Soil Carbon and Biochemical Indicators of Soil Quality as Affected by Different Conservation Agricultural and Weed Management Options
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Treatments
2.3. Crop Management
2.4. Collection and Preparation of the Soil Samples
2.5. Fractions of Carbon
2.6. Carbon Pools Based on Oxidizability
- -
- Very labile C (Pool I): SOC oxidizable under 12.0 N sulfuric acid;
- -
- Labile C (Pool II): The difference in SOC extracted between 18.0 and 12.0 N sulfuric acid;
- -
- Less labile C (Pool III): The difference in SOC extracted between 24.0 and 18.0 N sulfuric acid;
- -
- Non-Labile or recalcitrant C (Pool IV): Residual organic C after reaction with 24.0 N sulfuric acid when compared with total organic carbon.
2.7. Carbon Management Index (CMI)
2.8. Carbon Stock
2.9. Enzyme Activity
2.10. Soil Quality Index
2.11. Statistical Analysis
3. Results
3.1. Soil Organic Carbon (SOC)
3.2. Fractions of Carbon
3.3. Carbon Pools Based on Oxidizability
3.4. Carbon Management Index (CMI)
3.5. Enzyme Activities
3.6. SOC Stock
3.7. Soil Quality Index
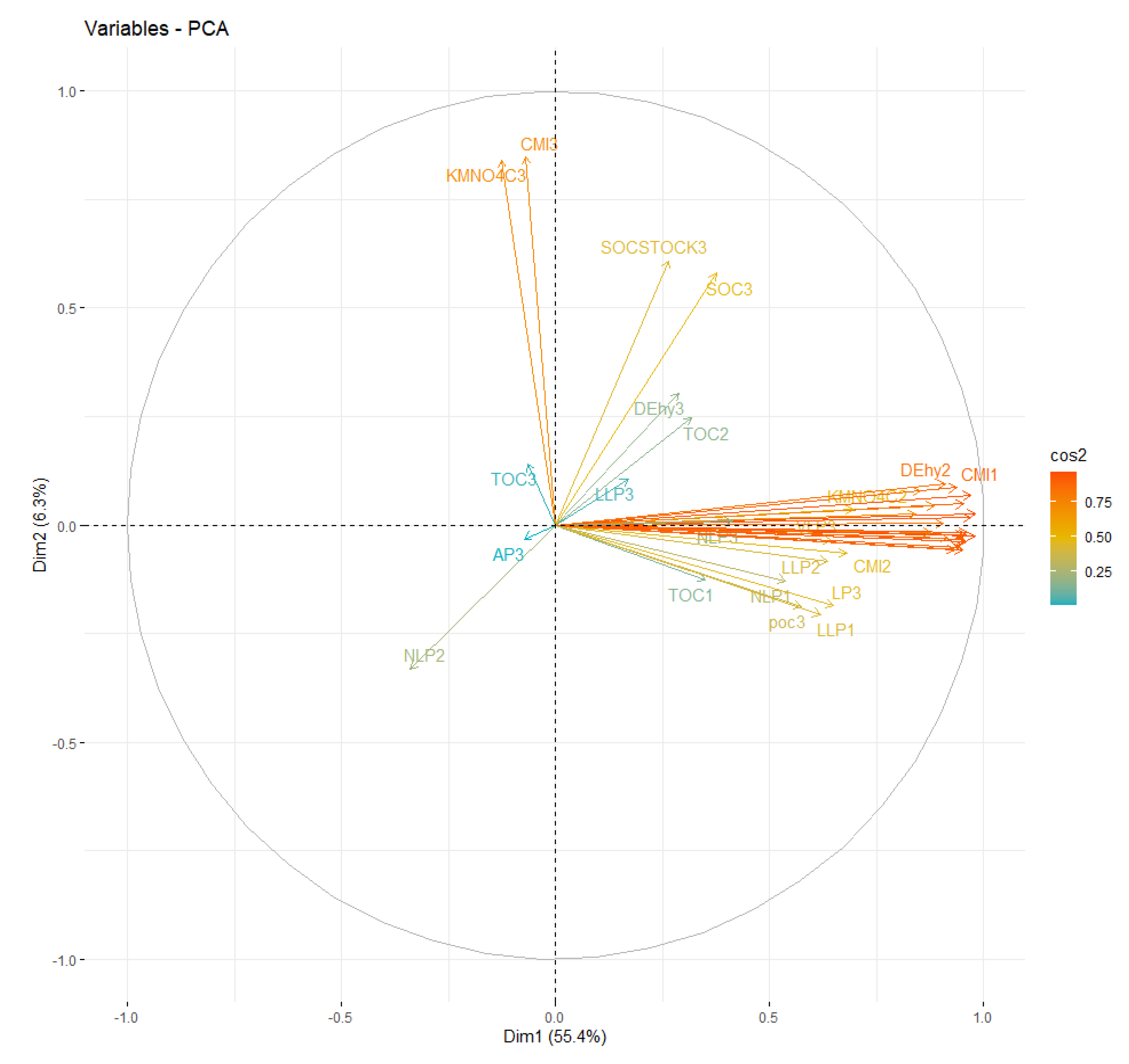
3.8. Selection of Minimum Data Set Attributes (MDS)
3.9. Scoring of Indicators
3.10. Soil Quality Index (SQI)
4. Discussion
4.1. Walkley and Black Carbon (SOC)
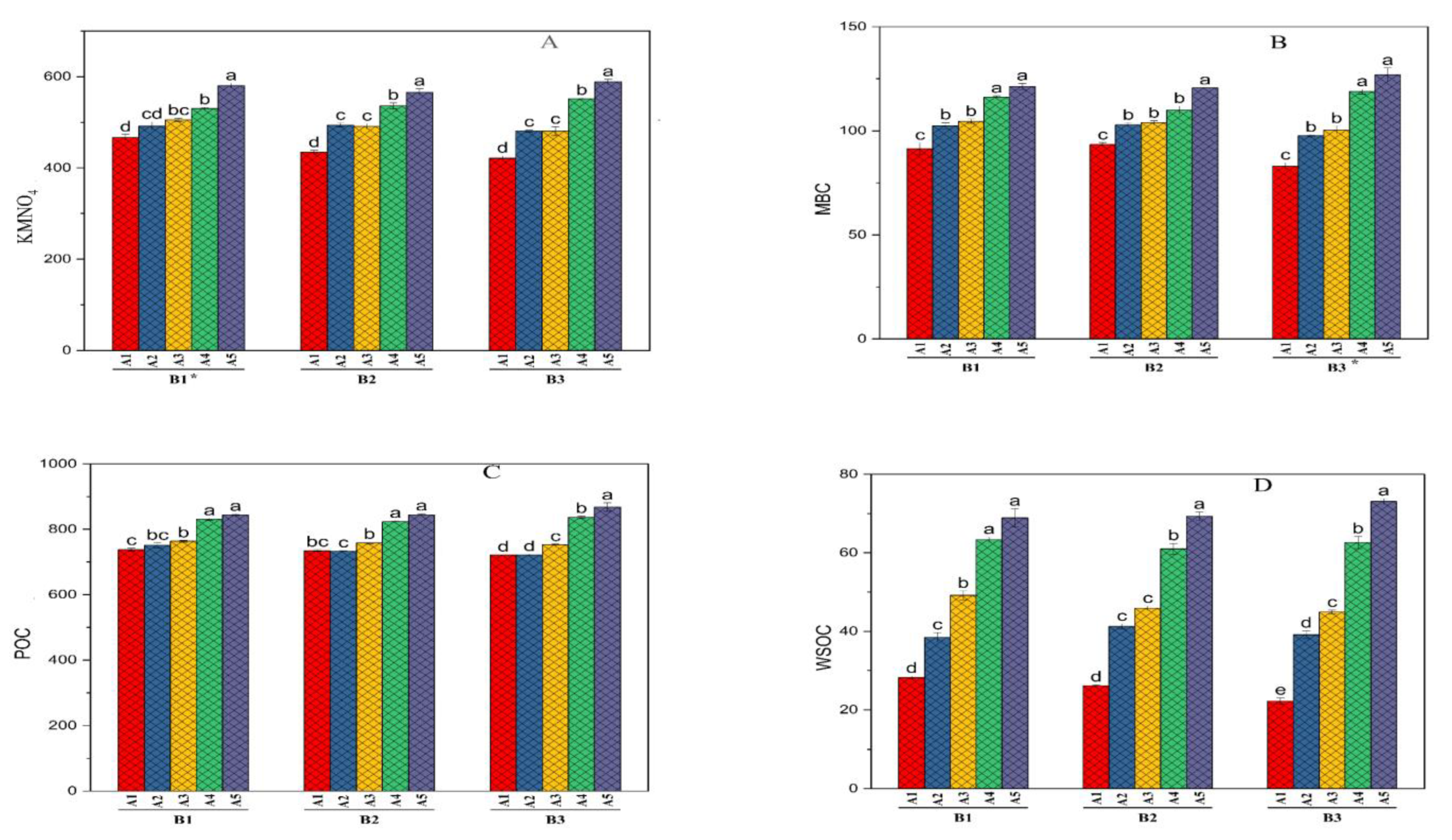
4.2. Permanganate Oxidizable Carbon (KMnO4-C)
4.3. Microbial Biomass Carbon (MBC)
4.4. Particulate Organic Carbon (POC)
4.5. Water Soluble Organic Carbon (WSOC)
4.6. Total Organic Carbon (TOC)
4.7. Carbon Pools Based on Oxidizability
4.8. Carbon Management Index (CMI)
4.9. Enzyme Activities
4.10. Soil Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, R.; Kukal, S.S.; Busari, M.A.; Arora, S.; Yadav, M. Sustainability issues on rice–wheat cropping system. Int. Soil Water Conserv. Res. 2016, 4, 64–74. [Google Scholar] [CrossRef]
- Dey, A.; Dwivedi, B.S.; Meena, M.C.; Datta, S.P. Dynamics of soil carbon and nitrogen under conservation agriculture in rice-wheat cropping system. Indian J. Fertil. 2018, 14, 12–26. [Google Scholar]
- Humphreys, E.; Kukal, S.S.; Christen, E.W.; Hira, G.S.; Singh, B.; Yadav, S.; Sharma, R.K. Halting the groundwater decline in north-west India: Which crop technologies will be winners? Adv. Agron. 2010, 109, 155–217. [Google Scholar]
- Singh, A.; Phogat, V.K.; Dahiya, R.; Batra, S.D. Impact of long-term zero till wheat on soil physical properties and wheat productivity under rice-wheat cropping system. Soil Tillage Res. 2014, 140, 98–105. [Google Scholar] [CrossRef]
- Hobbs, P.R.; Giri, G.S. Reduced and zero-tillage options for establishment of wheat after rice in South Asia. In Wheat: Prospects for Global Improvement; Paper No. 2; Springer: Dordrecht, The Netherlands, 1997; pp. 455–465. [Google Scholar]
- Gupta, R.K.; Naresh, R.K.; Hobbs, P.R.; Jiaguo, Z.; Ladha, J.K. Sustainability of post-green revolution agriculture: The rice-wheat cropping systems of the Indo-Gangetic Plains and China. In Improving the Productivity and Sustainability of Rice-Wheat Systems: Issues and Impacts; Ladha, J.K., Hill, J.E., Duxbury, J.M., Gupta, R.K., Buresh, R.J., Eds.; Special Publication. 65; Soil Science Society of America: Madison, WI, USA, 2003; pp. 1–25. [Google Scholar]
- Bhatt, R.; Singh, P.; Sharma, S. Changes in Soil Organic Pool and Carbon Preservation Capacity of Macro and Micro aggregates in Response to Land Use Change in North Western India. J. Soil Sci. Plant Nutr. 2023, 23, 2849–2867. [Google Scholar] [CrossRef]
- Dwivedi, B.S.; Singh, V.K.; Shukla, A.K.; Meena, M.C. Optimizing dry and wet tillage for rice on a Gangetic alluvial soil: Effect on soil characteristics, water use efficiency and productivity of the rice-wheat system. Eur. J. Agron. 2012, 43, 155–165. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Hill, J.E. Direct seeding of rice in Asia: Emerging issues and strategic research needs for the 21st century. In Direct Seeding: Research Issues and Opportunities. Proceedings Inernational Workshop on Direct Seeding in Asian Rice Systems: Strategic Research Issues and Oppertunities; Pandey, S., Mortimer, S., Wade, L., Tuong, T.P., Lopez, K., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philipines, 2002; pp. 15–43. [Google Scholar]
