Potential Mechanism of Optimal Tillage Layer Structure for Improving Maize Yield and Enhancing Root Growth in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
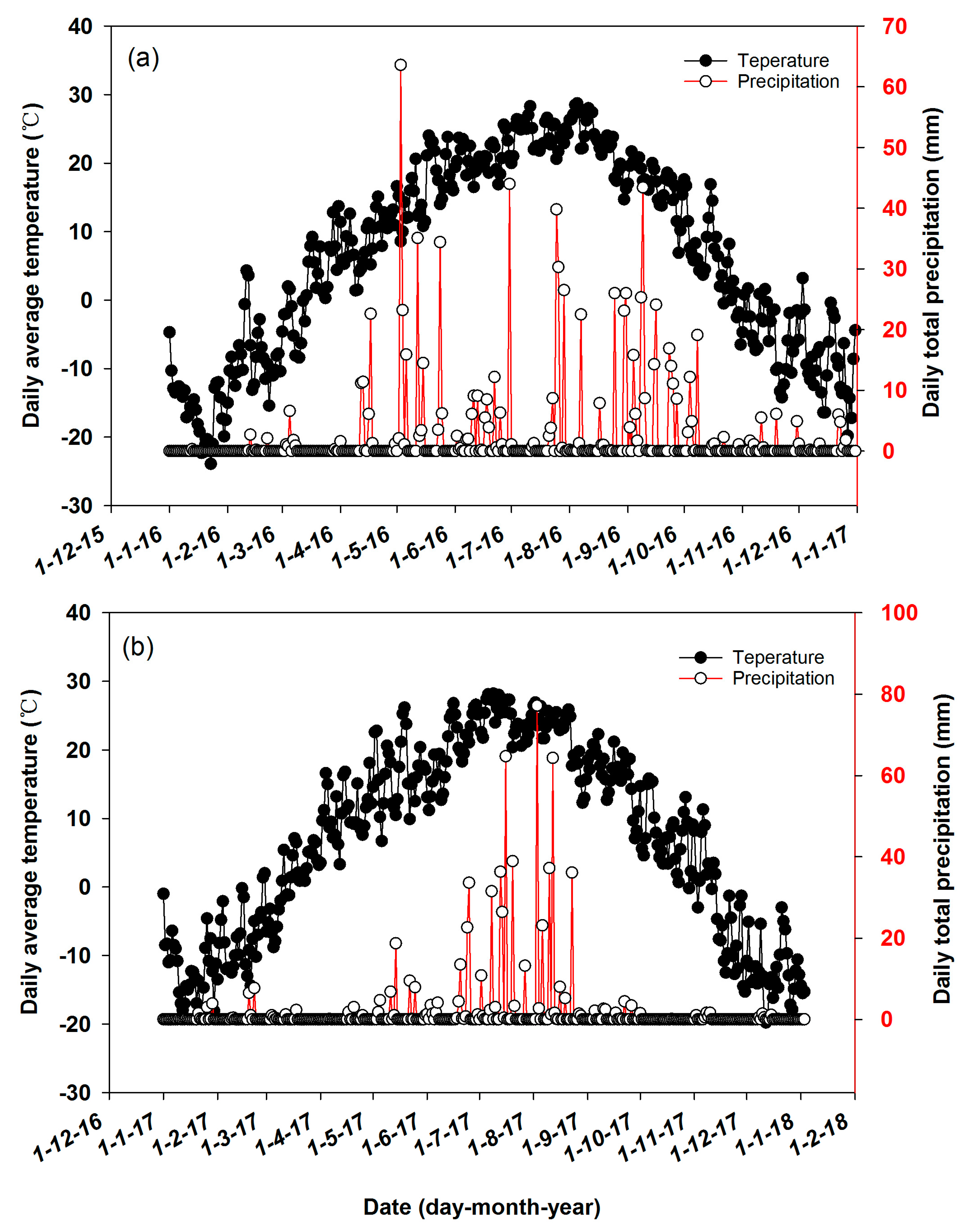
2.1. Site Description
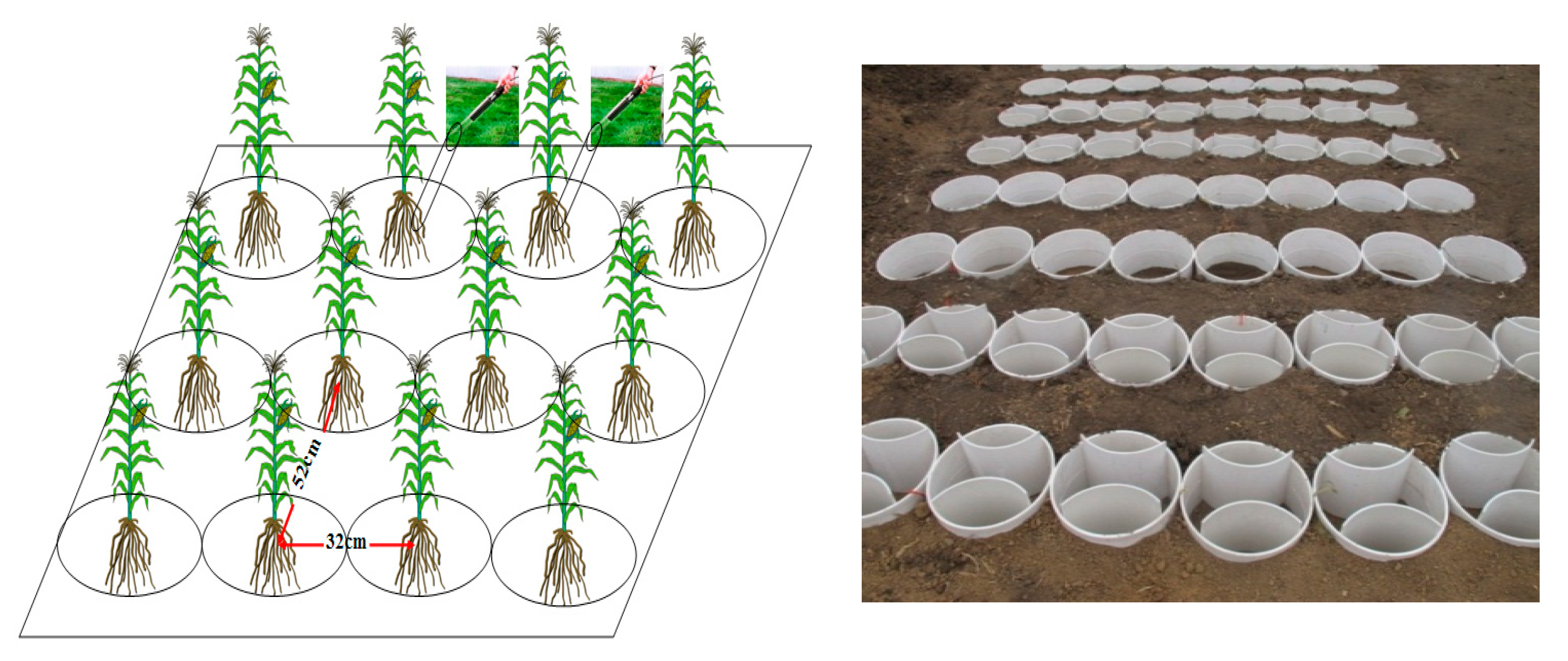
2.2. Experimental Design
2.3. Root Sampling
2.4. Soil Physical Parameters
2.5. Soil Nutrient Parameters
2.6. Root Morphology and Physiology
2.7. Maize Grain Yield
2.8. Data Analysis
3. Results
3.1. Effect of Different Tillage Layer Structures on Soil Physical Properties
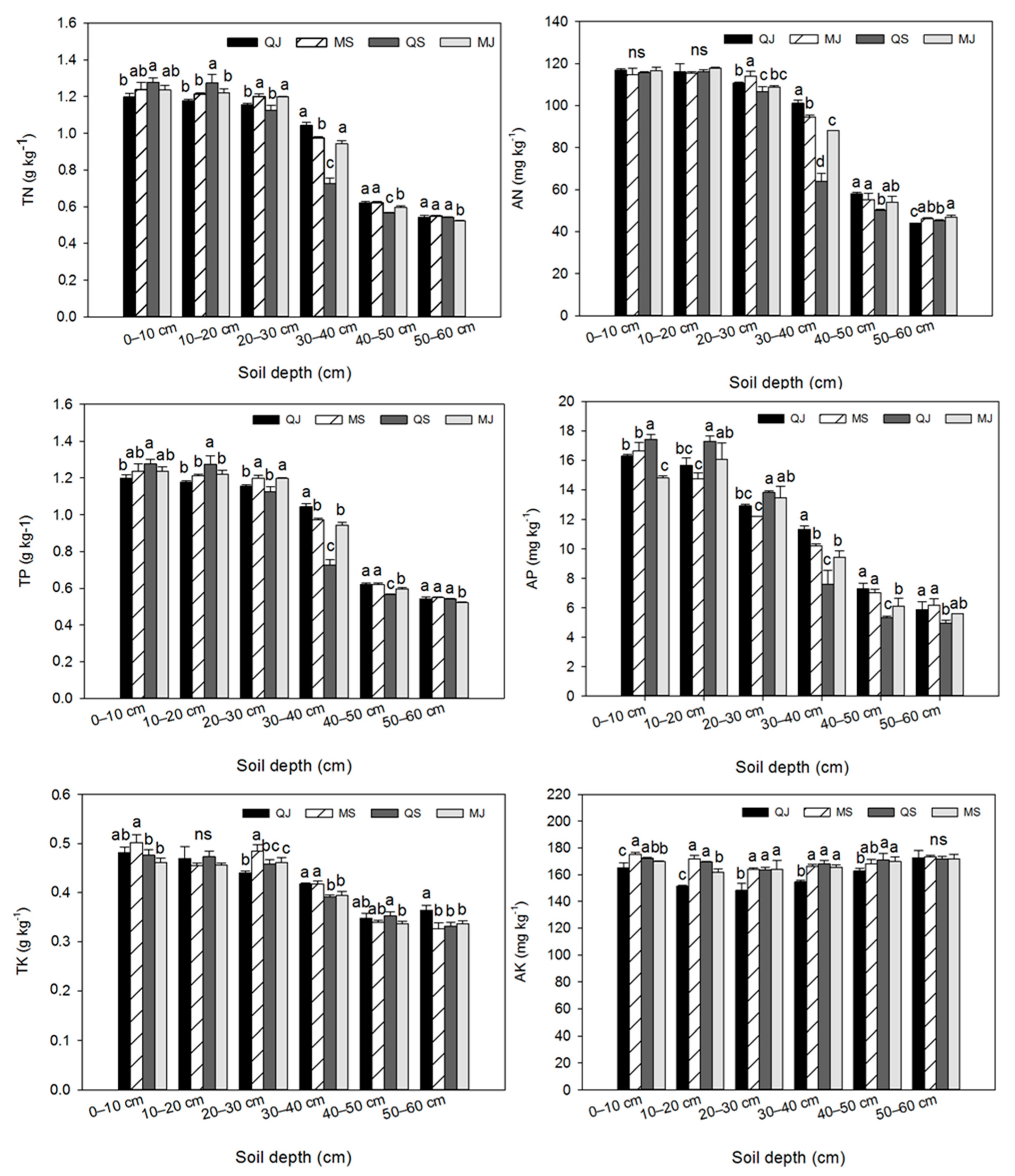
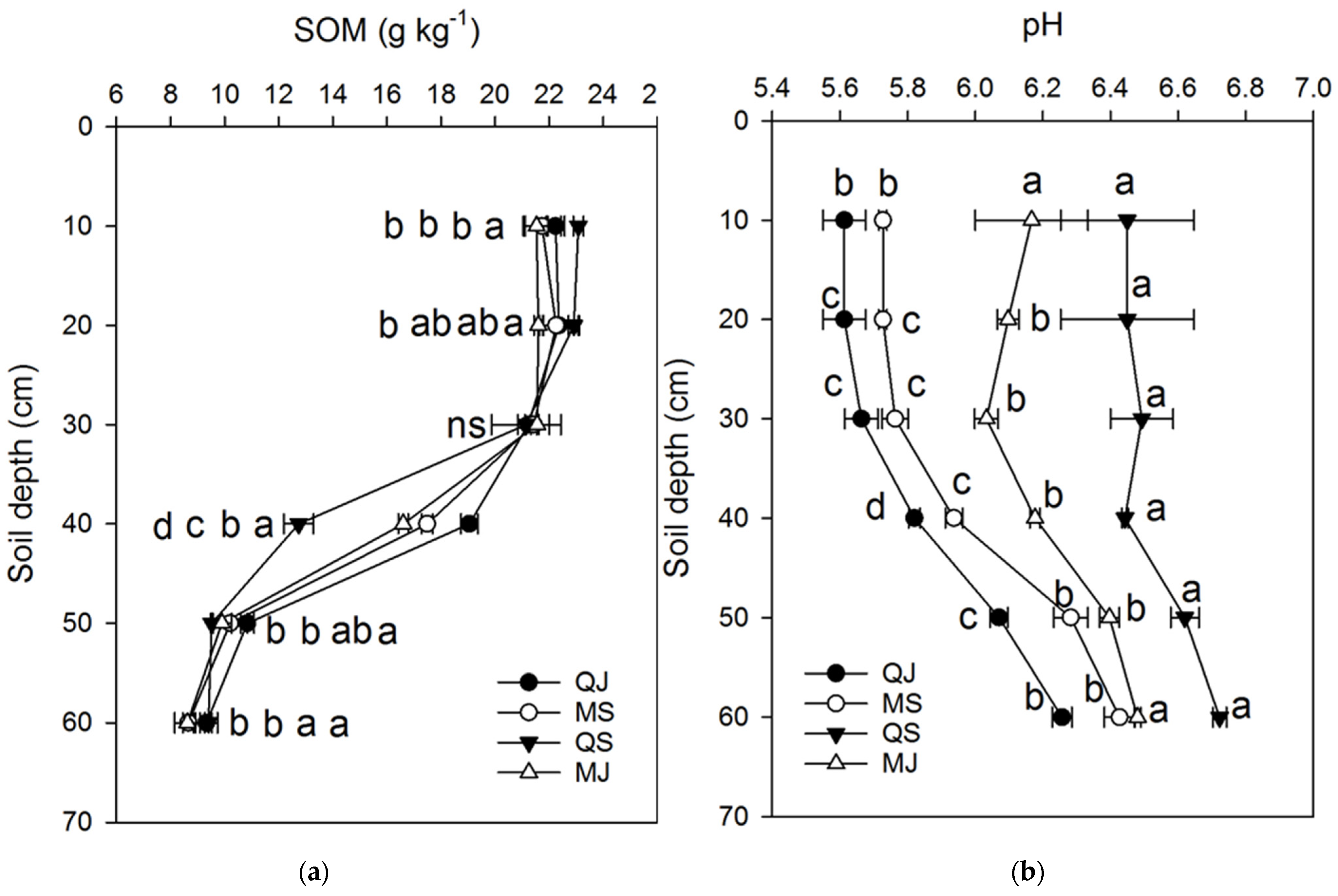
3.2. Tillage’s Effect on Soil Nutrients
3.3. Effect of Different Tillage Layer Structures on Root Morphology
3.4. Tillage’s Effect on Root Physiological Properties
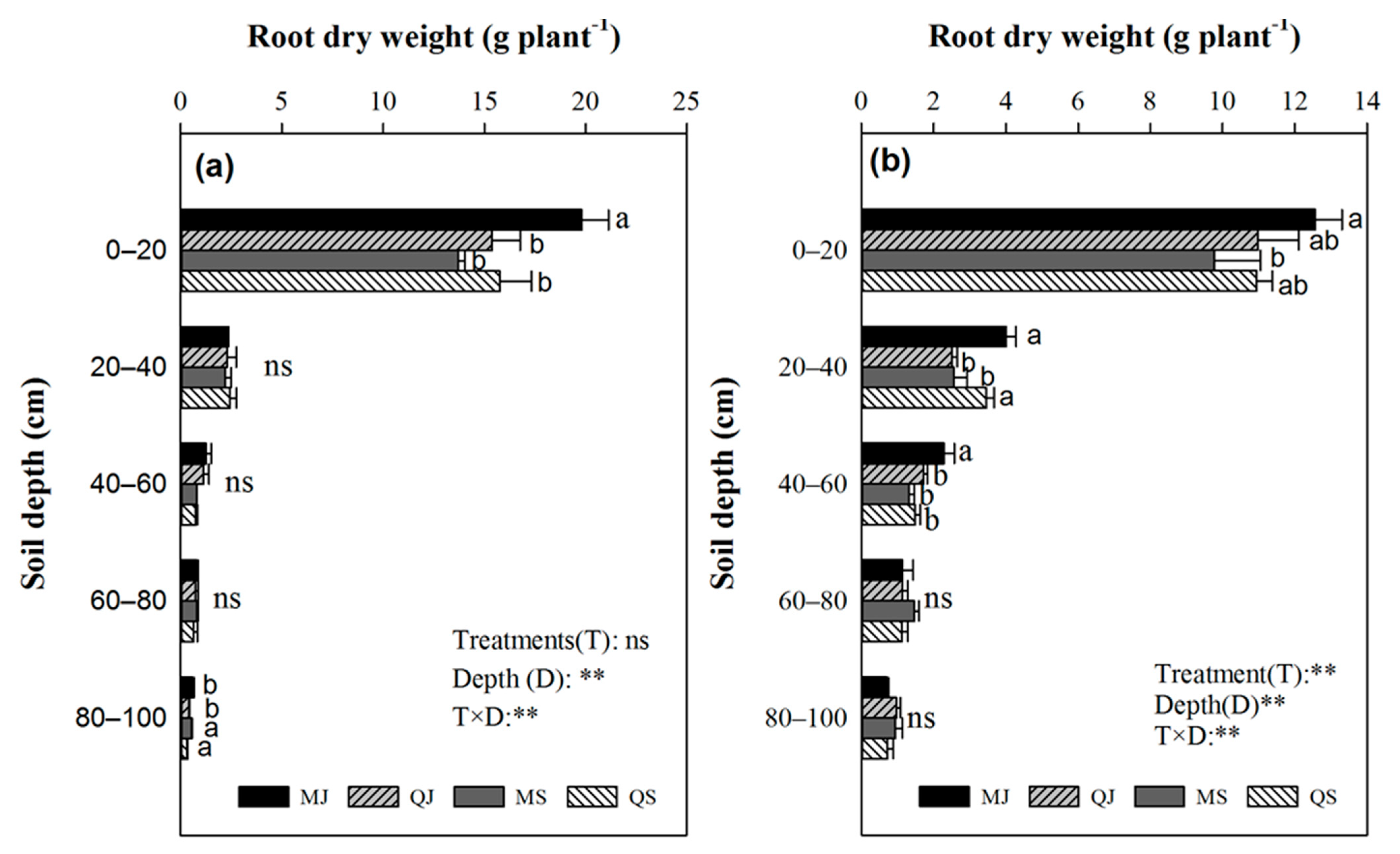
3.5. Tillage’s Effect on Root Dry Weight and Yield
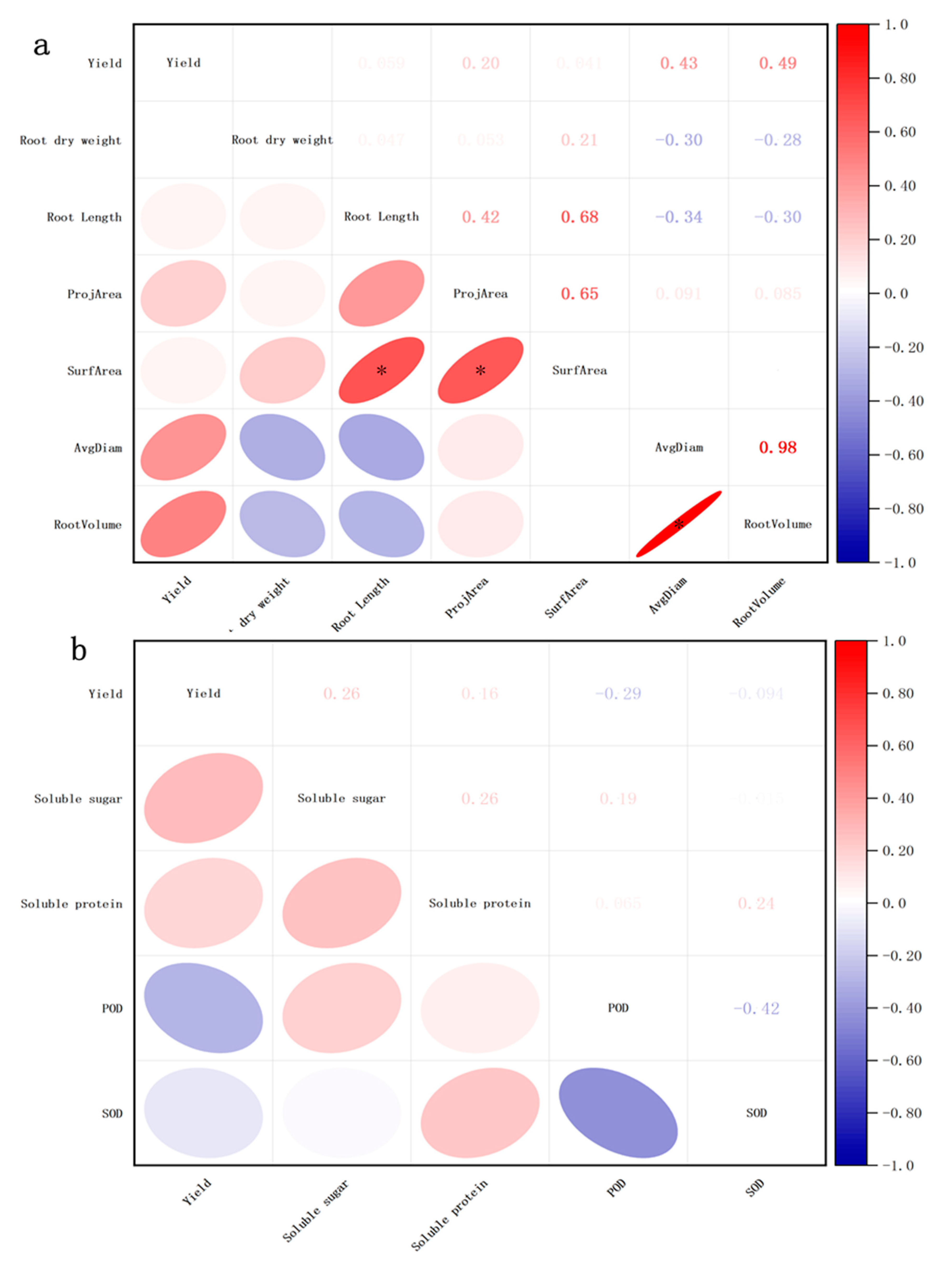
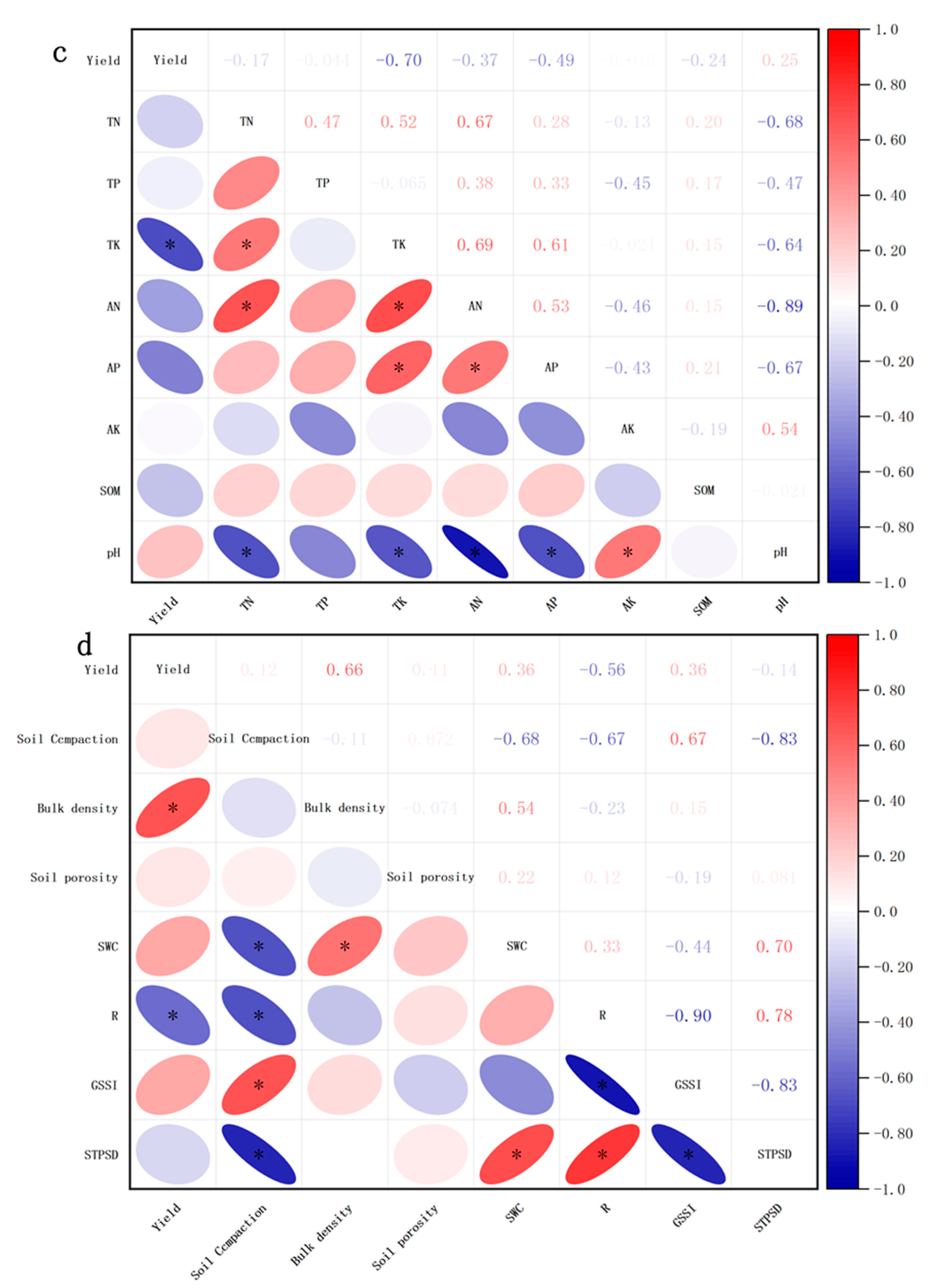
3.6. Correlation Analysis of Yield and Other Parameters
4. Discussion
4.1. Soil Physical Properties
4.2. Soil Nutrient Characteristics
4.3. Root Morphological and Physiological Traits
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, Q.; Li, G.; Yang, F.; Cui, Z.; Zhang, E.; Yang, F. Eleven-year mulching and tillage practices alter the soil quality and bacterial community composition in Northeast China. Arch. Agron. Soil Sci. 2022, 68, 1274–1289. [Google Scholar] [CrossRef]
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Cai, D.X.; Hoogmoed, W.B.; Oenema, O.; Perdok, U.D. Developments in conservation tillage in rain-fed regions of North China. Soil Tillage Res. 2007, 93, 239–250. [Google Scholar] [CrossRef]
- Sharma, A.R.; Biswas, A.K. Benefits and Limitations. In Conservation Agriculture in India: A Paradigm Shift for Sustainable Production; Taylor & Francis: London, UK, 2022. [Google Scholar]
- Smil, V. Who Will Feed China? China Q. 1995, 143, 801–813. [Google Scholar] [CrossRef]
- Liu, J.; Lu, X.S. Study on Sustainable Development Strategy of China: Sustainable Utilization of Land Resource; China Agricultural Press: Beijing, China, 2001. [Google Scholar]
- da Silva, G.F.; Calonego, J.C.; Luperini, B.C.O.; Chamma, L.; Alves, E.R.; Rodrigues, S.A.; Putti, F.F.; da Silva, V.M.; de Almeida Silva, M. Soil—Plant Relationships in Soybean Cultivated under Conventional Tillage and Long-Term No-Tillage. Agronomy 2022, 12, 697. [Google Scholar] [CrossRef]
- Basamba, T.A.; Amézquita, E.; Singh, B.R.; Rao, I.M. Effects of tillage systems on soil physical properties, root distribution and maize yield on a Colombian acid-savanna Oxisol. Acta Agric. Scand. 2006, 56, 255–262. [Google Scholar] [CrossRef]
- Lal, R. Residue management, conservation tillage and soil restoration for mitigating greenhouse effect by CO2 enrichment. Soil Tillage Res. 1997, 43, 81107. [Google Scholar] [CrossRef]
