G.; López-Díaz, M.L.; Moreno, G. Lessons Learnt: Cereal Crops within Walnut Plantations in Mediterranean Spain. 2017. 26p. Available online: http://www.agforward.eu/index.php/en/silvoarable-systems-in-spain.htm (accessed on 12 October 2017).
- Kanzler, M.; Mirck, J. Lessons Learnt—Alley Cropping in Germany; 2017; 12p. Available online: http://agforward.eu/index.php/en/alley-cropping-systems-in-germany.html (accessed on 28 July 2017).
- Wolton, R.J.; Pollard, K.A.; Goodwin, A.; Norton, L. Regulatory Services Delivered by Hedges: The Evidence Base; Report of Defra project LM0106; Defra: London, UK, 2014.
- Maudsley, M.; Seeley, B.; Lewis, O. Spatial distribution patterns of predatory arthropods within an English hedgerow in early winter in relation to habitat variables. Agric. Ecosyst. Environ. 2002, 89, 77–89. [Google Scholar] [CrossRef]
- Wickramasinghe, L.P.; Harris, S.; Jones, G.; Vaughan, N. Bat activity and species richness on organic and conventional farms: Impact of agricultural intensification. J. Appl. Ecol. 2003, 40, 984–993. [Google Scholar] [CrossRef]
- Graham, L.; Gaulton, R.; Gerard, F.; Staley, J.T. The influence of hedgerow structural condition on wildlife habitat provision in farmed landscapes. Biol. Conserv. 2018, 220, 122–131. [Google Scholar] [CrossRef]
- de Snoo, G.R.; Scheidegger, N.M.I.; de Jong, F.M.W. Vertebrate wildlife incidents with pesticides: A european survey. Pestic. Sci. 1999, 55, 47–54. [Google Scholar] [CrossRef]
- Staley, J.T.; Botham, M.S.; Amy, S.R.; Hulmes, S.; Pywell, R.F. Experimental evidence for optimal hedgerow cutting regimes for Brown hairstreak butterflies. Insect Conserv. Divers. 2017, 11, 213–218. [Google Scholar] [CrossRef]
- Merot, P. The influence of hedgerow systems on the hydrology of agricultural catchments in a temperate climate. Agronomie 1999, 19, 655–669. [Google Scholar] [CrossRef]
- Borin, M.; Passoni, M.; Thiene, M.; Tempesta, T. Multiple functions of buffer strips in farming areas. Eur. J. Agron. 2010, 32, 103–111. [Google Scholar] [CrossRef]
- Caubel-Forget, V.; Grimaldi, C.; Rouault, F. Contrasted dynamics of nitrate and chloride in groundwater submitted to the influence of a hedge. C. R. Acad. Sci. Ser. IIA Earth Planet. Sci. 2001, 332, 107–113. [Google Scholar] [CrossRef]
- Ford, H.; Healey, J.R.; Webb, B.; Pagella, T.F.; Smith, A.R. How do hedgerows influence soil organic carbon stock in livestock-grazed pasture? Soil Use Manag. 2019, 35, 576–584. [Google Scholar] [CrossRef]
- Pointereau, P.; Colon-Solagro, F. Reflecting Environmental Land Use Needs into EU Policy: Preserving and Enhancing the Environmental Benefits of Unfarmed Features on EU Farmland; Case Study report France; Institute for European Environmental Policy IEEP: Stevenage, UK, 2008. [Google Scholar]
- Dickie, I.E.A. The Economic Case for Investment in Natural Capital in England. Final Report for the Natural Capital Committee; Land Use appendix; Defra: London, UK, 2015.
