Effects of Forest Management Approach on Carbon Stock and Plant Diversity: A Case Study from Karnali Province, Nepal
Abstract
1. Introduction
2. Methodology
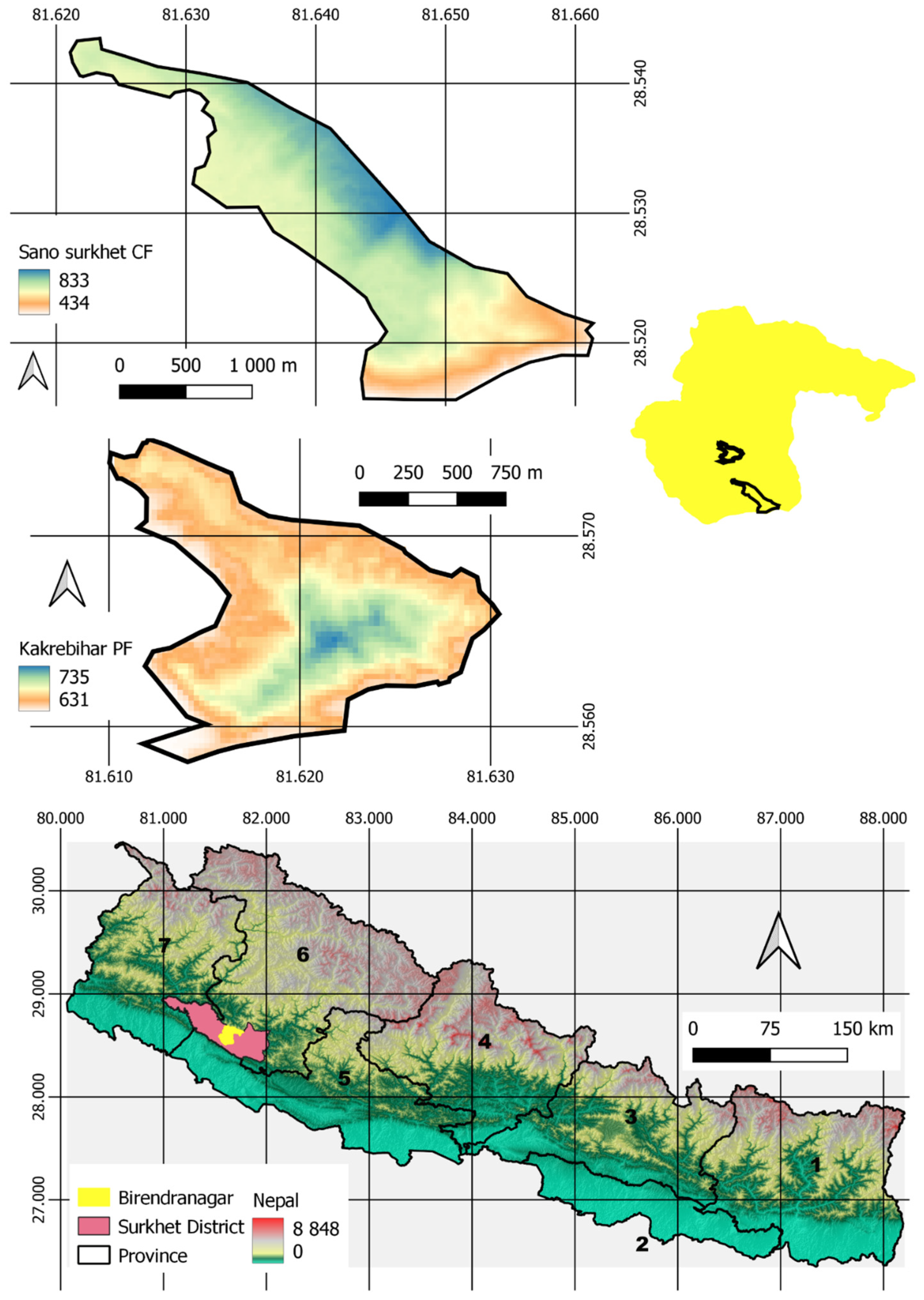
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
2.3.1. Carbon Stock Estimation
