Effects of Revetments on Nitrification and Denitrification Potentials in the Urban River–Riparian Interface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Field Sampling
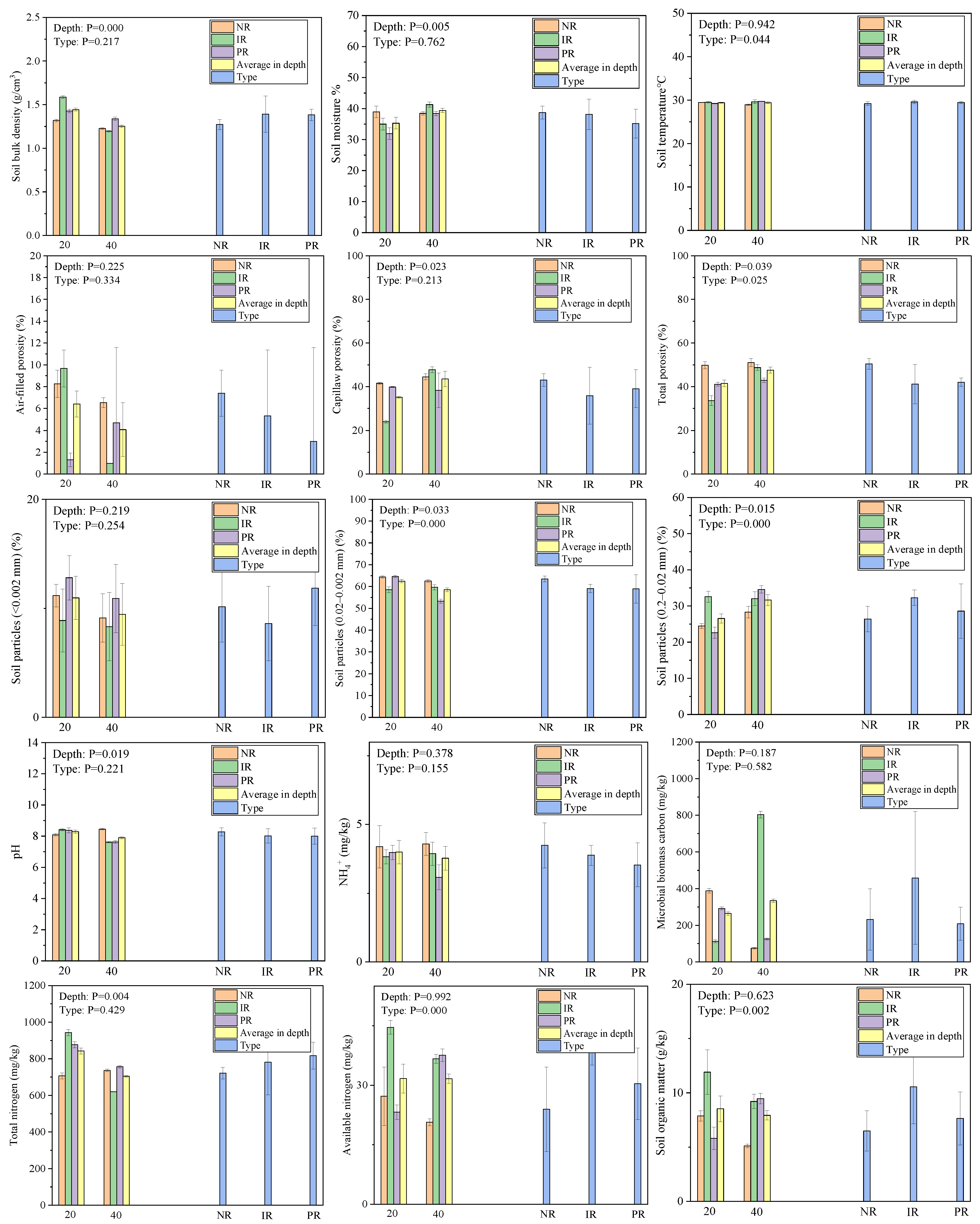
2.2. Soil Properties
2.3. Plant Biomass Measurements
2.4. Nitrification and Denitrification Potentials
2.5. Statistical Analysis
3. Results
3.1. Nitrification and Denitrification Potential Characteristics
3.2. Soil Properties and Plant Biomass Characteristics
3.3. Correlation between Soil Properties and the Potential for Nitrification and Denitrification
4. Discussion
4.1. Effect of Revetment on Nitrification Potential
4.2. Effect of Revetment on Denitrification Potential
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Wu, Y.; Gu, B. Urban rivers as hotspots of regional nitrogen pollution. Environ. Pollut. 2015, 205, 139–144. [Google Scholar] [CrossRef]
- Shaaban, M.; Wu, Y.; Khalid, M.S.; Peng, Q.-A.; Xu, X.; Wu, L.; Younas, A.; Bashir, S.; Mo, Y.; Lin, S.; et al. Reduction in soil N2O emissions by pH manipulation and enhanced nosZ gene transcription under different water regimes. Environ. Pollut. 2018, 235, 625–631. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Fan, H.; Tang, C. Intense denitrification and sewage effluent result in enriched 15 N in N2O from urban polluted rivers. J. Hydrol. 2020, 608, 127631. [Google Scholar] [CrossRef]
- Yan, L.; Xie, C.; Xu, X.; Che, S. The influence of revetment types on soil denitrification in the adjacent tidal urban riparian zones. J. Hydrol. 2019, 574, 398–407. [Google Scholar] [CrossRef]
- Yan, L.; Xie, C.; Liang, A.; Jiang, R.; Che, S. Effects of revetments on soil denitrifying communities in the urban river-riparian interface. Chemosphere 2021, 263, 128077. [Google Scholar] [CrossRef]
- Johnson, M.; Wilby, R. Seeing the landscape for the trees: Metrics to guide riparian shade management in river catchments. Water Resour. Res. 2015, 51, 3754–3769. [Google Scholar] [CrossRef]
- Trauth, N.; Musolff, A.; Knöller, K.; Kaden, U.S.; Keller, T.; Werban, U.; Fleckenstein, J.H. River water infiltration enhances denitrification efficiency in riparian groundwater. Water Res. 2018, 130, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Mayer, P.M.; Reynolds, S.K.; McCutchen, M.D.; Canfield, T.J. Meta-analysis of nitrogen removal in riparian buffers. J. Environ. Qual. 2007, 36, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Velez, J.D.; Harvey, J.W.; Cardenas, M.B.; Kiel, B. Denitrification in the Mississippi River network controlled by flow through river bedforms. Nat. Geosci. 2015, 8, 941–945. [Google Scholar] [CrossRef]
- Hester, E.T.; Brooks, K.E.; Scott, D.T. Comparing reach scale hyporheic exchange and denitrification induced by instream restoration structures and natural streambed morphology. Ecol. Eng. 2018, 115, 105–121. [Google Scholar] [CrossRef]
- Woodward, K.B.; Fellows, C.S.; Conway, C.L.; Hunter, H.M. Nitrate removal, denitrification and nitrous oxide production in the riparian zone of an ephemeral stream. Soil Biol. Biochem 2009, 41, 671–680. [Google Scholar] [CrossRef]
