Abstract
Soil organic carbon (SOC) is the major constituent of the soil organic matter. SOC stocks are determined by several factors such as altitude, slope, aspect, canopy cover, and vegetation type. Using the Third National Forest Inventory (2010–2014) data of Nepal, we assessed SOC status in forests at a national scale for the better understanding of the SOC distribution within Nepal. In this study, we estimated SOC against different factors and tested the spatial distribution of SOC using analysis of variance (ANOVA). The results showed that the forests located at a higher altitude have higher SOC accumulation. In particular, broadleaved forests exhibit a higher amount of carbon stock compared to other forest types. Moreover, forests with a larger canopy cover, located on a higher slope, and with a cooler aspect are associated with a higher accumulation of SOC. The SOC stock in the forest varies according to altitude, slope, aspect, canopy cover, and forest type, which might be attributed to the change in the microclimate of the area. The significant increase in SOC amount with the increase in slope, altitude, and crown cover helps to understand the extent of SOC distribution in forests. Broadleaved forests with a larger canopy cover in the higher altitude region have a higher SOC retention potential, which is likely to contribute to mitigating the impacts of climate change by sinking more carbon into the soil.
1. Introduction
Forest ecosystems are globally very important carbon reservoirs, storing 46% of the total terrestrial carbon [1]. Forest soils act as carbon sinks due to their higher organic matter content [2], which contains roughly 55–60% of soil organic carbon (SOC) [3]. Forest soil retains more than 40% of the organic carbon of the terrestrial ecosystem globally [4,5]. Thus, the soil is considered one of the most crucial carbon pools in the terrestrial system, and organic carbon in the soil is a highly important natural resource that needs to be restored, enhanced, and improved [6] as it improves the physical, chemical, biological, and ecological properties of the soil [3,7].
According to Global Forest Resource Assessment 2020, while 192 countries and territories (99 percent of the world’s forests) reported on biomass estimation, only 76 countries and territories (representing 66 percent of the world’s forests) reported information on forest carbon [8]. SOC dynamics and its storage in tropical forests persistently exhibit uncertainty in the global carbon cycle [9]. Understanding the dynamics and distribution of SOC in forest soils is essential to better predict the forest SOC [10]. However, the dynamics of the SOC stock are poorly quantified, largely due to a lack of direct field measurements [11]. The estimations presented in this study provide a baseline for estimating future changes in soil C stocks in Nepal, and for assessing their vulnerability to key global change drivers, thereby informing future actions aimed at the conservation and management of C stocks [12].
Maintaining higher levels of SOC is an indicator of good soil health [13] that increases soil fertility [14], whereas the loss of SOC contributes to soil and land degradation [15,16], global warming [17], and negative effects on soil functions [15]. Therefore, SOC is considered a key environmental indicator for several global environmental issues, including land degradation, and food security [18,19]. However, SOC is in a declining trend worldwide. Asia is the most affected region, losing 33.5% of the world’s SOC, and Nepal also shows a similar trend [18].
SOC stocks are the functions of several factors including the amount of above ground litter fall and root turnover, forest types, tree species diversity, canopy cover, soil conditions and vegetative cover, soil properties and moisture, soil depth, altitude, slope, aspect, and climate [20,21,22,23,24,25,26,27,28,29,30,31,32]. These predictor variables affect SOC stocks either positively or negatively. In particular, altitude has a positive relation with SOC stocks [33,34,35] while slope, warmer aspect, and crown cover have a negative relation [24,28,36]. The relationships between SOC stocks and these variables are not always consistent. Some studies reported a negative relation of SOC with altitude, e.g., [37,38], while others revealed a positive relation with slope, e.g., [24,39,40].
Vegetation—a source of organic matter—has a strong effect on accumulation of SOC stocks [41]. More than 41%ent of the land of Nepal is covered by forests [42], holding 37.8% carbon as SOC [43]. The forests distributed throughout the country, with considerable topographic and climatic variations, are expected to retain a different amount of SOC. Several studies have assessed SOC in the different forest types of Nepal; however, they are limited to a small area with lesser climatic and altitudinal variation [18,28,29].
Thus, a better understanding of the status of SOC under different forest types at the national scale is lacking. Only the third national level forest resource assessment (2010–2014) of Nepal has assessed SOC from the permanent sample plots so far. This study, using the nationwide SOC data, aims to answer the following questions. (1) How do altitude, aspect, slope, and canopy cover affect SOC stock distribution in the forests of Nepal? (2) Does the SOC stock vary among forest types in Nepal? The SOC status in forests located in various physiographic conditions will provide a better understanding of the distribution of SOC in the different types and will be supportive to adopt the appropriate forest management strategies to store more SOC in forests in the future.
2. Materials and Methods
2.1. Study Area
The study covers the entire forest area of Nepal (Figure 1). Of the total land area of the country, 86% is covered by hills and high mountains, and the remaining 14% is a flat- and lowland, located at less than 300 m altitude. Wide altitudinal variations and diverse climatic conditions have created four main physiographic zones, i.e., Terai (lowlands), mid hills, high mountains, and high Himal [44], and have also influenced the composition of flora and fauna [45]. Stainton [46] classified 35 forest types in Nepal, which are further broadly categorized into 10 major groups based on the altitudinal range [45].
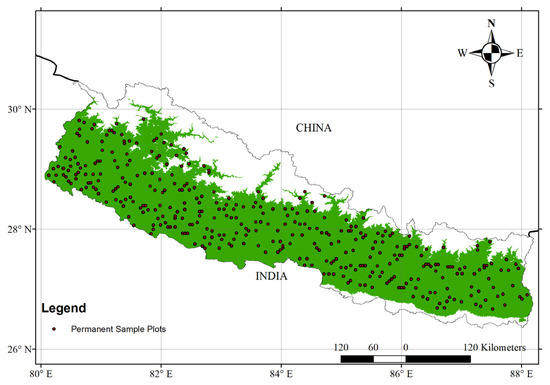
Figure 1.
Study area map showing permanent sample plots distributed throughout the forests of Nepal. Green color on the map indicated forest cover in Nepal.
2.2. Forest Types
In Nepal, there are three categories of classification of forest type based on species composition, structure, and altitude. This study included forest types mentioned by the Department of Forest Research and Survey, Nepal (DFRS), and Shrestha [43,47] as Group I and Group II, respectively. Group I includes 15 different forest types classified by the DFRS [43] based on tree species composition. They are (1) Shorea robusta forest (S), (2) Terai mixed hardwood forest (TMH), (3) lower mixed hardwood forest (LMH), (4) Pinus roxburghi forest (Pr), (5) Pinus wallichiana forest (Pw), (6) Quercus forest (Q), (7) upper mixed hardwood forest (UMH), (8) Abies forest (A), (9) Cedrus deodara forest (Ce), (10) Betula utilis forest (Bu), (11) Picea smithiana forest (Ps), (12) Cupressus torulosa forest(Ct), (13) Tsuga dumusa forest (Td), (14) Juglans wallichiana forest (Jw), and (15) Acacia catechu/Dalbergia sisso forest (AC/DS). Group II includes broadleaved forest, mixed forest, and coniferous forest, as classified by Shrestha [47] based on structural features. Table 1 shows the forest types included in this study, together with location attributes (altitude, aspect, and slope) and canopy cover for each of the forest types. Out of 15 forest types in Group I, only 7 forest types were included in the study (Table 1).

Table 1.
Forest types included in the study, and corresponding locality factors (altitude, aspect, and slope) and canopy cover of each of the forest types. TMH: Terai mixed hardwood forest; LMH: lower mixed hardwood forest; S: Shorea robusta forest; Pr: Pinus roxburghi forest; Pw: Pinus wallichiana forest (Pw); UMH = upper mixed hardwood forest; and Q: Quercus forest.
2.3. Data Collection
The primary data used in this study were acquired from the third national forest inventory (NFI) conducted during 2010–2014. The NFI adopted a two-phase systematic sampling design, composed of 450 clusters containing 1553 Permanent Sample Plots (PSPs) allocated systematically in the entire forest area. Data were collected only from the accessible sample plots (slope up to 100% or 45°). This is the first NFI in Nepal that collected soil samples to analyze the soil organic carbon of the forests. Four soil pits were established in the four cardinal directions in each PSP to collect soil samples. In each cardinal direction, soil pits of appropriate size within a 2 m × 2 m area were dug at a 21 m distance from the PSP center. Soil samples were collected from three different horizons (1–10 cm, 10–20 cm, and 20–30 cm) from each soil plot dug outside the peripheries of the PSPs [48]. A study reported that about 53% of the SOC stock over 1 m depth was held in the top soils, i.e., up to 30 cm depth [22].
2.4. Soil Organic Carbon (SOC) Analysis
For SOC analysis, four soil samples of the same horizon of the subplots were mixed. Each PSP had 3 soil samples taken from three different soil horizons. The Walkley–Black wet combustion method [49] together with titration was applied in the soil laboratory of the DFRS in Nepal to analyze soil organic carbon. The method is based on wet oxidation where the organic carbon in the soil is determined via oxidation with a mixture of potassium dichromate and sulfuric acid [50]. In addition, dry combustion and a LECO CHN Analyzer were used in the Metla Soil Laboratory, Finland, to assure the quality of the laboratory test. The soil organic carbon analyzed in the soil laboratory was later estimated on a per hectare basis [48].
Due to the inappropriateness of sites for soil collection, soil samples were not collected from all of the clusters and PSPs during the NFI. This study used only 1059 PSPs out of 1553 after eliminating non-soil PSPs and outlier PSPs for SOC analysis in different forest types. The lack of a full representation of the SOC samples in the study may cause some bias in the results.
