Abstract
Our study examined the impact of grazing by Hungarian Grey cattle on plant communities in grasslands restored and established through different methods. The grasslands were established and restored in 2009 using five methods: (I) naturally regenerating fallow, (II) hay-mulch addition, (III) seeded grassland following soil preparation, (IV) grazing on abandoned alfalfa fields, and (V) overseeded fallow initiated in 1989. From 2009 to 2011, all sites were uniformly mowed, after which they were grazed using free-ranging Hungarian Grey cattle starting in 2012. This project aims to restore traditional land use and dry grassland grazing in the region. Phytosociological recordings were conducted in 2012, the first year following grazing initiation, and again in 2020, nine years later. We assessed the botanical composition and grassland management value across sites in a 260-hectare experimental area within the Pannonian biogeographic region. The current study seeks to determine how different grassland restoration techniques have influenced the botanical composition and grassland management values of pastures and to identify the most suitable restoration methods. Statistical analyses were conducted using R to assess species count differences across four vegetation categories. The results indicate that the overseeded fallow established in 1989 and the hay-mulch addition method were the most effective techniques. Directly seeded grasslands and abandoned alfalfa fields differed most significantly from these areas. Across all types, species richness and total cover increased relative to the initial conditions in 2012, and the naturalness of the species composition also improved. Returning to traditional land use improved the ecological state of all sites. Restoration of the grazed areas proved optimal over the elapsed period, with two years of mowing followed by grazing. Grazing with Hungarian Grey cattle, a low-impact rustic breed, yielded successful grassland restoration outcomes. Among the restoration methods, direct seeding and the use of alfalfa fields proved uneconomical and ineffective in promoting the desired species composition. Natural regeneration and, particularly, the hay-mulch addition method, were the most effective for conservation and grassland management.
1. Introduction
Grasslands constitute a significant proportion of the Earth’s vegetated landscapes, making their maintenance, conservation, and proper management crucial [1,2,3]. In Hungary, 15% of agricultural land is grassland covering about 800,000 hectares, and 60% of them have been included in the Natura 2000 network. Many natural grasslands are global biodiversity hotspots [4,5,6,7,8,9,10], and the Eurasian region, known for its abundant and species-rich grasslands [11], is especially noteworthy. According to Hobohm and Bruchmann [12], 18.1% of Europe’s endemic vascular plants inhabit grassland ecosystems, with climatic factors playing a key role in shaping this diversity [13,14,15,16,17].
Beyond preserving biodiversity, they play a pivotal role in mitigating climate change, notably through their high capacity for global carbon sequestration [18,19,20,21]. In addition to these two current areas, an extensive review by Lyon et al. [2] highlights grasslands’ multifunctional roles, encompassing not only ecosystem restoration but also critical services in fodder production, erosion control, water management, and providing habitats for pollinators and wildlife. Additionally, these landscapes hold substantial cultural and social value [22].
However, many natural and semi-natural grasslands are found in areas with poor grazing potential, making them more vulnerable to climate change. Zhang et al. [23] provide evidence that drought is a significant factor in the decline of grassland biodiversity and multifunctionality. For instance, the cold spring of 2018 resulted in substantial losses among the Przewalski horse and Hungarian aurochs populations in the Hortobágy region due to limited forage availability [24]. Dry grasslands, in particular, are fragile ecosystems prone to severe wind erosion, especially when disturbed by grazing [1,25,26].
These findings suggest that managing grazing pressure in line with the principle of optimal disturbance is often essential. In the Pannonian region, grasslands have been heavily affected by the expansion of agricultural land over centuries. In Eastern Europe alone, grassland area has declined by at least 50% in the past 200 years [27]. A summary by Bíró et al. [28] shows the loss of eight natural and semi-natural grassland types in Hungary, with the least reduction occurring in saline grasslands (39%), which are unsuitable for agriculture, while other grassland types have lost 85–98% of their area. Similar reductions have been reported for steppes and forest steppes in western regions [29]. Many of these grasslands have been converted to forest, cropland, or intensive pasture or abandoned, much like in other regions of Europe [30,31].
Recently, grassland establishment and maintenance have gained renewed attention [32,33]. Restoration techniques vary widely, including direct seeding with diverse seed mixes and hay mulch addition, which can be effective [33,34,35]. Each method has distinct advantages; for instance, hay mulching reduces weed encroachment and enhances species richness [36,37] by inhibiting light-demanding weeds, protecting the soil from erosion and deflation, and providing a favorable microclimate for target species [38,39]. Choosing the right seed mix is also critical for conservation; ideally, native species that match the local site conditions should be selected [40,41]. Additional management practices, such as mowing, mulching, and grazing, are often required to promote restoration success. Mowing, particularly in the early stages, curbs weed growth and allows the establishment of other species, thus preserving species richness in degraded grasslands [42,43].
Grazing is especially suitable for grassland management once the grassland structure is established [44]. However, inappropriate grazing often promotes species that hinder or resist grazing due to traits, such as high silica content or dense, thorny, or toxic tissues, which animals avoid [45].
The choice of animal species, breed, and grazing season is crucial for biodiversity preservation and restoration. The size and grazing abilities of animals significantly impact vegetation. For instance, larger animals tend to avoid shrubs [46], while robust cattle breeds consume more shrub biomass [47,48,49]. Grazing impacts numerous plant species, particularly taller grasses [50]. Pauler et al. [48] found that herbivores’ feed selection drives diversity within grazed vegetation, influenced by forage quality [51,52]. They also observed that herbivores preferred plants with specific leaf area (SLA), high, leaf nitrogen, and phosphorus, but avoided those with leaf dry matter content (LDMC), high or defense mechanisms (e.g., thistles) [48]. In general, cattle avoid woody plants due to their toughness and low digestibility [53]. However, studies suggest that robust cattle graze more evenly and selectively across landscapes [54]. De Vries and Daleboudt [55] analyzed bite rates in different vegetative structures in Agrostis/Festuca and Lolium pastures, finding that cattle’s’ selectivity aligned with maximizing daily energy intake. McGeough et al. [56] reported that robust cattle are well-suited for extended grazing seasons, reducing the need for mechanical harvesting and enhancing farm efficiency.
The goal of this study was to restore traditional land use through grazing in low-biomass dry grasslands using Hungarian Grey cattle, a heritage breed adapted to the Pannonian region.
The research seeks to address the following questions:
- Is grazing with Hungarian Grey cattle indeed the most suitable method for enhancing species composition in Pannonian dry grasslands, even with the application of various grassland restoration techniques?
- How do species richness and grassland composition vary with different restoration methods in terms of ecological quality and potential utilization?
- Will the effects of different grassland establishment techniques persist, and if so, to what extent, and how will they manifest in the grassland composition during two years of mowing followed by grazing management?
- How effective and economically viable was the use of direct seeding and alfalfa sowing on the restored areas?
Our hypothesis was that grassland establishment types would continue to show substantial differences even following establishment and grazing periods. Among these, the direct-seeded area was predicted not to approach natural conditions, even after the study period. Grazing with Hungarian Grey cattle, established to restore traditional land use practices, is expected to greatly contribute to improving species composition in pastures.
2. Materials and Methods
2.1. Study Area
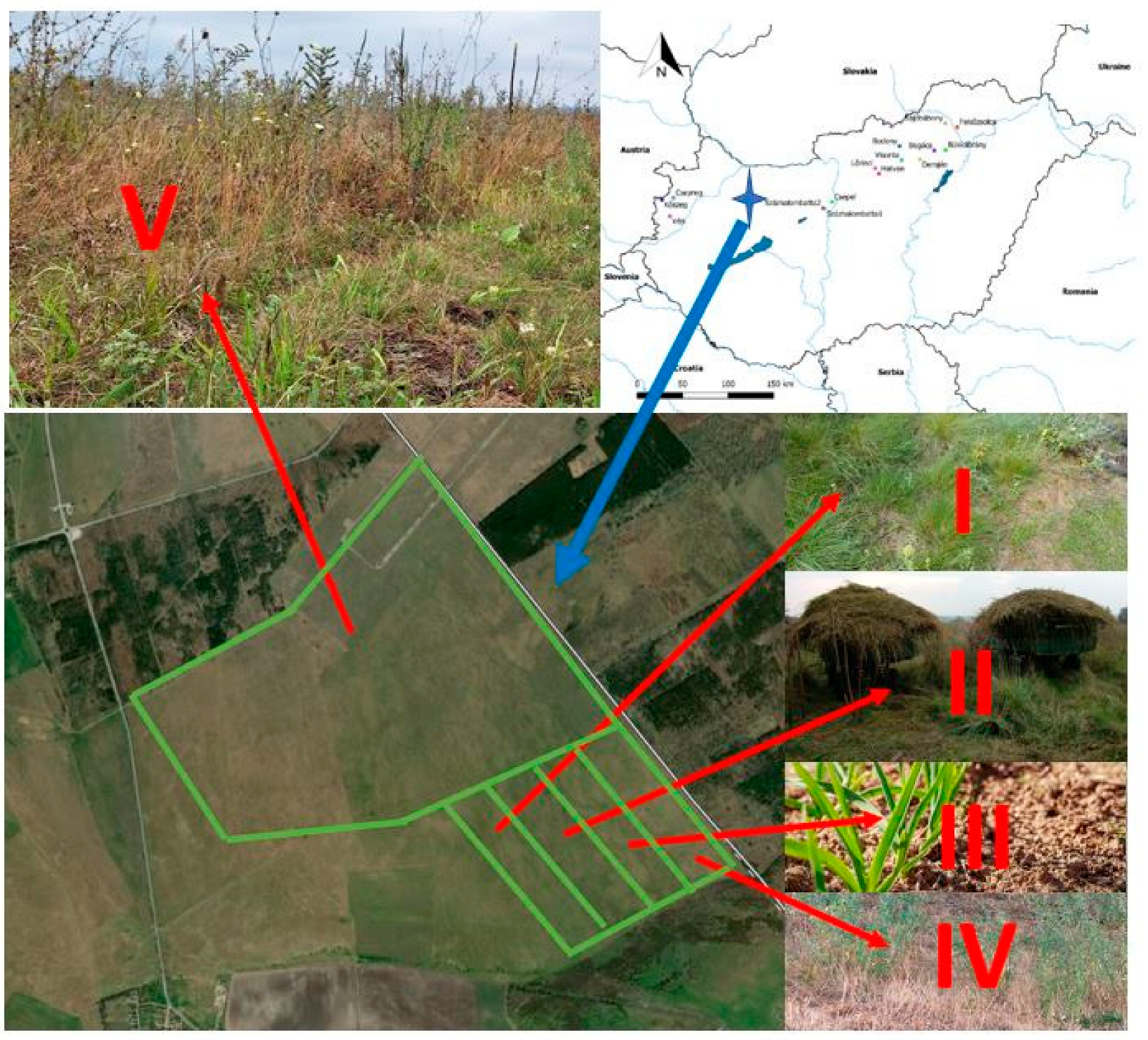
The research was conducted in the Páskom area (Reznek-dűlő, Túzok-rét), northeast of Zámoly, in the Zámoly Basin, Hungary (Figure 1). The soil of the area is rendzina [57]. The original vegetation of the area is slope steppe, Salvio nemorosae-Festucetum rupicolae Zólyomi ex Soó 1964, which usually contains many species with a Pontic and continental distribution and are generally rich in dioecious species, with a high diversity of species. The area is owned by the Pro Vértes Nature Conservation Foundation, which was plowed by the Zámoly Cooperative in the 1980s and used as cropland until 2000. Before the 1980s, this land was historically used as pasture due to its poor soil productivity. After purchasing the land, the Pro Vértes Foundation attempted restoration with small-game-friendly seed mixtures, though these attempts largely failed due to weather conditions. Considering that the Zámoly Basin’s Great Bustard population inhabited the area until the early 1980s, the site was re-grassed.

Figure 1.
The location of the sample sites in Hungary (I–V: Treatments).
