Artificial Light at Night Reduces the Surface Activity of Earthworms, Increases the Growth of a Cover Crop and Reduces Water Leaching
Abstract
:1. Introduction
2. Materials and Methods
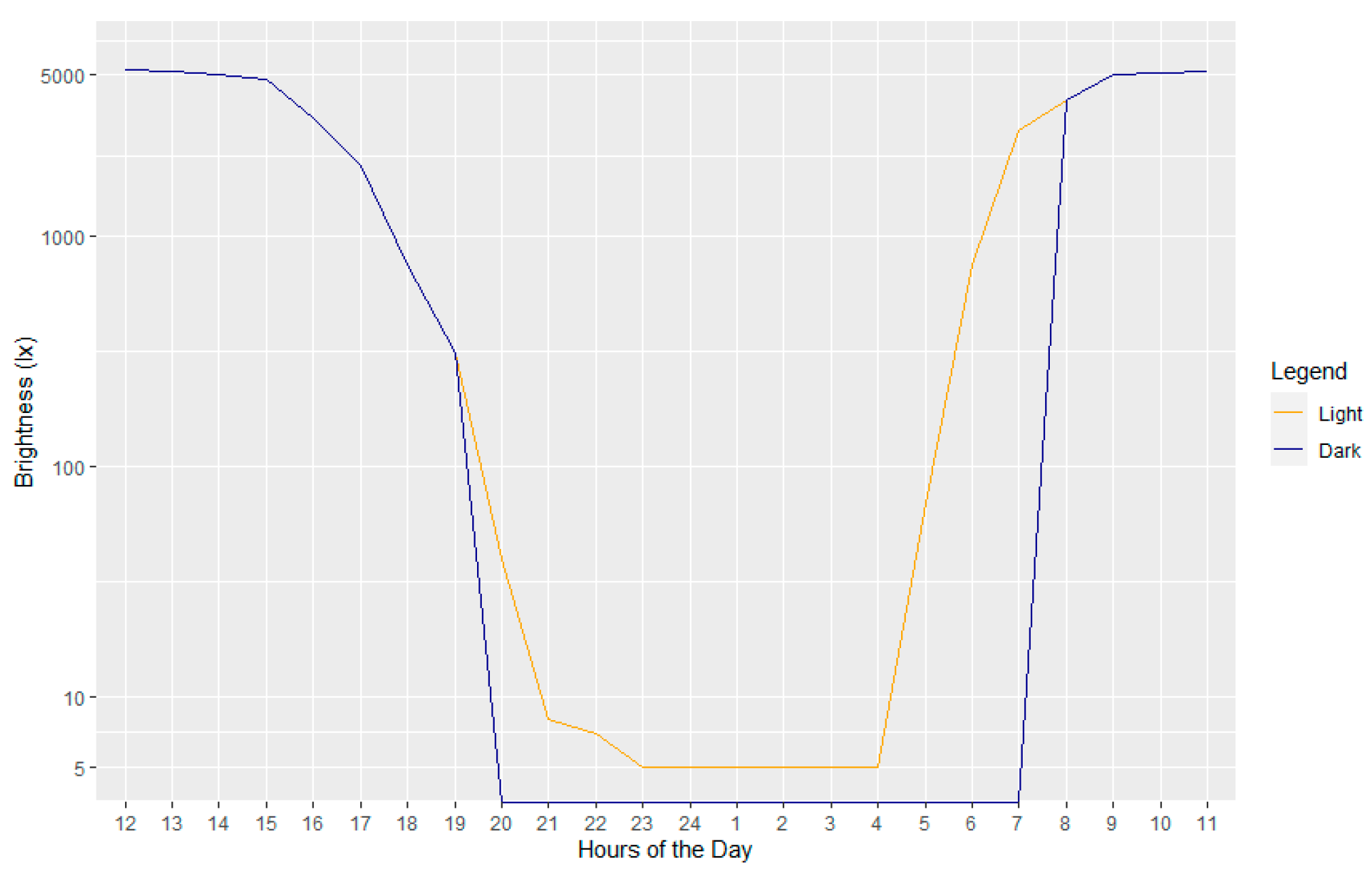
2.1. Experimental Setup
- ALAN: complete darkness (D) vs. artificial light pollution (L);
- Earthworms (EW): L. terrestris present (EW+) vs. absent (EW−);
- Plant species: sowing Phacelia tanacetifolia alone (P) vs. in combination with Artemisia artemisiifolia (M);
- Sowing depth: surface-sown (0) vs. 5 cm deep (5).
