Geochemical Features of Ground Ice from the Faddeevsky Peninsula Eastern Coast (Kotelny Island, East Siberian Arctic) as a Key to Understand Paleoenvironmental Conditions of Its Formation
Abstract
:1. Introduction
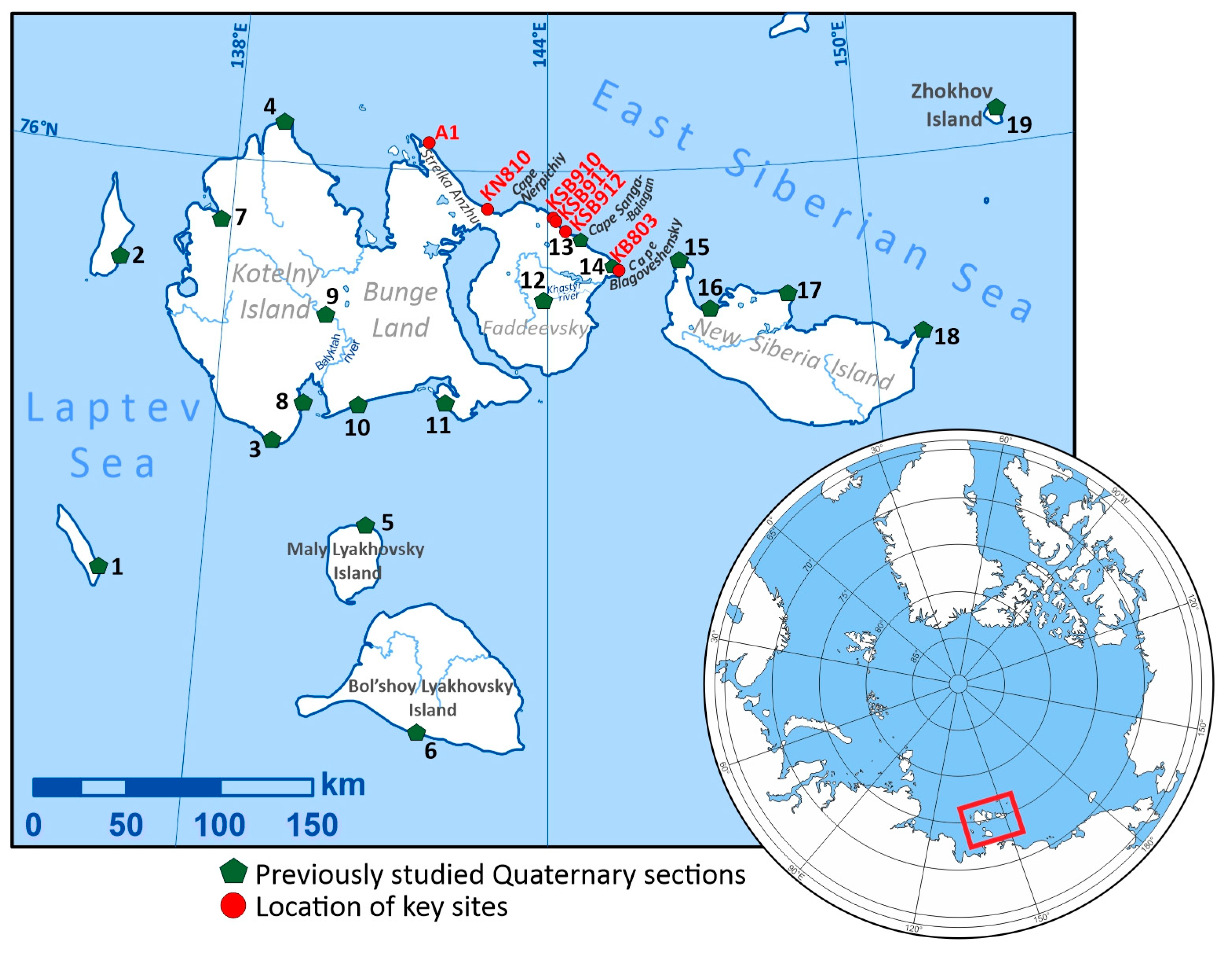
2. Study Area
3. Methods
3.1. Fieldwork and Sample Preparation
3.2. Stable Isotope Composition
3.3. Dissolved Gas Analysis, DOC, and DIC Measurements
3.4. Ion Composition, DIN Measurments
3.5. Fluorescence Measurements of Dissolved Organic Matter Molecular Composition
4. Results
4.1. Stable δ18O and δD Isotopes
4.2. Carbon Cycle Parameters (DOC, DIC, CH4, C2–C5 Gases)
4.3. Ion Composition and DIN
4.4. Fluorescent DOM (fDOM) Composition and Fluorescent Indices
5. Discussion
5.1. Paleoclimate Record from Ice Wedges of the Faddeevsky Coast Based on δ18O–δD Composition
5.2. Ion Composition of Ground Ice for Paleoenvironmental Reconstructions
5.3. DOC, DIC, and Methane Features in Different Types of Ground Ice
5.4. DOM Fluorescence Properties of Ground Ice
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liljedahl, A.K.; Boike, J.; Daanen, R.P.; Fedorov, A.N.; Frost, G.V.; Grosse, G.; Hinzman, L.D.; Iijma, Y.; Jorgenson, J.C.; Matveyeva, N.; et al. Pan-Arctic ice-wedge degradation in warming permafrost and its influence on tundra hydrology. Nat. Geosci. 2016, 9, 312–318. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, L.; Kunitsky, V.; Grosse, G.; Wetterich, S.; Meyer, H.; Schwamborn, G.; Babiy, O.; Derevyagin, A.; Siegert, C. Sedimentary characteristics and origin of the Late Pleistocene Ice Complex on north-east Siberian Arctic coastal lowlands and islands—A review. Quatern. Intern. 2011, 241, 3–25. [Google Scholar] [CrossRef] [Green Version]
- Streletskaya, I.D.; Pismeniuk, A.A.; Vasiliev, A.A.; Gusev, E.A.; Oblogov, G.E.; Zadorozhnaya, N.A. The ice-rich permafrost sequences as a paleoenvironmental archive for the Kara Sea region (Western Arctic). Front. Earth Sci. 2021, 9, 723382. [Google Scholar] [CrossRef]
- Meyer, H.; Dereviagin, A.Y.; Siegert, C.; Schirrmeister, L.; Hubberten, H.W. Paleoclimate reconstruction on Big Lyakhovsky Island, North Siberia—Hydrogen and oxygen isotopes in ice wedges. Permafr. Periglac. Process. 2002, 13, 91–105. [Google Scholar] [CrossRef]
- Vasil’chuk, Y.K. Oxygen Isotope Composition of Ground Ice Application to Paleogeocryological Reconstructions; Theoretical Problems Department, Russian Academy of Sciences and Lomonosov’s Moscow University Publ.: Moscow, Russia, 1992; p. 420. [Google Scholar]
- Streletskaya, I.D.; Vasiliev, A.A.; Oblogov, G.E.; Tokarev, I.V. Reconstruction of paleoclimate of Russian Arctic in Late Pleistocene–Holocene on the basis of isotope study of ice wedges. Kriosf. Zemli 2015, 19, 86–94. [Google Scholar]