The region receives an average annual precipitation of 560–600 mm, with an aridity index of 1.15–1.20 and an average annual temperature of 9.8–10 °C. The site, located at an altitude of approximately 140 m above sea level, experiences an average of 1950 h of sunshine annually and belongs to the Császár water catchment area [57]. The weather and exposure conditions of the sample areas are consistent, with northwest–southeast orientations on slightly sloping terrain (2–3%) with uniform soil.
The study area is divided into two major sections: the northern Túzok-rét, constituting the study site V, where overseeding (seeding without soil preparation, where the former vegetation remains) was conducted in 1989; and the southern Reznek-dűlő, where four parallel grassland restoration methods commenced in the 1990s.
2.2. Sample Plots
The sample plots consisted of the following restoration treatments:
Treatment I (Spontaneously regenerating the abandoned field): Previously used as cropland until 2000, abandoned and left to spontaneous grassland development (33.18 ha).
Treatment II (Hay mulching): An abandoned former cropland, hay mulched with hay collected from a nearby natural grassland (27.79 ha).
Treatment III (Direct-seeded area): In 2009, following complete soil preparation, the area was seeded with a 60 kg/ha seed mix (Lolium perenne 25%, Bromus inermis 15%, Festuca pratensis 10%, Dactylis glomerata 10%, Festuca rubra 15%, Festuca arundinacea 25%) (18 ha).
Treatment IV (Abandoned alfalfa field): Sown with alfalfa in 2009 and subsequently abandoned (30 ha).
Treatment V (Overseeded abandoned field): An area that was overseeded with a 40 kg/ha seed mix (Lolium perenne 10%, Bromus inermis 20%, Dactylis glomerata 40%, Festuca arundinacea 30%) in 1989 and intermittently grazed or mowed from 1982 to 2009 (150.83 ha).
Following two years of mowing (2010–2011), all areas were grazed by Hungarian Grey cattle under a free-ranging system from 2012, at a moderate stocking density of 0.5–0.7 livestock units per hectare [58].
2.3. Sampling
Three times a year, in May, June and September, seven randomly selected 2 × 2 m Braun-Blanquet [59] phytosociological quadrats were sampled for each restoration treatment, recording species cover as a percentage. Quadrats were placed along the length of each treatment strip, spaced 10 m apart from the northern edge. Species nomenclature follows Király [60].
2.4. Data Analysis
Sample sites were evaluated based on species conservation value categories following Simon [61], and social behavior types (plant strategies), as described by Borhidi [62] and the following life-form categories by Pignatti [63] were applied:
- Perennial Species:
H scap: species with ascending stems
H caesp: tussock-forming species
H ros: rosette-forming perennials
H rept: perennials with stolons, runners, or rhizomes
H bienn: biennial species
G bulb: geophytes with bulbs
G rhiz: rhizomatous, creeping geophytes
- Annual Species:
T scap: annual species with ascending stems
T ros: rosette-forming annual species
T caesp: annual tussock species
- Dwarf Shrubs:
Ch rept: creeping-stem dwarf shrubs
Ch succ: succulent dwarf shrubs
The coverage values of each grass species were calculated from the total coverage percentage per area.
The forage values [64,65,66,67] of various plots were calculated by the following formula:
FV: Forage value of the vegetation plots
a, b, c: categories of forage values of species
A, B, C: coverage of species
x: total coverage of species
The grassland production was estimated by Balázs-method [66,67] using the following formula:
P: yield [Kg/ha]
M: grass height [cm]
s: stubble height [cm]
BM: grass 400 [kg/ha]; alfalfa 470 [kg/ha]
b: coverage [%]
With the knowledge of the average grass height and the total coverage, the annual yield and the animal capacity of the grasslands could be estimated. The following data should be taken into account: 60 kg/day of green weight and a 210-day grazing season for cattle, 7 kg/day of green weight and a 210-day grazing season for sheep.
Statistical analyses were conducted using R version 4.2.3 (R Core Team, 2023) to assess species count differences across four vegetation categories (Fabaceae, Grass, Other, and Weed) commonly used in grassland surveys within five treatments (I–V) for the years 2012 and 2020 (Supplementary Material).
The data were pre-processed by removing observations with zero abundance, followed by aggregation based on treatment, year, category, and replication.
A two-way Analysis of Variance (ANOVA) was performed independently for each treatment within each year to examine differences in species counts among categories. Significant ANOVA results were followed by Tukey’s Honest Significant Difference (Tukey HSD) post hoc test to conduct pairwise comparisons among categories within each treatment. In addition to the ANOVA and post hoc analyses, an Additive Main Effects and Multiplicative Interaction (AMMI) model was applied to evaluate genotype-environment interactions, specifically to understand how different species categories responded to various treatments across years. This model allowed us to partition the variance and assess both main effects and interaction effects, providing a more nuanced view of treatment responses across species categories. A heatmap was generated using the pheatmap package to visualize the clustering patterns of species counts across treatments and years, aiding in the identification of categories with similar response profiles. To compare the total species number per treatment, we conducted a non-parametric Kruskal–Wallis test, due to the non-normal distribution of species counts, followed by a Bonferroni-adjusted post hoc test to control for multiple comparisons. This approach ensured a robust statistical comparison across treatments while accounting for potential Type I errors in multiple tests. The statistical and visualization analyses were conducted using various R packages: ggplot2 for plotting, dplyr for data manipulation, multcompView for post hoc test interpretation, readxl for data import, vegan for diversity analysis, agricolae for non-parametric testing and multiple comparisons, pheatmap for generating heatmaps to explore clustering patterns in species counts, and ggstatsplot for enhanced visualizations with statistical overlays. Descriptive statistics, including mean and standard deviation, were calculated to summarize species richness and abundance for each category within each treatment and year. All statistical tests were conducted with a significance threshold set at p < 0.05 [68,69].
3. Results
A total of 134 vascular plant species were recorded across two survey periods. The total coverage values increased in all sample areas during the study period (Figure 2). In 2012, four species were present across all five study areas: Dactylis glomerata, Festuca arundinacea, Potentilla argentea, and Trifolium pratense. By 2020, these were joined by Agrostis stolonifera, Agrimonia eupatoria, Bromus hordaceus, Festuca pseudovina, Elymus repens, Trifolium repens, Medicago lupulina, Achillea collina, and Veronica arvensis. In 2012, Treatment V (the overseeded abandoned field) had the highest species richness, with 77 species, based on quadrat sampling. Treatment II (hay-mulched area) also exhibited notable diversity with 44 species. The overseeded grassland (V) retained the highest absolute species richness across both survey periods (77 in 2012 and 75 in 2020), though Bromus inermis and Erodium cicutarium were absent in 2020.

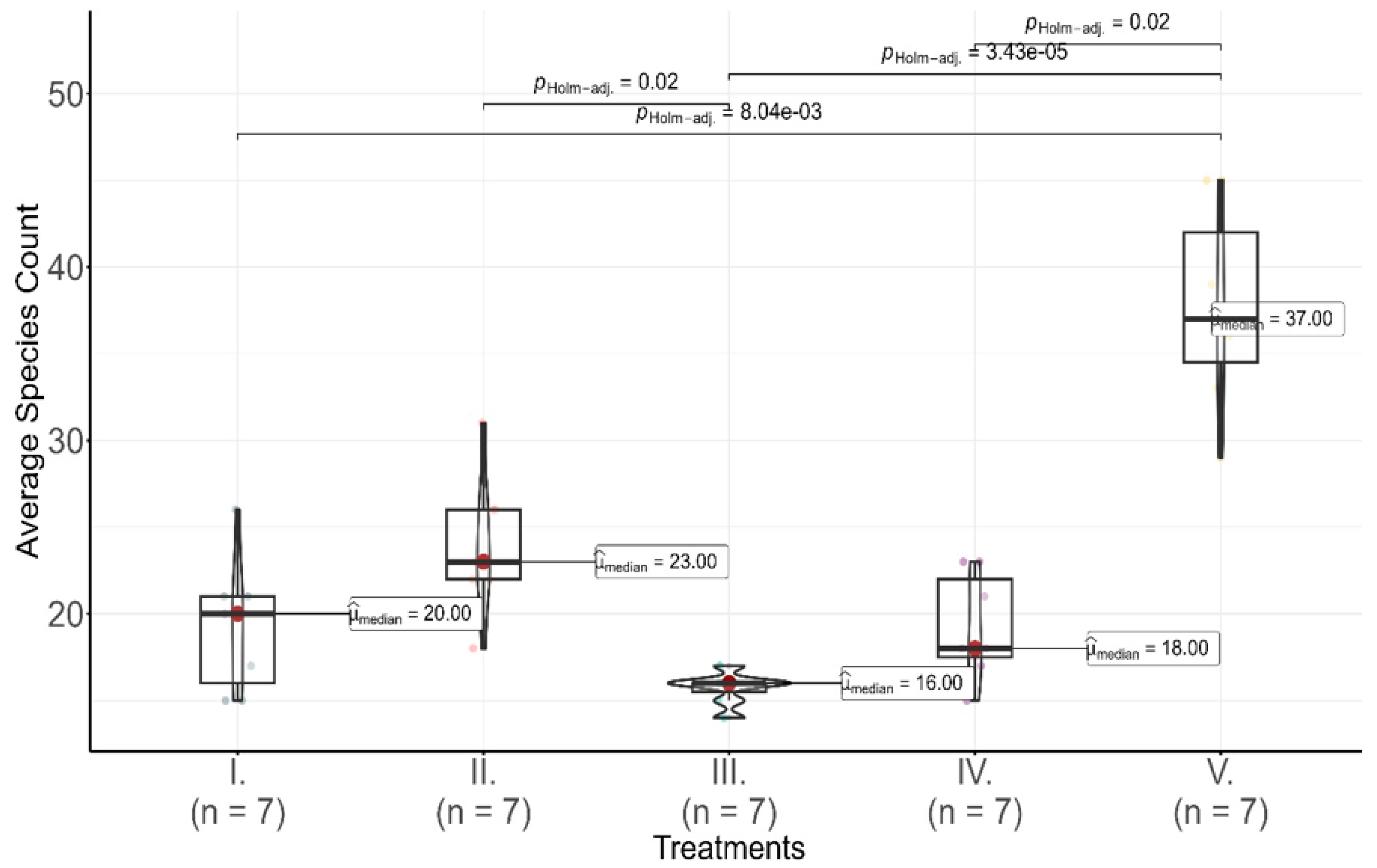
Figure 2.
Average species count per treatment in 2012 across five pasture treatments (I to V), represented with violin and box plots showing the distribution of species counts. Horizontal bars indicate statistically significant pairwise differences between treatments, determined by Dunn’s test with Holm adjustment at the p = 0.05 level. The adjusted p-values are annotated on the bars, providing a measure of significance for the observed differences in species counts. The letter n denotes the number of observed coenological quadrats sampled per treatment, with each treatment having seven observations (n = 7).
In 2012, both species abundance (χ2 = 10.71 p = 0.03 p) and species richness (χ2 = 24.99 p < 0.000) varied significantly across the five different pasture treatments (I. to V.), with a significant difference in abundance distributions and species count confirmed by a Kruskal–Wallis test. This finding suggests non-uniform abundance and distributions across treatments, indicating that different management strategies influenced specific species compositions (Figure 2).
Species abundance was generally low across treatments, with all treatments displaying a median abundance close to or at 1.0%, reflecting that the majority of species had relatively low abundance levels. Treatment I displayed an average abundance of 0.64% ± 1.92, with a median of 1.14%. The three most dominant species in this treatment were Poa angustifolia (11.43 ± 2.44%), Festuca pseudovina (10.0 ± 2.89%), and Dactylis glomerata (7.0 ± 2.83%), which together comprised a significant portion of the observed abundance distribution. Treatment II had an average species abundance of 0.64% ± 1.90, with a median of 0.88%, reflecting generally low abundance with some sporadic higher values. The most abundant species observed here were Festuca pseudovina (10.57 ± 1.99%), Poa angustifolia (9.29 ± 3.45%), and Festuca rupicola (6.86 ± 4.18%), contributing substantially to the observed variance in this treatment group. Treatment III exhibited a lower mean abundance of 0.51% ± 2.02, with a median of 1.0%, suggesting high variance in species abundance, though generally low values. The dominant species in this treatment included Festuca arundinacea (12.43 ± 2.51%), Poa angustifolia (9.71 ± 4.15%), and Bromus inermis (9.29 ± 3.45%). Treatment IV had a mean abundance of 0.57% ± 1.91, with a median of 1.14%. The dominant species in this treatment were Agropyron repens (9.71 ± 7.67%), Poa angustifolia (7.43 ± 2.7%), and Achillea collina (7.14 ± 5.84%), indicating specific responses to the applied treatment conditions. Lastly, Treatment V showed the highest mean abundance at 0.67% ± 1.63, with a median of 0.71%, underscoring that while most species had low abundance, a few exhibited higher values. The most dominant species in this treatment were Festuca pseudovina (13.86 ± 2.04%), Festuca rupicola (6.14 ± 2.04%), and Achillea pannonica (3.71 ± 1.7%).
