Field-Scale Floating Treatment Wetlands: Quantifying Ecosystem Service Provision from Monoculture vs. Polyculture Macrophyte Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Floating Treatment Wetlands (FTWs) and Experimental Strategy
2.2. Data Analysis
3. Results
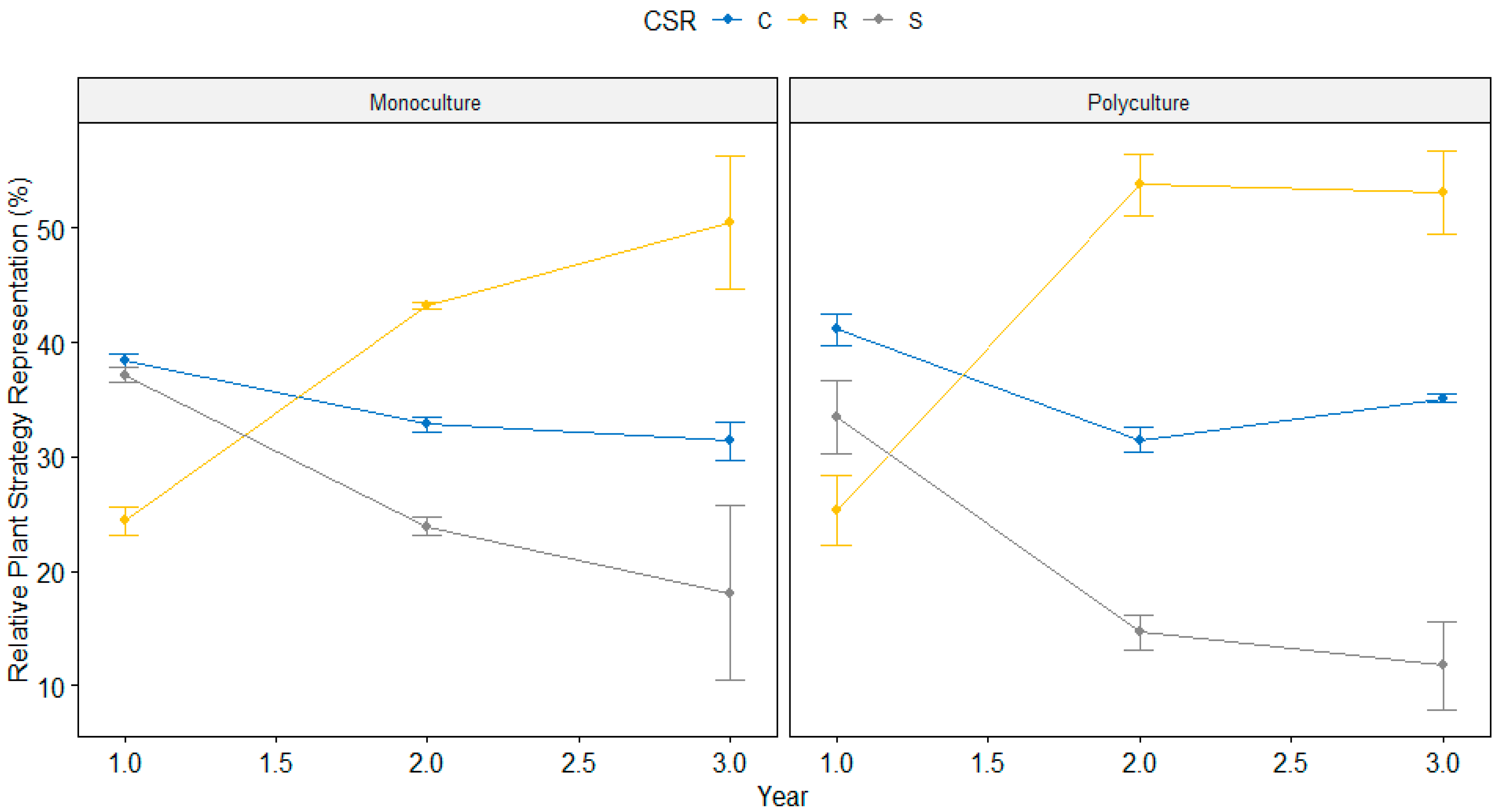
3.1. Macrophyte Community Composition Changes
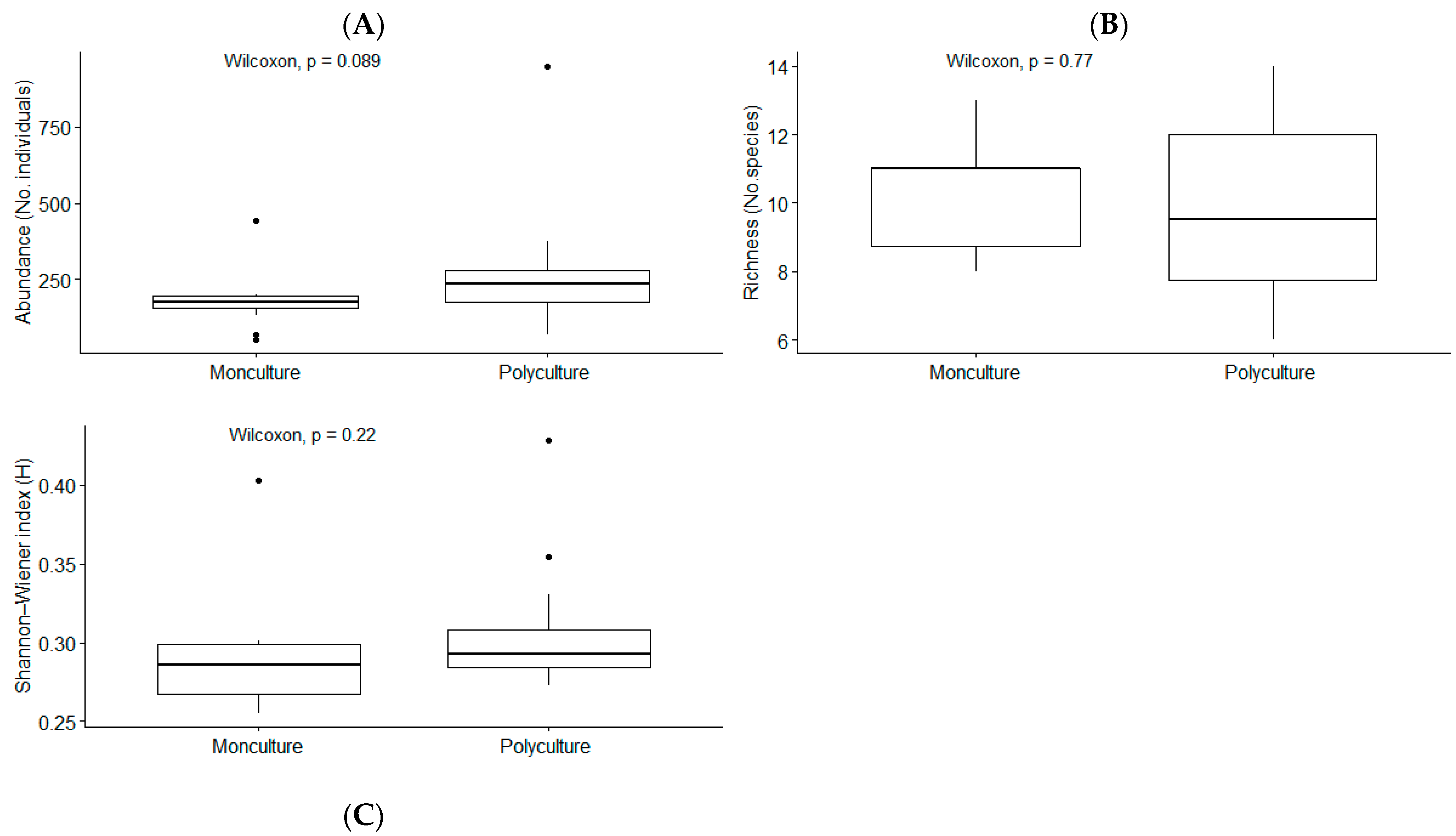
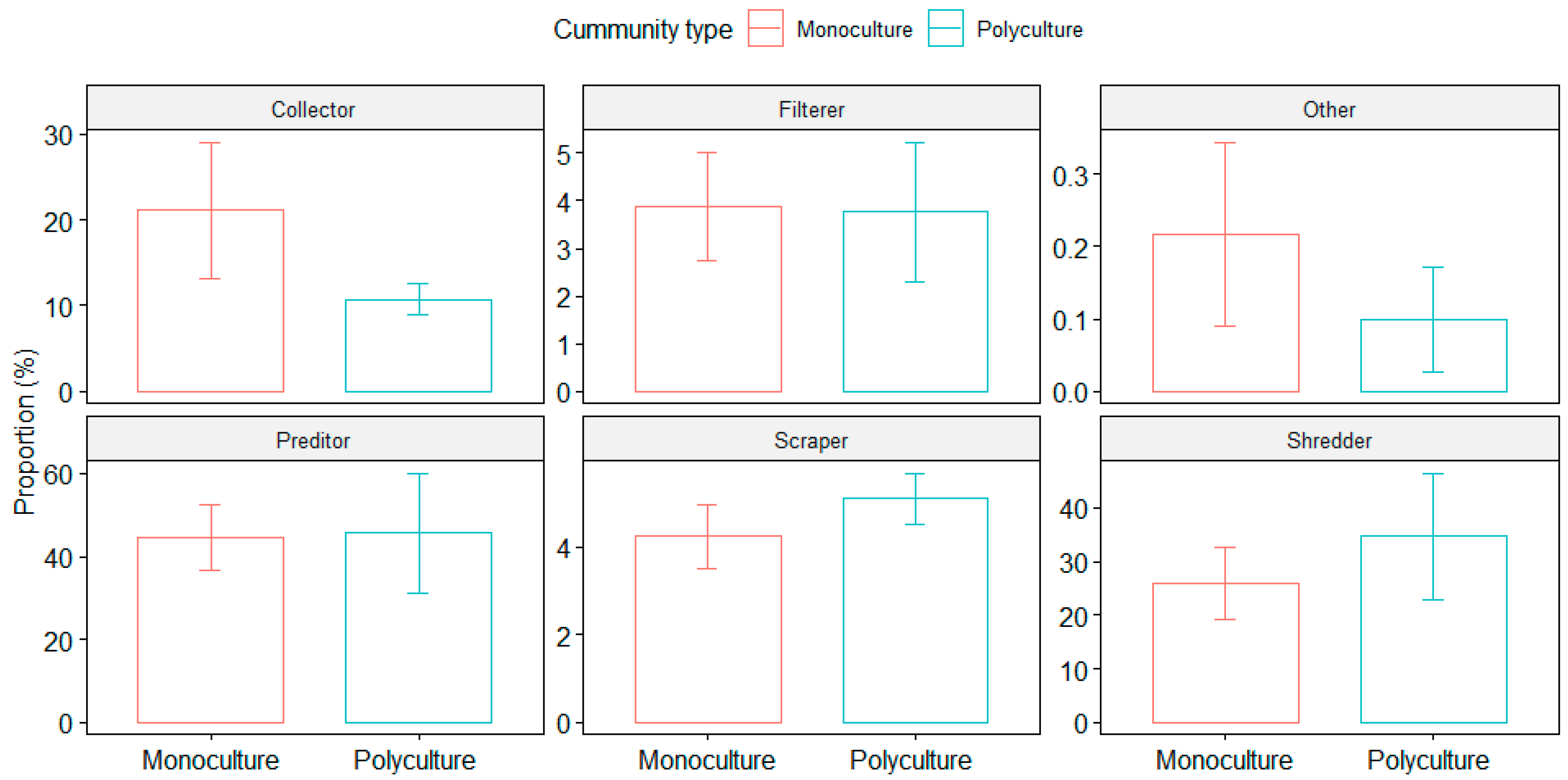
3.2. Macroinvertebrate Community Composition
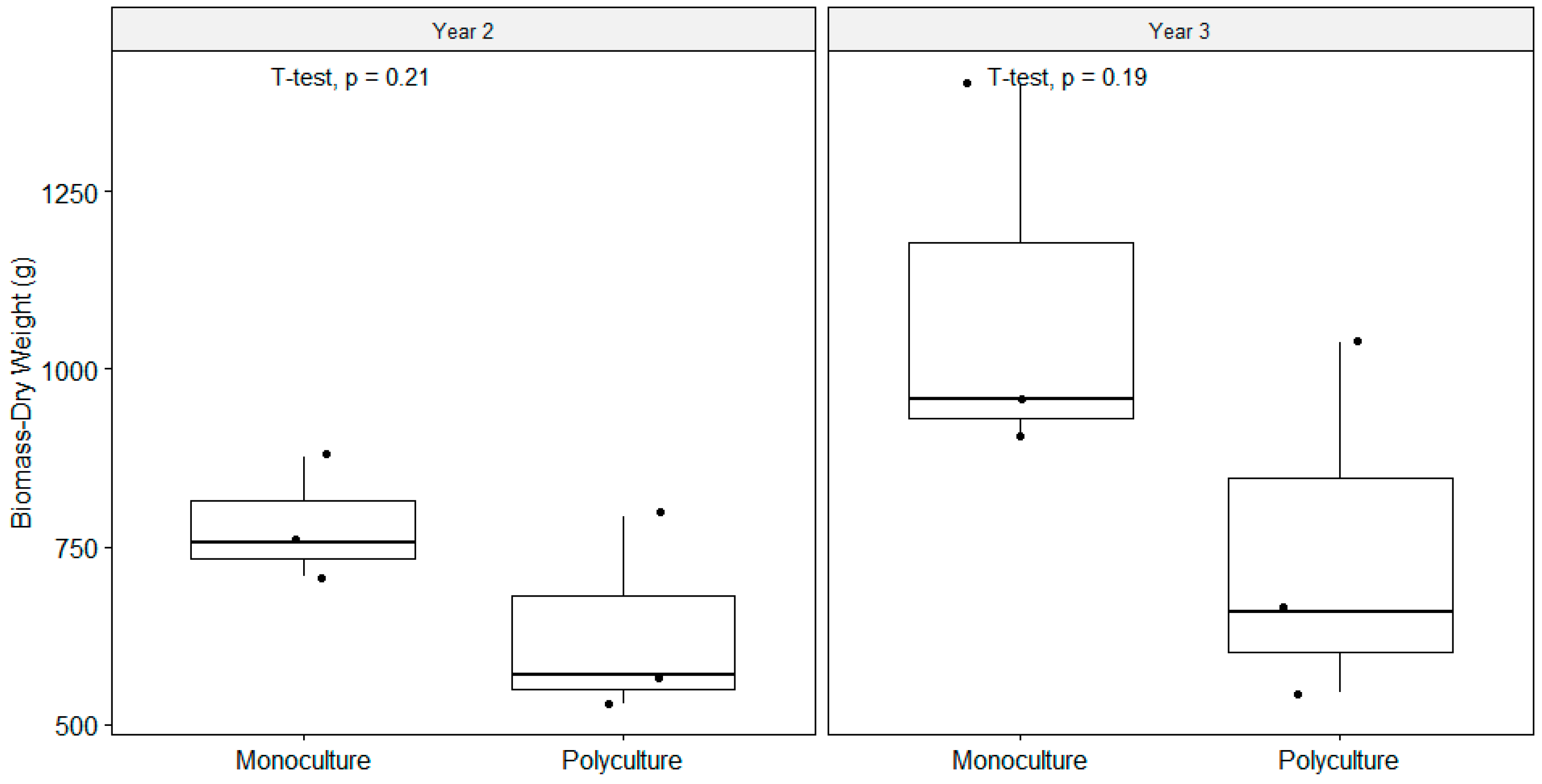
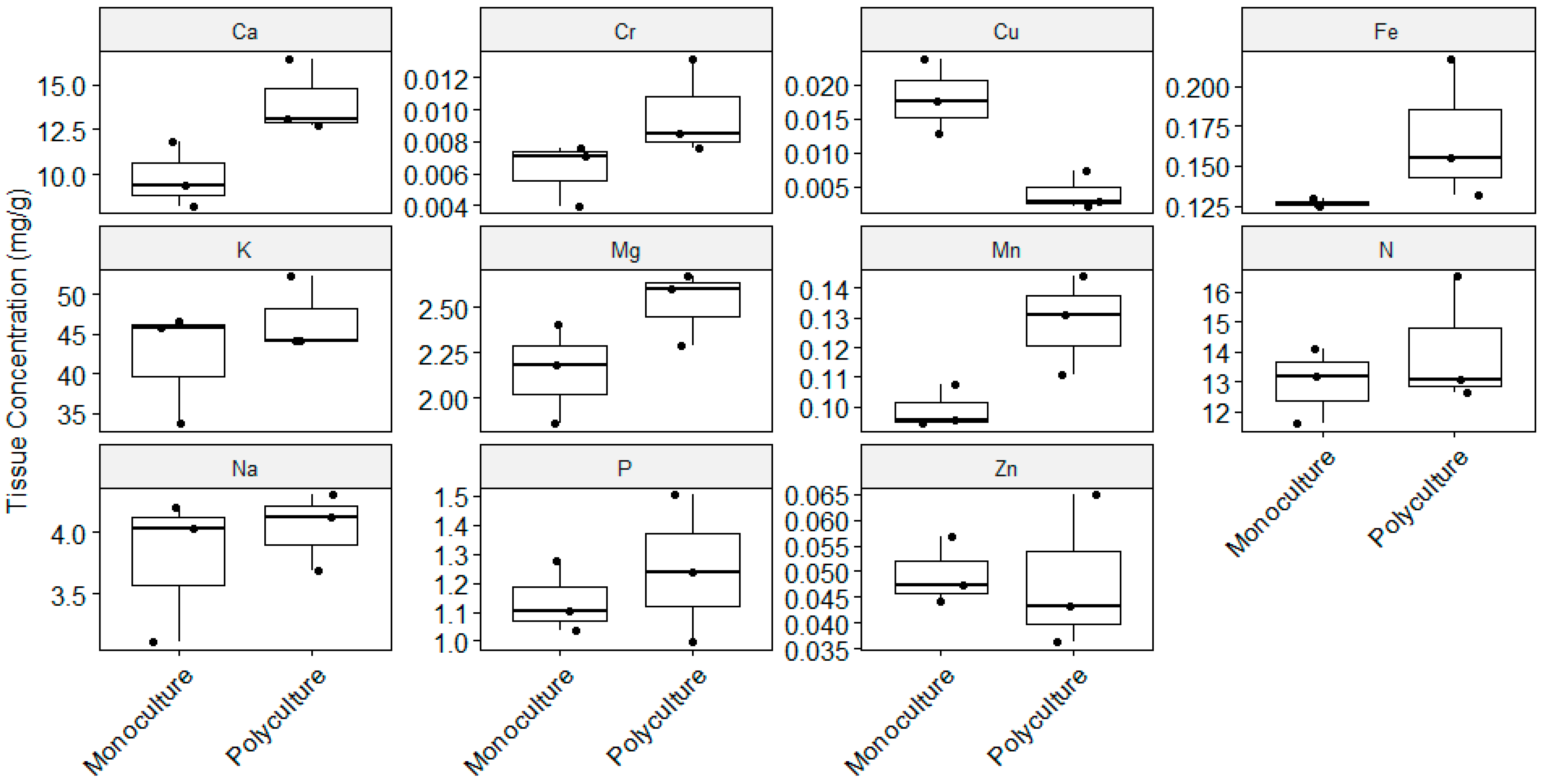
3.3. Plant Biomass and Nutrient Tissue Concentration
4. Discussion
4.1. Plant Community Succession
4.2. Macroinvertebrate Communities and Habitat Provision by FTWs
4.3. Resource Recovery
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berger, E.; Haase, P.; Kuemmerlen, M.; Leps, M.; Schaefer, R.B.; Sundermann, A. Water quality variables and pollution sources shaping stream macroinvertebrate communities. Sci. Total. Environ. 2017, 587, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Bunn, S.E. Grand challenge for the future of freshwater ecosystems. Front. Environ. Sci. 2016, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Grizzetti, B.; Lanzanova, D.; Liquete, C.; Reynaud, A.; Cardoso, A.C. Assessing water ecosystem services for water resource management. Environ. Sci. Policy 2016, 61, 194–203. [Google Scholar] [CrossRef]