- Fellows, C.S.; Hunter, H.M.; Eccleston, C.E.A.; Hayr, R.W.D.; Rassam, D.W.; Beard, N.J.; Bloesch, P.M. Denitrification potential of intermittently saturated floodplain soils from a subtropical perennial stream and an ephemeral tributary. Soil Biol. Biochem. 2011, 43, 324–332. [Google Scholar] [CrossRef]
- Yan, L.; Xie, C.; Xu, X.; Che, S. Effects of revetment type on the spatial distribution of soil nitrification and denitrification in adjacent tidal urban riparian zones. Ecol. Eng. 2019, 132, 65–74. [Google Scholar] [CrossRef]
- Riya, S.; Takeuchi, Y.; Zhou, S.; Terada, A.; Hosomi, M. Nitrous oxide production and mRNA expression analysis of nitrifying and denitrifying bacterial genes under floodwater disappearance and fertilizer application. Environ. Sci. Pollut. Res. Int. 2017, 24, 15852–15859. [Google Scholar] [CrossRef]
- Meng, Y.; He, Z.; Liu, B.; Chen, L.; Lin, P.; Luo, W. Soil Salinity and Moisture Control the Processes of Soil Nitrification and Denitrification in a Riparian Wetlands in an Extremely Arid Regions in Northwestern China. Water 2020, 12, 2815. [Google Scholar] [CrossRef]
- Baggs, E.; Richter, M.; Cadisch, G.; Hartwig, U. Denitrification in grass swards is increased under elevated atmospheric CO2. Soil Biol. Biochem. 2003, 35, 729–732. [Google Scholar] [CrossRef]
- Cui, P.; Fan, F.; Yin, C.; Song, A.; Huang, P.; Tang, Y.; Zhu, P.; Peng, C.; Li, T.; Wakelin, S.A.; et al. Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol. Biochem. 2016, 93, 131–141. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Lu, L.; Jia, Z. Urease gene-containing Archaea dominate autotrophic ammonia oxidation in two acid soils. Environ. Microbiol. 2013, 15, 1795–1809. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Lou, H.; Wang, D.; Deng, H.; Wang, C. Denitrification controls in urban riparian soils: Implications for reducing urban nonpoint source nitrogen pollution. Environ. Sci. Pollut. Res. Int. 2014, 21, 10174–10185. [Google Scholar] [CrossRef]
- He, J.; Hu, H.; Zhang, L. Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biol. Biochem. 2012, 55, 146–154. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, L.; Dai, Y.; Di, H.; He, J. PH-dependent distribution of soil ammonia oxidizers across a large geographical scale as revealed by high-throughput pyrosequencing. J. Soils Sediments 2013, 13, 1439–1449. [Google Scholar] [CrossRef]
- Kaden, U.; Fuchs, E.; Hecht, C.; Hein, T.; Rupp, H.; Scholz, M.; Schulz-Zunkel, C. Advancement of the Acetylene Inhibition Technique Using Time Series Analysis on Air-Dried Floodplain Soils to Quantify Denitrification Potential. Geosciences 2020, 10, 431. [Google Scholar] [CrossRef]
