Disentangling the Belowground Web of Biotic Interactions in Temperate Coastal Grasslands: From Fundamental Knowledge to Novel Applications
Abstract
:1. Introduction
2. Heterogeneity of Environmental Conditions in Coastal Grasslands
3. Diversity in Biological Interactions in Coastal Grasslands
4. Mycorrhizal Symbiosis in Coastal Grasslands
4.1. Mycorrhizal vs. Non-Mycorrhizal Plants
4.2. Mycorrhizal Fungal Community Structure
4.3. Mycorrhizal Symbiosis in Resource Acquisition
4.4. Common Mycorrhizal Networks
4.5. Mycorrhizal Symbiosis in Environmental Resilience
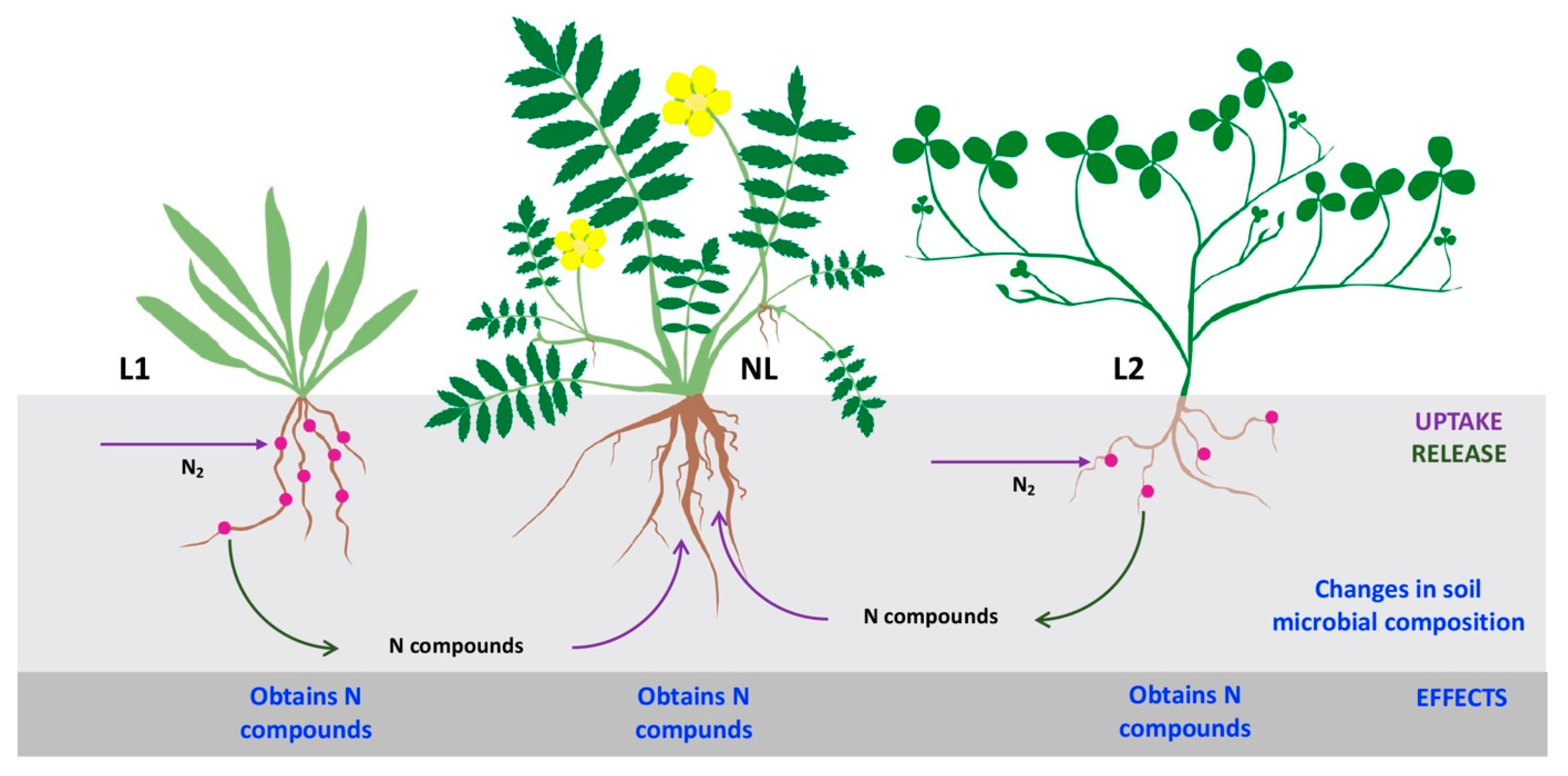
5. Rhizobial Symbiosis in Coastal Grasslands
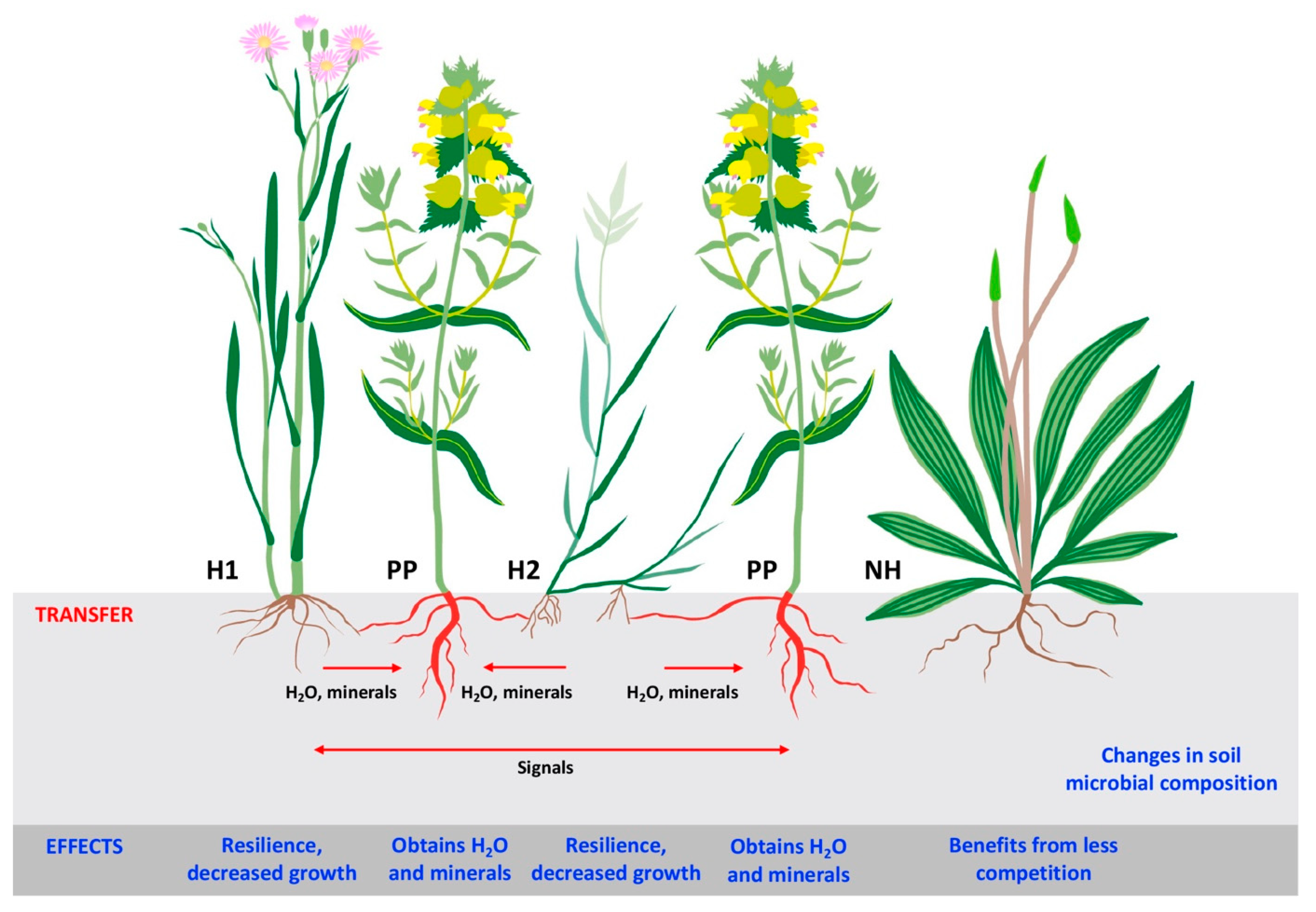
6. Plant–Parasitic Plant Interactions in Coastal Grasslands
7. Plant–Plant Interactions in Coastal Grasslands
8. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Zheng, J.; An, Y.; Xin, X.; Xu, D.; Yan, R.; Xu, L.; Shen, B.; Hou, L. Grassland Ecosystem Progress: A Review and Bibliometric Analysis Based on Research Publication over the Last Three Decades. Agronomy 2023, 13, 614. [Google Scholar] [CrossRef]
- Bai, J.; Wang, X.; Jia, J.; Zhang, G.; Wang, Y.; Zhang, S. Denitrification of soil nitrogen in coastal and inland salt marshes with different flooding frequencies. Phys. Chem. Earth Parts A/B/C 2017, 97, 31–36. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J.; Bartušková, A.; Ott, J.P. Belowground plant traits and their ecosystem functions along aridity gradients in grasslands. Plant Soil 2023, 1–10. [Google Scholar] [CrossRef]
- Hanisch, M.; Schweiger, O.; Cord, A.F.; Volk, M.; Knapp, S. Plant functional traits shape multiple ecosystem services, their trade-offs and synergies in grasslands. J. Appl. Ecol. 2020, 57, 1535–1550. [Google Scholar] [CrossRef]
- In ‘t Zandt, D.; Hoekstra, N.J.; de Caluwe, H.; Cruijsen, P.M.J.M.; Visser, E.J.W.; de Kroon, H. Plant life-history traits rather than soil legacies determine colonization of soil patches in a multi-species grassland. J. Ecol. 2022, 110, 889–901. [Google Scholar] [CrossRef]
- Delbosc, P.; Lagrange, I.; Rozo, C.; Bensettiti, F.; Bouzillé, J.-B.; Evans, D.; Lalanne, A.; Rapinel, S.; Bioret, F. Assessing the conservation status of coastal habitats under Article 17 of the EU Habitats Directive. Biol. Conserv. 2021, 254, 108935. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands-more important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Feurdean, A.; Ruprecht, E.; Molnár, Z.; Hutchinson, S.M.; Hickler, T. Biodiversity-rich European grasslands: Ancient, forgotten ecosystems. Biol. Conserv. 2018, 228, 224–232. [Google Scholar] [CrossRef]
- Revillini, D.; Gehring, C.A.; Johnson, N.C. The role of locally adapted mycorrhizas and rhizobacteria in plant–soil feedback systems. Funct. Ecol. 2016, 30, 1086–1098. [Google Scholar] [CrossRef] [Green Version]
- Ford, H.; Garbutt, A.; Jones, D.L.; Jones, L. Impacts of grazing abandonment on ecosystem service provision: Coastal grassland as a model system. Agric. Ecosyst. Environ. 2012, 162, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Karlsons, A.; Druva-Lusite, I.; Osvalde, A.; Necajeva, J.; Andersone-Ozola, U.; Samsone, I.; Ievinsh, G. Adaptation strategies of rare plant species to heterogeneous soil conditions on a coast of a lagoon lake as revealed by analysis of mycorrhizal symbiosis and mineral constituent dynamics. Environ. Exp. Biol. 2017, 15, 113–126. [Google Scholar]
- Karlsons, A.; Osvalde, A.; Ievinsh, G. Growth and mineral nutrition of two Triglochin species from saline wetlands: Adaptation strategies to conditions of heterogeneous mineral supply. Environ. Exp. Biol. 2011, 9, 83–90. [Google Scholar]
- Pärtel, M.; Bruun, H.H.; Sammul, M. Biodiversity in temperate European grasslands: Origin and conservation. In Integrating Efficient Grassland Farming and Biodiversity, Proceedings of the 13th International Occasional Symposium of the European Grassland Federation; Grassland Science in Europe, Tartu, Estonia, 29–31 August 2005; Lillak, R., Viiralt, R., Linke, A., Geherman, V., Eds.; European Grassland Federation: Tartu, Estonia, 2005; Volume 10, pp. 1–14. [Google Scholar]
- Daleo, P.; Alberti, J.; Chaneton, E.J.; Iribarne, O.; Tognetti, P.M.; Bakker, J.D.; Borer, E.T.; Bruschetti, M.; MacDougall, A.S.; Pascual, J.; et al. Environmental heterogeneity modulates the effect of plant diversity on the spatial variability of grassland biomes. Nat. Commun. 2023, 14, 1809. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Fantinato, E.; Janssen, J.; Bioret, F.; Acosta, A.; Prisco, I.; Tzonev, R.; Marcenò, C.; Rodwell, J.; Buffa, G. Biogeographic variability of coastal perennial grasslands at the European scale. Appl. Veg. Sci. 2018, 21, 312–321. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, L.J.; Tomassen, H.B.; Roelofs, J.G.; Bobbink, R. Effects of nitrogen enrichment on coastal dune grassland: A mesocosm study. Environ. Pollut. 2005, 138, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G. Biological basis of biological diversity: Physiological adaptations of plants to heterogeneous habitats along a sea coast. Acta Univ. Latv. 2006, 710, 53–79. [Google Scholar]
- Dronova, I. Environmental heterogeneity as a bridge between ecosystem service and visual quality objectives in management, planning and design. Landsc. Urban Plan. 2017, 163, 90–106. [Google Scholar] [CrossRef]
