The Pollen Representation of Vegetation and Climate Along an Altitudinal Gradient on the Eastern Tibetan Plateau
Abstract
:1. Introduction
2. Materials and Methods
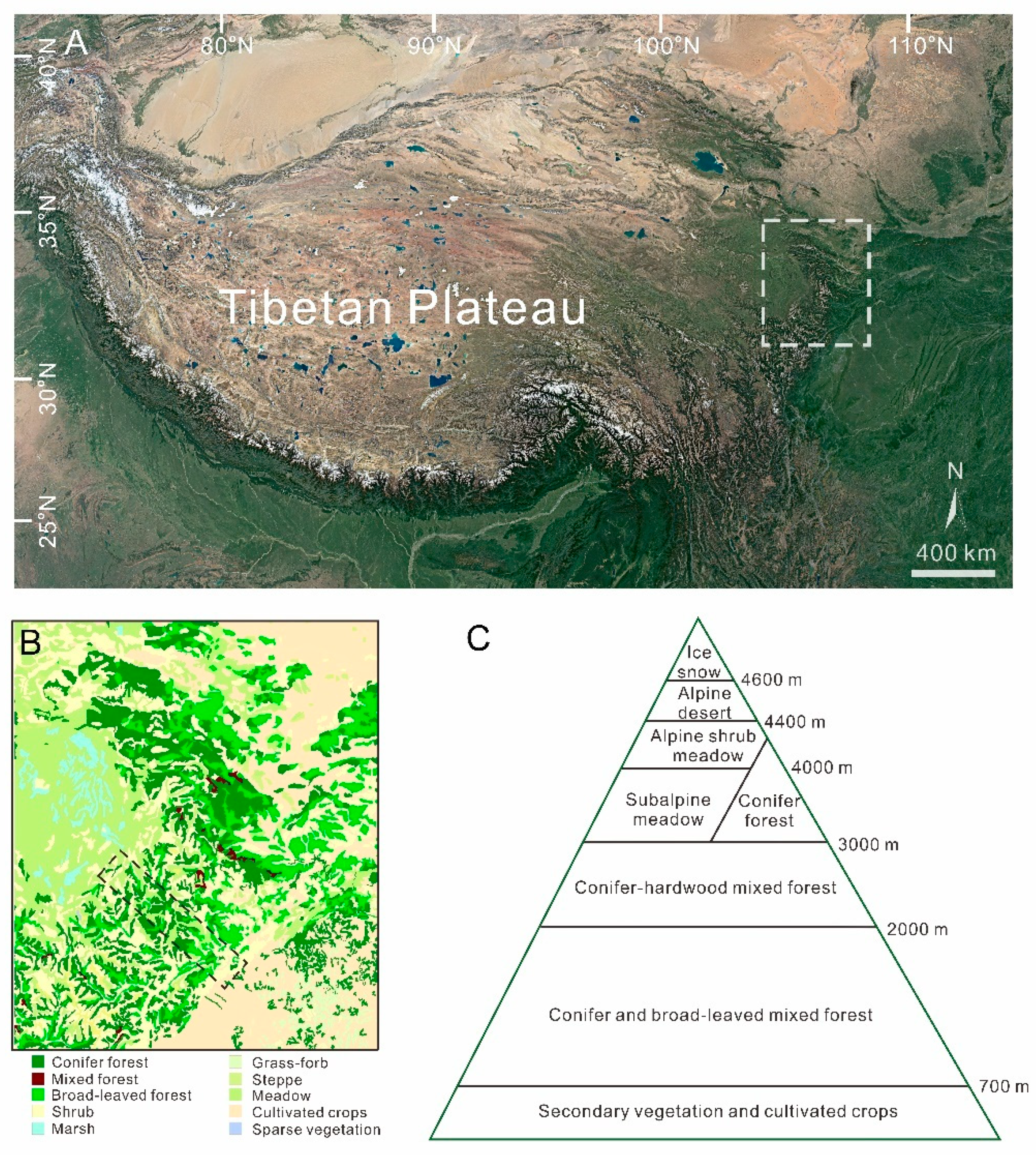
2.1. Regional Setting
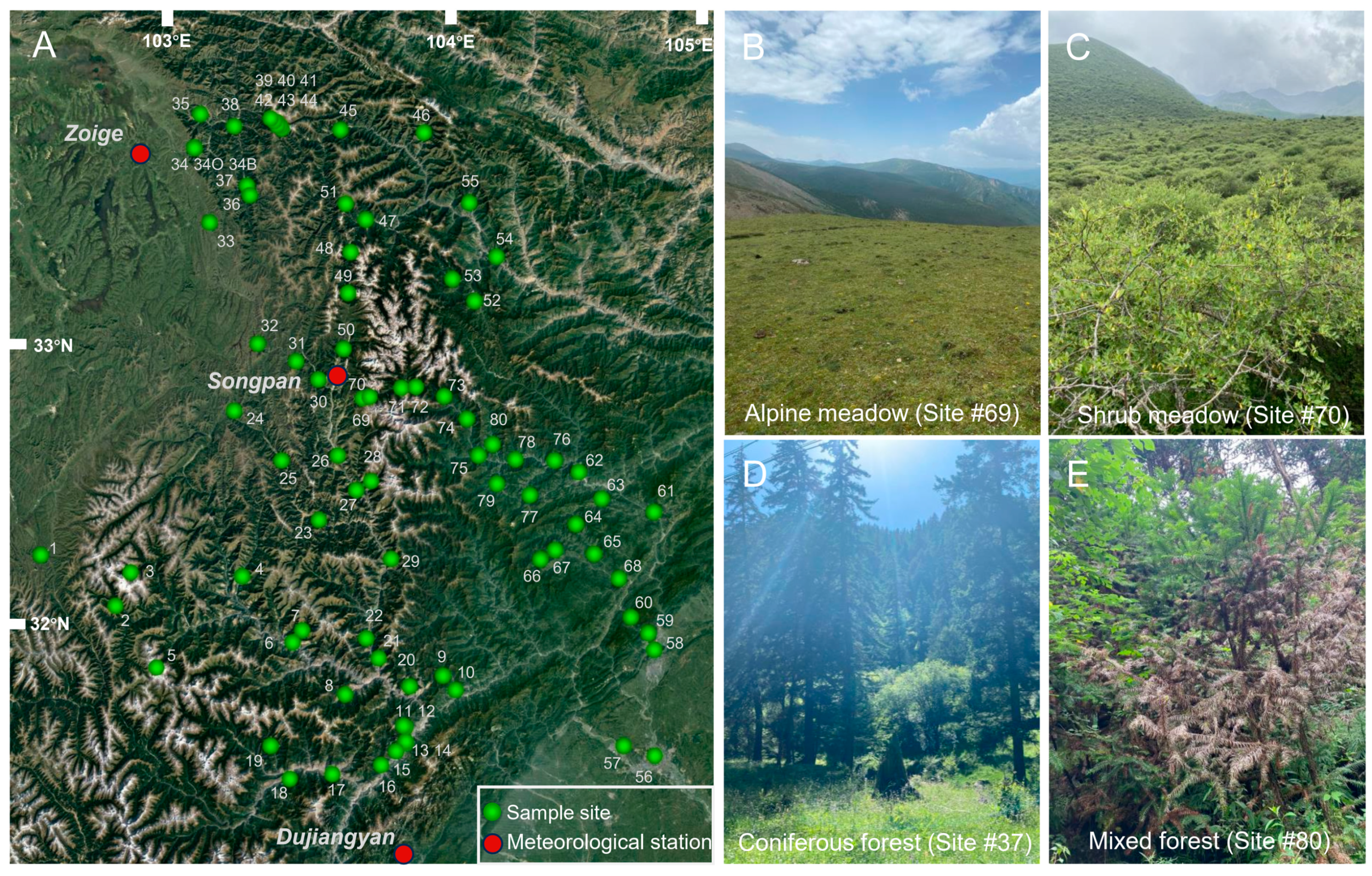
2.2. Sample Collection and Vegetation Survey
2.3. Pollen Analysis and Data Processing
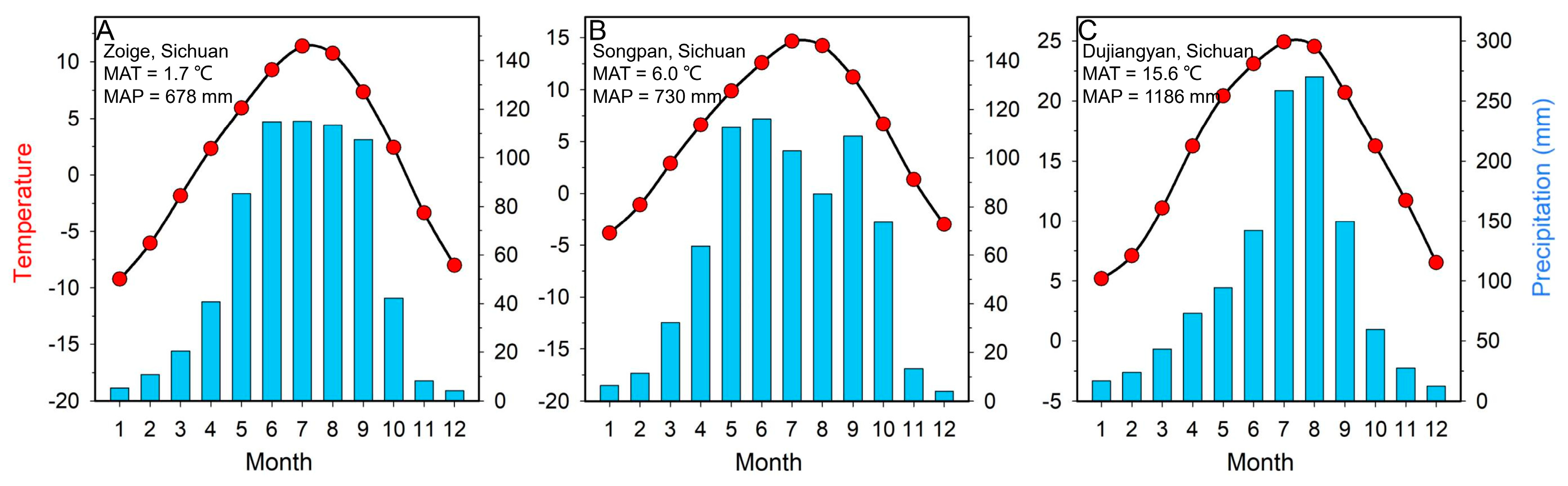
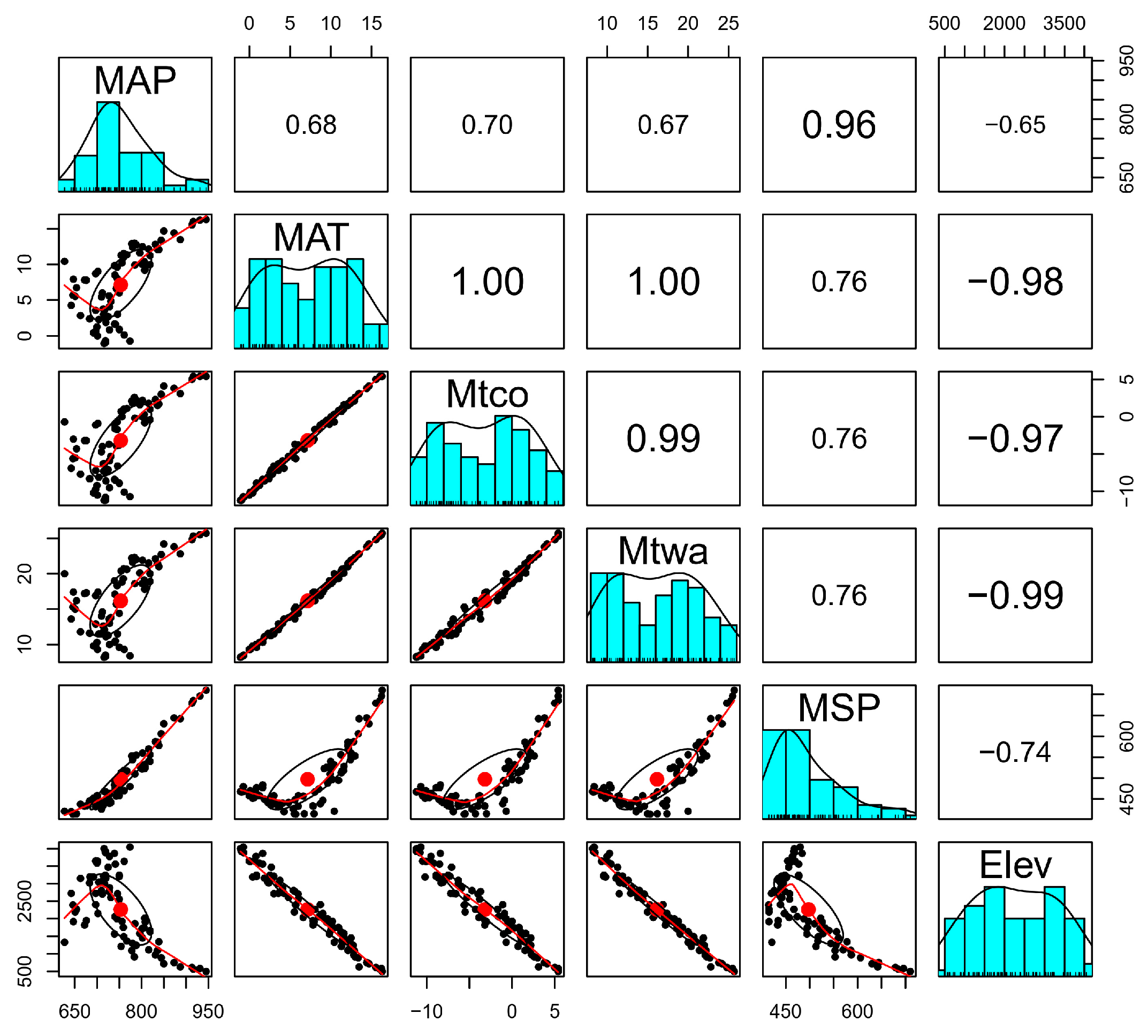
2.4. Modern Climate Data
2.5. Multivariate Analyses
3. Results
3.1. Vegetation Investigation Results
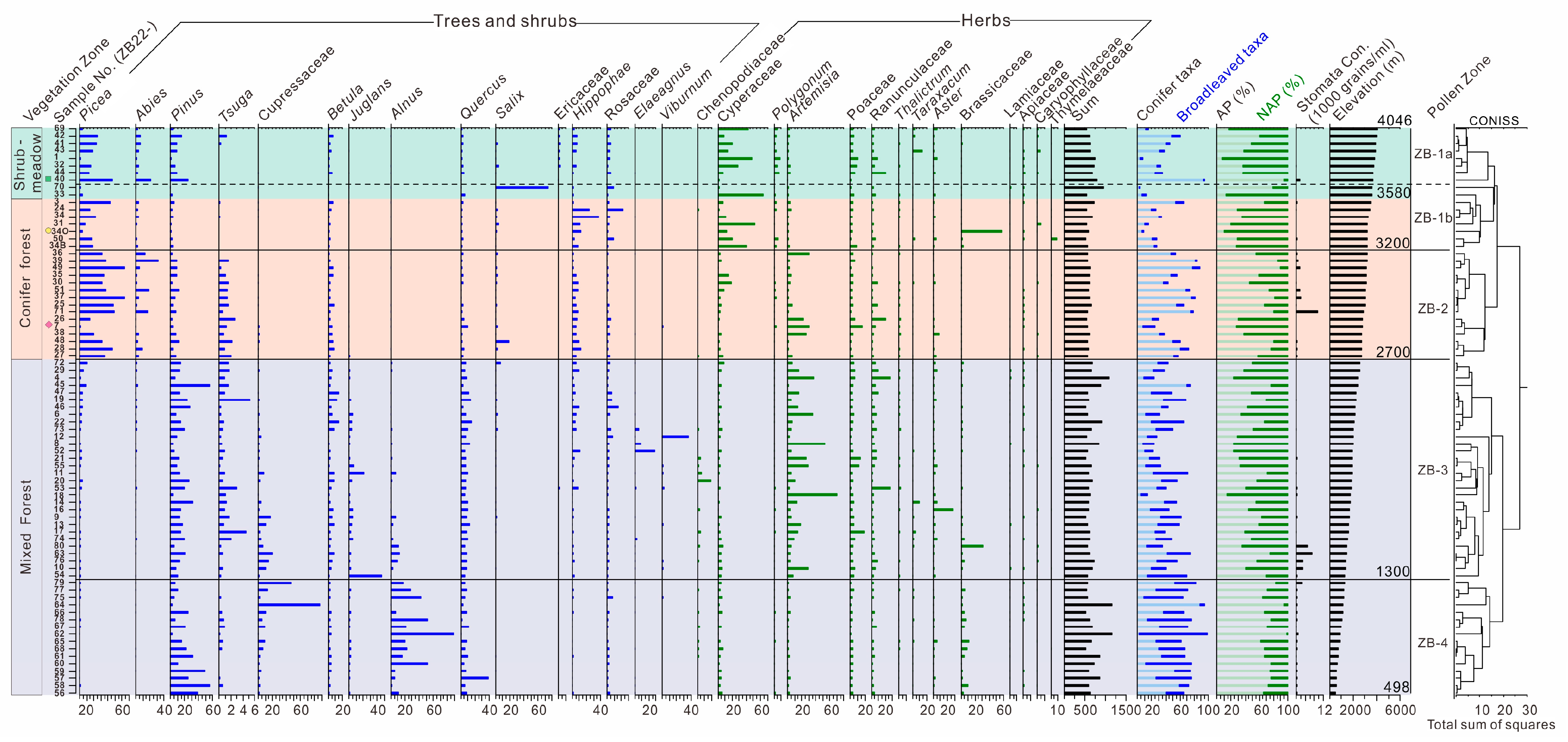
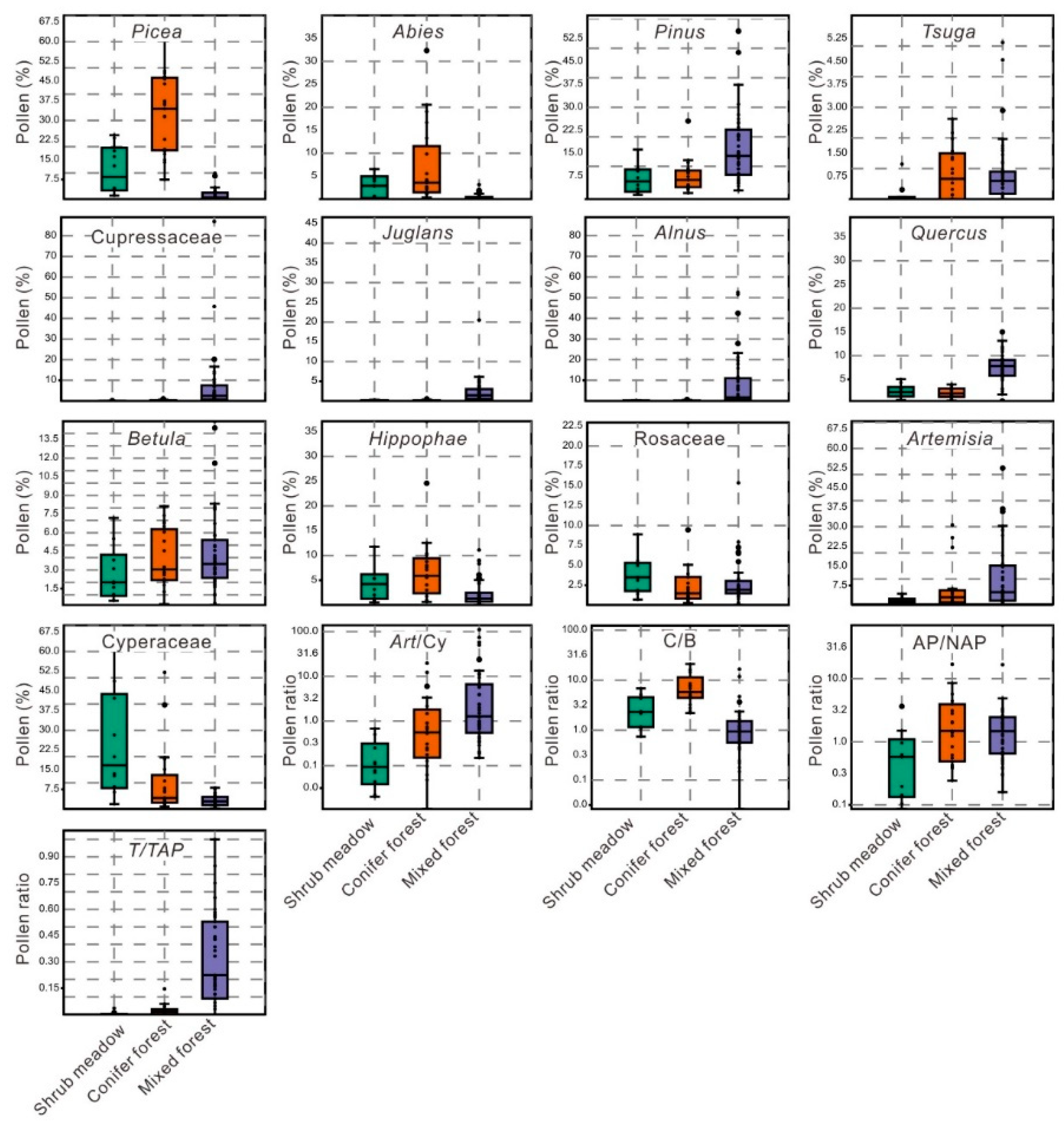
3.2. Pollen Assemblages in the Three Vegetation Types
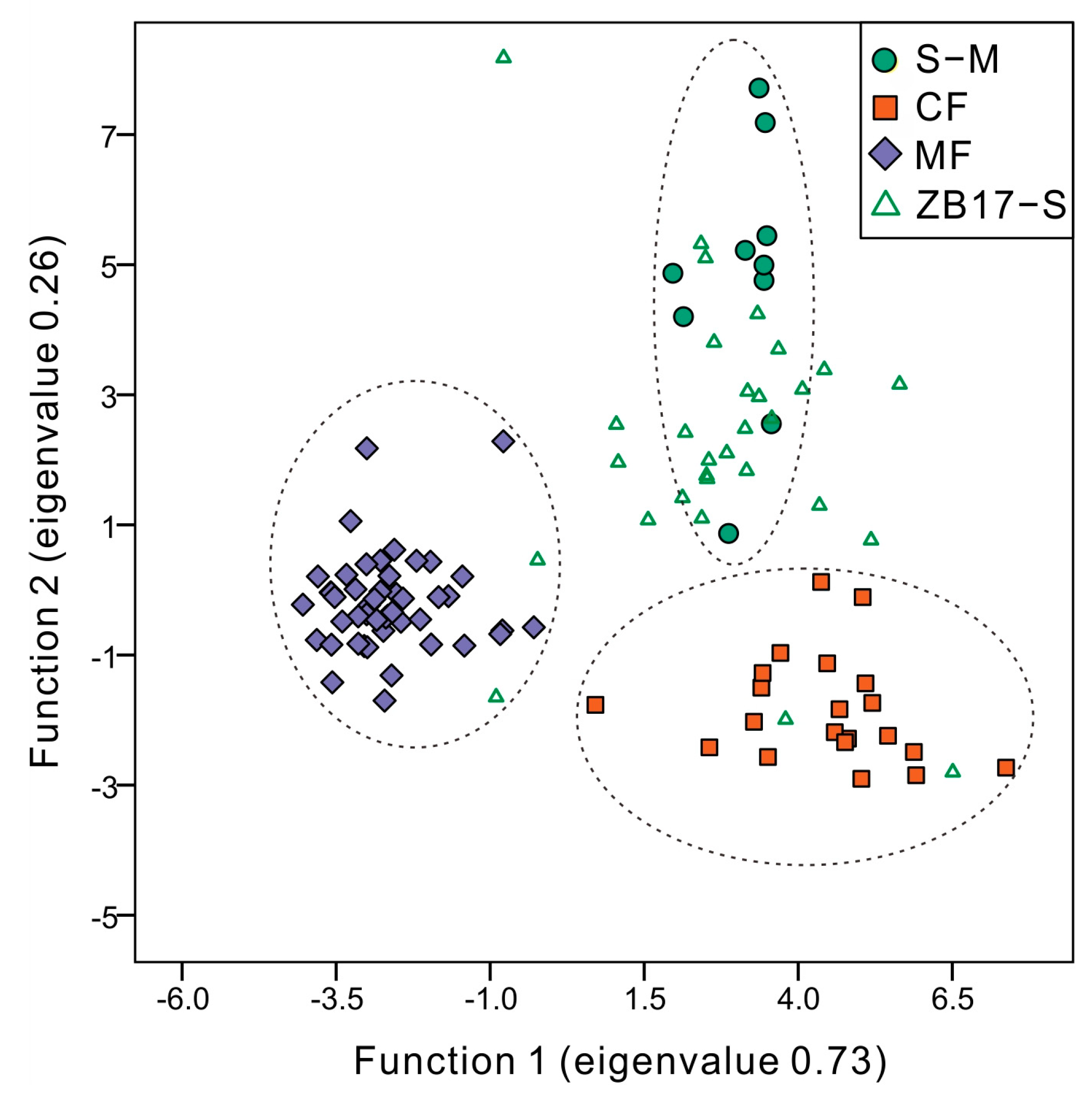
3.3. Numerical Analysis Results
4. Discussion
4.1. Modern Pollen Representation of Altitudinal Vegetation Types
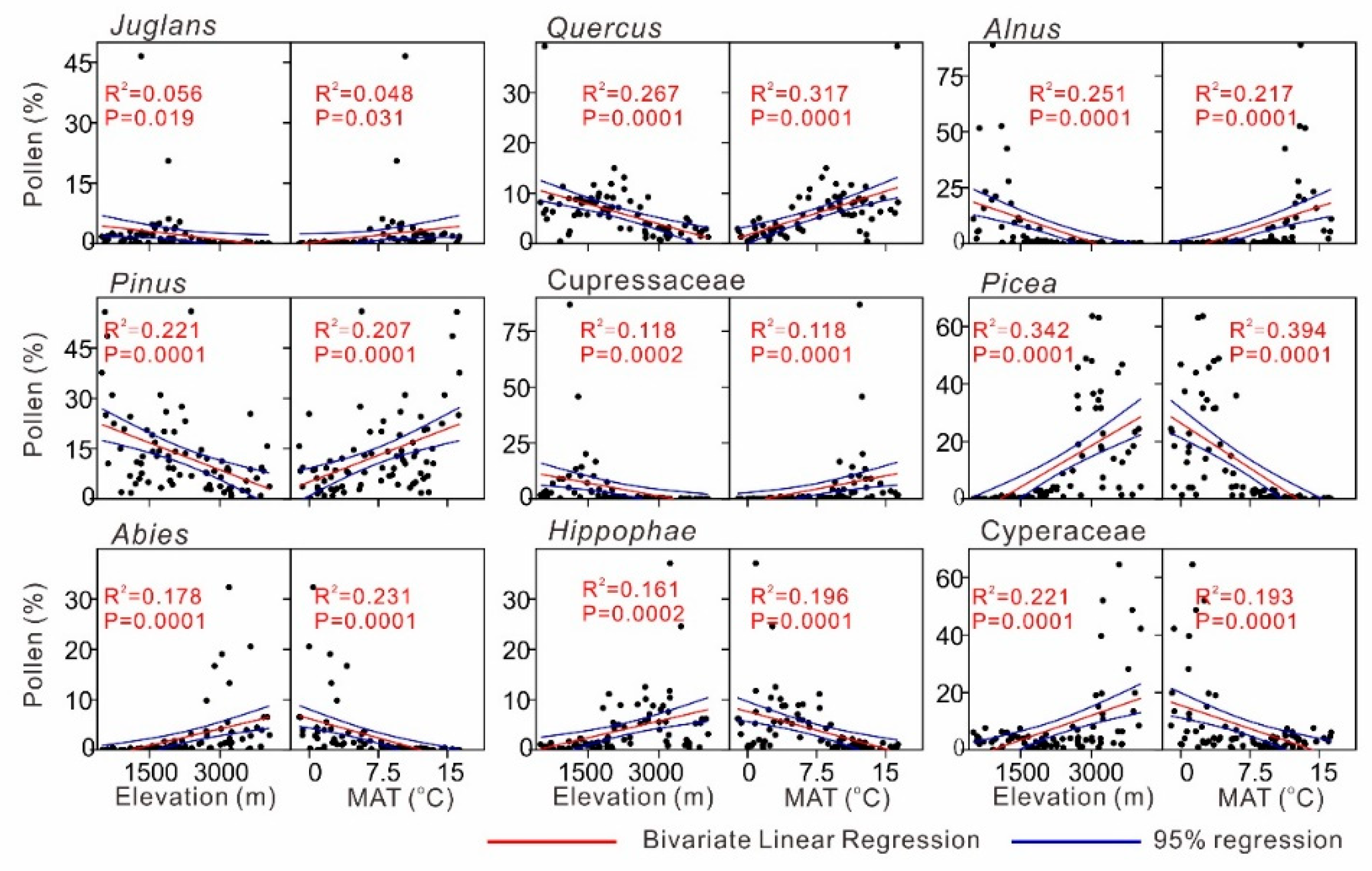
4.2. Pollen–Climate Relationships
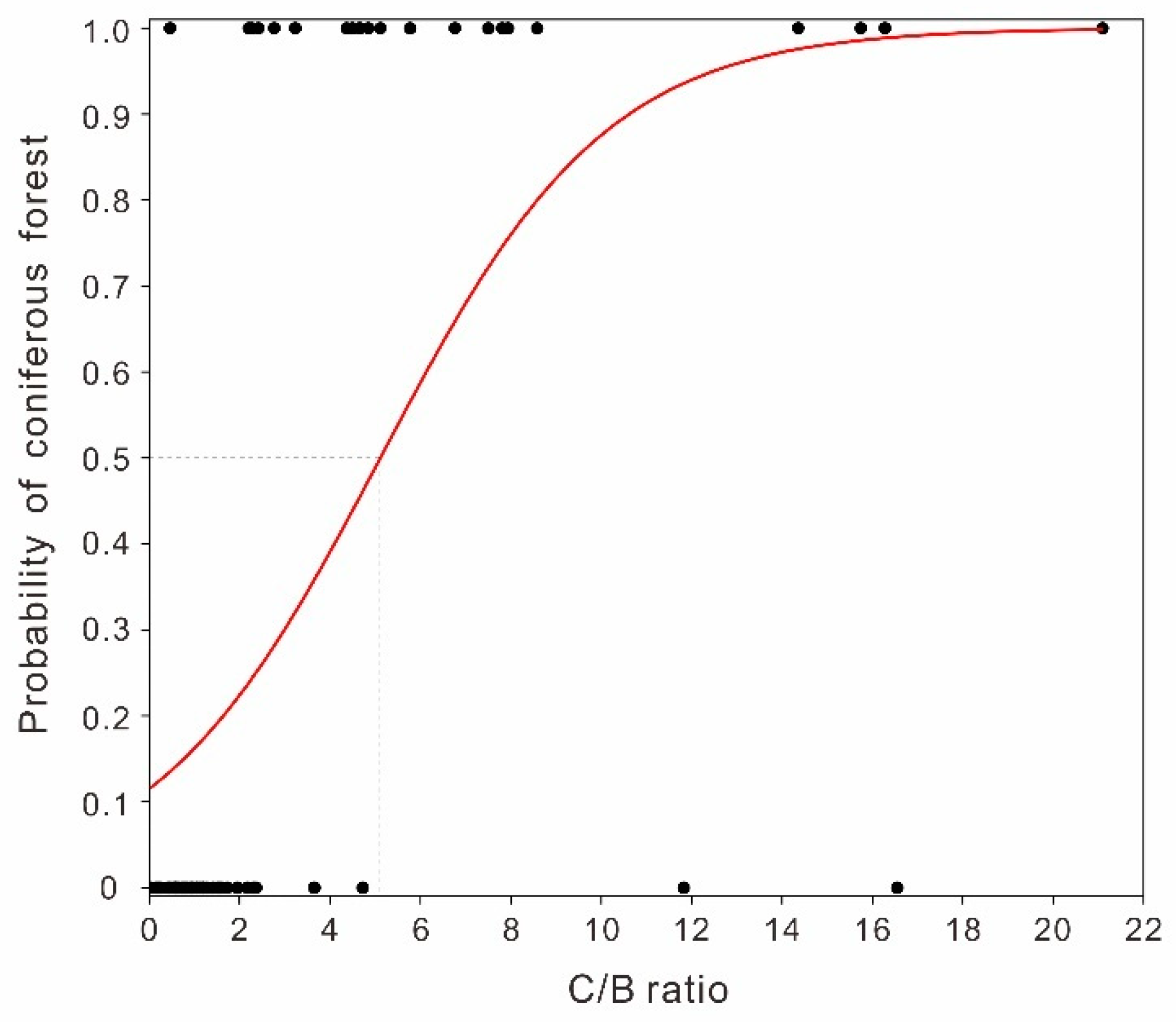
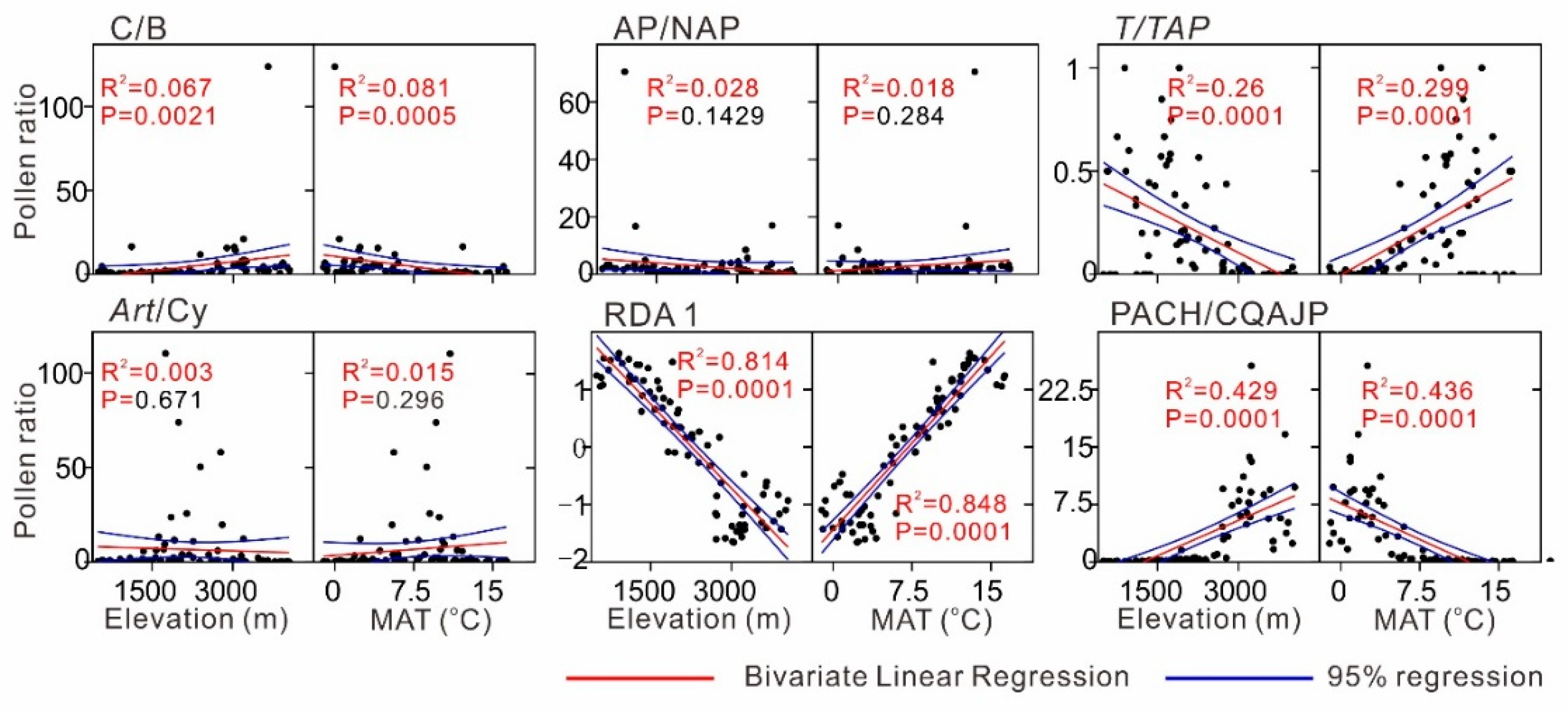
4.3. Implications of Pollen Ratios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Rahbek, C.; Borregaard, M.K.; Antonelli, A.; Colwell, R.K.; Holt, B.G.; Nogues-Bravo, D.; Rasmussen, C.M.Ø.; Richardson, K.; Rosing, M.T.; Whittaker, R.J.; et al. Building mountain biodiversity: Geological and evolutionary processes. Science 2019, 365, 1114–11191. [Google Scholar] [CrossRef] [PubMed]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Lézine, A.-M.; Izumi, K.; Kageyama, M.; Achoundong, G. A 90,000-year record of Afromontane forest responses to climate change. Science 2019, 363, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tzedakis, P.C.; Li, Q.; Qin, F.; Cui, Q.Y.; Liang, C.; Birks, H.J.B.; Liu, Y.L.; Zhang, Z.Y.; Ge, J.Y.; et al. Evolution of vegetation and climate variability on the Tibetan Plateau over the past 1.74 Myr. Sci. Adv. 2020, 6, eaay6193. [Google Scholar] [CrossRef]
- Dearing, J.A.; Bullock, S.; Costanza, R.; Dawson, T.P.; Edwards, M.E.; Poppy, G.M.; Smith, G.M. Navigating the Perfect Storm: Research Strategies for Socialecological Systems in a Rapidly Evolving World. Environ. Manag. 2012, 49, 767–775. [Google Scholar] [CrossRef]
