A Tale of Two Continents (and a Few Islands): Ecology and Distribution of Late Pleistocene Sloths
Abstract
:1. Introduction
2. Regions with Distinct Sloth Faunas
2.1. Temperate North America
2.2. Southern Mexico and Central America
2.3. Northern South America
2.4. West Coast of South America
2.5. Brazilian Intertropical Region
2.6. Andes and Altiplano
2.7. Pampas and Patagonia
3. Sloth Paleoecology
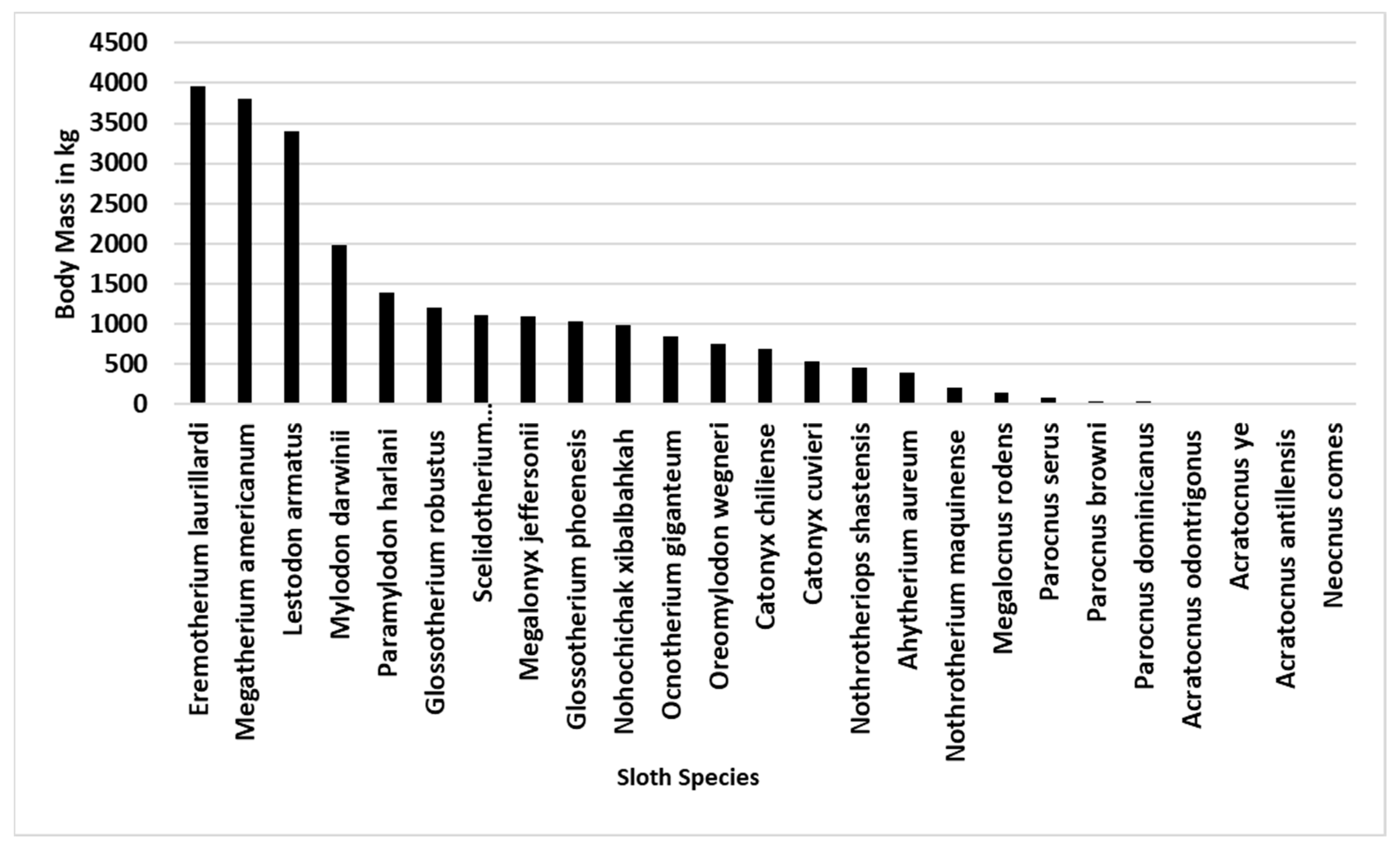
4. Synoptic Overview of Late Pleistocene Ground Sloths
4.1. Family Megatheriidae
4.1.1. Megatherium americanum—Pampas (Figure 2A)
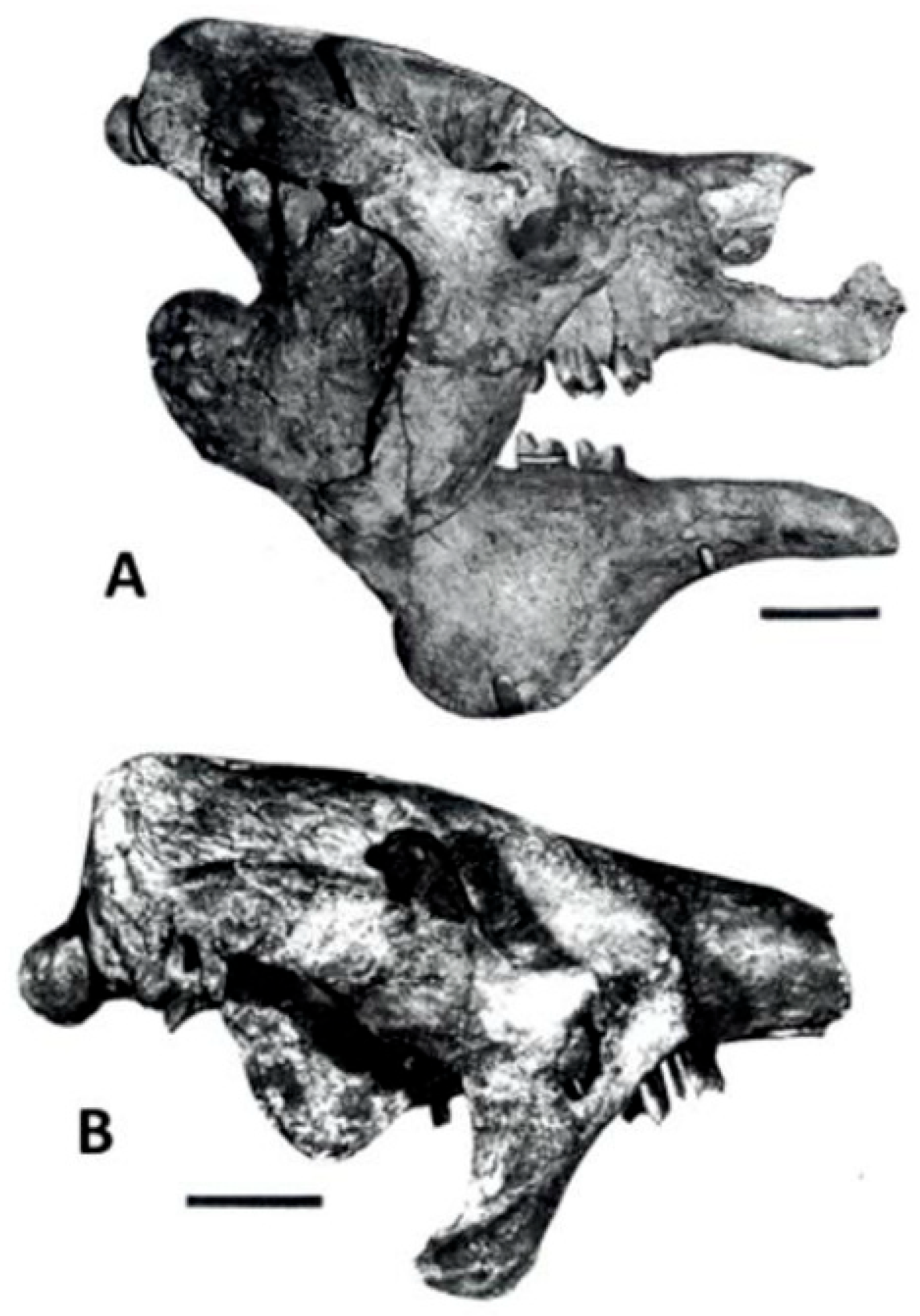
4.1.2. Megatherium celendinense—Andes and Altiplano
4.1.3. Megatherium urbinai—West Coast of South America
4.2. Family Megalonychidae
4.2.1. Megalonyx jeffersonii—Temperate North America (Figure 3A)

4.2.2. Nohochichak xibalbahkah—Southern Mexico and Central America
4.2.3. Xibalaonyx (X. oviceps, X. microcaninus, X. exinferis) Temperate North America, Southern Mexico, and Central America
4.2.4. Aff. Xibalbaonyx—Northern South America
4.2.5. Meizonyx salvadorensis—Southern Mexico and Central America (Figure 3B)
4.2.6. Megistonyx oreobios—Andes and Altiplano
4.2.7. Diabolotherium nordenskioldi—Andes and Altiplano
4.2.8. Ahytherium aureum—Brazilian Intertropical Region (Figure 3C)
4.2.9. Australonyx aquae—Brazilian Intertropical Region (Figure 3D)
4.3. Caribbean Megalonychids
4.4. Family Mylodontidae
4.4.1. Mylodon darwinii—Pampas and Patagonia (Figure 5A)

4.4.2. Glossotherium robustum—Pampas and Patagonia
4.4.3. Glossotherium tropicorum—West Coast South America (Figure 5B)
4.4.4. Glossotherium phoenesis—Brazilian Intertropical Region
4.4.5. Lestodon armatus—Pampas and Patagonia (Figure 5C)
4.4.6. Paramylodon harlani—Temperate North America (Figure 5D)
4.4.7. Oreomylodon wegneri—Andes and Altiplano
4.4.8. Ocnotherium giganteum—Brazilian Intertropical Region
4.4.9. Mylodonopsis ibseni—Brazilian Intertropical Region
4.4.10. Scelidotherium leptocephalum—Pampas and Patagonia (Figure 6A)

4.4.11. Catonyx cuvieri—Brazilian Intertropical Region
4.4.12. Catonyx cuvieri—Pampas, and Patagonia
4.4.13. Catonyx chiliense—West Coast South America (Figure 6B)
4.4.14. Valgipes bucklandi—Brazilian Intertropical Region