2.5. Data Analysis
Based on the findings of previous studies [29,30,31,36,51,52], forest types, altitude, aspect, slope, and canopy cover were selected to assess SOC variation in the forests of Nepal. To analyze the variables, altitude was divided into four groups (<1000, 1000–2000, 2000–3000, and 3000–4000 m), slope into four groups (<8.5°, 8.5–19°, 19–31°, and 31–45°) and forest canopy cover into three groups (<40, 40–69, and ≥70%). The category of altitude is based on the forest types including tropical forests, sub-tropical forests, temperate forests, etc., [45], whereas the categories of slope and canopy cover are based on the forest resource assessment of Nepal [48,53].
An ANOVA test was employed for data analysis. Before applying the ANOVA test, the SOC data were transformed using “BoxCoxTransformation” function in R-package (version 4.3.1) “e1071” [54] to normalize their distribution. The transformation method is used on a non-normal dependent variable to convert it into normal distribution for the statistical test. After the ANOVA test, Tukey’s post hoc test (pairwise test) was applied to see the significant difference between the two variables. All data were analyzed in the R program [55]. Due to the unequal number of subplots in a cluster, the mean per hectare of SOC and standard error were analyzed using Equations (1)–(3).
where:
- tci = total SOC in a ith cluster;
- n = number of sub plots;
- N = number of cluster plots;
- Mc = mean cluster size = n/N;
- msi = mean SOC in the ith cluster, i.e., tci/Mc.
The distribution of SOC based on forest types, altitude, aspect, slope, and canopy cover is depicted in the figures for visual interpretation using the “ggplot2” package in R. Although there were 15 forest types under Group I, we used only 7 forest types (see Table 1). The excluded 8 forest types contained ≤6 cluster plots and were considered to have an insufficient sample size.
3. Results
3.1. Distribution of SOC along the Altitude of the Forests
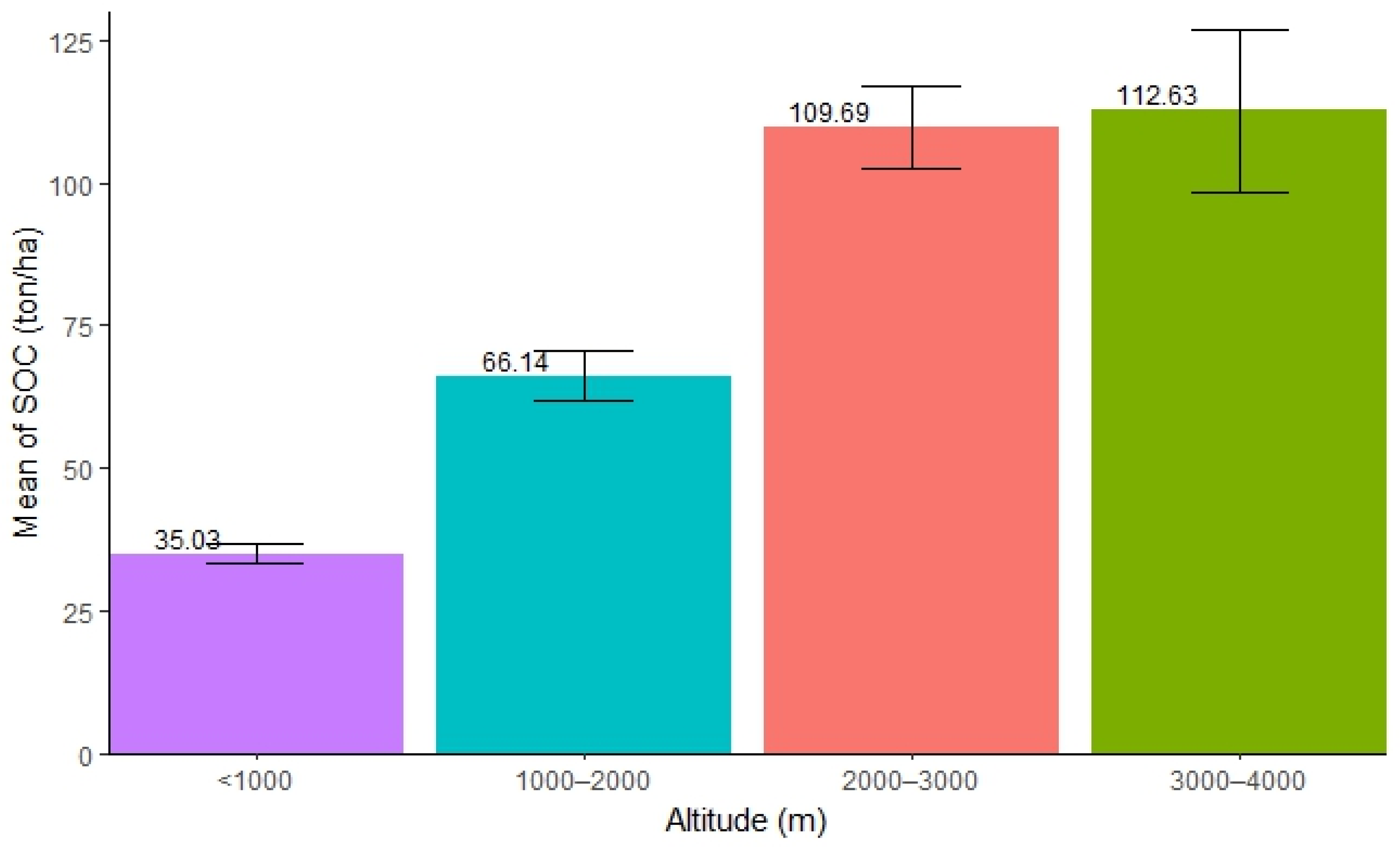
The distribution of SOC along the altitudinal ranges varied. The highest SOC concentration was found in the altitude ranging from 3000 to 4000 m (mean = 112.63 ton/ha, SE ± 14.15), followed by 2000–3000 m (mean = 109.69 ton/ha, SE ± 7.20), and 1000–2000 m (mean = 66.15 ton/ha, SE ± 4.34), while the lowest was found in forests with an altitude below 1000 m (mean = 35.03 ton/ha, SE ± 1.70). The result shows that an increase in altitude helps accumulate a substantial amount of SOC in forests (Figure 2). The ANOVA test showed that there was significant difference in SOC stocks along the altitudinal gradient in the forest (Table 2). Further, Tukey’s test (pairwise comparison) showed that the distribution of SOC in the altitudinal ranges 3000–4000 m and 2000–3000 m (p = 0.99) was not significantly different. Compared to the two lower altitudinal ranges (<1000 m and 1000–2000 m), both of them exhibited a significantly higher amount of SOC.
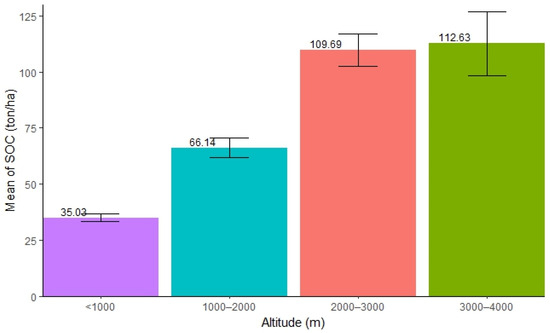
Figure 2.
Mean distribution of SOC along different altitudinal ranges.

Table 2.
ANOVA table for the SOC of different altitudinal ranges in forests.