- Markgraf, V. Pollen Dispersal in a Mountain Area. Grana 1980, 19, 127–146. [Google Scholar] [CrossRef]
- Schüler, L.; Hemp, A.; Behling, H. Relationship between vegetation and modern pollen-rain along an elevational gradient on Kilimanjaro, Tanzania. Holocene 2014, 24, 702–713. [Google Scholar] [CrossRef]
- Wei, H.C.; Ma, H.Z.; Zheng, Z.; Pan, A.D.; Huang, K.Y. Modern pollen assemblages of surface samples and their relationships to vegetation and climate in the northeastern Qinghai-Tibetan Plateau, China. Rev. Palaeobot. Palynol. 2011, 163, 237–246. [Google Scholar] [CrossRef]
- Zhao, Y.; Herzschuh, U. Modern pollen representation of source vegetation in the Qaidam Basin and surrounding mountains, north-eastern Tibetan Plateau. Veg. Hist. Archaeobot. 2009, 18, 245–260. [Google Scholar] [CrossRef]
- Pelánková, B.; Chytrý, M. Surface pollen–vegetation relationships in the forest-steppe, taiga and tundra landscapes of the Russian Altai Mountains. Rev. Palaeobot. Palynol. 2009, 157, 253–265. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Shen, J.; Wang, S.M. Spatial variation of modern pollen from surface lake sediments in Yunnan and southwestern Sichuan Province, China. Rev. Palaeobot. Palynol. 2011, 165, 224–234. [Google Scholar] [CrossRef]
- Fang, Y.M.; Ma, C.M.; Mao, L.M.; Zhu, C.; Zhang, W.Q. Surface pollen spectra from Shennongjia Mountains, central China: An interpretation aid to Quaternary pollen deposits. Rev. Palaeobot. Palynol. 2015, 214, 40–50. [Google Scholar] [CrossRef]
- Wei, H.C.; Zhao, Y. Surface pollen and its relationships with modern vegetation and climate in the Tianshan Mountains, northwestern China. Veg. Hist. Archaeobot. 2016, 25, 19–27. [Google Scholar] [CrossRef]
- Ali, S.N.; Quamar, M.F.; Dubey, J.; Morthekai, P.; Bisht, P.; Pandey, P.; Shekhar, M.; Ghosh, R. Surface pollen distribution in alpine zone of the higher Himalaya: A case study from the Kalla glacier valley, India. Bot. Lett. 2020, 167, 340–352. [Google Scholar] [CrossRef]
- Cañellas-Boltà, N.; Rull, V.; Vigo, J.; Mercadé, A. Modern pollen—Vegetation relationships along an altitudinal transect in the central Pyrenees (southwestern Europe). Holocene 2009, 19, 1185–1200. [Google Scholar] [CrossRef]