- Jat, H.S.; Datta, A.; Sharma, P.C.; Kumar, V.; Yadav, A.K.; Choudhary, M.; Choudhary, V.; Gathala, M.K.; Sharma, D.K.; Jat, M.L.; et al. Assessing soil properties and nutrient availability under conservation agriculture practices in a reclaimed sodic soil in cereal-based systems of north-west India. Arch. Agron. Soil Sci. 2018, 64, 531–545. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Das, T.K.; Sudhishri, S.; Dudwal, B.; Sharma, A.R.; Bhatia, A.; Singh, G. Conservation agriculture effects on soil organic carbon accumulation and crop productivity under a rice–wheat cropping system in the western Indo-Gangetic Plains. Eur. J. Agron. 2015, 70, 11–21. [Google Scholar] [CrossRef]
- Sharma, S.; Thind, H.S.; Sidhu, H.S.; Jat, M.L.; Parihar, C.M. Effects of crop residue retention on soil carbon pools after 6 years of rice-wheat cropping system. Environ. Earth Sci. 2019, 78, 296. [Google Scholar] [CrossRef]
- Chen, H.; Hou, R.; Gong, Y.; Li, H.; Fan, M.; Kuzyakov, Y. Effects of 11 years of conservation tillage on soil organic matter fractions in wheat monoculture in Loess Plateau of China. Soil Tillage Res. 2009, 106, 85–94. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Saikia, R.; Sharma, S.; Thind, H.S.; Sidhu, H.S. Temporal changes in biochemical indicators of soil quality in response to tillage, crop residue and green manure management in a rice-wheat system. Ecol. Indic. 2019, 103, 383–394. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar]
- Guo, L.J.; Zhang, Z.S.; Wang, D.D.; Li, C.F.; Cao, C.G. Effects of short-term conservation management practices on soil organic carbon fractions and microbial community composition under a rice-wheat rotation system. Biol. Fertil. Soils 2015, 51, 65–75. [Google Scholar] [CrossRef]
- Li, C.F.; Yue, L.X.; Kou, Z.K.; Zhang, Z.S.; Wang, J.P.; Cao, C.G. Short-term effects of conservation management practices on soil labile organic carbon fractions under a rape–rice rotation in central China. Soil Tillage Res. 2012, 119, 31–37. [Google Scholar] [CrossRef]
- Janzen, H.H. Soil organic matter characteristics after long-term cropping to various spring wheat rotations. Can. J. Soil Sci. 1987, 67, 845–856. [Google Scholar] [CrossRef]
- Briedis, C.; de Moraes Sá, J.C.; Lal, R.; Tivet, F.; Franchini, J.C.; de Oliveira Ferreira, A.; da Cruz Hartman, D.; Schimiguel, R.; Bressan, P.T.; Inagaki, T.M.; et al. How does no-till deliver carbon stabilization and saturation in highly weathered soils? Catena 2018, 163, 13–23. [Google Scholar] [CrossRef]
- Liu, Z.; He, T.; Lan, Y.; Yang, X.; Meng, J.; Chen, W. Maize stover biochar accelerated urea hydrolysis and short-term nitrogen turnover in soil. BioResources 2017, 12, 6024–6039. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC); Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 498–540. [Google Scholar]
- Alnaimy, M.A.; Elrys, A.S.; Zelenakova, M.; Pietrucha-Urbanik, K.; Merwad, A.R.M. The Vital Roles of Parent Material in Driving Soil Substrates and Heavy Metals Availability in Arid Alkaline Regions: A Case Study from Egypt. Water 2023, 15, 2481. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Hons, F.M. Soil-profile distribution of primary and secondary plant-available nutrients under conventional and no tillage. Soil Tillage Res. 1996, 39, 229–239. [Google Scholar] [CrossRef]
- Prashar, P.; Shah, S. Impact of fertilizers and pesticides on soil microflora in agriculture. Sustain. Agric. Rev. 2016, 14, 331–361. [Google Scholar]
- Walkley, A.J.; Black, T.A. An examination of the wet digestion method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Vieira, F.C.B.; Bayer, C.; Zanatta, J.A.; Dieckow, J.; Mielniczuk, J.; He, Z.L. Carbon management index based on physical fractionation of soil organic matter in an Acrisol under long-term no-till cropping systems. Soil Tillage Res. 2007, 96, 195–204. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial biomass measurements in forest soils: Determination of kc values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils. Soil Biol. Biochem. 1987, 19, 689–696. [Google Scholar] [CrossRef]
- McGill, W.B.; Cannon, K.B.; Robertson, J.A.; Cook, F.D. Dynamics of soil microbial biomass and water-soluble organic C in Breton L after 50 years of cropping to two rotations. Can. J. Soil Sci. 1986, 66, 1–19. [Google Scholar] [CrossRef]