- Pywell, R.F.; Heard, M.S.; Woodcock, B.A.; Hinsley, S.; Ridding, L.; Nowakowski, M.; Bullock, J.M. Wildlife-friendly farming increases crop yield: Evidence for ecological intensification. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151740. [Google Scholar] [CrossRef] [PubMed]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlöf, M.; Smith, H.G.; Kleijn, D. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—A meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, J.; Berg, Å.; Hiron, M.; Pärt, T.; Eggers, S. Grass buffer strips benefit invertebrate and breeding skylark numbers in a heterogeneous agricultural landscape. Agric. Ecosyst. Environ. 2013, 181, 101–107. [Google Scholar] [CrossRef]
- Williams, N.M.; Ward, K.L.; Pope, N.; Isaacs, R.; Wilson, J.; May, E.A.; Ellis, J.; Daniels, J.; Pence, A.; Ullmann, K.; et al. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol. Appl. 2015, 25, 2119–2131. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat Management to Conserve Natural Enemies of Arthropod Pests in Agriculture. Annu. Rev. Èntomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Jonsson, M.; Straub, C.S.; Didham, R.K.; Buckley, H.L.; Case, B.S.; Hale, R.J.; Gratton, C.; Wratten, S.D. Experimental evidence that the effectiveness of conservation biological control depends on landscape complexity. J. Appl. Ecol. 2015, 52, 1274–1282. [Google Scholar] [CrossRef]
- Vickery, J.A.; Feber, R.E.; Fuller, R.J. Arable field margins managed for biodiversity conservation: A review of food resource provision for farmland birds. Agric. Ecosyst. Environ. 2009, 133, 1–13. [Google Scholar] [CrossRef]
- Wolton, R.J.; Morris RK, A.; Pollard, K.A.; Dover, J. Understanding the Combined Biodiversity Benefits of the Component Features of Hedges; Report of Defra project BD5214; Bright Angel Coastal Consultants Ltd.: Stamford, CT, USA, 2013. [Google Scholar]
- DEFRA. Comparison of New and Existing Agri-Environment Scheme Options for Biodiversity Enhancement on Arable Land (Project Number BD1624); CEH: Wallingford, UK, 2007.
- Marshall, E.; Moonen, A. Field margins in northern Europe: Their functions and interactions with agriculture. Agric. Ecosyst. Environ. 2002, 89, 5–21. [Google Scholar] [CrossRef]
- Carvell, C.; Bourke, A.F.; Osborne, J.L.; Heard, M.S. Effects of an agri-environment scheme on bumblebee reproduction at local and landscape scales. Basic Appl. Ecol. 2015, 16, 519–530. [Google Scholar] [CrossRef]
- Heard, M.; Carvell, C.; Carreck, N.; Rothery, P.; Osborne, J.; Bourke, A. Landscape context not patch size determines bumble-bee density on flower mixtures sown for agri-environment schemes. Biol. Lett. 2007, 3, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Alison, J.; Duffield, S.J.; Noordwijk, C.G.E.; Morecroft, M.D.; Marrs, R.H.; Saccheri, I.J.; Hodgson, J.A. Spatial targeting of habitat creation has the potential to improve agri-environment scheme outcomes for macro-moths. J. Appl. Ecol. 2016, 53, 1814–1822. [Google Scholar] [CrossRef]
- Pywell, R.; Warman, E.; Carvell, C.; Sparks, T.; Dicks, L.; Bennett, D.; Wright, A.; Critchley, C.; Sherwood, A. Providing foraging resources for bumblebees in intensively farmed landscapes. Biol. Conserv. 2005, 121, 479–494. [Google Scholar] [CrossRef]
- Pringle, H.E.; Grice, P.V.; Siriwardena, G.M. Impacts of Environmental Stewardship on Bird Populations in Farmland 2002–2017; Report to Natural England; Natural England: London, UK, 2020.
- Falloon, P.; Powlson, D.; Smith, P. Managing field margins for biodiversity and carbon sequestration: A Great Britain case study. Soil Use Manag. 2004, 20, 240–247. [Google Scholar] [CrossRef]
- Keulemans, W.; Bylemans, D.; De Coninck, B. Farming without plant protection products: Can we grow without fungicides, pesticides and herbicides? In Panel for the Future of Science and Technology; STOA, Ed.; European Parliament: Brussels, Belgium, 2019. [Google Scholar]
- Bengtsson, J.; Ahnström, J.; Weibull, A.-C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Birkhofer, K.; Bezemer, T.M.; Bloem, J.; Bonkowski, M.; Christensen, S.; Dubois, D.; Ekelund, F.; Fließbach, A.; Gunst, L.; Hedlund, K.; et al. Long-term organic farming fosters below and aboveground biota: Implications for soil quality, biological control and productivity. Soil Biol. Biochem. 2008, 40, 2297–2308. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. Biol. Sci. 2017, 284, 20171711. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Kreuger, J.; Peterson, M.; Lundgren, E. Agricultural inputs of pesticide residues to stream and pond sediments in a small catchment in Southern Sweden. Bull. Environ. Contam. Toxicol. 1999, 62, 55–62. [Google Scholar] [CrossRef]
- Maeder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil Fertility and Biodiversity in Organic Farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- DEFRA. SCARAB/TALISMAN Completion Studies; Report for Defra project PS0243; DEFRA: London, UK, 2000.