- Chi, C.T.; Xiao, X.Y.; Wang, J.J.; Ke, R.; Jia, B.Y. The vertical distribution of modern pollen in the southeastern edge of the Tibetan Plateau, China. Palynology 2023, 48, 2258941. [Google Scholar] [CrossRef]
- Holger, N.; Corinna, B.; Hermann, B. Vegetation/modern pollen rain relationship along an altitudinal transect between 1920 and 3185 m a.s.l. in the Podocarpus National Park region, southeastern Ecuadorian Andes. Rev. Palaeobot. Palynol. 2010, 159, 69–80. [Google Scholar]
- Yang, Z.J.; Zhang, Y.; Ren, H.B.; Yan, S.; Kong, Z.C.; Ma, K.P.; Ni, J. Altitudinal changes of surface pollen and vegetation on the north slope of the Middle Tianshan Mountains, China. J. Arid Land 2016, 8, 799–810. [Google Scholar] [CrossRef]
- Ma, Q.F.; Zhu, L.P.; Yang, R.M.; Huang, L.; Wang, J.B.; Tang, L.Y. Modern pollen distribution in moss samples along an elevational gradient in southeast Tibet. Ecol. Indic. 2023, 154, 110867. [Google Scholar] [CrossRef]
- Roy, I.; Ranhotra, P.S.; Shekhar, M.; Bhattacharyya, A.; Ghosh, R.; Sharma, Y.K. Modern Pollen-vegetation Relationships along the Vegetation Gradient in the Bhagirathi Valley, Western Himalaya, India. J. Geol. Soc. India 2021, 97, 571–578. [Google Scholar] [CrossRef]
- Cour, P.; Zheng, Z.; Duzer, D.; Calleja, M.; Yao, Z. Vegetational and climatic significance of modern pollen rain in northwestern Tibet. Rev. Palaeobot. Palynol. 1999, 104, 183–204. [Google Scholar] [CrossRef]
- Tong, G.B.; Yang, X.D.; Liu, Z.M.; Wang, S.M.; Zhao, H.G. Surface soil pollen distributions in the Yulong Mountain. Mar. Geol. Quat. Geol. 2003, 23, 103–107. (In Chinese) [Google Scholar]
- Cai, Y.; Wang, Y.; Jiang, F.C.; Li, C.Z. Characteristics of pollen assemblages in surface soils in the Maqu-Hongyuan area, Zoige Plateau, Northern Sichuan. J. Geomech. 2007, 13, 333–339. (In Chinese) [Google Scholar]
- Li, Y.W.; Xu, Q.H.; Zhang, S.R.; Li, Y.C.; Sun, Y.H.; Wang, T.; Shen, W.; Yang, X.L.; Zhang, R.C.; Wei, H.C. Significance of pollen assemblages for the vegetation composition of alpine shrub meadow in the Qinghai-Tibetan Plateau, China. Chin. Sci. Bull. 2019, 64, 2141–2150. (In Chinese) [Google Scholar] [CrossRef]