- Ievinsh, G. Where land meets sea: Biology of coastal soils. In Structure and Functions of Pedosphere; Giri, B., Kapoor, R., Wu, Q.-S., Varma, A., Eds.; Springer: Singapore, 2022; pp. 151–173. [Google Scholar]
- Bai, Y.; Cotfruto, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef]
- Jones, M.L.; Sowerby, A.; Williams, D.L.; Jones, R.E. Factors controlling soil development in sand dunes: Evidence from a coastal dune soil chronosequence. Plant Soil 2008, 307, 219–234. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhang, G.; Bai, J.; Xia, Z.; Wang, W.; Jia, J.; Wang, X.; Liu, X.; Cui, B. Desalinization via freshwater restoration highly improved microbial diversity, co-occurrence patterns and functions in coastal wetland soils. Sci. Total. Environ. 2021, 765, 142769. [Google Scholar] [CrossRef]
- Marks, B.M.; Chambers, L.; White, J.R. Effect of Fluctuating Salinity on Potential Denitrification in Coastal Wetland Soil and Sediments. Soil Sci. Soc. Am. J. 2016, 80, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Pausas, J.; Paterson, E. Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol. Biochem. 2011, 43, 1705–1713. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Hu, G.; Li, X.; Dong, Y.; Zhuge, Y.; He, H.; Zhang, X. Distinct accumulation of bacterial and fungal residues along a salinity gradient in coastal salt-affected soils. Soil Biol. Biochem. 2021, 158, 108266. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Otlewska, A.; Migliore, M.; Dybka-Stępień, K.; Manfredini, A.; Struszczyk-Świta, K.; Napoli, R.; Białkowska, A.; Canfora, L.; Pinzari, F. When Salt Meddles Between Plant, Soil, and Microorganisms. Front. Plant Sci. 2020, 11, 553087. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef] [Green Version]
- Berger, F.; Gutjahr, C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994. [Google Scholar] [CrossRef] [PubMed]
- Sportes, A.; Hériché, M.; Boussageon, R.; Noceto, P.-A.; van Tuinen, D.; Wipf, D.; Courty, P.E. A historical perspective on mycorrhizal mutualism emphasizing arbuscular mycorrhizas and their emerging challenges. Mycorrhiza 2021, 31, 637–653. [Google Scholar] [CrossRef]
- Druva-Lusite, I.; Ievinsh, G. Diversity of arbuscular mycorrhizal symbiosis in plants from coastal habitats. Environ. Exp. Biol. 2010, 8, 17–34. [Google Scholar]
- Mason, E. Note on the presence of mycorrhiza in the roots of salt marsh plants. New Phytol. 1928, 27, 193–195. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Janetta, K.; Ouziad, F.; Renne, B.; Nawrath, K.; Bothe, H. Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza 2001, 10, 175–183. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.; Merckx, V.; Tedersoo, L. FungalRoot: Global online database of plant mycorrhizal associations. New Phytol. 2020, 227, 955–966. [Google Scholar] [CrossRef]
- Öpik, M.; Moora, M.; Liira, J.; Zobel, M. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 2006, 94, 778–790. [Google Scholar] [CrossRef]
- Bothe, H. Arbuscular mycorrhiza and salt tolerance of plants. Symbiosis 2012, 58, 7–16. [Google Scholar] [CrossRef]
- Rosendahl, S.; Stukenbrock, E.H. Community structure of arbsicualr mycorrhizal fungi in undisturbed vegetation revealed by analyses of LSU rDNA sequences. Mol. Ecol. 2004, 13, 3179–3186. [Google Scholar] [CrossRef]
- Stukenbrock, E.H.; Rosendahl, S. Distribution of dominant arbuscular mycorrhizal fungi among five plant speccies in undisturbed vegetation of a coastal grassland. Mycorrhiza 2005, 15, 497–503. [Google Scholar] [CrossRef]
- Cui, X.; Hu, J.; Wang, J.; Yang, J.; Lin, X. Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in Eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 2016, 98, 140–149. [Google Scholar] [CrossRef]
- Guo, X.; Gong, J. Differential effects of abiotic factors and host plant traits on diversity and community composition of root-colonizing arbuscular mycorrhizal fungi in a salt-stressed ecosystem. Mycorrhiza 2014, 24, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.; Ezawa, T. Characterization of arbuscular mycorrhizal fungal communities with respect to zonal vegetation in a coastal dune ecosystem. Oecologia 2013, 173, 533–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munkvold, L.; Kjøller, R.; Vestberg, M.; Rosendahl, S.; Jakobsen, I. High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol. 2004, 164, 357–364. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Richard, F.; He, X.; Simard, S.W. Mycorrhizal networks: Des liaisons dangereuses? Trends Ecol. Evol. 2006, 21, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Barto, E.K.; Weidenhamer, J.D.; Cipollini, D.; Rillig, M.C. Fungal superhighways: Do common mycorrhizal networks enhance below ground communication? Trends Plant Sci. 2012, 17, 633–637. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Mariotte, P.; Meugnier, C.; Johnson, D.; Thébault, A.; Spiegelberger, T.; Buttler, A. Arbuscular mycorrhizal fungi reduce the differences in competitiveness between dominant and subordinate plant species. Mycorrhiza 2013, 23, 267–277. [Google Scholar] [CrossRef]
- Friede, M.; Unger, S.; Hellmann, C.; Beyschlag, W. Conditions Promoting Mycorrhizal Parasitism Are of Minor Importance for Competitive Interactions in Two Differentially Mycotrophic Species. Front. Plant Sci. 2016, 7, 1465. [Google Scholar] [CrossRef] [Green Version]