- Keeler, B.L.; Polasky, S.; Brauman, K.A.; Johnson, K.A.; Finlay, J.C.; O’Neill, A.; Kovacs, K.; Dalzell, B. Linking water quality and well-being for improved assessment and valuation of ecosystem services. Proc. Natl. Acad. Sci. USA 2012, 109, 18619–18624. [Google Scholar] [CrossRef]
- Nesshöver, C.; Assmuth, T.; Irvine, K.N.; Rusch, G.M.; Waylen, K.A.; Delbaere, B.; Haase, D.; Jones-Walters, L.; Keune, H.; Kovacs, E.; et al. The science, policy and practice of nature-based solutions: An interdisciplinary perspective. Sci. Total. Environ. 2017, 579, 1215–1227. [Google Scholar] [CrossRef]
- van Rees, C.B.; Jumani, S.; Abera, L.; Rack, L.; McKay, S.K.; Wenger, S.J. The potential for nature-based solutions to combat the freshwater biodiversity crisis. PLoS Water 2023, 2, e0000126. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating Wetlands: A Sustainable Tool for Wastewater Treatment. Clean-Soil Air Water 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Fletcher, J.; Willby, N.J.; Oliver, D.M.; Quilliam, R.S. Phytoremediation using Aquatic Plants. In Phytoremediation—In-Situ Applications; Shmaefsky, B.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 205–260. [Google Scholar]
- Chen, Z.; Cuervo, D.P.; Müller, J.A.; Wiessner, A.; Köser, H.; Vymazal, J.; Kästner, M.; Kuschk, P. Hydroponic root mats for wastewater treatment—A review. Environ. Sci. Pollut. Res. 2016, 23, 15911–15928. [Google Scholar] [CrossRef]