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Effects on Earthworm Surface Foraging Activity
3.2. Phacelia Germination and Growth
3.3. Effects on Water Infiltration and Leaching
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cinzano, P.; Falchi, F.; Elvidge, C.D.; Baugh, K.E. The artificial night sky brightness mapped from DMSP satellite Operational Linescan System measurements. Mon. Not. R. Astron. Soc. 2000, 318, 641–657. [Google Scholar] [CrossRef]
- Elgert, C.; Hopkins, J.; Kaitala, A.; Candolin, U. Reproduction under light pollution: Maladaptive response to spatial variation in artificial light in a glow-worm. Proc. Biol. Sci. 2020, 287, 20200806. [Google Scholar] [CrossRef] [PubMed]
- Longcore, T.; Rich, C. Ecological light pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Lian, X.; Jiao, L.; Zhong, J.; Jia, Q.; Liu, J.; Liu, Z. Artificial light pollution inhibits plant phenology advance induced by climate warming. Environ. Pollut. 2021, 291, 118110. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de Miguel, A.; Bennie, J.; Rosenfeld, E.; Dzurjak, S.; Gaston, K.J. First Estimation of Global Trends in Nocturnal Power Emissions Reveals Acceleration of Light Pollution. Remote Sens. 2021, 13, 3311. [Google Scholar] [CrossRef]
- Bennie, J.; Davies, T.W.; Duffy, J.P.; Inger, R.; Gaston, K.J. Contrasting trends in light pollution across Europe based on satellite observed night time lights. Sci. Rep. 2014, 4, 3789. [Google Scholar] [CrossRef] [PubMed]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef]
- Kyba, C.C.M.; Ruhtz, T.; Fischer, J.; Hölker, F. Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLoS ONE 2011, 6, e17307. [Google Scholar] [CrossRef]
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010, 25, 681–682. [Google Scholar] [CrossRef]
- Raap, T.; Pinxten, R.; Eens, M. Light pollution disrupts sleep in free-living animals. Sci. Rep. 2015, 5, 13557. [Google Scholar] [CrossRef]
- Eisenbeis, G.; Hänel, A. Light pollution and the imapct of artificial night lighting on insects. In Ecology of Cities and Towns: A Comparative Approach; McDonnel, M.J., Hahs, A.K., Breuste, J.H., Eds.; Cambridge University Press: New York, NY, USA, 2009; pp. 243–263. ISBN 978-0-511-65079-6. [Google Scholar]
- Macgregor, C.J.; Evans, D.M.; Fox, R.; Pocock, M.J.O. The dark side of street lighting: Impacts on moths and evidence for the disruption of nocturnal pollen transport. Glob. Chang. Biol. 2017, 23, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; Zoller, L.; Ryser, R.; Gerpe, C.; Hörler, M.; Fontaine, C. Artificial light at night as a new threat to pollination. Nature 2017, 548, 206–209. [Google Scholar] [CrossRef] [PubMed]
- van den Broeck, M.; de Cock, R.; van Dongen, S.; Matthysen, E. Blinded by the Light: Artificial Light Lowers Mate Attraction Success in Female Glow-Worms (Lampyris noctiluca L.). Insects 2021, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Kronfeld-Schor, N.; Dominoni, D.; de la Iglesia, H.; Levy, O.; Herzog, E.D.; Dayan, T.; Helfrich-Forster, C. Chronobiology by moonlight. Proc. Biol. Sci. 2013, 280, 20123088. [Google Scholar] [CrossRef] [PubMed]
- Bennie, J.; Davies, T.W.; Cruse, D.; Gaston, K.J. Ecological effects of artificial light at night on wild plants. J. Ecol. 2016, 104, 611–620. [Google Scholar] [CrossRef]
- Mittmannsgruber, M.; Kavassilas, Z.; Spangl, B.; Gruber, E.; Jagg, E.; Zaller, J.G. Artificial light at night reduces earthworm activity but increases growth of invasive ragweed. BMC Ecol. Evol. 2024, 24, 10. [Google Scholar] [CrossRef]
- Mcmunn, M.S.; Yang, L.H.; Ansalmo, A.; Bucknam, K.; Claret, M.; Clay, C.; Cox, K.; Dungey, D.R.; Jones, A.; Kim, A.Y.; et al. Artificial Light Increases Local Predator Abundance, Predation Rates, and Herbivory. Environ. Entomol. 2019, 48, 1331–1339. [Google Scholar] [CrossRef]