- MacLaren, C.; Storkey, J.; Menegat, A.; Metcalfe, H.; Dehnen-Schmutz, K. An ecological future for weed science to sustain crop production and the environment. A review. Agron. Sustain. Dev. 2020, 40, 1–29. [Google Scholar] [CrossRef]
- Pimentel, D.; McLaughlin, L.; Zepp, A.; Lakitan, B.; Kraus, T.; Kleinman, P.; Vancini, F.; Roach, W.J.; Graap, E.; Keeton, W.S.; et al. Environmental and Economic Effects of Reducing Pesticide Use. BioScience 1991, 41, 402–409. [Google Scholar] [CrossRef]
- Lechenet, M.; Bretagnolle, V.; Bockstaller, C.; Boissinot, F.; Petit, M.-S.; Petit, S.; Munier-Jolain, N.M. Reconciling Pesticide Reduction with Economic and Environmental Sustainability in Arable Farming. PLoS ONE 2014, 9, e97922. [Google Scholar] [CrossRef]
- Lampkin, N.H.; Pearce, B.D.; Leake, A.R.; Creissen, H.; Gerrard, C.L.; Girling, R.; Lloyd, S.; Padel, S.; Smith, J.; Smith, L.G.; et al. The Role of Agroecology in Sustainable Intensification. In Organic Research Centre; Report for the Land Use Policy Group; Food and Agriculture Organization: Rome, Italy, 2015. [Google Scholar]
- Kohler, F.; Verhulst, J.; Van Klink, R.; Kleijn, D. At what spatial scale do high-quality habitats enhance the diversity of forbs and pollinators in intensively farmed landscapes? J. Appl. Ecol. 2008, 45, 753. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Bugg, R.L.; Fay, J.P.; Thorp, R.W. The area requirements of an ecosystem service: Crop pollination by native bee communities in California. Ecol. Lett. 2004, 7, 1109–1119. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gem-Mill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Kremen, C.; Morales, J.M.; Bommarco, R.; Cunningham, S.A.; Carvalheiro, L.G.; Chacoff, N.P.; Dudenhöffer, J.H.; Greenleaf, S.S.; et al. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 2011, 14, 1062–1072. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.-L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2010, 14, 101–112. [Google Scholar] [CrossRef]
- Perović, D.; Gámez-Virués, S.; Börschig, C.; Klein, A.-M.; Krauss, J.; Steckel, J.; Rothenwöhrer, C.; Erasmi, S.; Tscharntke, T.; Westphal, C. Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J. Appl. Ecol. 2015, 52, 505–513. [Google Scholar] [CrossRef]
- Maskell, L.; Botham, M.; Henrys, P.; Jarvis, S.; Maxwell, D.; Robinson, D.; Rowland, C.; Siriwardena, G.; Smart, S.; Skates, J.; et al. Exploring relationships between land use intensity, habitat heterogeneity and biodiversity to identify and monitor areas of High Nature Value farming. Biol. Conserv. 2019, 231, 30–38. [Google Scholar] [CrossRef]
- Lawton, J.; Brotherton, P.N.M.; Brown, V.K.; Elphick, C.; Fitter, A.H.; Forshaw, J.; Haddow, R.; Hilborne, S.; Leafe, R.; Mace, G.; et al. Making Space for Nature: A Review of England’s Wildlife Sites and Ecological Network; Defra: London, UK, 2010.