4.5. Mylodontid Indet
4.6. Family Nothrotheriidae
4.6.1. Nothrotherium maquinense—Brazilian Intertropical Region, Pampus and Patagonia (Figure 7A)
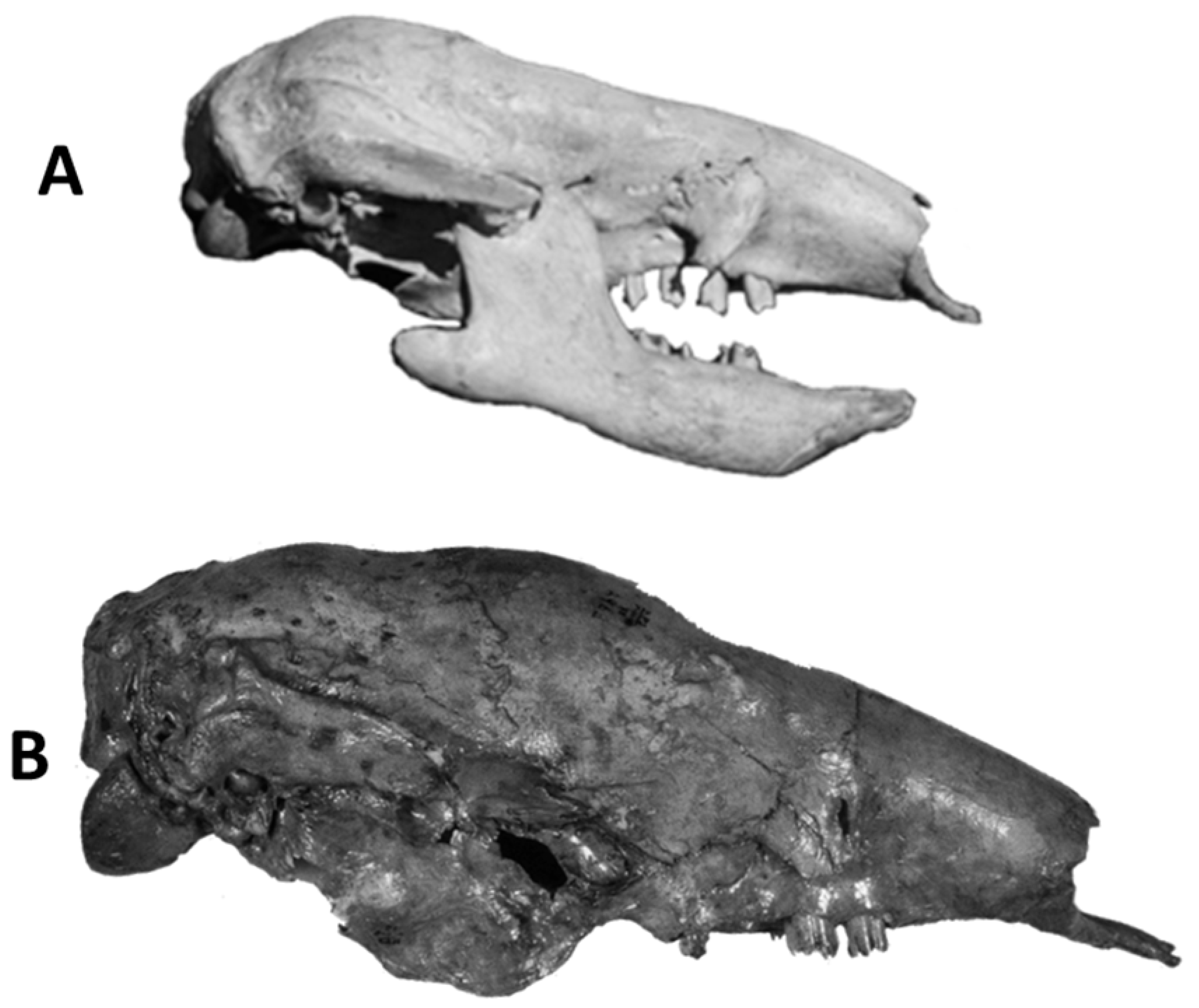
4.6.2. Nothrotherium escrivanense—Brazilian Intertropical Region
4.6.3. Nothrotheriops shastensis—Temperate North America, Southern Mexico, and Central America (Figure 7B)
4.6.4. Nothrotheriops sp.—Pampas and Patagonia
4.6.5. Nothropus—Pampas and Patagonia
5. Discussion
6. Extinction
Funding
Acknowledgments
Conflicts of Interest
References
- Vizcaino, S.F.; Cassini, G.H.; Toledo, N.; Bargo, M.S.; Patterson, B.P.; Costa, L.P. On the evolution of large size in mammalian herbivores of Cenozoic faunas of southern South America. In Bones, Clones, and Biomes; Patterson, B.D., Costa LP, L.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2012; pp. 76–101. [Google Scholar]
- MacPhee, R.D.E.; Iturralde-Vinent, M. First Tertiary land mammal from Greater Antilles: An Early Miocene sloth (Xenarthra, Megalonychidae) from Cuba. Am. Mus. Nat. Hist. Novit. 1994, 3094, 13. [Google Scholar]
- Viñola-Lopez, L.W.; Core Suárez, E.E.; Vélez-Juarbe, J.; Milan, J.A.; Bloch, J. The oldest known record of a ground sloth (Mammalia, Xenarthra, Folivora) from Hispaniola: Evolutionary and paleobiogeographical implications. J. Paleontol. 2022, 96, 684–691. [Google Scholar] [CrossRef]
- McDonald, H.G.; Carranza-Castañeda, O. Increased xenarthran diversity during the early stages of the Great American Biotic Interchange: A new genus and species of ground sloth (Mammalia, Xenarthra, Megalonychidae) from the Hemphillian (Late Miocene) of Jalisco, Mexico. J. Paleontol. 2017, 91, 1069–1082. [Google Scholar] [CrossRef] [Green Version]
- Steadman, D.W.; Martin, P.S.; MacPhee, R.D.E.; Jull, A.J.T.; McDonald, H.G.; Woods, C.A.; Iturralde-Vinent, M.; Hodgins, G.W.L. Asynchronous extinction of late Quaternary sloths on continents and islands. Proc. Natl. Acad. Sci. USA 2005, 102, 11763–11768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, N.; Adams, J. A GIS-based vegetation map of the world at the last glacial maximum 25,000-15,000 BP. Internet Archaeology 11. 2001. Available online: http://intarch.ac.uk/journal/issue11/rayadams_toc.html (accessed on 15 January 2023).