- Davies, T.W.; Bennie, J.; Gaston, K.J. Street lighting changes the composition of invertebrate communities. Biol. Lett. 2012, 8, 764–767. [Google Scholar] [CrossRef]
- Cesarz, S.; Eisenhauer, N.; Bucher, S.F.; Ciobanu, M.; Hines, J. Artificial light at night (ALAN) causes shifts in soil communities and functions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220366. [Google Scholar] [CrossRef]
- Nuutinen, V.; Butt, K.R.; Jauhiainen, L.; Shipitalo, M.J.; Sirén, T. Dew-worms in white nights: High-latitude light constrains earthworm (Lumbricus terrestris) behaviour at the soil surface. Soil Biol. Biochem. 2014, 72, 66–74. [Google Scholar] [CrossRef]
- Griffith, B.; Türke, M.; Weisser, W.W.; Eisenhauer, N. Herbivore behavior in the anecic earthworm species Lumbricus terrestris L.? Eur. J. Soil Biol. 2013, 55, 62–65. [Google Scholar] [CrossRef]
- Singh, J.; Schädler, M.; Demetrio, W.; Brown, G.G.; Eisenhauer, N. Climate change effects on earthworms—A review. Soil Org. 2019, 91, 114–138. [Google Scholar] [CrossRef] [PubMed]
- van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.J.; Brown, G.G.; de Deyn, G.B.; van Groenigen, K.J. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef] [PubMed]
- Forey, E.; Barot, S.; Decaëns, T.; Langlois, E.; Laossi, K.-R.; Margerie, P.; Scheu, S.; Eisenhauer, N. Importance of earthworm–seed interactions for the composition and structure of plant communities: A review. Acta Oecologica 2011, 37, 594–603. [Google Scholar] [CrossRef]
- Decaëns, T.; Mariani, L.; Betancourt, N.; Jiménez, J.J. Seed dispersion by surface casting activities of earthworms in Colombian grasslands. Acta Oecologica 2003, 24, 175–185. [Google Scholar] [CrossRef]
- Zaller, J.G.; Saxler, N. Selective vertical seed transport by earthworms: Implications for the diversity of grassland ecosystems. Eur. J. Soil Biol. 2007, 43, 86–91. [Google Scholar] [CrossRef]
- Schon, N.L.; Dominati, E.J. Valuing earthworm contribution to ecosystem services delivery. Ecosyst. Serv. 2020, 43, 101092. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Chang. Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Diallo, A.; Hoeffner, K.; Guillocheau, S.; Sorgniard, P.; Cluzeau, D. Combined effects of annual crop agricultural practices on earthworm communities. Appl. Soil Ecol. 2023, 192, 105073. [Google Scholar] [CrossRef]
- Crotty, F.V.; Stoate, C. The legacy of cover crops on the soil habitat and ecosystem services in a heavy clay, minimum tillage rotation. Food Energy Secur. 2019, 8, e00169. [Google Scholar] [CrossRef]
- Chami, B.; Niles, M.T.; Parry, S.; Mirsky, S.B.; Ackroyd, V.J.; Ryan, M.R. Incentive programs promote cover crop adoption in the northeastern United States. Agric. Environ. Lett. 2023, 8, e20114. [Google Scholar] [CrossRef]
- Euteneuer, P.; Wagentristl, H.; Steinkellner, S.; Fuchs, M.; Zaller, J.G.; Piepho, H.-P.; Butt, K.R. Contrasting effects of cover crops on earthworms: Results from field monitoring and laboratory experiments on growth, reproduction and food choice. Eur. J. Soil Biol. 2020, 100, 103225. [Google Scholar] [CrossRef]
- Roarty, S.; Hackett, R.A.; Schmidt, O. Earthworm populations in twelve cover crop and weed management combinations. Appl. Soil Ecol. 2017, 114, 142–151. [Google Scholar] [CrossRef]
- Bacq-Labreuil, A.; Crawford, J.; Mooney, S.J.; Neal, A.L.; Ritz, K. Cover crop species have contrasting influence upon soil structural genesis and microbial community phenotype. Sci. Rep. 2019, 9, 7473. [Google Scholar] [CrossRef] [PubMed]
- Bacq-Labreuil, A.; Crawford, J.; Mooney, S.J.; Neal, A.L.; Ritz, K. Phacelia (Phacelia tanacetifolia Benth.) affects soil structure differently depending on soil texture. Plant Soil 2019, 441, 543–554. [Google Scholar] [CrossRef]
- Gilbert, L. Phacelia tanacetifolia: A Brief Overview of a Potentially Useful Insectary Plant and Cover Crop. Available online: https://seriousaboutcamo.typepad.com/files/phacelia_farmer_version.pdf (accessed on 27 August 2024).
