Abstract
Conducted in Southern Italy’s Calabria region, this study aimed to repurpose olive wastes, which are still a source of valuable biomolecules including plant nutrients, flavonoids, polysaccharides, and phenolic compounds, into compost to be used in sustainable agriculture as fertilizers, in alternative to synthetic substances. The compost underwent chemical analysis and soil fertility testing to support eco-friendly agricultural practices. Factors like extraction process, waste composition, and percentage of waste in composting were studied for their impact. The research evaluated compost fertilizing effectiveness by analyzing soil chemical and biological properties 180 days after the application. The results demonstrated that the proportion of olive oil waste and the olive oil extraction method significantly impacted compost quality and its environmental footprint. All composts improved soil properties but to a different extent. Compost olive waste 3 (OWC3; 34% olive oil waste, 33% buffalo manure, and 33% straw) was the most effective in enhancing soil fertility. Compost olive waste 1 (OWC1), with the same olive waste percentage as compost olive waste 2 (OWC2) but from a different extraction process, outperformed OWC2 in enhancing soil fertility and microbial activity. The research highlighted the importance of organic matter addition to soil and the significant role of both raw material percentage and extraction process in compost quality. Life cycle assessment indicated that OWC3 had the lowest environmental impact and the highest fertilizing power. Composting represents a practical way to manage organic wastes and improve soil quality, providing essential nutrients for soil health and ecosystem functioning.
1. Introduction
The frequent or unregulated application of synthetic chemicals like nitrogen, phosphorus, and potassium to enhance plant growth leads to the deterioration of soil fertility and quality worldwide, a heightened risk of diseases due to the diminished nutritional and edible value of crops, and adverse impacts on human nutrition and health due to the low concentration of protein and micronutrients (e.g., Zn, Fe, Se, B, I) in crops, aggravating malnutrition, which affects 3.7 billion people, especially children [1,2]. Particularly, Mediterranean soils are under significant pressure due to various environmental (extreme events and climatic changes) and anthropogenic factors (unsustainable agriculture and land use transformation) [3]. Agricultural intensification in these regions is the major contributor to soil degradation, leading to substantial losses in soil organic carbon (SOC) and increased erosion. Intensive farming practices with heavy use of chemical fertilizers (urea, diammonium phosphate and potassium chloride, potassium nitrate, NPK) and pesticides (chlorpyrifos, carbendazim, alphametrin, dichlorvos) are accelerating the mineralization of organic matter, disrupting soil structure and decreasing soil biodiversity [4]. This not only depletes SOC but also reduces the soil’s ability to retain water and nutrients, making it more susceptible to erosion and degradation [5]. Moreover, the Mediterranean climate, with ever-increasingly hot, dry summers and mild, wet winters, is exacerbating these issues [6]. As a result, the soil’s fertility and productivity have declined over time, posing a threat to agriculture. The agricultural sector faces a dual challenge: the need to enhance crop productivity to meet the growing global food demand and the imperative to adopt sustainable practices that mitigate environmental degradation. The by-products of various agricultural and food processing activities represent a promising yet underutilized resource for addressing these challenges [7]. By transforming these wastes into fertilizers, it is potentially possible to reduce dependency on synthetic fertilizers, reduce waste disposal issues, and promote a circular economy. It is essential to adopt practices that enhance SOC levels and improve soil quality. These practices include the use of organic amendments to protect the soil from erosion, enhance biodiversity, and improve overall ecosystem resilience [8]. According to the latest update from the European Commission, Italian olive oil production reached 324,000 tons in the 2023/24 crop year, 80 percent of which is located in southern Italy, where Puglia represents the most important region, with about 370,000 ha, followed by Calabria (about 186,000 ha) and Sicily (about 160,000 ha) [9]. The olive oil extraction industry is a key agro-industrial sector in Mediterranean countries, playing a significant role in their economic and social development. Due to its nutritional benefits and rich bioactive compounds such as flavonoids, polyphenols, anthocyanins, and organic matter, olive oil remains a highly sought-after food product globally, with demand continually rising [10]. The extraction of olive oil can be performed using traditional pressing methods, three-phase centrifugal systems, or two-phase centrifugal systems. All these methods generate substantial amounts of waste and by-products. The extraction of one metric ton of olive oil using three-phase systems results in approximately 0.6 tons of olive solid waste (OSW) and around 1.5 cubic meters of olive wastewater (OWW) [11]. The two-phase process generates a semi-solid waste with higher moisture content [12], reducing the amount of olive wastewater. Olive solid residues were once deemed undesirable due to their environmental impact and the significant costs associated with their management and disposal concentrated in short period of production (October–November). These wastes are in the category of highly polluting and phytotoxic materials according to EU Waste Framework Directive (2008/98/EC) [13], but their richness in valuable compounds, such as organic matter, potassium and water-soluble carbohydrates, can be considered a potential to be exploited with the promotion of the new approach of a circular economy. Numerous studies have indicated that olive wastes, even if they are polluting, mainly related to phenolic compounds with allelopathic and phytotoxic effects, can be used raw or composted due to their high content of organic matter and nutrients to improve soil fertility, closing the residue–resource cycle [14,15,16]. The type and quantity of by-products and the way they are used can diversely affect key soil properties and crop responses [17]. Vignozzi et al. [18] in Mediterranean agro-ecosystems used by-products of the olive oil industry as organic amendments in comparison to organo-mineral fertilizer. Their results evidenced that the composted olive wastes induced the largest increase in soil TOC, TEC, and HC content. Compost and co-composts derived from olive mill wastes (OMWs) have been effectively used as organic fertilizers for horticultural crops [16], olive trees [19], and also as part of the substrate or growing media for ornamental plant culture [20]. Alfano et al. [21] also provided evidence of the disease-suppressive effect of compost from olive mill residues on several soil-borne plant pathogens. López-Piñeiro et al. [22], in a study conducted in Spain on an olive plantation, evidenced significant increases in organic carbon, total N, available P and K, and aggregate stability in olive-compost-amended soils. Leaf analysis of olive trees showed significant increases in N, P, and K concentrations in treated plots after the two first years of olive compost amendments. Also, a general increase in olive production was observed in the treated plots. On the basis of previous mentioned results, this manuscript delves into the potential and obstacles associated with the use of olive waste-based fertilizers. Despite the promising prospects, several obstacles hinder the widespread adoption of olive waste-based fertilizers. These include technical challenges in waste conversion processes, regulatory hurdles, market acceptance, and the need for comprehensive environmental impact assessments.