- Vivo, D.; Carmignotto, A.P. Holocene vegetation change and the mammal faunas of South America and Africa. J. Biogeogr. 2004, 31, 943–957. [Google Scholar] [CrossRef]
- Mayle, F.E. The Late Quaternary biogeographical history of South American seasonally dry tropical forests: Insights from palaeo-ecological data. In Neotropical Savannas and Seasonally Dry Forests; Pennington, R.T., Ratter, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 395–416. [Google Scholar]
- Anhuf, D.; Ledru, M.-P.; Behling, H.; Da Cruz, F.W., Jr.; Cordeiro, R.C.; Van der Hammen, T.; Karmann, I.; Marengo, J.A.; De Oliveira, P.E.; Pessenda, L.; et al. Paleo-environmental change in Amazonian and African rainforest during the LGM. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 239, 510–527. [Google Scholar] [CrossRef]
- Cartelle, C. Pleistocene mammals of the Cerrado and Caatinga of Brazil. In Mammals of the Neotropics; Eisenberg, J.F., Redford, K.H., Eds.; The University of Chicago Press: Chicago, IL, USA, 1999; pp. 27–46. [Google Scholar]
- Gallo, V.; Avilla, L.S.; Pereira, R.C.L.; Absolon, B.A. Distributional patterns of herbivore megamammals during the Late Pleistocene of South America. An. Acad. Bras. Ciências 2013, 85, 533–546. [Google Scholar] [CrossRef] [Green Version]
- Moreno, P.I.; Villagrán, C.; Marquet, P.A.; Marshall, L.G. Quaternary paleobiogeography of northern and central Chile. Rev. Chil. Hist. Nat. 1994, 67, 487–502. [Google Scholar]
- Varela, L.; Tambusso, P.S.; Patiño, S.J.; Di Giacomo, M.; Fariña, R.A. Potential distribution of fossil xenarthrans in South America during the Late Pleistocene: Co-occurrence and provincialism. J. Mamm. Evol. 2018, 25, 539–550. [Google Scholar] [CrossRef]
- Toledo, P.M. Descricão do Sincrânio de Eremotherium laurillardi Lund, 1842, Taxonomia e Paleobiogeografia. Unpublished. Master’s Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 1986; p. 127. [Google Scholar]
- Oliveria, E.V.; Dutra, T.L.; Zeltzer, F. Megaterldeos (Mammalia, Xenarthra) do Quaternario de Caçapava do Sul, Rio Grande do Sul, com consideracoes sobre a flora associada. Geol. Colomb. 2002, 27, 77–86. [Google Scholar]
- Corona, A.; Perea, D.; McDonald, H.G. Catonyx cuvieri (Xenarthra, Mylodontidae, Scelidotheriinae) from the Late Pleistocene of Uruguay with comments regarding the systematics of the subfamily. J. Vertebr. Paleontol. 2013, 33, 1214–1225. [Google Scholar] [CrossRef]
- Fariña, R.A.; Vizcaíno, S.F.; Bargo, M.S. Body mass estimations in Lujanian (Late Pleistocene-Early Holocene of South America) mammal megafauna. Mastozool. Neotrop. 1998, 5, 87–108. [Google Scholar]
- Christiansen, P.; Fariña, R.A. Mass estimation of two fossil ground sloths (Mammalia, Xenarthra: Mylodontidae). Senckenberg. Biol. 2003, 83, 95–101. [Google Scholar]
- McDonald, H.G. Paleoecology of extinct Xenarthrans and the Great American Biotic Interchange. Bull. Fla. Mus. Nat. Hist. 2005, 45, 313–333. [Google Scholar]
- Fariña, R.A.; Vizcaino, S.F.; De Iuliis, G. Megafauna, Giant Beasts of Pleistocene South America; Indiana University Press: Bloomington, Indiana, 2013. [Google Scholar]
- Fariña, R.A.; Vizcaino, S.F. Allometry of the bones of living and extinct armadillos (Xenarthra, Dasypoda). Z. Für Saugetierekunde 1997, 62, 65–70. [Google Scholar]
- Scott, K. Postcranial dimensions of ungulates as predictors of body mass. In Body Size in Mammalian Paleobiology: Estimation and Biological Implications; Damuth, J., MacFadden, B.J., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 301–355. [Google Scholar]
- Dantas, M.A.T.; Santos, A.M.A. Inferring the paleoecology of the Late Pleistocene giant ground sloths from the Brazilian Intertropical Region. J. S. Am. Earth Sci. 2022, 117, 103899. [Google Scholar] [CrossRef]
- Fariña, R.A. Trophic relationships among Lujanian mammals. Evol. Theory 1996, 11, 125–134. [Google Scholar]
- Casinos, A. Bipedalism and quadrupedalism in Megatherium: An attempt at biomechanical reconstruction. Lethaia 1996, 29, 87–96. [Google Scholar] [CrossRef]
- Brassey, C.A.; Gardiner, J.D. An advanced shape-fitting algorithm applied to quadrupedal mammals: Improving volumetric mass estimates. R. Soc. Open Sci. 2015, 2, 150302. [Google Scholar] [CrossRef] [Green Version]
- Bargo, M.S. The ground sloth Megatherium americanum: Skull shape, bite forces, and diet. Acta Palaeontol. Pol. 2001, 46, 173–192. [Google Scholar]
- Sanz-Pérez, D.; Fernández, M.H.; Tomassini, R.L.; Montalvo, C.I.; Gasparini, G.M.; Domingo, L. The Pampean region (Argentina) underwent larger variation in aridity than in temperature during the late Pleistocene: New evidence from the isotopic analysis of mammalian taxa. Quat. Sci. Rev. 2022, 286, 107555. [Google Scholar] [CrossRef]
- Amundson, R.; Austin, A.T.; Schuur, E.A.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W.T. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 2003, 17, 1031. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M. The carbon and nitrogen isotope composition of Australian grasses in relation to climate. Funct. Ecol. 2009, 23, 1040–1049. [Google Scholar] [CrossRef]
- Bocherens, H.; Cotte, M.; Bonini, R.A.; Straccia, P.; Scian, D.; Soibelzon, L.; Prevosti, F.J. Isotopic insight on paleodiet of extinct Pleistocene megafaunal Xenarthrans from Argentina. Gondwana Res. 2017, 48, 7–14. [Google Scholar] [CrossRef]
- Fariña, R.A.; Blanco, R.E. Megatherium, the stabber. Proc. R. Soc. Lond. B Biol. Sci. 1996, 263, 1725–1729. [Google Scholar]
- Prieto, A.R. Late Quaternary vegetational and climatic changes in the Pampa grassland of Argentina. Quat. Res. 1996, 45, 73–88. [Google Scholar] [CrossRef]
- Pujos, F. Megatherium celendinense sp. nov. from the Pleistocene of the Peruvian Andes and the phylogenetic relationships of megatheriines. Palaeontology 2006, 49, 285–306. [Google Scholar] [CrossRef]
- Philippi, R.A. Vorläufige nachricht über fossile säugethierknochen von Ulloma, Bolivia. Z. Der Dtsch. Geol. Ges. 1893, 45, 87–96. [Google Scholar]
- De Iuliis, G. On the taxonomic status of Megatherium sundti Philippi, 1893 (Mammalia: Xenarthra: Megatheriidae). Ameghiniana 2006, 43, 161–169. [Google Scholar]
- Pujos, F.; Salas, R. A new species of the genus Megatherium (Mammalia: Xenarthra: Megatheriidae) from the Pleistocene of Sacaco and Tres Ventanas. Palaeontology 2004, 47, 579–604. [Google Scholar] [CrossRef]
- Cartelle, C.; De Iuliis, G. Eremotherium laurillardi: The Panamerican late Pleistocene megatheriid sloth. J. Vertebr. Paleontol. 1995, 15, 830–841. [Google Scholar] [CrossRef]
- McDonald, H.G.; Lundelius, E.L., Jr. The giant ground sloth, Eremotherium laurillardi, (Xenarthra, Megatheriidae) in Texas. In Papers on Geology, Vertebrate Paleontology, and Biostratigraphy in Honor of Michael, O. Woodburne; Albright, L.B., III, Ed.; Museum of Northern Arizona Bulletin: Flagstaff, AZ, USA, 2009; Volume 65, pp. 407–421. [Google Scholar]
- Cruz, F.W., Jr.; Burns, S.J.; Karmann, I.; Sharp, W.D.; Vuille, M.; Ferrari, J.A. A stalagmite record of changes in atmospheric circulation and soil processes in the Brazilian subtropics during the Late Pleistocene. Quat. Sci. Rev. 2006, 25, 2749–2761. [Google Scholar] [CrossRef]
- Larmon, J.T.; McDonald, H.G.; Ambrose, S.; DeSantis, L.R.G.; Lucero, L.J. A year in the life of a giant ground sloth during the Last Glacial Maximum in Belize. Sci. Adv. 2019, 5, eaau1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Porta, J. Edentata Xenarthra del Pleistoceno de Colombia. Nota preliminar. Universidad Industrial de Santander, Publicaciones Cientificas. Bol. Geol. 1961, 6, 5–32. [Google Scholar]
- Solózano, A.; Rincón, A.D.; McDonald, H.G. A new mammal assemblage from the Late Pleistocene El Breal de Orocual, northeast of Venezuela. Nat. Hist. Mus. Los Angeles Cty. Sci. Ser. 2015, 42, 122–147. [Google Scholar]