In addition to species abundance, the average species count per treatment, a measure of species richness, showed significant differences across treatments. Calculated species richness (considering only species with non-zero abundance) revealed distinct patterns: Treatment I had a mean species count of 19.29 ± 3.95, indicating moderate species diversity. Treatment II had a slightly higher mean species count of 24.00 ± 4.12, suggesting a more diverse assemblage. Treatment III had the lowest mean species count of 15.71 ± 0.95, significantly lower than the other treatments, suggesting that this treatment supported fewer species overall. Treatment IV recorded a mean species count of 19.29 ± 3.09, similar to Treatment I, while Treatment V had the highest mean species count of 37.71 ± 5.91, highlighting that this treatment promoted substantially greater species richness.
These differences in species richness are further confirmed by Dunn’s pairwise test, adjusted using the Holm method, which indicated significant differences between treatments, particularly between Treatment III and Treatments I, II, IV, and V The pairwise comparisons also highlighted the significant difference between Treatment V and Treatments I, III, and IV (Holm-adjusted p-values of 0.02, 3.43 × 10−5, and 8.04 × 10−3, respectively), further supporting that Treatment V facilitated a higher level of species diversity compared to other treatments.
In summary, while all treatments in 2012 exhibited similar median values of species abundance, substantial variability was observed, as reflected by the standard deviations. The composition of dominant species varied across treatments, indicating that certain species adapted to specific pasture management strategies, resulting in significant distributional differences. The differences in species richness highlight that certain treatments, particularly Treatment V, facilitated greater species diversity, whereas Treatment III supported both lower abundance and richness. This pattern indicates that distinct management approaches may selectively benefit either a few dominant species (low richness) or a broader assemblage (higher richness), influencing the overall biodiversity across treatments.
In 2020, both species abundance and species richness varied across the five pasture treatments (I to V), though without a statistically significant difference in abundance (χ2 = 7.42 p = 0.12), the distribution of species showed significant differences (χ2 = 15.20 p < 0.000) across the five treatments. This result suggests that the treatments did not produce distinct abundance just distributions, contrasting with the patterns observed in 2012 (Figure 3).

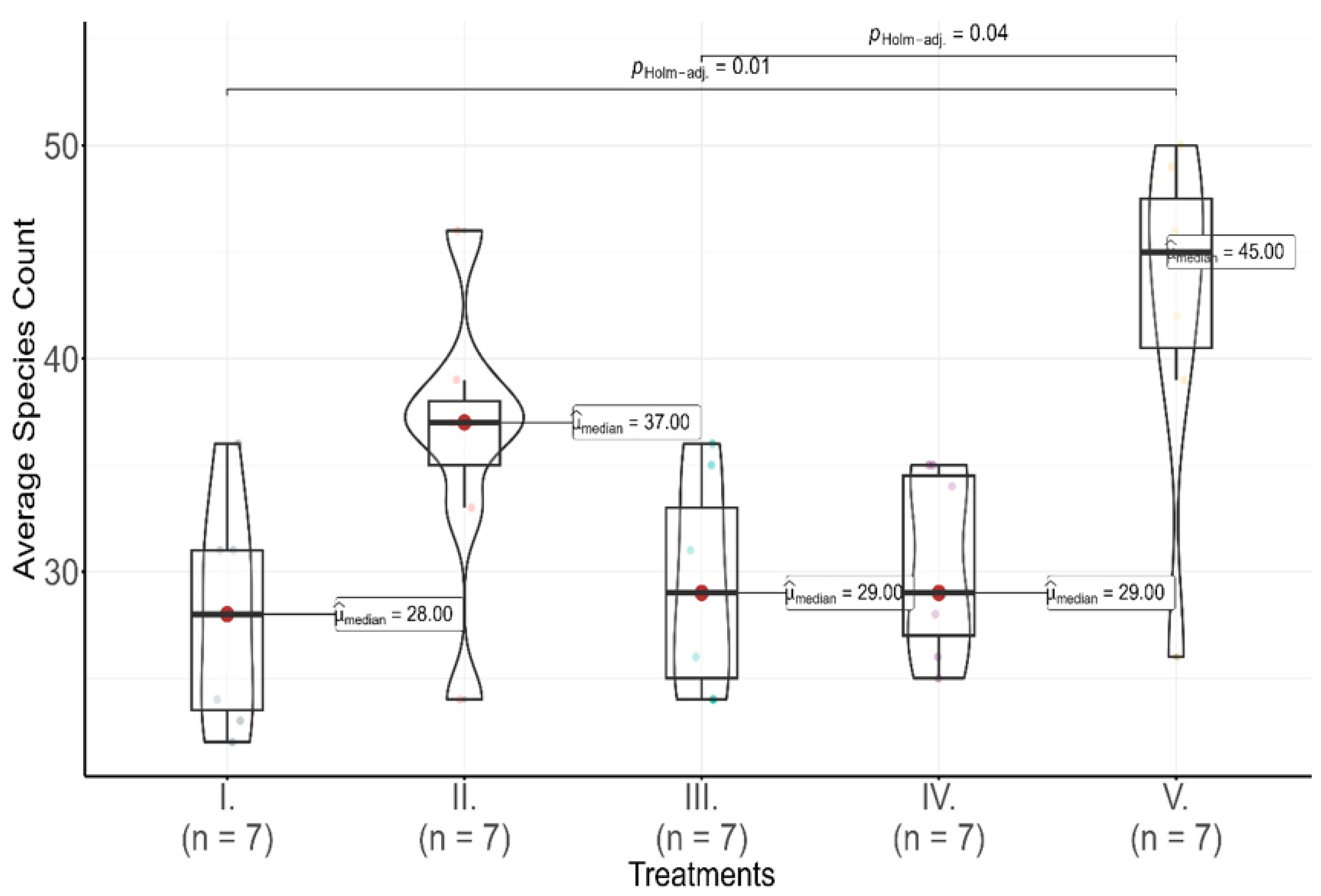
Figure 3.
Average species count per treatment in 2020 across five pasture treatments (I to V), represented with violin and box plots showing the distribution of species counts. Horizontal bars indicate statistically significant pairwise differences between treatments, determined by Dunn’s test with Holm adjustment at the p = 0.05 level. The adjusted p-values are annotated on the bars, providing a measure of significance for the observed differences in species counts. The letter n denotes the number of observed coenological quadrats sampled per treatment, with each treatment having seven observations (n = 7).
Species abundance remained generally low across treatments, with median values around 1.0%, indicating that most species were present at low abundance levels. However, notable species remained dominant within each treatment. For instance, Treatment I displayed a mean abundance of 0.72% ± 2.11 with a median of 1.0%. Dominant species included Festuca pseudovina (16.43 ± 2.44%), Festuca rupicola (7.86 ± 2.67%), and Plantago lanceolata (7.29 ± 3.5%). Treatment II showed a slightly higher mean abundance of 0.76% ± 2.15 and a median of 1.0%, with Festuca pseudovina (20.0 ± 4.08%), Achillea collina (5.86 ± 1.46%), and Plantago lanceolata (5.86 ± 2.27%) being most prevalent. Treatment III exhibited a mean abundance of 0.71% ± 2.02, with a median of 0.86%, where dominant species included Plantago lanceolata (14.57 ± 3.1%), Festuca pseudovina (12.71 ± 3.68%), and Achillea collina (5.86 ± 1.46%). Treatment IV had a mean abundance of 0.67% ± 1.92 with a median of 1.21% and was dominated by Festuca pseudovina (16.43 ± 2.44%), Cynodon dactylon (8.29 ± 2.36%), and Festuca rupicola (6.29 ± 1.89%). Finally, Treatment V recorded the highest mean abundance at 0.74% ± 1.66, with a median of 0.86%, where the dominant species were Festuca pseudovina (13.57 ± 5.56%), Festuca rupicola (5.0 ± 0.0%), and Achillea collina (4.71 ± 1.8%).
The average species count per treatment, showed significant differences across treatments, as indicated by a Kruskal–Wallis test result (χ2 = 10.8 p = 0.03). Treatment I had a mean species count of 27.86 ± 5.15, suggesting moderate species diversity. Treatment II displayed a higher mean species count of 36.14 ± 6.64, indicating increased richness. Treatment III recorded a mean species count of 29.29 ± 4.96, aligning with moderate diversity. Treatment IV had a mean species count of 30.29 ± 4.31, comparable to Treatments I and III. However, Treatment V, showed the highest mean species count at 42.43 ± 8.18, substantially greater than the other treatments, indicating that this treatment promoted significantly higher species richness. These patterns were reinforced by Dunn’s pairwise test with Holm adjustments, highlighting significant differences between Treatment V and Treatments I, III, and IV (Holm-adjusted p-values of 0.01, 0.04, and 0.04, respectively).
The 2020 results reveal consistent but low species abundance across treatments, with dominant species varying by treatment. In terms of species richness, Treatments II and V supported higher diversity, with Treatment V showing a particularly high mean species count, suggesting that this treatment promoted a more diverse assemblage. Contrastingly, the more moderate species counts observed in Treatments I, III, and IV highlight differences in how each pasture management strategy influenced community composition and biodiversity outcomes.
From a grassland management perspective, grass species were pivotal in this study, with five species—Dactylis glomerata, Elymus repens, Poa angustifolia, Festuca rupicola, and Festuca arundinacea—showing an average coverage exceeding 10%. Economically valuable legumes were not abundant, with Lotus corniculatus only appearing significantly in the overseeded abandoned field, averaging 3.89% cover, and Trifolium repens covering 8.39% in the alfalfa area. The dominance of Medicago sativa on the alfalfa field declined notably. Among other dicotyledons, only a few species exceeded 5% average cover: Achillea collina, Achillea pannonica, Sanguisorba minor, Scabiosa ochroleuca, and Taraxacum officinale, with Sanguisorba minor and Scabiosa ochroleuca confined to quadrats in the overseeded area.