- Houba, V.J.G.; van der Lee, J.J.; Novozamsky, I. Soil Analysis Procedures, Other Procedures (Soil and Plant Analysis. Part 5B); Wageningen Agricultural University: Wageningen, The Netherlands, 1995. [Google Scholar]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizable organic carbon fractions and soil quality changes in an Oxic Paleustalf under different pasture clays. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Dey, A.; Dwivedi, B.S.; Bhattacharyya, R.; Datta, S.P.; Meena, M.C.; Jat, R.K.; Gupta, R.K.; Jat, M.L.; Singh, V.K.; Das, D.; et al. Effect of conservation agriculture on soil organic and inorganic carbon sequestration and lability: A study from a rice-wheat cropping system on a calcareous soil of the eastern Indo-Gangetic Plains. Soil Use Manag. 2020, 36, 429–438. [Google Scholar] [CrossRef]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Andrews, S.S.; Mitchell, J.P.; Mancinelli, R.; Larlen, D.L.; Hartz, T.K.; Horwarth, W.R.; Pettygrove, G.S.; Scow, K.M.; Munk, D.S. On farm assessment of soil quality in California’s Central Valley. Agron. J. 2002, 94, 12–23. [Google Scholar]
- Brejda, J.J.; Moorman, T.B.; Karlen, D.L.; Dao, T.H. Identification of regional soil quality factors and indicators I. Central and southern high plains. Soil Sci. Soc. Am. J. 2000, 64, 2115–2124. [Google Scholar] [CrossRef]
- Wander, M.M.; Bollero, G.A. Soil quality assessment of tillage impacts on Illinois. Soil Sci. Soc. Am. J. 1999, 63, 961–971. [Google Scholar] [CrossRef]
- Fukumasu, J.; Jarvis, N.; Koestel, J.; Kätterer, T.; Larsbo, M. Relations between soil organic carbon content and the pore size distribution for an arable topsoil with large variations in soil properties. Eur. J. Soil Sci. 2022, 73, e13212. [Google Scholar] [CrossRef]
- Alam, M.K.; Islam, M.M.; Salahin, N.; Hasanuzzaman, M. Effect of tillage on soil properties under rice-wheat system. Sci. World J. 2014, 32, 231–246. [Google Scholar]
- Bhattacharyya, R.; Tuti, M.D.; Kundu, S.; Bisht, J.K.; Bhatt, J.C. Conservation tillage impacts on soil aggregation and carbon pools in a sandy clay loam soil of the Indian Himalayas. Soil Sci. Soc. Am. J. 2012, 76, 617–627. [Google Scholar] [CrossRef]
- Derpsch, R.; Franzluebbers, A.J.; Duiker, S.W.; Reicosky, D.C.; Koeller, K.; Friedrich, T.; Sturny, W.G.; Sá, J.C.M.; Weiss, K. Why do we need to standardize no-tillage research? Soil Tillage Res. 2014, 13, 16–22. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Culman, S.W.; Horwath, W.R.; Wade, J.; Cass, D.; Beniston, J.W.; Bowles, T.M.; Grandy, A.S.; Franzluebbers, A.J.; Schipanski, M.E.; et al. Comparison of permanganate-oxidizable carbon and mineralizable carbon for assessment of organic matter stabilization and mineralization. Soil Sci. Soc. Am. J. 2016, 80, 1352–1364. [Google Scholar] [CrossRef]
- Chivenge, P.P.; Murwira, H.K.; Giller, K.E.; Mapfumo, P.; Six, J. Long-term impact of reduced tillage and residue management on soil carbon stabilization: Implications for conservation agriculture on contrasting soils. Soil Tillage Res. 2007, 94, 328–337. [Google Scholar] [CrossRef]
- Shao, J.A.; Li, Y.; Wei, C.; Xie, D. Effects of land management practices on labile organic carbon fractions in rice cultivation. Chin. Geogr. Sci. 2009, 19, 241–248. [Google Scholar] [CrossRef]
- Prasad, J.; Rao, C.S.; Srinivas, K.; Jyothi, C.N.; Venkateswarlu, B.; Ramachandrappa, B.K.; Dhanapal, G.N.; Ravichandra, K.; Mishra, P.K. Effect of ten years of reduced tillage and recycling of organic matter on crop yields, soil organic carbon and its fractions in Alfisols of semi arid tropics of southern India. Soil Tillage Res. 2016, 156, 131–139. [Google Scholar] [CrossRef]
- Choudhary, M.; Datta, A.; Jat, H.S.; Yadav, A.K.; Gathala, M.K.; Sapkota, T.B.; Das, A.K.; Parbodh, C.; Sharma, P.C.; Jat, M.L.; et al. Changes in soil biology under conservation agriculture based sustainable intensification of cereal systems in Indo-Gangetic Plains. Geoderma 2018, 313, 193–204. [Google Scholar] [CrossRef]
