Soil Carbon Storage, Enzymatic Stoichiometry, and Ecosystem Functions in Indian Himalayan Legume-Diversified Pastures
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Research Site and Experiment
2.2. Collection of Plant and Soil Samples
2.3. Soil Organic Carbon Estimation and C Mineralization Study
2.4. Soil Enzyme Activity Measurement and Determination of Soil Functional Diversity Indices
2.5. Estimation of Indices for Ecosystem Function
2.6. Analytical Procedure of Data
3. Result
3.1. Soil pH, EC, Bulk Density, and Pasture Productivity
3.2. Total Soil Organic Carbon (TOC), Microbial Biomass C (MBC), Labile C (LC) and Recalcitrant C (RC)
3.3. Soil Organic Carbon Fractions
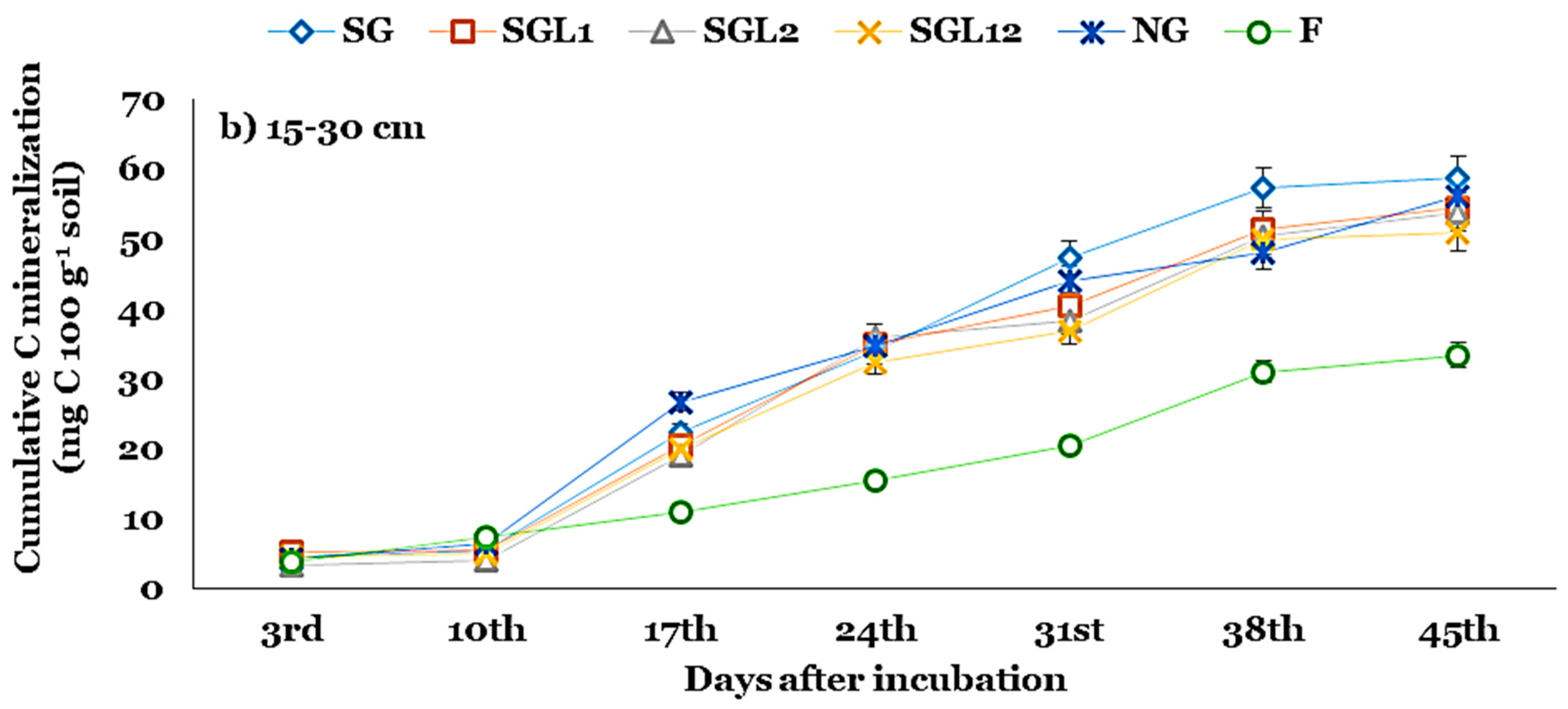
3.4. Soil Carbon Mineralization Kinetics
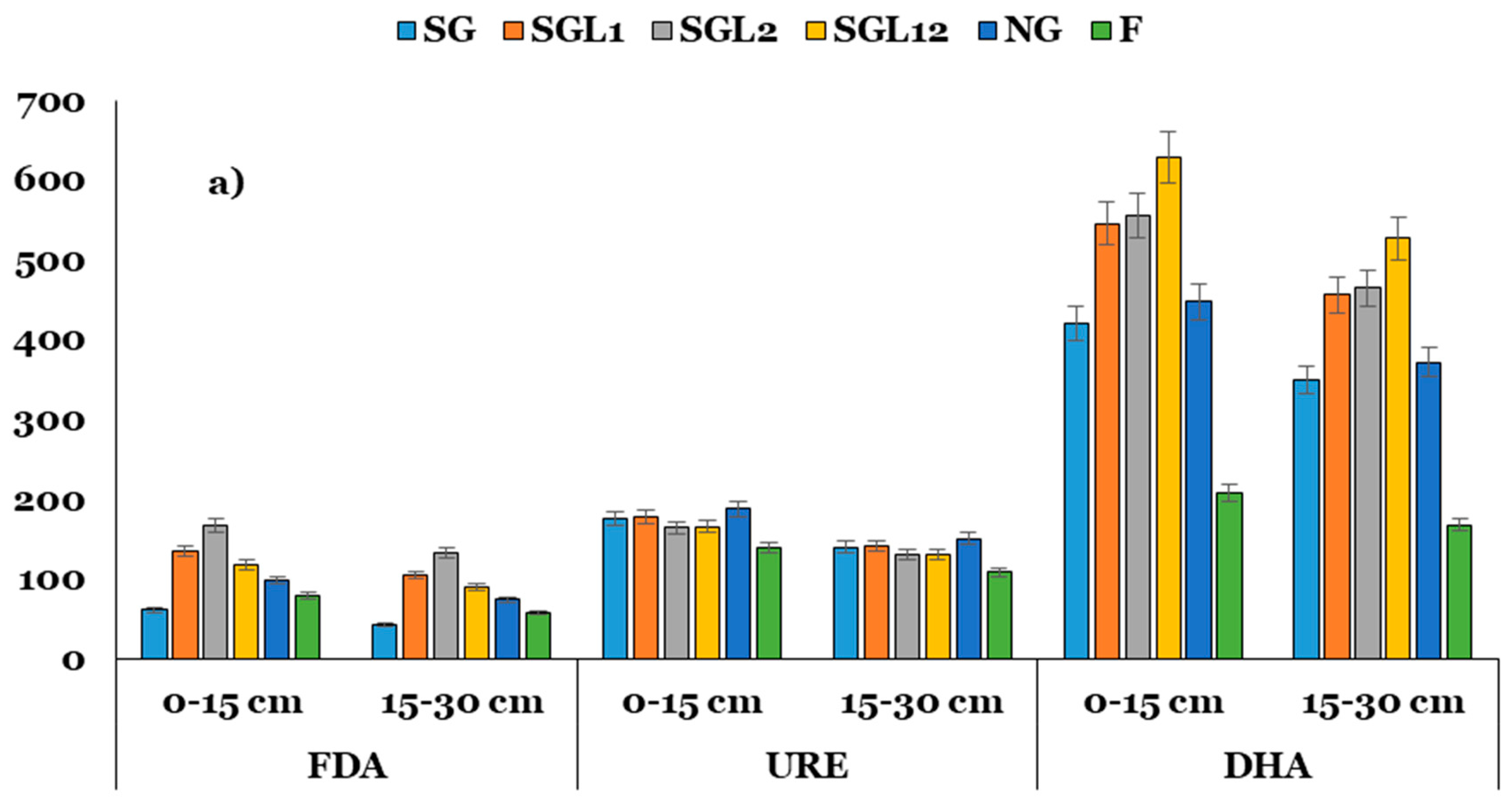
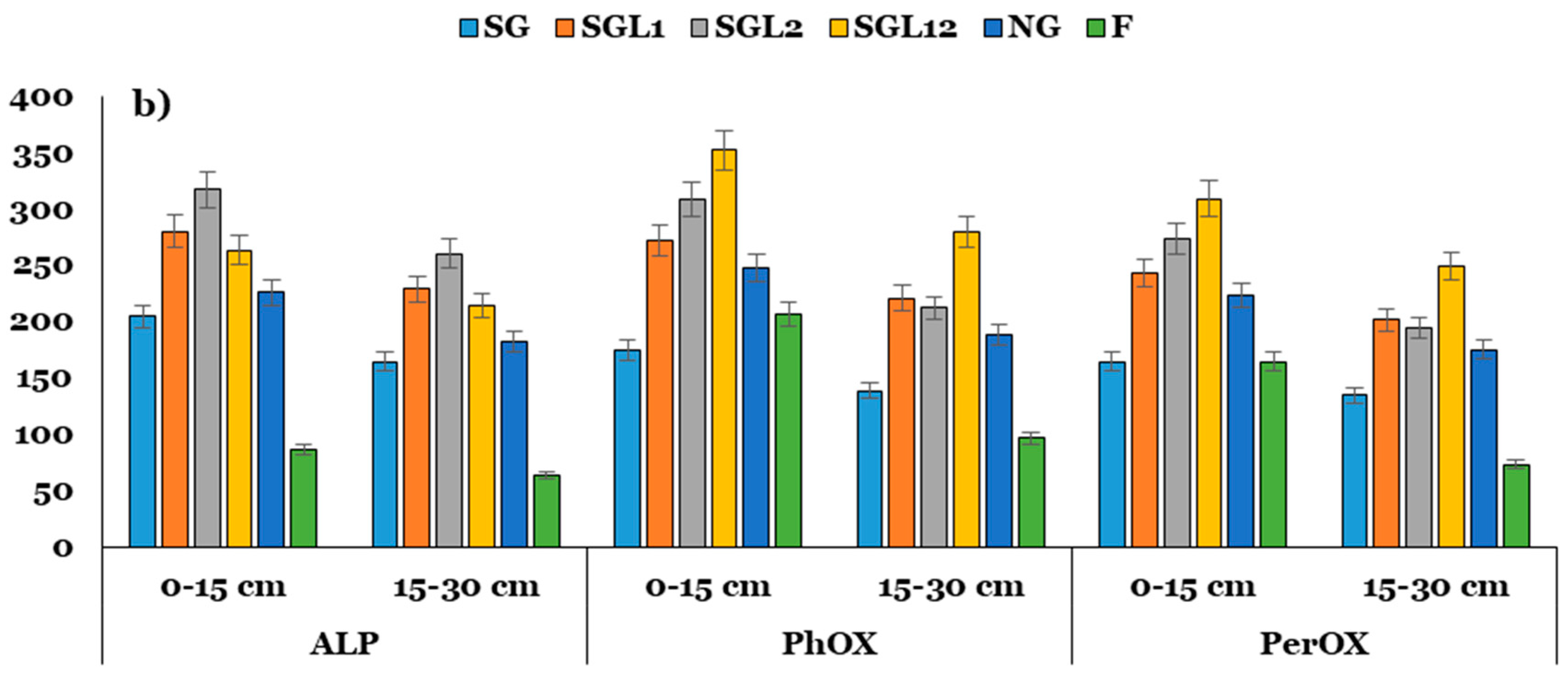
3.5. Enzyme Activities and Indices
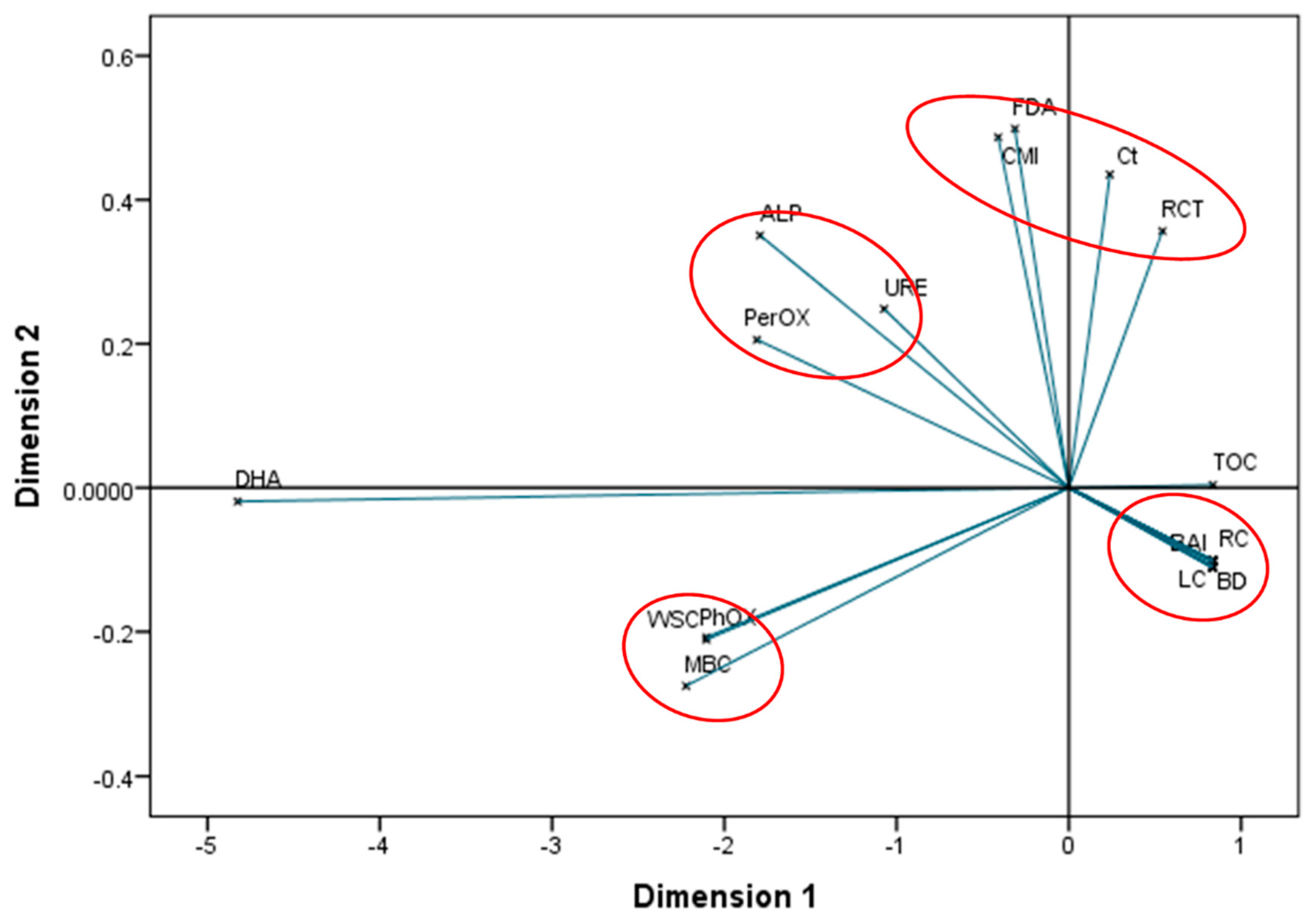
3.6. Stoichiometry of Soil Enzymes
3.7. Soil Organic Carbon Accumulation and Sequestration
3.8. Carbon Management Index (CMI), Biological Activity Index (BAI), and Ecorestoration Efficiency (ERE)
4. Discussion
4.1. Legume Diversification Shapes Soil Functional Diversity and Enzyme Stoichiometry
4.2. Legume Diversification Regulate Soil Carbon Pools and C Mineralization and Sequestration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mo, L.; Zohner, C.M.; Reich, P.B.; Liang, J.; de Miguel, S.; Nabuurs, G.-J.; Renner, S.S.; van den Hoogen, J.; Araza, A.; Herold, M.; et al. Integrated Global Assessment of the Natural Forest Carbon Potential. Nature 2023, 624, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fultz, L.M.; Moore-Kucera, J.; Acosta-Martínez, V.; Horita, J.; Strauss, R.; Zak, J.; Calderón, F.; Weindorf, D. Soil carbon sequestration potential in semi-arid grasslands in the Conservation Reserve Program. Geoderma 2017, 294, 80–90. [Google Scholar] [CrossRef]
