Urban Grassland Afforestation as a Public Land Management Tool for Environmental Improvement: The Example of Krakow (Poland)
Abstract
:1. Introduction
2. Materials and Methods
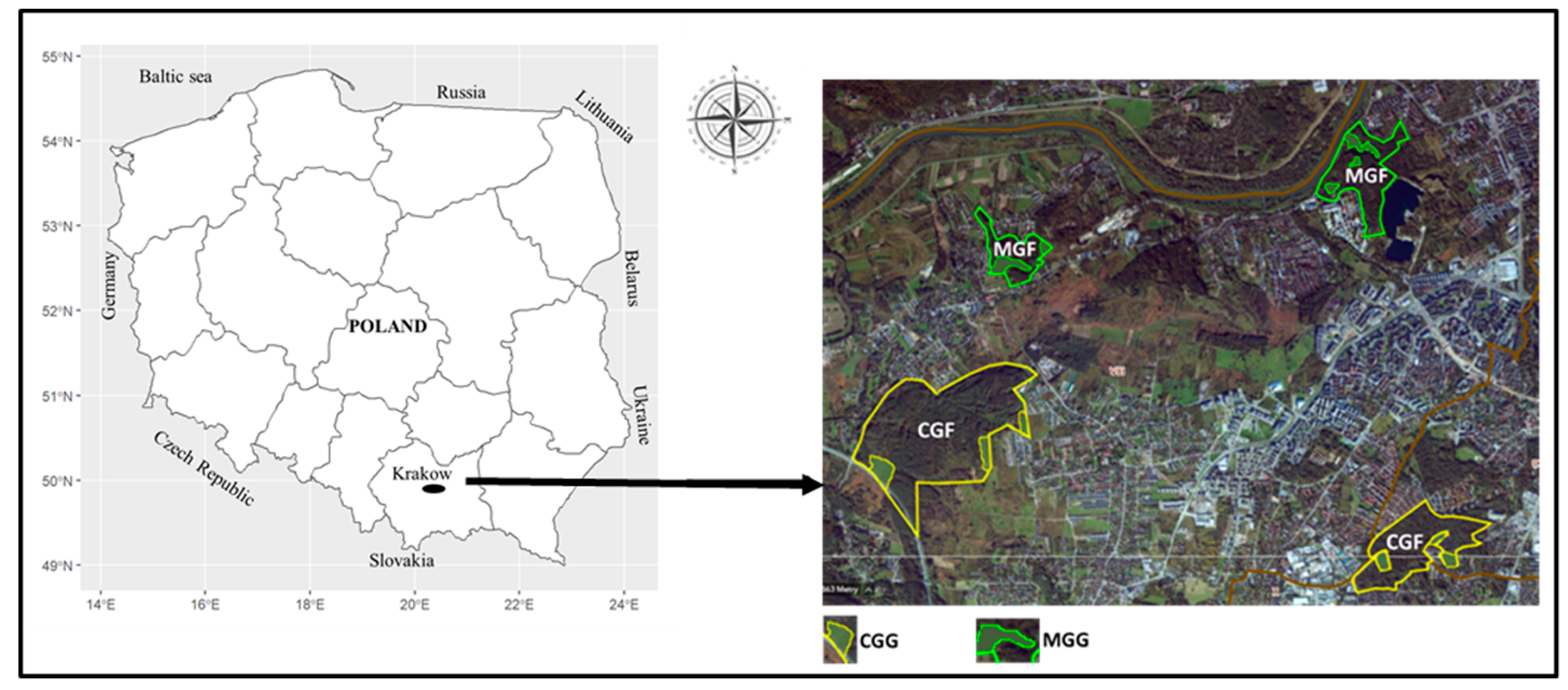
2.1. Study Area
2.2. Sampling
2.3. Methods
2.3.1. Field and Laboratory Analyses
2.3.2. Statistical Methods and A Horizon Development Index Calculation (ADI)
3. Results