- Opel, T.; Meyer, H.; Wetterich, S.; Laepple, T.; Dereviagin, A.; Murton, J. Ice wedges as archives of winter paleoclimate: A review. Permafr. Periglac. Process. 2018, 29, 199–209. [Google Scholar] [CrossRef]
- Porter, T.J.; Opel, T. Recent advances in paleoclimatological studies of Arctic wedge- and pore-ice stable-water isotope records. Permafr. Periglac. Process. 2020, 31, 429–441. [Google Scholar] [CrossRef]
- Savoskul, O.S. Ion Content of polygonal wedge ice on Bolshoi Lyakhov: A source of palaeoenvironmental information. Ann. Glaciol. 1995, 21, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, Y.; Miyamoto, C.; Matoba, S.; Iwahana, G.; Horiuchi, K.; Takahashi, Y.; Ohno, H. Ion concentrations in ice wedges: An innovative approach to reconstruct past climate variability. Earth Planet. Sci. Lett. 2019, 515, 58–66. [Google Scholar] [CrossRef]
- Campbell-Heaton, K.; Lacelle, D.; Fisher, D.; Pollard, W. Holocene ice wedge formation in the Eureka Sound Lowlands, high Arctic Canada. Quat. Res. 2021, 102, 175–187. [Google Scholar] [CrossRef]
- Streletskaya, I.D.; Vasiliev, A.A.; Oblogov, G.E.; Streletskiy, D.A. Methane content in ground ice and sediments of the Kara Sea coast. Geosciences 2018, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Semenov, P.B.; Pismeniuk, A.A.; Malyshev, S.A.; Leibman, M.O.; Streletskaya, I.D.; Shatrova, E.V.; Kizyakov, A.I.; Vanshtein, B.G. Methane and dissolved organic matter in the ground ice samples from Central Yamal: Implications to biogeochemical cycling and greenhouse gas emission. Geosciences 2020, 10, 450. [Google Scholar] [CrossRef]
- Brouchkov, A.; Fukuda, M. Preliminary measurements on methane content in permafrost, Central Yakutia, and some experimental data. Permafr. Periglac. Process. 2002, 13, 187–197. [Google Scholar] [CrossRef]
- Boereboom, T.; Samyn, D.; Meyer, H.; Tison, J.-L. Stable isotope and gas properties of two climatically contrasting (Pleistocene and Holocene) ice wedges from Cape Mamontov Klyk, Laptev Sea, northern Siberia. Cryosphere 2013, 7, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Yang, J.-W.; Yoon, H.; Byun, E.; Fedorov, A.; Ryu, Y.; Ahn, J. Greenhouse gas formation in ice wedges at Cyuie, central Yakutia. Permafrost Periglac. Process. 2019, 30, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Fellman, J.B.; Hood, E.; D’Amore, D.V.; Edwards, R.T.; White, D. Seasonal changes in the chemical quality and biodegradability of dissolved organic matter exported from soils to streams in coastal temperate rainforest watersheds. Biogeochemistry 2009, 95, 277–293. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Cory, R.M. Biological origins and fate of fluorescent dissolved organic matter in aquatic environments. In Aquatic Organic Matter Fluorescence; Coble, P.G., Lead, J.R., Eds.; Cambridge University Press: Cambridge, NY, USA, 2014; pp. 278–300. [Google Scholar]