- Dias Filho, M.B. Diagnostico’ das Pastagens no Brasil; Documentos; Embrapa: Brasília, Brazil, 2014. [Google Scholar]
- Gil, J.D.B.; Garrett, R.; Berger, T. Determinants of crop-livestock integration in Brazil: Evidence from the household and regional levels. Land Use Policy 2016, 59, 557–568. [Google Scholar] [CrossRef]
- Thornton, P.K.; Herrero, M. Adapting to climate change in the mixed crop and livestock farming systems in sub-Saharan Africa. Nat. Clim. Chang. 2015, 5, 830–836. [Google Scholar] [CrossRef]
- Cohn, A.S.; Mosnier, A.; Havlík, P.; Valin, H.; Herrero, M.; Schmid, E.; O’Hare, M.; Obersteiner, M. Cattle ranching intensification in Brazil can reduce global greenhouse gas emissions by sparing land from deforestation. Proc. Natl. Acad. Sci. USA 2014, 111, 7236–7241. [Google Scholar] [CrossRef]
- Assmann, J.M.; Anghinoni, I.; Martins, A.P.; de Andrade, S.E.V.G.; Cecagno, D.; Carlos, F.S.; de Faccio Carvalho, P.C. Soil carbon and nitrogen stocks and fractions in a long-term integrated crop–livestock system under no-tillage in southern Brazil. Agric. Ecosyst. Environ. 2014, 190, 52–59. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Schmid, B.; De Laender, F.; Eisenhauer, N.; Zhang, X.; Chen, H.; Craven, D.; De Boeck, H.J.; Hautier, Y.; Petchey, O.L.; et al. Biodiversity Promotes Ecosystem Functioning despite Environmental Change. Ecol. Lett. 2021, 25, 555–569. [Google Scholar] [CrossRef]
- Hagan, J.G.; Vanschoenwinkel, B.; Gamfeldt, L. We Should Not Necessarily Expect Positive Relationships between Biodiversity and Ecosystem Functioning in Observational Field Data. Ecol. Lett. 2021, 24, 2537–2548. [Google Scholar] [CrossRef]