- Qin, F.; Bunting, M.J.; Zhao, Y.; Li, Q.; Cui, Q.Y.; Ren, W.H. Relative pollen productivity estimates for alpine meadow vegetation, northeastern Tibetan Plateau. Veg. Hist. Archaeobot. 2020, 29, 447–462. [Google Scholar] [CrossRef]
- Zhang, X.S. Vegetation of China and Its Geographic Patterns; China Geology Publishing House: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Jiang, S. The meadows and forests of western mountains in Sichuan Province. Acta Bot. Sin. 1960, 9, 1136–1236. (In Chinese) [Google Scholar]
- Shen, C.M.; Tang, L.Y.; Wang, S.M.; Li, C.H.; Liu, K.B. Pollen records and time scale for the RM core of the Zoige Basin, northeastern Qinghai-Tibetan Plateau. Chin. Sci. Bull. 2005, 50, 553–562. [Google Scholar] [CrossRef]
- Jiang, Y.X. Ecological analysis of flora, species correlation and ordination of subalpine forest vegetation in Western Sichuan. Chin. J. Plant Ecol. 1982, 6, 281–301. (In Chinese) [Google Scholar]
- Faegri, K.; Iversen, J. Textbook of Pollen Analysis; John Wiley & Sons Ltd.: Chichester, UK, 1989. [Google Scholar]
- Tang, L.Y.; Mao, L.M.; Shu, J.W.; Li, C.H.; Shen, C.M.; Zhou, Z.Z. Atlas of Quaternary Pollen and Spores in China; Science Press: Beijing, China, 2017. [Google Scholar]
- Wang, F.X.; Qian, N.F.; Zhang, Y.L.; Yang, H.Q. Pollen Flora of China, 2nd ed.; Science Press: Beijing, China, 1995. (In Chinese) [Google Scholar]
- Grimm, E.C. CONISS—A Fortran-77 program for stratigraphically constrained cluster-analysis by the method of incremental sum of squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University, Evanston, Illinois, USA. 2023. Available online: https://CRAN.R-project.org/package=psych (accessed on 2 September 2024).
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Legendre, P.; Wiser, S.K.; Brotons, L. Using species combinations in indicator value analyses. Methods Ecol. Evol. 2012, 3, 973–982. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B.; Chessel, D. The ade4 Package--II: Two-table and K-table Methods. R News Forthcoming. 2007. Available online: https://cran.r-project.org/web/packages/ade4/citation.html (accessed on 2 November 2024).