- Colares, G.S.; Dell’Osbel, N.; Wiesel, P.G.; Oliveira, G.A.; Lemos, P.H.Z.; da Silva, F.P.; Lutterbeck, C.A.; Kist, L.T.; Machado, Ê.L. Floating treatment wetlands: A review and bibliometric analysis. Sci. Total. Environ. 2020, 714, 136776. [Google Scholar] [CrossRef]
- Fletcher, J.; Willby, N.; Oliver, D.M.; Quilliam, R.S. Resource recovery and freshwater ecosystem restoration—Prospecting for phytoremediation potential in wild macrophyte stands. Resour. Environ. Sustain. 2022, 7, 100050. [Google Scholar] [CrossRef]
- Yofukuji, K.Y.; Cardozo, A.L.P.; Quirino, B.A.; Aleixo, M.H.F.; Fugi, R. Macrophyte diversity alters invertebrate community and fish diet. Hydrobiologia 2021, 848, 913–927. [Google Scholar] [CrossRef]
- Hansen, J.P.; Sagerman, J.; Wikström, S.A. Effects of plant morphology on small-scale distribution of invertebrates. Mar. Biol. 2010, 157, 2143–2155. [Google Scholar] [CrossRef]
- Law, A.; Baker, A.; Sayer, C.; Foster, G.; Gunn, I.D.; Taylor, P.; Pattison, Z.; Blaikie, J.; Willby, N.J. The effectiveness of aquatic plants as surrogates for wider biodiversity in standing fresh waters. Freshw. Biol. 2019, 64, 1664–1675. [Google Scholar] [CrossRef]
- Hassall, C.; Hollinshead, J.; Hull, A. Environmental correlates of plant and invertebrate species richness in ponds. Biodivers. Conserv. 2011, 20, 3189–3222. [Google Scholar] [CrossRef]