- Gonzalez, A.; Germain, R.M.; Srivastava, D.S.; Filotas, E.; Dee, L.E.; Gravel, D.; Thompson, P.L.; Isbell, F.; Wang, S.; Kéfi, S.; et al. Scaling-up Biodiversity-ecosystem Functioning Research. Ecol. Lett. 2020, 23, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Bienes-Allas, R.; Gristina, L.; Cerdà, A.; Novara, A.; Pereira, P. Carbon input threshold for soil carbon budget optimization in eroding vineyards. Geoderma 2016, 271, 144–149. [Google Scholar] [CrossRef]
- Cong, W.F.; van Ruijven, J.; Mommer, L.; De Deyn, G.B.; Berendse, F.; Hoffland, E. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 2014, 102, 1163–1170. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Bhattacharyya, R.; Dwivedi, B.S.; Meena, M.C.; Agarwal, B.K.; Mahapatra, P.; Shahi, D.K.; Salwani, R.; Agnihorti, R. Temperature sensitivity of soil organic carbon decomposition as affected by long-term fertilization under a soybean based cropping system in a sub-tropical Alfisol. Agric. Ecosyst. Environ. 2016, 233, 202–213. [Google Scholar] [CrossRef]
- Hille, R.; Lambers, J.; Harpole, W.S.; Tilman, D. Species and mechanisms contributing to the positive diversity-productivity relationship. Ecol. Lett. 2004, 7, 661. [Google Scholar]
- Fornara, D.A.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Ferreira, A.C.C.; Leite, L.F.C.; de Araújo, A.S.F.; Eisenhauer, N. Land-use type effects on soil organic carbon and microbial properties in a semi-arid region of northeast Brazil. Land Degrad. Dev. 2016, 27, 171–178. [Google Scholar] [CrossRef]
- Novara, A.; Gristina, L.; Saladino, S.S.; Santoro, A.; Cerdà, A. Soil erosion assessment on tillage and alternative soil managements in a Sicilian vineyard. Soil Tillage Res. 2011, 117, 140–147. [Google Scholar] [CrossRef]
- Roa-Fuentes, L.L.; Martínez-Garza, C.; Etchevers, J.; Campo, J. Recovery of soil C and N in a tropical pasture: Passive and active restoration. Land Degrad. Dev. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.A.; Calvo-Magro, E.; Valentine, A. Benefits of the symbiotic association of shrubby legumes for the rehabilitation of degraded soils under Mediterranean climatic conditions. Land Degrad. Dev. 2016, 27, 395–405. [Google Scholar] [CrossRef]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible organic carbon fractions and soil quality changes in an oxic paleustalf under different pasture leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Brar, B.S.; Dheri, G.S.; Lal, R.; Singh, K.; Walia, S.S. Cropping system impacts on carbon fractions and accretion in typic ustochrept soil of Punjab, India. J. Crop. Improv. 2015, 29, 281–300. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Dick, R.P. (Ed.) Methods of Soil Enzymology; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 26. [Google Scholar]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil—V: A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, A.B.; Kumar, R.V.; Manna, M.C.; Bhattacharyya, R.; Rahman, M.M.; Sharma, P.; Rajput, P.S.; Misra, S. Soil enzymes and microbial elemental stoichiometry as bio-indicators of soil quality in diverse cropping systems and nutrient management practices of Indian Vertisols. Appl. Soil Ecol. 2020, 145, 103304. [Google Scholar] [CrossRef]
- Blair, N. Impact of cultivation and sugar-cane green trash management on carbon fractions and aggregate stability for a Chromic Luvisol in Queensland, Australia. Soil Tillage Res. 2000, 55, 183–191. [Google Scholar] [CrossRef]
- Ghosh, A.; Kumar, R.V.; Manna, M.C.; Singh, A.K.; Parihar, C.M.; Kumar, S.; Roy, A.K.; Koli, P. Eco-restoration of degraded lands through trees and grasses improves soil carbon sequestration and biological activity in tropical climates. Ecol. Eng. 2021, 162, 106176. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and productivity in a long-term grassland experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef]