- Kliszcz, A.; Puła, J.; Możdżeń, K.; Tatoj, A.; Zandi, P.; Stachurska-Swakoń, A.; Barabasz-Krasny, B. Wider Use of Honey Plants in Farming: Allelopathic Potential of Phacelia tanacetifolia Benth. Sustainability 2023, 15, 3061. [Google Scholar] [CrossRef]
- Pinke, G.; Giczi, Z.; Vona, V.; Dunai, É.; Vámos, O.; Kulmány, I.; Koltai, G.; Varga, Z.; Kalocsai, R.; Botta-Dukát, Z.; et al. Weed Composition in Hungarian Phacelia (Phacelia tanacetifolia Benth.) Seed Production: Could Tine Harrow Take over Chemical Management? Agronomy 2022, 12, 891. [Google Scholar] [CrossRef]
- Butt, K.R.; Nuutinen, V.; Sirén, T. Resource distribution and surface activity of adult Lumbricus terrestris L. in an experimental system. Pedobiologia 2003, 47, 548–553. [Google Scholar] [CrossRef]
- R Core Team 2023; R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016; ISBN 9783319242774. [Google Scholar]
- Hess, W.N. Photoreceptors of Lumbricus terrestris, with special reference to their distribution, structure, and function. J. Morphol. 1925, 41, 63–93. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Peres, G.; Tondoh, J.E.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Monoshyn, D.; Chibesa, M.C.; Puschenreiter, M.; Zaller, J.G.; Santner, J. Impact of earthworms on soil Si availability and wheat Si concentration in low- and high-Si soils. Appl. Soil Ecol. 2024, 201, 105483. [Google Scholar] [CrossRef]
- Euteneuer, P.; Wagentristl, H.; Steinkellner, S.; Scheibreithner, C.; Zaller, J.G. Earthworms affect decomposition of soil-borne plant pathogen Sclerotinia sclerotiorum in a cover crop field experiment. Appl. Soil Ecol. 2019, 138, 88–93. [Google Scholar] [CrossRef]
- Stracey, C.M.; Wynn, B.; Robinson, S.K. Light Pollution Allows the Northern Mockingbird (Mimus polyglottos) to Feed Nestlings After Dark. Wilson J. Ornithol. 2014, 126, 366–369. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Fisichelli, N.A.; Frelich, L.E.; Reich, P.B. Interactive effects of global warming and ‘global worming’ on the initial establishment of native and exotic herbaceous plant species. Oikos 2012, 121, 1121–1133. [Google Scholar] [CrossRef]
- Zaller, J.G.; Arnone, J.A. Interactions between plant species and earthworm casts in a calcareous grassland under elevated CO2. Ecology 1999, 80, 873–881. [Google Scholar] [CrossRef]
