Abstract
The cultivated soils in several semi-arid areas have very low organic matter due to climatic constraints that limit primary crop yield. Conservation tillage systems, outlined here as no tillage, no tillage with straw return and straw incorporation into the field, have been accepted as capable systems that preserve soil’s resources and sustain soil productivity. However, in semi-arid climates, there is presently no knowledge about the influence of different conservation tillage techniques on soil’s physical, chemical and biological properties at different soil depths in spring wheat fields and only little information about spring wheat yield in these management systems. Therefore, the present study was carried out with the objective of examining the impact of conservation tillage systems on soil properties (physical, chemical and biological) and spring wheat yield. The three conservation tillage treatments consisted of no tillage system (NT), wheat stubble return with no tillage (NTS) and straw incorporation with conventional tillage (CTS), as well as one conventional tillage (CT) control treatment, which were evaluated under randomized complete block design with three replications. The three conservation tillage treatments were compared with the conventional tillage control. Conservation tillage significantly increased the bulk density, gravimetric water content, water storage, hydraulic conductivity and soil aggregates and decreased the pore space and soil temperature compared to CT; however, no significant difference was found in the case of field capacity. Soil chemical properties in the 0–40 cm soil layer increased with conservation tillage compared to CT. Conservation tillage also notably increased the soil microbial counts, urease, alkaline phosphatase, invertase, cellulase and catalase activities relative to CT. Microbial biomasses (carbon and nitrogen) and wheat yield significantly elevated under conservation tillage compared to CT. Therefore, conservation tillage could significantly improve soil properties and maintain wheat yield for the research zone.
1. Introduction
The cultivated soils in several semi-arid areas have very low organic matter due to climatic constraints that limit primary crop yield [1]. Conventional tillage on these lands has led to soil degradation through soil erosion and fertility loss, thereby reducing crop yield. Improved soil management practices are, therefore, required to conserve soil resources and increase crop yields to feed the increasing global population [2]. In order to preserve the soil integrity, soil management techniques used in the agriculture sector should be oriented towards resource conservation [3]. Conservation tillage is a proven practice for increasing soil organic matter (SOM) in various climatic conditions. Through enhancing SOM, the conservation tillage may improve soil nitrogen mineralization, potentially minimizing crop nitrogen fertilizer demand in comparison to conventional tillage [4,5,6]. In accordance with Kassam et al. [7], conservation agriculture is based on three main interlinked ideas:
- (i)
- No or less soil inversion (no till with direct planting)
- (ii)
- Soil organic cover with crop straw (as a minimum 30%)
- (iii)
- Sustainable crop rotation (as a minimum three different crops)
In the current climate change scenario, the “soil management change” is significant to encourage soil and water conservation. Conservation tillage has revealed a great range of findings with respect to soil properties [8]. Therefore, investigation of the effects of these new soil tillage techniques on soil’s physical, chemical and biological properties is obligatory.
Physical quality of soil has been extensively approached by bulk density to estimate numerous soil processes or to evaluate soil carbon reserves [9]. Bulk density may be considered a significant parameter that reflects soil structure, being connected with total soil porosity [10]. Worldwide, there are plenty of studies that have depicted significant impacts of conservation tillage on soil physical properties by decreasing soil bulk density and temperature or increasing soil water content, water storage, hydraulic conductivity and aggregates [1,8,11,12]. Sithole et al. [13] reviewed the impacts of conservation tillage on soil properties, mostly by measuring soil’s physical, chemical and biological properties, and indicated strong improvement.
Chemical quality of soil has been broadly approached by soil nutrients to evaluate soil fertility. Soil nutrients improvement under conservation tillage was reported by [14,15,16,17]. Soil biochemical properties such as microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were significantly improved under conservation tillage [17,18,19,20]. Soil organic carbon is a valued soil indicator that sustains soil fertility, crop productivity and contributes to mitigate climate change and warrant food security. Conservation tillage has demonstrated to be a bright alternative to conventional tillage in long-term crop production and soil organic carbon sequestration. Conservation tillage decreases the carbon oxidation process and loss of soil organic carbon and facilitates soil organic carbon sequestration [21,22]. Soil organic matter (SOM) improvement in agro-ecosystems can mitigate changing climate by increasing sequestration of soil carbon whilst simultaneously enhancing soil productivity [23]. SOM improvement under conservation tillage over conventional tillage was reported by [23,24]. In a soil system, enzymes are the significant players in the biochemical processes of SOM recycling, and their activities are closely associated with SOM, microbial activity and soil physical properties. Additionally, during nutrient cycling and SOM decomposition, the soil enzymes act as obligatory catalysts and powerfully impact energy transformation, yield and environmental quality [25]. Tillage practice affects soil enzymes’ activities, thus influencing yield [26]. The adverse influence of conventional tillage has been well documented in soil enzymatic activities [27]. Conservation tillage improves enzymatic activities and microbial biomass [3,28]. Several studies have been conducted on the influence of conservation tillage on soil enzymes’ activities and showed a positive impact compared with conventional tillage [29,30,31]. Choudhary et al. [32] reported higher enzyme activities with residue retention compared to conventional tillage. Bergstrom et al. [33] showed higher microbial counts, urease, phosphatase, invertase, cellulase, dehydrogenase and catalase activities under conservation tillage.
Wheat is one of the vital cereal crops, and it makes a vast contribution to global food security. However, fulfilling global food requirements is becoming continually more challenging because of stagnant crop yields [12]. Accordingly, intensive tillage is used to attain higher yields. Regrettably, this approach has led to degrading soil and environmental quality [34,35]. As a substitute to conventional tillage, conservation tillage has demonstrated success in sustaining soil and increasing yields [36,37,38,39].
Studies on the impact of conservation tillage on selected soil physico-chemical properties have been conducted [8,30,34], but the knowledge is scanty on the impact of conservation tillage on soil’s physical, chemical and biological properties together in semi-arid areas, such as Dingxi Northwestern China belt. Moreover, in most of the studies, the soil sampling under conservation tillage was completed only at 0–10 cm soil depth; there is currently no knowledge about the influence of conservation tillage on soil properties at different soil depths. Therefore, soil sampling at different soil depths (0–10 cm, 10–20 cm and 20–40 cm) was completed. We hypothesized that conservation tillage would improve soil’s physical, chemical and biological properties and wheat yield. The objectives of the study were to evaluate (1) the impact of conservation tillage practices on soil bulk density, pore space, gravimetric water content, water storage, hydraulic conductivity, soil aggregates and soil temperature across different soil layers under wheat mono-cropping system conditions; (2) the influence of conservation tillage systems on soil total nitrogen, total phosphorous, total potassium, available nitrogen available phosphorous, available potassium, C:N ratio and pH at different soil depths; (3) the effects of conservation tillage measures on soil microbial counts, urease, alkaline phosphatase, invertase, cellulase and catalase activities and microbial biomasses (carbon and nitrogen); (4) the effect of conservation tillage techniques on spring wheat yield.
2. Materials and Methods
2.1. Research Site Description
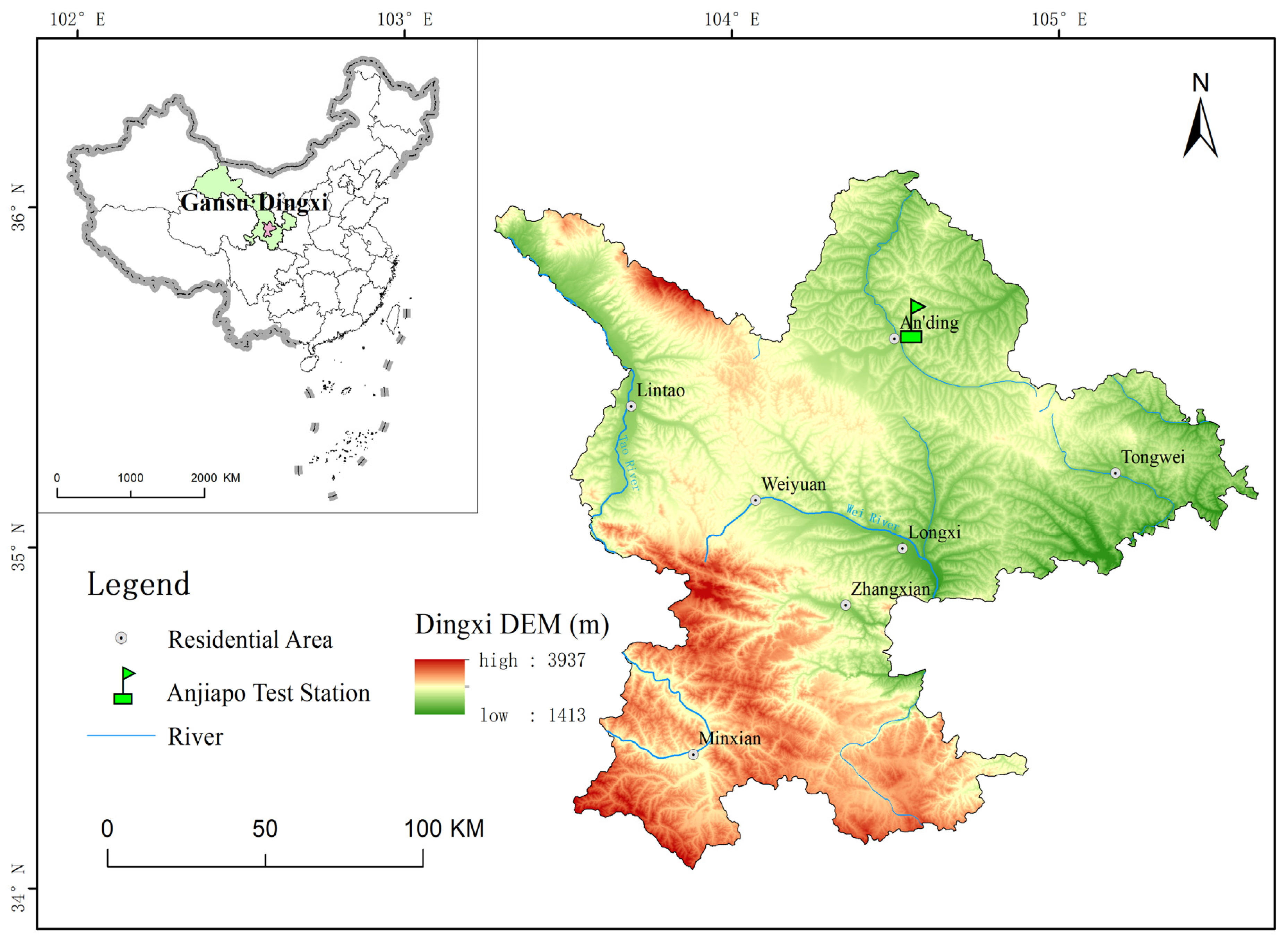
This field experiment was set up in the spring season of 2016 at the Dingxi under the supervision of the department of Soil and Water Conservation located at Gansu Province (35°34′53″ N, 104°38′30″ E longitude with an altitude of 2000 m above sea level), Northwestern region of China (Figure 1). The climatic conditions of study area are defined as semi-arid. Average temperatures of this region are 35 °C and −22 °C in the summer and winter months, respectively. Mean annual rainfall of study area is 400 mm with irregular distribution; mean annual radiation is 5930 MJ m−2 with 2480 h of sunshine per year, a frost-free period of 140 days, an annual temperature of 6.9 °C, an annual average evaporation of 1531 mm [40]. The soil type of research area is a Huangmian sandy loam in texture, low in organic carbon content with slightly alkaline pH and is classified as a Calcaric Cambisol [41]. A wide-ranging research location explanation has been provided in earlier studies [8,24,42]. Before the experiment, the study field was bare. Northern China, particularly Dingxi, had a long history of wheat farming and crop straws, mainly wheat stubbles, were continually removed earlier to the next crop cycle.
Figure 1.
Map of the study area in Anding, Gansu of China.