- Roscher, C.; Schumacher, J.; Baade, J.; Wilcke, W.; Gleixner, G.; Weisser, W.W.; Schmid, B.; Schulze, E.D. The role of biodiversity for element cycling and trophic interactions: An experimental approach in a grassland community. Basic Appl. Ecol. 2004, 5, 107–121. [Google Scholar] [CrossRef]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecol. 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The M icrobial E fficiency-M atrix S tabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.C.; DeLaune, R.D. Fungal and bacterial mediated denitrification in wetlands: Influence of sediment redox condition. Water Res. 2010, 44, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Feng, W.; Luo, Y.; Baldock, J.; Wang, E. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Glob. Chang. Biol. 2010, 23, 4430–4439. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Olander, L.P.; Vitousek, P.M. Regulation of soil phosphatase and chitinase activityby N and P availability. Biogeochemistry 2000, 49, 175–191. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M.; Claus, P. Phosphate inhibits acetotrophic methanogenesis on rice roots. Appl. Environ. Microbiol. 2000, 66, 828–831. [Google Scholar] [CrossRef]
- Li, L.; Qu, Z.; Jia, R.; Wang, B.; Wang, Y.; Qu, D. Excessive input of phosphorus significantly affects microbial Fe (III) reduction in flooded paddy soils by changing the abundances and community structures of Clostridium and Geobacteraceae. Sci. Total Environ. 2017, 607, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 229–243. [Google Scholar]
- Jian, S.Y.; Li, J.W.; Chen, J.; Wang, G.S.; Mayes, M.A.; Dzantor, K.; Hui, D.F.; Leo, Y.Q. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 10, 32–43. [Google Scholar] [CrossRef]