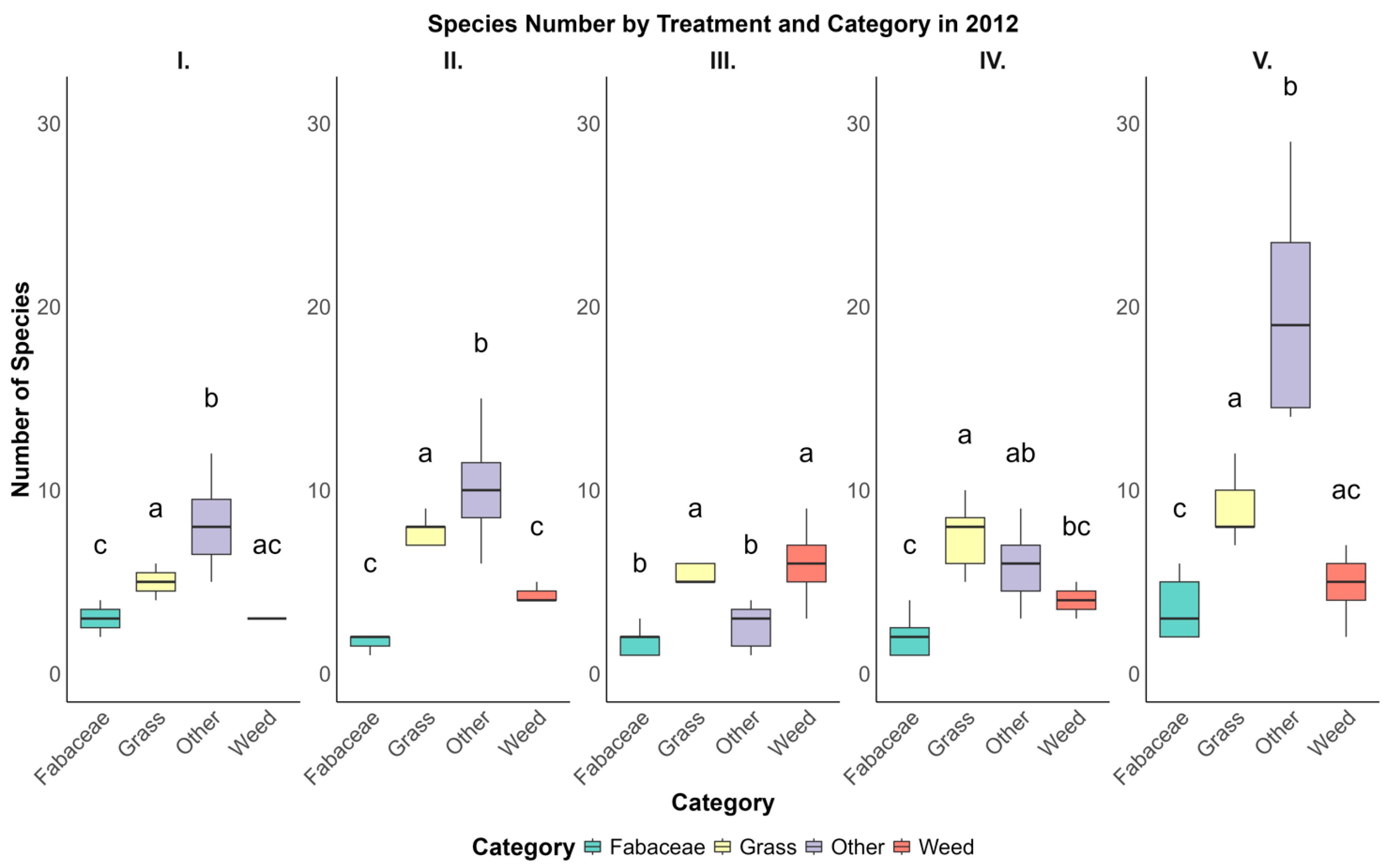
The analysis of the species number across various treatments and categories in 2012 and 2020 provides insights into how different types of vegetation respond to specific management practices over time. In this study, five treatments (I, II, III, IV, and V) were analyzed with each treatment, including the four primary categories of vegetation: Fabaceae, Grass, Other, and Weed. Descriptive statistics were calculated for each category within each treatment to provide a detailed overview of species distribution in terms of mean values and standard deviations (Figure 4).

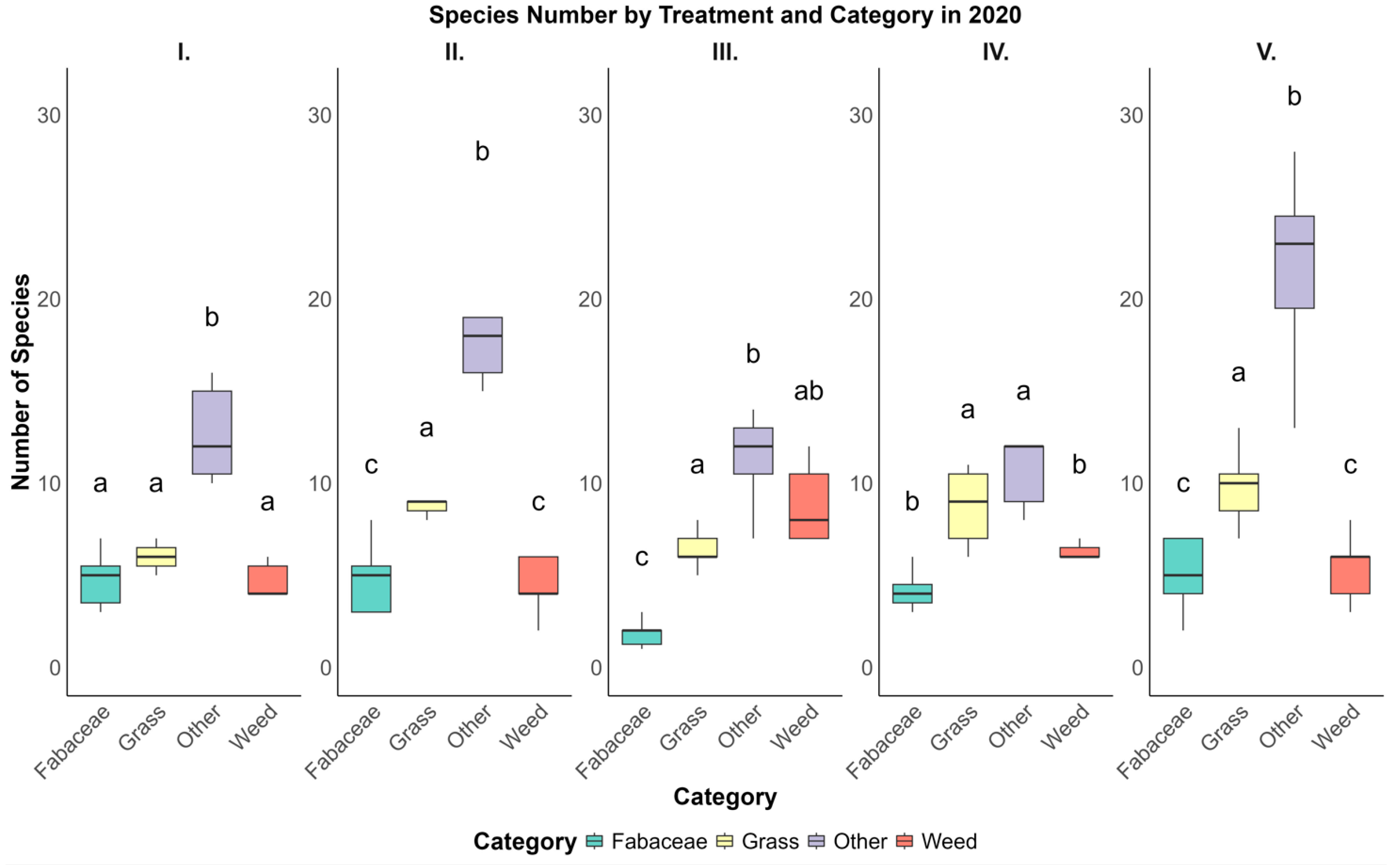
Figure 4.
Species distribution across vegetation categories under five pasture treatments (I–V) in 2012, showing notable differences in species numbers across categories. The ‘Grass’ category demonstrated relatively high species richness in multiple treatments, while “Fabaceae” generally exhibited lower counts. Letters above the boxplots denote statistically significant differences between categories within each treatment based on Tukey’s HSD test (p < 0.05).
In 2020, the distribution of species in Treatment I showed a particularly high mean species count in the “Other” category, with an average of 15.00 ± 4.50 species. This was considerably higher than the Fabaceae category in the same treatment, which averaged 4.20 ± 1.35 species. Such disparities between categories were evident across other treatments in 2020 as well. For instance, in Treatment IV, the mean species number for the “Other” category was 18.50 ± 4.50, again markedly higher than Fabaceae, which had a mean of 4.00 ± 1.20 species. The “Grass” and “Weed” categories also demonstrated variability in species numbers across treatments in 2020, although their values generally fell between those of Fabaceae and Other. In general, the category “Other” consistently displayed higher species numbers across treatments, suggesting that certain treatments in 2020 may have been more conducive to supporting diverse species in this category.
Conversely, in 2012, the patterns were somewhat different. For example, in Treatment II, the “Grass” category exhibited a relatively high mean species count of 8.00 ± 1.63, whereas “Fabaceae” was again lower, with an average of 3.00 ± 1.23 species. This trend in relatively lower species counts in the Fabaceae category continued across other treatments in 2012, indicating a consistent pattern where Fabaceae was less represented in terms of species number compared to Grass and Other. Treatment III in 2012 also showed a similar distribution, with the Grass category having a mean species number of 5.00 ± 1.35, which was higher than Fabaceae, averaging 2.80 ± 1.15 species. These findings suggest that the Fabaceae category may inherently have lower species diversity or that the environmental conditions or treatment practices in 2012 were not as favorable for its proliferation.
To further investigate the effect of the treatments and categories on species numbers, a two-way ANOVA was conducted for each year. In 2020, the analysis revealed a significant effect of treatment on species number, with an F-ratio of F(4, 24) = 15.42 and a p-value of <0.001, indicating a statistically significant impact of treatment type on species numbers. Similarly, there was a significant effect of category on species number, F(3, 24) = 18.33, p < 0.001, confirming that the category to which a species belongs also significantly influenced the number of species observed (Figure 5). This result highlights the importance of both treatment practices and the specific type of vegetation in determining biodiversity outcomes in 2020. In 2012, the ANOVA results displayed a comparable trend. The effect of treatment on species number was again significant, with F(4, 24) = 10.11 and a p-value of <0.001. The effect of the categories was also significant, with F(3, 24) = 12.75, p < 0.001, suggesting that both factors—treatment and category—were crucial determinants of species number in that year as well.

Figure 5.
Species richness across different vegetation categories under five pasture treatments (I–V) in 2020. Higher species numbers were observed in the ‘Other’ category, especially under Treatment I and IV, indicating a favorable response to these treatments. Letters above the boxplots represent significant differences between categories within each treatment, as determined by Tukey’s HSD test (p < 0.05).
To pinpoint specific differences between categories within each treatment, a post hoc analysis using Tukey’s HSD test was employed. This analysis provided more granular insights into how individual categories differed from one another within each treatment. In 2020, the results from Tukey’s test indicated that in Treatment I, the “Other” category, with a mean species number of 15.00 ± 4.50, had significantly higher species counts compared to the “Weed” category, which averaged 8.50 ± 2.50 species, with a p-value of 0.034. This significant difference suggests that Treatment I in 2020 was more favorable for species in the “Other” category than for those in the “Weed” category. Additionally, in Treatment IV, the “Other” category again had a notably higher mean species number (18.50 ± 4.50) than Fabaceae (4.00 ± 1.20), with a highly significant p-value of <0.01. These findings imply that certain treatments in 2020 may promote species diversity in categories such as “Other” more effectively than in others like Fabaceae.
The 2012 Tukey’s HSD test results revealed distinct patterns as well. In Treatment I, the Grass category, with an average species number of 8.00 ± 1.63, had significantly higher counts compared to Fabaceae, which had a mean of 3.00 ± 1.23 species (p = 0.049). This result indicates that in 2012, Treatment I supported a higher diversity of Grass species relative to Fabaceae. Similarly, in Treatment V, the “Weed” category, with an average of 6.50 ± 2.01 species, showed a significantly higher species count compared to “Fabaceae” (2.20 ± 0.98), with a p-value of 0.021. These differences highlight the variability in species support across treatments and suggest that specific treatments in 2012 may have favored weed species over Fabaceae.
In summary, this analysis reveals that both treatment type and category significantly influence species number, with notable differences observed between 2012 and 2020. The 2020 data generally show an increase in species numbers across most treatments compared to 2012, particularly in categories such as “Other” and “Weed.” This could be attributed to changes in environmental conditions or detailed treatment practices that may have been implemented between 2012 and 2020, potentially creating more favorable conditions for a wider variety of species. On the other hand, categories like Fabaceae consistently exhibited lower species counts across treatments and years, suggesting that this category may either have lower inherent species diversity or may not respond as positively to the treatments applied.