By overcoming these challenges, we can establish a foundation for more sustainable agricultural methods that align with global environmental objectives. Given the vital role of the olive sector in the Mediterranean region from socio-economic and cultural standpoints, particularly in relation to the healthy Mediterranean Diet, it is crucial to address the issue of olive waste, known for its rich content of phenolic compounds, fatty acids, tannins, and other pollutants that are detrimental to the agri-food production chain. Our research hypothesis posited that through composting processes, it is feasible to transform olive waste into a stabilized, sanitized product suitable for use as organic fertilizers. The primary objective of this study was to utilize olive waste, sourced from various oil extraction methods, to create compost for agricultural purposes. Prior to application, the composts underwent chemical analysis to ensure compliance with the marketability standards outlined in current Italian regulations (Legislative Decree no. 75/2010) and were tested for their impact on soil fertility and quality. Our aims were (1) to promote a more resilient and environmentally friendly agricultural system that maintains soil fertility by substituting chemical fertilizers with organic waste-derived alternatives, (2) to identify and select cost-effective soil quality indicators for highlighting eco-friendly agricultural practices, and (3) to investigate the factors affecting the quality and efficacy of compost obtained from the extraction process, the chemical composition of waste materials, and the proportion of olive waste utilized in composting. Additionally, we aimed to assess the effectiveness of compost as a soil amendment by monitoring changes in soil chemical and biochemical properties 180 days post-application, as well as to evaluate the overall impact of compost on soil and the environment using the life cycle assessment (LCA) methodology.
2. Materials and Methods
2.1. Raw Materials
The raw organic materials used for composting were olive waste collected in November 2022 from the traditional three-phase olive oil extraction process and from the two-phase centrifugation olive mill Decanter Multi Filter process (DMF) as well as straw as a structuring material. OWC1 consisted of 90% waste from olive oil (pulp and kernels of olives) + 10% straw; OWC2 consisted of 90% waste from olive oil + 10% straw; OWC3 consisted of 34% olive oil waste from the two-phase centrifugation olive mill Decanter process + 33% buffalo manure and 33% straw. The percentage of olive wastes to be used were derived from the results of previous composting experiments to evaluate the maximum olive waste percentage that can be composted (data not published).
2.2. Procedure for Compost Production
Three different composts were produced in separate bins of 300 liters. The mixtures consisted of 90% of olive oil waste (pulp and kernels of olives) from the three-phase olive oil extraction process + 10% straw for OWC1, 90% olive oil waste from a two-phase centrifugation olive mill (DMF) + 10% straw for OWC2, and 34% olive oil waste from a two-phase centrifugation olive mill Decanter + 33% buffalo manure and 33% straw for OWC3. The composting procedure was carried out three times, with the following settings: an initial mesophilic phase at 29 °C lasting 8 days, followed by a thermophilic phase at 50 °C for 20 days, and concluding with a mesophilic phase at 27 °C for 92 days. The rise in temperature resulted from vigorous microbial activity and adequate aeration, which supplied enough oxygen to boost biological activity and sustain aerobic conditions [23]. The subsequent steady temperature of 27 °C was due to reduced microbial activity and the diminished availability of organic material for decomposition. Throughout the process, moisture levels were kept at 50%, and oxygen levels exceeded 15%. These parameters were monitored daily with a probe placed in the center of the compost mass. Water was added as needed to maintain the 50% moisture level. To ensure aerobic conditions and facilitate decomposition into stable humus, the mixtures were turned daily, keeping the oxygen level above 15%. Complete decomposition and material stabilization were achieved within four months. The compost was then air-dried, crushed to pass through a 2 mm sieve, and thoroughly homogenized. The chemical analysis of the raw materials used for composting (Table 1) showed bulk density values ranging from 461 to 699 kg/m3. The highest bulk density (699 kg/m3) was observed in raw materials from the DMF oil production system, while the lowest (461 kg/m3) was found in broadleaf residues. The C/N ratio was highest in olive wastes from the DMF oil production system and lowest in broadleaf residues.

Table 1.
Inventory analyses of production of OWC1, OWC2 and OWC3.
2.3. Chemical Analysis of Compost
Chemical characterization of the initial waste materials and resulting composts was conducted following the methodologies outlined in the National Agency for Environmental Protection guidelines (ANPA) [24]. The rate of organic matter mineralization was determined by assessing the reduction in organic matter over time. Organic matter loss was calculated using the following formula:
Organic matter loss = (initial mass of carbon − final mass of carbon/initial mass of carbon) × 100
Fluorescein diacetate hydrolase (FDA) activity was measured using the method described by Adam and Duncan [25]. The absorbance was measured at 490 nm using a Shimadzu UV/Vis 1800 spectrophotometer. The enzyme activity was expressed as mg of fluorescein released per gram of dry compost [26]. Dehydrogenase activity (DHA) was determined as per the method of von Mersi and Schinner [27] and absorbance was measured at 490 nm. Water-soluble phenols (WSPs) were extracted from compost with water (1:10) and quantified using the Folin–Ciocalteu reagent [28], with tannic acid as standard. Compost samples were extracted with bi-distilled water (compost to water ratio of 1:10) at 25 °C for 24 h to determine ion concentrations via ion chromatography (Dionex ICS-1100, Thermo Fisher Scientific, Milan, Italy) [29]. Cation exchange capacity (CEC) was determined using an aqueous BaCl2 solution buffered to pH 7.0 to saturate the soil exchange complexes, as described by Mehlich et al. [30]. The maturity of composts was assessed by calculating the germination index of Cucumis sativus L seeds [31]. The GI (germination index) combines measures of relative seed germination (%) and relative root elongation (%) to estimate compost toxicity because germination and root elongation are considered the most sensitive parameters, capable of detecting low levels of toxicity which affect the root growth, as well as high toxicity levels which affect the germination [32]. Values higher than 60% indicate the non-phytotoxicity of compost [33].