2.2. Experimental Design and Setup
This research was conducted from March 2019 to July 2020. The treatment contains three conservation tillage systems, namely no tillage system (NT), wheat stubble return with no tillage (NTS) and straw incorporation with conventional tillage (CTS) and one conventional tillage (CT) control. The conservation tillage systems were compared with the conventional tillage control. These tillage systems have been practiced since 2016 (4 years), with continuous spring wheat cultivation. Descriptions of these tillage systems are presented in (Table 1). In the NT treated plots, after harvesting wheat, all stubbles were removed and crop planting was performed with a no tillage crop planter (Sazeh Kesht); nevertheless, in the NTS practice after harvesting the wheat, all stubble was returned to the field and crop sowing was completed with a no tillage crop planter. In order to manage CTS, after harvesting the wheat, crop residue was incorporated into the soil with moldboard plough followed by disc harrow and planting. For CT system, land cultivation was performed with moldboard plough at 20 cm depth, followed by disc harrow and planting in the absence of stubble. The research setup was a randomized complete block design (RCBD) with three replicates of each treatment, giving a total of 12 individual plots with an area of 24 m2. Furthermore, nitrogen and phosphorous using diammonium phosphate at the rate of (146 kg ha−1) and urea at the rate of (63 kg ha−1) were applied as basal doses. Meanwhile, potassium was not applied because the semi-arid Loess Plateau in China has appropriate soil potassium concentration, which was adequate to promote wheat growth [43,44]. Maintaining rows spaced 20 cm apart, spring wheat (cultivar Dingxi 42) was sown in mid-March at a rate of 187.5 kg seeds ha−1 and was harvested in late July of each year. In accordance with manufacturer’s instructions, herbicide glyphosate (30%) was used to control weeds, and, throughout the growing season, manual weeding was also conducted when required during studied years.

Table 1.
Tillage treatments and their description.
2.3. Data Collection
2.3.1. Soil Physical Properties
The disturbed and undisturbed soil samples including three soil layers (0–10 cm, 10–20 cm and 20–40 cm) from different experimental treatments (NT, NTS, CT and CTS) were collected with an auger of 4 cm diameter after harvesting stage in 2020 for the determination of soil physical properties. Five soil samples were taken from each individual plot involving three soil depths.
The determination of soil bulk density was completed from undisturbed core samples divided into 3 soil depths (0–10 cm, 10–20 cm and 20–40 cm). By using a core sampler (steel cylinders of 4 cm diameter and 3 cm in length), soil samples were sampled. According to procedure described by Campbell, [45], the sampled soil samples were processed. It was calculated by using Equation (1):
where BD = bulk density of soil (g cm−3), M = mass of the dry soil sample (gm), V = volume of sample (cm−3).
BD = M/V
Soil pore space was calculated from the bulk density of soil values and particle density of soil [45]. The soil porosity percent was calculated by using Equation (2):
P = [1 − (BD/Pd)] × 100
Gravimetric soil water content was measured using the oven-dry method by taking fresh soil sample and then dried for 72 h at 105 °C and weighed [46,47]. The calculation of soil water storage was completed from the gravimetric moisture content of soil, bulk density of soil, depth of soil and water density [46]. The soil water storage was calculated by using Equation (3):
where SWS = soil water storage (mm), SWC = gravimetric soil water content (%), BD = bulk density of soil (g cm−3), d = depth of soil (cm) and pw = density of water.
SWS = SWC × BD × d/ρw
The measurement of saturated soil hydraulic conductivity was conducted in situ at individual sub-plot to 0–40 cm soil depth using auger-hole method, using the Guelph Permeameter. Soil hydraulic conductivity determination was completed by taking three steady-state readings [48]. The measurements of soil temperature were conducted for three soil depths 0–10 cm, 10–20 cm and 20–40 cm at monthly intervals. Soil temperature was measured using a geothermometer [49,50]. The measurements of soil aggregates were conducted by using wet sieved method [51]. Field capacity was determined following the procedure as described by [52].
2.3.2. Soil Chemical Properties
In the experimental field, five soil samples at three soil depths 0–10 cm, 10–20 cm and 20–40 cm from different tillage plots were sampled and placed in a plastic bag and transported to laboratory for chemical analysis. Soil total nitrogen (TN), total phosphorous (TP), total potassium (TK), available nitrogen (AN), available phosphorous (AP) and available potassium (AK) were determined by using standard methods [49]. The determination of soil organic carbon was completed by using the modified Walkley–Black dichromate oxidation approach [53]. Light fraction organic carbon was isolated by density fractionation method [54], and determined with C and N analyzer (Elementar Vario MACRO CUBE). Soil organic matter was determined by using method as described by [55]. The soil pH and electrical conductivity were measured by using a pH meter and EC meter, respectively [49].
2.3.3. Soil Biological Properties
Soil biological properties were investigated in 2019 at crop harvest stage. The determination of soil microbial propagules (colony forming units, CFUs) was completed by using enumeration of luminescent colonies on agar media [56]. The activity of soil urease was analyzed on the basis of method described by Dick [57], the activity of soil alkaline phosphates following the procedures by Zhao and Jiang [58], soil invertase activity as described by Frankeberger and Johanson [59], the soil cellulase activity following the procedures of Guan [60], the soil catalase activity as described by Yan [61]. The microbial biomasses (carbon and nitrogen) were determined using the fumigation–extraction method [62].
2.3.4. Agronomic Traits
Spring wheat crop was harvested manually at the end of July in 2020 from all sub-plots by sampling an area of one meter square per plot. The agronomic attributes of biological yield, grain yield, seed m2, and thousand grain weights were determined. For biomass determination, the fresh wheat crop (biomass) samples were dried in an oven at 65 °C for 72 h and weighed [63]. At harvesting time, from all the sub-plots, including the one square meter, spring wheat grain yield was determined. The thousand grain weight was measured by randomly taking a sample of the whole grains using a seed counter, and dried at 70 °C for 48 h [63].
2.4. Statistical Analysis
The data obtained from the experiment were subjected to one-way factor interaction ANOVA. All statistical analyses were performed by using the linear model procedure of the suitable computer software program Statistical Package for Social Science (SPSS) window version 25.0 (IBM Corp., Chicago, IL, USA). The mean of different experimental treatments was separated by Duncan’s test at 5% significance level (p < 0.05). The obtained data are presented as the mean of three replicates with standard deviation.
3. Results
3.1. Soil Properties
The pre-sowing soil physical and chemical properties at different soil depths are shown in Table 2. The average over the different soil depths (0–10 cm, 10–20 cm and 20–40 cm), the pre-sowing soil BD, P, ST, SWC, SWS, Ks, pH, SOC, TN, TP and TK were 1.43 g cm−3, 46.81%, 6.20 °C, 14.96%, 48.84 mm, 0.52 mm ha−1, 8.41, 5.80 g kg−1, 0.60 g kg−1, 0.40 mg kg−1 and 18.47 g kg−1, respectively, and the texture of soil was sandy loam.

Table 2.
Pre-sowing soil physico-chemical properties in 2019.
3.2. Climatic Conditions
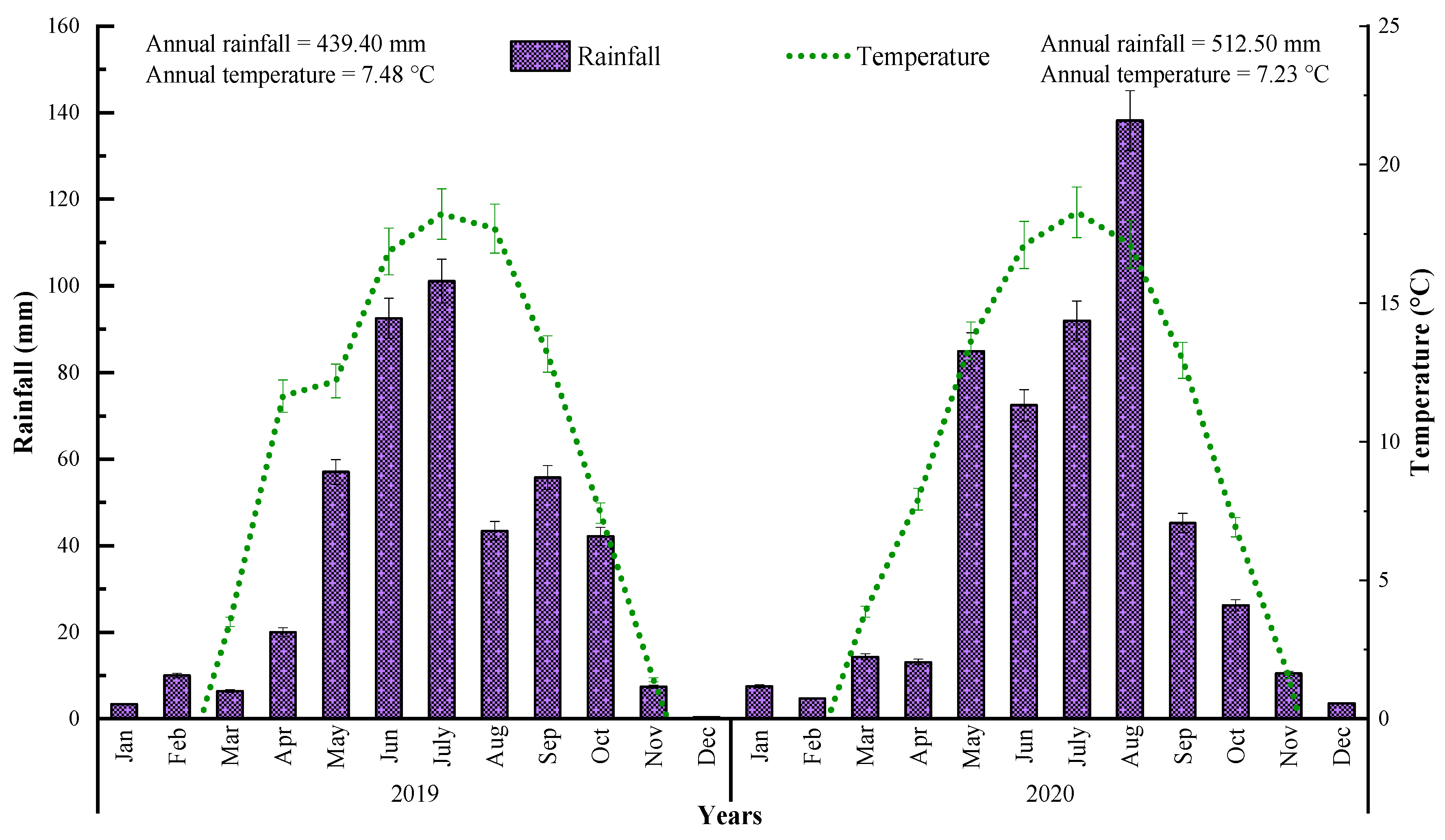
The average annual temperature in the years of 2019 and 2020 was 7.48 °C and 7.23 °C, respectively. The mean temperature throughout the 2 years was 7.35 °C (Figure 2). The mean annual rainfall in the years of 2019 and 2020 was 439.40 mm and 512.50 mm, respectively. The mean rainfall for the 2 years was 475.95 mm. Thus, in the first year, the water input was less, considered being mainly dry; nevertheless, rainfall was appropriate and more in the second year of the research period. Regarding the climate, 2020 might be considered the most appropriate season for spring wheat cultivation in Northwestern Loess plateau Dingxi (China).
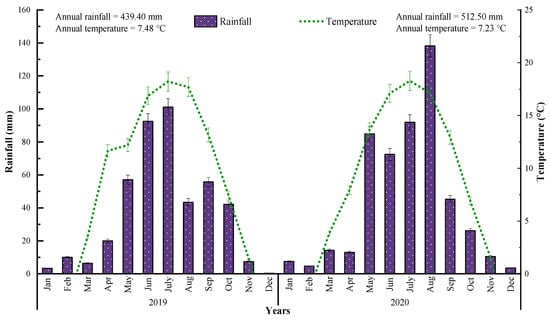
Figure 2.
Climatic conditions of the research area during 2019 and 2020. Error bars indicate standard error of mean values.
3.3. Effects of Conservation Tillage on Soil Physical Quality Indicators
The analysis of variance showed that soil physical properties BD, P, SWC, SWS, Ks, ST and soil aggregates were significantly influenced by conservation tillage systems at 0–10 cm and 10–20 cm depths (Table 3 and Figure 3). At 20–40 cm depth, conservation tillage did not show significant variation regarding the investigated soil physical properties except soil aggregates. At 0–10 cm depth, the NTS system had the highest values of BD, SWC, SWS, Ks and aggregates whilst the CT technique had lowest values; however, CT had maximum values of P and ST compared to conservative tillage treatments. The BD, SWC, SWS and Ks followed the trend of CT < NT < CTS < NTS, whereas the trend of P and ST was reverse as CT > NT > CTS > NTS. Moreover, the ST was highest under CT and CTS when the ambient temperature was high. The conservation tillage decreased the ST, demonstrating the temperature moderation effect.

Table 3.
Impact of conservation tillage on soil physical properties at different soil layers in 2020.