2.3.2. Quantifying Tree Species Diversity
2.4. Statistical Analyses
3. Results
3.1. Carbon Stock under Different Management Approaches
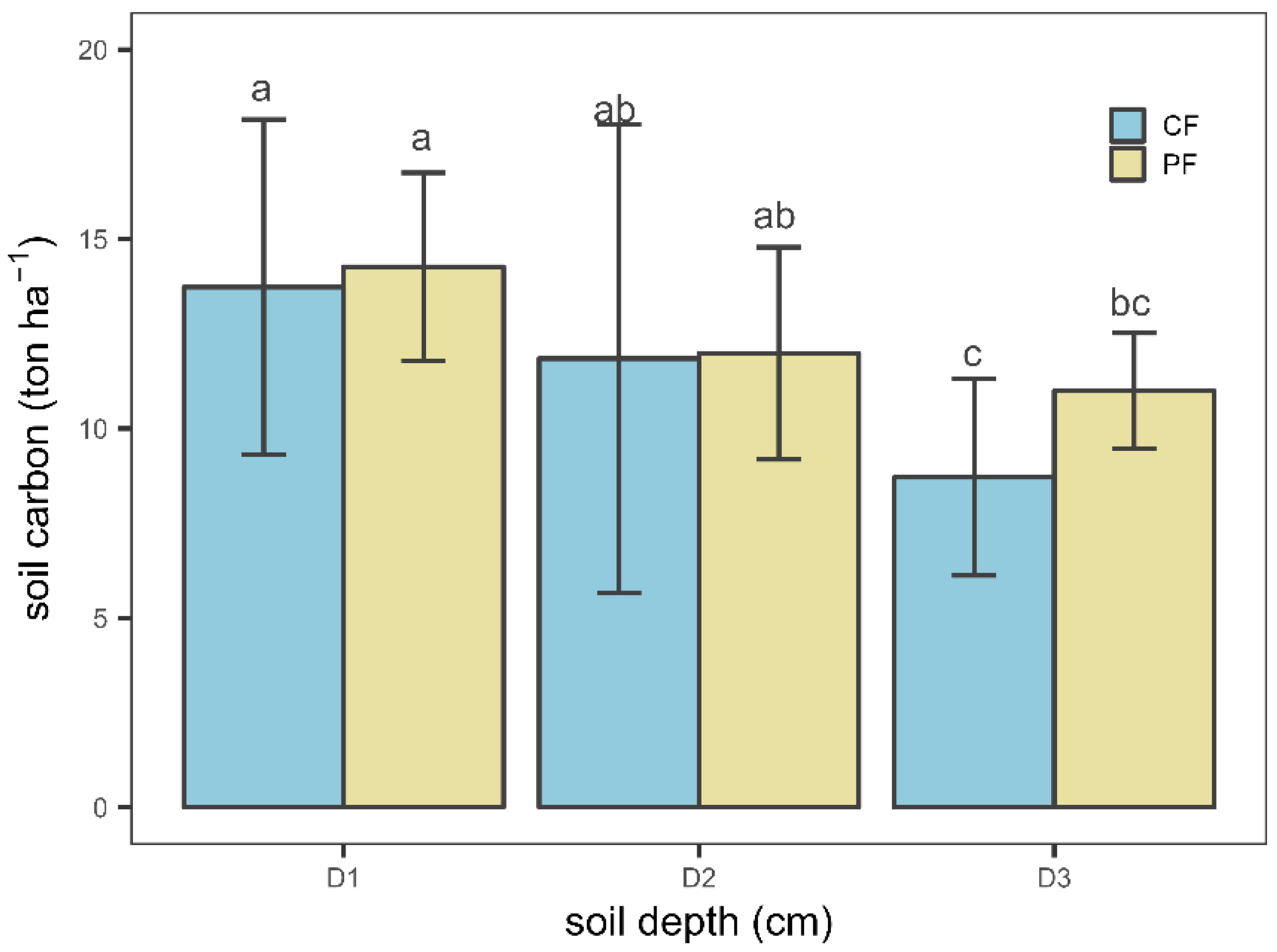
3.1.1. Soil Carbon Stock Variation among Soil Depth
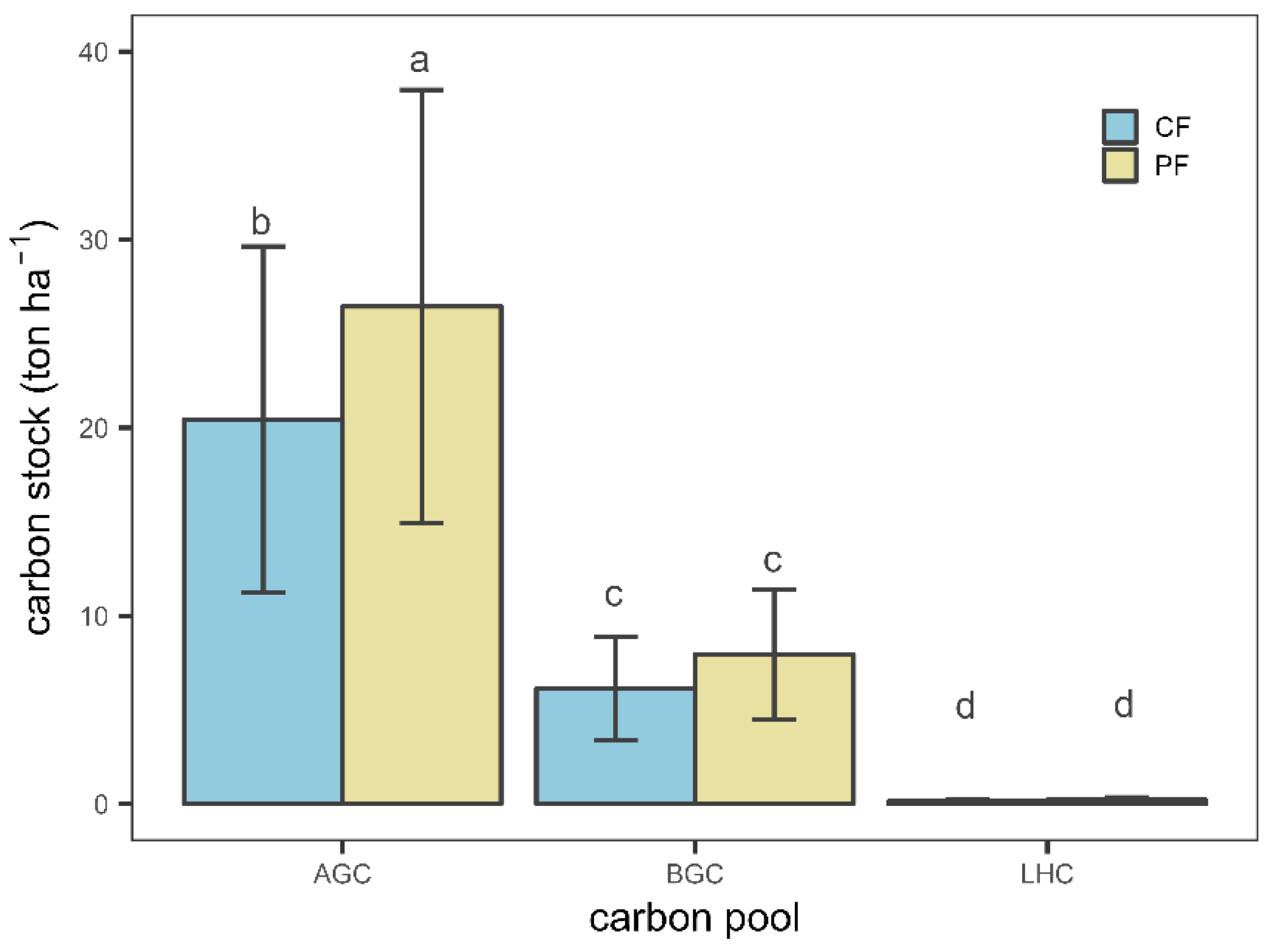