Results Based on Relative Ecological Indicators
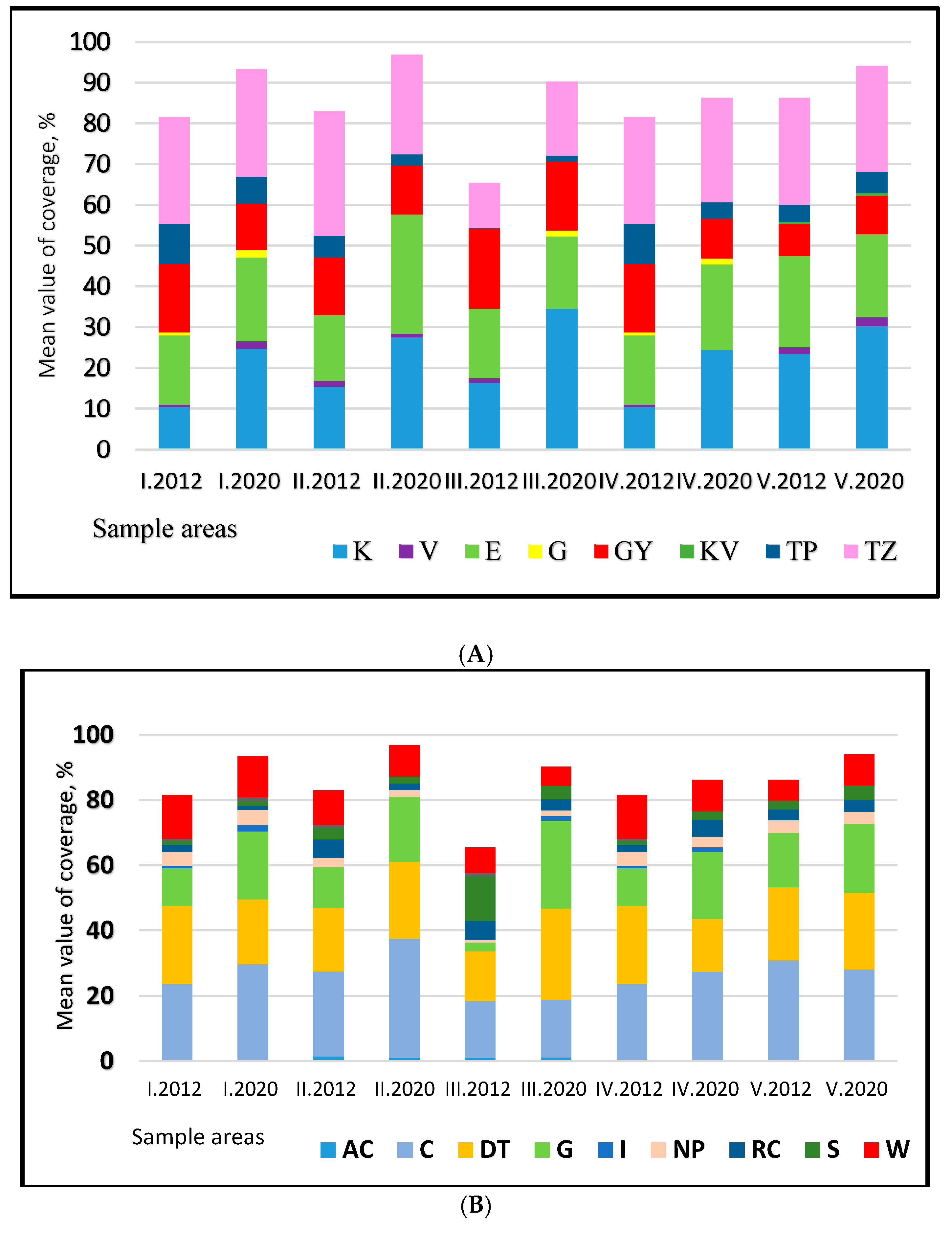
Across all sample areas, the proportion of natural disturbance-tolerant species (TZ) was the largest. Among the community-forming species, Festuca pseudovina and Poa angustifolia were prominent, while among natural disturbance-tolerant species, Achillea collina, Dactylis glomerata, and Lotus corniculatus were observed (Figure 6A). In 2012, Achillea collina, Dactylis glomerata, and Festuca arundinacea were dominant, and the dominance of Achillea collina and Dactylis glomerata persisted into 2020. Weed species (GY) were still significant in 2012, especially in the direct-sown (III) sample area. Stachys annua and Taraxacum officinale were notable in sample areas I, II, and IV in 2012, but their proportions decreased across all areas by 2020. Convolvulus arvensis and Cirsium arvense also appeared in 2012 with coverage values of 1–3%. Convolvulus arvensis was found in every sample area in 2012, though its proportion declined by 2020. Despite being a competitive weed, Cirsium arvense did not achieve significant dominance by 2020. The distribution of species conservation categories similarly reflected a predominance of natural disturbance-tolerant species (DT) across the sample areas (Figure 6B). Coverage values for ruderal competitors (RC) were high even in the direct-sown area. The proportion of competitive species (C), which are natural vegetation species, increased across all areas by 2020 (e.g., Agrostis stolonifera, Bromus inermis, Bromus erectus, Festuca pratensis, Festuca rupicola, Festuca pseudovina). Additionally, the abundance of generalist species (G) increased markedly.

Figure 6.
Distribution of species by conservation status in different treatments I–V: ((A): conservation status; (B): social behavior types) (K: companion species, V: protected species, KV: highly protected species, E: association-forming species, GY: weed species, G: economic plants, TP: pioneer species, TZ: disturbance tolerant; AC: aggressive competitors, C: competitors, DT: interference-tolerant plants in natural habitats, G: generalists, I: introduced and emerged crops, NP: natural pioneers, RC: rudimentary competitor of native flora, S: specialists, W: weeds).
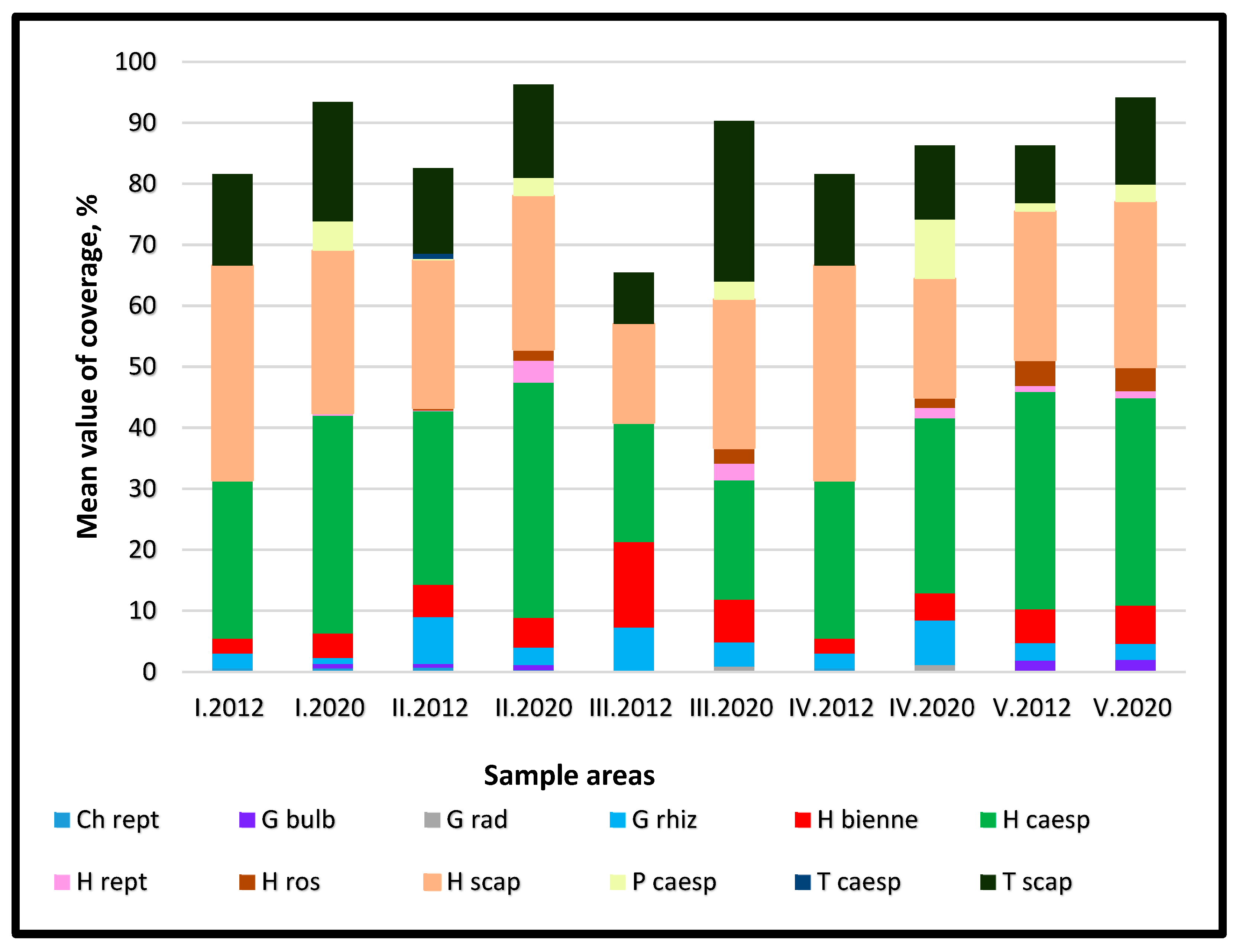
In terms of life forms based on Pignatti’s classification, perennial grasses (H caesp) dominated all five sample areas (Figure 7). In the spontaneously grassed area (I), Festuca rupicola, Poa angustifolia, and Dactylis glomerata represented the largest proportions of the grass-forming species (H caesp). Among grasses, the proportion of erect-stemmed species (H scap) was also notable. In the area covered with chopped hay mulch (II), grass-forming and erect-stemmed species were predominant, with rosette-forming perennials (H ros), represented by Taraxacum officinale and Plantago lanceolata, also present in 2012. The amount of rosette-forming perennials increased across all sample areas by 2020, with the abandoned Treatment IV (alfalfa field) showing the highest proportion. Among rhizomatous geophytes (G rhiz), Elymus repens had particularly high coverage values. In terms of life form diversity, the overseeded old fallow Treatment V (grassland) and the area with chopped Treatment II. (hay mulch) were the most varied. In the direct-sown area (III), Elymus repens was the most prominent among rhizomatous geophytes, while Bromus inermis, Dactylis glomerata, Poa angustifolia, and Festuca arundinacea were notable grass species. This sample area had the lowest diversity in life forms. As an indicator of grazing pressure, creeping-stemmed perennials (H rept) and rosette-forming species (H ros) were present in significant amounts. However, by 2020 these species did not become dominant. Coverage of Plantago lanceolata, a rosette-forming species, increased across all sample areas by 2020, whereas Taraxacum officinale coverage decreased, resulting in no net increase in the category’s coverage. Among creeping-stemmed species, Trifolium repens was the most significant, but it appeared at higher proportions only in the spontaneously grassed area (I) and the hay mulch-covered area (II) in 2020 and in the direct-sown grassland (III) in 2012.

Figure 7.
Distribution of species based on Pignatti’s life-form types across sample areas of different treatments I–V.
Estimates based on 2020 data indicated an increase in above-ground green biomass in most sample areas compared to 2012 (Table 1). The amount of green biomass increased in most sample areas from 2012 to 2020.

Table 1.
Expected green biomass by the Balázs method across sample areas (Treatments I–V; I: spontaneously grassed fallow, II: hay mulch area, III: sown grassland post-full soil preparation with two seed mixes, IV: abandoned alfalfa field undergoing transformation, V: 30-year-old oversown grassland subjected to mowing and grazing).