3.1. Basic Properties of Soils of Studied Stands
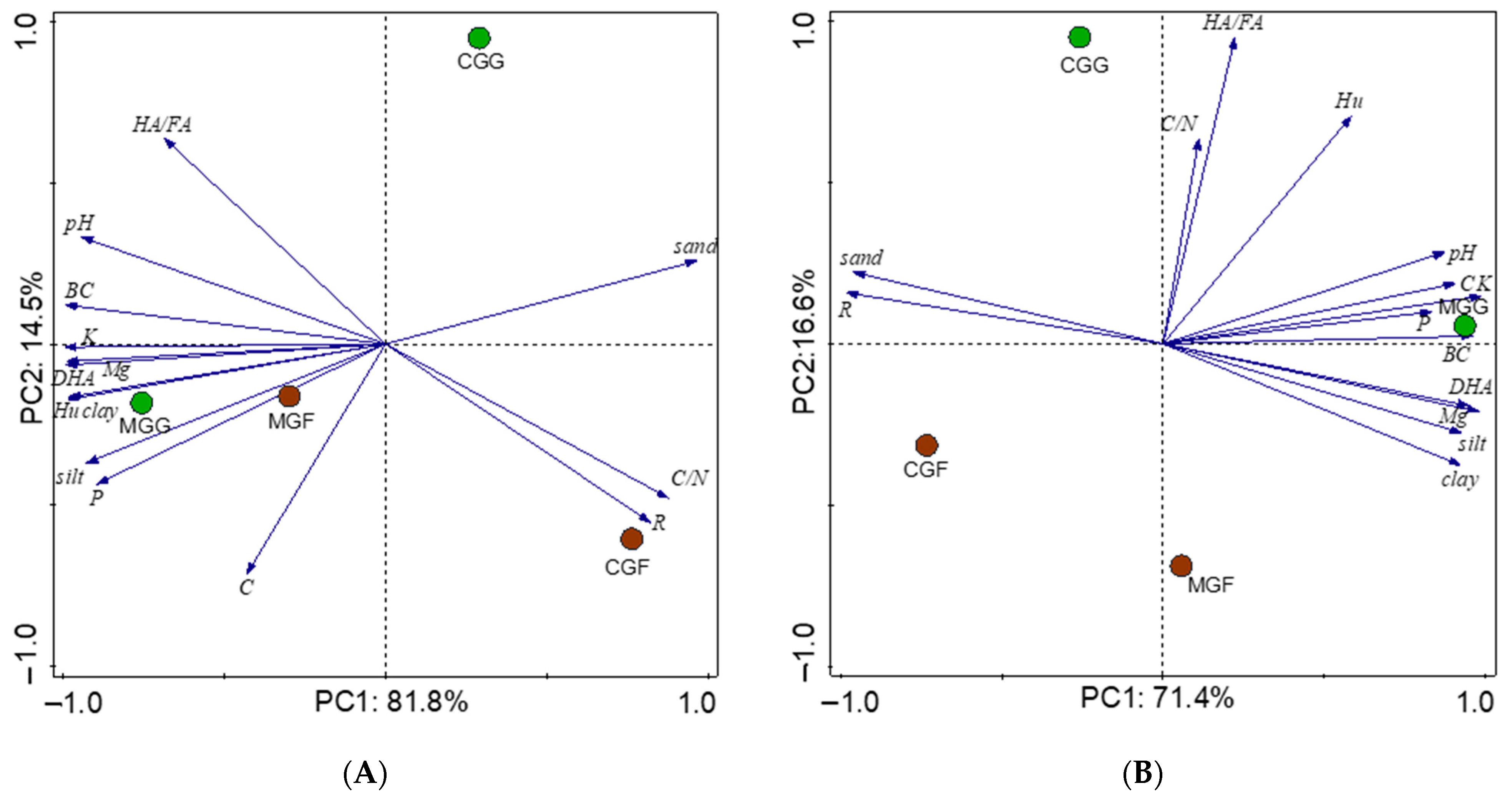
3.2. Results of the Principal Component Analysis
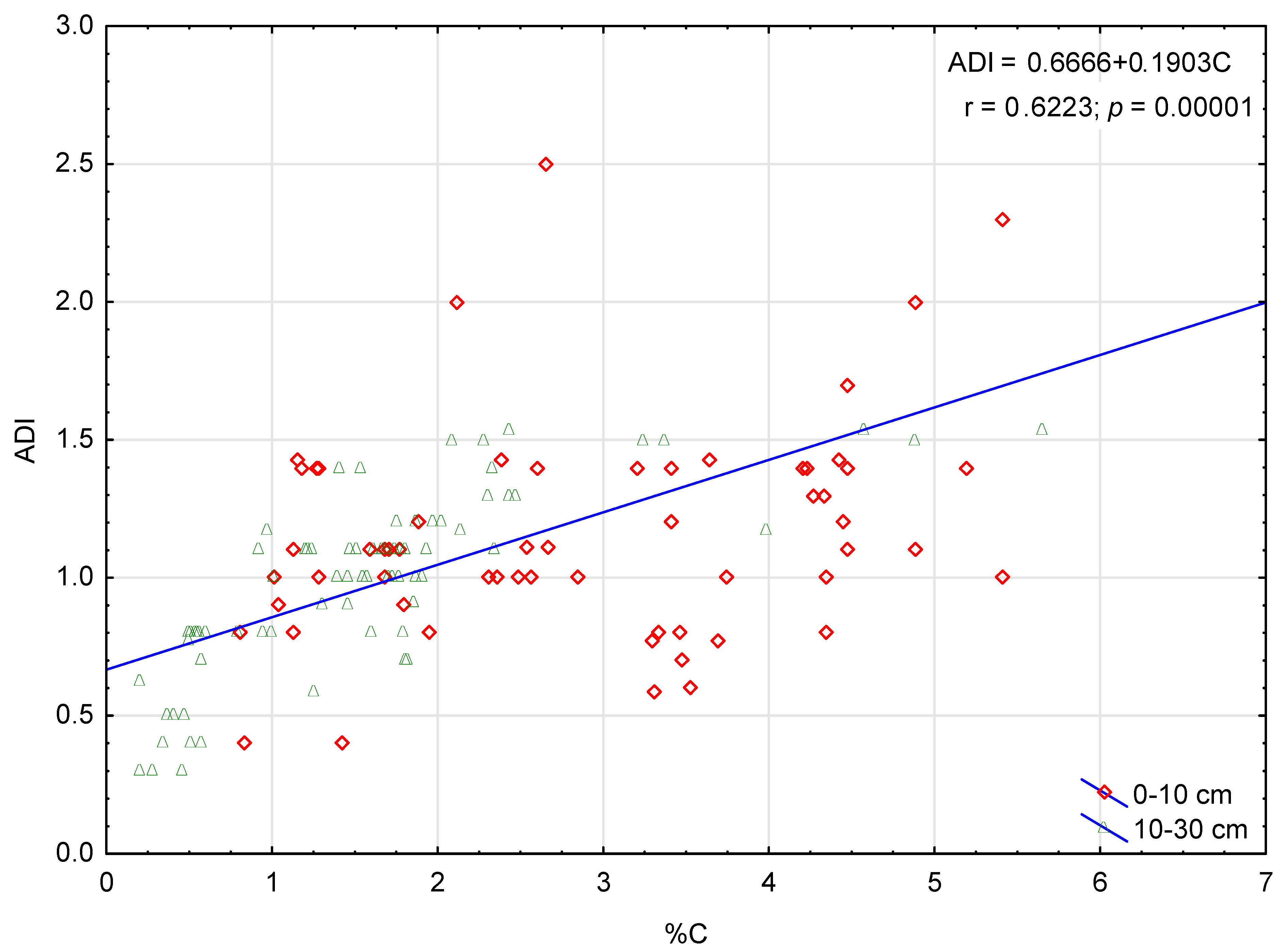
3.3. Soil Organic-Matter Parameters
4. Discussion
4.1. Effect of Afforestation on Selected Basic Soil Properties
4.2. Role of Afforested Stands in C Accumulation and Soil Organic-Matter Stabilisation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.F.; He, Z.-B.; Du, J.; Yang, J.-J.; Li, J. Impacts of afforestation on plant diversity, soil properties, and soil organic carbon storage in a semi-arid grassland of north western China. Catena 2016, 147, 300–307. [Google Scholar] [CrossRef]
- New EU Forest Strategy for 2030. Available online: https://environment.ec.europa.eu/strategy/forest-strategy_en (accessed on 30 March 2023).