- Mitsch, W.J. What is ecological engineering? Ecol. Eng. 2012, 45, 5–12. [Google Scholar] [CrossRef]
- Storkey, J.; Döring, T.; Baddeley, J.; Collins, R.; Roderick, S.; Jones, H.; Watson, C. Engineering a plant community to deliver multiple ecosystem services. Ecol. Applic. 2015, 25, 1034–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quilliam, R.S.; van Niekerk, M.A.; Chadwick, D.R.; Cross, P.; Hanley, N.; Jones, D.L.; Vinten, A.J.; Willby, N.; Oliver, D.M. Can macrophyte harvesting from eutrophic water close the loop on nutrient loss from agricultural land? J. Environ. Manag. 2015, 152, 210–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venables, W.N.; Smith, D.M. R Development Core Team. In An Introduction to R. A Programming Environment for Data Analysis and Graphics; R Development Core Team: Vienna, Austria, 2006. [Google Scholar]
- Cummins, K.W. Functional analysis of Stream Macroinvertebrates. In Limnology—Some New Aspects of Inland Water Ecology; Gökçe, D., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Fletcher, J.; Willby, N.; Oliver, D.M.; Quilliam, R.S. Floating treatment wetlands—Engineering nature-based solutions for ecosystem multifunctionality. SSRN 4358486 2023. [Google Scholar]