Anions including nitrate (NO3) and cations including ammonium (NH4+) were detected as reported in Muscolo et al. [29] by ion chromatography, using a chromatography system (Dionex ICS-1100). The ammonium N (NH4+-N) was determined according to the 920.03 A.O.A.C. method [34]. Briefly, OFs (organic fractions) were extracted with 1 M potassium chloride (KCl) at a 1:50 mass-to-volume ratio for 2 h; then, an aliquot of filtrate (20 mL) was dispensed into a digestion tube and analyzed in the Kjeldahl automatic instrument after adding 1 g of magnesium oxide (MgO). It is hereby established that nitrate N (NO3−-N) was determined with the same method, after reduction with 0.5 g of Devarda alloy (Carlo Erba, Milan, Italy). Organic nitrogen is determined by subtracting the ammonium and nitrate nitrogen (an optional test) from the total nitrogen. However, since nitrate nitrogen levels are generally very low, total nitrogen minus ammonium nitrogen will give a good estimate of organic nitrogen in most composts. The ON/TN ratio was mathematically calculated (Francisco da Silva et al., 2020) [35].
2.4. Analysis of Soil and Treatments
The experiments were conducted in an open field in Motta San Giovanni, Loc. Liso, Italy (x: 561023,1; y: 4204908,9; WGS 84 UTM Zone 33 N), where the soil is of the sandy loam (11.85% clay, 23.21% silt, and 64.94% sand) textural class according to FAO soil classification system [36]. The soils are slightly alkaline (pH 8.5) with total organic carbon and nitrogen content of 3.0% and 0.18%, respectively. Soils were divided in plots of 1 m square each and fertilized. Each compost was used in a quantity equivalent to 4.3 quintals per hectare. Each treatment was replicated six-fold. Unfertilized plots were used as control. The experiment was arranged in a randomized complete block design, with 3 parcels for each treatment. The experiments lasted six months and the results are the average of three independent experiments. During the experiment, the plots were irrigated to keep 70% of the field capacity for the vitality of soils; soil water content was monitored through a direct read soil pH/moisture meter—R181 (Hanna Instruments, Woonsocket, RI, USA). The soil samples were air-dried and sieved at 2 mm for chemical analysis, while fresh soil samples, also sieved at 2 mm, were utilized for microbiological analysis. The water content of the soil was determined on a gravimetric basis [37]. The corresponding equation related to the increase in the water-holding capacity of the treated soil over time was calculated. Particle size analysis was conducted using Bouyoucos’ method [38]. Dry matter content was determined by weighing samples after drying them for 24 h at 105 °C as described in Muscolo et al. [39]. The pH and electrical conductivity (EC) were measured according to the procedure described by Muscolo et al. [39]. Total organic carbon (TOC) content was determined using the Walkley–Black oxidimetric method [40], while total nitrogen (TN) was detected using the Kjeldahl digestion procedure [41], involving sulfuric acid at 380 °C.
Microbial biomass carbon (MBC) was quantified via the chloroform fumigation–extraction method outlined by Vance et al. [42], using field-moist samples equivalent to 20 g dry weight. Both fumigated and unfumigated soil extracts were analyzed for soluble organic carbon using the Walkley–Black method [40]. MBC was estimated by calculating the difference between the organic carbon extracted from fumigated and unfumigated soils, applying an extraction efficiency coefficient of 0.38 to convert soluble carbon into biomass carbon [42].
Microbial populations were extracted using the method described by Insam and Goberna [43]. Soil samples (2 g) were mixed with 30 glass beads and 20 mL of 0.90% Sodium Chloride (NaCl), then shaken at 4 °C for 1 h at 12,000× g to separate bacteria from solid particles. The supernatant was diluted with sterile one-fourth-strength Ringer solution to standardize the inoculum density. The soil bacterial population was estimated using Waksman’s method [44] with nutrient agar medium at a dilution of 105. The fungal population was estimated using the dilution plate method [45] with Martin’s Rose Bengal agar medium at a dilution of 103. The activities of FDA, DHA, ion concentrations, and CEC were determined as described in Section 2. All analyses were performed in triplicate.