- Liu, Y.; Heinen, R. Plant invasions under artificial light at night. Trends Ecol. Evol. 2024, 39, 703–705. [Google Scholar] [CrossRef]
- Milcu, A.; Schumacher, J.; Scheu, S. Earthworms (Lumbricus terrestris) affect plant seedling recruitment and microhabitat heterogeneity. Funct. Ecol. 2006, 20, 261–268. [Google Scholar] [CrossRef]
- Zaller, J.; Arnone, J. Earthworm and soil moisture effects on the productivity and structure of grassland communities. Soil Biol. Biochem. 1999, 31, 517–523. [Google Scholar] [CrossRef]
- Arnone, J.A.; Zaller, J.G. Earthworm effects on native grassland root system dynamics under natural and increased rainfall. Front. Plant Sci. 2014, 5, 152. [Google Scholar] [CrossRef]
- Agapit, C.; Gigon, A.; Puga-Freitas, R.; Zeller, B.; Blouin, M. Plant-earthworm interactions: Influence of age and proportion of casts in the soil on plant growth, morphology and nitrogen uptake. Plant Soil 2018, 424, 49–61. [Google Scholar] [CrossRef]
- Fründ, H.-C.; Graefe, U.; Tischer, S. Earthworms a Bioindicators of Soil Quality. In Biology of Earthworms; Karaca, A., Ed.; Springer: Berlin, Heidelberg, 2011; pp. 19–39. [Google Scholar]
- Bottinelli, N.; Henry-des-Tureaux, T.; Hallaire, V.; Mathieu, J.; Benard, Y.; Duc Tran, T.; Jouquet, P. Earthworms accelerate soil porosity turnover under watering conditions. Geoderma 2010, 156, 43–47. [Google Scholar] [CrossRef]
- Zaller, J.G.; Heigl, F.; Grabmaier, A.; Lichtenegger, C.; Piller, K.; Allabashi, R.; Frank, T.; Drapela, T. Earthworm-mycorrhiza interactions can affect the diversity, structure and functioning of establishing model grassland communities. PLoS ONE 2011, 6, e29293. [Google Scholar] [CrossRef] [PubMed]
- Zaller, J.G.; Wechselberger, K.F.; Gorfer, M.; Hann, P.; Frank, T.; Wanek, W.; Drapela, T. Subsurface earthworm casts can be important soil microsites specifically influencing the growth of grassland plants. Biol. Fertil. Soils 2013, 49, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Fonte, S.J.; Quintero, D.C.; Velásquez, E.; Lavelle, P. Interactive effects of plants and earthworms on the physical stabilization of soil organic matter in aggregates. Plant Soil 2012, 359, 205–214. [Google Scholar] [CrossRef]