3.2. Distribution of SOC along the Slope of the Forests
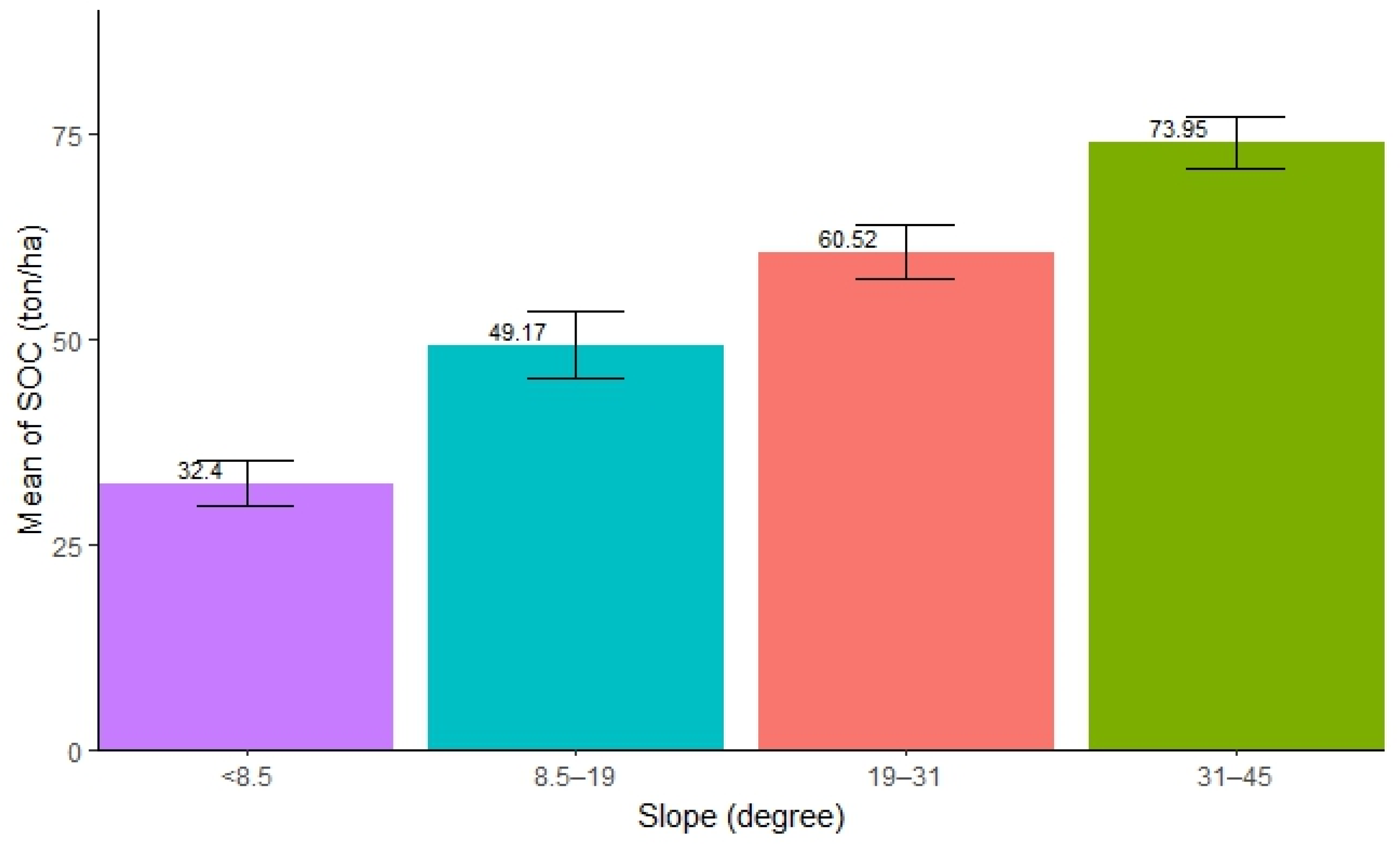
The distribution of SOC with varied slopes was found to be significantly different. The highest SOC concentration was observed in the slope range of 31–45° (mean = 73.95 ton/ha, SE ± 3.21), followed by 19–31° (mean = 60.52 ton/ha, SE ± 3.29), and 8.5–19° (mean = 49.17 ton/ha, SE ± 4.05), while the lowest was observed in forests with a slope below 8.5° (mean = 32.4 ton/ha, SE ± 2.71). The result shows that an increase in slope significantly contributes to the accumulation of SOC in forests (Figure 3). The ANOVA test showed that there was significant difference in SOC stocks along the slopes in the forest (Table 3). Further, the pairwise tests revealed that all pairs of slopes exhibited significant differences in SOC stocks (p < 0.05), except for the pairs (8.5°–19° and 19°–31°) and (19°–31° and 31°–45°).
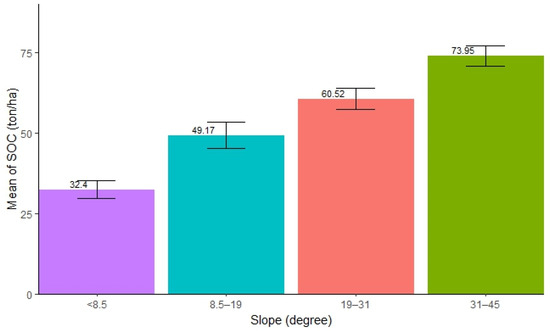
Figure 3.
Mean distribution of SOC along different slope ranges.

Table 3.
ANOVA table for the SOC of different slope ranges.
3.3. Distribution of SOC along the Aspect of the Forests
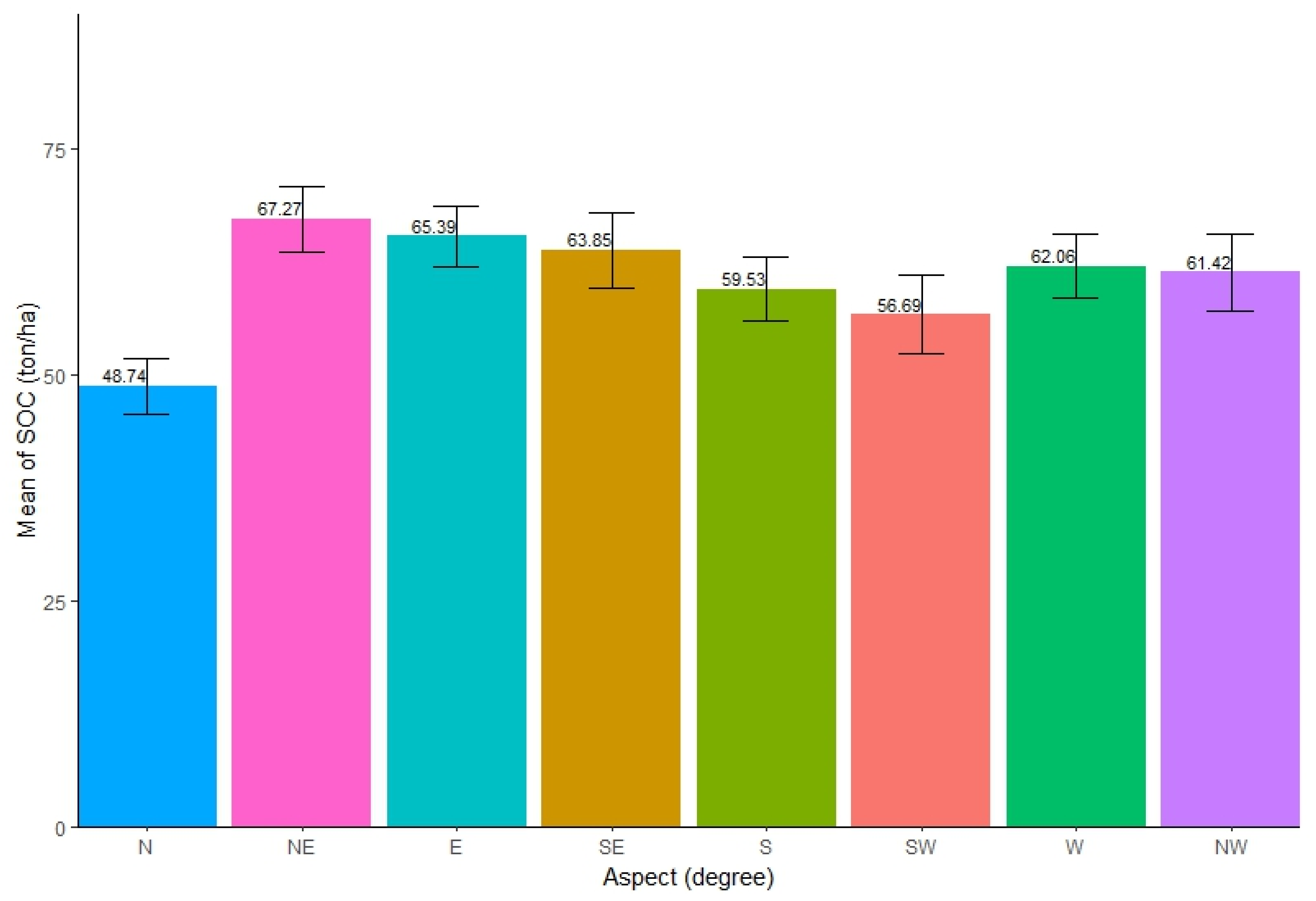
The distribution of SOC across varied aspects was found to be slightly different. The highest SOC (mean = 67.27 ton/ha, SE ± 3.58) concentration was found in the northeast (NE) aspect of the forests, while the north (N) aspect had the lowest SOC (mean = 48.74 ton/ha, SE ± 3.04). The SOC stocks among eight different aspects showed similar distribution (Figure 4). The ANOVA test showed that there was significant difference in SOC stocks among the different aspects in forests (Table 4). Further, Tukey’s pairwise tests showed that only the pair of N and E aspect and the pair of N and NE aspect had insignificant differences in SOC stocks (p < 0.05).
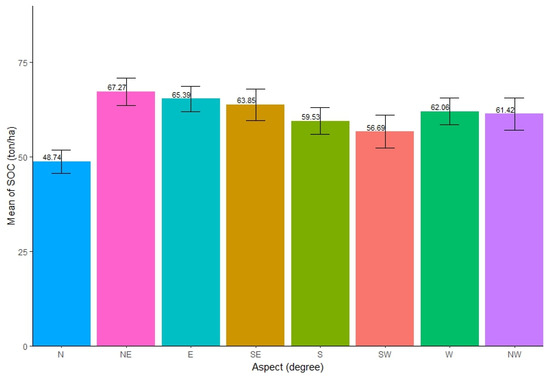
Figure 4.
Mean distribution of SOC along different aspects.

Table 4.
ANOVA table for the SOC of different aspects.