- Baskerville, M.; Reddy, N.; Ofosu, E.; Thevathasan, N.V.; Oelbermann, M. Vegetation Type does not Affect Nitrous Oxide Emissions from Riparian Zones in Agricultural Landscapes. Environ. Manag. 2021, 67, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Burger, M.; Doane, T.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Xie, C.; Jiang, R.; Liang, A.; Wu, H.; Che, S. Effects of revetments on soil ecosystems in the urban river-riparian interface. IScience 2022, 25, 105277. [Google Scholar] [CrossRef]
- O’Brien, J.; Dodds, W.; Wilson, K.; Murdock, J.; Eichmiller, J. Saturation of N cycling in Central Plains streams: ¹⁵N experiments across a broad gradient of nitrate concentrations. Biogeochemistry 2007, 84, 31–49. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroğlu, T.; Riaz, M.; Uslu, O.S. Seasonal Variations in Litter Layers’ Characteristics Control Microbial Respiration and Microbial Carbon Utilization Under Mature Pine, Cedar, and Beech Forest Stands in the Eastern Mediterranean Karstic Ecosystems. Microb. Ecol. 2022, 84, 153–167. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, H.; Shen, J.; He, J. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Borah, P.; Gogoi, N.; Mahanta, S.P. Seasonal Variation in Carbon Mineralization Kinetics, Microbial Biomass Carbon and Enzyme Activities in the Soils of Three Natural Ecosystems of Kaziranga National Park, Assam, North East India. J. Soil Sci. Plant Nutr. 2023, 23, 5300–5311. [Google Scholar] [CrossRef]
- Xie, C.; Yan, L.; Liang, A.; Jiang, R.; Man, Z.; Che, S. Seasonal and spatial characterisation of soil properties, nitrification and denitrification at the urban river-riparian interface with permeable revetments. Appl. Soil Ecol. 2022, 173, 104372. [Google Scholar] [CrossRef]
- Andrade, R.; Mancini, M.; Teixeira, A.; Silva, S.H.G.; Weindorf, D.C.; Chakraborty, S.; Guilherme, L.R.G.; Curi, N. Proximal sensor data fusion and auxiliary information for tropical soil property prediction: Soil texture. Geoderma 2022, 422, 115936. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil. V. A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Wen, L.; Wang, X.; Wu, Y. The effects of fencing on carbon stocks in the degraded alpine grasslands of the Qinghai-Tibetan Plateau. J. Environ. Manag. 2013, 128, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Weng, B.; Yang, Y.; Gong, X.; Li, M.; Yu, Z. Effects of the Freezing–Thawing Cycle Mode on Alpine Vegetation in the Nagqu River Basin of the Qinghai–Tibet Plateau. Water 2019, 11, 2122. [Google Scholar] [CrossRef]
- Liu, W.; Xiong, Z.; Liu, H.; Zhang, Q.; Liu, G. Catchment agriculture and local environment affecting the soil denitrification potential and nitrous oxide production of riparian zones in the Han River Basin, China. Agric. Ecosyst. Environ. 2016, 216, 147–154. [Google Scholar] [CrossRef]
- Hopfensperger, K.N. Effects of seasonal variation land cover on riparian denitrification along a mid-sized river. J. Freshw. Ecol. 2014, 29, 457–473. [Google Scholar] [CrossRef]
- Liu, W.; Yao, L.; Jiang, X.; Guo, L.; Cheng, X.; Liu, G. Sediment denitrification in Yangtze lakes is mainly influenced by environmental conditions but not biological communities. Sci. Total Environ. 2018, 616–617, 978–987. [Google Scholar] [CrossRef]