2.5. Environmental Impact Assessment
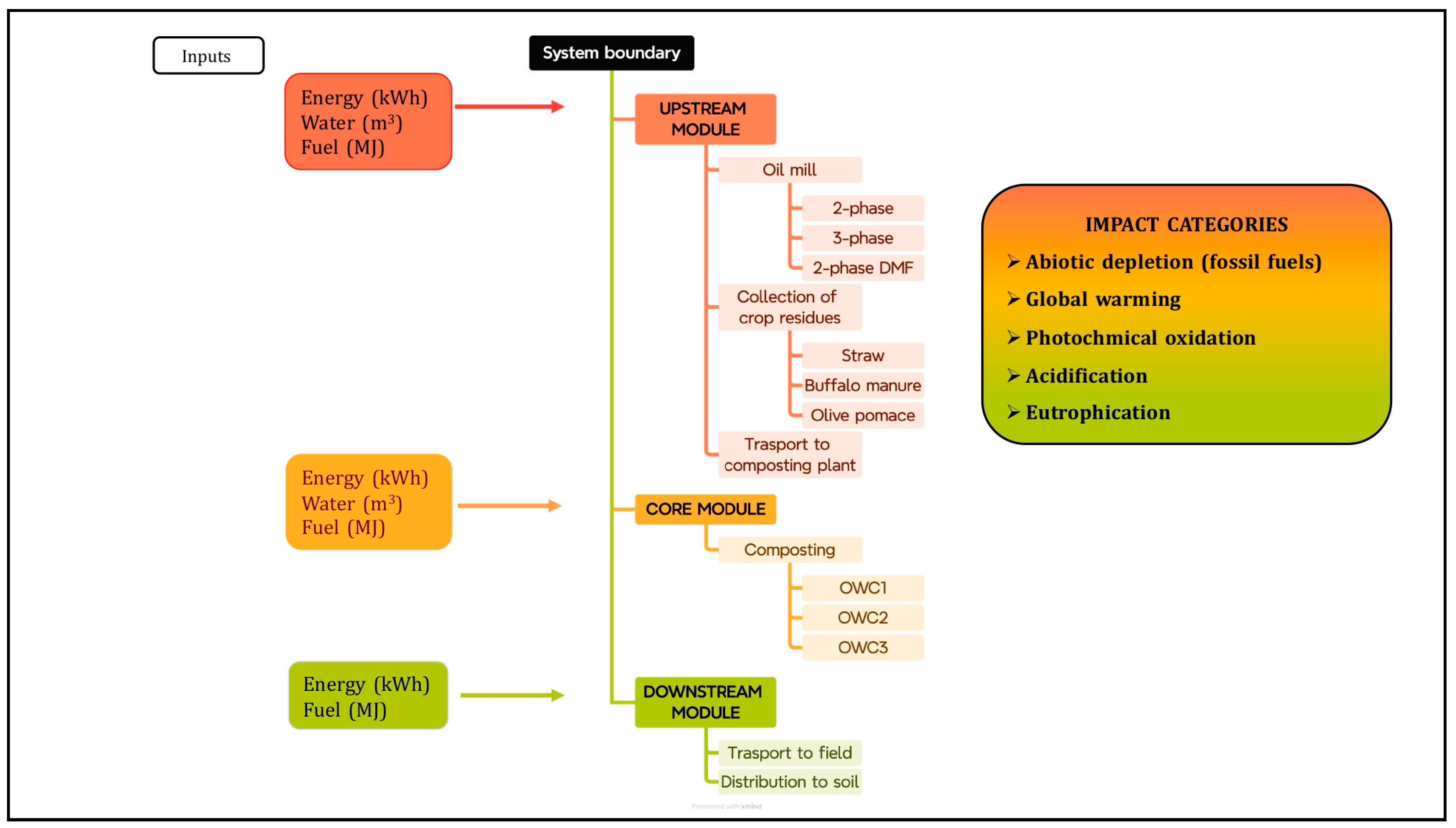
The environmental impact analysis was conducted using the life cycle assessment methodology, in accordance with ISO standards 14040 [46] and 14044 [47]. System boundaries were defined to determine what to include in the environmental impact assessment. The objective of the study was to compare the environmental impacts of three types of compost (OWC1, OWC2 and OWC3). The system boundaries and the input and output flows considered are depicted in Figure 1. The production process was divided into three modules:
The upstream module includes the extraction of raw materials, followed by the crushing stage in which pomace is produced, the collection of other agricultural residues useful for composting (straw, buffalo manure) and transport to the composting plant.
The core module includes the composting phase.
The downstream module includes the distribution of fertilizer to the soil.
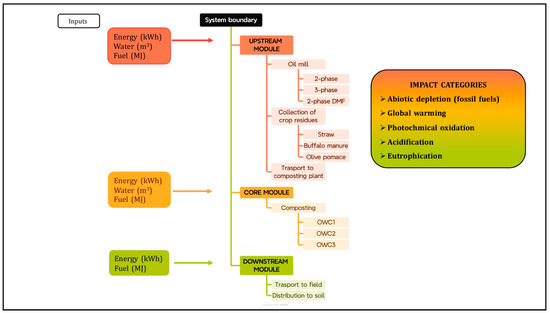
Figure 1.
System boundaries divided by upstream, core, and downstream modules for assessing the environmental impacts shown in the figure of OWC1, OWC2 and OWC3.
Figure 1.
System boundaries divided by upstream, core, and downstream modules for assessing the environmental impacts shown in the figure of OWC1, OWC2 and OWC3.
The inventory data for the study system were gathered through a specifically designed questionnaire to capture information on all inputs utilized in the production process. These data are detailed in Table 1.
Information concerning the upstream and core modules was collected for the various composts from the following locations:
- OWC1: Motta San Giovanni farm, Motta San Giovanni Reggio Calabria, Italy;
- OWC2: Mediterranea Foods Farm, Rizziconi, Reggio Calabria, Italy;
- OWC3: Nuovo Cilento farm in San Mauro Cilento, Salerno (SA), Italy.
Regarding the downstream module, data were collected from Orfei Farm in Motta San Giovanni, Loc. Liso, Italy.
In this study, priority was given to using primary data in terms of input material typologies and amount used. As reported by Pergola et al. [48], secondary data were additionally extrapolated from international databases of scientific importance and reliability like, Ecoinvent 3 [49] and ELCD. In particular, this was carried out for the production of diesel and electricity. For this study, impacts due to the construction of the composting facilities were not considered, only impacts due to the composting process. During the composting process, many types of gaseous compounds such as GHGs [methane (CH4), nitrous oxide (N2O), carbon dioxide (CO2)] and ammonia (NH3) are directly released into the atmosphere. The emissions of CH4, NH3 and N2O were not experimentally measured, but those reported by Pergola et al. [48] were 0.24 kg of CH4, 0.14 kg of NH3 and 0.12 kg of N2O per ton of feedstock. The SimaPro 9.0 software was used for assessing the environmental impacts, specifically applying the CML 2001 EU25 methodology. According to similar studies [50,51,52,53,54], the following impact categories were selected for this research:
- –
- Abiotic Depletion: This category focuses on the impact of mineral and fossil fuel extraction on human health and ecosystems. The abiotic depletion factor, calculated for each extraction, reflects the decreasing availability of these non-renewable resources. This global indicator is based on reserve concentrations and depletion rates.