- Ibrahim, K.M.; Muhammad, S.I. A review of afforestation efforts in Nigeria. Int. J. Adv. Res. Eng. Appl. Ascience 2016, 4, 24–37. Available online: https://www.researchgate.net/publication/291356792 (accessed on 3 April 2023).
- Nosetto, M.; Jobbagy, E.; Paruelo, J. Land-use change and water losses: The case of grassland afforestation across a soil textural gradient in central Argentina. Glob. Chang. Biol. 2005, 11, 1101–1117. [Google Scholar] [CrossRef]
- Navea, L.E.; Grant, M.; Domkec, G.M.; Hofmeistera, K.L.; Umakant Mishrae, U.; Perryc, C.H.; Waltersc, B.F.; Swanstonf, C.W. Refostation can sequster two petagrams of carbon in US topsoils in a century. Proc. Natl. Acad. Sci. USA 2018, 15, 2776–2781. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Xu, Z.H. Impacts of grassland afforestation with coniferous trees on soil phosphorus dynamics and associated microbial processes: A review. Forest Ecol. Manag. 2008, 255, 396–409. [Google Scholar] [CrossRef]
- Podwika, M.; Solek-Podwika, K.; Ciarkowska, K. Changes in the properties of grassland soils as a result of afforestation. iForest 2018, 11, 600–608. [Google Scholar] [CrossRef]
- Shi, S.-W.; Han, P.-F.; Zhang, P.; Ding, P.; Ma, C.-L. The Impact of Afforestation on Soil Organic Carbon Sequestration on the Qinghai Plateau, China. PLoS ONE 2015, 10, e0116591. [Google Scholar] [CrossRef]
- Hong, S.; Piao, S.; Chen, A.; Liu, Y.; Liu, L.; Peng, S.; Sardans, J.; Sun, Y.; Peñuelas, J.; Zeng, H. Afforestation neutralizes soil pH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef]
- Podwika, M.; Solek-Podwika, K.; Kaleta, D.; Ciarkowska, K. The Effect of Land-Use Change on Urban Grassland Soil Quality (Southern Poland). J. Soil Sci. Plant Nutr. 2020, 20, 473–483. [Google Scholar] [CrossRef]
- Chirino-Valle, I.; Davis, M.R.; Condrom, L.M. Impact of different tree species on soil phosphorus immediately following grassland afforestation. J. Soil Sci. Plant Nutr. 2016, 16, 477–489. [Google Scholar] [CrossRef]
- An, J.; Chang, H.; Han, S.H.; Khamzina, A.; Son, Y. Changes in basic soil properties and enzyme activities along an afforestation series on the dry Aral Sea Bed, Kazakhstan. Forest Sci. Technol. 2020, 16, 26–31. [Google Scholar] [CrossRef]
- Downey, A.E.; Groffman, P.M.; Mejía, G.A.; Cook, E.M.; Sritrairat, S.; Karty, R.; Palmer, M.I.; McPhearson, T. Soil Carbon Sequestration in Urban Afforestation Sites in NewYork City. Urban For. Urban Green. 2021, 65, 127342. [Google Scholar] [CrossRef]
- Hong, S.; Guodong, H.; Yin, G.; Piao, S.; Dybzinski, R.; Cong, N.; Li, X.; Wang, K.; Peńuelas, J.; Zeng, H.; et al. Divergent responses of soil organic carbon to afforestation. Nat. Sustain. 2020, 3, 694–700. [Google Scholar] [CrossRef]
- Korkanç, S.Y. Effects of afforestation on soil organic carbon and other soil properties. Catena 2014, 123, 62–69. [Google Scholar] [CrossRef]
- Holubík, O.; Podrázský, V.; Vopravil, J.; Khel, T.; Remeš, J. Effect of agricultural lands afforestation and tree species composition on the soil reaction, total organic carbon and nitrogen content in the uppermost mineral soil profile. Soil Water Res. 2014, 9, 192–200. [Google Scholar] [CrossRef]