- Hazra, K.K.; Ghosh, P.K.; Venkatesh, M.S.; Nath, C.P.; Kumar, N.; Singh, M.; Singh, J.; Nadarajan, N. Improving soil organic carbon pools through inclusion of summer mungbean in cereal-cereal cropping systems in Indo-Gangetic plain. Arch. Agron. Soil Sci. 2018, 64, 1690–1704. [Google Scholar] [CrossRef]
- Venkatesh, M.S.; Hazra, K.K.; Ghosh, P.K.; Praharaj, C.S.; Kumar, N. Long-term effect of pulses and nutrient management on soil carbon sequestration in Indo-Gangetic plains of India. Can. J. Soil Sci. 2013, 93, 127–136. [Google Scholar] [CrossRef]
- Virk, A.L.; Yadav, G.S.; Zhao, X.; Kan, Z.R.; Qi, J.Y.; Ahmad, N.; Lal, R.; Zhang, H.L. Role of Legumes in Managing Soil Organic Matter and Improving Crop Yield. In Soil Organic Matter and Feeding the Future; CRC Press: Boca Raton, FL, USA, 2021; pp. 259–278. [Google Scholar]




| Parameters | Soil pH | Soil EC | Bulk Density | Productivity | |||
|---|---|---|---|---|---|---|---|
| Soil Layer | |||||||
| Treatments # | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | |
| SG | 6.49 a | 6.86 a | 0.08 a | 0.04 a | 1.36 a | 1.38 a | 8.98 b |
| SGL1 | 6.87 a | 7.11 a | 0.10 a | 0.05 a | 1.33 a | 1.34 a | 10.59 a |
| SGL2 | 6.32 a | 6.37 a | 0.13 a | 0.12 a | 1.31 a | 1.39 a | 10.02 a |
| SGL12 | 6.78 a | 6.81 a | 0.07 a | 0.08 a | 1.33 a | 1.33 a | 9.48 ab |
| NG | 6.33 a | 6.97 a | 0.09 a | 0.06 a | 1.34 a | 1.36 a | 4.22 c |
| F | 6.54 a | 6.62 a | 0.12 a | 0.11 a | 1.40 a | 1.41 a | --- |
| Parameters | TOC | LC | RC | MBC | ||||
|---|---|---|---|---|---|---|---|---|
| Soil Layer | ||||||||
| Treatments # | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm |
| SG | 9.08 b | 7.99 b | 5.42 b | 4.47 b | 3.66 bc | 3.52 c | 257.71 b | 232.24 b |
| SGL1 | 10.71 ab | 9.98 ab | 6.04 a | 5.34 a | 4.67 b | 4.64 b | 295.92 ab | 278.94 a |
| SGL2 | 12.34 a | 7.26 b | 6.82 a | 3.86 c | 5.53 a | 3.41 c | 334.13 a | 215.26 b |
| SGL12 | 11.07 a | 11.07 a | 6.02 a | 5.91 a | 5.05 a | 5.16 a | 304.41 ab | 304.41 a |