- –
- Acidification: Acidifying substances negatively affect soil, groundwater, surface water, organisms, ecosystems, and materials. The impact of acidification is measured in sulfur dioxide (SO2) equivalent per kilogram of emissions. This category is relevant on both local and global scales.
- –
- Eutrophication: Eutrophication results from excessive levels of nitrogen dioxide (NO2), and phosphate ions (PO34−) and other nutrients in the environment, leading to increased production of plankton, algae, and aquatic plants. Is measured in PO4 equivalent per kilogram of emission.
- –
- Global Warming Potential: Greenhouse gas emissions contribute to climate change, impacting ecosystem health, human health, and material welfare. The Intergovernmental Panel on Climate Change (IPCC) developed a model to measure the global warming potential (GWP) over a 100-year period, expressed in kilograms of carbon dioxide (CO2) equivalent per emission.
- –
- Photochemical Oxidation: Photochemical ozone formation, a significant air pollution issue, occurs in urban areas with stagnant air and low humidity. This secondary pollutant results from complex photochemical reactions involving sunlight, nitrogen oxides (NOx), and volatile organic compounds (VOCs). Mainly produced by combustion engines and organic solvent use, photochemical ozone is also known as summer smog and is expressed in kilograms of ethylene equivalents.
2.6. Statistical Analysis
Significant differences were analyzed with Tukey multiple tests to compare all pairs of means. Simple descriptive analysis was applied to determine the average value of the quantitative variables. Normality of the data was confirmed using the Shapiro–Wilk test, justifying the use of parametric tests. Statistical analyses were performed using SYSTAT 8.0 software (SPSS Inc, Chicago, Illinois, USA). p values < 0.05 were considered significant as the probability levels. To explore the relationships among the different composts on the soil, we analyzed datasets using principal component analysis with XLStat. PCA is employed to condense large datasets by transforming variables into a smaller set of uncorrelated components, known as principal components. These components capture the maximum variance present in the original data, allowing for a simplified interpretation of complex relationships.
3. Results
3.1. Compost Characteristics
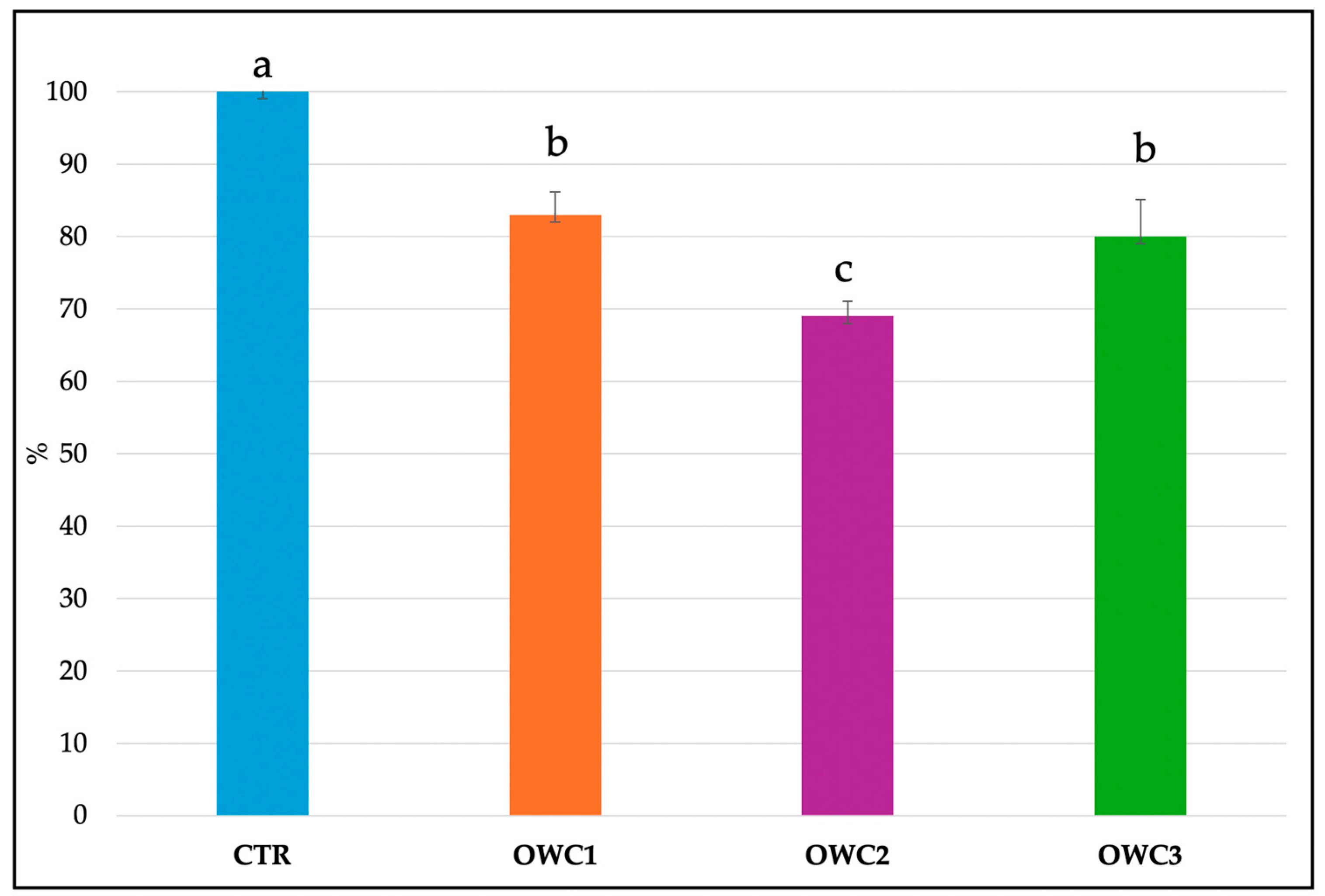
The composting procedure was repeated three times for each type of compost to ensure standardization, and the data confirmed the reproducibility of the process. After 120 days, the analysis revealed significant differences among the three composts produced using the same method (Table 2). OWC2 exhibited the most acidic pH and the highest EC value. OWC3 had the highest pH value (7.8), the highest content of TOC and TN, and the highest C/N WC. The C/N ratio exceeded 20 in all composts. The N-NH4+/N-NO3− ratio varied, being 11 in OWC2, 6.08 in OWC3, and 1.60 in OWC1. The organic nitrogen to total nitrogen (ON/TN) ratio was significantly higher in OWC1 and OWC2 (90% and 85%, respectively) compared to OWC3 (81%) (Table 2). All the composts were nutrient-rich, albeit to varying extents (Table 2). OWC2 had the highest content of nutrients, including the greatest amounts of NH4+ (0.28 mg/L), K+ (20.29 mg/L), Ca2+ (0.56 mg/L), SO42 (1.21 mg/L), and PO43− (2.14 mg/L) (Table 3). OWC2 contained more WSP than OWC1. OWC3 was the compost with the lowest amount of WSP. OWC3 had higher CEC and DHA than OWC2 and OWC1. Conversely OWC1 had the highest FDA activity (Table 4). Phytotoxicity, an indicator of compost maturity, expressed as GI (Figure 2), showed that composts OWC1 and OWC3, tested for seed germination, were not phytotoxic. The GI, detected 6 days after germination, in presence of the composts at 50% showed values higher than 80%, falling in the class of phytonutrient. These data agree with the global germination index showing values ranging from 71 to 85% that confirmed the non-phytotoxicity of the two composts [33].