3.4. Distribution of SOC along the Canopy Cover of the Forests
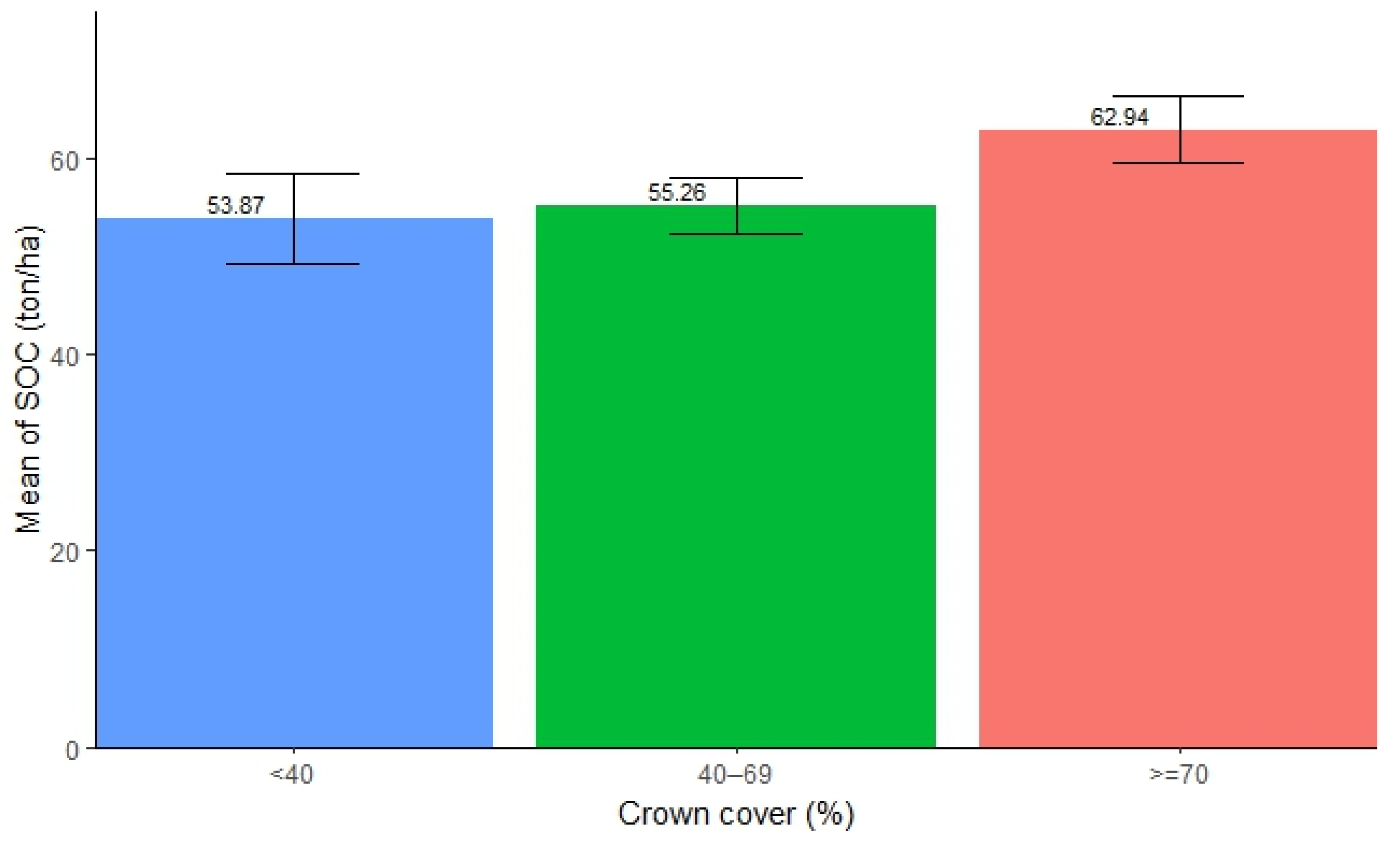
The SOC in forests with different canopy cover was found to be significantly different. The highest SOC concentration was found in the canopy cover exceeding 70% (mean = 62.94 ton/ha, SE ± 3.35), followed by 40–69% (mean = 55.26 ton/ha, SE ± 2.88), while the lowest was found in the canopy cover below 40% (mean = 53.87 ton/ha, SE ± 4.66). The result shows that an increase in canopy cover contributes to a higher accumulation of SOC in forests (Figure 5). However, the ANOVA test showed no significant difference in SOC stocks among the different canopy covers in forests (Table 5). Further, pairwise tests showed that none of the pairs displayed significant differences in SOC stocks (p > 0.05).
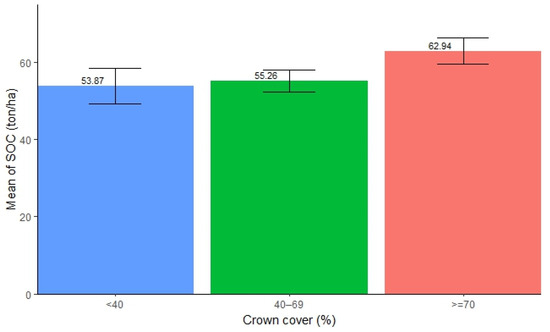
Figure 5.
Mean distribution of SOC along different canopy covers.

Table 5.
ANOVA table for the SOC of different canopy covers.
3.5. Distribution of SOC in the Forest Types (Group I)
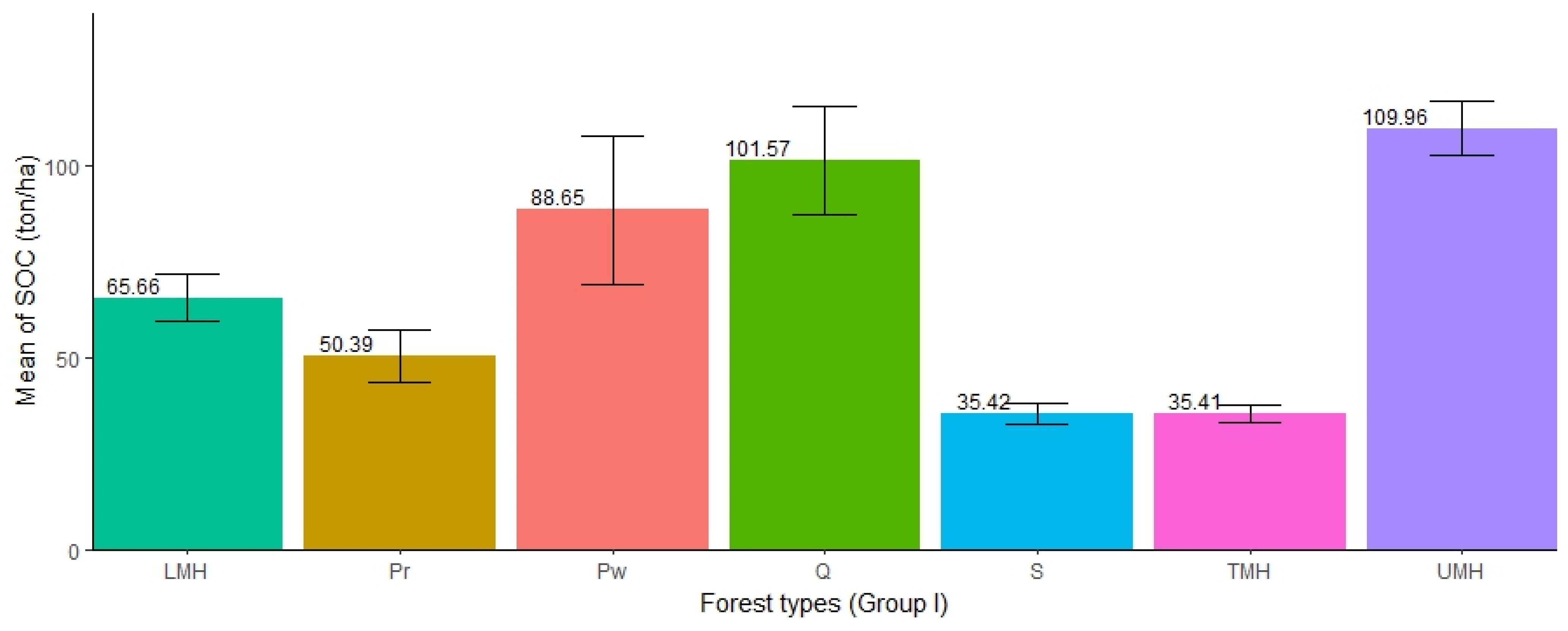
The distribution of SOC in the seven forest types was found to be different except for TMH and S forests, which had approximately the same amount of SOC (35.41 and 35.42 ton/ha). The highest mean SOC concentration was found in UMH forests (mean = 109.96 ton/ha, SE ± 6.96), followed by Q (mean = 101.57 ton/ha, SE ± 14.06), Pw (mean = 88.65 ton/ha, SE ± 19.5), LMH (65.66 ton/ha, SE ± 6.09), Pr (mean = 50.39 ton/ha, SE ± 6.91), TMH (mean = 35.41 ton/ha, SE ± 2.30), while the lowest was found in S (mean = 35.42 ton/ha, SE ± 2.64). The SOC stocks in UMH forests are three times higher than those in S forests. The ANOVA test showed that there was significant difference in SOC stocks among the forest types (Table 6). Furthermore, the pairwise test showed that out of 21 pairs of forest types, SOC stocks were significantly different in 12 pairs of forests (i.e., Q-LMH, S-LMH, TMH-LMH, UMH-LMH, Q-Pr, UMH-Pr, S-Pw, TMH-Pw, S-Q, TMH-Q, UMH-S, and UMH-TMH). The ANOVA result showed significant differences in SOC accumulation in different forest types (Figure 6).

Table 6.
ANOVA table for the SOC of different forest types (Group I).
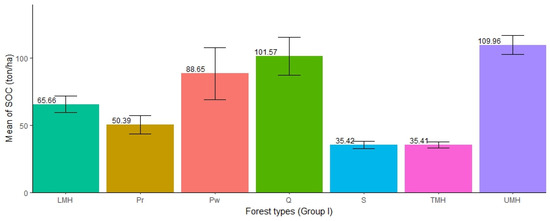
Figure 6.