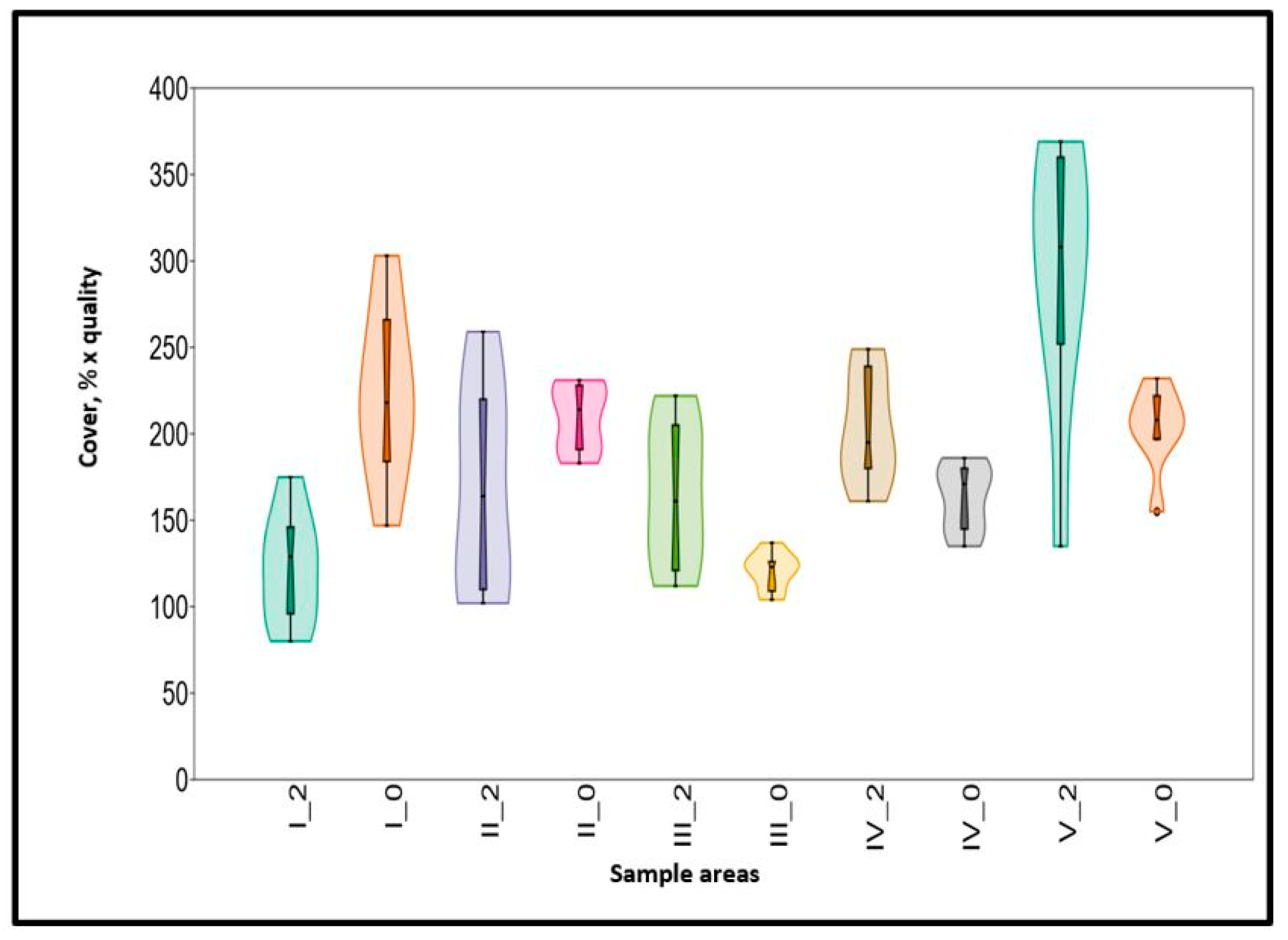
The quality of the fodder, forage value, which can be calculated based on the Balázs method, and is given by the product of the cover values of the rotating species and the quality values determined by Balázs, did not develop in parallel with the green biomass (Figure 8). In the area with abandoned alfalfa field treatment IV, it even decreased significantly. In the other sample areas, compared to 2012, these quality values leveled off by 2020.

Figure 8.
The development of forage values according to Balázs between 2012 (2) and 2020 (0) in all sample areas (treatment I–V).
4. Discussion
The study area, with poor soil productivity and situated in an extremely arid climate, reflects the significant impacts of recent climate change trends observed in the Carpathian Basin [70,71,72]. Pannonian grasslands increasingly exhibit trends similar to those in semi-desert or Mediterranean regions, where the abandonment of grasslands leads to drastic changes and vegetation degradation [73,74,75]. Originally classified as a dry grassland type, the study area was unsuitable for intensive agricultural use, likely hosting rocky grassland or semi-natural vegetation [76].
A detailed examination in 2012 revealed that diverse restoration techniques resulted in significant differences among the five sample areas. Only 3.7% of the species observed that year were present across all plots, consisting entirely of disturbance-tolerant species (Dactylis glomerata, Festuca arundinacea, Potentilla argentea, Trifolium pratense) [61,62]. By 2020, the number and proportion of shared species increased to 11%, with additional disturbance-tolerant species (Agrimonia eupatoria, Bromus hordeaceus, Elymus repens, Trifolium repens, Achillea collina) present. Two natural competitor species, Agrostis stolonifera and Festuca pseudovina, appeared in all sample areas, suggesting improved ecological stability, particularly due to the dominant Festuca pseudovina [76].
The area was mowed for two years following grassland restoration, significantly enhancing species richness across all sample sites. Studies show that mowing promotes vegetation establishment and stability, suppressing weeds and facilitating the establishment of introduced species [2,65,77,78]. Mowing also reduces litter, which can help establish target species in newly sown or overseeded areas. This practice is frequently used to control weeds, as natural species generally exhibit lower competitive capacity than introduced grasses [79,80,81,82]. Additionally, a nearby propagule source greatly benefited the sample areas.
Grazing following mowing further improved grassland structure, corroborating findings by Price et al. [3] that controlled grazing has positive effects on grassland dynamics. Conversely, vegetation can deteriorate with grazing cessation, leading to degradation. Grazing animals selectively consume green biomass, favoring certain plant groups, although this effect varies by site and climate. In fertile temperate areas, grazing often increases the abundance of grasses [82], while semi-arid regions, like savannas, experience a decrease in grasses under grazing [83,84,85]. Rodríguez et al. [86] showed that grass abundance increased on sheep-grazed plots in the Pyrenees, while forbs dominated cattle-grazed plots. In the current study areas, we observed a decrease in grass species diversity but an increase in their coverage values. Broad-leaved grasses (Dactylis glomerata, Elymus repens, Festuca arundinacea) declined under grazing, while narrow-leaved grasses (Festuca pseudovina, Festuca rupicola) increased. This dynamic, essential for restoring natural vegetation in semi-arid climates, illustrates that robust cattle grazing reduces tall, broad-leaved grasses while promoting the spread of lower, narrow-leaved grasses, aiding in natural vegetation restoration. Cynodon dactylon, a creeping species common to arid environments, also increased under grazing [63].
Grazing intensity is crucial for species replacement, as higher intensity creates more bare patches, raising weed invasion risks [86,87]. Weed reduction across all sample types indicates appropriate grazing pressure without overgrazing. Grasses are dominant, contributing to both ecological structure and grassland management value. The presence of Festuca species, particularly Festuca pseudovina and Festuca rupicola (the latter characterizes Salvinio nemorosae-Festucetosum rupicolae association), highlights their role in supporting potential native slope steppe vegetation [76]. Festuca arundinacea, a Eurasian forage grass widely planted in North America [88], also plays a significant role in overseeding in the Pannonian region. Records indicate its previous use in an overseeding effort in 1989, though detailed methods and species were unavailable for this study [88,89]. Elymus repens’ abundance and behavior are notable under grazing, as its ecological resilience and nutritional value make it valuable under arid climate conditions despite its weed classification by Borhidi [62] and Simon [61]. Elymus repens occurred in notable coverage in three plots in 2012 (II, III, and IV) and plays a structural role in abandoned alfalfa fields, where species richness remained low in both observations (44 and 70 species). Poa angustifolia became dominant in these fields, a species known for colonizing fallows [90].
An analysis of life forms following Pignatti’s classification further supports that the sample areas were not overgrazed, as creeping or rhizomatous perennials (H rept) and rosette-forming species (T ros and H ros) did not dominate [63,64,91,92]. Rosette perennials (H ros) were most abundant in the overseeded alfalfa field (IV), likely due to lower cover values and increased grazing pressure. These findings align with Tóth et al. [81], who also found that spontaneously recovering grasslands achieved the best ecological states, featuring high native species richness and cover similar to those observed in this study. Although seeded grasses initially suppressed unwanted species, natural target species representative of grassland ecosystems were slower to establish.
5. Conclusions
Addressing our initial hypothesis and research questions, we observed that grassland establishment types resulted in substantial differences. Although some differences between the various grassland types persisted by the end of the study period, especially post-grazing, the magnitude of these differences diminished over time. Grazing with Hungarian Grey cattle significantly enhanced species composition across all grassland types, notably increasing the abundance of ecologically valuable species. The coverage and agricultural value of each grassland type also showed improvement in parallel.
The critical question arises: will the key to restoring semi-arid and arid Pannonian grasslands be the method of grassland establishment or grazing with robust cattle breeds? Our findings suggest that grazing with Hungarian Grey cattle offers a balanced solution, akin to mixed cattle and sheep grazing systems seen in the Pyrenees [86], which maintains a balance between grass and forb species, allowing neither to dominate excessively.
Of the seeded grass species in the sown grasslands, Lolium perenne, Festuca pratensis, and Festuca rubra were absent as early as 2012, indicating their unsuitability for dry grasslands. Festuca pratensis appeared only once in sample site V in 2012 but disappeared by 2020. Bromus inermis persisted with low coverage in the direct-sown grassland (III) in both 2012 and 2020. Only Dactylis glomerata and Festuca arundinacea remained from the seed mixture, though these species were present across other sample areas as well. Therefore, the applied seed mix was uneconomical for dry grasslands. We recommend the use of Festuca species commonly found and structurally significant in dry grasslands (Festuca pseudovina and Festuca rupicola) alongside Dactylis glomerata and Festuca arundinacea.
Direct seeding and alfalfa-seeding grassland establishment approaches were also found to be inefficient. Instead, promoting natural grassland regeneration on fallow lands, especially when covered with hay mulch, proved the most effective restoration method and is recommended. From ecological, grassland management, and economic perspectives, these approaches offer the most viable solutions.
The restoration’s success is underscored by the massive occurrence of Orchis morio in the overseeded fallow area (V) by 2022 (Figure 9).

Figure 9.
Massive occurrence of Orchis morio in the overseeded fallow area.
Overall, the findings underscore the complex relationship between treatment practices and vegetation type in shaping biodiversity outcomes. These results suggest that some treatments may enhance species diversity in certain vegetation types while having limited effects on others. Future research could benefit from exploring the underlying ecological mechanisms that drive these differences, particularly examining how specific environmental conditions or management practices influence the growth and proliferation of different vegetation categories. Understanding these dynamics can inform more targeted conservation and management strategies, aimed at preserving or enhancing biodiversity across various ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/land13122135/s1.
Author Contributions
Conceptualization, K.P., S.S. and Z.W.; methodology, K.P., V.K., J.B. and S.S.; software, Z.K., P.P., F.P. and A.K.; formal analysis, Z.W. and E.S.-F.; investigation, K.P., E.S.-F., S.S. and I.T.-J.; writing—original draft preparation, K.P., V.K., E.S.-F., I.T.-J., Z.K., S.S. and J.B.; writing—review and editing, F.P., K.P. and E.S.-F.; supervision, S.S. and K.P.; funding acquisition, K.P. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by OTKA K-147342, the Strategic Research Found of the University of Veterinary Medicine Budapest (Grant No. SRF-002) and the Research Excellence Program of the Hungarian University of Agriculture and Life Sciences.