- Willby, N.J.; Pulford, I.D.; Flowers, T.H. Tissue nutrient signatures predict herbaceous-wetland community responses to nutrient availability. New Phytol. 2001, 152, 463–481. [Google Scholar] [CrossRef]
- Williams, J. Phytoremediation in wetland ecosystems: Progress, problems, and potential. Crit. Rev. Plant Sci. 2010, 21, 607. [Google Scholar] [CrossRef]
- Yuan, Y.K.; Huang, C.M. Investigation of the water purification efficiency of flood irrigation system by using flora succession as an index. Int. J. Phytoremediat. 2010, 12, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Thrippleton, T.; Bugmann, H.; Snell, R.S. Herbaceous competition and browsing may induce arrested succession in central European forests. J. Ecol. 2018, 106, 1120–1132. [Google Scholar] [CrossRef]
- Matthews, J.W.; Spyreas, G. Convergence and divergence in plant community trajectories as a framework for monitoring wetland restoration progress. J. Appl. Ecol. 2010, 47, 1128–1136. [Google Scholar] [CrossRef]
- Munford, K.E.; Asemaninejad, A.; Basiliko, N.; Mykytczuk, N.C.; Glasauer, S.; McGarry, S.; Watmough, S.A. Native plants facilitate vegetation succession on amended and unamended mine tailings. Int. J. Phytoremediat. 2022, 24, 963–974. [Google Scholar] [CrossRef]
- Williams, L.D.; Ahn, C. Plant community development as affected by initial planting richness in created mesocosm wetlands. Ecol. Eng. 2015, 75, 33–40. [Google Scholar] [CrossRef]