- Kellerman, A.M.; Hawkings, J.R.; Wadham, J.L.; Kohler, T.J.; Stibal, M.; Grater, E.; Marshall, M.; Hatton, J.E.; Beaton, A.; Spencer, R.G.M. Glacier outflow dissolved organic matter as a window into seasonally changing carbon sources: Leverett Glacier, Greenland. JGR Biogeosci. 2020, 125, e2019JG005161. [Google Scholar] [CrossRef]
- Vonk, J.E.; Mann, P.J.; Davydov, S.; Davydova, A.; Spencer, R.G.M.; Schade, J.; Sobczak, W.V.; Zimov, N.; Zimov, S.; Bulygina, E.; et al. High biolability of ancient permafrost carbon upon thaw. Geophys. Res. Lett. 2013, 40, 2689–2693. [Google Scholar] [CrossRef] [Green Version]
- Dubnick, A.; Sharp, M.; Barker, J.; Wadham, J.L.; Lis, G.P.; Telling, J.; Fitzsimmons, S.; Jackson, M. Characterization of dissolved organic matter (DOM) from glacial environments using total fluorescence spectroscopy and parallel factor analysis. Ann. Glaciol. 2010, 51, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Tumskoy, V.; Kuznetsova, T.V. Cryolithostratigraphy of the Middle Pleistocene to Holocene Deposits in the Dmitry Laptev Strait, Northern Yakutia. Front. Earth Sci. 2022, 10, 789421. [Google Scholar] [CrossRef]
- Tumskoy, V.E. Peculiarities of cryolithogenesis in Northern Yakutia (Middle Neopleistocene to Holocene). Kriosf. Zemli 2012, 16, 12–21. [Google Scholar]
- Vasil’chuk, Y.K.; Makeev, V.M.; Maslakov, A.A.; Budantseva, N.A.; Vasil’chuk, A.K. Late Pleistocene and Early Holocene winter air temperatures in Kotelny Island: Reconstructions using stable isotopes of ice wedges. Kriosf. Zemli 2019, 23, 13–28. [Google Scholar] [CrossRef]
- Schirrmeister, L.; Grosse, G.; Kunitsky, V.V.; Fuchs, M.C.; Krbetschek, M.; Andreev, A.; Herzschuh, U.; Barbyi, O.; Siegert, C.; Meyer, H.; et al. The mystery of Bunge Land (New Siberian Archipelago): Implications for its formation based on palaeoenvironmental records, geomorphology, and remote sensing. Quat. Sci. Rev. 2009, 29, 3598–3616. [Google Scholar] [CrossRef]
- Gasanov, S.S. Cryolithological Analysis; Nauka: Moscow, Russia, 1981; p. 196. [Google Scholar]
- Pavlova, E.Y.; Anisimov, M.A.; Dorozhkina, M.V.; Pitulko, V.V. The tracks of old glaciation on the Novaya Sibir Island (New Siberian Islands) and it natural conditions at the Late Pleistocene. Ice Snow 2010, 110, 85–92. [Google Scholar]
- Ivanova, V.V. Geochemical features of formation of massive ground ice bodies (New Siberia Islands, Siberian Arctic) as the evidence of their genesis. Kriosf. Zemli 2012, 6, 56–70. [Google Scholar]
- Anisimov, M.A.; Tumskoy, V.E.; Ivanova, V.V. The subsurface ice at Novosibirskie Islands as a relic of ancient glaciation. Mater. Glaciol. Res. 2006, 101, 143–145. [Google Scholar]