- Fabrizzi, K.P.; Garc, A.F.O.; Costa, J.L.; Picone, L.I. Soil water dynamics, physical properties and corn and wheat responses to minimum and no-tillage systems in the southern pampas of argentina. Soil Tillage Res. 2005, 81, 57–69. [Google Scholar] [CrossRef]
- Bala, M.I.; Zakharchenko, E. Which ways of soil tillage are the best for crops? In Proceedings of the Scientific-Practical Conference II International Scientific and Theoretical Conference «Science of XXI Century: Development, Main Theories and Achievements», Helsinki, Finland, 24 June 2022; Volume 1, pp. 80–82. [Google Scholar]
- Gebhardt, M.R.; Daniel, T.C.; Schweizer, E.E.; Allmaras, R.R. Conservation Tillage. Science 1985, 230, 625–630. [Google Scholar] [CrossRef]
- Blevins, R.L.; Smith, M.S.; Thomas, G.W.; Frye, W.W. Influence of conservation tillage on soil properties. J. Soil Water Conserv. 1983, 38, 301–305. [Google Scholar]
- Peigné, J.; Ball, B.C.; Roger-Estrade, J.; David, C. Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 2007, 23, 129–144. [Google Scholar] [CrossRef]
- Koller, K. Techniques of soil tillage. In Soil Tillage in Agroeco-Systems; El Titi, A., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–25. [Google Scholar]
- Johnson, A.M.; Hoyt, G.D. Changes to the soil environment under conservation tillage. HortTechnology 1999, 9, 380–393. [Google Scholar] [CrossRef]
- McVay, K.A.; Radcliffe, D.E.; Hargrove, W.L. Winter legume effects on soil properties and nitrogen fertilizer requirements. Soil Sci. Soc. Amer. J. 1989, 53, 1856–1862. [Google Scholar] [CrossRef]
- Wilson, G.F.; Lal, R.; Okigbo, B.N. Effects of cover crops on soil structure and on yield of subsequent arable crops grown under strip tillage on an eroded alfisol. Soil Tillage Res. 1982, 2, 223–250. [Google Scholar] [CrossRef]
- Frye, W.W.; Herbek, J.H.; Blevins, R.L. Legume cover crops in production of notillage corn. In Environmentally Sound Agriculture: Selected Papers from the 4th International Conference of the International Federation of Organic Agriculture Movements; Praeger Scientific: Cambridge, MA, USA, 1982; pp. 179–191. [Google Scholar]
- Aziz, I.; Mahmood, T.; Islam, K.R. Effect of long term no-till and conventional tillage practices on soil quality. Soil Tillage Res. 2013, 131, 28–35. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, J.; Gao, S.; Li, T.; Li, Y.; Vinay, N.; Mo, F.; Liao, Y.; Wen, X. Conservation tillage enhances crop productivity and decreases soil nitrogen losses in a rain-fed agroecosystem of the Loess Plateau, China. J. Clean. Prod. 2020, 274, 122854. [Google Scholar] [CrossRef]