- Parihar, C.M.; Jat, S.L.; Singh, A.K.; Datta, A.; Parihar, M.D.; Varghese, E.; Jat, M.L. Changes in carbon pools and biological activities of a sandy loam soil under medium-term conservation agriculture and diversified cropping systems. Eur. J. Soil Sci. 2018, 69, 902–912. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Das, T.K.; Pramanik, P.; Ganeshan, V.; Saad, A.A.; Sharma, A.R. Impacts of conservation agriculture on soil aggregation and aggregate-associated N under an irrigated agroecosystem of the Indo-Gangetic Plains. Nutr. Cycl. Agroecosyst. 2013, 96, 185–202. [Google Scholar] [CrossRef]
- Mishra, V.; Chowdhury, T.; Singh, A.P.; Gupta, S.B. Changes in biochemical properties of rice rhizosphere as influenced by tillage and herbicide application. Indian J. Weed Sci. 2013, 45, 231–234. [Google Scholar]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Soil quality response to long-term nutrient and crop management on a semi-arid Inceptisol. Agriculture, Ecosyst. Environ. 2007, 118, 130–142. [Google Scholar] [CrossRef]
- Wang, Q.; Bai, Y.; Gao, H.; He, J.; Chen, H.; Chesney, R.C.; Kuhn, N.J.; Li, H. Soil chemical properties and microbial biomass after 16 years of no-tillage farming on the Loess Plateau, China. Geoderma 2008, 144, 502–508. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhou, J.M.; Xiao, B.H. Characterization of dissolved organic matter derived from rice straw at different stages of decay. J. Soils Sediments 2010, 10, 915–922. [Google Scholar] [CrossRef]
- Dou, F.; Wright, A.L.; Hons, F.M. Sensitivity of labile soil organic carbon to tillage in wheat-based cropping systems. Soil Sci. Soc. Am. J. 2008, 72, 1445–1453. [Google Scholar] [CrossRef]
- Parihar, C.M.; Yadav, M.R.; Jat, S.L.; Singh, A.K.; Kumar, B.; Pradhan, S.; Chakraborty, D.; Jat, M.L.; Jat, R.K.; Saharawat, Y.S.; et al. Long term effect of conservation agriculture in maize rotations on total organic carbon, physical and biological properties of a sandy loam soil in north-western Indo-Gangetic Plains. Soil Tillage Res. 2016, 161, 116–128. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Stuedemann, J.A. Bermudagrass management in the southern Piedmont USA: III. Particulate and biologically active soil carbon. Soil Sci. Soc. Am. J. 2003, 67, 132–138. [Google Scholar] [CrossRef]
- Dou, F.; Hons, F.M. Tillage and nitrogen e ects on soil organic matter fractions in wheat-based systems. Soil Sci. Soc. Am. J. 2006, 70, 1896–1905. [Google Scholar] [CrossRef]
- Mondal, S.; Poonia, S.P.; Mishra, J.S.; Bhatt, B.P.; Karnena, K.R.; Saurabh, K.; Kumar, R.; Chakraborty, D. Short-term (5 years) impact of conservation agriculture on soil physical properties and organic carbon in a rice-wheat rotation in the Indo-Gangetic plains of Bihar. Eur. J. Soil Sci. 2019, 71, 1076–1089. [Google Scholar] [CrossRef]
- Jat, H.S.; Datta, A.; Choudhary, M.; Yadav, A.K.; Choudhary, V.; Sharma, P.C.; Gathala, M.K.; Jat, M.L.; McDonald, A. Effects of tillage, crop establishment and diversification on soil organic carbon, aggregation, aggregate associated carbon and productivity in cereal systems of semi-arid northwest India. Soil Tillage Res. 2019, 190, 128–138. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; De-la-Luz, M. Dynamics and Environmental Significance of Dissolved Organic Matter in Soil. Ph.D. Thesis, Regional Institute Limited, Palmerston North, New Zealand, 2004. [Google Scholar]
- Zhu, L.; Hu, N.; Zhang, Z.; Xu, J.; Tao, B.; Meng, Y. Short-term responses of soil organic carbon and carbon pool management index to different annual straw return rates in a rice-wheat cropping system. Catena 2015, 135, 283–289. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practices. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Wang, H.; Bao, L.; Zhou, J. Crop yields and soil organic carbon fractions as influenced by straw incorporation in a rice–wheat cropping system in south eastern China. Nutr. Cycl. Agroecosyst. 2018, 112, 61–73. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-term manure and fertilizer effects on soil organic matter fractions and microbes under a wheat–maize cropping system in northern China. Geoderma 2009, 149, 318–324. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Ouyang, Z. Effects of land use, climate, topography and soil properties on regional soil organic carbon and total nitrogen in the Upstream Watershed of Miyun Reservoir, North China. J. Environ. Sci. 2012, 24, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Zhao, Y.; Xu, X.; Hai, N.; Huang, B.; Deng, W. Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: A meta-analysis. Agric. Ecosyst. Environ. 2015, 204, 40–50. [Google Scholar] [CrossRef]