- Simard, S.W.; Durall, D.M. Mycorrhizal networks: A review of their extent, function, and importance. Can. J. Bot. 2004, 82, 1140–1165. [Google Scholar] [CrossRef]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Gorzelak, M.A.; Asay, A.K.; Pickles, B.J.; Simard, S.W. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 2015, 7, plv050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, R.; Horton, T.R. Mycorrhiza specificity: Its role in the development and function of common mycelial networks. In Mycorrhizal Networks; Horton, T.R., Ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 2015; pp. 1–39. [Google Scholar]
- Gilbert, L.; Johnson, D. Plant–plant communication through common mycorrhizal networks. In Advances in Botanical Research; Becard, G., Ed.; How Plants Communicate with their Biotic Environment; Academic Press: London, UK, 2017; Volume 82, pp. 83–97. [Google Scholar]
- Bidartondo, M.I. The evolutionary ecology of myco-heterotrophy. New Phytol. 2005, 167, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, X.; Yu, F. Non-host plants: Are they mycorrhizal networks players? Plant Divers. 2022, 44, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Boyno, G.; Demir, S. Plant-mycorrhizal communication and mycorrhizae in inter-plant communication. Symbiosis 2022, 86, 155–168. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, R.S.; Xu, J.F.; Li, J.; Shen, X.; Yihdego, W.G. Interplant Communication of Tomato Plants through Underground Common Mycorrhizal Networks. PLoS ONE 2010, 5, e13324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Wu, Y.; Liu, C.-C.; Liu, L.-W.; Ma, F.-F.; Wu, X.-Y.; Wu, M.; Hang, Y.-Y.; Chen, J.-Q.; Shao, Z.-Q.; et al. Identification of Arbuscular Mycorrhiza (AM)-Responsive microRNAs in Tomato. Front. Plant Sci. 2016, 7, 429. [Google Scholar] [CrossRef] [Green Version]
- Couzigou, J.-M.; Lauressergues, D.; André, O.; Gutjahr, C.; Bruno Guillotin, B.; Bécard, G.; Combier, J.-P. Positive gene regulation by a natural protective miRNA enables arbuscular mycorrhizal symbiosis. Cell Host Microbe 2017, 21, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Šečić, E.; Kogel, K.-H.; Ladera-Carmona, M.J. Biotic stress-associated microRNA families in plants. J. Plant Physiol. 2021, 263, 153451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular mycorrhizal fungi conducting the hyposphere bacterial orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef]
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef]
- Aliasgharzadeh, N.; Rastin, S.N.; Towfighi, H.; Alizadeh, A. Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza 2001, 11, 119–122. [Google Scholar] [CrossRef]
- Sonjak, S.; Udovič, M.; Wraber, T.; Likar, M.; Regvar, M. Diversity of halophytes and identification of arbuscular mycorrhizal fungi colonising their roots in an abandoned and sustained part of Sečovlje salterns. Soil Biol. Biochem. 2009, 41, 1847–1856. [Google Scholar] [CrossRef]
- Füzy, A.; Biró, B.; Tóth, T. Effect of saline soil parameters on endomycorrhizal colonisation of dominant halophytes in four Hungarian sites. Span. J. Agric. Res. 2010, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Füzy, A.; Biró, B.; Tóth, T.; Hildebrandt, U.; Bothe, H. Drought, but not salinity, determines the apparent effectiveness of halophytes colonized by arbuscular mycorrhizal fungi. J. Plant Physiol. 2008, 165, 1181–1192. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Correia, P.M.; Caçador, I.; Martins-Loução, M.A. Effects of salinity and flooding on the infectivity of salt marsh arbuscular mycorrhizal fungi in Aster tripolium L. Biol. Fertil. Soils 2003, 38, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Feng, G.; Li, X.; Zhang, F. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl. Soil Ecol. 2004, 26, 143–148. [Google Scholar] [CrossRef]
- Borde, M.; Dudhane, M.; Jite, P. Growth photosynthetic activity and antioxidant responses of mycorrhizal and non-mycorrhizal bajra (Pennisetum glaucum) crop under salinity stress condition. Crop. Prot. 2011, 30, 265–271. [Google Scholar] [CrossRef]
- Estrada, B.; Aroca, R.; Barea, J.M.; Ruiz-Lozano, J.M. Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci. 2013, 201–202, 42–51. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 96, 114–121. [Google Scholar] [CrossRef]
- Rahman, M.; Ali, M.; Alam, F.; Banu, M.; Anik, M.; Bhuiyan, M. Effect of Arbuscular Mycorrhizal Fungi on Germination, Nodulation and Sporulation of Lentil (Lens culinaris) at Different NaCl Levels. Bangladesh J. Microbiol. 2017, 34, 73–81. [Google Scholar] [CrossRef]
- Elhindi, K.M.; El-Din, A.S.; Elgorban, A. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci. 2017, 24, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashtebani, F.; Hajiboland, R.; Aliasgharzad, N. Characterization of salt-tolerance mechanisms in mycorrhizal (Claroideoglomus etunicatum) halophytic grass, Puccinellia distans. Acta Physiol. Plant. 2014, 36, 1713–1726. [Google Scholar] [CrossRef]
- Hammer, E.C.; Nasr, H.; Pallon, J.; Olsson, P.A.; Wallander, H. Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 2011, 21, 117–129. [Google Scholar] [CrossRef]
- Shokri, S.; Maadi, B. Effects of Arbuscular Mycorrhizal Fungus on the Mineral Nutrition and Yield of Trifolium alexandrinum Plants under Salinity Stress. J. Agron. 2009, 8, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Camprubí, A.; Abril, M.; Estaún, V.; Calvet, C. Contribution of arbuscular mycorrhizal symbiosis to the survival of psammophilic plants after sea water flooding. Plant Soil 2012, 351, 97–105. [Google Scholar] [CrossRef]