- Mychajliw, A.M.; Mohammed, R.S.; Rice, K.A.; Farrell, A.B.; Rincón, A.D.; McAfee, R.; McDonald, H.G.; Lindsey, E.L. The biogeography of “breas”: Contextualizing the taphonomy, ecology, and diversity of Trinidad’s asphaltic fossil record. Quat. Sci. Rev. 2020, 232, 21. [Google Scholar] [CrossRef]
- Lindsey, E.L.; Reyes, E.X.L.; Matzke, G.E.; Rice, K.A.; McDonald, H.G. A monodominant late-Pleistocene megafauna locality from Santa Elena, Ecuador: Insight on the biology and behavior of giant ground sloths. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 544, 109599. [Google Scholar] [CrossRef]
- Tito, G. New remains of Eremotherium laurillardi (Lund, 1842) (Megatheriidae, Xenarthra) from the coastal region of Ecuador. J. South Am. Earth Sci. 2008, 26, 424–434. [Google Scholar] [CrossRef]
- Edmund, A.G. A Late Pleistocene fauna from the Santa Elena Peninsula, Ecuador. Royal Ontario Museum. Life Sci. Div. 1965, 63, 1–21. [Google Scholar]
- Ficcarelli, G.; Coltorti, M.; Moreno-Espinosa, M.; Pieruccini, P.L.; Rook, L.; Torre, D. A model for the Holocene extinction of the mammal megafauna in Ecuador. J. South Am. Earth Sci. 2003, 15, 835–845. [Google Scholar] [CrossRef]
- de Melo França, L.; Dantas, M.A.T.; Bocchiglieri, A.; Cherckinsky, A.; de Souza Ribeiro, A.; Bocherens, H. Chronology and ancient feeding ecology of two upper Pleistocene megamammals from the Brazilian Intertropical Region. Quat. Sci. Rev. 2014, 99, 78–83. [Google Scholar] [CrossRef]
- Omena, É.C.; Silva, J.L.L.D.; Sial, A.N.; Cherkinsky, A.; Dantas, M.A.T. Late Pleistocene meso-megaherbivores from Brazilian Intertropical Region: Isotopic diet (δ 13C), niche differentiation, guilds and paleoenvironmental reconstruction (δ 13C, δ 18O). Hist. Biol. 2021, 33, 2299–2304. [Google Scholar] [CrossRef]
- Dantas, M.A.T.; Araújo, A.V.; Nogueira, E.E.; Silva, L.A.; Lessa, C.M.B.; Carvalho, J.C.; Alves, B.S.; Pansani, T.R.; Santos, V.G.; Silva, J.S. Novos registros de fósseis de preguiças gigantes terrícolas (Xenarthra, Tardigrada) em uma caverna de Andaraí, Bahia: Taxonomia e inferências sobre a distribuição geográfica durante o Pleistoceno final. An. 34 Congr. Bras. Espeleol. 2017, 567–573. [Google Scholar]
- Oliveira, J.F.; Asevedo, L.; Cherkinsky, A.; Dantas, M.A.T. Radiocarbon dating and integrative paleoecology (δ13C, stereomicrowear) of Eremotherium laurillardi (LUND, 1842) from midwest region of the Brazilian Intertropical Region. J. South Am. Earth Sci. 2020, 102, 102653. [Google Scholar] [CrossRef]
- McDonald, H.G.; Harington, C.R.; De Iuliis, G. The ground sloth, Megalonyx, from Pleistocene deposits of the Old Crow Basin, Yukon, Canada. Arctic 2000, 53, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Semken, H.A., Jr.; McDonald, H.G.; Graham, R.W.; Adrain, T.; Artz, J.A.; Baker, R.G.; Bryk, A.B.; Brenzel, D.J.; Bettis, E.A.; Clack, A.A.; et al. Paleoecology, taphonomy and potential family relationships of three contemporaneous Jefferson’s ground sloths (Megalonyx jeffersonii), West Tarkio Valley, Southwestern Iowa, USA. J. Vertebr. Paleontol. 2022, 42, e2124115. [Google Scholar] [CrossRef]
- McDonald, H.G.; Feranec, R.S.; Miller, N. First record of the extinct ground sloth, Megalonyx jeffersonii, (Xenarthra, Megalonychidae) from New York and contributions to its paleoecology. Quat. Int. 2019, 530–531, 42–46. [Google Scholar] [CrossRef]
- France, C.A.M.; Zelanko, P.M.; Kaufman, A.J.; Holtz, T.R. Carbon and nitrogen isotopic analysis of Pleistocene mammals from the Saltville Quarry (Virginia, USA): Implications for trophic relationships. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 249, 271–282. [Google Scholar] [CrossRef]
- Islebe, G.A.; Sánchez-Sánchez, O.; Valdéz-Hernández, H.M.; Weissenberger, H. Distribution of vegetation types. In Biodiversity and Conservation of the Yucatan Peninsula; Islebe, G.A., Calmé, S., León-Cortés, J.L., Schmook, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 39–53. [Google Scholar] [CrossRef]
- Barber, A.; Tun, J.; Crespo, M.B. A new approach on the bioclimatology and potential vegetation of the Yucatan Peninsula (Mexico). Phytocoenologia 2000, 31, 1–32. [Google Scholar] [CrossRef]
- McDonald, H.G.; Chatters, J.C.; Gaudin, T.J. A new genus of megalonychid ground sloth (Mammalia, Xenarthra) from the late Pleistocene of Quintana Roo, Mexico. J. Vertebr. Paleontol. 2017, 37, 14. [Google Scholar] [CrossRef]
- Stinnesbeck, S.R.; Frey, E.; Olguin, J.A.; González, A.G.; Morlet, A.V.; Stinnesbeck, W. Life and death of the ground sloth Xibalbaonyx oviceps from the Yucatán Peninsula, Mexico. Hist. Biol. 2021, 33, 2610–2626. [Google Scholar] [CrossRef]
- Stinnesbeck, S.R.; Frey, E.; Olguín, J.A.; Stinnesbeck, W.; Zell, P.; Mallison, H.; González, A.G.; Núñez, E.A.; Morlet, A.V.; Mta, A.T.; et al. Xibalbaonyx oviceps, a new megalonychid ground sloth (Folivora, Xenarthra) from the late Pleistocene of the Yucatán Peninsula, Mexico, and its paleobiogeographic significance. Paläontologische Z. 2017, 91, 245–271. [Google Scholar] [CrossRef]
- Schubert, C. Late Pleistocene Mérida glaciation, Venezuelan Andes. Boreas 1974, 3, 147–152. [Google Scholar] [CrossRef]
- Schubert, C. Evidencias de una glaciación antigua en la Sierra de Perijá, estado Zulia. Boletín Soc. Venez. Espeleol. 1975, 6, 71–75. [Google Scholar]
- Salgado-Laboreau, M.L. Cambios climáticos durante el cuaternario tardío paramero y su correlación con las tierras tropicales calientes. In El Medio Ambiente P’aramo; Salgado–Laboreau, M.L., Ed.; Actas del seminario de Mérida, Venezuela; Ediciones Centro de Estudios Avanzados: Córdoba, Argentina, 1979; pp. 55–66. [Google Scholar]
- McDonald, H.G.; Arroyo-Cabrales, J.; Alarcón-Durán, I.; Espinosa-Martínez, V. First record of Meizonyx salvadorensis (Mammalia, Xenarthra, Pilosa) from Mexico and its phylogenetic position within the Megalonychidae. J. Syst. Palaeontol. 2020, 18, 1829–1851. [Google Scholar] [CrossRef]
- Lozano-Garcia, M.S.; Ortega-Guerrero, B.; Caballero-Miranda, M.; Urrutia-Fucugauchi, J. Late Pleistocene and Holocene paleoenvironments of Chalco Lake, Central Mexico. Quat. Res. 1993, 40, 332–342. [Google Scholar] [CrossRef]
- Metcalfe, S.E.; O’Hara, S.L.; Caballero, M.; Davies, S.J. Records of Late Pleistocene–Holocene climate change in Mexico—A review. Quat. Sci. Rev. 2000, 19, 699–721. [Google Scholar] [CrossRef]
- Correa-Metrio, A.; Bush, M.B.; Hodell, D.A.; Brenner, M.; Escobar, J.; Guilderson, T. The influence of abrupt climate change on the ice-age vegetation of the Central American lowlands. J. Biogeogr. 2012, 39, 497–509. [Google Scholar] [CrossRef]
- Webb, S.D.; Perrigo, S.C. New megalonychid sloths from El Salvador. In The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas; Montgomery, G.G., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1985; pp. 113–120. [Google Scholar]
- McDonald, H.G.; Rincón, A.D.; Gaudin, T.J. A new genus of megalonychid sloth (Mammalia, Xenarthra) from the Late Pleistocene (Lujanian) of Sierra de Perija, Zulia State, Venezuela. J. Vertebr. Paleontol. 2013, 33, 1226–1238. [Google Scholar] [CrossRef]
- Stansell, N.D.; Polissar, P.J.; Abbott, M.B. Last glacial maximum equilibrium-line altitude and paleo–temperature reconstructions for the Cordillera de Mérida, Venezuelan Andes. Quat. Res. 2007, 67, 115–127. [Google Scholar] [CrossRef]
- Shockey, B.J.; Salas-Gismondi, R.; Baby, P.; Guyot, J.L.; Baltazar, M.C.; Huamán, L.; Clack, A.; Stucchi, M.; Pujos, F.; Emerson, J.M.; et al. New Pleistocene cave faunas of the Andes of Central Perú: Radiocarbon Ages and the Survival of Low Latitude, Pleistocene DNA. Palaeontol. Electron. 2009, 12, 15A, 15p. Available online: http://palaeo-electronica.org/2009_3/189/index.html (accessed on 15 January 2023).