- Görres, J.H.; Savin, M.C.; Amador, J.A. Soil micropore structure and carbon mineralization in burrows and casts of an anecic earthworm (Lumbricus terrestris). Soil Biol. Biochem. 2001, 33, 1881–1887. [Google Scholar] [CrossRef]
- Capowiez, Y.; Cadoux, S.; Bouchant, P.; Ruy, S.; Roger-Estrade, J.; Richard, G.; Boizard, H. The effect of tillage type and cropping system on earthworm communities, macroporosity and water infiltration. Soil Tillage Res. 2009, 105, 209–216. [Google Scholar] [CrossRef]
- Ehlers, W. Observations on earthworm channels and infiltration on tilled and untilled loess soil. Soil Sci. 1975, 119, 242–249. [Google Scholar] [CrossRef]
- Farenhorst, A.; Topp, E.; Bowman, B.; Tomlin, A. Earthworm burrowing and feeding activity and the potential for atrazine transport by preferential flow. Soil Biol. Biochem. 2000, 32, 479–488. [Google Scholar] [CrossRef]
- Munyankusi, E.; Gupta, S.C.; Moncrief, J.F.; Berry, E.C. Earthworm Macropores and Preferential Transport in a Long-Term Manure Applied Typic Hapludalf. J. Environ. Qual. 1994, 23, 773–784. [Google Scholar] [CrossRef]
- Brown, G.G.; Edwards, C.A.; Brussaard, L. How Earthworms Affect Plant Growth: Burrowing into the Mechanisms. In Earthworm Ecology, 2nd ed.; Edwards, C.A., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 13–49. ISBN 0-8493-1819-X. [Google Scholar]
- Edwards, C.A.; Arancon, N.Q. Biology and Ecology of Earthworms, 4th ed.; Springer: New York, NY, USA, 2022; ISBN 978-0-387-74942-6. [Google Scholar]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef]
- Craven, D.; Thakur, M.P.; Cameron, E.K.; Frelich, L.E.; Beauséjour, R.; Blair, R.B.; Blossey, B.; Burtis, J.; Choi, A.; Dávalos, A.; et al. The unseen invaders: Introduced earthworms as drivers of change in plant communities in North American forests (a meta-analysis). Glob. Chang. Biol. 2017, 23, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Frelich, L.E.; Hale, C.M.; Scheu, S.; Holdsworth, A.R.; Heneghan, L.; Bohlen, P.J.; Reich, P.B. Earthworm invasion into previously earthworm-free temperate and boreal forests. Biol. Invasions 2006, 8, 1235–1245. [Google Scholar] [CrossRef]
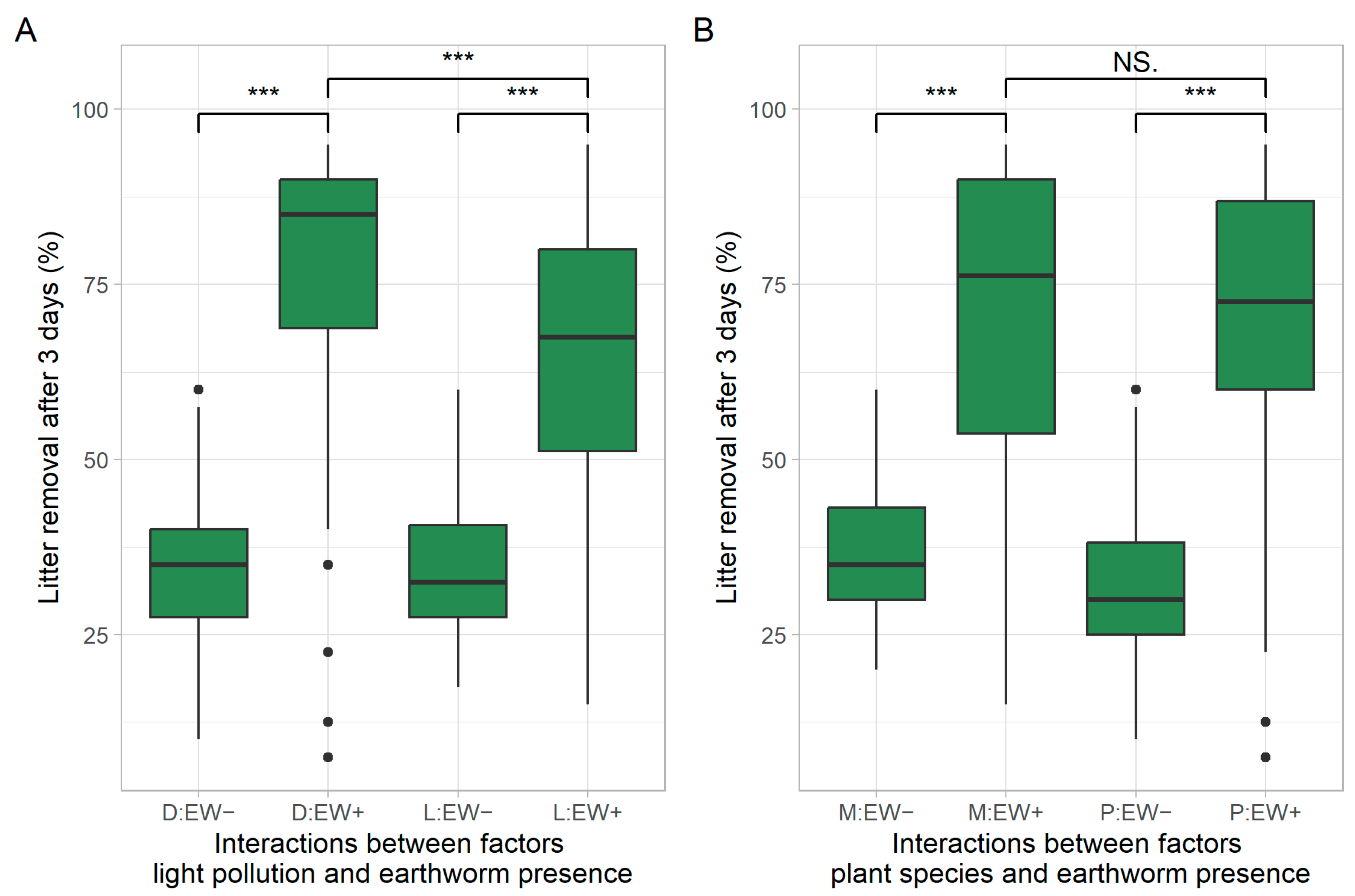

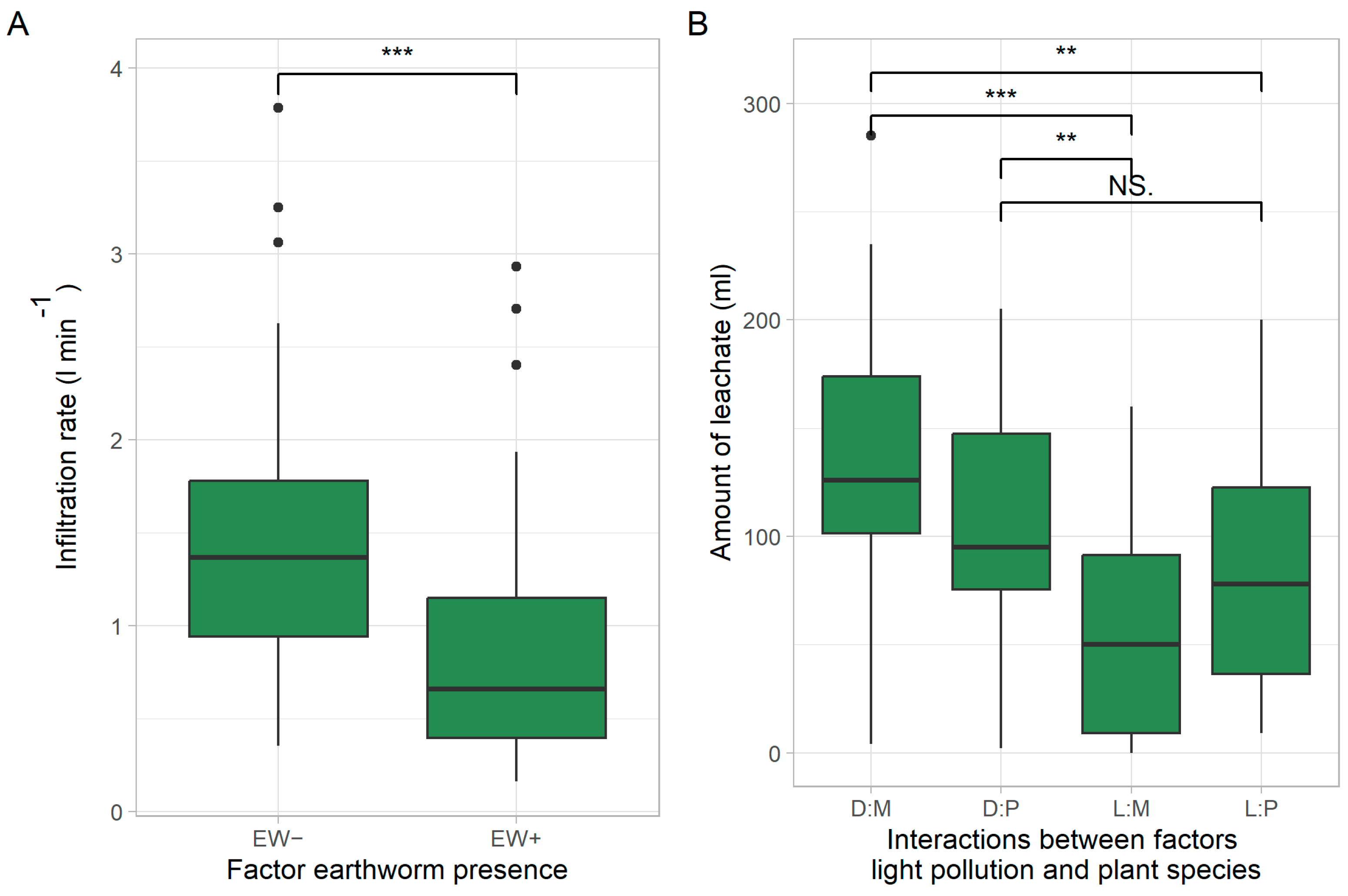