- Hartmond, U.; Schaesberg, N.V.; Graham, J.H.; Syvertsen, J.P. Salinity and flooding stress effects on mycorrhizal and non-mycorrhizal citrus rootstock seedlings. Plant Soil 1987, 104, 37–43. [Google Scholar] [CrossRef]
- Rutto, K.L.; Mizutani, F.; Kadoya, K. Effect of root-zone flooding on mycorrhizal and non-mycorrhizal peach (Prunus persica Batsch) seedlings. Sci. Hortic. 2002, 94, 285–295. [Google Scholar] [CrossRef]
- Zheng, F.-L.; Liang, S.-M.; Chu, X.-N.; Yang, Y.-L.; Wu, Q.-S. Mycorrhizal fungi enhance flooding tolerance of peach through inducing proline accumulation and improving root architecture. Plant Soil Environ. 2020, 66, 624–631. [Google Scholar] [CrossRef]
- Fougnies, L.; Renciot, S.; Müller, F.; Plenchette, C.; Prin, Y.; De Faria, S.M.; Bouvet, J.M.; Sylla, S.N.; Dreyfus, B.; Bâ, A.M. Arbuscular mycorrhizal colonization and nodulation improve flooding tolerance in Pterocarpus officinalis Jacq. seedlings. Mycorrhiza 2007, 17, 159–166. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, X.; Li, S. Effects of Arbuscular Mycorrhizal Fungi on Rice Growth Under Different Flooding and Shading Regimes. Front. Microbiol. 2021, 12, 756752. [Google Scholar] [CrossRef]
- Miller, S.P.; Sharitz, R.R. Manipulation of flooding and arbuscular mycorrhiza formation influences growth and nutrition of two semiaquatic grass species. Funct. Ecol. 2000, 14, 738–748. [Google Scholar] [CrossRef]
- Miller, S.P. Arbuscular mycorrhizal colonization of semi-aquatic grasses along a wide hydrologic gradient. New Phytol. 2000, 145, 145–155. [Google Scholar] [CrossRef]
- Ellis, J.R. Post Flood Syndrome and Vesicular-Arbuscular Mycorrhizal Fungi. J. Prod. Agric. 1998, 11, 200–204. [Google Scholar] [CrossRef]
- Vallino, M.; Fiorilli, V.; Bonfante, P. Rice flooding negatively impacts root branching and arbuscular mycorrhizal colonization, but not fungal viability. Plant Cell Environ. 2014, 37, 557–572. [Google Scholar] [CrossRef]
- Neto, D.; Carvalho, L.M.; Cruz, C.; Martins-Loução, M.A. How do mycorrhizas affect C and N relationships in flooded Aster tripolium plants? Plant Soil 2006, 279, 51–63. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Qiu, Q.; Xin, G.; Yang, Z.; Shi, S. Flooding Greatly Affects the Diversity of Arbuscular Mycorrhizal Fungi Communities in the Roots of Wetland Plants. PLoS ONE 2011, 6, e24512. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bao, X.; Björn, L.O.; Li, S.; Olsson, P.A. Response differences of arbuscular mycorrhizal fungi communities in the roots of an aquatic and a semiaquatic species to various flooding regimes. Plant Soil 2016, 403, 361–373. [Google Scholar] [CrossRef]
- Huang, G.-M.; Srivastava, A.K.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Exploring arbuscular mycorrhizal symbiosis in wetland plants with a focus on human impacts. Symbiosis 2021, 84, 311–320. [Google Scholar] [CrossRef]
- Deepika, S.; Kothamasi, D. Soil moisture—A regulator of arbuscular mycorrhizal fungal community assembly and symbiotic phosphorus uptake. Mycorrhiza 2015, 25, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zou, Y.-N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Elucidating the Mechanisms Underlying Enhanced Drought Tolerance in Plants Mediated by Arbuscular Mycorrhizal Fungi. Front. Microbiol. 2021, 12, 809473. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.-N.; Shu, B.; Wu, Q.-S. Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Sci. Hortic. 2023, 308, 111591. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Skiba, V.; Wainwright, M. Nitrogen transformation in coastal sands and dune soils. J. Arid. Environ. 1984, 7, 1–8. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bakker, R.; Verwaal, J.; Scheublin, T.R.; Rutten, M.; Van Logtestijn, R.; Staehelin, C. Symbiotic bacteria as a determinant of plant community structure and plant productivity in dune grassland. FEMS Microbiol. Ecol. 2006, 56, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.H. Rhizobia from wild legumes: Diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J. Biotechnol. 2001, 91, 143–153. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break crop benefits in temperate wheat production. Field Crop. Res. 2008, 107, 185–195. [Google Scholar] [CrossRef]
- Sindhu, S.; Dahiya, A.; Gera, R.; Sindhu, S.S. Mitigation of abiotic stress in legume-nodulating rhizobia for sustainable crop production. Agric. Res. 2020, 9, 444–459. [Google Scholar] [CrossRef]
- Benezech, C.; Doudement, M.; Gourion, B. Legumes tolerance to rhizobia is not always observed and not always deserved. Cell. Microbiol. 2020, 22, e13124. [Google Scholar] [CrossRef]
- Sugawara, M.; Takahashi, S.; Umehara, Y.; Iwano, H.; Tsurumaru, H.; Odake, H.; Suzuki, Y.; Kondo, H.; Konno, Y.; Yamakawa, T.; et al. Variation in bradyrhizobial NopP effector determines symbiotic incompatibility with Rj2-soybeans via effector-triggered immunity. Nat. Commun. 2018, 9, 3139. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Yang, Y.; Liu, P.; Xu, R.; Rensing, C.; Fu, X.; Liao, H. Genotype and rhizobium inoculation modulate the assembly of soybean rhizobacterial communities. Plant Cell Environ. 2019, 42, 2028–2044. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, S.; Shang, Y.; Brunel, B.; Li, S.; Zhao, Y.; Liu, Y.; Chen, W.; Wang, E.; Singh, R.P.; et al. Genomic diversity of chickpea-nodulating rhizobia in Ningxia (north central China) and gene flow within symbiotic Mesorhizobium muleiense populations. Syst. Appl. Microbiol. 2020, 43, 126089. [Google Scholar] [CrossRef]