| NG | 10.17 ab | 8.90 b | 5.54 b | 4.69 b | 4.63 b | 4.21 b | 283.19 b | 253.47 ab |
| F | 6.90 c | 6.40 c | 3.81 c | 3.42 c | 3.09 c | 2.98 d | 206.71 c | 195.00 c |
| Parameters | WSC | AC | POM-C | MOM-C | Decay Rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soil Layer | ||||||||||
| Treatments # | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm |
| SG | 247.99 a | 233.04 a | 1.04 c | 0.98 bc | 2.29 c | 2.09 cd | 3.62 cd | 3.57 cd | 2.40 × 10−2 a | 6.81 × 10−4 c |
| SGL1 | 257.82 a | 246.84 a | 1.13 b | 1.09 ab | 2.43 b | 2.28 b | 4.10 b | 4.09 b | 2.06 × 10−3 b | 5.63 × 10−4 d |
| SGL2 | 269.99 a | 223.46 a | 1.22 a | 0.94 c | 2.59 a | 1.96 d | 4.46 a | 4.73 a | 1.47 × 10−4 d | 3.99 × 10−4 e |
| SGL12 | 257.47 a | 255.73 a | 1.15 ab | 1.15 a | 2.42 b | 2.40 a | 4.54 a | 4.58 a | 2.12 × 10−4 c | 3.50 × 10−4 e |
| NG | 249.92 a | 236.54 a | 1.10 bc | 1.03 bc | 2.32 bc | 2.14 c | 3.95 c | 3.81 c | 2.42 × 10−3 b | 7.76 × 10−4 a |
| F | 222.70 b | 216.52 b | 0.92 d | 0.90 c | 1.95 d | 1.87 e | 3.42 d | 3.38 d | 2.31 × 10−3 b | 1.64 × 10−4 b |
| Parameters | H | SYI | CMI | BAI | ERE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soil Layers | ||||||||||
| Treatments # | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm |
| SG | 4.42 b | 4.59 b | 4.63 c | 4.95 c | 110.40 bc | 107.52 bc | 1.38 d | 1.66 c | 1.52 c | 1.79 d |
| SGL1 | 3.91 c | 3.96 c | 4.88 bc | 5.38 b | 118.58 b | 117.56 ab | 1.94 b | 2.41 a | 2.30 b | 2.84 b |
| SGL2 | 3.76 c | 3.72 c | 5.08 b | 5.34 b | 126.93 a | 104.02 bc | 2.13 a | 2.54 a | 2.70 a | 2.64 b |
| SGL12 | 5.17 a | 5.22 a | 6.65 a | 6.21 a | 120.42 ab | 123.19 a | 2.05 ab | 2.60 a | 2.47 b | 3.20 a |
| NG | 4.12 bc | 4.18 bc | 5.03 b | 5.54 b | 115.83 b | 112.02 b | 1.65 c | 2.02 b | 1.91 c | 2.26 c |
| F | 2.83 d | 2.67 d | 1.67 d | 1.68 d | 100.00 d | 100.00 c | 1.00 e | 1.00 d | 1.00 d | 1.00 e |