- Pires, L.F.; Borges JA, R.; Rosa, J.A.; Coope, M.; Heck, R.J.; Passoni, S.; Roque, W.L. Soil structure changes induced by tillage systems. Soil Tillage Res. 2017, 165, 66–79. [Google Scholar] [CrossRef]
- Or, D.; Ghezzehei, T.A. Modeling post-tillage soil structural dynamics: A review. Soil Tillage Res. 2002, 64, 41–59. [Google Scholar] [CrossRef]
- Vian, J.F.; Peigne, J.; Chaussod, R.; Roger-Estrade, J. Effects of four tillage systems on soil structure and soil microbial biomass in organic farming. Soil Use Manag. 2009, 25, 1–10. [Google Scholar] [CrossRef]
- Parkhomenko, G.; Kambulov, S.; Olshevskaya, A.; Babadzhanyan, A.; Gucheva, N.; Mekhantseva, I. The tillage effect on the change of soil structure[C]//IOP Conference Series: Earth and Environmental Science. IOP Publ. 2019, 403, 012144. [Google Scholar]
- Liu, Z.; Cao, S.; Sun, Z.; Wang, H.; Qu, S.; Lei, N.; He, J.; Dong, Q. Tillage effects on soil properties and crop yield after land reclamation. Sci. Rep. 2021, 11, 4611. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Johnson, H.P. The effect of tillage systems on pesticide in runoff from small watersheds. Trans. ASAE 1979, 22, 554–559. [Google Scholar] [CrossRef]
- Ferreras, L.; Costa, J.; Garcia, F.; Pecorari, C. Effect of no-tillage on some soil physical properties of a structural degraded Petrocalcic Paleudoll of the southern “Pampa” of Argentina. Soil Tillage Res. 2000, 54, 31–39. [Google Scholar] [CrossRef]
- Abascal, S.A.; Buschiazzo, D.E. Competition between wheat and weeds in two tillage and fertilization systems in the central pampas of argentina. Agrochim. Pisa 2005, 49, 1–9. [Google Scholar]
- Zhang, Y.; Tan, C.; Wang, R.; Li, J.; Wang, X. Conservation tillage rotation enhanced soil structure and soil nutrients in long-term dryland agriculture. Eur. J. Agron. 2021, 131, 126379. [Google Scholar] [CrossRef]
- Lal, R. Tillage and agricultural sustainability. Soil Tillage Res. 1991, 20, 133–146. [Google Scholar] [CrossRef]
- McGarry, D. Tillage and soil compaction. In Conservation Agriculture; Springer: Dordrecht, The Netherlands, 2003; pp. 307–316. [Google Scholar]
- Maurya, P.R.; Lal, R. Effects of no-tillage and ploughing on roots of maize and leguminous crops. Exp. Agric. 1980, 16, 185–193. [Google Scholar] [CrossRef]
- Dal Ferro, N.; Sartori, L.; Simonetti, G.; Berti, A.; Morari, F. Soil macro-and microstructure as affected by different tillage systems and their effects on maize root growth. Soil Tillage Res. 2014, 140, 55–65. [Google Scholar] [CrossRef]
- Lipiec, J.; Horn, R.; Pietrusiewicz, J.; Siczek, A. Effects of soil compaction on root elongation and anatomy of different cereal plant species. Soil Tillage Res. 2012, 121, 74–81. [Google Scholar] [CrossRef]
- Guan, D.; Al-Kaisi, M.M.; Zhang, Y.; Duan, L.; Tan, W.; Zhang, M.; Li, Z. Tillage practices affect biomass and grain yield through regulating root growth, root-bleeding sap and nutrients uptake in summer maize. Field Crop. Res. 2014, 157, 89–97. [Google Scholar] [CrossRef]