- Chen, W.F.; Wang, E.T.; Ji, Z.J.; Zhang, J.J. Recent development and new insight of diversification and symbiosis specificity of legume rhizobia: Mechanism and application. J. Appl. Microbiol. 2020, 131, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Wakelin, S.A.; Moot, D.J.; Blond, C.; Laugraud, A.; Ridgway, H.J. Trifolium repens and T. subterraneum modify their nodule microbiome in response to soil pH. J. Appl. Microbiol. 2021, 131, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.R.; Lau, J.A. When mutualisms matter: Rhizobia effects on plant communities depend on host plant population and soil nitrogen availability. J. Ecol. 2017, 106, 1046–1056. [Google Scholar] [CrossRef]
- Wielbo, J.; Marek-Kozaczuk, M.; Kubik-Komar, A.; Skorupska, A. Increased metabolic potential of Rhizobium spp. is associated with bacterial competitiveness. Can. J. Microbiol. 2007, 53, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Simms, E.L.; Taylor, D.; Povich, J.; Shefferson, R.P.; Sachs, J.; Urbina, M.; Tausczik, Y. An empirical test of partner choice mechanisms in a wild legume–rhizobium interaction. Proc. R. Soc. B Biol. Sci. 2006, 273, 77–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, A.R.; Sicardi, M.; Frioni, L. Competition for nodule occupancy between introduced and native strains of Rhizobium leguminosarum biovar trifolii. Biol. Fertil. Soils 2010, 46, 419–425. [Google Scholar] [CrossRef]
- Pahua, V.J.; Stokes, P.J.N.; Hollowell, A.C.; Regus, J.U.; Gano-Cohen, K.A.; Wendlandt, C.E.; Quides, K.W.; Lyu, J.Y.; Sachs, J.L. Fitness variation among host species and the paradox of inefective rhizobia. J. Evol. Biol. 2018, 31, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Basile, L.A.; Lepek, V.C. Legume–rhizobium dance: An agricultural tool that could be improved? Microb. Biotechnol. 2021, 14, 1917–1987. [Google Scholar] [CrossRef]
- Jin, L.; Sun, X.; Wang, X.; Shen, Y.; Hou, F.; Chang, S.; Wang, C. Synergistic interactions of arbuscular mycorrhizal fungi and rhizobia promoted the growth of Lathyrus sativus under sulphate salt stress. Symbiosis 2010, 50, 157–164. [Google Scholar] [CrossRef]
- García, I.; Mendoza, R. Lotus tenuis seedlings subjected to drought or waterlogging in a saline sodic soil. Environ. Exp. Bot. 2014, 98, 47–55. [Google Scholar] [CrossRef]
- Dūmiņš, K.; Andersone-Ozola, U.; Samsone, I.; Elferts, D.; Ievinsh, G. Growth and physiological performance of a coastal species Trifolium fragiferum as affected by interaction with Trifolium repens, NaCl treatment, and inoculation with rhizobia. Plants 2021, 10, 2196. [Google Scholar] [CrossRef] [PubMed]
- Gaile, L.; Andersone-Ozola, U.; Samsone, I.; Elferts, D.; Ievinsh, G. Modification of Growth and Physiological Response of Coastal Dune Species Anthyllis maritima to Sand Burial by Rhizobial Symbiosis and Salinity. Plants 2021, 10, 2584. [Google Scholar] [CrossRef] [PubMed]
- Chanway, C.P.; Holl, F.B.; Turkington, R. Effect of Rhizobium Leguminosarum Biovar Trifolii Genotype on Specificity between Trifolium Repens and Lolium Perenne. J. Ecol. 1989, 77, 1150–1160. [Google Scholar] [CrossRef]
- Bruning, B.; Rozema, J. Symbiotic nitrogen fixation in legumes: Perspectives for saline agriculture. Environ. Exp. Bot. 2013, 92, 134–143. [Google Scholar] [CrossRef]
- Andersone-Ozola, U.; Jēkabsone, A.; Purmale, L.; Romanovs, M.; Ievinsh, G. Abiotic stress tolerance of coastal accessions of a promising forage legume species, Trifolium fragiferum. Plants 2021, 10, 1552. [Google Scholar] [CrossRef]
- Ruņģis, D.E.; Andersone-Ozola, U.; Jēkabsone, A.; Ievinsh, G. Genetic diversity and structure of Latvian Trifolium fragiferum populations, a crop wild relative legume species, in the context of the Baltic Sea region. Diversity 2023, 15, 473. [Google Scholar] [CrossRef]
- Andersone-Ozola, U.; Jēkabsone, A.; Karlsons, A.; Romanovs, M.; Ievinsh, G. Soil chemical properties and mineral nutrition of Latvian accessions of Trifolium fragiferum, a crop wild relative plant species. Environ. Exp. Biol. 2021, 19, 245–254. [Google Scholar]
- Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Romanovs, M.; Ievinsh, G. Effect of Salinity on Growth, Ion Accumulation and Mineral Nutrition of Different Accessions of a Crop Wild Relative Legume Species, Trifolium fragiferum. Plants 2022, 11, 797. [Google Scholar] [CrossRef]
- Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Neiceniece, L.; Romanovs, M.; Ievinsh, G. Dependence on nitrogen availability and rhizobial symbiosis of different accessions of Trifolium fragiferum, a crop wild relative legume species, as related to physiological traits. Plants 2022, 11, 1141. [Google Scholar] [CrossRef]
- Kozieł, M.; Kalita, M.; Janczarek, M. Genetic diversity of microsymbionts nodulating Trifolium pratense in subpolar and temperate climate regions. Sci. Rep. 2022, 12, 12144. [Google Scholar] [CrossRef]
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for Swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Nickrent, D.L. Parasitic angiosperms: How often and how many? Taxon 2020, 69, 5–27. [Google Scholar] [CrossRef]
- Teixeira-Costa, L.; Davis, C.C. Life history, diversity, and distribution in parasitic flowering plants. Plant Physiol. 2020, 187, 32–51. [Google Scholar] [CrossRef]
- Parker, C. The parasitic weeds of the Orobanchaceae. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 313–344. [Google Scholar]