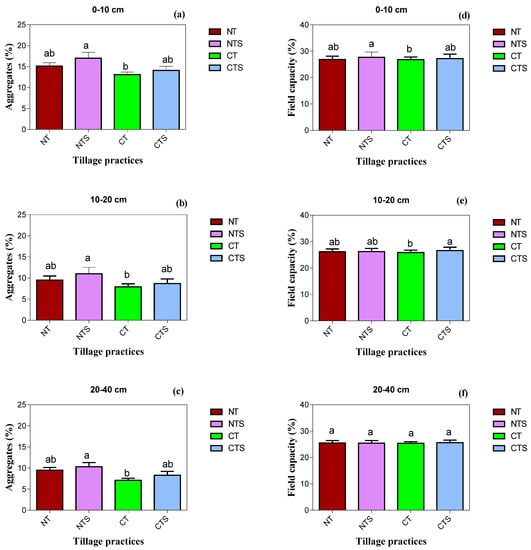
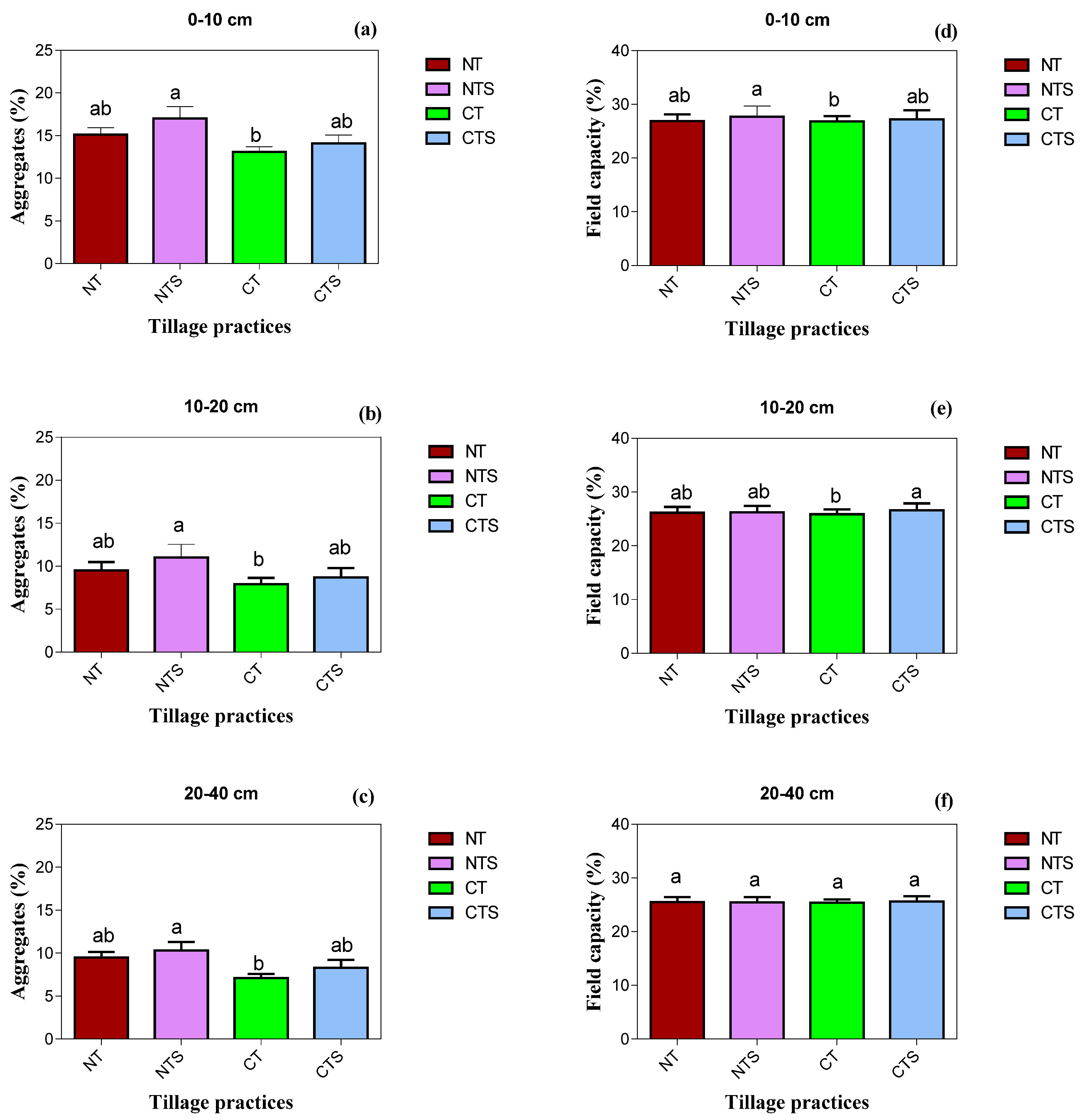
Figure 3.
Soil physical quality attributes under conservation tillage in 2020. Vertical error bars represent the corresponding standard error of mean values. Different lowercase letters in the same layer indicate significant differences amongst different treatments at p < 0.05 (Duncan’s test performed for mean separation). Note: (a–c) is the soil aggregates values as affected by the tillage techniques at different soil depths; (d–f) is the soil field capacity values under conservation tillage strategy at different soil layers.
Similar with depth above, at 10–20 cm depth, the NTS, CTS and NT management practices significantly varied BD, SWC, SWS, Ks and aggregates, the highest values being under the NTS system. Interestingly, maximum logarithmic Ks was associated with the NT technique at 10–20 cm depth. The maximum reductions in values of BD, SWC and SWS, Ks and aggregates were observed in CT. In addition, our results depicted that the highest SWS was associated with sub-surface 20–40 cm soil depth over 0–10 cm and 10–20 cm soil depth.
At 20–40 cm depth, NTS achieved the maximum soil aggregates, which was followed by NT, which was statistically on par with CTS, whereas CT related with minimum soil aggregates. The NTS, NT and CTS increased the soil aggregates by 17, 12 and 6.8%, respectively, compared with CT.
3.4. Conservation Tillage Influenced Soil Chemical Quality Indicators
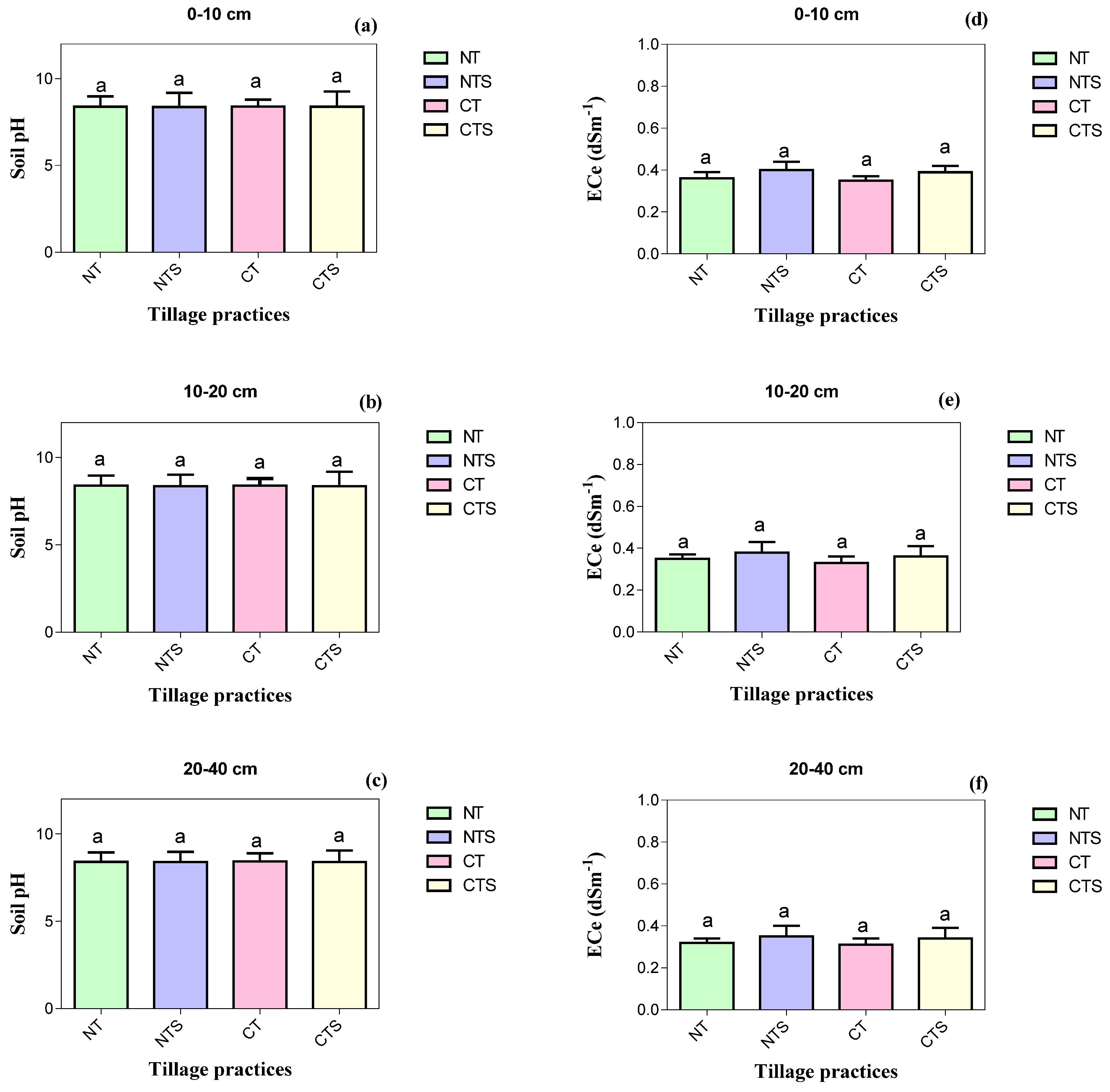
The concentration of soil chemical properties TN, TP, TK, AN, AP, AK, SOC, LFOC and SOM, pH and ECe at sub-surface soil depths was reduced over surface soil depths irrespective of treatments (Table 4 and Figure 4). At 0–10 cm depth, significantly higher values of TN (0.72 ± 0.03 g kg−1) and AN (45.33 ± 2.52 mg kg−1) were observed in CTS, whilst significantly higher values of TP (0.50 ± 0.03 g kg−1), AP (17.66 ± 1.68 mg kg−1), AK (250.43 ± 17.96 mg kg−1), SOC (11.81 ± 0.3 g kg−1), LFOC (1.29 ± 0.31 g kg−1) and SOM (13.85 ± 0.22 g kg−1) were noted in NTS over all other treatments. The maximum reductions in values of TN, TP, TK, AN, AP, AK, SOC, LFOC and SOM were observed in CT. These chemical attributes followed the trend of NTS > CTS > NT > CT. Moreover, different treatments had no statistically significant variation regarding TK, pH and ECe including all tested soil depths.

Table 4.
Soil chemical properties influenced by conservation tillage at different soil depths in 2020.
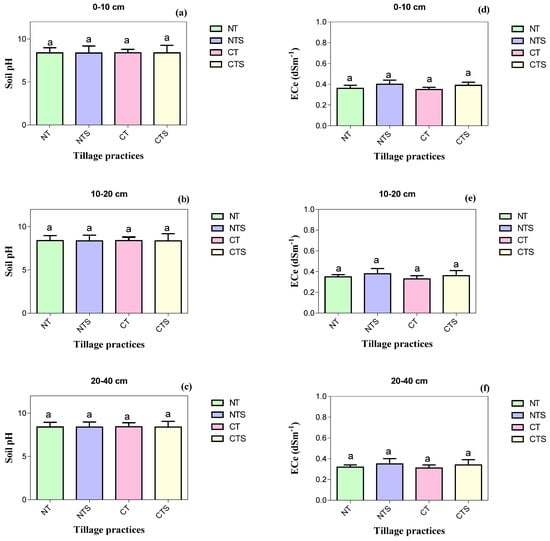
Figure 4.
Soil chemical quality attributes influenced by conservation tillage in 2020. Vertical error bars represent the corresponding standard error of mean values. Different lowercase letters in the same depth indicate significant differences amongst different treatments at p < 0.05 (Duncan’s test performed for mean separation). Note: (a–c) is the soil pH values as influenced by the tillage systems at different soil depths; (d–f) is the soil electrical conductivity values under conservation tillage at different soil layers.