- Samal, S.K.; Rao, K.K.; Poonia, S.P.; Kumar, R.; Mishra, J.S.; Prakash, V.; Mondal, S.; Dwivedi, S.K.; Bhatt, B.P.; Naik, S.K.; et al. Evaluation of long-term conservation agriculture and crop intensification in rice-wheat rotation of Indo-Gangetic Plains of South Asia: Carbon dynamics and productivity. Eur. J. Agron. 2017, 90, 198–208. [Google Scholar] [CrossRef]
- Kaiser, K.; Kalbitz, K. Cycling downwards–dissolved organic matter in soils. Soil Biol. Biochem. 2012, 52, 29–32. [Google Scholar] [CrossRef]
- Nandan, R.; Singh, V.; Singh, S.S.; Kumar, V.; Hazra, K.K.; Nath, C.P.; Poonia, S.; Malik, R.K.; Bhattacharyya, R.; McDonald, A. Impact of conservation tillage in rice–based cropping systems on soil aggregation, carbon pools and nutrients. Geoderma 2019, 340, 104–114. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 3, 3–17. [Google Scholar]
- Majumder, B.; Mandal, B.; Bandyopadhyay, P.K. Soil organic carbon pools and productivity in relation to nutrient management in a 20-year-old rice-berseem agroecosystem. Biol. Fertil. Soils 2008, 44, 451–561. [Google Scholar] [CrossRef]
- Ghosh, B.N.; Meena, V.S.; Alam, N.M.; Dogra, P.; Bhattacharyya, R.; Sharma, N.K.; Mishra, P.K. Impact of conservation practices on soil aggregation and the carbon management index after seven years of maize–wheat cropping system in the Indian Himalayas. Agric. Ecosyst. Environ. 2016, 216, 247–257. [Google Scholar] [CrossRef]
- Tirol-Padre, A.; Ladha, J.K. Assessing the reliability of permanganate- oxidizable carbon as an index of soil labile carbon. Soil Sci. Soc. Am. J. 2004, 68, 969–978. [Google Scholar] [CrossRef]
- Nannipieri, P. The potential use of soil enzymes as indicators of productivity, sustainability and pollution. In Soil Biota: Management in Sustainable Farming Systems; Pankhurst, C.E., Doube, B.M., Gupta, V.V., Grace, P.R., Eds.; CSIRO: Canberra, Australia, 1994; pp. 238–244. [Google Scholar]
- Caravaca, F.; Roldan, A. Assessing changes in physical and biological properties in a soil contaminated by oil sludges under semiarid Mediterranean conditions. Geoderma 2003, 117, 53–61. [Google Scholar] [CrossRef]
- Roldán, A.; Salinas-García, J.R.; Alguacil, M.M.; Diaz-Pereira, E.; Caravaca, F. Soil enzyme activities suggest advantages of conservation tillage practices in sorghum cultivation under subtropical conditions. Geoderma 2005, 129, 178–185. [Google Scholar] [CrossRef]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Balota, E.L.; Colozzi, A.; Andrade, D.S.; Dick, R.P. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Tillage Res. 2004, 77, 137–145. [Google Scholar] [CrossRef]
- Melero, S.; López-Garrido, R.; Murillo, J.M.; Moreno, F. Conservation tillage: Short-and long-term effects on soil carbon fractions and enzymatic activities under Mediterranean conditions. Soil Tillage Res. 2009, 104, 292–298. [Google Scholar] [CrossRef]
- Majumdar, B.; Saha, A.R.; Sarkar, S.; Maji, B.; Mahapatra, B.S. Effect of herbicides and fungicide application on fibre yield and nutrient uptake by jute (Corchorus olitorius) residual nutrient status and soil quality. Indian J. Agric. Sci. 2010, 80, 878–883. [Google Scholar]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. Adv. Agron. 2019, 156, 1–107. [Google Scholar]
- Abrol, V.; Sharma, P.; Sankar, G.R.; Sharma, M.; Chandra, R.; Sharma, V. Soil management effects on soil quality and crop performance in dry sub-humid inceptisols of India. Indian J. Soil Conserv. 2015, 43, 47–57. [Google Scholar]



| Treatment | Crop | Crop Establishment Method | Previous Crop Residue Removed or Retained |
|---|---|---|---|
| T1 (Conventional agriculture) CTR-CTW | Rice | Conventional-transplanted rice (CTR) | Wheat residue removed (−WR) |
| Wheat | Conventional-tilled (CT) wheat | Rice residue removed (−RR) | |
| T2 (Partial CA 1) CTR-ZTW-ZTG | Rice | Conventional-transplanted rice | Green gram residue incorporated |
| Wheat | Zero-till (ZT) wheat | Rice residue removed (−RR) | |
| Green gram | Zero-tilled green gram | Wheat residue removed (−WR) | |
| T3 (Partial CA 2) CTDSR -CTW-ZTG | Rice | Conventional-tilled direct seeded rice | Green gram residue incorporated |