Table 2.
Physical–chemical properties of composts OWC1, OWC2 and OWC3 120 days after the composting process.

Table 3.
Cation and anion concentration (mg/L) detected in OWC1, OWC2 and OWC3 at the end of the composting process (120 days).

Table 4.
Chemical and biochemical characteristics of composts OWC1, OWC2 and OWC3 120 days after the composting process.
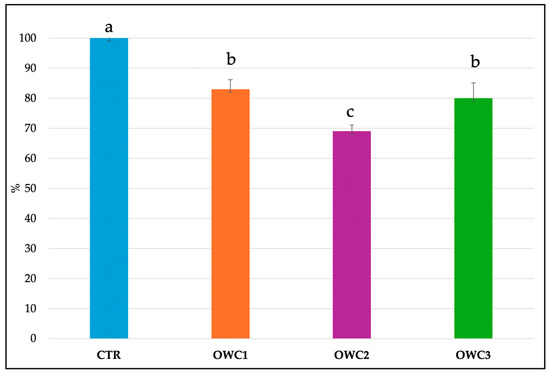
Figure 2.
Global germination index of Cucumis sativus L. (%) in the presence of selected concentrations of the different composts. Data are the mean of 3 replications ± standard deviation. Different letters indicate significant differences (Turkey’s test p < 0.05).
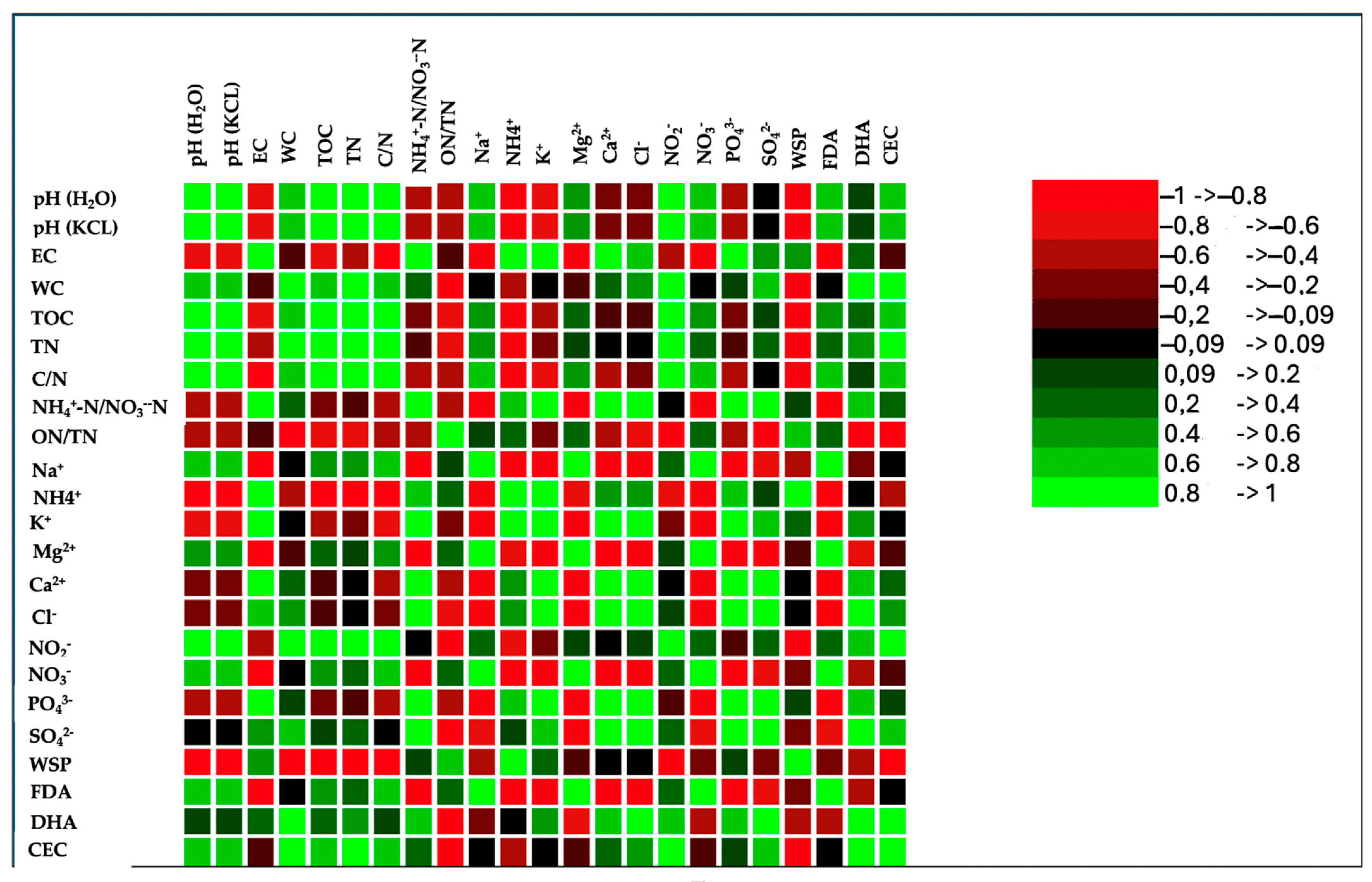