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ter Braak, C.J.F.; Prentice, I.C. A Theory of Gradient Analysis. In Advances in Ecological Research; Begon, M., Fitter, A.H., Ford, E.D., Macfadyen, A., Eds.; Academic Press: Cambridge, MA, USA, 1988; Volume 18, pp. 271–317. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Verdonschot, P.F.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.; Elphick, C. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Simpson, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://github.com/vegandevs/vegan (accessed on 2 November 2024).
- Li, Q.; Ge, Q.S.; Tong, G.B. Modern pollen-vegetation relationship based on discriminant analysis across an altitudinal transect on Gongga Mountain, eastern Tibetan Plateau. Chin. Sci. Bull. 2012, 57, 4600–4608. [Google Scholar] [CrossRef]
- Shen, C.M.; Liu, K.B.; Tang, L.Y.; Overpeck, J.T. Numerical Analysis of Modern and Fossil Pollen Data from the Tibetan Plateau. Ann. Am. Asssoc. Geeogr. 2008, 98, 755–772. [Google Scholar] [CrossRef]
- Zhao, K.L.; Li, X.Q. Modern pollen and vegetation relationships in the Yili Basin, Xinjiang, NW China. Chin. Sci. Bull. 2013, 58, 4133–4142. [Google Scholar] [CrossRef]
- Cao, X.Y.; Herzschuh, U.; Ni, J.; Zhao, Y.; Böhmer, T. Spatial and temporal distributions of major tree taxa in eastern continental Asia during the last 22,000 years. Holocene 2015, 25, 79–91. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Herzschuh, U. Reliability of pollen ratios for environmental reconstructions on the Tibetan Plateau. J. Biogeogr. 2007, 34, 1265–1273. [Google Scholar] [CrossRef]
- Pan, T.; Dai, E.; Wu, S.H. Relationship between surface pollen and modern vegetation in Southwestern China. J. Mt. Sci. 2010, 7, 176–186. [Google Scholar] [CrossRef]
- Li, J.F.; Xie, G.; Yang, J.; Ferguson, D.K.; Liu, X.-D.; Liu, H.; Wang, Y.-F. Asian Summer Monsoon changes the pollen flow on the Tibetan Plateau. Earth-Sci. Rev. 2020, 202, 103114. [Google Scholar] [CrossRef]
- Li, W.Q.; Yao, Z.J. Study on Late Quaternary Vegetation and Environment in North and Middle Tropics of China; China Ocean Press: Beijing, China, 1993. (In Chinese) [Google Scholar]