| Wheat | Conventional-tilled wheat | Rice residue removed (−RR) | |
| Green gram | Zero-tilled green gram | Wheat residue removed (−WR) | |
| T4 (Partial CA 3) ZTDQSR-ZTW-ZTG | Rice | Zero-till direct seeded rice | Green gram residue retained as brown manure |
| Wheat | Zero-tilled wheat | Rice residue retained as mulch in wheat (+RR) | |
| Green gram | Zero-tilled green gram | Wheat straw removed(-WR) | |
| T5 (Full CA) ZTDSR-ZTW-ZTG | Rice | Zero-till direct seeded rice | Green gram residue retained as brown manure crop |
| Wheat | Zero-tilled wheat | Rice residue retained as mulch in wheat (+RR) | |
| Green gram | Zero-tilled green gram | Wheat residue retained (+WR) | |
| Weed Management (WM) Treatments | |||
| Treatment | Rice | Wheat | |
| Chemical weed control (Herbicide) | Pre-emergence application of Pendimethalin 30 EC @1250 mL ha−1 and post-emergence application after 25 days of sowing, bispyribac-sodium 25 g a.i./ha | Pre-emergence application of Pendimethlin 30 EC @1250 mL ha−1 and after 30 days of sowing, clodinafop (15%) + metsulfuron methyl (1%) 60 + 4 g a.i./ha was applied as post-emergence with knap sac sprayer fitted with flat fan boom nozzle. | |
| Integrated weed management (IWM) | Chemical weed control + 1 hand weeding | Chemical weed control + 1 hand weeding | |
| Weedycheck | No weed control | No weed control | |
| Treatments | SOC (g kg−1) | TOC (g kg−1) | CMI | SOC Stocks (Mg C ha−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| WM | CA | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm |
| Herbicide | Conventional Agriculture | 4.59 ± 0.17 c | 2.86 ± 0.07 b | 1.12 ± 0.02 ab | 0.89 ± 0.01 a | 100.0 ± 0 d | 100.0 ± 0 a | 5 ± 0.2 b | 3.59 ± 0.1 b |
| Partial CA1 | 4.97 ± 0.12 bc | 3.09 ± 0.08 b | 1.12 ± 0.02 ab | 0.86 ± 0.02 a | 103.4 ± 0.14 cd | 109.8 ± 4.6 a | 5.62 ± 0.18 b | 3.73 ± 0.11 b | |
| Partial CA2 | 5.1 ± 0.13 bc | 3.17 ± 0.08 b | 1.08 ± 0.01 b | 0.88 ± 0.03 a | 108.9 ± 1.77 bc | 115.7 ± 3 a | 5.67 ± 0.18 b | 3.87 ± 0.08 b | |
| Partial CA3 | 5.85 ± 0.14 ab | 3.64 ± 0.09 a | 1.18 ± 0.01 a | 0.89 ± 0.02 a | 113.9 ± 1.27 b | 111.1 ± 5.1 a | 6.91 ± 0.13 a | 4.41 ± 0.11 a | |
| Full CA | 6.38 ± 0.23 a | 3.97 ± 0.09 a | 1.15 ± 0.02 a | 0.93 ± 0.01 a | 123.7 ± 0.92 a | 119.8 ± 3.73 a | 7.21 ± 0.32 a | 4.71 ± 0.09 a | |
| IWM | Conventional Agriculture | 4.74 ± 0.17 c | 2.94 ± 0.07 b | 1.17 ± 0.03 a | 0.72 ± 0.11 b | 92.9 ± 1.59 c | 106.7 ± 0.23 a | 5.23 ± 0.21 b | 3.68 ± 0.09 b |
| Partial CA1 | 4.98 ± 0.12 c | 3.09 ± 0.08 b | 1.11 ± 0.02 b | 0.85 ± 0.04 ab | 105.5 ± 0.15 b | 109.7 ± 0.19 a | 5.59 ± 0.18 b | 3.83 ± 0.12 b | |
| Partial CA2 | 5.12 ± 0.13 bc | 3.18 ± 0.08 b | 1.04 ± 0 c | 0.87 ± 0.02 ab | 106.2 ± 2.1 b | 108.6 ± 3.56 a | 5.77 ± 0.11 b | 3.89 ± 0.13 b | |
| Partial CA3 | 5.98 ± 0.15 ab | 3.72 ± 0.09 a | 1.15 ± 0.02 ab | 0.93 ± 0.02 a | 115.4 ± 2.72 a | 109.0 ± 1.58 a | 7 ± 0.15 a | 4.51 ± 0.11 a | |
| Full CA | 6.44 ± 0.21 a | 4 ± 0.1 a | 1.17 ± 0.01 ab | 0.89 ± 0.01 a | 121.7 ± 3.02 a | 113.5 ± 6.58 a | 7.18 ± 0.15 a | 4.69 ± 0.12 a | |
| Weedy Check | Conventional Agriculture | 4.78 ± 0.23 b | 2.98 ± 0.07 b | 1.14 ± 0.02 bc | 0.91 ± 0.01 a | 89.7 ± 2.14 c | 89.3 ± 4.33 b | 5.14 ± 0.24 b | 3.71 ± 0.08 b |
| Partial CA1 | 4.97 ± 0.12 b | 3.09 ± 0.08 b | 1.11 ± 0 c | 0.87 ± 0.01 a | 102.9 ± 1.66 b | 104.7 ± 2.41 ab | 5.62 ± 0.17 b | 3.76 ± 0.1 b | |
| Partial CA2 | 5.07 ± 0.12 b | 3.15 ± 0.08 b | 1.2 ± 0.01 ab | 0.92 ± 0.01 a | 103.9 ± 0.16 b | 110.6 ± 4.08 a | 5.64 ± 0.18 b | 3.9 ± 0.07 b | |
| Partial CA3 | 6.22 ± 0.21 a | 3.87 ± 0.09 a | 1.21 ± 0.01 a | 0.89 ± 0.02 a | 118.4 ± 1.58 a | 118.2 ± 3.86 a | 7.05 ± 0.1 a | 4.67 ± 0.12 a | |
| Full CA | 6.51 ± 0.28 a | 4.05 ± 0.1 a | 1.15 ± 0.01 abc | 0.89 ± 0.03 a | 125.6 ± 0.79 a | 121.9 ± 5.63 a | 7.21 ± 0.21 a | 4.67 ± 0.14 a | |
| Treatments | WSOC (mg kg−1) | KMnO4-C (mg kg−1) | POC (mg kg−1) | MBC (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| WM | CA | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm |
| Herbicide | Conventional Agriculture | 28.24 ± 0.38 d | 23.04 ± 0.7 d | 466.9 ± 5.9 d | 270.9 ± 9.08 b | 737.6 ± 4.52 c | 482.1 ± 2.8 c | 91.42 ± 2.79 c | 87.26 ± 0.49 c |
| Partial CA1 | 38.46 ± 1.25 c | 32.73 ± 0.54 c | 491.7 ± 9.2 cd | 287.9 ± 4.88 ab | 750.9 ± 7.38 bc | 507.3 ± 3.22 b | 102.44 ± 1.56 b | 87.41 ± 1.05 c | |