3.1.2. Biomass Carbon Variation within and between Management Approaches
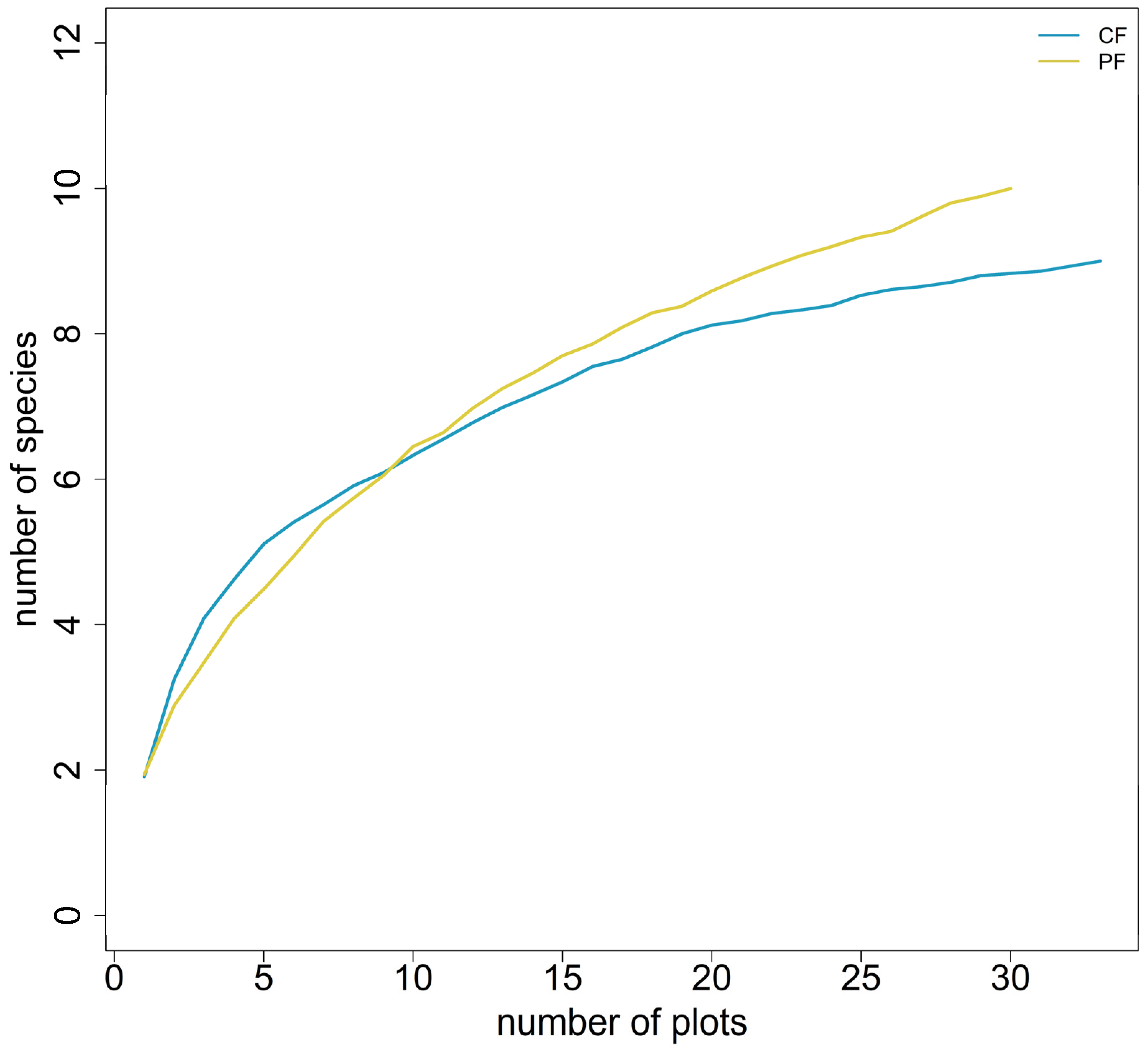
3.2. Species Richness and Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies: Kanagawa, Japan, 2006.
- Banskota, K.; Karky, B.S.; Skutsch, M. Reducing Carbon Emissions through Community-Managed Forests in the Himalaya; International Centre for Integrated Mountain Development (ICIMOD): Kathmandu, Nepal, 2007; ISBN 9789291150588. [Google Scholar]
- United Nations Framework Convention on Climate Change (UNFCCC) Kyoto Protocol to the Convention on Climate Change; United Nations: Bonn, Germany, 1997.