- Lü, H.Y.; Wu, N.Q.; Liu, K.B.; Zhu, L.P.; Yang, X.D.; Yao, T.D.; Wang, L.; Li, Q.; Liu, X.Q.; Shen, C.M.; et al. Modern pollen distributions in Qinghai-Tibetan Plateau and the development of transfer functions for reconstructing Holocene environmental changes. Quat. Sci. Rev. 2011, 30, 947–966. [Google Scholar] [CrossRef]
- Shen, C.M.; Liu, K.B.; Tang, L.Y.; Jonathan, T.O. Quantitative relationships between modern pollen rain and climate in the Tibetan Plateau. Rev. Palaeobot. Palynol. 2006, 140, 61–77. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Duo, L.; Pang, Y.Z.; Felde, V.A.; Birks, H.H.; Birks, H.J.B. Modern pollen assemblages and their relationships to vegetation and climate in the Lhasa Valley, Tibetan Plateau, China. Quat. Int. 2018, 467, 210–221. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Cui, Q.Y.; Qin, F.; Zheng, Z.; Xiao, X.Y.; Ma, C.M.; Felde, V.A.; Liu, Y.L.; Li, Q.; et al. Temperature reconstructions for the last 1.74-Ma on the eastern Tibetan Plateau based on a novel pollen-based quantitative method. Glob. Planet. Chang. 2021, 199, 103433. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Guo, Z.; Fang, K.; Li, Q.; Cao, X. Abrupt vegetation shifts caused by gradual climate changes in central Asia during the Holocene. Sci. China Earth Sci. 2017, 60, 1317–1327. [Google Scholar] [CrossRef]
- An, Z.S.; Clemens, S.C.; Shen, J.; Qiang, X.K.; Jin, Z.D.; Sun, Y.B.; Prell, W.L.; Luo, J.J.; Wang, S.M.; Xu, H.; et al. Glacial-Interglacial Indian Summer Monsoon Dynamics. Science 2011, 333, 719–723. [Google Scholar]
- Miao, Y.F.; Fang, X.M.; Sun, J.M.; Xiao, W.J.; Yang, Y.H.; Wang, X.L.; Farnsworth, A.; Huang, K.Y.; Ren, Y.L.; Wu, F.L.; et al. A new biologic paleoaltimetry indicating Late Miocene rapid uplift of northern Tibet Plateau. Science 2022, 378, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Y.; Leroy, S.A.G.; Yang, S.L.; Zhang, E.L.; Wang, L.; Yang, X.X.; Rioual, P. Synchronous strengthening of the Indian and East Asian monsoons in response to global warming since the last deglaciation. Geophys. Res. Lett. 2019, 46, 3944–3952. [Google Scholar] [CrossRef]