| Treatments # | C Accumulation (Mg C ha−1) | C Sequestration (Mg C ha−1) |
|---|---|---|
| SG | 34.97 c | 14.72 c |
| SGL1 | 41.48 ab | 18.66 b |
| SGL2 | 39.33 bc | 17.93 b |
| SGL12 | 44.03 a | 20.31 a |
| NG | 38.57 bc | 17.88 b |
| F | 27.94 d | 12.77 d |
| Variables | DHA:FDA | DHA:URE | DHA:ALP | DHA: PhOX | DHA: PerOX |
|---|---|---|---|---|---|
| C accumulation | −0.592 NS | −0.642 * | −0.771 * | −0.796 ** | −0.801 ** |
| C sequestration | −0.634 * | −0.517 NS | −0.738 * | −0.799 ** | −0.792 ** |
| C mineralization | 0.287 NS | 0.935 ** | 0.678 * | 0.559 NS | 0.627 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, A.; Ahmad, S.; Singh, A.K.; Jha, P.; Kumar Yadav, R.; Jiménez Ballesta, R.; Bhatt, S.S.; Biradar, N.; Hamid Mir, N. Soil Carbon Storage, Enzymatic Stoichiometry, and Ecosystem Functions in Indian Himalayan Legume-Diversified Pastures. Land 2024, 13, 452. https://doi.org/10.3390/land13040452
Ghosh A, Ahmad S, Singh AK, Jha P, Kumar Yadav R, Jiménez Ballesta R, Bhatt SS, Biradar N, Hamid Mir N. Soil Carbon Storage, Enzymatic Stoichiometry, and Ecosystem Functions in Indian Himalayan Legume-Diversified Pastures. Land. 2024; 13(4):452. https://doi.org/10.3390/land13040452
Chicago/Turabian StyleGhosh, Avijit, Suheel Ahmad, Amit K. Singh, Pramod Jha, Rajendra Kumar Yadav, Raimundo Jiménez Ballesta, Sheeraz Saleem Bhatt, Nagaratna Biradar, and Nazim Hamid Mir. 2024. "Soil Carbon Storage, Enzymatic Stoichiometry, and Ecosystem Functions in Indian Himalayan Legume-Diversified Pastures" Land 13, no. 4: 452. https://doi.org/10.3390/land13040452
APA StyleGhosh, A., Ahmad, S., Singh, A. K., Jha, P., Kumar Yadav, R., Jiménez Ballesta, R., Bhatt, S. S., Biradar, N., & Hamid Mir, N. (2024). Soil Carbon Storage, Enzymatic Stoichiometry, and Ecosystem Functions in Indian Himalayan Legume-Diversified Pastures. Land, 13(4), 452. https://doi.org/10.3390/land13040452