- Bostelmann, E.; López, P.; Salas-Gismondi, R.; Mena, F. First record of Diabolotherium cf. nordenskioldi, Kraglievich 1926, (Mammalia, Tardigrada, Megalonychidae), from the Late Pleistocene of Chile. Ameghiniana 2011, 48, 146. [Google Scholar]
- Pujos, F.; De Iuliis, G.; Argot, C.C.; Werdelin, L. A peculiar climbing Megalonychidae from the Pleistocene of Peru and its implication for sloth history. Zool. J. Linn. Soc. 2007, 149, 179–235. [Google Scholar] [CrossRef] [Green Version]
- Furlow, J.J. The systematics of the American species of Alnus (Betulaceae) I. Rhodora 1979, 81, 1–121. [Google Scholar]
- Weng, C.; Bush, M.B.; Chepstow-Lusty, A.J. Holocene changes of Andean alder (Alnus acuminata) in highland Ecuador and Peru. J. Quat. Sci. 2004, 19, 685–691. [Google Scholar] [CrossRef]
- MacPhee, R.D.E.; White, J.L.; Woods, C.A. New megalonychid sloths (Phyllophaga, Xenarthra) from the Quaternary of Hispaniola. Am. Mus. Novit. 2000, 3303, 32. [Google Scholar] [CrossRef]
- White, J.; MacPhee, R. The sloths of the West Indies: A systematic and phylogenetic overview. In Biogeography of the West Indies: Patterns and Perspectives, 2nd ed.; Woods, C.A., Sergile, F.E., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 201–235. [Google Scholar]
- Rega, E.; McFarlane, D.A.; Lundberg, J.; Christenson, K. A new Megalonychid sloth from the Late Wisconsinan of the Dominican Republic. Caribb. J. Sci. 2002, 38, 11–19. [Google Scholar]
- McAfee, R.K.; Beery, S.M.; Rimoli, R.; Almonte, J.; Lehman, P.; Cooke, S.B. New species of the ground sloth Parocnus from the late Pleistocene–early Holocene of Hispaniola. Vertebr. Anat. Morphol. Palaeontol. 2021, 9, 52–82. [Google Scholar] [CrossRef]
- McAfee, R.K. Feeding mechanics and dietary implications in the fossil sloth Neocnus (Mammalia: Xenarthra: Megalonychidae) from Haiti. J. Morphol. 2011, 272, 1204–1216. [Google Scholar] [CrossRef]
- White, J.L. Indicators of locomotor habits in xenarthrans: Evidence for locomotor heterogeneity among fossil sloths. J. Vertebr. Paleontol. 1993, 13, 230–242. [Google Scholar] [CrossRef]
- Arredondo, C.; Villavicencio, R. Tafonomía del depósito arqueológico Solapa del Megalocnus en el noroeste de Villa Clara, Cuba. Rev. Biol. 2004, 18, 160–171. [Google Scholar]
- Antúnez, C.A.; Suárez, R.R. Evidencias directas de herbivorismo en coprolitos de perezosos extintos (Mammalia: Pilosa: Megalonychidae) de Cuba. Rev. Jardín Botánico Nac. 2013, 34–35, 67–73. [Google Scholar]
- Kraglievich, L. Contribucio’n al conocimiento de Mylodon darwini Owen y especies afines. Rev. Mus. Plata 1934, 34, 255–292. [Google Scholar]
- Bargo, M.S.; Toledo, N.; Vizcaíno, S.F. Muzzle of South American Pleistocene ground sloths (Xenarthra, Tardigrada). J. Morphol. 2006, 267, 248–263. [Google Scholar] [CrossRef]
- Brambilla, L.; Haro, J.A. A comparative study of the postcranial skeleton of Patagonian and Pampean specimens of the Pleistocene giant sloth genus Mylodon Owen, 1839 (Xenarthra, Pilosa) and its implications. Hist. Biol. 2022, 1–10. [Google Scholar] [CrossRef]
- Brandoni, D.; Ferrero, B.S.; Brunetto, A. Mylodon darwini Owen (Xenarthra, Mylodontidae) from the Late Pleistocene of Mesopotamia, Argentina, with remarks on individual variability, paleobiology, paleobiogeography, and paleoenvironment. J. Vertebr. Paleontol. 2010, 30, 1547–1558. [Google Scholar] [CrossRef]
- López-Mendoza, P.; Mena-Larraín, F. Extinct ground sloth dermal bones and their role in the taphonomic research of caves: The case of Baño Nuevo-1 (Andean Central Patagonia, Chile). Rev. Mex. Cienc. Geológicas 2011, 28, 519–532. [Google Scholar]
- Moore, D.M. Post-glacial vegetation in the South Patagonian territory of the giant ground sloth, Mylodon. Bot. J. Linn. Soc. 1978, 77, 177–202. [Google Scholar] [CrossRef]
- Markgraf, V. Late Pleistocene faunal extinctions in southern Patagonia. Science 1985, 228, 1110–1112. [Google Scholar] [CrossRef]
- Heusser, C.J.; Borrero, L.A.; Lanata, J.A. Late glacial vegetation at Cueva del Mylodon. Anales del Instituto de la Patagonia. Punía Arenas 1992, 21, 97–102. [Google Scholar]
- van Geel, B.; van Leeuwen, J.F.N.; Nooren, K.; Mol, D.; den Ouden, N.; van der Knaap, P.W.O.; Seersholm, F.V.; Rey-Iglesia, A.; Lorenzen, E.D. Diet and environment of Mylodon darwinii based on pollen of a Late-Glacial coprolite from the Mylodon Cave in southern Chile. Rev. Palaeobot. Palynol. 2022, 296, 104549. [Google Scholar] [CrossRef]
- Tejada, J.V.; Flynn, J.J.; MacPhee, R.; O’Connell, T.C.; Cerling, T.E.; Bermudez, L.; Capuñay, C.; Wallsgrove, N.E.; Popp, B.N. Isotope data from amino acids indicate Darwin’s ground sloth was not an herbivore. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Scillato-Yane, G.J.; Carlini, A.A.; Vizcaíno, S.F.; Juareguizar, E.O. Los Xenarthros. In Evolucion Biológica y Climática de la Region Pampeana durante los últimos Cinco Millones de Años; Alberdi, M.T., Leone, G., Tonni, E.P., Eds.; Un ensayo de Correlacion con el Mediterráneo Occidental. Monógrafías 12; CSIC: Madrid, Spain, 1995; pp. 183–209. [Google Scholar]
- Esteban, G.I. Revision de los Mylodontinae Cuaternarios (Edentata-Tardigrada) de Argentina, Bolivia y Uruguay. Sistematica, Filogenia, Paleobiologıa, Paleozoogeograf´ıa y Paleoecolog’ıa. Ph.D. Thesis, Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucuman, Tucuman, Argentina, 1996; p. 314. [Google Scholar]
- McAfee, R.K. Reassessment of the cranial characters of Glossotherium and Paramylodon (Mammalia: Xenarthra: Mylodontidae). Zool. J. Linn. Soc. 2009, 155, 885–903. [Google Scholar] [CrossRef] [Green Version]
- Mones, A. Palaeovertebrata sudamericana. Catalogo sistematico de los vertebrados fosiles de America del Sur. Parte I Lista preliminar y bibliografıa. Cour. Forsch. Senckenberg 1986, 82, 1–625. [Google Scholar]
- Bargo, M.S. El aparato masticatorio de los perezosos terrestres (Xenarthra, Tardigrada) del Pleistoceno de la Argentina. Morfometrıá y biomecánica. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, 2001. [Google Scholar]
- Bargo, M.S.; De Iuliis, G.; Vizcaíno, S.F. Hypsodonty in Pleistocene ground sloths. Acta Palaeontol. Pol. 2006, 51, 53–61. [Google Scholar]
- Bargo, M.S.; Vizcaíno, S.F.; Archuby, F.M.; Blanco, R.E. Limb bone proportions, strength and digging in some Lujanian (late Pleistocene-early Holocene) mylodontid ground sloths (Mammalia, Xenarthra). J. Vertebr. Paleontol. 2000, 20, 601–610. [Google Scholar] [CrossRef]
- Vizcaíno, S.F.; Zdrate, M.; Bargo, M.S.; Dondas, A. Pleistocene burrows in the Mar del Plata area (Argentina) and their probable builders. Acta Palaeontol. Pol. 2001, 46, 289–301. [Google Scholar]
- Tonni, E.P.; Cione, A.L. Los mamiferos como indicadores de cambios climáticos en el Cuaternario de la Región Pampeana de la Argentina. In Climas Cuaternąrtos en América del Sur; Argo1lo, J., Mourguiart, P., Eds.; Orstom: La Paz, Bolivia, 1995; pp. 319–326. [Google Scholar]
- Tonni, E.P.; Cione, A.L. Did the Argentine Pampean ecosystem exist in the Pleistocene? Curr. Res. Pleistocene 1997, 14, 131–133. [Google Scholar]
- McNab, B.K. Energetics, population biology, and distribution of xenarthrans, living and extinct. In The Evolution and Ecology of Armadillos, Sloths and Vermilinguas; Montgomery, G.G., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1985; pp. 219–232. [Google Scholar]
- Czerwonogora, A.; Fariña, R.A.; Tonni, E.P. Diet and isotopes of Late Pleistocene ground sloths: First results for Lestodon and Glossotherium (Xenarthra, Tardigrada). Neues Jahrb. Für Geol. Und Paläontologie 2011, 262, 257–266. [Google Scholar] [CrossRef]