- Acharya, K.P.; Dangi, R.B.; Tripathi, D.M.; Bushley, B.R.; Bhandary, R.R.; Bhattarai, B. Ready for REDD? Taking Stock of Experience, Opportunities and Challenges in Nepal; Nepal Foresters Association: Kathmandu, Nepal, 2009; ISBN 9789937219679. [Google Scholar]
- Sah, S.; Sharma, S.; Mandal, R.A. Comparison of Carbon Stock in Chure, Bhawar and Terai, Nepal. Int. J. Sci. Eng. Res. 2019, 10, 80–93. [Google Scholar]
- Pandey, H.P.; Bhusal, M. A Comparative Study on Carbon Stock in Sal (Shorea Robusta) Forest in Two Different Ecological Regions of Nepal. Banko Janakari 2016, 26, 24–31. [Google Scholar] [CrossRef]
- Bhusal, S.; Bhattarai, A. An Assessment of Carbon Stick Variation in Chure Area of Arghakhanchi District, Nepal. Int. J. Adv. Res. Biol. Sci. 2020, 8, 1–5. [Google Scholar]
- Winjum, J.K.; Dixon, R.K.; Schroeder, P.E. Estimating the Global Potential of Forest and Agroforest Management Practices to Sequester Carbon. Water Air Soil Pollut. 1992, 64, 213–227. [Google Scholar] [CrossRef]
- Bhatta, S.; Poudel, A.; KC, Y.B. A Comparative Study of Carbon Stocks in the Sal Forest (Shorea Robusta) in Core and Buffer Zones of Shuklaphanta National Park, Nepal. For. J. Inst. For. Nepal 2021, 18, 52–60. [Google Scholar] [CrossRef]
- Haris, A.A.; Chhabra, V.; Biswas, S. Carbon Sequestration for Mitigation of Climate Change-a Review. Agric. Rev. 2013, 34, 129–136. [Google Scholar]
- Muradian, R.; Arsel, M.; Pellegrini, L.; Adaman, F.; Aguilar, B.; Agarwal, B.; Corbera, E.; Ezzine de Blas, D.; Farley, J.; Froger, G.; et al. Payments for Ecosystem Services and the Fatal Attraction of Win-Win Solutions. Conserv. Lett. 2013, 6, 274–279. [Google Scholar] [CrossRef]
- Carlson, K.M.; Curran, L.M. REDD Pilot Project Scenarios: Are Costs and Benefits Altered by Spatial Scale? Environ. Res. Lett. 2009, 4, 31–34. [Google Scholar] [CrossRef]
- MFSC. Nepal Biodiversity Strategy and Action Plan (2004–2014); Ministry of Forest and Soil Conservation, Government of Nepal: Kathmandu, Nepal, 2014.
- DoFSC Forestry Database of Nepal. Available online: https://www.dofsc.gov.np/ (accessed on 21 August 2021).
- Gautam, A.P.; Bhujel, K.B.; Chhetri, R. Political Economy of Forest Tenure Reform Implementation in Nepal: The Case of Protected Forests. J. For. Livelihood 2017, 15, 71–86. [Google Scholar] [CrossRef]
- MFSC. Guideline for Inventory of Community Forests; Ministry of Forests and Soil Conservation (MFSC), Department of Forests, Community and Private Forest Division: Kathmandu, Nepal, 2004.
- Sharma, E.R.; Pukkala, T. Volume Equations and Biomass Prediction of Forest Trees in Nepal; Ministry of Forests and Soil Conservation; Forest Survey and Statistics Division: Kathmandu, Nepal, 1990.
- Khanal, Y.; Sharma, R.; Upadhyaya, C. Soil and Vegetation Carbon Pools in Two Community Forests of Palpa District, Nepal. Banko Janakari 2010, 20, 34–40. [Google Scholar] [CrossRef]
- Subedi, B.P.; Pandey, S.S.; Pandey, A.; Rana, E.B.; Bhattarai, S.; Banskota, T.R.; Charmakar, S.; Tamrakar, R. Forest Carbon Stock Measurement: Guidelines for Measuring Carbon Stocks in Community-Managed Forests; ANSAB, FECOFUN, ICIMOD: Kathmandu, Nepal, 2010; ISBN 9789937226127. [Google Scholar]
- MPFS. Master Plan for Forestry Sector; Government of Nepal, Ministry of Forests and Soil Conservation: Kathmandu, Nepal, 1989.