| Partial CA2 | 49.17 ± 1.1 b | 38.96 ± 0.66 b | 505.1 ± 3.88 bc | 305.4 ± 1.84 ab | 763.4 ± 2.41 b | 518.9 ± 4.41 b | 104.66 ± 1 b | 89.03 ± 0.59 bc | |
| Partial CA3 | 63.34 ± 0.67 a | 58.06 ± 0.88 a | 530.2 ± 2.32 b | 290.9 ± 6.37 ab | 830.5 ± 3.1 a | 544.2 ± 1.9 a | 116.12 ± 0.7 a | 95.77 ± 1.85 b | |
| Full CA | 68.9 ± 2.31 a | 58.94 ± 0.79 a | 580.3 ± 5.1 a | 313.8 ± 2.18 a | 844.6 ± 2.47 a | 553.1 ± 5.36 a | 121.32 ± 1.45 a | 105.48 ± 2.27 a | |
| IWM | Conventional Agriculture | 26.14 ± 0.28 d | 23.52 ± 0.58 e | 435.0 ± 3.46 d | 272.2 ± 10.11 a | 735.3 ± 2.42 bc | 481.9 ± 2.3 c | 93.36 ± 0.98 c | 87.58 ± 1.4 c |
| Partial CA1 | 41.26 ± 0.62 c | 33.24 ± 1.12 d | 494.0 ± 4.41 c | 289.1 ± 5.83 a | 732.4 ± 2.51 c | 509.6 ± 5.2 b | 102.91 ± 0.95 b | 87.94 ± 1.74 c | |
| Partial CA2 | 45.89 ± 0.53 c | 39.3 ± 0.57 c | 491.2 ± 5.67 c | 287.3 ± 2.98 a | 758.1 ± 3.34 b | 522.5 ± 6.36 b | 103.86 ± 0.94 b | 93.04 ± 0.58 bc | |
| Partial CA3 | 61.03 ± 1.39 b | 58.64 ± 0.67 b | 535.8 ± 6.47 b | 286.9 ± 2.62 a | 823.4 ± 2.44 a | 548.0 ± 4.57 a | 109.97 ± 1.97 b | 101.04 ± 1.34 ab | |
| Full CA | 69.3 ± 1.1 a | 64.13 ± 0.88 a | 566.0 ± 7.37 a | 303.1 ± 11.32 a | 844.7 ± 3.19 a | 556.5 ± 2.68 a | 120.65 ± 0.09 a | 106.66 ± 1.67 a | |
| Weedy Check | Conventional Agriculture | 22.2 ± 0.86 e | 21.43 ± 0.64 d | 421.0 ± 5.27 d | 233.6 ± 17.24 c | 721.6 ± 1.08 d | 481.3 ± 9.53 c | 82.98 ± 1.68 c | 82.01 ± 1.18 b |
| Partial CA1 | 39.14 ± 1.02 d | 30.9 ± 0.53 c | 481.1 ± 2.52 c | 275.3 ± 0.5 b | 721.6 ± 2.56 d | 499.2 ± 3.87 bc | 97.59 ± 0.45 b | 84.7 ± 2.05 b | |
| Partial CA2 | 44.92 ± 0.48 c | 37.01 ± 0.67 b | 480.2 ± 9.52 c | 293.8 ± 4.63 ab | 752.7 ± 1.89 c | 518.0 ± 0.55 b | 100.34 ± 1.93 b | 85.95 ± 2.58 b | |
| Partial CA3 | 62.63 ± 1.54 b | 60.55 ± 0.97 a | 551.2 ± 2 b | 306.1 ± 4.49 ab | 836.7 ± 3.58 b | 557.3 ± 5.96 a | 118.82 ± 1.16 a | 102.64 ± 1.67 a | |
| Full CA | 73.08 ± 0.72 a | 63.35 ± 0.4 a | 588.5 ± 5.6 a | 314.5 ± 7.96 a | 867.8 ± 13.16 a | 567.2 ± 1.56 a | 126.85 ± 3.35 a | 108.62 ± 0.88 a | |
| Treatments | Very Labile Pool | Labile Pool | Less Labile Pool | Non-Labile Pool | |||||
|---|---|---|---|---|---|---|---|---|---|
| WM | CA | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm |
| Herbicide | Conventional Agriculture | 2.12 ± 0.04 b | 1.31 ± 0.05 b | 1.64 ± 0.04 c | 1.07 ± 0.04 b | 0.84 ± 0.04 a | 0.47 ± 0.04 a | 4.64 ± 0.52 a | 2.84 ± 0.22 a |
| Partial CA1 | 2.18 ± 0.1 b | 1.44 ± 0.04 b | 1.82 ± 0.05 bc | 1.19 ± 0.04 ab | 0.85 ± 0.04 a | 0.49 ± 0.03 a | 4.92 ± 0.56 a | 1.73 ± 0.12 b | |
| Partial CA2 | 2.4 ± 0.1 b | 1.5 ± 0.05 b | 1.85 ± 0.05 bc | 1.2 ± 0.04 ab | 0.86 ± 0.04 a | 0.48 ± 0.04 a | 4.94 ± 0.56 a | 1.74 ± 0.11 b | |
| Partial CA3 | 2.97 ± 0.03 a | 1.85 ± 0.06 a | 1.97 ± 0.05 ab | 1.29 ± 0.04 a | 0.92 ± 0.06 a | 0.52 ± 0.03 a | 5.65 ± 0.66 a | 1.8 ± 0.12 b | |
| Full CA | 3.33 ± 0.13 a | 2.04 ± 0.05 a | 2.08 ± 0.05 a | 1.36 ± 0.04 a | 0.98 ± 0.05 a | 0.56 ± 0.02 a | 6.01 ± 0.7 a | 1.83 ± 0.12 b | |
| IWM | Conventional Agriculture | 2.23 ± 0.06 c | 1.38 ± 0.05 b | 1.69 ± 0.04 c | 1.1 ± 0.04 b | 0.82 ± 0.04 a | 0.47 ± 0.03 a | 4.69 ± 0.6 a | 2.91 ± 0.23 a |
| Partial CA1 | 2.36 ± 0.05 c | 1.45 ± 0.04 b | 1.8 ± 0.04 bc | 1.17 ± 0.04 ab | 0.85 ± 0.04 a | 0.46 ± 0.02 a | 4.94 ± 0.56 a | 1.71 ± 0.12 b | |
| Partial CA2 | 2.45 ± 0.04 c | 1.52 ± 0.05 b | 1.81 ± 0.05 bc | 1.18 ± 0.04 ab | 0.88 ± 0.06 a | 0.5 ± 0.03 a | 4.98 ± 0.57 a | 1.73 ± 0.11 b | |
| Partial CA3 | 2.97 ± 0.09 b | 1.92 ± 0.03 a | 1.98 ± 0.05 ab | 1.29 ± 0.04 a | 0.9 ± 0.05 a | 0.51 ± 0.03 a | 5.69 ± 0.66 a | 1.79 ± 0.13 b | |
| Full CA | 3.39 ± 0.1 a | 2.11 ± 0.02 a | 2.06 ± 0.05 a | 1.35 ± 0.04 a | 0.98 ± 0.05 a | 0.56 ± 0.02 a | 6 ± 0.7 a | 1.82 ± 0.14 b | |
| Weedy Check | Conventional Agriculture | 2.25 ± 0.12 b | 1.4 ± 0.04 b | 1.71 ± 0.04 c | 1.12 ± 0.04 b | 0.86 ± 0.05 a | 0.48 ± 0.03 a | 4.79 ± 0.54 a | 1.7 ± 0.13 a |
| Partial CA1 | 2.36 ± 0.04 b | 1.46 ± 0.05 b | 1.76 ± 0.04 c | 1.15 ± 0.04 b | 0.87 ± 0.03 a | 0.5 ± 0.03 a | 4.91 ± 0.56 a | 1.7 ± 0.12 a | |
| Partial CA2 | 2.35 ± 0.09 b | 1.45 ± 0.04 b | 1.85 ± 0.05 bc | 1.2 ± 0.04 ab | 0.89 ± 0.05 a | 0.51 ± 0.03 a | 4.97 ± 0.57 a | 1.74 ± 0.11 a | |