- Iriondo, M.H.; García, N.O. Climatic variations in the Argentine plains during the last 18,000 years. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1993, 101, 209–220. [Google Scholar] [CrossRef]
- Iriondo, M. Climatic changes in the South American plains: Records of a continent-scale oscillation. Quat. Int. 1999, 57, 93–112. [Google Scholar] [CrossRef]
- Tonni, E.P.; Cione, A.L.; Figini, A.J. Predominance of arid climates indicated by mammals in the pampas of Argentina during the Late Pleistocene and Holocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 147, 257–281. [Google Scholar] [CrossRef]
- Hoffstetter, R. Les mammifères pléistocènes de la République de l’Equateur. Mémoires Société Géologique Fr. 1952, 66, 1–391. [Google Scholar]
- Román-Carrión, J.; Brambilla, L. Comparative skull osteology of Oreomylodon wegneri (Xenarthra, Mylodontinae): Defining the taxonomic status of the Ecuadorian endemic mylodontid. J. Vertebr. Paleontol. 2019, 39, e1674860. [Google Scholar] [CrossRef]
- Cartelle, C.; De Iuliis, G.; Boscaini, A.; Pujos, F. Anatomy, possible sexual dimorphism, and phylogenetic affinities of a new mylodontine sloth from the Late Pleistocene of intertropical Brazil. J. Syst. Palaeontol. 2019, 17, 1957–1988. [Google Scholar] [CrossRef]
- Henriques, D.D.R. Os fosseis de Lestodon Gervais, 1855 (Edentata, Mylodontidae) da Colecão de Paleovertebrados do Museu Nacional/UFRJ. Estudo Morfologico e Comparativo. Unpublished Mater’s Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 1992; p. 182. [Google Scholar]
- Vargas -Peixoto, D.; Colusso, C.S.; Da-Rosab, Á.A.S.; Kerber, L. A new record of Lestodon armatus Gervais 1855 (Xenarthra, Mylodontidae) from the Quaternary of southern Brazil and remarks on its postcranial anatomy. Hist. Biol. 2021, 33, 159–175. [Google Scholar] [CrossRef]
- Naples, V.L. The feeding mechanism in the Pleistocene ground sloth, Glossotherium. Nat. Hist. Mus. Los Angeles Cty. Contrib. Sci. 1989, 415, 1–23. [Google Scholar] [CrossRef]
- Pérez-Crespo, V.A.; Carbot-Chanona, G.; Morales-Puente, P.; Cienfuegos-Alvarado, E.; Otero, F.J. Paleoambiente de la Depresión Central de Chiapas, con base en isótopos estables de carbono y oxígeno. Rev. Mex. Cienc. Geológicas 2015, 32, 273–282. [Google Scholar]
- Díaz-Sibaja, R.; Jiménez-Hidalgo, E.; Ponce-Saavedra, J.; García-Zepeda, M.L. A combined mesowear analysis of Mexican Bison antiquus shows a generalist diet with geographical variation. J. Paleontol. 2018, 92, 1130–1139. [Google Scholar] [CrossRef]
- McDonald, H.G.; Pelikan, S. Mammoths and mylodonts: Exotic species from two different continents in North American Pleistocene faunas. Quat. Int. 2006, 142–143, 229–241. [Google Scholar] [CrossRef]
- Ruez, D.R., Jr. Diet of Pleistocene Paramylodon harlani (Xenarthra: Mylodontidae): Review of methods and preliminary use of carbon isotopes. Tex. J. Sci. 2005, 57, 329–344. [Google Scholar]
- Coltrain, J.B.; Harris, J.M.; Cerling, T.E.; Ehleringer, J.R.; Dearing, M.D.; Ward, J.; Allen, J. Rancho La Brea stable isotope biogeochemistry and its implications for the palaeoecology of late Pleistocene, coastal southern California. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 205, 199–219. [Google Scholar] [CrossRef]
- Gilmour, D.M.; Butler, V.L.; O’Connor, J.E.; Davis, E.B.; Culleton, B.J.; Kennett, D.J.; Hodgins, G. Chronology and ecology of late Pleistocene megafauna in the northern Willamette Valley, Oregon. Quat. Res. 2015, 83, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Clapperton, C.M. Glacial geomorphology. Quaternary glacial sequence and paleoclimatic inferences in the Ecuadorian Andes. In International Geomorphology 1986, Part II; Gardiner, V., Ed.; Wiley: London, UK, 1987; pp. 843–870. [Google Scholar]
- Porter, S.C. Pleistocene glaciation in the southern Lake District of Chile. Quat. Res. 1981, 24, 269–292. [Google Scholar] [CrossRef]
- Ficcarelli, G.; Azzaroli, A.; Bertini, A.; Coltorti, M.; Mazza, P.; Mezzabotta, C.; Torre, D. Hypothesis on the cause of extinction of the South American mastodonts. J. S. Am. Earth Sci. 1997, 10, 29–38. [Google Scholar] [CrossRef]
- Cartelle, C. Estudo comparativo do rádio e esqueleto da máo de Glossotherium (Ocnotherum) giganteum Lund, 1842. An. Acad. Bras. Ciências 1980, 52, 359–377. [Google Scholar]
- Cartelle, C. Um novo Mylodontinae (Edentata, Xenarthra) do Pleistoceno final da região intertropical Brasileira. An. Acad. Bras. Ciências 1991, 63, 161–170. [Google Scholar]
- Dantas, M.A.T.; Zucon, M.H. Occurrence of Catonyx cuvieri (Lund, 1839) (Tardigrada, Scelidotheriinae) in Late Pleistocene-Holocene of Brazil. Rev. Bras. Paleontol. 2007, 10, 129–132. [Google Scholar] [CrossRef]
- Dantas, M.A.T.; Cherkinsky, A.; Bocherens, H.; Drefahl, M.; Bernardes, C.; França, L.M. Isotopic paleoecology of the Pleistocene megamammals from the Brazilian Intertropical Region: Feeding ecology (d13C), niche breadth and overlap. Quat. Sci. Rev. 2017, 170, 152–163. [Google Scholar] [CrossRef]
- Dantas, M.A.T.; Porpino, K.D.; Bauermann, S.G.; Prata, A.D.N.; Cozzuol, M.A.; Kinoshita, A.; Barbosa, J.H.O.; Baffa, O. Megafauna do Pleistoceno superior de Sergipe, Brasil: Registros taxonômicos e cronológicos. Rev. Brasil. Paleo. 2011, 14, 311–320. [Google Scholar] [CrossRef]
- Bergqvist, L.P.; Gomide, M.; Cartelle, C.; Capilla, R. Faunas locais e mamíferos pleistocênicos de Itapipoca/Ceará, Taperoa/Paraíba, e Campina Grande/Paraíba. Estudo comparativo, Bioestratinomico e Paleoambiental. Rev. Univ. Guarulhos: Geociências 1997, 2, 23–32. [Google Scholar]
- Curvello, C.M.A.; Faure, M.; Hugueney, M.; Mourer-Chauviré, C. A fauna pleistocênica do Piauí (Nordeste do Brasil): Relações paleoecológicas e biocronológicas. Fundhamentos 1996, 3, 56–103. [Google Scholar]
- Born, P.A.; Dias Neto, C.D.M.; Pellaes, F. Registro de Mamíferos Pleistocênicos no Estado de Alagoas, Nordeste do Brasil; Boletim de Resumo, Congresso Brasileiro de Paleontologia: Brasília, Brazil, 2003; p. 81. [Google Scholar]
- Vasconcelos, A.G.; Vilaboim, L.S.; Kraemer, B.M. New occurrence of Pleistocenic mammals in Chapada Diamantina, Brazil. In A Paleoenvironmental Studies Collaboration; Boletim de Resumo, Congreso Latinoamericano de Paleontología de Vertebrados, 3: Neuquén, Argentina, 2008; p. 258. [Google Scholar]
- Ghilardi, A.M.; Fernandes, M.A.; Bichuette, M.E. Megafauna from the late Pleistocene-Holocene deposits of the upper Ribeira karst area, southeast Brazil. Quat. Int. 2011, 245, 369–378. [Google Scholar] [CrossRef]
- Ubilla, M.; Perea, D. Quaternary fossil vertebrates of Uruguay: A biostratigraphic, biogeographic and climate overview. Quat. S. Am. Antarct. Penins. 1999, 12, 75–90. [Google Scholar]
- Martínez, S.; Ubilla, M. El cuaternario en Uruguay. In Cuencas Sedimentarias de Uruguay, Cenozoico; Veroslavsky, G., Ubilla, M., Martínez, S., Eds.; Ediciones DIRAC, Facultad de Ciencias: Montevideo, Uruguay, 2004; pp. 195–227. [Google Scholar]
- Ubilla, M. Late Pleistocene of South America. In Encyclopedia of Quaternary Science; Elias, S.A., Ed.; Royal Holloway University of London: Egham, UK; Elsevier: Surrey, England, 2007; pp. 3175–3189. [Google Scholar]
- Miño-Boilini, A.R.; Carlini, A.A.; Chiesa, J.O.; Lucero, N.P.; Zurita, A.E. First record of Scelidodon chiliense (Lydekker) (Phyllophaga, Scelidotheriinae) from the Lujanian stage (late Pleistocene-early Holocene) of Argentina. Neues Jahrb. Für Geol. Paläontologie-Abh. 2009, 253, 373–381. [Google Scholar] [CrossRef]