- Feng, X.; Hao, Y.; Latifmanesh, H.; Rattan, L.; Cao, T.; Guo, J.; Deng, A.; Song, Z.; Zhang, W. Effects of subsoiling tillage on soil properties, maize root distribution, and grain yield on mollisols of Northeastern China. Agron. J. 2018, 110, 1607–1615. [Google Scholar] [CrossRef]
- Himmelbauer, M.L.; Sobotik, M.; Loiskandl, W. No-tillage farming, soil fertility and maize root growth. Arch. Agron. Soil Sci. 2012, 58 (Suppl. 1), S151–S157. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, L.; Wang, Y.; Ruan, R.; Xiong, P.; Peng, X.H. Bio-tillage improves soil physical properties and maize growth in a compacted Vertisol by cover crops. Soil Sci. Soc. Am. J. 2022, 86, 324–337. [Google Scholar] [CrossRef]
- Farahani, E.; Emami, H.; Forouhar, M. Effects of tillage systems on soil organic carbon and some soil physical properties. Land. Degrad. Dev. 2022, 33, 1307–1320. [Google Scholar] [CrossRef]
- Bhuyan, S.I.; Chakma, B.; Laskar, I. Degradation of soil physical properties due to modernization of tillage techniques: A recent man made crisis to agro-ecology in North East India. Proc. Int. Acad. Ecol. Environ. Sci. 2022, 12, 113–119. [Google Scholar]
- Lv, L.; Gao, Z.; Liao, K.; Zhu, Q.; Zhu, J.J. Impact of conservation tillage on the distribution of soil nutrients with depth. Soil Tillage Res. 2023, 225, 105527. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P. Reducing and eliminating tillage in order to promote sustainable agriculture. He Pharma Innov. J. 2022, SP-11, 183–188. [Google Scholar]
- Upa, J. The effect of previous crop soil cultivation on the yield of grain maize and winter wheat in the drier area of southern moravia. Rostl. Vyrob. 2000, 46, 113–117. [Google Scholar]
- Chen, S.; Yang, P.; Zhang, Y.; Dong, W.; Hu, C.; Oenema, O. Responses of cereal yields and soil carbon sequestration to four long-term tillage practices in the North China plain. Agronomy 2022, 12, 176. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Vera, C.L.; Malhi, S.S. Effects of water deficit and supplemental irrigation on winter wheat growth, grain yield and quality, nutrient uptake, and residual mineral nitrogen in soil. Commun. Soil Sci. Plant Anal. 2015, 36, 1405–1419. [Google Scholar] [CrossRef]
- Cai, L.; Guo, Z.; Zhang, J.; Liu, X. No tillage and residue mulching method on bacterial community diversity regulation in a black soil region of Northeastern China. PLoS ONE 2021, 16, e0256970. [Google Scholar] [CrossRef] [PubMed]
- Strudley, M.W.; Green, T.R.; James, I.I. Tillage effects on soil hydraulic properties in space and time: State of the science. Soil Tillage Res. 2008, 99, 4–48. [Google Scholar] [CrossRef]