Data Availability Statement
The original contributions presented in this study are included in the Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Péter Penksza was employed by the company Anton Paar Hungary. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Maestre, F.T.; Le Bagousse-Pinguet, Y.; Delgado-Baquerizo, M.; Eldridge, D.J.; Saiz, H.; Berdugo, M.; Gozalo, B.; Ochoa, V.; Guirado, E.; García-Gómez, M.; et al. Grazing and ecosystem service delivery in global drylands. Science 2022, 378, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.G.; Török, P.; Hermann, J.-M.; Kiehl, K.; Kirmer, A.; Kollmann, J.; Overbeck, G.E.; Tischew, S.; Allen, E.B.; Bakker, J.D.; et al. Challenges and opportunities for grassland restoration: A global perspective of best practices in the era of climate change. Glob. Ecol. Conserv. 2023, 46, e02612. [Google Scholar] [CrossRef]
- Price, J.N.; Sitters, J.; Ohlert, T.; Tognetti, P.M.; Brown, C.S.; Seabloom, E.W.; Borer, E.T.; Prober, S.M.; Bakker, E.S.; MacDougall, A.S.; et al. Evolutionary History of Grazing and Resources Determine Herbivore Exclusion Effects on Plant Diversity. Nat. Ecol. Evol. 2022, 6, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Dengler, J.; Bergmeier, E.; Willner, W.; Chytrý, M. Towards a consistent classification of European grasslands. J. Veg. Sci. 2013, 16, 518–520. [Google Scholar] [CrossRef]
- Wilson, J.B.; Peet, R.K.; Dengler, J.; Pärtel, M. Plant species richness: The world records. J. Veg. Sci. 2012, 23, 796–802. [Google Scholar] [CrossRef]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Wesche, K.; Ambarlı, D.; Kamp, J.; Török, P.; Treiber, J.; Dengler, J. The Palaearctic steppe biome: A new synthesis. Biodivers. Conserv. 2016, 25, 2197–2231. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Habel, J.C.; Dengler, J.; Janišová, M.; Török, P.; Wellstein, C.; Wiezik, M. European grassland ecosystems: Threatened hotspots of biodiversity. Biodivers. Conserv. 2013, 22, 2131–2138. [Google Scholar] [CrossRef]
- Cremene, C.; Groza, G.; Rakosy, L.; Schileyko, A.A.; Baur, A.; Erhardt, A.; Baur, B. Alterations of steppe-like grasslands in Eastern Europe: A threat to regional biodiversity hotspots. Conserv. Biol. 2005, 19, 1606–1618. Available online: http://edoc.unibas.ch/dok/A5249054 (accessed on 4 April 2024). [CrossRef]
- Conant, R.T.; Paustian, K.; Elliott, E.T. Grassland management and conversion into grassland: Effect on soil carbon. Ecol. Appl. 2001, 11, 343–355. Available online: https://api.semanticscholar.org/CorpusID:84726423 (accessed on 4 April 2024). [CrossRef]
- Hobohm, C.; Bruchmann, I. Endemische Gefäßpflanzen und ihre Habitate in Europa: Plädoyer für den Schutz der Grasland-Ökosysteme. Berichte Der Reinhold-Tüxen-Ges. 2009, 21, 142–161. [Google Scholar]
- Collins, S.L.; Chung, Y.A.; Baur, L.E.; Hallmark, A.; Ohlert, T.J.; Rudgers, J.A. Press–pulse interactions and long-term community dynamics in a Chihuahuan Desert grassland. J. Veg. Sci. 2020, 31, 722–732. [Google Scholar] [CrossRef]
- Cleland, E.E.; Collins, S.L.; Dickson, T.L.; Farrer, E.C.; Gross, K.L.; Gherardi, L.A.; Hallett, L.M.; Hobbs, R.J.; Hsu, J.S.; Turnbull, L.; et al. Sensitivity of grassland plant community composition to spatial vs. temporal variation in precipitation. Ecology 2013, 94, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.P.; Gornish, E.S.; Copeland, S. Climate-driven diversity loss in a grassland community. Proc. Natl. Acad. Sci. USA 2015, 112, 8672–8677. [Google Scholar] [CrossRef]
- Jonas, J.L.; Buhl, D.A.; Symstad, A.J. Impacts of weather on long-term patterns of plant richness and diversity vary with location and management. Ecology 2015, 96, 2417–2432. [Google Scholar] [CrossRef]
- Bartha, S.; Szabó, G.; Csete, S.; Purger, D.; Házi, J.; Csathó, A.I.; Campetella, G.; Canullo, R.; Chelli, S.; Tsakalos, J.L.; et al. High-Resolution Transect Sampling and Multiple Scale Diversity Analyses for Evaluating Grassland Resilience to Climatic Extremes. Land 2022, 11, 378. [Google Scholar] [CrossRef]
- Dong, S.; Shang, Z.; Gao, J.; Boone, R.B. Enhancing sustainability of grassland ecosystems through ecological restoration and grazing management in an era of climate change on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2020, 287, 106684. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Wen, Y.; Ou, J. Quantitative analysis of the contributions of climatic and human factors to grassland productivity in northern China. Ecol. Indic. 2019, 103, 542–553. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; He, Y.; Shao, J.; Hu, Z.; Liu, R.; Hosseinibai, S. Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: A meta-analysis. Glob. Change Biol. 2017, 23, 1167–1179. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Evans, J.P.; McCabe, M.F.; De Jeu, R.A.; van Dijk, A.I.; Dolman, A.J.; Saizen, I. Changing climate and overgrazing are decimating Mongolian steppes. PLoS ONE 2013, 8, e57599. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582 . [Google Scholar] [CrossRef]
- Zhang, M.; Delgado-Baquerizo, M.; Li, G.; Isbell, F.; Wang, Y.; Hautier, Y.; Wang, Y.; Xiao, Y.; Cai, J.; Pan, X.; et al. Experimental impacts of grazing on grassland biodiversity and function are explained by aridity. Nat Commun. 2023, 14, 5040. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, V.; Ozogány, K.; Sándor, S.; Vegvari, Z.; Czető, C.; Nyírő, B.; Szabados, T.; Széles, L.; Barta, Z. Analysis habitat use, activity, and bogy condition scores of condition scores of Przewalski’s horses ib Hortobágy National Park, Hungary. Nat. Conserv. Res. 2019, 4 (Suppl. S2), 31–40. [Google Scholar] [CrossRef]
- Du, H.Q.; Zuo, X.A.; Li, S.; Wang, T.; Xue, X. Wind erosion changes induced by different grazing intensities in the desert steppe, Northern China. Agr. Ecosyst. Environ. 2019, 274, 1–13. [Google Scholar] [CrossRef]
- Zheng, M.; Song, J.; Ru, J.; Zhou, Z.; Zhong, M.; Jiang, L.; Hui, D.; Wan, S. Effects of grazing, wind erosion, and dust deposition on plant community composition and structure in a temperate steppe. Ecosystems 2021, 24, 403–420. [Google Scholar] [CrossRef]
- Török, P.; Dengler, J. Palaearctic Grasslands in Transition: Overarching Patterns and Future Prospects. Grasslands of the World: Diversity, Management and Conservation. In Grasslands of the World: Diversity, Management and Conservation; CRC Press: Boca Raton, FL, USA, 2018; pp. 15–26. [Google Scholar]
- Biró, M.; Bölöni, J.; Molnár, Z. Use of long-term data to evaluate loss and endangerment status of Natura 2000 habitats and effects of protected areas. Conserv. Biol. 2018, 32, 660–671. [Google Scholar] [CrossRef]
- Erdős, L.; Ambarlı, D.; Anenkhonov, O.A.; Bátori, Z.; Cserhalmi, D.; Kiss, M.; Kröel-Dulay, G.; Liu, H.; Magnes, M.; Molnár, Z.; et al. The edge of two worlds: A new review and synthesis on Eurasian forest-steppes. Appl. Veg. Sci. 2018, 21, 345–362. [Google Scholar] [CrossRef]
- Kuemmerle, T.; Levers, C.; Erb, K.; Estel, S.; Jepsen, M.R.; Müller, D.; Plutzar, C.; Stürck, J.; Verkerk, P.J.; Verburg, P.H.; et al. Hotspots of land use change in Europe. Environ. Res. Lett 2016, 11, 064020. [Google Scholar] [CrossRef]
- Watson, J.E.; Jones, K.R.; Fuller, R.A.; Di Marco, M.; Segan, D.B.; Butchart, S.H.; Allan, J.R.; McDonald-Madden, E.; Venter, O. Persistent disparities between recent rates of habitat conversion and protection and implications for future global conservation targets. Conserv. Lett. 2016, 9, 413–421. [Google Scholar] [CrossRef]
- Török, P.; Kelemen, A.; Valkó, O.; Deák, B.; Lukács, B.; Tóthmérész, B. Lucerne dominated fields recover native grass diversity without intensive management actions. J. Appl. Ecol. 2011, 48, 257–264. [Google Scholar] [CrossRef]
- Jongepierová, I.; Mitchley, J.; Tzanopoulos, J. A field experiment to recreate species rich hay meadows using regional seed mixtures. Biol. Conserv. 2007, 139, 297–305. [Google Scholar] [CrossRef]
- Török, P.; Deák, B.; Vida, E.; Valkó, O.; Lengyel Sz Tóthmérész, B. Restoring grassland biodiversity: Sowing low-diversity seed mixtures can lead to rapid favourable changes. Biol. Conserv. 2010, 143, 806–812. [Google Scholar] [CrossRef]
- Török, P.; Miglécz, T.; Valkó, O.; Kelemen, A.; Tóth, K.; Lengyel, S.; Tóthmérész, B. Fast recovery of grassland vegetation by a combination of seed mixture sowing and low-diversity hay transfer. Ecol. Eng. 2012, 44, 133–138. [Google Scholar] [CrossRef]
- Wagner, M.; Hulmes, S.; Hulmes, L.; Redhead, J.W.; Nowakowski, M.; Pywell, R.F. Green hay transfer for grassland restoration: Species capture and establishment. Restor. Ecol. 2020, 29, e13259. [Google Scholar] [CrossRef]
- Rasran, L.; Vogt, K.; Jensen, K. Seed content and conservation evaluation of hay material of fen grasslands. J. Nat. Conserv. 2006, 14, 34–45. [Google Scholar] [CrossRef]
- Donath, T.; Bissels, S.; Hölzel, N.; Otte, A. Large scale application of diaspore transfer with plant material in resoration practice- Impact of seed and microsite limitation. Biol. Conserv. 2007, 138, 224–234. [Google Scholar] [CrossRef]
- Nagy, G. A gyephasználati lehetőségek sokoldalúsága. Gyepgazdálkodási Közlemények 2008, 6, 5–7. [Google Scholar]
- Mijnsbrugge, V.K.; Bischoff, A.; Smith, B. A question of origin: Where and how to collect seed for ecological restoration. Basic Appl. Ecol. 2010, 11, 300–311. [Google Scholar] [CrossRef]
- Kaulfuß, F.; Rosbakh, S.; Reisch, C. Grassland restoration by local seed mixtures: New evidence from a practical 15-year restoration study. Appl. Veg. Sci. 2022, 25, e12652. [Google Scholar] [CrossRef]
- Billeter, R.; Peintinger, M.; Diemer, M. Restoration of montane fen meadows by mowing remains possible after 4–35 years of abandonment. Acta Bot. Helv. 2007, 117, 1–13. [Google Scholar] [CrossRef]
- Házi, J.; Penksza, K.; Bartha, S.; Hufnagel, L.; Tóth, A.; Gyuricza, C.; Szentes, S. Cut mowing and grazing Effects with grey cattle on plant species composition in case of Pannon wet grasslands. Appl. Ecol. Environ. Res. 2012, 10, 223–231. [Google Scholar] [CrossRef]
- Kiss, T.; Lévai, P.; Ferencz, Á.; Szentes, S.; Hufnagel, L.; Nagy, A.; Balogh, Á.; Pintér, O.; Saláta, D.; Házi, J.; et al. Change of composition and diversity of species and grassland management between different grazing intensity—In Pannonian dry and wet grasslands. Appl. Ecol. Environ. Res. 2011, 9, 197–230. [Google Scholar] [CrossRef]