- Segura, C.; Jiménez, M.N.; Fernández-Ondoño, E.; Navarro, F.B. Effects of afforestation on plant diversity and soil quality in semiarid SE Spain. Forests 2021, 12, 1730. [Google Scholar] [CrossRef]
- Qiu, Z.; Shi, C.; Zhao, M.; Wang, K.; Zhang, M.; Wang, T.; Shi, F. Improving Effects of Afforestation with Different Forest Types on Soil Nutrients and Bacterial Community in Barren Hills of North China. Sustainability 2022, 14, 1202. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Felson, A.J.; Wood, S.A.; Hallett, R.A.; Strickland, M.S.; Bradford, M.A. Positive effects of afforestation efforts on the health of urban soils. Forest Ecol. Manag. 2014, 313, 266–273. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Tang, Z.; Shangguand, Z.; Chang, F.; Jia, F.; Chen, Y.; He, X.; Shi, W.; Deng, L. Effects of grassland afforestation on structure and function of soil bacterial and fungal communities. Sci. Total Environ. 2019, 676, 396–406. [Google Scholar] [CrossRef]
- Kukuļs, I.; Kļaviņš, M.; Nikodemus, O. Changes in soil organic matter and soil humic substances following the afforestation of former agricultural lands in the boreal-nemoral ecotone (Latvia). Geoderma Reg. 2019, 16, e00213. [Google Scholar] [CrossRef]
- Mongil-Manso, J.; Navarro-Hevia, J.; San Martín, R. Impact of Land Use Change and Afforestation on Soil Properties in a Mediterranean Mountain Area of Central Spain. Land 2022, 11, 1043. [Google Scholar] [CrossRef]
- Sokołowska, J.; Józefowska, A.; Woźnica, K.; Zaleski, T. Succession from meadow to mature forest: Impacts on soil biological, chemical and physical properties—Evidence from the Pieniny Mountains, Poland. Catena 2020, 189, 104503. [Google Scholar] [CrossRef]
- Strand, L.T.; Fjellstad, W.; Jackson-Blake, L.; De Wit, H.A. Afforestation of a pasture in Norway did not result in higher soil carbon, 50 years after planting. Land. Urban Plan. 2021, 207, 104007. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Gorlach, E.; Mazur, T. Agricultural Chemistry: Basics of Nutrition and Principles of Fertilization; PWN: Warsaw, Poland, 2001; p. 125. (In Polish) [Google Scholar]
- Kowalenko, C. Assessment of Leco CNS-2000 analyzer for simultaneously measuring total carbon, nitrogen, and sulphur in soil. Commun. Soil Sci. Plant Anal. 2001, 32, 13–14. [Google Scholar] [CrossRef]
- Sohi, S.P.; Powlson, D.S.; Gaunt, J.L. A procedure for isolating soil organic matter fractions suitable for modelling. Soil Sci. Soc. Am. J. 2001, 65, 1121–1128. [Google Scholar] [CrossRef]
- Calderoni, G.; Schnitzer, M. Effects of age on the chemical structure of paleosol humic acids and fulvic acids. Geochim. Cosmochim Acta 1984, 48, 2045–2051. [Google Scholar] [CrossRef]
- Tan, K.T. Soil Sampling, Preparation and Analysis; Taylor &Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Statistica (Data Analysis Software System), version 13.3; StatSoft Inc.: Tulsa, OK, USA, 2019.
- ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar] [CrossRef]
- Mazurek, R.; Kowalska, J.; Gasiorek, M.; Setlak, M. Micromorphological and physico-chemical analyses of cultural layers in the urban soil of a medieval city—A case study from Krakow, Poland. Catena 2016, 141, 73–84. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Effects of afforestation on phosphorus dynamics and biological properties in a New Zealand grassland soil. Plant Soil 2000, 220, 151–163. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Miechówka, A. The role of bilberry and Alpine lady-fern in soil formation within the Carpathian subalpine spruce forest stands. Geoderma 2017, 305, 162–172. [Google Scholar] [CrossRef]
- Xie, H.; Tang, Y.; Yu, M.; Wang, G.G. The effects of afforestation tree species mixing on soil organic carbon stock, nutrients accumulation, and understory vegetation diversity on reclaimed coastal lands in Eastern China. Global Ecol Cons. 2021, 26, e01478. [Google Scholar] [CrossRef]
- Chang, R.; Fu, B.; Liu, G.; Wang, S.; Yao, X. The effects of afforestation on soil organic and inorganic carbon: A case study of the Loess Plateau of China. Catena 2012, 95, 145–152. [Google Scholar] [CrossRef]
- Vesterdal, L.; Rosenoqvist, L.; Van der Salm, K.; Hansen, K.; Gronenberg, B.-J.; Johansson, M. Carbon Sequestration in Soil and Biomass Following Afforestation: Experiences from Oak and Norway Spruce Chronosequences in Denmark, Sweden and the Netherlands.19-53. In Environmental Effects of Afforestation in North-Western Europe; From Field Observations to Decision Support Edited by Gerrit, W.; Heil, Bart Muys, Karin Hansen; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Recio-Vazquez, L.; Almendros, G.; Knicker, H.; Carral, P.; Álvarez, A.-M. Multivariate statistical assessment of functional relationships between soil physical descriptors and structural features of soil organic matter in Mediterranean ecosystems. Geoderma 2014, 230–231, 95–117. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Miechówka, A. The effect of understory on cation binding reactions and aluminium behaviour in acidic soils under spruce forest stands (Southern Poland). Biogeochemistry 2019, 143, 55–66. [Google Scholar] [CrossRef]
- Sokołowska, J.; Józefowska, A.; Zaleski, T. Humus horizon development during natural forest succession process in the Polish Carpathians. J. Mt. Sci. 2022, 19, 647–661. [Google Scholar] [CrossRef]
| Basic Properties of 0–10 cm Horizons of Soils from Studied Stands | ||||
| Parameters/Stands | CGF | CGG | MGF | MGG |
| Sand | b | b | a | a |
| Silt | a | a | b | c |
| Clay | a | a | b | b |
| pH | a | b | bc | c |
| BC | a | b | c | d |
| DHA | a | a | b | c |
| P | a | a | a | a |
| K | a | a | a | b |
| Mg | a | ab | c | d |
| Basic Properties of 10–30 cm Horizons of Soils from Studied Stands | ||||
| Parameters/Stands | CGF | CGG | MGF | MGG |
| Sand | c | c | ab | a |
| Silt | a | a | ab | b |
| Clay | a | a | bc | c |
| pH | a | b | b | b |
| BC | a | b | b | c |
| DHA | a | a | ab | b |
| P | a | a | a | a |
| K | a | b | ab | b |
| Mg | a | a | b | c |
| Parameters/Stands | CGF | CGG | MGF | MGG |
|---|---|---|---|---|
| 0–10 cm | ||||
| Soil colour | 10YR 2-4/2-3 | 10YR 2-4/1-2 | 10YR 2-4/1-4 | 10YR 2-4/2-4 |
| ADI | 1.06 a | 0.93 a | 1.41 ab | 1.59 b |
| C | b | a | ab | b |
| C/N | b | a | a | a |
| R | ab | a | ab | b |
| HA/FA | a | b | ab | b |
| Hu | a | a | a | b |
| 10–30 cm | ||||
| Soil colour | 10YR 4-5/2-4 | 10YR 3-4/2-3 | 10YR 4-5/3-8 | 10YR 3-4/4 |
| ADI | 0.73 a | 0.95 ab | 1.06 b | 1.35 c |
| C | a | a | a | b |
| C/N | a | 5.00 a | a | b |
| R | a | a | a | b |
| HA/FA | a | b | a | b |
| Hu | a | b | c | d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podwika, M.; Ciarkowska, K.; Solek-Podwika, K. Urban Grassland Afforestation as a Public Land Management Tool for Environmental Improvement: The Example of Krakow (Poland). Land 2023, 12, 1042. https://doi.org/10.3390/land12051042
Podwika M, Ciarkowska K, Solek-Podwika K. Urban Grassland Afforestation as a Public Land Management Tool for Environmental Improvement: The Example of Krakow (Poland). Land. 2023; 12(5):1042. https://doi.org/10.3390/land12051042
Chicago/Turabian StylePodwika, Miłosz, Krystyna Ciarkowska, and Katarzyna Solek-Podwika. 2023. "Urban Grassland Afforestation as a Public Land Management Tool for Environmental Improvement: The Example of Krakow (Poland)" Land 12, no. 5: 1042. https://doi.org/10.3390/land12051042