- Hill, R.L.; Cruse, R.M. Tillage effects on bulk density and soil strength of two Mollisols. Soil Sci. Soc. Am. J. 1985, 49, 1270–1273. [Google Scholar] [CrossRef]
- Logsdon, S.D.; Karlen, D.L. Bulk density as a soil quality indicator during conversion to no-tillage. Soil Tillage Res. 2004, 78, 143–149. [Google Scholar] [CrossRef]
- Anderson, E.L. Corn root growth and distribution as influenced by tillage and nitrogen fertilization. Agron. J. 1987, 79, 544–549. [Google Scholar] [CrossRef]
- Barber, S.A. Effect of tillage practice on corn (Zea mays L.) root distribution and morphology. Agron. J. 1971, 63, 724–726. [Google Scholar] [CrossRef]
- Qin, R.; Noulas, C.; Herrera, J.M. Morphology and distribution of wheat and maize roots as affected by tillage systems and soil physical parameters in temperate climates: An overview. Arch. Agron. Soil Sci. 2018, 64, 747–762. [Google Scholar] [CrossRef]
- Mosaddeghi, M.R.; Mahboubi, A.A.; Safadoust, A. Short-term effects of tillage and manure on some soil physical properties and maize root growth in a sandy loam soil in western Iran. Soil Tillage Res. 2009, 104, 173–179. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, Y.; Mu, X.; Liu, K.; Li, C. Effects of tillage on soil physical properties and root growth of maize in loam and clay in central China. Plant Soil Environ. 2013, 59, 295–302. [Google Scholar] [CrossRef]
- Thierfelder, C.; Matemba-Mutasa, R.; Rusinamhodzi, L. Yield response of maize (Zea mays L.) to conservation agriculture cropping system in Southern Africa. Soil Tillage Res. 2015, 146, 230–242. [Google Scholar] [CrossRef]
- Rashidi, M.; Keshavarzpour, F. Effect of different tillage methods on grain yield and yield components of maize (Zea mays L.). Int. J. Agric. Biol. 2007, 9, 274–277. [Google Scholar]
- Aikins, S.H.M.; Afuakwa, J.J.; Owusu-Akuoko, O. Effect of four different tillage practices on maize performance under rain-fed conditions. Agric. Biol. J. N. Am. 2012, 3, 25–30. [Google Scholar] [CrossRef]
- Hussain, I.; Olson, K.R.; Ebelhar, S.A. Impacts of tillage and no-till on production of maize and soybean on an eroded Illinois silt loam Soil. Soil Tillage Res. 1999, 52, 37–49. [Google Scholar] [CrossRef]
- Khurshid, K.; Iqbal, M.; Arif, M.S.; Nawaz, A. Effect of tillage and mulch on soil physical properties and growth of maize. Int. J. Agric. Biol. 2006, 8, 593–596. [Google Scholar]
- Kladivko, E.J. Tillage systems and soil ecology. Soil Tillage Res. 2001, 61, 61–76. [Google Scholar] [CrossRef]
- Grewal, K.S.; Singh, D.; Mehta, S.C.; Singh, Y.P.; Kuhad, M.S. Effect of long-term fertilizer application on crop yields, nutrient uptake and available soil nutrients under different cropping sequences. Crop Res. 2000, 20, 426–436. [Google Scholar]