- Gallardo, L.I.; Carnevali, R.P.; Porcel, E.A.; Poi, A.S.G. Does the effect of aquatic plant types on invertebrate assemblages change across seasons in a subtropical wetland? Limnetica 2017, 36, 87–98. [Google Scholar]
- Hyseni, C.; Heino, J.; Bini, L.M.; Bjelke, U.; Johansson, F. The importance of blue and green landscape connectivity for biodiversity in urban ponds. Basic Appl. Ecol. 2021, 57, 129–145. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G.; Melo, F.J.; Hernández, V.J.; González-Portela, R.E. Long-term assessment at field scale of Floating Treatment Wetlands for improvement of water quality and provision of ecosystem services in a eutrophic urban pond. Sci. Total. Environ. 2017, 584, 561–571. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 135–189. [Google Scholar]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Printz, B.; Lutts, S.; Hausman, J.F.; Sergeant, K. Copper trafficking in plants and its implication on cell wall dynamics. Front. Plant Sci. 2016, 7, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karstens, S.; Langer, M.; Nyunoya, H.; Čaraitė, I.; Stybel, N.; Razinkovas-Baziukas, A.; Bochert, R. Constructed floating wetlands made of natural materials as habitats in eutrophicated coastal lagoons in the Southern Baltic Sea. J. Coast. Conserv. 2021, 25, 44. [Google Scholar] [CrossRef]
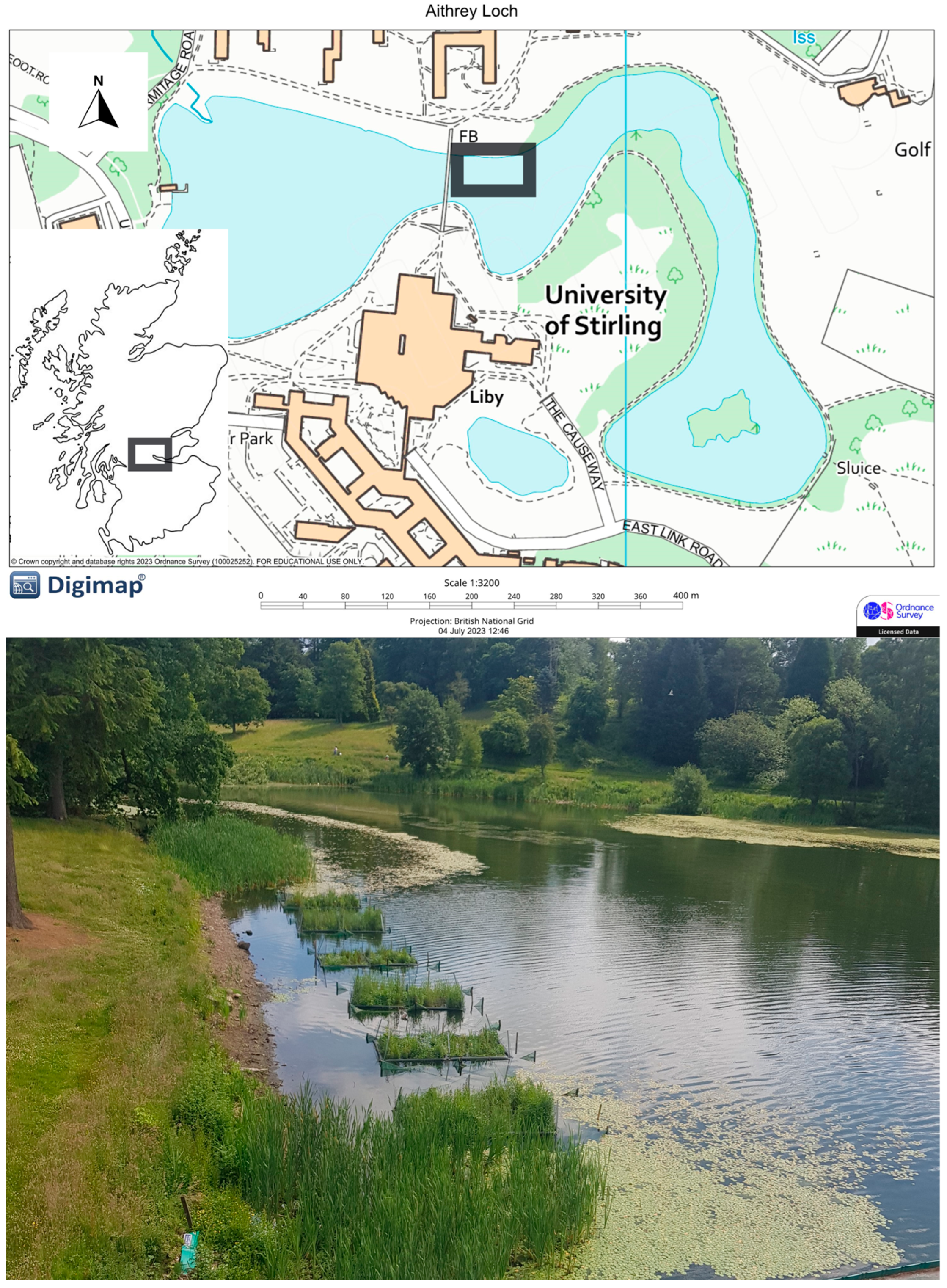


| Ecosystem Service | Measurable Variable | Sample Timing | ||