- Basilyan, A.E.; Nikolskiy, P.A.; Maksimov, F.E.; Kuznetsov, V.Y. Age of cover glaciation of the New Siberian Islands based on 230Th/U-dating of mollusk shells. In The Structure and History of the Development of the Lithosphere; Paulsen: Moscow, Russia, 2010; pp. 506–514. [Google Scholar]
- Romanenko, F.A.; Nikolaev, V.I.; Arkhipov, V.V. Changes in the isotopic composition of natural ice in the East Siberian Sea: A geographical aspect. Ice Snow 2011, 113, 93–104. [Google Scholar]
- Wetterich, S.; Meyer, H.; Fritz, M.; Mollenhauer, G.; Rethemeyer, J.; Kizyakov, A.I.; Schirrmeister, L.; Opel, T. Northeast Siberian permafrost ice-wedge stable isotopes depict pronounced Last Glacial Maximum winter cooling. Geophys. Res. Lett. 2021, 48, e2020GL092087. [Google Scholar] [CrossRef]
- Vasil’chuk, Y.K.; Makeev, V.M.; Maslakov, A.A.; Budantseva, N.A.; Vasil’chuk, A.C.; Chizhova, J.N. The oxygen isotope composition of Late Pleistocene and Holocene ice wedges of Kotelny Island. Dokl. Earth Sci. 2018, 482, 1216–1220. [Google Scholar] [CrossRef]
- Trufanov, G.V.; Belousov, K.N.; Vakulenko, A.S. Materials to stratigraphy of Cenozoic deposits of New Siberian Archipelago. In Tertiary Continental Strata of the North-East of Asia; Baranova, Y.P., Shilo, N.A., Eds.; Nauka: Novosibirsk, Russia, 1979; pp. 30–40. [Google Scholar]
- Alekseev, M.N.; Drushchits, V.A. Climatic events of the Kazantsevo interglacial and Holocene of the Eastern part of the Russian shelf and Siberia. BKIChP 2001, 64, 78–88. [Google Scholar]
- Pismeniuk, A.A.; Streletskaya, I.D.; Kozachek, A.V.; Malyshev, S.A.; Semenov, P.B.; Yarzhembovsky, Y.D. Ground ice of the coast of the East Siberian Sea: New data on its origin and paleoenvironmental conditions. In Proceedings of the All-Russian Conference with International Participation: The Global Problems of the Arctic and Antarctic, Arkhangelsk, Russia, 3–5 November 2020; pp. 139–143. [Google Scholar]
- Pavlova, E.Y.; Ivanova, V.V.; Meyer, H.; Pitulko, V.V. The oxygen isotope composition of fossil ice as a climate proxy: Case study of the northern New Siberian Islands and the western Yana-Indigirka lowland. In Proceedings of the IX All-Russian Conf. on the Quaternary, V.B. Sochava Institute of Geography, Irkutsk, Russia, 15–20 September 2015; pp. 349–351. [Google Scholar]
- Bassinot, F.C.; Labeyrie, L.L.; Vincent, E.; Quidelleur, X.; Shackleton, N.J.; Lancelot, Y. The astronomical theory of climate and the age of the Brunhes-Matuyama magnetic reversal. Earth Planet. Sci. Lett. 1994, 126, 91–108. [Google Scholar] [CrossRef]