- Haruna, S.I.; Anderson, S.H.; Udawatta, R.P.; Glark, J.J.; Phillips, N.C.; Cui, S.; Gao, Y. Improving soil physical properties through the use of cover crops: A review. Agrosystems Geosci. Environ. 2020, 3, e20105. [Google Scholar] [CrossRef]
- Comegna, V.; Ruggiero, C.; Amato, M.; D’Anna, F.; Sommella, A.; Santini, A. Physical and hydrophysical properties of a vertic ustorthents soil of southern italy as affected by some cultivation systems. Agric. Mediterr. 1990, 120, 159–169. [Google Scholar]
- Haynes, R.J.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosystems 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Toor, M.D.; Adnan, M.; Rehman, F.U.; Tahir, R.; Pareek, V. Nutrients and their importance in agriculture crop production: A review. Ind. J. Pure App. Biosci. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Boincean, B.; Dent, D. Crop rotation. In Farming the Black Earth; Springer: Cham, Switzerland, 2019; pp. 89–124. [Google Scholar]
- Malobane, M.E.; Nciizah, A.D.; Mudau, F.N.; Wakindiki, I.I.C. Tillage, crop rotation and crop residue management effects on nutrient availability in a sweet sorghum-based cropping system in marginal soils of South Africa. Agronomy 2020, 10, 776. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Karlen, D.L.; Hurley, E.; Andrews, S.; Cambardella, C.; Meek, M.; Duffy, M. Crop rotation effects on soil quality at three northern corn/soybean locations. Staff. Gen. Res. Pap. Arch. 2006, 6, 1–6. [Google Scholar] [CrossRef]
- Li, F.R.; Zhao, W.Z.; Liu, J.L.; Huang, Z.G. Degraded vegetation and wind erosion influence soil carbon, nitrogen and phosphorus accumulation in sandy grasslands. Plant Soil 2009, 317, 79. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Singer, J.W. The Use of Cover Crops to Manage Soil. In Soil Management: Building a Stable Base for Agriculture; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 2011; pp. 321–337. [Google Scholar]
- Haruna, S.I.; Nkongolo, N.V. Influence of cover crop, tillage, and crop rotation management on soil nutrients. Agriculture 2020, 10, 225. [Google Scholar] [CrossRef]
- Ruffo, M.L.; Bollero, G.A. Modeling rye and hairy vetch residue decomposition as a function of degree days and decomposition days. Agron. J. 2020, 95, 900–907. [Google Scholar] [CrossRef]
- Smith, M.S.; Frye, W.W.; Varco, J.J. Legume winter cover crops. Adv. Soil Sci. 1987, 7, 95–139. [Google Scholar]
- Yang, J.; Zhang, H.; Zhang, J. Root morphology and physiology in relation to the yield formation of rice. J. Integr. Agric. 2012, 11, 920–926. [Google Scholar] [CrossRef]
- Richner, W.; Liedgens, M.; Bürgi, H.; Soldati, A.; Stamp, P. Root image analysis and interpretation. In Root Methods; Springer: Berlin/Heidelberg, Germany, 2000; pp. 305–341. [Google Scholar]