|---|---|---|---|---|
| Year 1 (2019) | Year 2 (2020) | Year 3 (2021) | ||
| Habitat provision | Macroinvertebrates (abundance and diversity) | - | August (summer) | - |
| Plant community stability | Abundance (Domin scale) | June (summer) | August (summer) | September (late summer) |
| Biomass | Dry weight | - | September (late summer) | September (late summer) |
| Resource recovery | Tissue concentration and standing stocks | - | September (late summer) | |
| Ratio Name | Ratio of Feeding Groups | Thresholds and Explanations | Interpretations |
|---|---|---|---|
| Habitat stability index | filterers + scrapers shredders + gatherers | Ratio bigger than 0.5 indicates that suspended fine particulate organic matter is greater than entrained fine particulate matter | Filtering collectors require stable locations and scrapers require surfaces that remain in a stable position facing upwards |
| Shredder index | shredders collectors + filterers | A ratio of >0.5 in autumn/winter, and of 0.25 in spring/summer, indicates that CPOM availability for shredders is greater than FPOM availability for collectors | CPOM food support for shredders > than FPOM for collectors |
| Filtering collector index | filterers collectors | A ratio of <0.50 indicates that suspended FPOM load is less than stored (entrained) FPOM | FPOM food for collectors at higher density and/or better quality than storage FPOM |