Distribution of SOC in different forest types (Group I).
3.6. Distribution of SOC in the Forest Types (Group II)
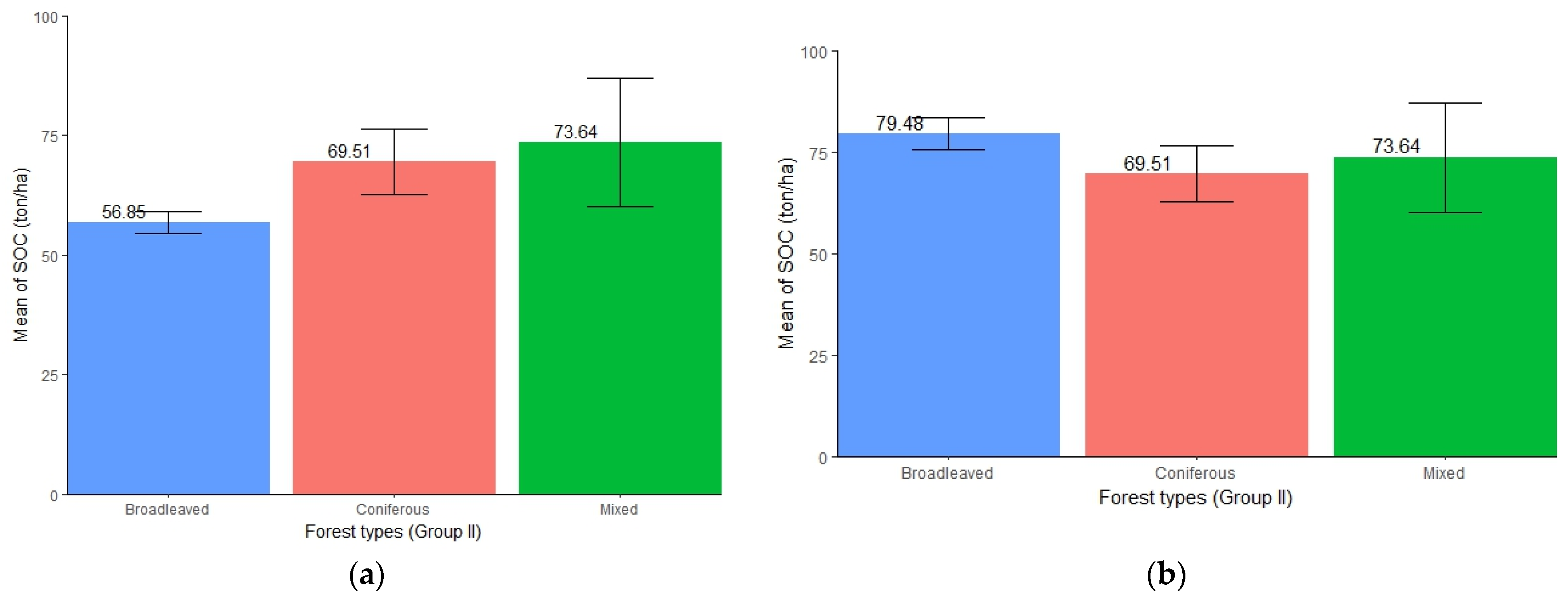
The three different forest types exhibited varied amount of SOC accumulation. When considering all of the altitudinal ranges of the study area, mixed forests (mean = 73.64 ton/ha, SE ± 13.42) were found to store the highest amount of SOC followed by coniferous forests (mean = 69.51 ton/ha, SE ± 6.89) and broadleaved forests (mean = 56.85 ton/ha, SE ± 2.31) (Figure 7a). While assessing SOC above 657 m altitude (the range of both broadleaved and coniferous forests), a higher concentration of SOC was found in broadleaved forest (mean = 79.48 ton/ha, SE ± 4.01) followed by mixed and coniferous forests (Figure 7b). However, the ANOVA test showed no significant differences in SOC stocks among the three forest types (p > 0.05) in either case (Table 7 and Table 8).
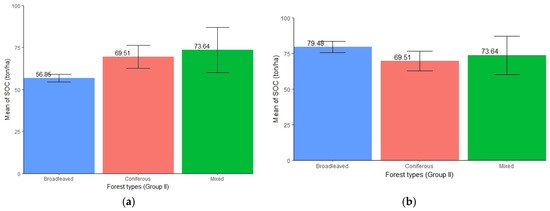
Figure 7.
(a) Distribution of SOC in different forest types (Group II) representing all altitudinal ranges. (b) Distribution of SOC in different forest types (Group II) representing altitude > 657 m, i.e., where the presence of coniferous forests is seen.

Table 7.
ANOVA table for the SOC of different forest types (Group II).

Table 8.
ANOVA table for the SOC of different forest types (Group II, alt > 657 m).
4. Discussion
4.1. Effects of Altitude, Slope, Aspect, and Canopy Cover to SOC Stocks in Forests
Our study shows a positive relation between altitude and SOC stocks. Several studies have also reported a similar positive correlation [33,34,35,56,57,58,59]. However, it is important to note that this relationship is found to be case-specific. Contrastingly, some studies reported that the stabilization of SOC increases at lower altitude compared to higher [37,38]. Along with altitude, several other factors such as topography, exposure, climate, parent material, and vegetation influence soil carbon stocks [20,60,61,62]. In particular, altitude is an important factor for regulating soil organic matter decomposition [51], resulting in an impact on SOC stocks. It does not directly affect the ecosystem, but is an indicator of climatic functions [63]. SOC distribution depends on the altitude-induced variation in climatic variables (temperature and precipitation). SOC stocks increase with altitude due to slow soil organic matter decomposition at the higher elevation sites [25,64]. In Nepal, temperature decreases by 6 °C for every 1000 m increase in elevation [65], which supports our result that a lower temperature at a higher altitude contributes to the higher SOC stocks.
Likewise, slope is an important predictor variable for estimating SOC [66]. Our result shows the effect of slope on SOC stocks. SOC accumulation in the forest soil increases with an increase in slope. Other previous studies [24,39,40] reported the same positive relation between slope and SOC. However, an inverse relationship between SOC and slope has also been reported [36,67], which could be attributed to the erosive down-hill transport of leaf litter and soil debris. It is also reported that the differences in climatic variables (temperature and moisture), aspects, and plant species affect SOC distribution regardless of slope [68]. Our result is supported by the fact that land surface temperature decreases with the increase in slope, influencing the incidence angle and reflectivity of solar radiation [69]. This leads to a lower rate of decomposition due to a decrease in temperature, contributing to more SOC retention [23].
The aspect of the forest is also one of the variables affecting SOC stocks due to a difference in temperature depending on the duration of the sun light received. North-facing slopes are generally cooler, more moist, and densely vegetated than other aspects, and thus accumulate a higher amount of SOC [36,70]. Our result shows a similar trend to previous studies except in the N aspect. Contrary to other studies [32,36,68,70], the N aspect shows the lowest amount of SOC in our study. Sample plots representing the north aspect in our study had the lowest mean altitude (801.71 m) whereas other aspects, namely, NE, E, SE, S, SW, W, and NW had 1379.36 m, 1299.98 m, 1402.72 m, 1267.41 m, 1205.67 m, 1370.06 m, and 1385.65 m, respectively. A lower altitude, and thus a higher temperature, on the N aspect does not contribute more to SOC accumulation. A lower amount of SOC stocks in the N aspect is not due to the aspect itself but rather to the selection of sample plots (i.e., a sampling error). However, there was no significant difference in SOC stocks except for the N–E and N–NE aspects. Aspect does have an effect on SOC stocks as the temperature of the soil differs according to the aspect. But this condition applies well when the study area has a lower altitudinal range. In the areas with larger altitudinal variation, altitude can be a strong predictor compared to other variables, and is likely to cancel out the microclimatic effect due to the aspect [71].
Furthermore, our result shows a positive relationship between SOC and forest canopy cover. This result corroborates the findings reported by Gebeyehu et al. and Kara [24,52]. Canopy cover does not affect directly the change in the amount of SOC but influences the microclimate. The change in the microclimate of the soil habitat due to the alteration in canopy cover includes temperature, soil moisture, light, and wind speed [72]. These factors affect the activities of fauna and organisms in the soil [72], leading to variations in soil respiration intensity and the formation of SOC [73,74]. In fact, an increase in tree canopy cover helps reduce soil temperature [75,76,77] and slows down the rate of litter decomposition [78], resulting in more SOC retention.
The effect of topographical factors (altitude, slope, and aspect) on SOC is primarily observed due to the variation in climate. Therefore, the manipulation of the topographical variables is not easy under forest management activities. However, canopy cover management can be performed to increase SOC in forests.
4.2. SOC Accumulation of SOC under Different Forest Types under Group I and Group II
The amount of SOC increases along the altitudinal gradient [49,50,51]. This implies that forests at a higher altitude are likely to possess a higher amount of SOC stocks compared to forests at a lower altitude. Our study also shows similar findings. Moreover, the distribution of SOC in different forest types situated at various altitudinal gradients differed substantially in our study.