| Partial CA3 | 3.26 ± 0.05 a | 2.03 ± 0.04 a | 2.06 ± 0.05 ab | 1.34 ± 0.04 a | 0.92 ± 0.05 a | 0.53 ± 0.03 a | 5.82 ± 0.68 a | 1.82 ± 0.15 a | |
| Full CA | 3.43 ± 0.04 a | 2.13 ± 0.07 a | 2.13 ± 0.06 a | 1.38 ± 0.04 a | 0.97 ± 0.05 a | 0.55 ± 0.02 a | 6.12 ± 0.72 a | 1.84 ± 0.12 a | |
| Treatments | Dehydrogenase Activity | Alkaline Phosphate Activity | Soil Quality Index | |||
|---|---|---|---|---|---|---|
| WM | CA | 0–7.5 cm | 7.5–15 cm | 0–7.5 cm | 7.5–15 cm | |
| Herbicide | Conventional Agriculture | 91.17 ± 0.42 d | 72.5 ± 1.54 c | 168.34 ± 1.67 c | 111.67 ± 1.67 bc | 0.70 ± 0.005 d |
| Partial CA1 | 113.67 ± 2.29 c | 80.48 ± 0.63 bc | 175.0 ± 2.89 bc | 108.34 ± 1.67 c | 0.79 ± 0.009 c | |
| Partial CA2 | 115.82 ± 2.13 c | 87.52 ± 3.27 b | 185.0 ± 2.89 b | 115.0 ± 5 bc | 0.80 ± 0.011 c | |
| Partial CA3 | 128.27 ± 0.87 b | 100.19 ± 0.85 a | 208.34 ± 1.67 a | 130.0 ± 0 ab | 0.86 ± 0.024 b | |
| Full CA | 140.71 ± 3.24 a | 101.83 ± 1.47 a | 216.67 ± 6.67 a | 145.0 ± 2.89 a | 0.95 ± 0.012 a | |
| IWM | Conventional Agriculture | 87.58 ± 0.72 d | 72.03 ± 1.03 c | 163.67 ± 1.86 b | 116.67 ± 4.41 b | 0.65 ± 0.021 d |
| Partial CA1 | 111.75 ± 0.64 c | 82.12 ± 0.82 bc | 175.0 ± 2.89 b | 118.34 ± 1.67 b | 0.77 ± 0.014 c | |
| Partial CA2 | 111.27 ± 1.25 c | 91.98 ± 4.48 ab | 178.34 ± 1.67 b | 118.34 ± 1.67 b | 0.78 ± 0.014 c | |
| Partial CA3 | 128.75 ± 1.46 b | 100.43 ± 0.41 a | 221.67 ± 1.67 a | 141.67 ± 1.67 a | 0.86 ± 0.002 b | |
| Full CA | 141.43 ± 1.1 a | 99.96 ± 0.24 a | 230.0 ± 5.78 a | 141.67 ± 1.67 a | 0.92 ± 0.022 ab | |
| Weedy Check | Conventional Agriculture | 86.62 ± 1.05 d | 72.97 ± 0 b | 163.34 ± 3.34 b | 110.0 ± 5.78 b | 0.65 ± 0.003 d |
| Partial CA1 | 106.73 ± 0.87 c | 83.06 ± 2.45 b | 171.67 ± 1.67 b | 110.0 ± 5.78 b | 0.76 ± 0.002 c | |
| Partial CA2 | 111.04 ± 1.34 c | 95.03 ± 3.27 a | 170.0 ± 0.2 b | 113.34 ± 1.67 b | 0.77 ± 0.005 c | |
| Partial CA3 | 136.4 ± 1.81 b | 101.13 ± 0.41 a | 235.0 ± 2.89 a | 146.67 ± 4.41 a | 0.92 ± 0.014 ab | |
| Full CA | 147.65 ± 0.87 a | 102.77 ± 2.49 a | 250.0 ± 0.24 a | 141.67 ± 7.27 a | 0.97 ± 0.003 a | |
| Component Matrix | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | KMnO4C | WSOC | POC | MBC | TOC | CMI | DHA | APA | SOC | Stock | VLC | LC | LLC | RC |
| 1 | 0.975 | 0.831 | 0.981 | 0.971 | 0.819 | 0.511 | 0.970 | 0.878 | 0.892 | 0.777 | 0.888 | 0.973 | 0.756 | 0.668 |
| 2 | −0.151 | 0.492 | −0.191 | 0.271 | 0.793 | −0.240 | 0.163 | −0.003 | −0.046 | −0.075 | 0.039 | −0.092 | −0.216 | −0.354 |
| PC Number | Eigenvalue | % Variance | Cumulative Percent | Weight of Each PC | Indicators Selected for SQI |
|---|---|---|---|---|---|
| PC1 | 9.761 | 75.082 | 75.082 | 0.89 | KMnO4-C |
| PC2 | 1.094 | 8.414 | 83.496 | 0.10 | TOC |
| Parameter | MBC | KMnO4-C | CMI | Dehydrogenase | Labile-C |
|---|---|---|---|---|---|
| MBC | 1.00 | 0.96 | 0.91 | 0.96 | 0.94 |
| KMnO4-C | 0.96 | 1.00 | 0.99 | 0.97 | 0.95 |
| CMI | 0.91 | 0.99 | 1.00 | 0.95 | 0.92 |
| Dehydrogenase | 0.96 | 0.97 | 0.95 | 1.00 | 0.97 |
| Labile –C | 0.94 | 0.95 | 0.92 | 0.97 | 1.00 |
| Total | 4.77 | 4.86 | 4.77 | 4.85 | 4.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, G.; Sharma, K.R.; Bhatt, R.; Singh, J.; Wani, O.A.; Dewidar, A.Z.; Mattar, M.A. Soil Carbon and Biochemical Indicators of Soil Quality as Affected by Different Conservation Agricultural and Weed Management Options. Land 2023, 12, 1783. https://doi.org/10.3390/land12091783
Singh G, Sharma KR, Bhatt R, Singh J, Wani OA, Dewidar AZ, Mattar MA. Soil Carbon and Biochemical Indicators of Soil Quality as Affected by Different Conservation Agricultural and Weed Management Options. Land. 2023; 12(9):1783. https://doi.org/10.3390/land12091783
Chicago/Turabian StyleSingh, Gobinder, Kuldeep Raj Sharma, Rajan Bhatt, Jagdeep Singh, Owais Ali Wani, Ahmed Z. Dewidar, and Mohamed A. Mattar. 2023. "Soil Carbon and Biochemical Indicators of Soil Quality as Affected by Different Conservation Agricultural and Weed Management Options" Land 12, no. 9: 1783. https://doi.org/10.3390/land12091783
APA StyleSingh, G., Sharma, K. R., Bhatt, R., Singh, J., Wani, O. A., Dewidar, A. Z., & Mattar, M. A. (2023). Soil Carbon and Biochemical Indicators of Soil Quality as Affected by Different Conservation Agricultural and Weed Management Options. Land, 12(9), 1783. https://doi.org/10.3390/land12091783