| Top-down predator index | predators all functional feeding groups | Predator:prey ratio 0.10–0.20 to total macroinvertebrate population | This level of a predator population density (or biomass) is supported by sufficient prey to support them |
| Habitat Stability Index | Shedder Index | Filtering Collector Index | Top-Down Predator Index | |
|---|---|---|---|---|
| Monoculture | 0.19 | 8.36 | 0.23 | 0.42 |
| Polyculture | 0.21 | 9.95 | 0.24 | 0.44 |
| All Communities | 0.20 | 9.16 | 0.24 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fletcher, J.; Willby, N.; Oliver, D.M.; Quilliam, R.S. Field-Scale Floating Treatment Wetlands: Quantifying Ecosystem Service Provision from Monoculture vs. Polyculture Macrophyte Communities. Land 2023, 12, 1382. https://doi.org/10.3390/land12071382
Fletcher J, Willby N, Oliver DM, Quilliam RS. Field-Scale Floating Treatment Wetlands: Quantifying Ecosystem Service Provision from Monoculture vs. Polyculture Macrophyte Communities. Land. 2023; 12(7):1382. https://doi.org/10.3390/land12071382
Chicago/Turabian StyleFletcher, Jonathan, Nigel Willby, David M. Oliver, and Richard S. Quilliam. 2023. "Field-Scale Floating Treatment Wetlands: Quantifying Ecosystem Service Provision from Monoculture vs. Polyculture Macrophyte Communities" Land 12, no. 7: 1382. https://doi.org/10.3390/land12071382