- Ghuman, B.S.; Sur, H.S. Tillage and residue management effects on soil properties and yields of rainfed maize and wheat in a subhumid subtropical climate. Soil Tillage Res. 2001, 58, 1–10. [Google Scholar] [CrossRef]
- Chassot, A. Early Growth of Roots and Shoots of Maize as Affected by Tillage-Induced Changes in Soil Physical Properties; ETH Zurich: Zürich, Switzerland, 2000. [Google Scholar]
- Ren, B.; Li, X.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J.W. Soil physical properties and maize root growth under different tillage systems in the North China Plain. Crop J. 2018, 6, 669–676. [Google Scholar] [CrossRef]
- Anderson, E.L. Tillage and N fertilization effects on maize root growth and root: Shoot ratio. Plant Soil 1988, 108, 245–251. [Google Scholar] [CrossRef]
- Cai, H.; Ma, W.; Zhang, X.; Ping, J.Q.; Yan, X.G.; Liu, J.Z.; Yuan, J.C.; Wang, L.C.; Ren, J. Effect of subsoil tillage depth on nutrient accumulation, root distribution, and grain yield in spring maize. Crop J. 2014, 2, 297–307. [Google Scholar] [CrossRef]
- DeFelice, M.S.; Carter, P.R.; Mitchell, S.B. Influence of tillage on corn and soybean yield in the United States and Canada. Crop Manag. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Raimbault, B.A.; Vyn, T.J. Crop rotation and tillage effects on corn growth and soil structural stability. Agron. J. 1991, 83, 979–985. [Google Scholar] [CrossRef]
- Miao, F.; Li, Y.; Cui, S.; Jagadamma, S.; Yang, G. Soil extracellular enzyme activities under long-term fertilization management in the croplands of China: A meta-analysis. Nutr. Cycl. Agroecosystem 2019, 114, 125–138. [Google Scholar] [CrossRef]



| VT | R1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Root Length (cm) | Root Proj. Area (cm2) | Root Surf. Area (cm2) | Root Avg. Diam. (mm) | Root Volume (cm3) | Root Length (cm) | Root Proj. Area (cm2) | Root Surf. Area (cm2) | Root Avg. Diam (mm) | Root Volume (cm3) | ||
| Year (Y) | 2016 | 1286.20 a | 47.00 a | 34.17 a | 0.76 b | 12.14 b | 1385.8 a | 47.51 a | 35.02 a | 0.75 b | 44.76 a |
| 2017 | 1051.14 b | 38.09 b | 27.59 b | 1.44 a | 62.91 a | 1059.5 b | 38.30 b | 27.63 b | 1.35 a | 18.40 b | |
| Depth (D) | 0–20 | 1512.00 a | 58.47 a | 42.51 a | 1.27 a | 42.72 b | 1693.5 a | 58.90 a | 43.12 a | 1.19 ab | 43.11 a |
| 20–40 | 1105.04 b | 38.91 b | 28.34 b | 0.72 b | 23.63 c | 1118.7 b | 39.06 b | 28.58 b | 0.71 d | 19.90 e | |
| 40–60 | 1089.93 c | 38.57 c | 28.02 c | 0.82 b | 18.25 d | 1111.0 b | 39.02 b | 28.51 b | 0.97 c | 29.89 c | |
| 60–80 | 1069.63 d | 38.39 cd | 27.83 cd | 1.42 a | 41.64 b | 1100.0 c | 38.87 bc | 28.29 c | 1.03 bc | 37.14 b | |
| 80–100 | 1066.75 d | 38.36 d | 27.73 d | 1.27 a | 61.38 a | 1089.6 d | 38.69 c | 28.12 d | 1.36 a | 27.87 d | |
| Treatment (T) | QJ | 1209.67 a | 42.65 a | 31.24 a | 0.97 b | 38.82 b | 1219.2 b | 42.88 b | 31.31 b | 1.05 a | 30.87 b |