In Group I, our findings show that forest types located at higher altitudes have a higher amount of SOC stocks than forest types at lower altitudes. In particular, UMH and Q forests, located at higher altitudes in Nepal, have higher SOC concentrations compared to other major forest types at lower regions. Similar results (for Q forests) have also been reported by Seikh et al. [38]. The significant difference in the amount of SOC accumulation in the different forest types is possibly due to the cumulative effect of altitude, slope, aspect, canopy cover, and forest disturbance (e.g., forest harvesting), which ultimately influence temperature and microbial activities. The effect of altitude, slope, aspect (except for the N aspect), and canopy cover on SOC stocks found in this study is similar to that reported in previous studies [24,33,34,39,40,56,57,58,59]. Furthermore, forest harvesting affects the depletion of SOC stocks [79,80]. However, it depends on the accessibility to the forest which is difficult at higher altitudes due to the rugged terrain compared to lower regions. Forest disturbances by humans (tree cutting, bush cutting, litter collection, lopping, and cattle grazing) are lower at higher altitudes [40,77], which is likely to increase SOC in forests at higher altitudes.
In Group II, when considering all altitudinal ranges, the study result shows that mixed forests have the highest SOC stocks, followed by coniferous and broadleaved forests (Figure 7a). The result is similar to the findings reported by Devi [81]. Mixed forests are found in higher regions, i.e., starting from 657 m altitude (Table 1). The higher amount of SOC stocks in mixed forests might be the result of the higher amount of SOC stocks in the broadleaved and coniferous forests lying at higher altitudes. In contrast to our study, Dulamsuren [82] reported the lowest amount of SOC stocks in mixed forests compared to other forests. The difference in altitudinal ranges between the two studies, i.e., 88–3993 m in our study and 1300–1500 m in Dulamsuren [82], could be the reason for the dissimilar result.
Moreover, our result demonstrates higher SOC stocks in coniferous forests than broadleaved forests. The finding is similar to the result reported by Vhiti et al. [83]. In our study, coniferous forests are found only at higher altitudes (i.e., altitude > 869 m), whereas broadleaved forests are found at lower altitudes up to 88 m in our study (Table 1). The variation in altitude difference—resulting in temperature difference—between coniferous and broadleaved forests could have influenced the average SOC stocks. We found a lower amount of SOC in the lower altitudinal region, which corroborates the finding reported by Devi [81]. A lower amount of SOC in broadleaved forests, particularly in lower regions, might be due to increased microbial activity at warmer temperatures [22], resulting in a higher rate of CO2 release due to the rapid decomposition of soil organic matter.
On the other hand, the result shows that broadleaved forests have a higher SOC accumulation than coniferous and mixed forests when similar altitudinal regions are considered (Figure 7b), i.e., altitude > 657 m. Similar findings have been reported by various previous studies, e.g., [31,38,83,84]. The large canopy cover of broadleaved forests—compared to coniferous forests in our study (Table 1)—may be the reason for the former holding more SOC, as a larger tree canopy cover helps reduce soil temperature [75,76] and lowers the rate of decomposition of organic matter, leading to more SOC retention [23]. Furthermore, mixed forests are the result of disturbance [85,86], and they are likely to release a large amount of SOC into the atmosphere due to either heterotrophic respiration or in combination with fire [87,88,89]. This could be a possible reason for the lower amount of SOC in mixed forests than in broadleaved forests in the same region. Maintaining broadleaved forests at higher altitudes could support SOC retention, thus contributing to climate change mitigation, besides providing multiple benefits to people.
4.3. SOC Retention by Forests and its Implication in Climate Change Mitigation
Plants (above and below ground parts) are the main source of soil organic matter [90], of which 58% is assumed to be occupied by soil organic carbon [91]. Thus, forests play an important role in SOC retention. However, the amount of SOC depends on the characteristics of tree species, forest type, and the locality where they are present. Our result shows that altitude, aspect, slope, and canopy cover influence SOC accumulation in forests.
This study confirms that different forest types have different levels of SOC retention potential. Firstly, broadleaved forests have higher SOC retention than coniferous forests. Secondly, our study argues that forests at higher altitudes have a higher potential for SOC retention. Although temperature decreases with an increase in altitude, recent studies show that the rate of temperature increase in Nepal is higher in higher altitudinal regions [92,93], which might have implications for the storage of SOC in forests at higher altitudes.
5. Conclusions
SOC stocks in forests vary with altitude, aspect, slope, canopy cover, and forest type. This variation could be attributed to changes in the microclimate (soil temperature and moisture) of the area influenced by these locality factors and stand attributes. The significant increase in SOC amount with the increase in slope, altitude, and crown cover helps to understand the extent of SOC distribution in forests.
In general, forests at higher altitudes store more SOC than forests at lower regions. In particular, broadleaved forests at higher altitudes are the largest SOC reservoirs, compared to mixed and coniferous forests, provided that there is minimal human disturbance. As Nepal is facing a problem with the increasing rate of temperature in higher altitude regions [92], this can plausibly result in increased carbon emissions from forest soils in these regions. Broadleaved forests with a larger canopy cover in higher altitude regions have a higher SOC retention potential, which is likely to contribute to mitigating the impact of climate change by sinking more carbon into the soil. Further research is needed to assess the mitigation potential of forests at higher altitudes in the context of a changing climate.
Author Contributions
R.M. and P.R.N. contributed to designing the study. R.M. contributed to data acquisition, data analysis, and the drafting of the manuscript. P.R.N. contributed to the drafting and final revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data that support the findings of this study are available from the Forest Research and Training Centre (FRTC), Kathmandu, Nepal, but not publicly accessed due to the data sharing protocol of the FRTC. However, data can be obtained by following a formal process of written application with supporting documents.
Acknowledgments
The Authors are thankful to FRTC, Kathmandu, for the provision of data and to the reviewers for their constructive comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Watson, R.T.; Noble, I.R.; Bolin, B.; Ravindranath, N.; Verardo, D.J.; Dokken, D.J. Land Use, Land-Use Change, and Forestry: A Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Dey, S.K. A preliminary estimation of carbon stock sequestrated through rubber (Hevea brasiliensis) plantation in north eastern region of India. Indian For. 2005, 131, 1429–1436. [Google Scholar]
- Montanarella, L.; Pennock, D.; Mckenzie, N.; Alavipanah, S.K.; Alegre, J.; Alshankiti, A.; Arrouays, D.; Aulakh, M.S.; Badraoui, M.; Costa, I.D.S.B.; et al. Status of the World’s Soil Resources; Intergovernmental Technical Panel on Soils; Food and Agriculture Organization: Rome, Italy, 2015. [Google Scholar]
- IPCC. Climate Change 2007—The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Smith, P. Global Climate Change and Pedogenic Carbonates; Lal, R., Kimble, J.M., Stewart, B.A., Eswaran, H., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001. [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Lal, R. Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Forest Resource Assessment 2020: Main Report; FAO: Rome, Italy, 2020. [Google Scholar]
- Sayer, E.J.; Lopez-Sangil, L.; Crawford, J.A.; Bréchet, L.M.; Birkett, A.J.; Baxendale, C.; Castro, B.; Rodtassana, C.; Garnett, M.H.; Weiss, L.; et al. Tropical forest soil carbon stocks do not increase despite 15 years of doubled litter inputs. Sci. Rep. 2019, 9, 18030. [Google Scholar] [CrossRef]
- Yu, F.; Liu, Q.; Fan, C.; Li, S. Modeling the vertical distribution of soil organic carbon in temperate forest soils on the basis of solute transport. Front. For. Glob. Chang. 2023, 6, 1228145. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, C.; Zhou, Z.; Zhou, G.; Hu, X.; Jiang, L.; Li, Y.; Liu, G.; Ji, C.; Zhao, S.; et al. Increasing soil carbon stocks in eight permanent forest plots in China. Biogeosciences 2020, 17, 715–726. [Google Scholar] [CrossRef]
- Doblas-Miranda, E.; Rovira, P.; Brotons, L.; Martínez-Vilalta, J.; Retana, J.; Pla, M.; Vayreda, J. Soil carbon stocks and their variability across the forests, shrublands and grasslands of peninsular Spain. Biogeosciences 2013, 10, 8353–8361. [Google Scholar] [CrossRef]
- Casci, T. Evolution: Arabidopsis’ hidden potential. Nat. Rev. Genet. 2008, 9, 248. [Google Scholar] [CrossRef]
- Iwasaki, S.; Endo, Y.; Hatano, R. The effect of organic matter application on carbon sequestration and soil fertility in upland fields of different types of Andosols. Soil Sci. Plant Nutr. 2017, 63, 200–220. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil Organic Carbon—An Appropriate Indicator to Monitor Trends of Land and Soil Degradation within the SDG Framework? German Environment Agency: Dessau, Germany, 2016; Volume 147.