- Porter, C.; Morin, P.; Howat, I.; Noh, M.-J.; Bates, B.; Peterman, K.; Keesey, S.; Schlenk, M.; Gardiner, J.; Tomko, K.; et al. ArcticDEM, Version 3, Harvard Dataverse, V1. Available online: https://doi.org/10.7910/DVN/OHHUKH (accessed on 10 November 2022).
- Veremeeva, A.; Nitze, I.; Günther, F.; Grosse, G.; Rivkina, E. Geomorphological and climatic drivers of thermokarst lake area increase trend (1999–2018) in the Kolyma Lowland Yedoma region, North-Eastern Siberia. Remote Sens. 2021, 13, 178. [Google Scholar] [CrossRef]
- Yamamoto, S.; Alcauskas, J.B.; Crozier, T.E. Solubility of methane in distilled water and seawater. J. Chem. Eng. 1976, 21, 78–80. [Google Scholar] [CrossRef]
- Abrams, M.A. Significance of hydrocarbon seepage relative to sub-surface petroleum generation and entrapment. Mar. Petrol. Geol. 2005, 22, 457–478. [Google Scholar] [CrossRef]
- Trivittayasil, V.; Tsuta, M.; Kokawa, M.; Yoshimura, M.; Sugiyama, J.; Fujita, K.; Shibata, M. Method of determining the optimal dilution ratio for fluorescence fingerprint of food constituents. Biosci. Biotechnol. Biochem. 2015, 79, 652–657. [Google Scholar] [CrossRef] [Green Version]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of aquatic fulvic acids for determination of precursor organic material and general structural properties. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Murphy, K.R.; Butler, K.D.; Spencer, R.G.M.; Stedmon, C.A.; Boehme, J.R.; Aiken, G.R. Measurement of dissolved organic matter fluorescence in aquatic environments: An interlaboratory comparison. Environ. Sci. Technol. 2010, 24, 9405–9412. [Google Scholar] [CrossRef]
- Bro, R. PARAFAC. Tutorial and applications. Chemometr. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- He, W.; Hur, J. Conservative behavior of fluorescence EEM-PARAFAC components in resin fractionation processes and its applicability for characterizing dissolved organic matter. Water Res. 2015, 83, 217–226. [Google Scholar] [CrossRef]
- Parr, T.B.; Ohno, T.; Cronan, C.S.; Simon, K.S. comPARAFAC: A library and tools for rapid and quantitative comparison of dissolved organic matter components resolved by Parallel Factor Analysis. Limnol. Oceanogr.-Meth. 2014, 12, 114–125. [Google Scholar] [CrossRef]
- Wetterich, S.; Tumskoy, V.; Rudaya, N.; Andreev, A.; Opel, T.; Meyer, H.; Schirrmeister, L.; Hüls, M. Ice Complex formation in arctic East Siberia during the MIS3 Interstadial. Quatern. Sci. Rev. 2014, 84, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Wetterich, S.; Tumskoy, V.; Rudaya, N.; Kuznetsov, V.; Maksimov, F.; Opel, T.; Meyer, H.; Andreev, A.A.; Schirrmeister, L. Ice Complex permafrost of MIS5 age in the Dmitry Laptev Strait coastal region (East Siberian Arctic). Quatern. Sci. Rev. 2016, 147, 298–311. [Google Scholar] [CrossRef] [Green Version]