- Wang, X.L.; Yang, S.T.; Mannaerts, C.M.; Gao, Y.F.; Guo, J.X. Spatially explicit estimation of soil denitrification rates and land use effects in the riparian buffer zone of the large Guanting reservoir. Geoderma 2009, 150, 240–252. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, S.; Yao, L.; Liu, G.; Zhang, Q.; Liu, W. Topography and land use effects on spatial variability of soil denitrification and related soil properties in riparian wetlands. Ecol. Eng. 2015, 83, 437–443. [Google Scholar] [CrossRef]
- Park, E.J.; Sul, W.J.; Smucker, A.J.M. Glucose additions to aggregates subjected to drying/wetting cycles promote carbon sequestration and aggregate stability. Soil Biol. Biochem. 2007, 39, 2758–2768. [Google Scholar] [CrossRef]
- Lima, I.B.T.; Ramos, F.M.; Bambace, L.A.W.; Rosa, R.R. Methane emissions from large dams as renewable energy resources: A developing nation perspective. Mitig. Adapt. Strateg. Glob. Chang. 2008, 13, 193–206. [Google Scholar] [CrossRef]
- Barton, L.; Gleeson, D.; Maccarone, L.; Zúñiga, L.; Murphy, D. Is liming soil a strategy for mitigating nitrous oxide emissions from semi-arid soils? Soil Biol. Biochem. 2013, 62, 28–35. [Google Scholar] [CrossRef]
- Song, H.; Che, Z.; Cao, W.; Huang, T.; Wang, J.; Dong, Z. Changing roles of ammonia-oxidizing bacteria and archaea in a continuously acidifying soil caused by over-fertilization with nitrogen. Environ. Sci. Pollut. Res. Int. 2016, 23, 11964–11974. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xu, Z.; He, J. Ammonia-Oxidizing Archaea Play a Predominant Role in Acid Soil Nitrification. Adv. Agron. 2014, 125, 261. [Google Scholar]
- Gundersen, P.; Lauren, A.; Finer, L.; Ring, E.; Koivusalo, H.; Sætersdal, M.; Weslien, J.O.; Sigurdsson, B.D.; Högbom, L.; Laine, J.; et al. Environmental services provided from riparian forests in the Nordic countries. Ambio 2010, 39, 555–566. [Google Scholar] [CrossRef]
- Poblador, S.; Lupon, A.; Sabate, S.; Sabater, F. Soil water content drives spatiotemporal patterns of CO2 and N2O emissions from a Mediterranean riparian forest soil. Biogeosciences 2017, 14, 4195–4208. [Google Scholar] [CrossRef]
- Zhu, M.; De Boeck, H.J.; Xu, H.; Chen, Z.; Lv, J.; Zhang, Z. Seasonal variations in the response of soil respiration to rainfall events in a riparian poplar plantation. Sci. Total Environ. 2020, 747, 141222. [Google Scholar] [CrossRef]
- Ueda, M.; Tokuchi, N.; Hiura, T. Soil nitrogen pools and plant uptake at sub-zero soil temperature in a cool temperate forest soil: A field experiment using 15N labeling. Plant Soil 2015, 392, 205–214. [Google Scholar] [CrossRef]