| MJ | 1195.24 b | 42.61 a | 31.04 b | 1.30 a | 40.63 a | 1218.9 b | 42.82 b | 31.29 b | 1.09 a | 31.98 a | |
| MS | 1072.87 c | 42.42 ab | 30.59 c | 1.15 ab | 32.38 b | 1232.8 a | 43.08 a | 31.46 a | 1.03 a | 30.85 b | |
| QS | 1196.90 b | 42.49 b | 30.66 c | 0.99 b | 38.26 c | 1219.0 b | 42.85 b | 31.24 b | 1.03 a | 32.62 a | |
| analysis of variance | Y | * | * | * | * | * | * | * | * | * | * |
| D | * | * | * | * | * | * | * | * | * | * | |
| T | * | * | * | * | * | * | * | * | ns | * | |
| Y×D | * | * | * | * | * | * | * | * | * | * | |
| Y×T | * | * | * | * | * | ns | * | ns | ns | * | |
| D×T | * | ns | * | * | * | ns | ns | ns | ns | * | |
| Y×D×T | * | ns | ns | * | * | ns | ns | ns | ns | * | |
| Soluble Sugar % | Soluble Protein mg·g−¹ | POD u·g−¹ | SOD u·g−¹ | ||
|---|---|---|---|---|---|
| Year (Y) | 2016 | 0.003 a | 7.323 a | 330.14 a | 223.62 a |
| 2017 | 0.003 a | 8.254 a | 313.50 a | 270.42 a | |
| Stage (S) | VT | 0.003 a | 7.914 a | 327.52 a | 241.16 a |
| R1 | 0.003 a | 7.663 a | 316.12 a | 252.88 a | |
| Treatment (T) | QJ | 0.005 a | 7.634 bc | 309.13 b | 251.74 a |
| MJ | 0.002 bc | 8.503 bc | 267.04 b | 235.86 a | |
| MS | 0.003 bc | 11.204 a | 411.24 a | 260.27 a | |
| QS | 0.001 c | 3.815 c | 299.86 b | 240.22 a | |
| analysis of variance | Y | ns | ns | ns | ns |
| S | ns | ns | ns | ns | |
| T | * | * | * | ns | |
| Y×S | ns | ns | ns | * | |
| Y×T | ns | ns | ns | ns | |
| S×T | ns | ns | ns | * | |
| Y×S×T | ns | ns | ns | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Li, R.; Sui, P.; Wang, H.; Ren, Y.; Yuan, Y.; Tian, S.; Zhou, S.; Liu, W.; Luo, Y.; et al. Potential Mechanism of Optimal Tillage Layer Structure for Improving Maize Yield and Enhancing Root Growth in Northeast China. Land 2023, 12, 1798. https://doi.org/10.3390/land12091798
Zheng H, Li R, Sui P, Wang H, Ren Y, Yuan Y, Tian S, Zhou S, Liu W, Luo Y, et al. Potential Mechanism of Optimal Tillage Layer Structure for Improving Maize Yield and Enhancing Root Growth in Northeast China. Land. 2023; 12(9):1798. https://doi.org/10.3390/land12091798
Chicago/Turabian StyleZheng, Hongbing, Ruiping Li, Pengxiang Sui, Hao Wang, Ying Ren, Ye Yuan, Shengtao Tian, Siqi Zhou, Wuren Liu, Yang Luo, and et al. 2023. "Potential Mechanism of Optimal Tillage Layer Structure for Improving Maize Yield and Enhancing Root Growth in Northeast China" Land 12, no. 9: 1798. https://doi.org/10.3390/land12091798
APA StyleZheng, H., Li, R., Sui, P., Wang, H., Ren, Y., Yuan, Y., Tian, S., Zhou, S., Liu, W., Luo, Y., & Zheng, J. (2023). Potential Mechanism of Optimal Tillage Layer Structure for Improving Maize Yield and Enhancing Root Growth in Northeast China. Land, 12(9), 1798. https://doi.org/10.3390/land12091798