- Banwart, S.A.; Black, H.; Cai ZuCong, C.Z.; Gicheru, P.T.; Joosten, H.; Victoria, R.L.; Milne, E.; Noellemeyer, E.; Pascual, U. The global challenge for soil carbon. In Soil Carbon: Science, Management and Policy for Multiple Benefits; CABI: Wallingford, UK, 2014; pp. 1–9. [Google Scholar] [CrossRef]
- von Lützow, M.; Kögel-Knabner, I. Temperature sensitivity of soil organic matter decomposition-what do we know? Biol. Fertil. Soils 2009, 46, 1–15. [Google Scholar] [CrossRef]
- Prăvălie, R.; Nita, I.A.; Patriche, C.; Niculiță, M.; Birsan, M.V.; Roșca, B.; Bandoc, G. Global changes in soil organic carbon and implications for land degradation neutrality and climate stability. Environ. Res. 2021, 201, 111580. [Google Scholar] [CrossRef] [PubMed]
- IPBES. The IPBES Assessment Report on Land Degradation and Restoration; Secretariate of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystems Services: Bonn, Germany, 2018. [Google Scholar]
- Zhou, X.Y.; Zhang, C.Y.; Guo, G.F. Effects of climate change on forest soil organic carbon storage: A review. Chin. J. Appl. Ecol. 2010, 21, 1867–1874. [Google Scholar]
- Labaz, B.; Galka, B.; Bogacz, A.; Waroszewski, J.; Kabala, C. Factors influencing humus forms and forest litter properties in the mid-mountains under temperate climate of southwestern Poland. Geoderma 2014, 230–231, 265–273. [Google Scholar] [CrossRef]
- Hounkpatin, O.K.L.; Op de Hipt, F.; Bossa, A.Y.; Welp, G.; Amelung, W. Soil organic carbon stocks and their determining factors in the Dano catchment (Southwest Burkina Faso). Catena 2018, 166, 298–309. [Google Scholar] [CrossRef]
- Zinn, Y.L.; Andrade, A.B.; Araujo, M.A.; Lal, R. Soil organic carbon retention more affected by altitude than texture in a forested mountain range in Brazil. Soil Res. 2018, 56, 284–295. [Google Scholar] [CrossRef]
- Gebeyehu, G.; Soromessa, T.; Bekele, T.; Teketay, D. Carbon stocks and factors affecting their storage in dry Afromontane forests of Awi Zone, northwestern Ethiopia. J. Ecol. Environ. 2019, 43, 1–18. [Google Scholar] [CrossRef]
- Tashi, S.; Singh, B.; Keitel, C.; Adams, M. Soil carbon and nitrogen stocks in forests along an altitudinal gradient in the eastern Himalayas and a meta-analysis of global data. Glob. Chang. Biol. 2016, 22, 2255–2268. [Google Scholar] [CrossRef]
- Yimer, F.; Ledin, S.; Abdelkadir, A. Soil organic carbon and total nitrogen stocks as affected by topographic aspect and vegetation in the Bale Mountains, Ethiopia. Geoderma 2006, 135, 335–344. [Google Scholar] [CrossRef]
- Andivia, E.; Rolo, V.; Jonard, M.; Formánek, P.; Ponette, Q. Tree species identity mediates mechanisms of top soil carbon sequestration in a Norway spruce and European beech mixed forest. Ann. For. Sci. 2016, 73, 437–447. [Google Scholar] [CrossRef]
- Reyna-Bowen, L.; Lasota, J.; Vera-Montenegro, L.; Vera-Montenegro, B.; Błońska, E. Distribution and factors influencing organic carbon stock in mountain soils in Babia Góra National Park, Poland. Appl. Sci. 2019, 9, 3070. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, Q.; Qin, Y.; Cao, J.; Li, H.; Zhao, Y. Soil organic carbon as functions of slope aspects and soil depths in a semiarid alpine region of Northwest China. Catena 2017, 152, 94–102. [Google Scholar] [CrossRef]
- Pradhan, B.M.; Awasthi, K.D.; Bajracharya, R.M. Soil organic carbon stocks under different forest types in pokhare khola sub-watershed: A case study from Dhading district of Nepal. WIT Trans. Ecol. Environ. 2012, 157, 535–546. [Google Scholar] [CrossRef]
- Shapkota, J.; Kafle, G. Variation in soil organic carbon under different forest types in Shivapuri Nagarjun National Park, Nepal. Scientifica 2021, 2021, 1382687. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Ghimire, P. Assessment of Soil Organic Carbon Stock of Churia Broad Leaved Forest of Nawalpur District, Nepal. Grassroots J. Nat. Resour. 2019, 2, 45–52. [Google Scholar] [CrossRef]
- Dieleman, W.I.J.; Venter, M.; Ramachandra, A.; Krockenberger, A.K.; Bird, M.I. Soil carbon stocks vary predictably with altitude in tropical forests: Implications for soil carbon storage. Geoderma 2013, 204–205, 59–67. [Google Scholar] [CrossRef]
- Badía, D.; Ruiz, A.; Girona, A.; Martí, C.; Casanova, J.; Ibarra, P.; Zufiaurre, R. The influence of elevation on soil properties and forest litter in the Siliceous Moncayo Massif, SW Europe. J. Mt. Sci. 2016, 13, 2155–2169. [Google Scholar] [CrossRef]
- Ali, S.; Begum, F.; Hayat, R.; Bohannan, B.J.M. Variation in soil organic carbon stock in different land uses and altitudes in Bagrot Valley, Northern Karakoram. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 551–561. [Google Scholar] [CrossRef]
- Jakšić, S.; Ninkov, J.; Milić, S.; Vasin, J.; Živanov, M.; Jakšić, D.; Komlen, V. Influence of slope gradient and aspect on soil organic carbon content in the region of Niš, Serbia. Sustainability 2021, 13, 8332. [Google Scholar] [CrossRef]
- Bangroo, S.A.; Najar, G.R.; Rasool, A. Effect of altitude and aspect on soil organic carbon and nitrogen stocks in the Himalayan Mawer Forest Range. Catena 2017, 158, 63–68. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Kumar, M.; Bussmann, R.W. Altitudinal variation in soil organic carbon stock in coniferous subtropical and broadleaf temperate forests in Garhwal himalaya. Carbon Balance Manag. 2009, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Saimun, M.S.R.; Karim, M.R.; Sultana, F.; Arfin-Khan, M.A.S. Multiple drivers of tree and soil carbon stock in the tropical forest ecosystems of Bangladesh. Trees For. People 2021, 5, 100108. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Ouyang, Z. Effects of land use, climate, topography and soil properties on regional soil organic carbon and total nitrogen in the Upstream Watershed of Miyun Reservoir, North China. J. Environ. Sci. 2012, 24, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Yajuan, W.; Meiying, L.; Ji, W.; Xiaohong, D.; Yanlong, H. The effects of vegetation communities on soil organic carbon stock in an enclosed desert-steppe region of northern China. Soil Sci. Plant Nutr. 2022, 68, 284–294. [Google Scholar] [CrossRef]
- FRTC. National Land Cover Monitoring System of Nepal; Forest Research and Training Center: Kathmandu, Nepal, 2022.
- DFRS. State of Nepal’s Forests; Forest Resource Assessment (FRA) Nepal, Department of Forest Research and Survey (DFRS): Kathmandu, Nepal, 2015.
- LRMP. Summary Report: Land Resources Mapping Project; Survey Department, HMGN and Kenting Earth Sciences: Kathmandu, Nepal, 1986. [Google Scholar]
- MoFSC. Nepal Biodiversity Strategy; Ministry of Forests and Soil Conservation: Kathmandu, Nepal, 2002.
- Stainton, J.D.A. Forests of Nepal; John Murray: London, UK, 1972; Volume 22. [Google Scholar]
- Shrestha, T.B. Classification of Nepalese Forests and Their Distribution in Protected Areas. Initiation 1970, 2, 1–9. [Google Scholar] [CrossRef]
- DFRS/FRA. Terai Forests of Nepal; Forest Resource Assessment Nepal Project/Department of Forest Research and Survey. Department of Forest Research and Survey: Kathmandu, Nepal, 2014; p. 160.
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Slepetiene, A.; Slepetys, J.; Liaudanskiene, I. Standard and modified methods for soil organic carbon determination in agricultural soils. Agron. Res. 2008, 6, 543–554. [Google Scholar]
- Manojlović, M.; Čabilovski, R.; Sitaula, B. Soil organic carbon in Serbian Mountain soils: Effects of land use and altitude. Polish J. Environ. Stud. 2011, 20, 977–986. [Google Scholar]
- Kara, Ö.; Bolat, I.; Çakiroǧlu, K.; Öztürk, M. Plant canopy effects on litter accumulation and soil microbial biomass in two temperate forests. Biol. Fertil. Soils 2008, 45, 193–198. [Google Scholar] [CrossRef]
- DFRS. Middle Mountains Forests of Nepal: Forest Resource Assessment (FRA); Department of Forest Research and Survey: Kathmandu, Nepal, 2015; ISBN 9789937889629.
- Dimitriadou, E.; Hornik, K.; Leisch, F.; Meyer, D.; Weingessel, A. e1071: Misc Functions of the Department of Statistics (e1071), TU Wien. R Package Version 1.6-1. 2012. Available online: http://CRAN.R-project.org/package=e1071 (accessed on 23 November 2022).
- Andy Bunn, M.K. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; pp. 11–18. Available online: http://www.r-project.org (accessed on 16 November 2022).