- Li, Y.; Chapman, S.; Nicol, G.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Hestrin, R.; Weber, P.; Pett-Ridge, J.; Lehmann, J. Plants and mycorrhizal symbionts acquire substantial soil nitrogen from gaseous ammonia transport. New Phytol. 2021, 231, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Junk, W.J. The flood pulse concept in river-floodplain systems. Canad. J. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Shrestha, J.; Niklaus, P.A.; Pasquale, N.; Huber, B.; Barnard, R.L.; Frossard, E.; Schleppi, P.; Tockner, K.; Luster, J. Flood pulses control soil nitrogen cycling in a dynamic river floodplain. Geoderma 2014, 228–229, 14–24. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Or, D. Hydration and diffusion processes shape microbial community organization and function in model soil aggregates. Water Resour. Res. 2015, 51, 9804–9827. [Google Scholar] [CrossRef]
- Gebre, M.; Earl, H. Effects of Growth Medium and Water Stress on Soybean [Glycine max (L.) Merr.] Growth, Soil Water Extraction and Rooting Profiles by Depth in 1-m Rooting Columns. Front. Plant Sci. 2020, 11, 487. [Google Scholar] [CrossRef]
- Groh, T.; Davis, M.; Isenhart, T.; Jaynes, D.; Parkin, T. In Situ Denitrification in Saturated Riparian Buffers. J. Environ. Qual. 2019, 48, 376–384. [Google Scholar] [CrossRef]
- Roley, S.S.; Tank, J.L.; Williams, M.A. Hydrologic connectivity increases denitrification in the hyporheic zone restored floodplains of an agricultural stream. J. Geophys. Res. Biogeosci. 2012, 117, G00N04. [Google Scholar] [CrossRef]




| NP | DP | |
|---|---|---|
| Moisture | −0.279 | 0.471 * |
| Temperature | −0.414 | 0.090 |
| pH | 0.179 | −0.129 |
| AB | −0.679 *** | 0.553 ** |
| UB | −0.217 | 0.419 |
| MBC | −0.129 | 0.032 |
| TN | −0.242 | 0.246 |
| SOM | −0.303 | −0.125 |
| AN | −0.535 * | −0.022 |
| NH4+ | 0.548 * | −0.226 |
| TP | 0.512 * | −0.305 |
| CP | 0.246 | −0.017 |
| AFP | 0.313 | −0.452 * |
| BD | −0.285 | 0.184 |
| Particles (<0.002 mm) | −0.155 | 0.306 |
| Particles (0.02 mm–0.002 mm) | 0.386 | −0.112 |
| Particles (0.2 mm–0.02 mm) | −0.236 | −0.129 |
| Particles (2.0 mm–0.2 mm) | −0.110 | 0.317 |
| Environmental Factors | Direct Effects | Indirect Effects | Total Effects | |
|---|---|---|---|---|
| Nitrification | AB | −0.450 | 0.016 | −0.434 |
| TP | 0.060 | 0.000 | 0.060 | |
| SOM | 0.390 | −0.234 | 0.156 | |
| AN | −0.450 | −0.184 | −0.634 | |
| NH4+ | 0.200 | 0.000 | 0.200 | |
| Denitrification | AB | 0.630 | −0.122 | 0.508 |
| Moisture | 0.200 | −0.208 | −0.008 | |
| AFP | −0.110 | 0.000 | −0.110 | |
| SOM | −0.350 | 0.000 | −0.350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, Z.; Xie, C.; Qin, Y.; Che, S. Effects of Revetments on Nitrification and Denitrification Potentials in the Urban River–Riparian Interface. Land 2024, 13, 333. https://doi.org/10.3390/land13030333
Man Z, Xie C, Qin Y, Che S. Effects of Revetments on Nitrification and Denitrification Potentials in the Urban River–Riparian Interface. Land. 2024; 13(3):333. https://doi.org/10.3390/land13030333
Chicago/Turabian StyleMan, Zihao, Changkun Xie, Yifeng Qin, and Shengquan Che. 2024. "Effects of Revetments on Nitrification and Denitrification Potentials in the Urban River–Riparian Interface" Land 13, no. 3: 333. https://doi.org/10.3390/land13030333