In sub-surface 10–20 cm depth, TN, TP, AN, AP, AK, SOC, LFOC and SOM depicted significant differences under different conservation tillage practices. The CTS had maximum values of TN, TP and AK, whilst NTS had the highest values of AN, AP, SOC, LFOC and SOM. The lowest TN, TP, AN, AP, AK, SOC, LFOC and SOM values were associated with CT.
Similar to the depth above, at 20–40 cm, the conservation tillage system had a marked influence on TN, AN, AP, AK, SOC and LFOC. The NTS had the highest values of TN and SOC, whilst CTS had maximum values of AN, AP, AK and LFOC. The NTS, CTS and NT practices were better for improving soil chemical quality indicators at all investigated soil layers; however, the influence of conservation tillage decreases with increasing soil depth.
3.5. Impact of Conservation Tillage on Soil Biological Quality Indicators
In the surface soil layer with a depth of 0–10 cm, soil microbial counts, urease, alkaline phosphatase, invertase, cellulase and catalase activities were significantly influenced by the different tillage techniques. The highest values of all investigated biological properties were reached in the NTS system followed by NT treatment, whilst the lowest values were associated with the CT strategy. Compared with the CT system, the NTS, NT and CT treatments notably increased the soil microbial counts by 17%, 13% and 9%, respectively, soil urease activity by 7.6%, 5.9% and 2.2%, respectively, soil alkaline phosphatase activity by 28.3%, 22.4% and 14%, respectively, soil invertase activity by 29.8%, 15.9% and 6.9%, respectively, soil cellulase activity by 5.3%, 4.6% and 3.7% and soil catalase activity by 5.2%, 3.2% and 2%, respectively.
At the soil layer with 10–20 cm depth, conservation tillage had a significant effect on soil microbial counts and soil enzymes’ activities (urease and catalase) except for alkaline phosphatase, invertase and catalase. The NTS had the highest microbial counts whilst urease activity was highest in NTS system, whereas the catalase activity noted in NT treatment was highest. Interestingly, although at 10–20 cm depth, soil catalase and urease depicted the same trend as that of average surface soil layer with 0–10 cm depth; a small effect happened with the tillage measures with CTS having the minimum catalase and urease activity.
Almost the same as with the depth above, at 20–40 cm, the conservation tillage system had a notable effect on catalase activity. The no till system achieved the maximum catalase activity, whereas CT related with minimum activity. The soil (microbial counts, urease, alkaline phosphatase and invertase) was not noticeably affected under NT, NTS and CTS treatments. The impact of conservation tillage practices declined with increasing soil depth, at a soil layer of 20–40 cm depth regarding soil microbial counts, urease, alkaline phosphatase and invertase, as indicated by the data (Table 5).

Table 5.
Response of soil microbial counts (104 CFU g−1 soil), urease (mg [NH3-N] g−1 d−1), alkaline phosphatase (mg [phenol] g−1 d−1), invertase (mg [glucose] g−1 d−1), cellulase (mg [glucose] g−1 d−1) and catalase (mL [0.1 mol L−1 KMnO4] g−1 h−1) activities to conservation tillage technique at different soil depths in 2019.
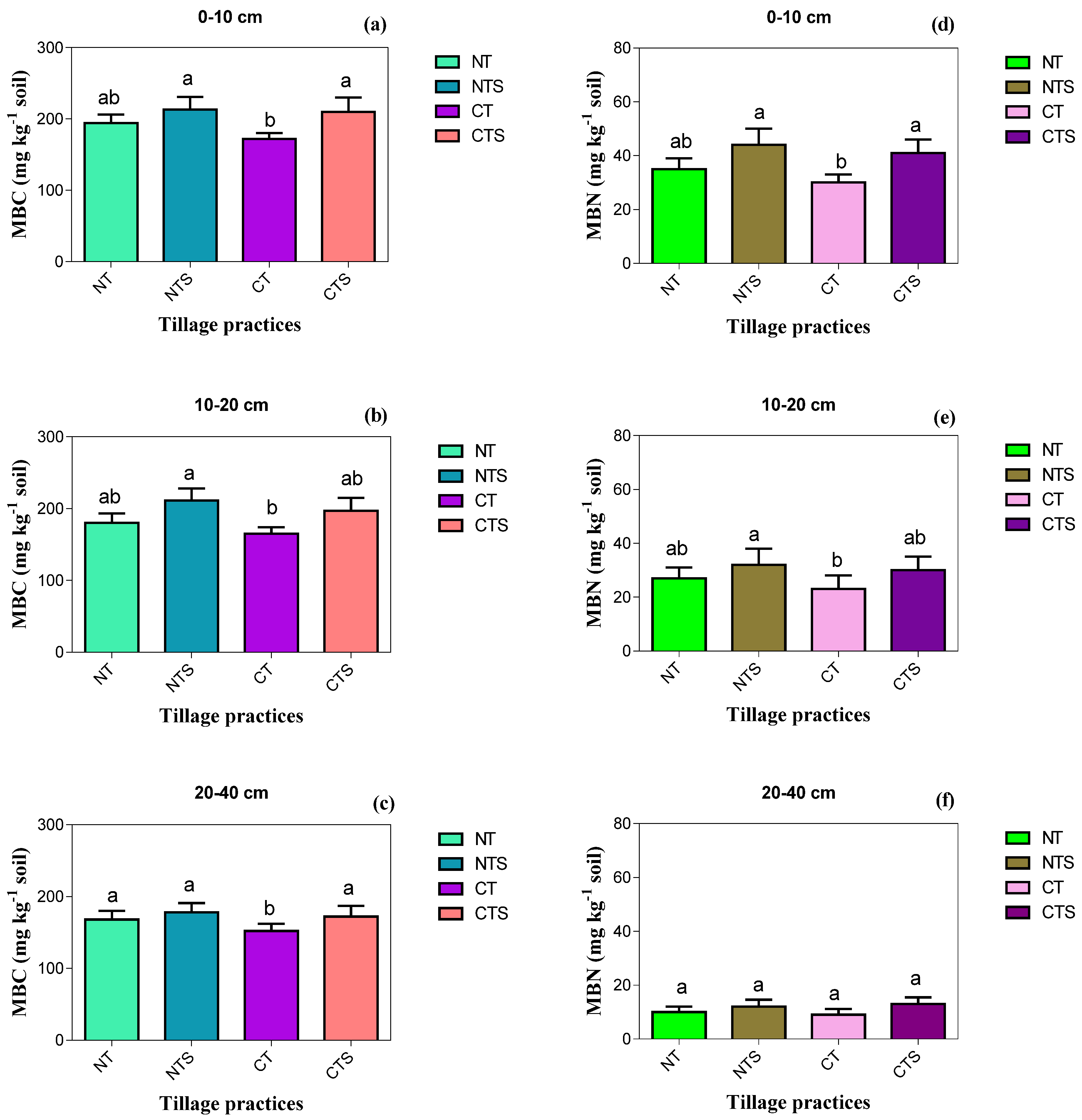
Conservation tillage practices have a significant impact (p < 0.05) on microbial biomasses (MBC and MBN) at the surface soil layer with depth of 0–10 cm. MBC was in the order of CT (172 mg kg−1 soil) < NT (194 mg kg−1 soil) < CTS (210 mg kg−1 soil) < NTS (215 mg kg−1 soil) and MBN followed the order of NTS (45 mg kg−1 soil) > CTS (41 mg kg−1 soil) > NT (35 mg kg−1 soil) > CT (30 mg kg−1 soil). In sub-surface 10–20 cm soil depth, MBC and MBN were notably higher in NTS compared with the other tillage systems, and the lowest concentrations of MBC and MBN were registered in the CT technique. The trend of MBC and MBN at sub-surface 10–20 cm soil depth was the same as the above soil layer 0–10 cm, being NTS > CTS > NT > CT. In addition, the valuable impact of tillage strategies on MBC and MBN was not reflected at 20–40 cm soil depth (Figure 5).
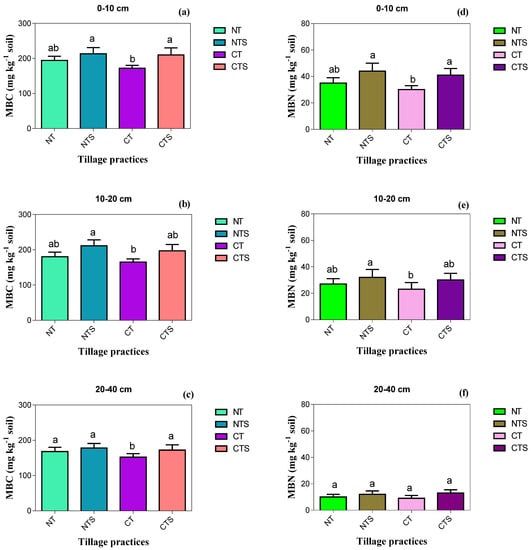
Figure 5.
Effect of conservation tillage on soil biochemical indicators in 2019. Vertical error bars represent the corresponding standard error of mean values. Different lowercase letters in the same layer indicate significant differences (Duncan’s 0.05) amongst different tillage techniques. Note: (a–c) is the soil microbial biomass carbon (mg kg−1 soil) as influenced by the tillage techniques at different soil layers; (d–f) is the soil microbial biomass nitrogen (mg kg−1 soil) under treatments at different soil depths.
3.6. Influences of Conservation Tillage on Crop Agronomy Traits
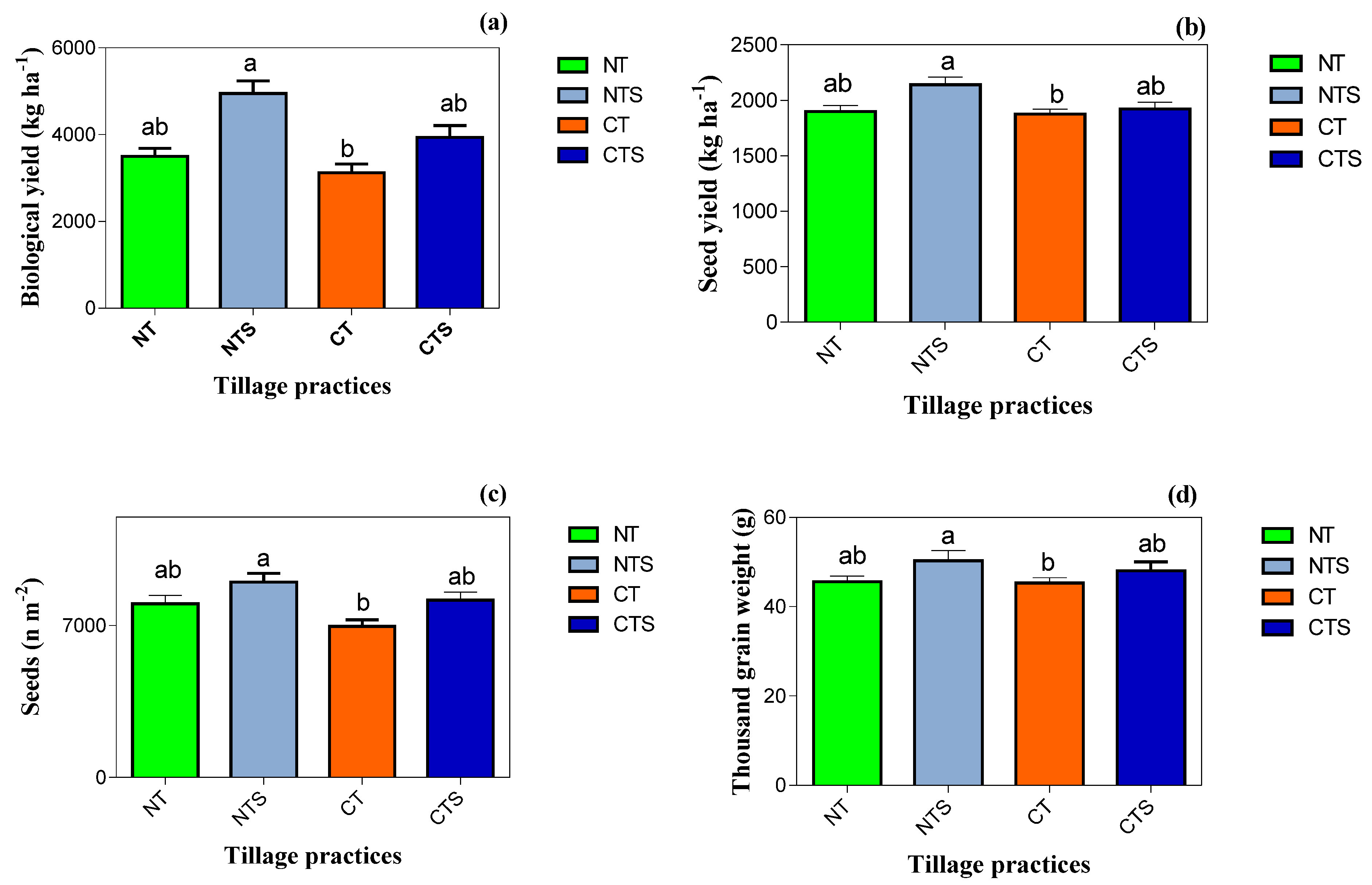
Tillage systems have varied effects on crop agronomic traits, namely biological yield, grain yield, number of seeds m−2 and thousand grain weight (Figure 6). The NTS produced significantly (p < 0.05) highest yield and yield-attributing traits, which was followed by the CTS treatment, whilst lowest agronomic attributes were associated with the CT system. The biomass productivity ranged from a highest value of 4945 ± 319 kg ha−1 to a lowest value of 3123 ± 247 kg ha−1, whilst grain yield ranged from a highest value of 2140 ± 180 kg ha−1 to a lowest value of 1877 ± 145 kg ha−1. In addition, the different conservation tillage practices NTS, CTS and NT increased the number of seeds m−2 by 35, 30 and 13% compared with the CT technique. Moreover, the thousand seed weight followed the trend of (CT < NT < CTS < NTS). The NTS increased the thousand seed weight by 9% compared with CT.