- Fritz, M.; Opel, T.; Tanski, G.; Herzschuh, U.; Meyer, H.; Eulenburg, A.; Lantuit, H. Dissolved organic carbon (DOC) in Arctic ground ice. Cryosphere 2015, 9, 737–752. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Inamdar, S.; Scott, D. Comparison of two PARAFAC models of dissolved organic matter fluorescence for a Mid-Atlantic forested watershed in the USA. J. Ecosyst. 2013, 2013, 532424. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, Y.; Cory, R.M.; Nishioka, J.; Kuma, K.; Tanoue, E.; Jaffe, R. Fluorescence characteristics of dissolved organic matter in the deep waters of the Okhotsk Sea and the northwestern North Pacific Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 1478–1485. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, R.D.; Yen, J.H.; Grizzard, T.J. Characterizing natural organic material from the Occoquan Watershed (Northern Virginia, US) using fluorescence spectroscopy and PARAFAC. Sci. Total Environ. 2006, 361, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G.; Green, S.; Blough, N.V.; Gagosian, R.B. Characterization of dissolved organic matter in the Black Sea by fluorescence spectroscopy. Nature 1990, 348, 432–435. [Google Scholar] [CrossRef]
- Wetterich, S.; Kizyakov, A.; Fritz, M.; Wolter, J.; Mollenhauer, G.; Meyer, H.; Fuchs, M.; Aksenov, A.; Matthes, H.; Schirrmeister, L.; et al. The cryostratigraphy of the Yedoma cliff of Sobo-Sise Island (Lena delta) reveals permafrost dynamics in the central Laptev Sea coastal region during the last 52 kyr. Cryosphere 2020, 14, 4525–4551. [Google Scholar] [CrossRef]
- Domine, F.; Sparapani, R.; Ianniello, A.; Beine, H.J. The origin of sea salt in snow on Arctic Sea ice and in coastal regions. Atmos. Chem. Phys. 2004, 4, 2259–2271. [Google Scholar] [CrossRef] [Green Version]
- Vasil’chuk, Y.K. Geochemical composition of underground ice in the north of the Russian Arctic. Arkt. Antarkt. 2016, 2, 99–115. [Google Scholar] [CrossRef]
- Patris, N.; Delmas, R.J.; Legrand, M.; Angelis, M.D.; Ferron, F.A.; Stiévenard, M.; Jouzel, J. First sulfur isotope measurements in central Greenland ice cores along the preindustrial and industrial periods. J. Geophys. Res. Atmos. 2002, 107, ACH 6-1–ACH 6-11. [Google Scholar] [CrossRef]
- Leck, C.; Persson, C. The central Arctic Ocean as a source of dimethyl sulfide Seasonal variability in relation to biological activity. Tellus B Chem. Phys. Meteorol. 1996, 48, 156–177. [Google Scholar] [CrossRef]
- Jones, E.L.; Hodson, A.J.; Thornton, S.F.; Redeker, K.R.; Rogers, J.; Wynn, P.M.; Dixon, T.J.; Bottrell, S.H.; O’Neill, H.B. Biogeochemical processes in the active layer and permafrost of a High Arctic fjord valley. Front. Earth Sci. 2020, 8, 342. [Google Scholar] [CrossRef]