- FAO. Carbon Sequestration Options Under the Clean Development Mechanism to Address Land Degradation; FAO: Roma, Italy, 2000. [Google Scholar]
- MacDicken, K. A Guide to Monitoring Carbon Storage in Forestry and Agro-Forestry Projects; Winrock International: Arlington, TX, USA, 1997. [Google Scholar]
- Mclean, E.O. Soil PH and Lime Requirement. Methods of Soil Analysis. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 119–224. ISBN 978-0-89118-204-7. [Google Scholar]
- Awasthi, K.D.; Singh, B.R.; Sitaula, B.K. Profile Carbon and Nutrient Levels and Management Effect on Soil Quality Indicators in the Mardi Watershed of Nepal. Acta Agric. Scand. Sect. B Soil Plant Sci. 2005, 55, 192–204. [Google Scholar] [CrossRef]
- Pearson, T.R.H.; Brown, S.L.; Birdsey, R.A. Measurement Guidelines for the Sequestration of Forest Carbon; US Department of Agriculture, Forest Service, Northern Research Station: Washington, DC, USA, 2007; Volume 18.
- Ugland, K.I.; Gray, J.S.; Ellingsen, K.E. The Species-Accumulation Curve and Estimation of Species Richness. J. Anim. Ecol. 2003, 72, 888–897. [Google Scholar] [CrossRef]
- Magurran, A. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.0-10. 2013. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 June 2023).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Technical Report Number 019. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes. R Package Version 1.1-2. 2014. Available online: https://CRAN.R-project.org/package=RColorBrewer (accessed on 10 June 2023).
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2013. Available online: http://www.R-project.org/ (accessed on 22 March 2022).
- Gurung, M.B.; Bigsby, H.; Cullen, R.; Manandhar, U. Estimation of Carbon Stock under Different Management Regimes of Tropical Forest in the Terai Arc Landscape, Nepal. For. Ecol. Manag. 2015, 356, 144–152. [Google Scholar] [CrossRef]
- Khadka, G.B.; Mandal, R.A.; Mathema, A.B. Comparison Growing Stock, Carbon Stock and Biodiversity in and Around Banke National Park, Nepal. Int. J. Adv. Res. Bot. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Mandal, R.A.; Dutta, I.C.; Jha, P.K.; Karmacharya, S. Relationship between Carbon Stock and Plant Biodiversity in Collaborative Forests in Terai, Nepal. ISRN Botany 2013, 1–7. [Google Scholar] [CrossRef]
- Department of Forest Research and Survey (DFRS) State of Nepal’s Forests. Forest Resource Assessment (FRA) Nepal; Department of Forest Research and Survey (DFRS): Kathmandu, Nepal, 2015.