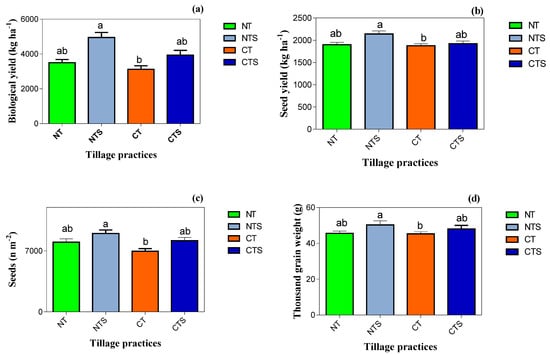
Figure 6.
Spring wheat yield and yield-attributing traits under different tillage systems in 2020. Vertical error bars represent the corresponding standard error of mean values. Lowercase letters indicate the least significant difference (Duncan’s 0.05) amongst treatments. Note: (a,b) are the biological yield and grain yield under treatments; (c,d) are the wheat number of seeds m−2 and thousand grain weight as affected by the tillage techniques.
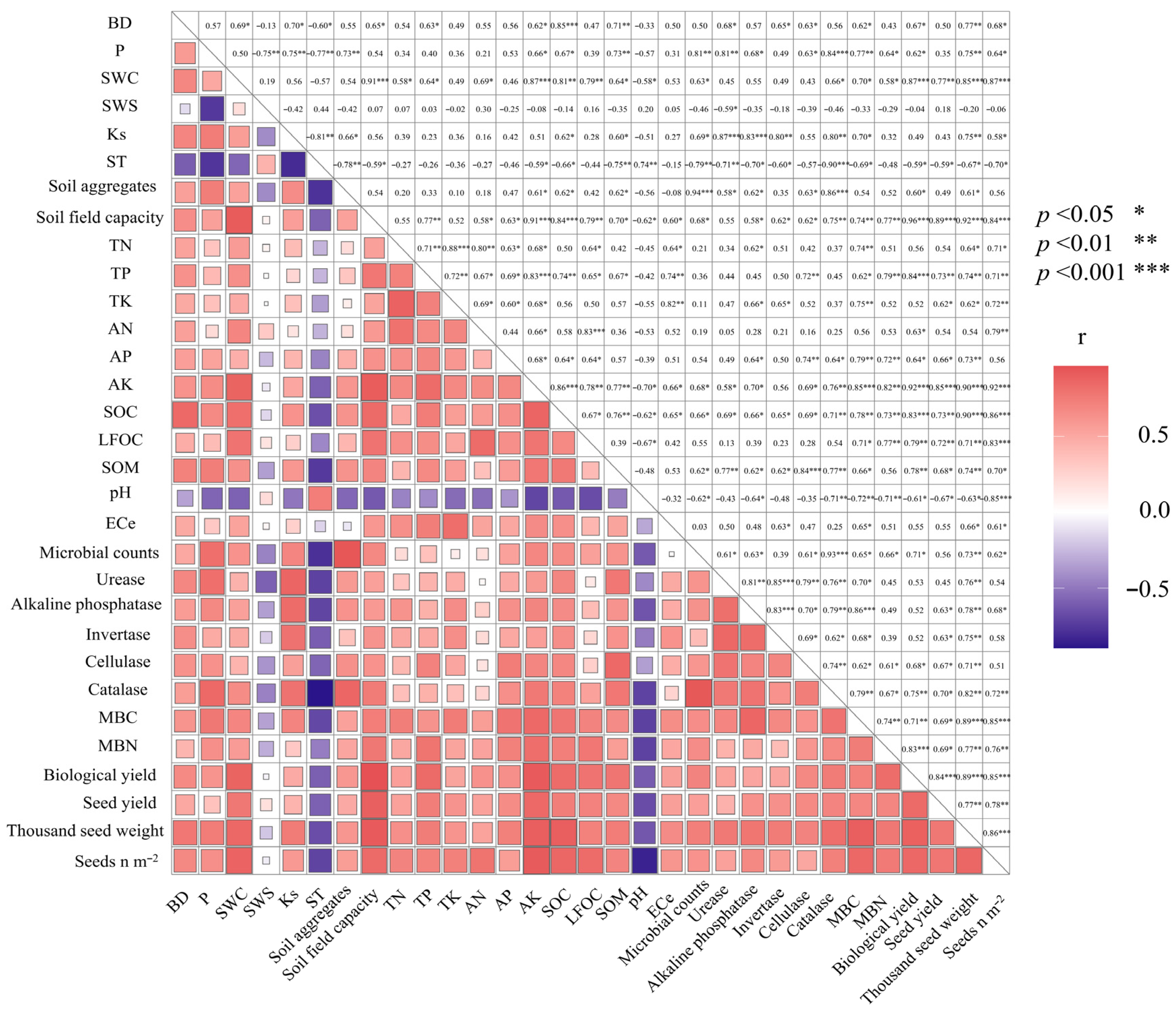
The correlations amongst the spring wheat seed yield and yield-attributing attributes are shown in (Figure 7). As expected, the correlations between grain yield and other yield-attributing attributes were significantly positive. In addition, our study showed that there is a significant positive correlation between soil nutrients and enzymes’ activity with seed yield. Furthermore, a significant positive correlation was observed between soil nutrients and enzymes’ activity.
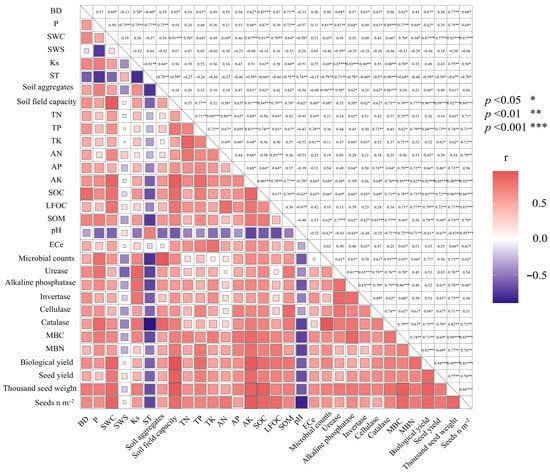
Figure 7.
Heat map correlation of wheat agronomic traits and soil properties. Indicates significance at: * p < 0.05; ** p < 0.010; *** p < 0.00010. The abbreviated words stand for BD = soil bulk density; P = soil porosity; SWC = soil gravimetric water content; SWS = soil water storage; Ks = saturated hydraulic conductivity; ST = soil temperature; TN = total nitrogen; TP = total phosphorous; TK = total potassium; AN = available nitrogen; AP =available phosphorous; AK = available potassium; SOC = soil organic carbon; LFOC = light fraction organic carbon; TN = total nitrogen; AN = available nitrogen; TP = total phosphorous; AP = available phosphorous; TK = total potassium; AK = available potassium; pH = soil pH; ECe = soil electrical conductivity; MBC = microbial biomass carbon; MBN = microbial biomass nitrogen; Seed n m−2 = number of seed per meter square.
4. Discussion
4.1. Effect of Treatments on Soil Physical Properties
Within the recent research, the maximum soil BD under NTS and NT practices was related to less soil disturbance by eliminating preceding soil tillage, which resulted in soil compactness and may also be associated with residue return in NTS, which led to soil influenced by raindrops beating [64]. Under CTS treatment, the higher BD was due to traffic physical compaction by direct heavy machinery [15]. Our results are in contrast with similar research by Higashi et al. [65], who observed that conservative tillage might decrease soil BD. Additionally, the results of the current study are similar to Higashi et al. [66], who stated that conservation tillage had higher soil BD over CT. Moreover, CTS and NT increased the soil porosity because of the accumulation of organic matter under conservation tillage measures, which led to decreased soil BD and augmented soil pore space [66,67]. Furthermore, including different soil depths, the calculated soil porosity ranged from 44.10 to 49.44% in the standard range of soil pore space for agronomic soils [68]. The results of this study are similar to [68], where the soil porosity ranged from 47 to 52% after four years of research. Normally, soil porosity was reduced with a reduction in soil depth [64,69]. Our results are in contrast with [70], who reported that CT amplified porosity over reduced soil tillage. Additionally, SWC and SWS were noticeably higher under NTS and CTS treatments due to retention of residues. Residues mainly improved the water availability and infiltration [71]. Our results are in agreement with [47], who declared that residues application markedly improved SWC and SWS. Normally, presence of straw preserves more water over a CT system [72]. Furthermore, our findings demonstrated higher SWC and SWS under NT than CT because of the earth flip inherent to CT techniques, resulting in a high loss of water owing to evaporation. This loss of water is sidestepped in the NT system, lacking the prerequisite to add residue. Previous research depicted the same findings, where conservation tillage achieved the highest SWC and SWS over CT [71].
Moreover, the Ks values remarkably varied across the treatments, being highest in NTS and NT whilst lowest in CT and CTS. The contradictions in soil Ks values may be owing to the complexity of the differences in the soil physical environment caused by tillage operations. In soil tillage treatments such as CT and CTS, the lowermost soil Ks may be attributed to the aggregate’s destruction and macroporosity reduction [73]. The positive results of NTS and NT on soil Ks were not amazing as the universal idea is that an increase in soil compaction is stereotypically associated with minimum conductivity. Soil Ks determined by effective macroporosity are the root tubes produced by aggregate faces and herbaceous vegetation. Soil Ks does not hang on the absolute macroporosity value but on the vertical interconnection that this macroporosity presents, which is cracked by soil tillage practice. Therefore, although tillage can increase macroporosity, most of this macroporosity is not active. However, NT can decrease macroporosity, but it shows a much higher vertical interconnection. This explains that CT grants inferior Ks compared to the NT system, which is particularly noteworthy in the surface soil layers, which are the most influenced by soil management (tillage type: CT or CTS). Our results are in line with Pikul et al. [74], who confirmed a rise in Ks under NTS and NT over CT, perhaps due to the stubble retention that modified the soil structure and, hence, aggregates’ stability [67].