- Hatcher, M.J.; Dick, J.T.; Dunn, A.M. Diverse effects of parasites in ecosystems: Linking interdependent processes. Front. Ecol. Environ. 2012, 10, 186–194. [Google Scholar] [CrossRef]
- Hegenauer, V.; Körner, M.; Albert, M. Plants under stress by parasitic plants. Curr. Opin. Plant Biol. 2017, 38, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.C.; Watkinson, A.R. The host range and selectivity of a parasitic plant: Rhinanthus minor L. Oecologia 1989, 78, 401–406. [Google Scholar] [CrossRef]
- Clarke, C.R.; Timko, M.P.; Yoder, J.I.; Axtell, M.J.; Westwood, J.H. Molecular Dialog Between Parasitic Plants and Their Hosts. Annu. Rev. Phytopathol. 2019, 57, 279–299. [Google Scholar] [CrossRef]
- Seel, W.E.; Press, M.C. Influence of the host on three sub-arctic annual facultative root hemiparasites. I. Growth, mineral accumulation and above-ground dry-matter partitioning. New Phytol. 1993, 125, 131–138. [Google Scholar] [CrossRef]
- Rowntree, J.K.; Cameron, D.D.; Preziosi, R.F. Genetic variation changes the interactions between the parasitic plant-ecosystem engineer Rhinanthus and its hosts. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1380–1388. [Google Scholar] [CrossRef] [Green Version]
- Chaudron, C.; Mazalová, M.; Kuras, T.; Malenovský, I.; Mládek, J. Introducing ecosystem engineers for grassland biodiversity conservation: A review of the effects of hemiparasitic Rhinanthus species on plant and animal communities at multiple trophic levels. Perspect. Plant Ecol. Evol. Syst. 2021, 52, 125633. [Google Scholar] [CrossRef]
- Heer, N.; Klimmek, F.; Zwahlen, C.; Fischer, M.; Hölzel, N.; Klaus, V.H.; Kleinebecker, T.; Prati, D.; Boch, S. Hemiparasite-density effects on grassland plant diversity, composition and biomass. Perspect. Plant Ecol. Evol. Syst. 2018, 32, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Watson, D.M. Parasitic plants as facilitators: More Dryad than Dracula? J. Ecol. 2009, 97, 1151–1159. [Google Scholar] [CrossRef]
- Watson, D.M.; McLellan, R.C.; Fontúrbel, F.E. Functional Roles of Parasitic Plants in a Warming World. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 25–45. [Google Scholar] [CrossRef]
- DiGiovanni, J.P.; Wysocki, W.P.; Burke, S.; Duvall, M.R.; Barber, N.A. The role of hemiparasitic plants: Influencing tallgrass prairie quality, diversity, and structure. Restor. Ecol. 2017, 25, 405–413. [Google Scholar] [CrossRef]
- Delavault, P.; Montiel, G.; Brun, G.; Puvreau, J.-B.; Thoiron, S.; Simier, P. Communication between host plants and parasitic plants. In Advances in Botanical Research; Becard, G., Ed.; How Plants Communicate with their Biotic Environment; Academic Press: London, UK, 2017; Volume 82, pp. 55–82. [Google Scholar]
- Smith, J.D.; Mescher, M.C.; De Moraes, C.M. Implications of bioactive solute transfer from hosts to parasitic plants. Curr. Opin. Plant Biol. 2013, 16, 464–472. [Google Scholar] [CrossRef]
- Kim, G.; LeBlanc, M.L.; Wafula, E.K.; Depamphilis, C.W.; Westwood, J.H. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 2014, 345, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Westwood, J.H.; Kim, G. RNA mobility in parasitic plant—Host interactions. RNA Biol. 2017, 14, 450–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-Y.; Shimizu, K.; Brown, J.; Aoki, K.; Westwood, J.H. Mobile Host mRNAs Are Translated to Protein in the Associated Parasitic Plant Cuscuta campestris. Plants 2022, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lu, Q.-W.; Yang, B.-F.; Zagorchev, L.; Li, J.-M. Integrated small RNA, mRNA, and degradome sequencing reveals the important role of miRNAs in the interactions between parasitic plant Cuscuta australis and its host Trifolium repens. Sci. Hortic. 2021, 289, 110548. [Google Scholar] [CrossRef]
- Tešitel, J.; Li, A.-I.; Knotková, K.; McLellan, R.; Badaranayake, P.C.G.; Watson, D.M. The bright side of parasitic plants: What are they good for? Plant Physiol. 2021, 185, 1309–1324. [Google Scholar] [CrossRef]
- Hartenstein, M.; Albert, M.; Krause, K. The plant vampire diaries: A historic perspective on Cuscuta research. J. Exp. Bot. 2023, 74, 2944–2955. [Google Scholar] [CrossRef]
- Watkinson, A.R.; Davy, A.J. Population biology of salt marsh and sand dune annuals. Vegetatio 1985, 62, 487–497. [Google Scholar] [CrossRef]
- Singh, D.; Jha, B. The isolation and identification of salt-responsive novel microRNAs from Salicornia brachiata, an extreme halophyte. Plant Biotechnol. Rep. 2014, 8, 325–336. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Yang, X.; Wu, H.; Zhu, H.; Zhang, H. miRNA–mRNA integrated analysis reveals roles for miRNAs in a typical halophyte, Reaumuria soongorica, during seed germination under salt stress. Plants 2020, 9, 351. [Google Scholar] [CrossRef] [Green Version]
- Baek, D.; Chun, H.J.; Kang, S.; Shin, G.; Park, S.J.; Hong, H.; Kim, C.; Kim, D.H.; Lee, S.Y.; Kim, M.C.; et al. A Role for Arabidopsis miR399f in Salt, Drought, and ABA Signaling. Mol. Cells 2016, 39, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shen, J.; Xu, Q.; Dong, J.; Song, L.; Wang, W.; Shen, F. Long noncoding RNA lncRNA354 functions as a competing endogenous RNA of miR160b to regulate ARF genes in response to salt stress in upland cotton. Plant Cell Environ. 2021, 44, 3302–3321. [Google Scholar] [CrossRef]
- Makkar, H.; Arora, S.; Khuman, A.K.; Chaudhary, B. Target-Mimicry-Based miR167 Diminution Confers Salt-Stress Tolerance During In Vitro Organogenesis of Tobacco (Nicotiana tabacum L.). J. Plant Growth Regul. 2022, 41, 1462–1480. [Google Scholar] [CrossRef]