- Lobato, C.; Varela, L.; Tambusso, P.S.; Miño-Boilini, A.; Clavijo, L.; Farina, R.A. Presence of the ground sloth Valgipes bucklandi (Xenarthra, Folivora, Scelidotheriinae) in southern Uruguay during the Late Pleistocene: Ecological and biogeographical implications. Quat. Int. 2021, 601, 104–115. [Google Scholar] [CrossRef]
- Pereira, I.C.S.; Dantas, M.A.T.; Ferreira, R.L. Record of the giant sloth Valgipes bucklandi (Lund, 1839) (Tardigrada, Scelidotheriinae) in Rio Grande do Norte state, Brazil, with notes on taphonomy and paleoecology. J. S. Am. Earth Sci. 2013, 43, 42–45. [Google Scholar] [CrossRef]
- Woodburne, M.O. A Late Pleistocene occurrence of the collared peccary, Dicotyles tajacu, in Guatemala. J. Mammal. 1969, 50, 121–125. [Google Scholar] [CrossRef]
- Hatt, R.T. Faunal and archaeological researches in Yucatan Caves. Cranbrook Inst. Sci. Bull. 1953, 33, 1–119. [Google Scholar]
- Gazin, C.L. Exploration for the remains of giant ground sloths in Panama. Smithson. Rep. 1957, 1956, 341–354. [Google Scholar]
- Lucas, S.G. Late Pleistocene mammals from El Hatillo, Panama. Rev. Geológica América Cent. 2014, 50, 139–151. [Google Scholar]
- de Paula Couto, C.D. On two small Pleistocene ground-sloths. An. Acad. Bras. Ciências 1971, 43, 500–513. [Google Scholar]
- Cartelle, C.; Fonseca, J.S. Contribuição ao melhor conhecimento da pequena preguiça terrícola Nothrotherium maquinense (Lund) Lydekker, 1889. Lundiana. Int. J. Biodivers. 1982, 2, 127–182. [Google Scholar]
- Pujos, F. Nouvelles donn6es sur le genre Nothrotherium Lydekker, 1889 et validit6 des espéces N. maquinense (Lund, 1839) et N. escrivanense (Reinhardt, 1878). Geobios 2001, 34, 349–356. [Google Scholar] [CrossRef]
- Perea, D. Nothrotherium cf. N. maquinense (Xenarthra, Tardigrada) en la Formación Sopas (Pleistoceno tardío de Uruguay). Rev. Soc. Urug. Geol. 2007, 14, 5–9. [Google Scholar]
- Ubilla, M.; Corona, A.; Rinderknecht, A.; Perea, D.; Verde, M. Marine Isotope Stage 3 (MIS 3) and continental beds of northern Uruguay (Sopas Formation): Paleontology, chronology, and climate. In Marine Isotope Stage 3 in Southern South America, 60 ka B.P.-30 ka B.P; Gasparini, G., Rabassa, J., Deschamps, C., Tonni, E.P., Eds.; Springer Earth System Sciences: Berlin/Heidelberg, Germany, 2016; pp. 183–205. [Google Scholar]
- Schulthess, B. Beiträge zur Kenntniss der Xenarthra auf Grund der “Santiago Roth’schen Sammlung” des Zoologischen Museum der Universität Zürich, das Skelett der Hand und des Fusses der Xenarthra, etc. Mémoires Soc. Paléontologique Suisse 1920, 44, 1–120. [Google Scholar]
- Poinar, H.N.; Hofreiter, M.; Spaulding, W.G.; Martin, P.S.; Stankiewicz, B.A.; Bland, H.; Evershed, R.P.; Possnert, G.; Pääbo, S. Molecular coproscopy: Dung and diet of the extinct ground sloth Nothrotheriops shastensis. Science 1998, 281, 402–406. [Google Scholar] [CrossRef] [Green Version]
- Hansen, R.M. Shasta ground sloth food habits, Rampart Cave, Arizona. Palaeobiology 1978, 4, 302–319. [Google Scholar] [CrossRef]
- Laudermilk, J.D.; Munz, P.A. Plants in the dung of Nothrotherium from Gypsum Cave, Nevada. Carnegie Inst. Wash. Publ. No. 1934, 453, 29–37. [Google Scholar]
- Thompson, R.S.; Van Devender, T.R.; Martin, P.S.; Foppe, T.; Long, A. Shasta ground sloth (Nothrotheriops shastense Hoffstetter) at Shelter Cave, New Mexico: Environment, diet, and extinction. Quat. Res. 1980, 14, 360–376. [Google Scholar] [CrossRef]
- Gill, F.L.; Crump, M.P.; Schouten, R.; Bull, I.D. Lipid analysis of a ground sloth coprolite. Quat. Res. 2009, 72, 284–288. [Google Scholar] [CrossRef]
- McDonald, H.G.; Jefferson, G.T. Distribution and habitat of Nothrotheriops (Xenarthra, Nothrotheridae) in the Pleistocene of North America. In: Wang X and Barnes LG (Editors.)–Geology and Vertebrate Palaeontology of Western and Southern North America, Contributions in Honor of David P. Whistler. Nat. Hist. Mus. Los Angeles Cty. Sci. Ser. No. 2008, 41, 313–331. [Google Scholar]
- Iuliis, D.; McDonald, H.G.; Stanchly, N.; Spenard, J.; Powis, T.G. Nothrotheriops shastensis (Sinclair, 1905) from Actun Lak: First record of Nothrotheridae (Mammalia, Xenarthra, Pilosa) from Belize. Ameghiniana 2015, 52, 153–171. [Google Scholar] [CrossRef]
- Warter, J.K. Late Pleistocene plant communities—Evidence from the Rancho La Brea tar pits. In Symposium Proceedings on Plant Communities of Southern California; California Native Plant Society Special Publication: Pasadena, CA, USA, 1976; Volume 2, pp. 32–39. [Google Scholar]
- Shaw, C.A.; Quinn, J.P. Rancho La Brea: A look at coastal southern California’s past. Calif. Geol. 1986, 39, 123–133. [Google Scholar]
- Bonde, A.M. Palaeoecology of Late Pleistocene megaherbivores: Stable isotope reconstruction of environment, climate, and response. Ph.D. Thesis, University of Nevada, Las Vegas, NV, USA, 2013; p. 213. [Google Scholar]
- McDonald, H.G. Paleoecology of the extinct Shasta ground sloth, Nothrotheriops shastensis, (Xenarthra, Nothrotheriidae): The physical environment. Bull. New Mex. Mus. Nat. Hist. Sci. 2022, 88, 33–43. [Google Scholar]
- Brandoni, D.; Vezzosi, R.I. Nothrotheriops sp. (Mammalia, Xenarthra) from the Late Pleistocene of Argentina: Implications for the dispersion of ground sloths during the Great American Biotic Interchange. Boreas 2019, 48, 879–890. [Google Scholar] [CrossRef]
- Brandoni, D.; McDonald, H.G. An enigmatic Nothrotheriinae (Xenarthra, Tardigrada) from the Pleistocene of Argentina. Ameghiniana 2015, 52, 294–302. [Google Scholar] [CrossRef]
- Cione, A.L.; Gasparini, G.M.; Soibelzon, E.; LH, L.H.S.; Tonni, E.P. 2015. The Great American biotic interchange. a South American perspective. In Springer Brief Monographies in Earth System Sciences. South America and the Southern Hemisphere; Rabassa, J., Lohmann, G., Notholt, J., Mysak, L.A., Unnithan, V., Eds.; Springer: Berlin, Germany, 1997. [Google Scholar] [CrossRef]
- Vucetich, M.G.; Deschamps, C.M.; Pérez, M.E. The first capybaras (Rodentia, Caviidae, Hydrochoerinae) involved in the Great American Biotic Interchange. Ameghiniana 2015, 52, 324–333. [Google Scholar] [CrossRef]
- Graham, R.W. Quaternary mammal communities: Relevance of the individualistic response and non-analogue faunas. Paleontol. Soc. Pap. 2005, 11, 141–158. [Google Scholar] [CrossRef]
- Semken, H.A., Jr.; Graham, R.W.; Stafford, T.W., Jr. AMS 14C analysis of Late Pleistocene non-analog faunal components from 21 cave deposits in southeastern North America. Quat. Int. 2010, 217, 240–255. [Google Scholar] [CrossRef]
- Janzen, D.H. Latent extinction—The living dead. In Encyclopedia of Biodiversity; Academic Press: Salt Lake City, NJ, USA, 2001; Volume 3, pp. 689–699. [Google Scholar]
- Janzen, D.H.; Martin, P.S. Neotropical anachronisms: What the gomphotheres ate. Science 1982, 215, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, P.R., Jr.; Galetti, M.; Jordano, P. Seed dispersal anachronisms: Rethinking the fruits extinct megafauna ate. PLoS ONE 2008, 3, e1745. [Google Scholar] [CrossRef] [Green Version]
- Valiente-Banuet, A.; Aizen, M.A.; Alcantara, J.M.; Arroyo, J.; Cocucci, A.; Galetti, M.; Garcıa, M.B.; Garcıa, D.; Gomez, J.M.; Jordano, P.; et al. Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 2015, 29, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Owen-Smith, R.N. Megaherbivores, The influence of very large body size on ecology. In Cambridge Studies in Ecology; Cambridge University Press: London, UK, 1988. [Google Scholar]
- McDonald, H.G. Biogeography and paleoecology of ground sloths in California, Arizona, and Nevada. San Bernardino Cty. Mus. Assoc. Q. 1996, 43, 61–65. [Google Scholar]