Our findings revealed that NTS and CTS reduced ST due to residues accumulation. An earlier study stated that conservation tillage strategies reduced ST compared with the CT system [75]. Moreover, our results also revealed that the highest ST under CT over NT may be owing to differences in surface; under NT, the soil surface is moderately shielded by straw remnants from the previous crops, prompting the soil to absorb the least solar radiation [76]. In addition, under CT treatment, the tillage depth makes the soil porous, and, accordingly, the soil possibly has lesser thermal conductivity [77]. This inconsistency points to higher heat retention for CT. This is consistent with numerous previous studies globally that indicated maximum ST for CT over NT [8,50]. Nevertheless, our results are in contrast with [78], who declared that residues treatments had a maximum ST. Our results recommend that conservation tillage reduced ST. This was mainly because of augmented SWC availability with the conservation tillage [8]. Moreover, conservation tillage improved the infiltration rate, which led to facilitated water movement towards the lowermost soil depths and lessened ST [8]. Soil substance, soil structure and energy conservation are based on soil aggregates. Overall soil quality can be directly determined by soil aggregates quality as well as quantity. Under NTS, NT and CTS, higher soil aggregates may be accredited to the formation of SOM, which can improve the soil aggregates’ formation, and, particularly, higher soil aggregates under NTS and NT might be due to the no soil inversion by eliminating preceding soil tillage, which resulted in stopping breakdown of soil aggregates [24]. Higher soil aggregates under NTS and CTS treatments may be due to retention of straw [24]. However, the CT treatment powerfully disturbs the soil, which can decrease the degree of soil aggregate and soil aggregates’ stability [79]. Our findings are inconsistent with Yeboah et al. [80], who confirmed a rise in soil aggregates under NTS and NT over CT. Deprived soil aggregates structure as well as stability can decrease infiltration of water and decrease soil carbon and essential nutrients in macro- and microaggregates. Higher soil aggregation can be attained by decreasing soil inversion and increasing straw retention via conservation tillage [30]. Moreover, a non-significant difference was noted in the case of field capacity under NTS, NT and CTS treatments. This lack of sensitivity to NTS, CTS and NT is not surprising. Field capacity chiefly depends on clay content [80], which would not be significantly influenced by soil tillage management practices. These findings are in line with those of [24], who stated that conservation tillage did not affect soil water retention characteristics. Additionally, our results depicted a positive correlation amongst spring wheat productivity and yield-attributing attributes due to certain climatic and genetic factors [81].
4.2. Effect of Treatments on Soil Chemical Properties
Soil conservation tillage is a significant approach to increase SOM, soil fertility and lastly increase crop productivity [1,20,30,75]. Nevertheless, continuous plowing, excessive use of fertilizers and poor soil and water management techniques have led to a serious soil fertility decline, which impedes crop yields.
For the current research, the NTS, CTS and NT practices pointedly improved soil TN, TP, AN, AP, AK, SOC, LFOC and SOM. This agrees with results reported by Sadiq et al. [8] and Wulanningtyas et al. [14]. Higher soil TN and AN under NTS and CTS than CT might be due to nitrogen immobilization and microbial biomass in stubble [82]. These findings are in agreement with [20,65,83], who observed the maximum accumulation of nitrogen components under conservation agriculture over CT. Moreover, straw can reduce nitrogen volatilization and leaching losses by minimizing the ST and finally increasing soil TN and AN [50]. The maximum TN and AN under NTS, CTS and NT might be connected to greater biological activity. The nitrification process boosted the SOM transformation to soil nitrogen [82]. Overall, under NTS, CTS and NT, the higher TN and AN levels were because of higher soil compaction, which limited water movement and led to a decline in leaching losses. Moreover, the highest soil TP, AP and AK in NTS and CTS were attributed to residue return, which releases essential soil nutrients [71,83]. The solubility of soil phosphorus is recognized to be improved by enhancing SOM and declining pH [24] by acidifying the rhizosphere soil. After straw retention to the soil, a rise in availability of soil phosphorus might arise by reducing the phosphorus adsorption to mineral surfaces [24]. This agrees with results reported by Zhao et al. [30] and Sadiq et al. [8]. Additionally, carbon dioxide and organic acids produced by SOM might increase availability of fixed potassium. After straw retention to the soil, a rise in potassium availability might occur by reducing the potassium adsorption to clay mineral surfaces [24]; this concurs with the results noted by [83]. Our results suggest that conservation tillage increased SOC and LFOC. The maximum SOC and LFOC under NTS, CTS and NT were because of environmental conditions that augmented the decomposition of crop straw [84]. However, the lowest SOC and LFOC under CT were because of ploughing and were starved of residue, which led to soil structure deterioration and might have increased the carbon mineralization and decomposition rates, which encouraged carbon loss [15]. Moreover, NT treatment lessened the macro-aggregates disintegration, which held carbon and contributed to a rise in SOC and LFOC [65]. The CT operation exposes SOC and LFOC to air, which results in higher organic carbon oxidation; thus, depressing tillage operation favors organic carbon accumulation under no till [85]. The role of crop stubble retention and straw incorporation on improving SOC and LFOC was also reported by many researchers [14,85]. Our results depicted that NTS, CTS and NT practices increased SOM. A past study reported that conservation tillage increased SOM in the 0–10, 10–20 and 20–40 cm depths [24]. The maximum SOM values under conservation tillage were due to stubble retention and no till practice. Adoption of stubble retention and no till are the efforts to improve SOM because these strategies reduced soil erosion and decreased runoff of dissolved soil matter. Conventional tillage operation tends to increase the organic matter loss rate primarily by hastening microbial decomposition, altering soil structural stability and reducing the SOM amount [24]. These results are in agreement with the findings of [24]. Our results demonstrated that the valuable impact of conservation tillage practices on TN, TP and SOM were not reflected at 20–40 cm soil depth, which is consistent with the findings of [24,83]. Additionally, TK and ECe were not influenced by tillage at the different investigated soil depths; this is perhaps as a result of the short-term stubble application, and, possibly, straw needs more time for TK and ECe dynamics [1]; this concurs with the results noted by [1,8]. The soil pH was also not significantly affected by tillage; however, it tended to be lesser in NTS, CTS and NT over CT because of the acidifying as well as root exudation results of the mineralization of SOM [86]. The minimum soil pH is a temporary impact mainly due to the soil microbes’ respiratory process as well as decomposition of stubbles that yield the organic acids and lead to a reduced soil pH [86]. These findings are in line with those of [8], who stated that conservation tillage decreases the soil pH but with a non-significant difference.
Additionally, the CTS system was expected to increase the soil nutrients accumulation and SOC, LFOC and SOM over NTS; however, the findings rather depicted a reduction trend. The cause might be as a result of the slow straw decomposition rate, thereby affecting the nutrients’ accumulation in the soil system [30]. In addition, our results showed that NT implementation reduced the soil nutrient losses over CT. The CT practices in agriculture can generally lead to a reduction in soil nutrients accumulation due to the soil structure destruction and aggravated decomposition of SOM [24].
4.3. Effect of Treatments on Soil Biological Properties
Microbial populations and enzyme activities are vital for sustaining soil quality by mediating the SOM revenue processes and nutrient cycling [24]. Conservation tillage, e.g., NTS, CTS and NT, influences microbial populations and enzyme activities, as is evident from microbial populations and extracellular enzyme activities, such as urease, alkaline phosphatase, invertase, cellulase and catalase. The overall microbial populations and enzyme activities under NTS-, CTS- and NT-based conservation tillage scenarios were higher compared to CT practices at different investigated soil depths. This agrees with the results reported by Zhao et al. [30]. It is chiefly attributable to no soil disturbance and straw retention, which delivers an appropriate environment for microbes by moderating soil temperature and moisture over the CT system [26]. Stubble serves as a carbon source that facilitates microbial growth and enzyme activities and macroaggregates formation through soil and straw interactions [20,24]. This result is similar to that of Han et al. [20], wherein urease, alkaline phosphatase, invertase, cellulase and catalase activity increased with residue return. These enzymes catalyzed the residue carbon to active organic carbon conversion and augmented soil respiration.
It is well known that MBC and MBN are more sensitive to farming practices over SOM dynamics [30]. Dynamics in MBC and MBN are significant soil quality indicators because of the influence of soil management strategies on soil attributes [20,24,26]. Moreover, MBC and MBN are more sensitive indexes for soil fertility evaluation over any other essential nutrient. In the current study, NTS, CTS and NT significantly increased MBC and MBN at surface 0–10 cm and sub-surface 10–20 cm soil depth compared to the CT-based system; it can be described that straw presence in NTS and CTS created appropriate environments for microbial growth [87] as crop straw sustains temperature, substrate availability and moisture for good microbial growth [24], in agreement with Han et al. [20] and Zhao et al. [30]. Stubble acts as a readily available food source and also offers an extensive nutrients range to microorganisms, which led to higher biomasses (MBC and MBN) [87]. Moreover, our results exhibited that there is a significant positive correlation between soil chemical properties and biological properties with spring wheat productivity. Additionally, a significant positive correlation was noted amongst soil chemical properties with biological properties. This concurs with results reported by [1,8,30].
4.4. Effect of Treatments on Crop Agronomic Attributes
Crop stubble has the potential to improve the availability of essential plant nutrients and facilitate the crops’ production [1,8]. Conservation tillage strategies, especially the NT system, have many advantages, including increased soil physicochemical as well as biological quality indicators and improved crop productivity [14]. Irrespective of these benefits, continuous NT practice caused an accumulation of surface straw, which in turn led to build-up of SOM and accumulation of essential nutrients compared to the CT technique [15]. Additionally, nutrient cycling and their availability to plants are influenced by long-term soil management techniques [11].
Conservation tillage management practices influence spring wheat agronomic traits to different extents. Significant improvement was recorded for agronomic traits of the spring wheat crop studied under NTS, CTS and NT measures. The improvement in spring wheat yield traits was attributed to several factors. Firstly, significant variation amongst the different conservation tillage treatments, e.g., NTS, CTS and NT, might be due to their essential soil nutrients retaining the ability of suitable crop utilization, as found in other studies [20,65]. Secondly, residues contain a considerable amount of SOM, which improves soil health or quality attributes, leading to increased crop yield traits [24]. Thirdly, the optimistic influence of NTS, CTS and NT measures on spring wheat productivity as well as yield attributes was because of better soil physical and hydrological conditions [88]. The results of recent research are in agreement with the results of [8,14] as they recorded that residue return and straw incorporation and the NT technique improved crop production over CT under semi-arid climatic conditions. Conversely, our findings are in contrast with the results of [89], who observed that conservation tillage did not significantly increase the wheat agronomic attributes. Additionally, the current study findings are also in contrast with the results of [65], who took the view that the practice of no soil inversion did not markedly increase the wheat yield attributes. It has been known that the practice of no till farming could benefit from a long-term non-stop no till practice [89]. Moreover, it is also documented that NTS and CTS might result in noteworthy nitrogen immobilization, thus leading to reduced crop productivity [82]. The strong influence of NTS, CTS and NT was noticed in this study over CT.
5. Conclusions
Based on the 2 years of comprehensive scientific study data, we conclude that conservation tillage systems had a significant positive impact on soil’s physical, chemical and biological properties and spring wheat yield. The conservation tillage systems NT, NTS and CTS improved the soil bulk density, gravimetric water content, soil water storage, saturated hydraulic conductivity and reduced the soil porosity and soil temperature compared with conventional tillage. Moreover, these conservative tillage treatments not only increased the TN, TP, TK, AN, AP, AK, SOC, LFOC and SOM but also enhanced soil microbial counts, urease, alkaline phosphatase, invertase, cellulase and catalase activities compared with conventional tillage. Straw implementation either coupled with no tillage or conventional tillage systems markedly improved the spring wheat agronomic traits compared to conventional tillage practice. Consequently, stubble return with no till and straw incorporation with conventional tillage should be recommended and promoted between the smallholder farmer systems to increase soil quality and sustainability under spring wheat agroecosystems.
Author Contributions
M.S. designed the research; M.S. and J.Y. collected the field and laboratory data; J.Y. and M.S. analyzed data and wrote the article. The study field has been managed by G.L., with ploughing, fertilizing, sowing, weeding and harvesting. N.R., M.M.T., L.Y., M.Z., Y.L., A.S. and B.M. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was honored to be supported by the “Key R&D projects in Gansu Province” (22YF7FA116); “Excellent doctoral program in Gansu Province” (22JR5RA843); the “Innovation star” project of excellent graduate students in Gansu Province (2022CXZX-641); the Industrial support plan project, China (2021CYZC-15); the Key Talent Projects in Gansu Province, China (LRYCZ-2020-1) and Industrial support projects for colleges and universities in Gansu Province, China (2022CYZC-41).
Data Availability Statement
Not applicable.
Acknowledgments
We admiringly acknowledge the plan of Gansu Province key research and development (18 YF1NA070), Collective innovation team project (2018C-16) of University in Gansu Province, China.
Conflicts of Interest
The authors declare no conflict of interest. Regarding this manuscript, titled changes in soil properties and crop yield under sustainable conservation tillage systems in spring wheat agroecosystems, Jianyu Yuan and Mahran Sadiq et al. have no known competing financial interests or personal associations that could have appeared to affect the work included in this manuscript.