- Dalmolin, R.S.D.; Gonçalves, C.N.; Dick, D.P.; Knicker, H.; Klamt, E.; Kögel-Knabner, I. Organic matter characteristics and distribution in Ferralsol profiles of a climosequence in southern Brazil. Eur. J. Soil Sci. 2006, 57, 644–654. [Google Scholar] [CrossRef]
- Sousa Neto, E.; Carmo, J.B.; Keller, M.; Martins, S.C.; Alves, L.F.; Vieira, S.A.; Piccolo, M.C.; Camargo, P.; Couto, H.T.Z.; Joly, C.A.; et al. Soil-atmosphere exchange of nitrous oxide, methane and carbon dioxide in a gradient of elevation in the coastal Brazilian Atlantic forest. Biogeosciences 2011, 8, 733–742. [Google Scholar] [CrossRef]
- Zech, M.; Hörold, C.; Leiber-Sauheitl, K.; Kühnel, A.; Hemp, A.; Zech, W. Buried black soils on the slopes of Mt. Kilimanjaro as a regional carbon storage hotspot. Catena 2014, 112, 125–130. [Google Scholar] [CrossRef]
- Garten, C.T.; Hanson, P.J. Measured forest soil C stocks and estimated turnover times along an elevation gradient. Geoderma 2006, 136, 342–352. [Google Scholar] [CrossRef]
- Tsui, C.C.; Chen, Z.S.; Hsieh, C.F. Relationships between soil properties and slope position in a lowland rain forest of southern Taiwan. Geoderma 2004, 123, 131–142. [Google Scholar] [CrossRef]
- Pichler, V.; Gömöryová, E.; Leuschner, C.; Homolák, M.; Abrudan, I.V.; Pichlerová, M.; Střelcová, K.; Di Filippo, A.; Sitko, R. Parent material effect on soil organic carbon concentration under primeval european beech forests at a regional scale. Forests 2021, 12, 405. [Google Scholar] [CrossRef]
- Fissore, C.; Giardina, C.P.; Kolka, R.K.; Trettin, C.C.; King, G.M.; Jurgensen, M.F.; Barton, C.D.; Mcdowell, S.D. Temperature and vegetation effects on soil organic carbon quality along a forested mean annual temperature gradient in North America. Glob. Chang. Biol. 2008, 14, 193–205. [Google Scholar] [CrossRef]
- Hanawalt, R.B.; Wittaker, R.H. Altitudinally coordinated patterns of soils and vegetation in the San Jacinto mountains, California. Soil Sci 1976, 121, 114–124. [Google Scholar] [CrossRef]
- Schindlbacher, A.; De Gonzalo, C.; Díaz-Pinés, E.; Gíorra, P.; Matthews, B.; Inclán, R.; Zechmeister-Boltenstern, S.; Rubio, A.; Jandl, R. Temperature sensitivity of forest soil organic matter decomposition along two elevation gradients. J. Geophys. Res. Biogeosciences 2010, 115, G03018. [Google Scholar] [CrossRef]
- Jha, P.K. Environment and Man in Nepal; Craftsman Press: Bangkok, Thailand, 1992. [Google Scholar]
- Nanko, K.; Hashimoto, S.; Miura, S.; Ishizuka, S.; Sakai, Y.; Levia, D.F.; Ugawa, S.; Nishizono, T.; Kitahara, F.; Osone, Y.; et al. Assessment of soil group, site and climatic effects on soil organic carbon stocks of topsoil in Japanese forests. Eur. J. Soil Sci. 2017, 68, 547–558. [Google Scholar] [CrossRef]
- Kobler, J.; Zehetgruber, B.; Dirnböck, T.; Jandl, R.; Mirtl, M.; Schindlbacher, A. Effects of aspect and altitude on carbon cycling processes in a temperate mountain forest catchment. Landsc. Ecol. 2019, 34, 325–340. [Google Scholar] [CrossRef]
- Xue, R.; Yang, Q.; Miao, F.; Wang, X.; Shen, Y. Slope aspect influences plant biomass, soil properties and microbial composition in alpine meadow on the Qinghai-Tibetan Plateau. J. Soil Sci. Plant Nutr. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Peng, X.; Wu, W.; Zheng, Y.; Sun, J.; Hu, T.; Wang, P. Correlation analysis of land surface temperature and topographic elements in Hangzhou, China. Sci. Rep. 2020, 10, 10451. [Google Scholar] [CrossRef] [PubMed]
- Ajami, M.; Heidari, A.; Khormali, F.; Gorji, M.; Ayoubi, S. Environmental factors controlling soil organic carbon storage in loess soils of a subhumid region, northern Iran. Geoderma 2016, 281, 1–10. [Google Scholar] [CrossRef]
- Malla, R.; Neupane, P.R.; Köhl, M. Modelling Soil Organic Carbon as a Function of Topography and Stand Variables. Forests 2022, 13, 1391. [Google Scholar] [CrossRef]
- Cannell, M.G.R. Forest canopies: Edited by Margaret D. Lowman and Nalini M. Nadkarni. pp. 624. Academic Press, 1995. US$69.95. ISBN 0 12 457650 9. Endeavour 1995, 19, 133. [Google Scholar] [CrossRef]
- Harte, J.; Rawa, A.; Price, V. Effects of manipulated soil microclimate on mesofaunal biomass and diversity. Soil Biol. Biochem. 1996, 28, 313–322. [Google Scholar] [CrossRef]
- Bokhorst, S.; Wardle, D.A. Microclimate within litter bags of different mesh size: Implications for the ‘arthropod effect’ on litter decomposition. Soil Biol. Biochem. 2013, 58, 147–152. [Google Scholar] [CrossRef]
- Lozano-Parra, J.; Pulido, M.; Lozano-Fondón, C.; Schnabel, S. How do soil moisture and vegetation covers influence soil temperature in drylands of Mediterranean regions? Water 2018, 10, 1747. [Google Scholar] [CrossRef]
- Agena, A. Effects of three tree species on microclimate and soil amelioration in the central rift valley of Ethiopia. J. Soil Sci. Environ. Manag. 2014, 5, 62–71. [Google Scholar] [CrossRef]
- Breshears, D.D.; Nyhan, J.W.; Heil, C.E.; Wilcox, B.P. Effects of woody plants on microclimate in a semiarid woodland: Soil temperature and evaporation in canopy and intercanopy patches. Int. J. Plant Sci. 1998, 159, 1010–1017. [Google Scholar] [CrossRef]
- Çömez, A.; Güner, Ş.T.; Tolunay, D. The effect of stand structure on litter decomposition in Pinus sylvestris L. stands in Turkey. Ann. For. Sci. 2021, 78, 19. [Google Scholar] [CrossRef]
- Ortiz, C.A.; Lundblad, M.; Lundström, A.; Stendahl, J. The effect of increased extraction of forest harvest residues on soil organic carbon accumulation in Sweden. Biomass Bioenergy 2014, 70, 230–238. [Google Scholar] [CrossRef]
- James, J.; Page-Dumroese, D.; Busse, M.; Palik, B.; Zhang, J.; Eaton, B.; Slesak, R.; Tirocke, J.; Kwon, H. Effects of forest harvesting and biomass removal on soil carbon and nitrogen: Two complementary meta-analyses. For. Ecol. Manag. 2021, 485, 118935. [Google Scholar] [CrossRef]
- Devi, A.S. Influence of trees and associated variables on soil organic carbon: A review. J. Ecol. Environ. 2021, 45, 5. [Google Scholar] [CrossRef]
- Dulamsuren, C. Organic carbon stock losses by disturbance: Comparing broadleaved pioneer and late-successional conifer forests in Mongolia’s boreal forest. For. Ecol. Manag. 2021, 499, 119636. [Google Scholar] [CrossRef]
- Chiti, T.; Díaz-Pinés, E.; Rubio, A. Soil organic carbon stocks of conifers, broadleaf and evergreen broadleaf forests of Spain. Biol. Fertil. Soils 2012, 48, 817–826. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Shin, J.; Yim, J.; Kang, J. Assessing the carbon storage of soil and litter from national forest inventory data in South Korea. Forests 2020, 11, 1318. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Hauck, M.; Mühlenberg, M. Vegetation at the taiga forest-steppe borderline in the western Khentey Mountains, northern Mongolia. Ann. Bot. Fenn. 2005, 42, 411–426. [Google Scholar]
- Dorjsuren, C. Anthropogenic succession in larch forests of Mongolia. Biol. Resourc. Nat. Cond. Mong. 2009, 50, 1–260. [Google Scholar]
- Isaev, A.S.; Korovin, G.N.; Bartalev, S.A.; Ershov, D.V.; Janetos, A.; Kasischke, E.S.; Shugart, H.H.; French, N.H.F.; Orlick, B.E.; Murphy, T.L. Using remote sensing to assess Russian forest fire carbon emissions. Clim. Chang. 2002, 55, 235–249. [Google Scholar] [CrossRef]
- De Groot, W.J.; Pritchard, J.M.; Lynham, T.J. Forest floor fuel consumption and carbon emissions in Canadian boreal forest fires. Can. J. For. Res. 2009, 39, 367–382. [Google Scholar] [CrossRef]
- Randerson, J.T.; Liu, H.; Flanner, M.G.; Chambers, S.D.; Jin, Y.; Hess, P.G.; Pfister, G.; Mack, M.C.; Treseder, K.K.; Welp, L.R.; et al. The impact of boreal forest fire on climate warming. Science 2006, 314, 1130–1132. [Google Scholar] [CrossRef]
- Crow, S.E.; Lajtha, K.; Filley, T.R.; Swanston, C.W.; Bowden, R.D.; Caldwell, B.A. Sources of plant-derived carbon and stability of organic matter in soil: Implications for global change. Glob. Chang. Biol. 2009, 15, 2003–2019. [Google Scholar] [CrossRef]
- Kerven, G.L.; Menzies, N.W.; Geyer, M.D. Soil carbon determination by high temperature combustion—A comparison with dichromate oxidation procedures and the influence of charcoal and carbonate carbon on the measured value. Commun. Soil Sci. Plant Anal. 2000, 31, 1935–1939. [Google Scholar] [CrossRef]
- MoFE. Third National Communication to the United Nations Framework Convention on Climate Change October 2019; Ministry of Forests and Environment: Kathmandu, Nepal, 2019.
- Darjee, K.B.; Neupane, P.R.; Köhl, M. Do Local Perceptions of Climate Variability and Changes Correspond to Observed Climate Changes? A Comparative Study from Nepal as One of the Most Climate-Vulnerable Countries. Weather Clim. Soc. 2022, 14, 205–222. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).