- Holland, K.M.; Porter, T.J.; Criscitiello, A.S.; Froese, D.G.; Kokelj, S. Marine aerosol contribution to ice-wedge geochemistry: Potential for paleo-sea ice and paleogeography reconstructions in the Canadian Arctic. In Proceedings of the AGU Fall Meeting, Online, 1–17 December 2020. [Google Scholar]
- Schüpbach, S.; Fischer, H.; Bigler, M.; Erhardt, T.; Gfeller, G.; Leuenberger, D.; Stowasser, O.; Mulvaney, R.; Abram, N.; Fleet, L.; et al. Greenland records of aerosol source and atmospheric lifetime changes from the Eemian to the Holocene. Nat. Commun. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynn, P.M.; Hodson, A.J.; Heaton, T.H.E.; Chenery, S.R. Nitrate production beneath a High Arctic glacier, Svalbard. Chem. Geol. 2007, 244, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Makeev, V.M.; Arslanov, K.A.; Baranovskaya, O.F.; Kosmodamiansky, D.P.; Tertychnaya, T.V. Late Pleistocene and Holocene stratigraphy, geochronology, and paleogeography of Kotelny Island. Bull. Quat. Comm. 1989, 58, 58–69. [Google Scholar]
- Yang, Y.; Guo, X.; Wang, Q.; Jin, H.; Yun, H.; Wu, Q. Dissolved organic carbon (DOC) in ground ice on northeastern Tibetan Plateau. Front. Earth Sci. 2022, 10, 782013. [Google Scholar] [CrossRef]
- Spencer, R.G.M.; Mann, P.J.; Dittmar, T.; Eglinton, T.I.; McIntyre, C.; Holmes, R.M.; Zimov, N.; Stubbins, A. Detecting the signature of permafrost thaw in Arctic rivers. Geophys. Res. Lett. 2015, 42, 2830–2835. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Ping, C.-L.; Macdonald, R.W. Mobilization pathways of organic carbon from permafrost to arctic rivers in a changing climate. Geophys. Res. Lett. 2007, 34, L13603. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Rivkina, E.; Petrovskaya, L.; Vishnivetskaya, T.; Krivushin, K.; Shmakova, L.; Tutukina, M.; Meyers, A.; Kondrashov, F. Metagenomic analyses of the late Pleistocene permafrost—Additional tools for reconstruction of environmental conditions. Biogeosciences 2016, 13, 2207–2219. [Google Scholar] [CrossRef] [Green Version]









| Site | Type of Ice | δ18O (‰) | δD (‰) | d-excess |
|---|---|---|---|---|
| KSB911 | Holocene IW | −24.2 to −24.0; −23.8 * | −183.7 to −180.9; −182.3 | 7.0 to 11,3; 9.8 |
| KB910 | Holocene IW | −23.2 to −21.3; −22.5 | −176.7 to −161.0; −170.3 | 9.1 to 10.7; 9.9 |
| KB803 | Holocene IW | −25.3 to −21.7; −24.2 | −190.2 to −163.4; −182.6 | 7.9 to 12.7; 11.3 |
| KN810 | Late Pleistocene IW | −31.0 to −29.3; −30.2 | −240.6 to −226.5; −234.0 | 7.2 to 8.8; 7.9 |
| KSB912 | TGI | −18.2 to −11.2; −14.2 | −139.5 to −95.8; −111.6 | −6.2 to 5.9; 1.8 |
| A1 | Lens ice | −14.1 | −122.1 | −9.5 |
| Site | Type of Ice | DOC, mg/L | DIC, mg/L | CH4, μmol/L | Total HCG, ppm | kW, % |
|---|---|---|---|---|---|---|
| KSB911 | Holocene IW | 9.3 to 13.6; 11.4 * | 2.1 to 4.9; 3.5 | 0.50 to 1.55; 1.06 | 12.6 | 0.7 |