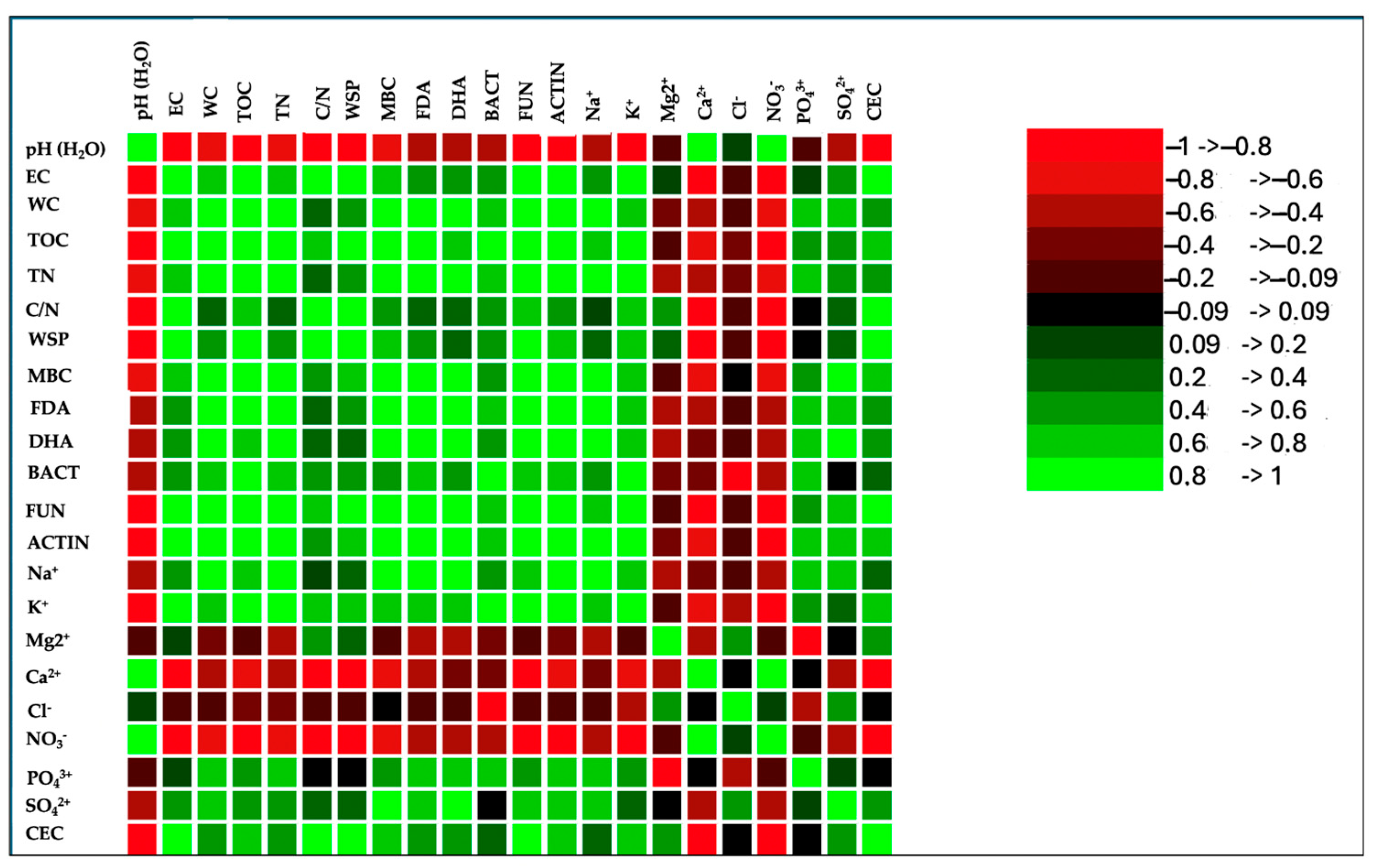
The correlation matrix of the wastes’ physical–chemical properties revealed strong and positive relationships among various parameters (Figure 3). The Pearson correlation coefficient also highlighted relationships among cations and anions and between cations and anions (Figure 3). Phosphate positively correlated with all cations and anions, except for sodium, magnesium, nitrite, and nitrate; the latter two showed an inverse correlation with phosphate. Sulphate correlated positively with calcium and, to a lesser extent, with potassium, and negatively with magnesium. A positive correlation was also evident between phosphate and chloride (Figure 3).
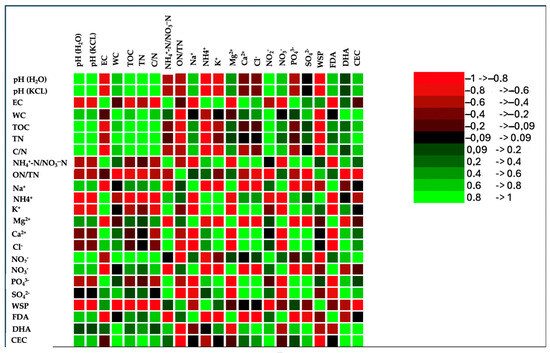
Figure 3.