| Ground Litter Removal | ||||
|---|---|---|---|---|
| Parameter | Df | Sum Sq | F Value | Pr (>F) |
| ALAN | 1 | 1770 | 7.828 | 0.006 ** |
| Sowing depth | 1 | 41 | 0.181 | 0.671 |
| Plant species | 1 | 155 | 0.684 | 0.409 |
| Earthworms | 1 | 84,875 | 375.418 | <0.001 *** |
| Iteration | 2 | 537 | 1.188 | 0.307 |
| Soil moisture | 1 | 92 | 0.407 | 0.524 |
| Soil temperature | 1 | 747 | 3.305 | 0.070 |
| ALAN × sowing depth | 1 | 402 | 1.776 | 0.184 |
| ALAN × plant species | 1 | 4114 | 18.196 | <0.001 *** |
| ALAN × earthworms | 1 | 4067 | 17.988 | <0.001 *** |
| Sowing depth × plant species | 1 | 123 | 0.544 | 0.461 |
| Sowing depth × earthworms | 1 | 3 | 0.014 | 0.905 |
| Plant species × earthworms | 1 | 1012 | 4.476 | 0.035 * |
| Residuals | 255 | 57,651 | ||
| Germination (%) | Height (Mean cm pot−1) | Biomass (Mean g pot−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Df | Sum Sq | F Value | Pr (>F) | Df | Sum Sq | F Value | Pr (>F) | Df | Sum Sq | F Value | Pr (>F) |
| ALAN | 1 | 0.079 | 1.656 | 0.202 | 1 | 666.7 | 7.064 | 0.012 * | 1 | 0.001 | 0.117 | 0.735 |
| Sowing depth | 1 | 0.090 | 1.892 | 0.173 | 1 | 217.0 | 2.299 | 0.140 | 1 | 0.082 | 15.752 | <0.001 *** |
| Plant spp. | 1 | 0.040 | 0.846 | 0.361 | 1 | 7.1 | 0.075 | 0.786 | 1 | 0.021 | 3.952 | 0.056 |
| Earthworms (EW) | 1 | 0.485 | 10.154 | 0.002 ** | 1 | 209.3 | 2.217 | 0.147 | 1 | 0.026 | 5.076 | 0.031 * |
| Soil moisture | 1 | 0.094 | 1.980 | 0.163 | 1 | 387.6 | 4.107 | 0.051 | 1 | 0.112 | 21.607 | <0.001 *** |
| Soil temperature | 1 | 0.056 | 1.163 | 0.284 | 1 | 0.4 | 0.004 | 0.950 | 1 | 0.000 | 0.001 | 0.978 |
| ALAN × depth | 1 | 0.017 | 0.361 | 0.550 | 1 | 522.8 | 5.540 | 0.025 * | 1 | 0.022 | 4.194 | 0.049 * |
| ALAN × plant spp. | 1 | 0.014 | 0.301 | 0.585 | 1 | 32.5 | 0.345 | 0.561 | 1 | 0.004 | 0.836 | 0.368 |
| ALAN × EW | 1 | 0.007 | 0.145 | 0.704 | 1 | 1.3 | 0.014 | 0.907 | 1 | 0.006 | 1.074 | 0.308 |
| Depth × plant spp. | 1 | 0.041 | 0.864 | 0.355 | 1 | 27.2 | 0.288 | 0.595 | 1 | 0.040 | 7.715 | 0.009 ** |
| Depth × EW | 1 | 0.117 | 2.456 | 0.121 | 1 | 30.9 | 0.327 | 0.572 | 1 | 0.004 | 0.792 | 0.380 |
| Plant spp. × EW | 1 | 0.131 | 2.737 | 0.102 | 1 | 59.0 | 0.626 | 0.435 | 1 | 0.032 | 6.084 | 0.019 * |