References
- Roy, D.; Datta, A.; Jat, H.; Choudhary, M.; Sharma, P.; Singh, P.; Jat, M. Impact of long term conservation agriculture on soil quality under cereal based systems of North West India. Geoderma 2022, 405, 115391. [Google Scholar] [CrossRef]
- Ma, L.; Kong, F.; Lv, X.; Wang, Z.; Zhou, Z.; Meng, Y. Responses of greenhouse gas emissions to different straw management methods with the same amount of carbon input in cotton field. Soil Tillage Res. 2021, 213, 105126. [Google Scholar] [CrossRef]
- Liu, X.; Peng, C.; Zhang, W.; Li, S.; An, T.; Xu, Y.; Ge, Z.; Xie, N.; Wang, J. Subsoiling tillage with straw incorporation improves soil microbial community characteristics in the whole cultivated layers: A one-year study. Soil Tillage Res. 2022, 215, 105188. [Google Scholar] [CrossRef]
- Sharma, S.; Thind, H.; Sidhu, H.; Jat, M.; Parihar, C. Effects of crop residue retention on soil carbon pools after 6 years of rice–wheat cropping system. Environ. Earth Sci. 2019, 78, 296. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, Z.; Feng, G.; Huang, M.; Shi, X. Experimental study on the potential use of bundled crop straws as subsurface drainage material in the newly reclaimed coastal land in Eastern China. Water 2018, 10, 31. [Google Scholar] [CrossRef]
- Canisares, L.P.; Grove, J.; Miguez, F.; Poffenbarger, H. Long-term no-till increases soil nitrogen mineralization but does not affect optimal corn nitrogen fertilization practices relative to inversion tillage. Soil Tillage Res. 2021, 213, 105080. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of conservation agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Sadiq, M.; Li, G.; Rahim, N.; Tahir, M.M. Sustainable Conservation Tillage Technique for Improving Soil Health by Enhancing Soil Physicochemical Quality Indicators under Wheat Mono-Cropping System Conditions. Sustainability 2021, 13, 8177. [Google Scholar] [CrossRef]
- Vereecken, H.; Schnepf, A.; Hopmans, J.W.; Javaux, M.; Or, D.; Roose, T.; Vanderborght, J.; Young, M.; Amelung, W.; Aitkenhead, M. Modeling soil processes: Review, key challenges, and new perspectives. Vadose Zone J. 2016, 15, vzj2015.09.0131. [Google Scholar] [CrossRef]
- Hernanz, J.; Peixoto, H.; Cerisola, C.; Sánchez-Girón, V. An empirical model to predict soil bulk density profiles in field conditions using penetration resistance, moisture content and soil depth. J. Terramechanics 2000, 37, 167–184. [Google Scholar] [CrossRef]
- Vizioli, B.; Cavalieri-Polizeli, K.M.V.; Tormena, C.A.; Barth, G. Effects of long-term tillage systems on soil physical quality and crop yield in a Brazilian Ferralsol. Soil Tillage Res. 2021, 209, 104935. [Google Scholar] [CrossRef]
- Singh, D.; Mishra, A.K.; Patra, S.; Mariappan, S.; Singh, N. Near-saturated soil hydraulic conductivity and pore characteristics as influenced by conventional and conservation tillage practices in North-West Himalayan region, India. Int. Soil Water Conserv. Res. 2021, 9, 249–259. [Google Scholar] [CrossRef]
- Sithole, N.J.; Magwaza, L.S.; Mafongoya, P.L. Conservation agriculture and its impact on soil quality and maize yield: A South African perspective. Soil Tillage Res. 2016, 162, 55–67. [Google Scholar] [CrossRef]
- Wulanningtyas, H.S.; Gong, Y.; Li, P.; Sakagami, N.; Nishiwaki, J.; Komatsuzaki, M. A cover crop and no-tillage system for enhancing soil health by increasing soil organic matter in soybean cultivation. Soil Tillage Res. 2021, 205, 104749. [Google Scholar] [CrossRef]
- Jat, H.; Datta, A.; Sharma, P.; Kumar, V.; Yadav, A.; Choudhary, M.; Choudhary, V.; Gathala, M.; Sharma, D.; Jat, M. Assessing soil properties and nutrient availability under conservation agriculture practices in a reclaimed sodic soil in cereal-based systems of North-West India. Arch. Agron. Soil Sci. 2018, 64, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Nandan, R.; Singh, V.; Singh, S.S.; Kumar, V.; Hazra, K.K.; Nath, C.P.; Poonia, S.; Malik, R.K.; Bhattacharyya, R.; McDonald, A. Impact of conservation tillage in rice–based cropping systems on soil aggregation, carbon pools and nutrients. Geoderma 2019, 340, 104–114. [Google Scholar] [CrossRef]
- Piazza, G.; Pellegrino, E.; Moscatelli, M.C.; Ercoli, L. Long-term conservation tillage and nitrogen fertilization effects on soil aggregate distribution, nutrient stocks and enzymatic activities in bulk soil and occluded microaggregates. Soil Tillage Res. 2020, 196, 104482. [Google Scholar] [CrossRef]
- Choudhary, M.; Datta, A.; Jat, H.S.; Yadav, A.K.; Gathala, M.K.; Sapkota, T.B.; Das, A.K.; Sharma, P.C.; Jat, M.L.; Singh, R. Changes in soil biology under conservation agriculture based sustainable intensification of cereal systems in Indo-Gangetic Plains. Geoderma 2018, 313, 193–204. [Google Scholar] [CrossRef]
- Njaimwe, A.N.; Mnkeni, P.N.; Muchaonyerwa, P.; Chiduza, C.; Wakindiki, I.I. Sensitivity of selected chemical and biological soil quality parameters to tillage and rotational cover cropping at the Zanyokwe Irrigation Scheme, South Africa. S. Afr. J. Plant Soil 2018, 35, 321–328. [Google Scholar] [CrossRef]
- Han, Y.; Ma, W.; Zhou, B.; Yang, X.; Salah, A.; Li, C.; Cao, C.; Zhan, M.; Zhao, M. Effects of Straw-Return Method for the Maize–Rice Rotation System on Soil Properties and Crop Yields. Agronomy 2020, 10, 461. [Google Scholar] [CrossRef]
- Kan, Z.-R.; Ma, S.-T.; Liu, Q.-Y.; Liu, B.-Y.; Virk, A.L.; Qi, J.-Y.; Zhao, X.; Lal, R.; Zhang, H.-L. Carbon sequestration and mineralization in soil aggregates under long-term conservation tillage in the North China Plain. Catena 2020, 188, 104428. [Google Scholar] [CrossRef]
- Modak, K.; Ghosh, A.; Bhattacharyya, R.; Biswas, D.R.; Das, T.K.; Das, S.; Singh, G. Response of oxidative stability of aggregate-associated soil organic carbon and deep soil carbon sequestration to zero-tillage in subtropical India. Soil Tillage Res. 2019, 195, 104370. [Google Scholar] [CrossRef]
- Martín-Lammerding, D.; Gabriel, J.L.; Zambrana, E.; Santín-Montanyá, I.; Tenorio, J.L. Organic Amendment vs. Mineral Fertilization under Minimum Tillage: Changes in Soil Nutrients, Soil Organic Matter, Biological Properties and Yield after 10 Years. Agriculture 2021, 11, 700. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, R.; Luo, Z.; Li, L.; Cai, L.; Li, G.; Xie, J. Contributions of long-term tillage systems on crop production and soil properties in the semi-arid Loess Plateau of China. J. Sci. Food Agric. 2016, 96, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, M.C.; Secondi, L.; Marabottini, R.; Papp, R.; Stazi, S.; Mania, E.; Marinari, S. Assessment of soil microbial functional diversity: Land use and soil properties affect CLPP-MicroResp and enzymes responses. Pedobiologia 2018, 66, 36–42. [Google Scholar] [CrossRef]
- Jat, H.S.; Datta, A.; Choudhary, M.; Sharma, P.C.; Dixit, B.; Jat, M.L. Soil enzymes activity: Effect of climate smart agriculture on rhizosphere and bulk soil under cereal based systems of north-west India. Eur. J. Soil Biol. 2021, 103, 103292. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Barut, Z.; Ortas, I.; Gok, M.; Demirbas, A.; Tulun, Y.; Akpinar, C. Impacts of different tillage practices on some soil microbiological properties and crop yield under semi-arid Mediterranean conditions. Int. J. Plant Prod. 2011, 5, 237–254. [Google Scholar]
- Srinivasa Rao, C.; Grover, M.; Kundu, S.; Desai, S. Soil Enzymes. In Encyclopedia of Soil Science, 3rd ed.; Lal, R., Ed.; Taylor & Francis: Singapore, 2017; pp. 2100–2107. [Google Scholar] [CrossRef]
- Jat, H.S.; Choudhary, M.; Datta, A.; Yadav, A.K.; Meena, M.D.; Devi, R.; Gathala, M.K.; Jat, M.L.; McDonald, A.; Sharma, P.C. Temporal changes in soil microbial properties and nutrient dynamics under climate smart agriculture practices. Soil Tillage Res. 2020, 199, 104595. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, G.; Wang, H.; Lu, D.; Chen, X.; Zhou, J. Effects of full straw incorporation on soil fertility and crop yield in rice-wheat rotation for silty clay loamy cropland. Agronomy 2019, 9, 133. [Google Scholar] [CrossRef]
- Govaerts, B.; Mezzalama, M.; Sayre, K.D.; Crossa, J.; Nicol, J.M.; Deckers, J. Long-term consequences of tillage, residue management, and crop rotation on maize/wheat root rot and nematode populations in subtropical highlands. Appl. Soil Ecol. 2006, 32, 305–315. [Google Scholar] [CrossRef]
- Choudhary, M.; Jat, H.S.; Datta, A.; Yadav, A.K.; Sapkota, T.B.; Mondal, S.; Meena, R.; Sharma, P.C.; Jat, M.L. Sustainable intensification influences soil quality, biota, and productivity in cereal-based agroecosystems. Appl. Soil Ecol. 2018, 126, 189–198. [Google Scholar] [CrossRef]
- Bergstrom, D.; Monreal, C.; Tomlin, A.; Miller, J. Interpretation of soil enzyme activities in a comparison of tillage practices along a topographic and textural gradient. Can. J. Soil Sci. 2000, 80, 71–79. [Google Scholar] [CrossRef]
- Alhassan, A.-R.M.; Yang, C.; Ma, W.; Li, G. Influence of conservation tillage on Greenhouse gas fluxes and crop productivity in spring-wheat agroecosystems on the Loess Plateau of China. PeerJ 2021, 9, e11064. [Google Scholar] [CrossRef]
- Rahman, M.M.; Aravindakshan, S.; Hoque, M.A.; Rahman, M.A.; Gulandaz, M.A.; Rahman, J.; Islam, M.T. Conservation tillage (CT) for climate-smart sustainable intensification: Assessing the impact of CT on soil organic carbon accumulation, greenhouse gas emission and water footprint of wheat cultivation in Bangladesh. Environ. Sustain. Indic. 2021, 10, 100106. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Ferreira, J.F.; Wang, M.; Na, J.; Huang, J.; Liang, Z. Long-term combined effects of tillage and rice cultivation with phosphogypsum or farmyard manure on the concentration of salts, minerals, and heavy metals of saline-sodic paddy fields in Northeast China. Soil Tillage Res. 2022, 215, 105222. [Google Scholar] [CrossRef]
- Fang, Y.; Van Zwieten, L.; Rose, M.T.; Vasileiadis, S.; Donner, E.; Vancov, T.; Rigg, J.L.; Weng, Z.H.; Lombi, E.; Drigo, B. Unraveling microbiomes and functions associated with strategic tillage, stubble, and fertilizer management. Agric. Ecosyst. Environ. 2022, 323, 107686. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; Dong, Y.; Lapen, D.R.; Liu, J.; Chen, W. Subsoiling and conversion to conservation tillage enriched nitrogen cycling bacterial communities in sandy soils under long-term maize monoculture. Soil Tillage Res. 2022, 215, 105197. [Google Scholar] [CrossRef]
- Mašková, L.; Simmons, R.W.; Deeks, L.K.; De Baets, S. Best Management Practices to Alleviate Deep-Seated Compaction in Asparagus (Asparagus officinalis) Interrows (UK). Soil Tillage Res. 2021, 213, 105124. [Google Scholar] [CrossRef]
- Xingchen, D.; Zhang, J.; Huizhen, Q.; Zhang, H.; Chaoyue, L.; Delei, D.; Qirong, S.; Zhongjun, J. Chronic nitrogen fertilization modulates competitive interactions among microbial ammonia oxidizers in a loess soil. Pedosphere 2019, 29, 24–33. [Google Scholar]