- Lotfi, A.; Pervaiz, T.; Jiu, S.; Faghihi, F.; Jahanbakhshian, Z.; Khorzoghi, E.G.; Fang, J.; Seyedi, S.M. Role of microRNAs and their target genes in salinity response in plants. Plant Growth Regul. 2017, 82, 377–390. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, C.; Zheng, C.; Yao, Y.; Du, Y. Advances in the regulation of plant salt-stress tolerance by miRNA. Mol. Biol. Rep. 2022, 49, 5041–5055. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, G.M. Transpiration and dry matter allocation in the angiosperm root parasite Cynomorium coccineum L. and two of its halophytic hosts. Biol. Plant. 1993, 35, 603–608. [Google Scholar] [CrossRef]
- Ouf, S.A. Mycological studies on the angiosperm root parasite Cynomorium coccineum L. and two of its halophytic hosts. Biol. Plant. 1993, 35, 591–602. [Google Scholar] [CrossRef]
- Pennings, S.C.; Callaway, R.M. Impact of a Parasitic Plant on the Structure and Dynamics of Salt Marsh Vegetation. Ecology 1996, 77, 1410–1419. [Google Scholar] [CrossRef] [Green Version]
- Zagorchev, L.; Stöggl, W.; Teofanova, D.; Li, J.; Kranner, I. Plant Parasites under Pressure: Effects of Abiotic Stress on the Interactions between Parasitic Plants and Their Hosts. Int. J. Mol. Sci. 2021, 22, 7418. [Google Scholar] [CrossRef]
- Veste, M.; Todt, H.; Breckle, S.-W. Influence of halophytic hosts on their parasites—The case of Plicosepalus acaciae. AoB Plants 2015, 7, plu084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewell, B.J. Parasite Facilitates Plant Species Coexistence in a Coastal Wetland. Ecology 2008, 89, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, G.M. Ecophysiology of the holoparasitic angiosperm Cistanche phelypaea (Orobancaceae) in a coastal salt marsh. Turk. J. Bot. 2013, 37, 908–919. [Google Scholar] [CrossRef]
- Callaway, R.M.; Walker, L.R. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Montesinos, D. Plant–plant interactions: From competition to facilitation. Web Ecol. 2015, 15, 1–2. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Schandry, N.; Becker, C. Allelopathic Plants: Models for Studying Plant–Interkingdom Interactions. Trends Plant Sci. 2020, 25, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; Van Kleunen, M. Effect of allelopathy on plant performance: A meta-analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef]
- Delory, B.M.; Delaplace, P.; Fauconnier, M.-L.; du Jardin, P. Root-emitted volatile organic compounds: Can they mediated belowground plant-plant interactions? Plant Soil 2016, 402, 1–26. [Google Scholar] [CrossRef] [Green Version]
- da Silva, E.R.; Overbeck, G.E.; Soares, G.L.G. Something old, something new in allelopathy review: What grassland ecosystems tell us. Chemoecology 2017, 27, 217–231. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Ding, L.; Kong, C.-H. Allelopathy and Allelochemicals in Grasslands and Forests. Forests 2023, 14, 562. [Google Scholar] [CrossRef]
- Dudley, S.A.; Murphy, G.P.; File, A.L. Kin recognition and competition in plants. Funct. Ecol. 2013, 27, 898–906. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K.; Ishizaki, S.; Wetzel, W.; Evans, R.Y. Kin recognition affects plant communication and defence. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123062. [Google Scholar] [CrossRef] [Green Version]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Lin, Y.; Berger, U.; Grimm, V.; Ji, Q. Differences between symmetric and asymmetric facilitation matter: Exploring the interplay between modes of positive and negative plant interactions. J. Ecol. 2012, 100, 1482–1491. [Google Scholar] [CrossRef] [Green Version]
- Vogt, D.R.; Murrell, D.J.; Stoll, P. Testing Spatial Theories of Plant Coexistence: No Consistent Differences in Intra- and Interspecific Interaction Distances. Am. Nat. 2010, 175, 73–84. [Google Scholar] [CrossRef]
- Dudley, S.A. Plant cooperation. AoB Plants 2015, 7, plv113. [Google Scholar] [CrossRef] [Green Version]
- Weigelt, A.; Schumacher, J.; Walther, T.; Bartelheimer, M.; Steinlein, T.; Beyschlag, W. Identifying mechanisms of competition in multi-species communities. J. Ecol. 2007, 95, 53–64. [Google Scholar] [CrossRef]
- Grant, K.; Kreyling, J.; Heilmeier, H.; Beierkuhnlein, C.; Jentsch, A. Extreme weather events and plant–plant interactions: Shifts between competition and facilitation among grassland species in the face of drought and heavy rainfall. Ecol. Res. 2014, 29, 991–1001. [Google Scholar] [CrossRef]
- Le Bagousse-Pinguet, Y.; Maalouf, J.-P.; Touzard, B.; Michalet, R. Importance, but not intensity of plant interactions relates to species diversity under the interplay of stress and disturbance. Oikos 2014, 123, 777–785. [Google Scholar] [CrossRef]
- Ungar, I.A. Are biotic factors significant in influencing the distribution of halophytes in saline habitats? Bot. Rev. 1998, 64, 176–199. [Google Scholar] [CrossRef]
- Samsone, I.; Ievinsh, G. Different plant species accumulate various concentration of Na+ in a sea-affected coastal wetland during a vegetation season. Environ. Exp. Biol. 2018, 16, 117–127. [Google Scholar]
- Gagné, J.-M.; Hoyle, G. Facilitation of Leymus mollis by Honckenya peploides on coastal dunes in subarctic Quebec, Canada. Can. J. Bot. 2001, 79, 1327–1331. [Google Scholar]
- Franks, S.J.; Peterson, C.J. Burial disturbance leads to facilitation among coastal dune plants. Plant Ecol. 2003, 168, 13–21. [Google Scholar] [CrossRef]