- Zhao, K.L.; Zhou, X.Y.; Ji, M.; Li, X.Q. Palynological Evidence of Late Holocene Paleo-Monsoon in Eastern Pamir. Geophys. Res. Lett. 2019, 46, 10015–10023. [Google Scholar] [CrossRef]
- Wahl, R.E. Pollen surface samples for paleoenvironmental reconstruction from the coast and transverse ranges of southern California. Madroño 2003, 50, 286–299. [Google Scholar]
- Zhao, Y.; Li, F.R.; Hou, Y.T.; Sun, J.H.; Zhao, W.W.; Tang, Y.; Li, H. Surface pollen and its relationships with modern vegetation and climate on the Loess Plateau and surrounding deserts in China. Rev. Palaeobot. Palynol. 2012, 181, 47–53. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Z.C.; Zhao, W.W. Holocene vegetation and climate histories in the eastern Tibetan Plateau: Controls by insolation-driven temperature or monsoon-derived precipitation changes? Quat. Sci. Rev. 2011, 30, 1173–1184. [Google Scholar] [CrossRef]
- Liu, H.Y.; Cui, H.T.; Pott, R.; Speier, M. The surface pollen of the woodland–steppe ecotone in southeastern Inner Mongolia, China. Rev. Palaeobot. Palynol. 1999, 105, 237–250. [Google Scholar] [CrossRef]
- Herzschuh, U.; Winter, K.; Wünnemann, B.; Li, S. A general cooling trend on the central Tibetan Plateau throughout the Holocene recorded by the Lake Zigetang pollen spectra. Quat. Int. 2006, 154–155, 113–121. [Google Scholar] [CrossRef]
- Huang, X.Z.; Chen, C.Z.; Jia, W.N.; An, C.B.; Zhou, A.F.; Zhang, J.W.; Jin, M.; Xia, D.S.; Chen, F.H.; Grimm, E.C. Vegetation and climate history reconstructed from an alpine lake in central Tienshan Mountains since 8.5 ka BP. Paleogeogr. Paleoclimatol. Paleoecol. 2015, 432, 36–48. [Google Scholar] [CrossRef]
- Lü, H.Y.; Wu, N.Q.; Yang, X.D.; Shen, C.M.; Zhu, L.P.; Wang, L.; Li, Q.; Xu, D.K.; Tong, G.B.; Sun, X.J. Spatial pattern of Abies and Picea surface pollen distribution along the elevation gradient in the Qinghai–Tibetan Plateau and Xinjiang, China. Boreas 2008, 37, 254–262. [Google Scholar] [CrossRef]

| Vegetation | Pollen Taxa | Specificity | Fidelity | IndVal | p-Value | Significant |
|---|---|---|---|---|---|---|
| Shrub meadow | Ericaceae | 0.9785 | 0.6000 | 0.766 | 0.001 | *** |
| Apiaceae | 0.7098 | 0.8000 | 0.754 | 0.005 | ** | |
| Coniferous forest | Thymelaeaceae | 0.8573 | 0.2857 | 0.495 | 0.161 | - |