- McDonald, H.G.; Ray, C.E. The ground sloth, Megalonyx, from the North Atlantic Continental Shelf. Proc. Biol. Soc. Wash. 1990, 103, 1–5. [Google Scholar]
- Aires, A.S.S.; Lopes, R.P. Representativity of Quaternary mammals from the southern Brazilian continental shelf. Rev. Bras. Paleontol. 2012, 15, 57–66. [Google Scholar] [CrossRef]
- Fray, C.; Ewing, M. Pleistocene sedimentation and fauna of the Argentine shelf. I. Wisconsin sea level as indicated in Argentine Continental shelf sediments. Proc. Acad. Nat. Sci. USA Phila. 1963, 115, 113–126. [Google Scholar]
- Varela, L.; Fariña, R. Co-occurrence of mylodontid sloths and insights on their potential distributions during the late Pleistocene. Quat. Res. 2016, 85, 66–74. [Google Scholar] [CrossRef]
- Hershkovitz, P. A geographic classification of Neotropical Mammals. Fieldiana Zool. 1958, 36, 581–620. [Google Scholar]
- Anderson, B.J. The historical development of the Tension Zone concept in the Great Lakes Region of North America. Great Lakes Bot. 2005, 44, 127–138. [Google Scholar]
- Lopes, R.P.; Pereira, J.C. On the presence of Megatherium Cuvier, 1796 (Xenarthra, Pilosa) in fossiliferous deposits of the coastal plain of southern Brazil. Rev. Bras. Paleontol. 2019, 22, 38–52. [Google Scholar] [CrossRef]
- Stinnesbeck, S.R.; Frey, E.; Stinnesbeck, W. New insights on the palaeogeographic distribution of the Late Pleistocene ground sloth genus Xibalbaonyx along the Mesoamerican Corridor. J. S. Am. Earth Sci. 2018, 85, 108–120. [Google Scholar] [CrossRef]
- McDonald, H.G. A Systematic Review of the Plio-Pleistocene Scelidotherinae Ground Sloth (Mammalia: Xenarthra: Mylodontidae). Ph.D Thesis, University of Toronto, Toronto, ON, Canada, 1987; p. 478. [Google Scholar]
- Casamiquela, R. Los vertebrados jurásicos de la Argentina y Chile. Actas IV Congr. Lat. Zool. 1970, 2, 873–890. [Google Scholar]
- Zurita, A.E.; Carlini, A.A.; Scillato-Yané, G.J.; Tonni, E.P. Mamíferos extintos del Cuaternario de la Provincia de Chaco (Argentina) y su relación con aquellos del este de la región pampeana y de Chile. Rev. Geológica De Chile 2004, 31, 65–87. [Google Scholar] [CrossRef]
- Iuliis, D.; Cartelle, C.; McDonald, H.G.; Pujos, F. The Mylodontine ground sloth Glossotherium tropicorum from the late Pleistocene of Ecuador and Peru. Pap. Palaeontol. 2017, 3, 613–636. [Google Scholar] [CrossRef]
- De Iuliis, G.; Boscaini, A.; Pujos, F.R.F.; McAfee, R.K.; Cartelle, C.; Tsuji, L.J.; Rook, L. On the status of the giant mylodontine sloth Glossotherium wegneri (Spillmann, 1931) (Xenarthra, Folivora) from the late Pleistocene of Ecuador. Comptes Rendus Palevol 2020, 19, 215–232. [Google Scholar] [CrossRef]
- Redmond, B.G.; McDonald, H.G.; Greenfield, H.J.; Burt, M.L. New evidence for Late Pleistocene human exploitation of Jefferson’s ground sloth (Megalonyx jeffersonii) from northern Ohio, USA. World Archaeol. 2012, 44, 75–101. [Google Scholar] [CrossRef]
- Bustos, D.J.; Jakeway, T.M.; Urban, V.T.; Holliday, B.; Fenerty, D.A.; Raichlen, M.; Budka, S.C.; Reynolds, B.D.; Allen, D.W.; Love, V.L.; et al. Footprints preserve terminal Pleistocene hunt? Human-Sloth Interactions in North America. Sci. Adv. 2018, 4, eaar7621. [Google Scholar] [CrossRef] [Green Version]
- Dantas, M.A.T.; de Queiroz, A.N.; Santos, V.D.; Cozzuol, M.A. An anthropogenic modification in an Eremotherium tooth from northeastern Brazil. Quat. Int. 2012, 253, 107–109. [Google Scholar] [CrossRef]
- Meltzer, D.J. Overkill, glacial history, and the extinction of North America’s ice age megafauna. Proc. Natl. Acad. Sci. USA 2020, 117, 28555–28563. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.; Faith, J.T. The paleoecological impact of grazing and browsing: Consequences of the late Quaternary large herbivore extinctions. In The Landscape Ecology of Fire, Analysis and Synthesis. The Ecology of Browsing and Grazing II, Ecological Studies; Gordon, I.J., Prins, H.H.T., Eds.; Springer Nature Switzerland AG: Basel, Switzerland, 2019; Volume 239, pp. 61–79. [Google Scholar] [CrossRef]
- Gill, J.L.; Williams, J.W.; Jackson, S.T.; Lininger, K.B.; Robinson, G.S. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science 2009, 326, 1100–1103. [Google Scholar] [CrossRef] [Green Version]
- Pauli, J.N.; Mendoza, J.E.; Steffan, S.A.; Carey, C.C.; Weimer, P.J.; Peery, M.Z. A syndrome of mutualism reinforces the lifestyle of a sloth. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couvreur, M.; Christiaen, B.; Verheyen, K.; Hermy, M. Large herbivores as mobile links between isolated nature reserves through adhesive seed dispersal. Appl. Veg. Sci. 2004, 7, 229–236. [Google Scholar] [CrossRef]
- Lenz, L.W. Seed dispersal in Yucca brevifolia (Agavaceae)-present and past, with consideration of the future of the species. Aliso A J. Syst. Florist. Bot. 2001, 20, 61–74. [Google Scholar] [CrossRef] [Green Version]

| TAXON | TNA | SMCA | NSA | WCSA | AA | BIR | PP | CI |
|---|---|---|---|---|---|---|---|---|
| Megatheriidae | ||||||||
| Megatherium americanum | X | |||||||
| Megatherium celendinense | X | |||||||
| Megatherium medinae | X | |||||||
| Megatherium sundti | X | |||||||
| Megatherium tarijense | X | |||||||
| Megatherium urbinai | X | |||||||
| Eremotherium laurillardi | X | X | X | X | X | |||
| Megalonychidae | ||||||||
| Megalonyx jeffersonii | X | |||||||
| Nohochichak xibalbahkah | X | |||||||
| Xibalbaonyx oviceps | X | G | ||||||
| Xibalbaonyx exinferis | X | |||||||
| Xibalbaonyx microcaninus | X | |||||||
| Meizonyx salvadorensis | X | |||||||
| Megistonyx oreobios | X | |||||||
| Diabolotherium nordenskioldi | X | |||||||
| Ahytherium aureum | X | |||||||
| Australonyx aquae | X | |||||||
| Megalocnus rodens | X (H) | |||||||
| Parocnus serus | X (H) | |||||||
| Parocnus browni | X (C) | |||||||
| Parocnus dominicanus | X (H) | |||||||
| Acratocnus odontrigonus | X (P) | |||||||
| Acratocnus ye | X (H) | |||||||
| Acratocnus simorhynchus | X (H) | |||||||
| Acratocnus antillensis | X (C) | |||||||
| Neocnus comes | X (H) | |||||||
| Neocnus dousman | X (H) | |||||||
| Neocnus toupiti | X (H) | |||||||
| Neocnus major | X (C) | |||||||
| Neocnus gliriformis | X (C) | |||||||
| Mylodontidae | ||||||||
| Glossotherium robustum | X | |||||||
| Glossotherium tropicorum | X | |||||||
| Glossotherium phoenesis | X | |||||||
| Mylodon darwinii | X | |||||||
| Lestodon armatus | X | |||||||
| Paramylodon harlani | X | |||||||
| Oreomylodon wegneri | X | |||||||
| Ocnotherium giganteum | X | |||||||
| Mylodonopsis ibseni | X | |||||||
| Scelidotherium leptocephalum | X | |||||||
| Catonyx cuvieri | X | |||||||
| Catonyx chiliense | X | |||||||
| Valgipes bucklandi | X | |||||||
| Nothrotheriidae | ||||||||
| Nothrotheriops shastensis | X | X | G | |||||
| Nothropus priscus | X | |||||||
| Nothrotherium maquinense | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonald, H.G. A Tale of Two Continents (and a Few Islands): Ecology and Distribution of Late Pleistocene Sloths. Land 2023, 12, 1192. https://doi.org/10.3390/land12061192
McDonald HG. A Tale of Two Continents (and a Few Islands): Ecology and Distribution of Late Pleistocene Sloths. Land. 2023; 12(6):1192. https://doi.org/10.3390/land12061192
Chicago/Turabian StyleMcDonald, H. Gregory. 2023. "A Tale of Two Continents (and a Few Islands): Ecology and Distribution of Late Pleistocene Sloths" Land 12, no. 6: 1192. https://doi.org/10.3390/land12061192
APA StyleMcDonald, H. G. (2023). A Tale of Two Continents (and a Few Islands): Ecology and Distribution of Late Pleistocene Sloths. Land, 12(6), 1192. https://doi.org/10.3390/land12061192