- Kurz, W.A.; Dymond, C.C.; White, T.M.; Stinson, G.; Shaw, C.H.; Rampley, G.J.; Smyth, C.; Simpson, B.N.; Neilson, E.T.; Trofymow, J.A.; et al. CBM-CFS3: A Model of Carbon-Dynamics in Forestry and Land-Use Change Implementing IPCC Standards. Ecol. Model. 2009, 220, 480–504. [Google Scholar] [CrossRef]
- Nautiyal, N.; Singh, V. Carbon Stock Potential of Oak and Pine Forests in Garhwal. J. Pharmacogn. Phytochem. 2013, 2, 43–48. [Google Scholar]
- Joshi, R.; Singh, H.; Chhetri, R.; Poudel, S.R.; Rijal, S. Carbon sequestration potential of community forests: A comparative analysis of soil organic carbon stock in community managed forests of Far-Western Nepal. Eurasian J. Soil Sci. 2021, 10, 96–104. [Google Scholar] [CrossRef]
- Cambi, M.; Certini, G.; Neri, F.; Marchi, E. The Impact of Heavy Traffic on Forest Soils: A Review. For. Ecol. Manag. 2015, 338, 124–138. [Google Scholar] [CrossRef]
- Trujillo, W.; Amezquita, E.; Fisher, M.J.; Lal, R. Soil Organic Carbon Dynamics and Land Use in the Colombian Savannas I. Aggregate Size Distribution; Soil Proce; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351415767. [Google Scholar]
- Börjesson, G.; Bolinder, M.A.; Kirchmann, H.; Kätterer, T. Organic Carbon Stocks in Topsoil and Subsoil in Long-Term Ley and Cereal Monoculture Rotations. Biol. Fertil. Soils 2018, 54, 549–558. [Google Scholar] [CrossRef]
- Acharya, K.P. Conserving Biodiversity and Improving Livelihoods: The Case of Community Forestry in Nepal. In Proceedings of the The International Conference on Rural Livelihoods, Forests and Biodiversit, Bonn, Germany, 9–23 May 2003; pp. 19–23. [Google Scholar]
- Baral, S.K.; Katzensteiner, K. Disturbance Gradient in a Central Mid-Hill Community Forest of Nepal. Group 2009, 19, 3–10. [Google Scholar]
- Roberts, M.R. Response of the Herbaceous Layer to Natural Disturbance in North American Forests. Can. J. Bot. 2004, 82, 1273–1283. [Google Scholar] [CrossRef]
- Smith, G.F.; Gittings, T.; Wilson, M.; French, L.; Oxbrough, A.; O’Donoghue, S.; O’Halloran, J.; Kelly, D.L.; Mitchell, F.J.G.; Kelly, T.; et al. Identifying Practical Indicators of Biodiversity for Stand-Level Management of Plantation Forests. Biodivers. Conserv. 2008, 17, 991–1015. [Google Scholar] [CrossRef]