| Mixed forest | Juglans | 0.9476 | 0.9574 | 0.952 | 0.001 | *** |
| Cupressaceae | 0.9621 | 0.9362 | 0.949 | 0.001 | *** | |
| Alnus | 0.9714 | 0.9149 | 0.943 | 0.001 | *** | |
| Elaeagnus | 0.9493 | 0.4681 | 0.667 | 0.03 | * | |
| Viburnum | 1.0000 | 0.1915 | 0.438 | 0.12 | - | |
| Shrub meadow and coniferous forest | Picea | 0.9677 | 1.0000 | 0.984 | 0.001 | *** |
| Abies | 0.9560 | 0.9355 | 0.946 | 0.001 | *** | |
| Salix | 0.9548 | 0.8710 | 0.912 | 0.002 | ** | |
| Caryophyllaceae | 0.8970 | 0.6129 | 0.741 | 0.009 | ** | |
| Coniferous forest and mixed forest | Tsuga | 0.9190 | 0.7941 | 0.854 | 0.002 | ** |
| Actual Group | No. of Samples | Predicted Group | ||
|---|---|---|---|---|
| Shrub Meadow | Coniferous Forest | Mixed Forest | ||
| Shrub meadow | 10 | 9 (90%) | 1 (10%, no.42) | 0 |
| Coniferous forest | 21 | 0 | 20 (95.2%) | 1 (4.8%, no.26) |
| Mixed forest | 47 | 0 | 0 | 47 (100%) |
| Alpine meadow of [24] | 30 | 23 (76.7%) | 5 (16.7%) | 3 (6.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, W.; Liu, M.; Qin, F.; Li, Q.; Yi, G.; Chen, W.; Li, S.; Liu, Z.; Peng, Q.; Liang, C.; et al. The Pollen Representation of Vegetation and Climate Along an Altitudinal Gradient on the Eastern Tibetan Plateau. Land 2024, 13, 1866. https://doi.org/10.3390/land13111866
Ren W, Liu M, Qin F, Li Q, Yi G, Chen W, Li S, Liu Z, Peng Q, Liang C, et al. The Pollen Representation of Vegetation and Climate Along an Altitudinal Gradient on the Eastern Tibetan Plateau. Land. 2024; 13(11):1866. https://doi.org/10.3390/land13111866
Chicago/Turabian StyleRen, Weihe, Min Liu, Feng Qin, Quan Li, Guitian Yi, Weiyu Chen, Shuming Li, Zijian Liu, Qing Peng, Chen Liang, and et al. 2024. "The Pollen Representation of Vegetation and Climate Along an Altitudinal Gradient on the Eastern Tibetan Plateau" Land 13, no. 11: 1866. https://doi.org/10.3390/land13111866
APA StyleRen, W., Liu, M., Qin, F., Li, Q., Yi, G., Chen, W., Li, S., Liu, Z., Peng, Q., Liang, C., & Zhao, Y. (2024). The Pollen Representation of Vegetation and Climate Along an Altitudinal Gradient on the Eastern Tibetan Plateau. Land, 13(11), 1866. https://doi.org/10.3390/land13111866