- Chinese Soil Taxonomy Cooperative Research Group. Chinese Soil Taxonomy (Revised Proposal); Institute of Soil Science, Chinese Academy of Sciences: Beijing, China, 1995. [Google Scholar]
- Xu, A.; Li, L.; Coulter, J.A.; Xie, J.; Gopalakrishnan, S.; Zhang, R.; Luo, Z.; Cai, L.; Liu, C.; Wang, L. Long-Term Nitrogen Fertilization Impacts on Soil Bacteria, Grain Yield and Nitrogen Use Efficiency of Wheat in Semiarid Loess Plateau, China. Agronomy 2020, 10, 1175. [Google Scholar] [CrossRef]
- Lingling, L.; Renzhi, Z.; Zhuzhu, L.; Weili, L.; Junhong, X.; Liqun, C.; Bellotti, B. Evolution of soil and water conservation in rain-fed areas of China. Int. Soil Water Conserv. Res. 2014, 2, 78–90. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Zhang, X.-Z.; Yi, W.; Xu, X.-F.; Han, Z.-H. Key minerals influencing apple quality in Chinese orchard identified by nutritional diagnosis of leaf and soil analysis. J. Integr. Agric. 2015, 14, 864–874. [Google Scholar] [CrossRef]
- Campbell, D. Determination and use of soil bulk density in relation to soil compaction. In Developments in Agricultural Engineering; Elsevier: Amsterdam, The Netherlands, 1994; Volume 11, pp. 113–139. [Google Scholar]
- O’Kelly, B.C. Accurate determination of moisture content of organic soils using the oven drying method. Dry. Technol. 2004, 22, 1767–1776. [Google Scholar] [CrossRef]
- Czyz, E.; Dexter, A. Soil physical properties under winter wheat grown with different tillage systems at selected locations. Int. Agrophys. 2008, 22, 191–200. [Google Scholar]
- Reynolds, W.; Carter, M.; Gregorich, E. Saturated hydraulic properties: Well permeameter. In Soil Sampling Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1025–1042. [Google Scholar]
- Lu, R. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Rani, A.; Bandyopadhyay, K.; Krishnan, P.; Sarangi, A.; Datta, S. Effect of tillage, residue and nitrogen management on soil mineral nitrogen dynamics and nitrogen use efficiency of wheat crop in an inceptisol. J. Agric. Phys. 2017, 17, 16–30. [Google Scholar]
- Yang, X.-M.; Wander, M.M. Temporal changes in dry aggregate size and stability: Tillage and crop effects on a silty loam Mollisol in Illinois. Soil Tillage Res. 1998, 49, 173–183. [Google Scholar] [CrossRef]
- Shi, X.; Yu, D.; Warner, E.; Pan, X.; Petersen, G.; Gong, Z.; Weindorf, D. Soil database of 1: 1,000,000 digital soil survey and reference system of the Chinese genetic soil classification system. Soil Surv. Horiz. 2004, 45, 129–136. [Google Scholar] [CrossRef]
- Nelson, D.A.; Sommers, L. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods, 5.3; Soil Science Society of America: Madison, WI, USA, 1983; Volume 9, pp. 539–579. [Google Scholar]
- Gregorich, E.; Ellert, B. Light Fraction and Macroorganic. Soil Sampl. Methods Anal. 1993, 397, 397–407. [Google Scholar]
- Davies, B.E. Loss-on-ignition as an estimate of soil organic matter. Soil Sci. Soc. Am. J. 1974, 38, 150–151. [Google Scholar] [CrossRef]
- Li, F.D.; Yu, Z.N.; He, S.J. Experimental Technique of Agricultural Microbiology; China Agriculture Press: Beijing, China, 1996. [Google Scholar]
- Dick, R.P.; Burns, R.G. A brief history of soil enzymology research. Methods Soil Enzymol. 2011, 9, 1–34. [Google Scholar]
- Zhao, L.; Jiang, Y. Discussion on measurements of soil phosphates. Chin. J. Soil Sci. 1986, 17, 138–141. [Google Scholar]
- Frankeberger, W.; Johanson, J. Method of measuring invertase activity in soils. Plant Soil 1983, 74, 301–311. [Google Scholar] [CrossRef]
- Guan, S. Soil Enzymes and Their Research Methods; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Yan, C. Soil Fertility and Its Research Method; Science Press: Beijing, China, 1988. [Google Scholar]
- Sparling, G.; West, A. Modifications to the flmigation-extraction technique to permit simultaneous extraction and estimation of soil microbial c and n. Commun. Soil Sci. Plant Anal. 1988, 19, 327–344. [Google Scholar] [CrossRef]
- Demuner-Molina, G.; Cadena-Zapata, M.; Campos-Magaña, S.G.; Zermeño-González, A.; Sánchez-Pérez, F.d.J. Efecto de labranza y mejoradores de suelo en humedad y desarrollo radicular. Tecnol. Cienc. Agua 2014, 5, 123–130. [Google Scholar]
- Gathala, M.K.; Ladha, J.; Saharawat, Y.S.; Kumar, V.; Kumar, V.; Sharma, P.K. Effect of tillage and crop establishment methods on physical properties of a medium-textured soil under a seven-year rice−wheat rotation. Soil Sci. Soc. Am. J. 2011, 75, 1851–1862. [Google Scholar] [CrossRef]
- Khorami, S.S.; Kazemeini, S.A.; Afzalinia, S.; Gathala, M.K. Changes in soil properties and productivity under different tillage practices and wheat genotypes: A short-term study in Iran. Sustainability 2018, 10, 3273. [Google Scholar] [CrossRef]
- Higashi, T.; Yunghui, M.; Komatsuzaki, M.; Miura, S.; Hirata, T.; Araki, H.; Kaneko, N.; Ohta, H. Tillage and cover crop species affect soil organic carbon in Andosol, Kanto, Japan. Soil Tillage Res. 2014, 138, 64–72. [Google Scholar] [CrossRef]
- Ordoñez-Morales, K.D.; Cadena-Zapata, M.; Zermeño-González, A.; Campos-Magaña, S. Effect of tillage systems on physical properties of a clay loam soil under oats. Agriculture 2019, 9, 62. [Google Scholar] [CrossRef]
- Martínez, E.; Fuentes, J.-P.; Silva, P.; Valle, S.; Acevedo, E. Soil physical properties and wheat root growth as affected by no-tillage and conventional tillage systems in a Mediterranean environment of Chile. Soil Tillage Res. 2008, 99, 232–244. [Google Scholar] [CrossRef]
- Sasal, M.C.; Andriulo, A.E.; Taboada, M.A. Soil porosity characteristics and water movement under zero tillage in silty soils in Argentinian Pampas. Soil Tillage Res. 2006, 87, 9–18. [Google Scholar] [CrossRef]
- Khan, S.; Shah, A.; Nawaz, M.; Khan, M. Impact of different tillage practices on soil physical properties, nitrate leaching and yield attributes of maize (Zea mays L.). J. Soil Sci. Plant Nutr. 2017, 17, 240–252. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Shen, Q. Influence of straw amendment on soil physicochemical properties and crop yield on a consecutive mollisol slope in Northeastern China. Water 2018, 10, 559. [Google Scholar] [CrossRef]
- Jabro, J.; Stevens, W.; Iversen, W.; Evans, R. Bulk density, water content, and hydraulic properties of a sandy loam soil following conventional or strip tillage. Soil Tillage Res. 2011, 27, 765–768. [Google Scholar] [CrossRef]
- Singh, Y.; Bhardwaj, A.; Singh, S.; Singh, R.; Chaudhary, D.; Saxena, A.; Singh, V.; Singh, S.P.; Kumar, A. Effect of rice (Oryza sativa)-establishment methods, tillage practices in wheat (Triticum aestivum) and fertilization on soil physical properties and rice-wheat system productivity on a silty day Mollisol of Uttaranchal. Indian J. Agric. Sci. 2002, 72, 200–205. [Google Scholar]
- Pikul, J.L.; Aase, J.K. Water infiltration and storage affected by subsoiling and subsequent tillage. Soil Sci. Soc. Am. J. 2003, 67, 859–866. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Shi, H.; Hu, Q.; Zhang, Y.; Leng, X. Effect of biodegradable film mulching on crop yield, soil microbial and enzymatic activities, and optimal levels of irrigation and nitrogen fertilizer for the Zea mays crops in arid region. Sci. Total Environ. 2021, 776, 145970. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Sun, H.; Zhang, X. Effects of different cultivation practices on soil temperature and wheat spike differentiation. Cereal Res. Commun. 2009, 37, 575–584. [Google Scholar] [CrossRef]
- Sarkar, S.; Singh, S. Interactive effect of tillage depth and mulch on soil temperature, productivity and water use pattern of rainfed barley (Hordium vulgare L.). Soil Tillage Res. 2007, 92, 79–86. [Google Scholar] [CrossRef]
- Yang, H.; Feng, J.; Zhai, S.; Dai, Y.; Xu, M.; Wu, J.; Shen, M.; Bian, X.; Koide, R.T.; Liu, J. Long-term ditch-buried straw return alters soil water potential, temperature, and microbial communities in a rice-wheat rotation system. Soil Tillage Res. 2016, 163, 21–31. [Google Scholar] [CrossRef]
- Yeboah, S.; Lamptey, S.; Zhang, R. Effects of Different Tillage and Straw Management Systems on Soil Aggregation and Crop Yield in Rainfed Loess Plateau. Adv. Agric. Sci. 2018, 6. [Google Scholar]
- Xu, G. Path analysis of main factors affecting field moisture capacity. J. Agric. Sci. YanBian Univ. 1997, 4, 432–435. [Google Scholar]
- Debiase, G.; Montemurro, F.; Fiore, A.; Rotolo, C.; Farrag, K.; Miccolis, A.; Brunetti, G. Organic amendment and minimum tillage in winter wheat grown in Mediterranean conditions: Effects on yield performance, soil fertility and environmental impact. Eur. J. Agron. 2016, 75, 149–157. [Google Scholar] [CrossRef]
- Morris, N.; Miller, P.; Orson, J.; Froud-Williams, R. The adoption of non-inversion tillage systems in the United Kingdom and the agronomic impact on soil, crops and the environment—A review. Soil Tillage Res. 2010, 108, 1–15. [Google Scholar] [CrossRef]
- Huang, G.; Luo, Z.; Li, L.; Zhang, R.; Li, G.; Cai, L.; Xie, J. Effects of stubble management on soil fertility and crop yield of rainfed area in Western Loess Plateau, China. Appl. Environ. Soil Sci. 2012, 2012, 256312. [Google Scholar] [CrossRef]
- Wang, W.; Sardans, J.; Wang, C.; Pan, T.; Zeng, C.; Lai, D.Y.; Bartrons, M.; Peñuelas, J. Straw application strategy to optimize nutrient release in a southeastern China rice cropland. Agronomy 2017, 7, 84. [Google Scholar] [CrossRef]
- Yeboah, S.; Zhang, R.; Cai, L.; Li, L.; Xie, J.; Luo, Z.; Liu, J.; Wu, J. Tillage effect on soil organic carbon, microbial biomass carbon and crop yield in spring wheat-field pea rotation. Plant Soil Environ. 2016, 62, 279–285. [Google Scholar] [CrossRef]
- Neugschwandtner, R.; Liebhard, P.; Kaul, H.; Wagentristl, H. Soil chemical properties as affected by tillage and crop rotation in a long-term field experiment. Plant Soil Environ. 2014, 60, 57–62. [Google Scholar] [CrossRef]
- Jat, H.; Datta, A.; Choudhary, M.; Sharma, P.C.; Yadav, A.; Choudhary, V.; Gathala, M.; Jat, M.L.; McDonald, A. Climate Smart Agriculture practices improve soil organic carbon pools, biological properties and crop productivity in cereal-based systems of North-West India. Catena 2019, 181, 104059. [Google Scholar] [CrossRef]
- Mazzoncini, M.; Sapkota, T.B.; Barberi, P.; Antichi, D.; Risaliti, R. Long-term effect of tillage, nitrogen fertilization and cover crops on soil organic carbon and total nitrogen content. Soil Tillage Res. 2011, 114, 165–174. [Google Scholar] [CrossRef]
- Gwenzi, W.; Gotosa, J.; Chakanetsa, S.; Mutema, Z. Effects of tillage systems on soil organic carbon dynamics, structural stability and crop yields in irrigated wheat (Triticum aestivum L.)–cotton (Gossypium hirsutum L.) rotation in semi-arid Zimbabwe. Nutr. Cycl. Agroecosyst. 2009, 83, 211–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).