- Jackson, J.K.; Stapleton, C.; Jeanrenaud, J.-P. Manual of Afforestation in Nepal; Nepal-United Kingdom Forestry Research Project, Department of Forest: Kathmandu, Nepal, 1994.
- Charmakar, S.; Oli, B.N.; Joshi, N.R.; Maraseni, T.N.; Atreya, K. Forest Carbon Storage and Species Richness in FSC Certified and Non-Certified Community Forests in Nepal. Small-Scale For. 2021, 20, 199–219. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Nisbet, K.; Stone, M.; Seibold, S. Decaying Forest Wood Releases a Whopping 10.9 Billion Tonnes of Carbon Each Year. This Will Increase under Climate Change. Conversat. Biol. Divers. 2022, 8, 8–11. [Google Scholar]
- Antal, T.; Erika, S.; Arezoo, M.H. Wet Air Oxidation of Aqueous Wastes. In Wastewater Treatment Engineering; Mohamed, S., Ed.; IntechOpen: Rijeka, Croatia, 2015; Chapter 6. [Google Scholar]

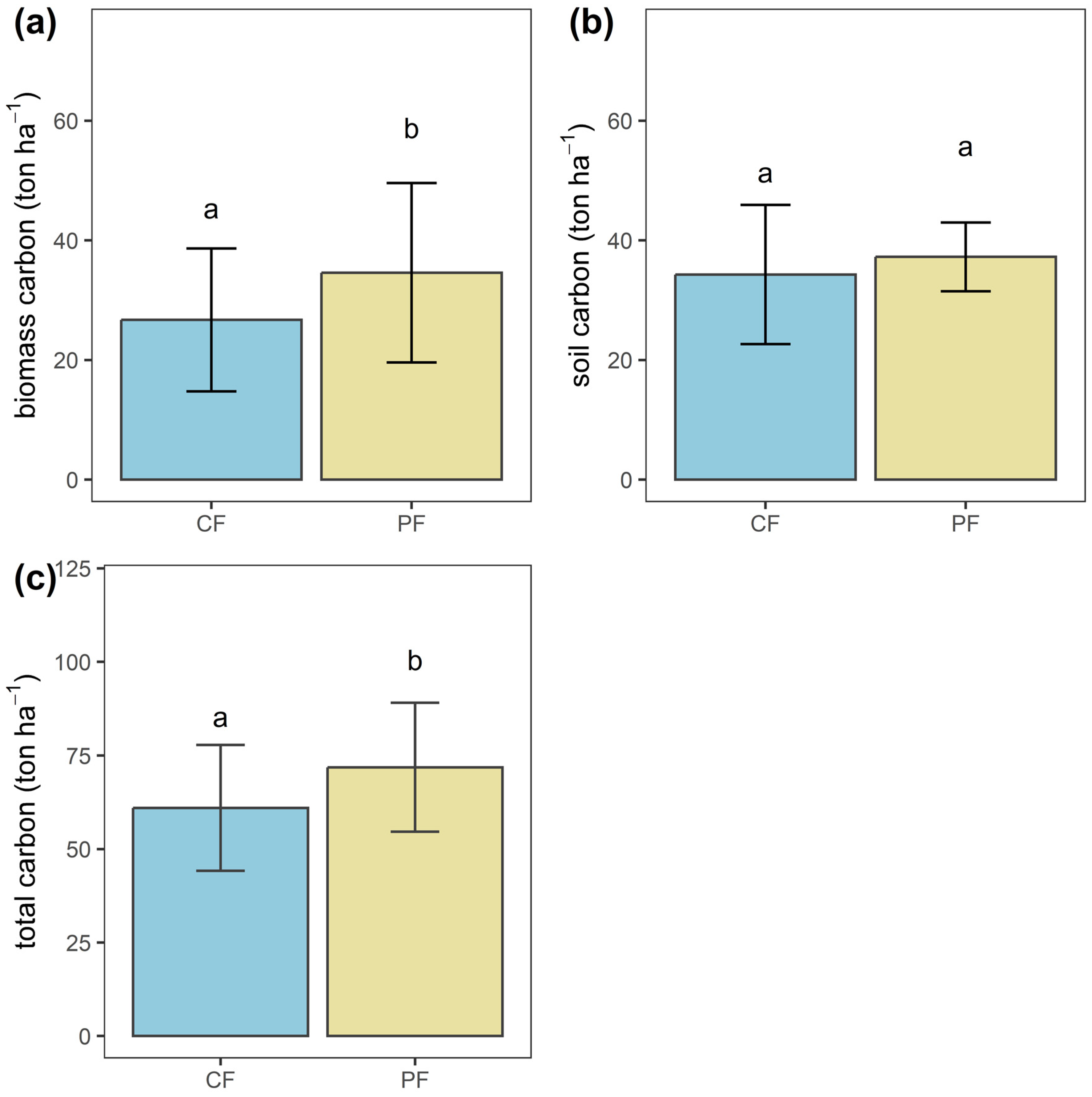


| Management Approach | Mean Carbon Stock (ton ha−1) | Standard Deviation | Min | Max |
|---|---|---|---|---|
| CF | 61.00 | 16.82 | 38.04 | 98.32 |
| PF | 71.85 | 17.22 | 25.65 | 121.60 |
| Management Approaches | Diversity Indices | |||
|---|---|---|---|---|
| Richness (S) | Number of Individuals | Simpson Index (1-D) | Shannon Diversity (H′) | |
| CF | 9 | 230 | 0.61 | 1.3 |
| PF | 10 | 279 | 0.59 | 1.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamsal, P.; Aryal, K.R.; Adhikari, H.; Paudel, G.; Maharjan, S.K.; Khatri, D.J.; Sharma, R.P. Effects of Forest Management Approach on Carbon Stock and Plant Diversity: A Case Study from Karnali Province, Nepal. Land 2023, 12, 1233. https://doi.org/10.3390/land12061233
Lamsal P, Aryal KR, Adhikari H, Paudel G, Maharjan SK, Khatri DJ, Sharma RP. Effects of Forest Management Approach on Carbon Stock and Plant Diversity: A Case Study from Karnali Province, Nepal. Land. 2023; 12(6):1233. https://doi.org/10.3390/land12061233
Chicago/Turabian StyleLamsal, Puspa, Kamal Raj Aryal, Hari Adhikari, Gayatri Paudel, Surya Kumar Maharjan, Dinesh Jung Khatri, and Ram P. Sharma. 2023. "Effects of Forest Management Approach on Carbon Stock and Plant Diversity: A Case Study from Karnali Province, Nepal" Land 12, no. 6: 1233. https://doi.org/10.3390/land12061233
APA StyleLamsal, P., Aryal, K. R., Adhikari, H., Paudel, G., Maharjan, S. K., Khatri, D. J., & Sharma, R. P. (2023). Effects of Forest Management Approach on Carbon Stock and Plant Diversity: A Case Study from Karnali Province, Nepal. Land, 12(6), 1233. https://doi.org/10.3390/land12061233