- Concepción, E.D.; Díaz, M.; Kleijn, D.; Báldi, A.; Batáry, P.; Clough, Y.; Gabriel, D.; Herzog, F.; Holzschuh, A.; Knop, E.; et al. Interactive effects of landscape context constrain the effectiveness of local agri-environmental management. J. Appl. Ecol. 2012, 49, 695–705. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Fuller, R.J.; Norton, L.R.; Feber, R.E.; Johnson, P.J.; Chamberlain, D.E.; Joys, A.C.; Mathews, F.; Stuart, R.C.; Townsend, M.C.; Manley, W.J.; et al. Benefits of organic farming to biodiversity vary among taxa. Biol. Lett. 2005, 1, 431–434. [Google Scholar] [CrossRef]
- Kazemi, H.; Klug, H.; Kamkar, B. New services and roles of biodiversity in modern agroecosystems: A review. Ecol. Indic. 2018, 93, 1126–1135. [Google Scholar] [CrossRef]
- Buzhdygan, O.Y.; Petermann, J.S. Multitrophic biodiversity enhances ecosystem functions, services and ecological intensification in agriculture. J. Plant Ecol. 2023, 16, rtad019. [Google Scholar] [CrossRef]
- Heroldova, M.; Michalko, R.; Suchomel, J.; Zejda, J. Influence of no-tillage versus tillage system on common vole (Microtus arvalis) population density. Pest Manag. Sci. 2018, 74, 1346–1350. [Google Scholar] [CrossRef]
- Harper, J.K.; Roth, G.W.; Garalejić, B.; Škrbić, N. Programs to promote adoption of conservation tillage: A Serbian case study. Land Use Policy 2018, 78, 295–302. [Google Scholar] [CrossRef]
- Keenleyside, C.B.; Maskell, L.C.; Smart, S.M.; Siriwardena, G.M.; Alison, J. Environment and Rural Affairs Monitoring & Modelling Programme (ERAMMP): Report-25: SFS Evidence Review Annex-4B—Building Ecosystem Resilience in Improved Farmland; Report to Welsh Government (Contract C210/2016/2017); UKCEH: Bangor, UK, 2020. [Google Scholar]
- Alahmad, A.; Decocq, G.; Spicher, F.; Kheirbeik, L.; Kobaissi, A.; Tetu, T.; Dubois, F.; Duclercq, J. Cover crops in arable lands increase functional complementarity and redundancy of bacterial communities. J. Appl. Ecol. 2019, 56, 651–664. [Google Scholar] [CrossRef]
- Cardina, J.; Herms, C.P.; Doohan, D.J. Crop rotation and tillage system effects on weed seedbanks. Weed Sci. 2009, 50, 448–460. [Google Scholar] [CrossRef]
- Haenke, S.; Kovács-Hostyánszki, A.; Fründ, J.; Batáry, P.; Jauker, B.; Tscharntke, T.; Holzschuh, A. Landscape configuration of crops and hedgerows drives local syrphid fly abundance. J. Appl. Ecol. 2014, 51, 505–513. [Google Scholar] [CrossRef]
- Morandin, L.A.; Kremen, C. Bee Preference for Native versus Exotic Plants in Restored Agricultural Hedgerows. Restor. Ecol. 2012, 21, 26–32. [Google Scholar] [CrossRef]
- Boatman, N.D. (Ed.) Field Margins—Integrating Agriculture and Conservation; BCPC Monograph No 58; British Crop Protection Council: Farnham, UK, 1994. [Google Scholar]
- Hinsley, S.; Bellamy, P. The influence of hedge structure, management and landscape context on the value of hedgerows to birds: A review. J. Environ. Manag. 2000, 60, 33–49. [Google Scholar] [CrossRef]
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P. Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–132. [Google Scholar] [CrossRef]
- Kovář, P.; Vaššová, D.; Hrabalíková, M. Mitigation of surface runoff and erosion impacts on catchment by stone hedgerows. Soil Water Res. 2011, 6, 153–164. [Google Scholar] [CrossRef]
- Smith, R.G.; Gross, K.L.; Robertson, G.P. Effects of Crop Diversity on Agroecosystem Function: Crop Yield Response. Ecosystems 2008, 11, 355–366. [Google Scholar] [CrossRef]
- Vincent-Caboud, L.; Peigné, J.; Casagrande, M.; Silva, E.M. Overview of Organic Cover Crop-Based No-Tillage Technique in Europe: Farmers’ Practices and Research Challenges. Agriculture 2017, 7, 42. [Google Scholar] [CrossRef]
- Bernal, M.; Alburquerque, J.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).