| Residuals | 77 | 3.675 | 33 | 2925.5 | 31 | 0.161 | ||||||
| Water Infiltration (L min−1) | Leachate Amount (mL) | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Df | Sum Sq | F Value | Pr (>F) | Sum Sq | F Value | Pr (>F) |
| ALAN | 1 | 0.479 | 1.307 | 0.257 | 53,047 | 16.253 | <0.001 *** |
| Sowing depth | 1 | 1.297 | 3.541 | 0.064 | 27 | 0.008 | 0.927 |
| Plant spp. | 1 | 0.001 | 0.003 | 0.954 | 230 | 0.070 | 0.792 |
| Earthworms | 1 | 9.452 | 25.813 | <0.001 *** | 6644 | 2.036 | 0.158 |
| Soil moisture | 1 | 2.190 | 5.981 | 0.017 * | 1720 | 0.527 | 0.470 |
| Soil temperature | 1 | 0.577 | 1.575 | 0.213 | 3870 | 1.186 | 0.280 |
| ALAN × sowing depth | 1 | 0.156 | 0.425 | 0.517 | 10,695 | 3.277 | 0.074 |
| ALAN × plant spp. | 1 | 0.346 | 0.946 | 0.334 | 15,946 | 4.886 | 0.030 * |
| ALAN × earthworms | 1 | 0.015 | 0.040 | 0.842 | 1836 | 0.563 | 0.456 |
| Sowing depth × plant spp. | 1 | 0.412 | 1.126 | 0.292 | 2150 | 0.659 | 0.420 |
| Sowing depth × earthworms | 1 | 0.721 | 1.968 | 0.165 | 1780 | 0.545 | 0.463 |
| Plant spp. × earthworms | 1 | 0.814 | 2.223 | 0.140 | 7495 | 2.296 | 0.134 |
| Residuals | 77 | 28.197 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavassilas, Z.; Mittmannsgruber, M.; Gruber, E.; Zaller, J.G. Artificial Light at Night Reduces the Surface Activity of Earthworms, Increases the Growth of a Cover Crop and Reduces Water Leaching. Land 2024, 13, 1698. https://doi.org/10.3390/land13101698
Kavassilas Z, Mittmannsgruber M, Gruber E, Zaller JG. Artificial Light at Night Reduces the Surface Activity of Earthworms, Increases the Growth of a Cover Crop and Reduces Water Leaching. Land. 2024; 13(10):1698. https://doi.org/10.3390/land13101698
Chicago/Turabian StyleKavassilas, Zenia, Marion Mittmannsgruber, Edith Gruber, and Johann G. Zaller. 2024. "Artificial Light at Night Reduces the Surface Activity of Earthworms, Increases the Growth of a Cover Crop and Reduces Water Leaching" Land 13, no. 10: 1698. https://doi.org/10.3390/land13101698
APA StyleKavassilas, Z., Mittmannsgruber, M., Gruber, E., & Zaller, J. G. (2024). Artificial Light at Night Reduces the Surface Activity of Earthworms, Increases the Growth of a Cover Crop and Reduces Water Leaching. Land, 13(10), 1698. https://doi.org/10.3390/land13101698