| KB910 | Holocene IW | 3.5 to 7.6; 5.0 | 2.0 to 5.7; 3.6 | 0.02 to 0.29; 0.11 | 1.5 | 6.7 |
| KB803 | Holocene IW | 7.0 to 1.3; 8.6 | 1.0 to 3.2; 2.2 | 0.13 to 2.27; 0.80 | 11.4 | 1.1 |
| KN810 | Late Pleistocene IW | 10.5 to 17.7; 13.5 | 3.4 to 6; 4.2 | 0.02 to 0.19; 0.07 | 1.2 | 25.7 |
| KSB912 | TGI | 1.7 to 6.5; 4.2 | 0.9 to 1.3; 1.1 | 0.07 to 0.59; 0.23 | 3.4 | 1.7 |
| A1 | Lens ice | 13.7 | 4.5 | 0.01 to 0.07; 0.05 | 0.7 | 5.4 |
| Site | Type of Ice | Na+ | NH4+ | K+ | ssCa2+ | nssCa2+ | Mg2+ | Cl− | ssSO42− | nssSO42− | TDS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KSB911 | Holocene IW | 19.8 | 2.1 | 5.4 | 0.5 | 8.5 | 5.2 | 37.9 | 3.9 | 3.5 | 27.6 |
| KSB910 | Holocene IW | 16.3 | 0.5 | 2.7 | 0.4 | 5.8 | 5.0 | 49.9 | 3.2 | 3.5 | 30.7 |
| KB803 | Holocene IW | 23.5 | 0.8 | 2.3 | 0.6 | 3.2 | 3.3 | 53.1 | 4.6 | 4.2 | 67.0 |
| KN810 | Late Pleistocene IW | 17.7 | 1.1 | 1.8 | 0.4 | 3.2 | 3.9 | 31.6 | 3.5 | 32.1 | 101.1 |
| KSB912 | TGI | 24.0 | 2.8 | 2.1 | 0.6 | 3.7 | 2.6 | 35.7 | 4.8 | 21.3 | 52.9 |
| A1 | Lens ice | 41.0 | 0.8 | 2.6 | 1.0 | 0.7 | 1.7 | 42.5 | 8.1 | 0 | 344.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pismeniuk, A.; Semenov, P.; Veremeeva, A.; He, W.; Kozachek, A.; Malyshev, S.; Shatrova, E.; Lodochnikova, A.; Streletskaya, I. Geochemical Features of Ground Ice from the Faddeevsky Peninsula Eastern Coast (Kotelny Island, East Siberian Arctic) as a Key to Understand Paleoenvironmental Conditions of Its Formation. Land 2023, 12, 324. https://doi.org/10.3390/land12020324
Pismeniuk A, Semenov P, Veremeeva A, He W, Kozachek A, Malyshev S, Shatrova E, Lodochnikova A, Streletskaya I. Geochemical Features of Ground Ice from the Faddeevsky Peninsula Eastern Coast (Kotelny Island, East Siberian Arctic) as a Key to Understand Paleoenvironmental Conditions of Its Formation. Land. 2023; 12(2):324. https://doi.org/10.3390/land12020324
Chicago/Turabian StylePismeniuk, Anfisa, Petr Semenov, Alexandra Veremeeva, Wei He, Anna Kozachek, Sergei Malyshev, Elizaveta Shatrova, Anastasiia Lodochnikova, and Irina Streletskaya. 2023. "Geochemical Features of Ground Ice from the Faddeevsky Peninsula Eastern Coast (Kotelny Island, East Siberian Arctic) as a Key to Understand Paleoenvironmental Conditions of Its Formation" Land 12, no. 2: 324. https://doi.org/10.3390/land12020324
APA StylePismeniuk, A., Semenov, P., Veremeeva, A., He, W., Kozachek, A., Malyshev, S., Shatrova, E., Lodochnikova, A., & Streletskaya, I. (2023). Geochemical Features of Ground Ice from the Faddeevsky Peninsula Eastern Coast (Kotelny Island, East Siberian Arctic) as a Key to Understand Paleoenvironmental Conditions of Its Formation. Land, 12(2), 324. https://doi.org/10.3390/land12020324