- Aboling, S. Do Poisonous Plants in Pastures Communicate Their Toxicity? Meta-Study and Evaluation of Poisoning Cases in Central Europe. Animals 2023, 13, 3795. [Google Scholar] [CrossRef] [PubMed]
- Hessle, A.; Rutter, M.; Wallin, K. Effect of breed, season and pasture moisture gradient on foraging behaviour in cattle on semi-natural grasslands. Appl. Anim. Behav. Sci. 2008, 111, 108–119. [Google Scholar] [CrossRef]
- Hessle, A.; Wissman, J.; Bertilsson, J.; Burstedt, E. Effect of breed of cattle and season on diet selection and defoliation of competitive plant species in semi-natural grasslands. Grass Forage Sci. 2008, 63, 86–93. [Google Scholar] [CrossRef]
- Pauler, C.M.; Isselstein, J.; Braunbeck, T.; Schneider, M.K. Influence of Highland and Production-Oriented Cattle Breeds on Pasture Vegetation: A Pairwise Assessment across Broad Environmental Gradients. Agric. Ecosyst. Environ. 2019, 284, 106585. [Google Scholar] [CrossRef]
- Pauler, C.M.; Isselstein, J.; Suter, M.; Berard, J.; Braunbeck, T.; Schneider, M.K. Choosy grazers: Influence of plant traits on forage selection by three cattle breeds. Funct. Ecol. 2020, 34, 980–992. [Google Scholar] [CrossRef]
- Spiegal, S.; Estell, R.E.; Cibils, A.F.; James, D.K.; Peinetti, H.R.; Browning, D.M.; Romig, K.B.; Gonzalez, A.L.; Lyons, A.J.; Bestelmeyer, B.T. Seasonal divergence of landscape use by heritage and conventional cattle on desert rangeland. Rangel. Ecol. Manag. 2019, 72, 590–601. [Google Scholar] [CrossRef]
- Poetsch, E.M.; Resch, R.; Haeusler, J.; Steinwidder, A. Productivity and floristic diversity of a continuous grazing system on short swards in mountainous regions of Austria. Grassl. Sci. Eur. 2010, 19, 139–141. [Google Scholar]
- Mandaluniz, N.; Aldezabal, A.; Oregui, L.M. Diet selection of beef cattle on Atlantic grassland-heathland mosaic: Are heathers more preferred than expected? Livest. Sci. 2011, 138, 49–55. [Google Scholar] [CrossRef]
- Fraser, M.D.; Theobald, V.J.; Griffiths, J.B.; Morris, S.M.; Moorby, J.M. Comparative diet selection by cattle and sheep grazing two contrasting heathland communities. Agric. Ecosyst. Environ. 2009, 129, 182–192. [Google Scholar] [CrossRef]
- Koczura, M.; Martin, B.; Bouchon, M.; Turille, G.; Berard, J.; Farruggia, A.; Kreuzer, M.; Coppa, M. Grazing behaviour of dairy cows on biodiverse mountain pastures is more influenced by slope than cow breed. Animal 2019, 13, 2594–2602. [Google Scholar] [CrossRef]
- De Vries, W.M.F.; Daleboudt, C. Foraging strategy of cattle in patchy grassland. Oecologia 1994, 100, 98–106. [Google Scholar] [CrossRef] [PubMed]
- McGeough, E.J.; Cattani, D.J.; Koscielny, Z.; Hewitt, B.; Ominski, K.H. Annual and perennial forages for fall/winter grazing in western Canada. Can. J. Plant Sci. 2018, 98, 247–254. [Google Scholar] [CrossRef]
- Dövényi, Z. (szerk.) Magyarország Kistájaink a Katasztere, 2nd ed.; Akadémiai Kiadó: Budapest, Hungary, 2010. [Google Scholar]
- Boglárka, U.; Lajos, J.; László, S.; Levente, I.V.; Penksza, A.; Szentes, S.; Házi, J.; Sutyinszki, Z.; Tth, A.; Penksza, K. Telepített és felújított gyepek, parlagok összehasonlító botanikai, gyepgazdálkodási vizsgálata [Coenological and (economical) forage value comparison of seminatural and man-made grasslands in Hungary]. Anim. Welf. Ethol. Hous. Syst. 2014, 10, 85–106. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie; Wien: New York, NY, USA, 1964; pp. 2–865. [Google Scholar]
- Király, G. Új Magyar Füvészkönyv. Magyarország Hajtásos Növényei. határozókulcsok [New Hungarian Herbal. The Vascular Plants of Hungary. Identification Key]. Király, G., Ed.; Aggteleki Nemzeti Park Igazgatóság: Jósvafő, Hungary, 2009. [Google Scholar]
- Simon, T. A Magyar Edényes Flóra Határozója [Definier of the Hungarian Vascular Flora]; Tankönyvkiadó: Budapest, Hungary, 2000. [Google Scholar]
- Borhidi, A. Social behavior types, the naturalness and relative ecological indicator values of the higher plants in the Hungarian Flora. Acta Bot. Acad. Sci. Hung. 1995, 39, 97–181. [Google Scholar]
- Pignatti, S. Valori di bioindicazione delle piante vascolari della flora d’Italia. Braun Blanquetia 2005, 39, 1–97. [Google Scholar]
- Briemle, G.; Nitsche, S.; Nitsche, L. Nutzungswertzahlen für Gefäßpflanzen des Grünlandes. Schriftenreihe für Vegetationskunde; Bundesamt für Naturschutz: Bonn, Germany, 2005; pp. 203–225. [Google Scholar]
- Házi, J.; Penksza, K.; Barczi, A.; Szentes, S.; Pápay, G. Effects of Long-Term Mowing on Biomass Composition in Pannonian Dry Grasslands. Agronomy 2022, 12, 1107. [Google Scholar] [CrossRef]
- Balázs, F. A Gyepek Botanikai és Gazdasági Értékelése [Botanical and Economic Assessment of Grasslands]; Mezőgazdasági Kiadó: Budapest, Hungary, 1960; pp. 3–28. [Google Scholar]
- Klapp, E.; Boeker, P.; König, F.; Stählin, A. Wertzahlen der Grünlandpflanzen. Grünland 1953, 2, 38–40. [Google Scholar]
- Ushey, K.; Allaire, J.; Tang, Y. Reticulate: Interface to ‘Python’. R Package Version 1.38.0, 2024. Available online: https://github.com/rstudio/reticulate (accessed on 13 May 2024).
- Waskom, M. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Bartholy, J.; Pongrácz, R. Tendencies of extreme climate indicates based on daily precipitation in the Carpathian Basin for the 20th century. Időjárás 2005, 109, 1–20. [Google Scholar]
- Bartholy, J.; Pongrácz, R. Comparing tendencies of some temperature and precipitation on global and regional scales. Időjárás 2006, 110, 35–48. [Google Scholar]
- Bartholy, J.; Pongrácz, R. Regional analysis of extreme temperature and precipitation indices for the Carpathian Basin from 1946 to 2001. Glob. Planet. Change 2007, 57, 83–95. [Google Scholar] [CrossRef]
- Peco, B.; Sánchez, A.M.; Azcárate, F.M. Abandonment in grazing systems: Consequences for vegetation and soil. Agric. Ecosyst. Environ. 2006, 113, 284–294. [Google Scholar] [CrossRef]
- Catorci, A.; Cesaretti, S.; Gatti, R. Biodiversity conservation: Geosynphytosociology as a tool of analysis and modelling of grassland systems. Hacquetia 2009, 8, 129–146. Available online: https://ojs.zrc-sazu.si/hacquetia/article/view/2909 (accessed on 4 April 2024). [CrossRef]
- Catorci, A.; Piermarteri, K.; Penksza, K.; Házi, J.; Tardella, F.M. Filtering effect of temporal niche fluctuation and amplitude of environmental variations on the trait-related flowering patterns: Lesson from sub-Mediterranean grasslands. Sci. Rep. 2017, 7, 12034. [Google Scholar] [CrossRef]
- Borhidi, A.; Kevey, B.; Lendvai, G.; Seregélyes, T. Plant Communities of Hungary; Akadémiai Kiadó: Budapest, Hungary, 2012. [Google Scholar]
- Deák, B.; Tóthmérész, B. A kaszálás hatása a Hortobágy Nyírőlapos csetkákás társulásában [Effect of cutting on a Bolboschoenetum maritime eleochariosum association in the Nyirőlapos (Hortobágy)]. Természetvédelmi Közlemények 2007, 13, 179–186. [Google Scholar]
- Deák, B.; Valkó, O.; Kelemen, A.; Török, P.; Miglécz, T.; Ölvedi, T.; Lengyel Sz Tóthmérész, B. Litter and graminoid biomass accumulation suppresses weedy forbs in grassland restoration. Plant Biosyst. 2011, 145, 730–737. [Google Scholar] [CrossRef]
- Prach, K.; Pyšek, P. Using spontaneous succession for restoration of human-disturbed habitats: Experience from Central Europe. Ecol. Eng. 2001, 17, 55–62. [Google Scholar] [CrossRef]
- McLachlan, S.M.; Knispel, A.L. Assessment of long-term tallgrass prairie restoration in Manitoba, Canada. Biol. Conserv. 2005, 124, 75–88. [Google Scholar] [CrossRef]
- Tóth, E.; Deák, B.; Valkó, O.; Kelemen, A.; Miglécz, T.; Tóthmérész, B.; Török, P. Livestock type is more crucial than grazing intensity:traditional cattle and sheep grazing in short-grass steppes. Land Degrad. Dev. 2018, 29, 231–239. [Google Scholar] [CrossRef]
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. mowing: A meta-analysis of biodiversity benefits for grassland management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Török, P.; Lindborg, R.; Eldridge, D.; Pakeman, R. Grazing effects on vegetation: Biodiversity, management, and restoration. Appl. Veg. Sci. 2024, 27, e12794. [Google Scholar] [CrossRef]
- Ding, J.; Eldridge, D.J. The success of woody plant removal depends on encroachment stage and plant traits. Nat. Plants 2023, 9, 58–67. [Google Scholar] [CrossRef]
- Carboni, L.J.; Yahdjian, L.; Oñatibia, G.R. Effects of livestock grazing intensification on plant communities of Patagonian drylands increase with increasing aridity. Appl. Veg. Sci. 2023, 26, e12754. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ibanez, M.; Chocarro, C.; Sebastià, M.T. Livestock species rather than grazing intensity shape plant guild proportions in interaction with multiple environmental drivers in grassland from the Pyrenees. Appl. Veg. Sci. 2023, 26, e12724. [Google Scholar] [CrossRef]
- Kovacsics-Vári, G.; Sonkoly, J.; Tóth, K.; McIntosh-Buday, A.; Cando, P.D.; Törő-Szijgyártó, V.; Balogh, N.; Suntaxi, L.R.G.; Ami, F.D.E.; Demeter, L.; et al. Intensity-dependent effects of cattle and sheep grazing in sand grasslands—Does livestock type really matter? Appl. Veg. Sci. 2023, 26, e12727. [Google Scholar] [CrossRef]
- Scott, B.; Nelson, M.; Coon, J.J.; Schacht, W.H.; Miller, J.R. Cattle select against the invasive grass tall fescue in heterogeneous pastures managed with prescribed fire. Grass Forage Sci. 2019, 74, 486–495. [Google Scholar] [CrossRef]
- Tulloch, A.I.T.; Healy, A.; Silcock, J.; Wardle, G.M.; Dickman, C.R.; Frank, A.S.K.; Aubault, H.; Barton, K.; Greenville, A.C. Long-term livestock exclusion increases plant richness and reproductive capacity in arid woodlands. Ecol. Appl. 2023, 33, e2909. [Google Scholar] [CrossRef]
- Szemán, L. Ökológiai Gyepgazdálkodás [Ecological Grassland Management]. In Ökológiai Gazdálkodás: Általános Kérdések, Növénytermesztés, Állattenyésztés; Radics, L., Ed.; Dinasztia: Budapest-Gödöllő, Hungary, 2001; pp. 153–166. [Google Scholar]
- Amiaud, B.; Touzard, B.; Bonis, A.; Bouzillé, J.B. After grazing exclusion, is there any modification of strategy for two guerrilla species: Elymus repens (L.) Gould and Agrostis stolonifera (L.)? Plant Ecol. 2008, 197, 107–117. [Google Scholar] [CrossRef]
- Zimmermann, Z.; Szabó, G.; Bartha, S.; Szentes, S.; Penksza, K. Juhlegeltetés hatásainak természetvédelmi célú vizsgálata legelt és művelésből kivont gyepek növényzetére. AWETH 2011, 7, 234–262. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).