- Martínez, M.L.; García-Franco, J.G. Plant-plant interactions in coastal dunes. In Coastal Dunes, Ecology and Conservation; Martínez, M.L., Psuty, N.P., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2004; Volume 171, pp. 205–220. [Google Scholar]
- Yu, Z.; Zhang, Q.; Yang, H.; Tang, J.; Weiner, J.; Chen, X. The effects of salt stress and arbuscular mycorrhiza on plant neighbour effects and self-thinning. Basic Appl. Ecol. 2012, 13, 673–680. [Google Scholar] [CrossRef]
- Subrahmaniam, H.J.; Libourel, C.; Journet, E.-P.; Morel, J.-B.; Muños, S.; Niebel, A.; Raffaele, S.; Roux, F. The genetics underlying natural variation of plant–plant interactions, a beloved but forgotten member of the family of biotic interactions. Plant J. 2018, 93, 747–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wilson, G.W.T.; Cobb, A.B.; Zhang, Y.; Liu, L.; Zhang, X.; Sun, F. Mycorrhizal and rhizobial interactions influence model grassland plant community structure and productivity. Mycorrhiza 2022, 32, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G. Halophytic Clonal Plant Species: Important Functional Aspects for Existence in Heterogeneous Saline Habitats. Plants 2023, 12, 1728. [Google Scholar] [CrossRef] [PubMed]
- Tomilov, A.; Tomilova, N.; Yoder, J.I. In vitro haustorium development in roots and root cultures of the hemiparasitic plant Triphysaria versicolor. Plant Cell Tissue Organ Cult. 2004, 77, 257–265. [Google Scholar] [CrossRef]
- Takeda, N.; Handa, Y.; Tsuzuki, S.; Kojima, M.; Sakakibara, H.; Kawaguchi, M. Gibberellins Interfere with Symbiosis Signaling and Gene Expression and Alter Colonization by Arbuscular Mycorrhizal Fungi in Lotus japonicus. Plant Physiol. 2015, 167, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Ho-Plágaro, T.; Tamayo-Navarette, N.I.; García-Garrido, J.M. Functional analysis of plant genes related to arbuscular mycorrhizal symbiosis using Agrobacterium rhizogenes-mediated root transformation and hairy root production. In Hairy Root Cultures Based Applications; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Rhizosphere Biology; Springer Nature: Singapore, 2020; pp. 191–215. [Google Scholar]
- Singh, J.; Kumar, K.; Verma, P.K. Functional characterization of genes involved in legume nodulation using hairy root cultures. In Hairy Root Cultures Based Applications; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Rhizosphere Biology; Springer Nature: Singapore, 2020; pp. 217–228. [Google Scholar]
- Lin, P.; Zhang, M.; Wang, M.; Li, Y.; Liu, J.; Chen, Y. Inoculation with arbuscular mycorrhizal fungus modulates defense-related genes expression in banana seedlings susceptible to wilt disease. Plant Signal. Behav. 2021, 16, e1884782. [Google Scholar] [CrossRef]
- He, X.; Zhang, Q.; Li, B.; Jin, Y.; Jiang, L.; Wu, R. Network mapping of root–microbe interactions in Arabidopsis thaliana. npj Biofilms Microbiomes 2021, 7, 72. [Google Scholar] [CrossRef]
- Lee, K.K.; Kim, H.; Lee, Y.-H. Cross-kingdom co-occurrence networks in the plant microbiome: Importance and ecological interpretations. Front. Microbiol. 2022, 13, 953300. [Google Scholar] [CrossRef]



| Species | Presence in Coastal Habitats 1 | Salinity Tolerance 1 | Presence in eHALOPH Database (Life Form) 2 |
|---|---|---|---|
| Anthyllis vulneraria subsp. maritima (Hagen) Corb. (syn. Anthyllis maritima Schweigg. ex K.G.Hagen) | 0 | 1 | – |
| Lathyrus palustris L. | 3 | 3 | hydrohalophyte |
| Lotus maritimus L. | 6 | 3 | – |
| Lotus tenuis Waldst. and Kit. ex Willd. | 7 | 4 | hydrohalophyte |
| Melilotus albus Medik. | 1 | 2 | annual |
| Melilotus altissimus Thuill. | 1 | 3 | – |
| Melilotus dentatus (Waldts. and Kit.) Pers. | 4 | 4 | annual |
| Ononis spinosa L. | 4 | 2 | – |
| Trifolium fragiferum L. | 7 | 3 | herbaceous perennial |
| Trifolium pratense L. | 1 | 2 | – |
| Trifolium repens L. | 1 | 2 | – |
| Species | Presence in Coastal Habitats 1 | Salinity Tolerance 1 | Presence in Coastal Habitats 1 | Mycorrhizal Status 2 |
|---|---|---|---|---|
| Euphrasia nemorosa (Pers.) Wettst. | 1 | 2 | – | NM |
| Euphrasia stricta J.P.Wolff ex J.F.Lehm. | 1 | 2 | – | NM |
| Melampyrum arvense L. | 0 | 1 | – | NM |
| Odontites litoralis Fr. | 10 | 4 | parasite | NM |
| Odontites vernus (Bellardi) Dumort. | 3 | 2 | – | NM |
| Odontites vulgaris Moench | 2 | 2 | – | NM |
| Pedicularis palustris L. | 3 | 2 | – | NM-AM |
| Rhinanthus minor L. | 1 | 2 | – | NM |
| Rhinanthus serotinus (Schön) Oborny (syn. R. angustifolius C.C.Gmel.) | 1 | 2 | – | NM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ievinsh, G. Disentangling the Belowground Web of Biotic Interactions in Temperate Coastal Grasslands: From Fundamental Knowledge to Novel Applications. Land 2023, 12, 1209. https://doi.org/10.3390/land12061209
Ievinsh G. Disentangling the Belowground Web of Biotic Interactions in Temperate Coastal Grasslands: From Fundamental Knowledge to Novel Applications. Land. 2023; 12(6):1209. https://doi.org/10.3390/land12061209
Chicago/Turabian StyleIevinsh, Gederts. 2023. "Disentangling the Belowground Web of Biotic Interactions in Temperate Coastal Grasslands: From Fundamental Knowledge to Novel Applications" Land 12, no. 6: 1209. https://doi.org/10.3390/land12061209
APA StyleIevinsh, G. (2023). Disentangling the Belowground Web of Biotic Interactions in Temperate Coastal Grasslands: From Fundamental Knowledge to Novel Applications. Land, 12(6), 1209. https://doi.org/10.3390/land12061209



_Kazoglou.png)