Correlation matrix (Pearson(n)) of chemical and biological properties and anions and cations at the end of the composting process (120 days). Green color and its shades, in the correlation matrix, indicate a positive correlation, signifying that the variables move in the same direction. On the other hand, the red color and its gradients represent an inverse correlation, suggesting that the variables move in the opposite direction.
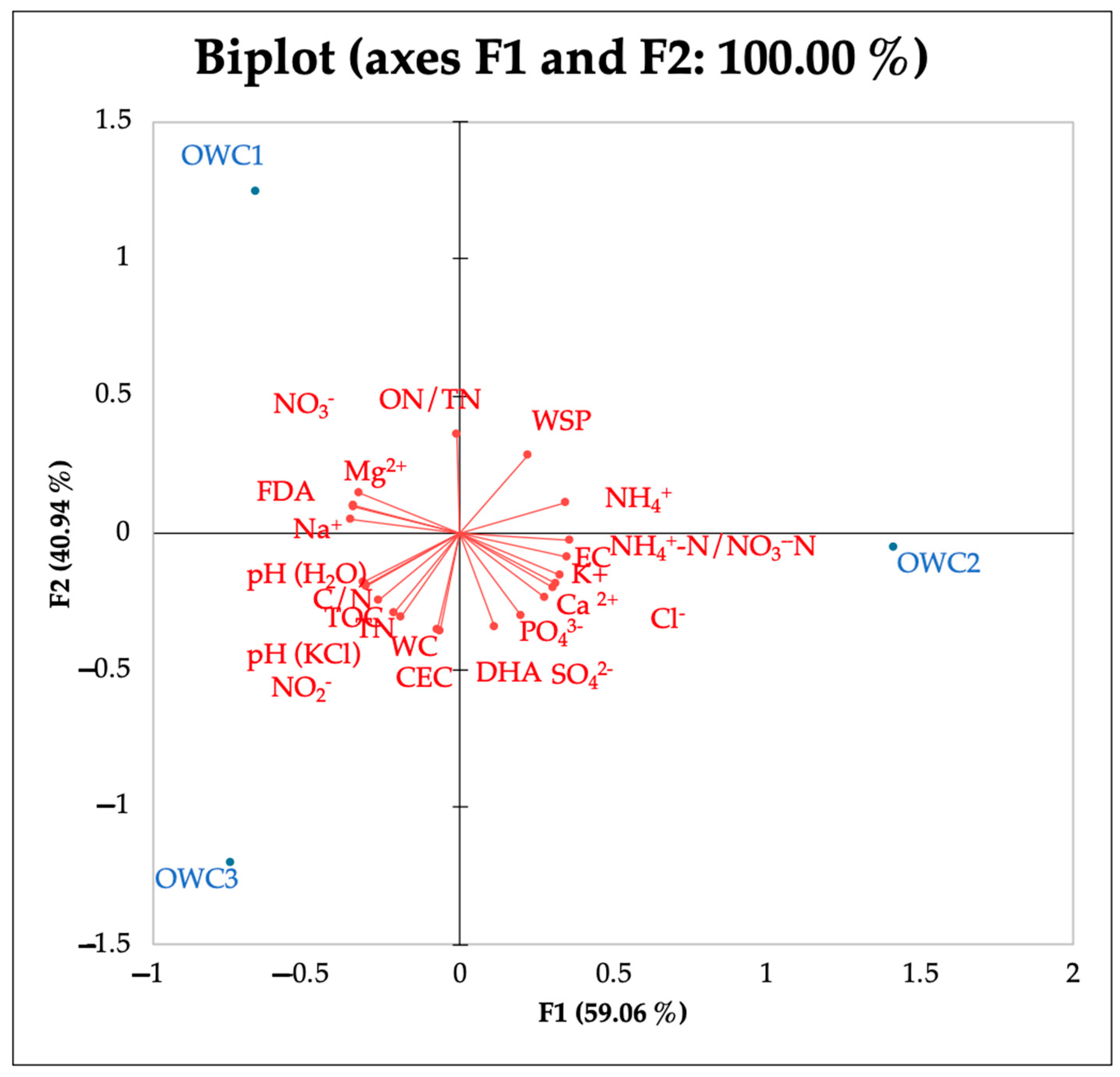
PCA analysis showed that OWC1 is most influenced by variables pointing upwards to the left quadrant in the biplot, such as NO3−, ON/TN and Mg2+. The position of OWC2 along the F1 axis indicates a strong correlation with NH4+, NH4+-N/NO3−-N, Cl−, EC, K+, PO43−, and SO42−. The position of OWC3 on the lower left quadrant indicates a strong correlation with Na+, pH and CEC (Figure 4).
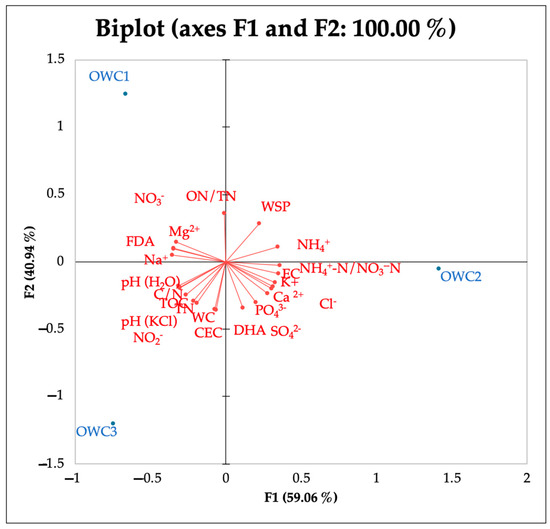
Figure 4.
PCA of chemical and biological properties and anions and cations detected at the end of the composting process (120 days).
3.2. Compost Effects on Soil
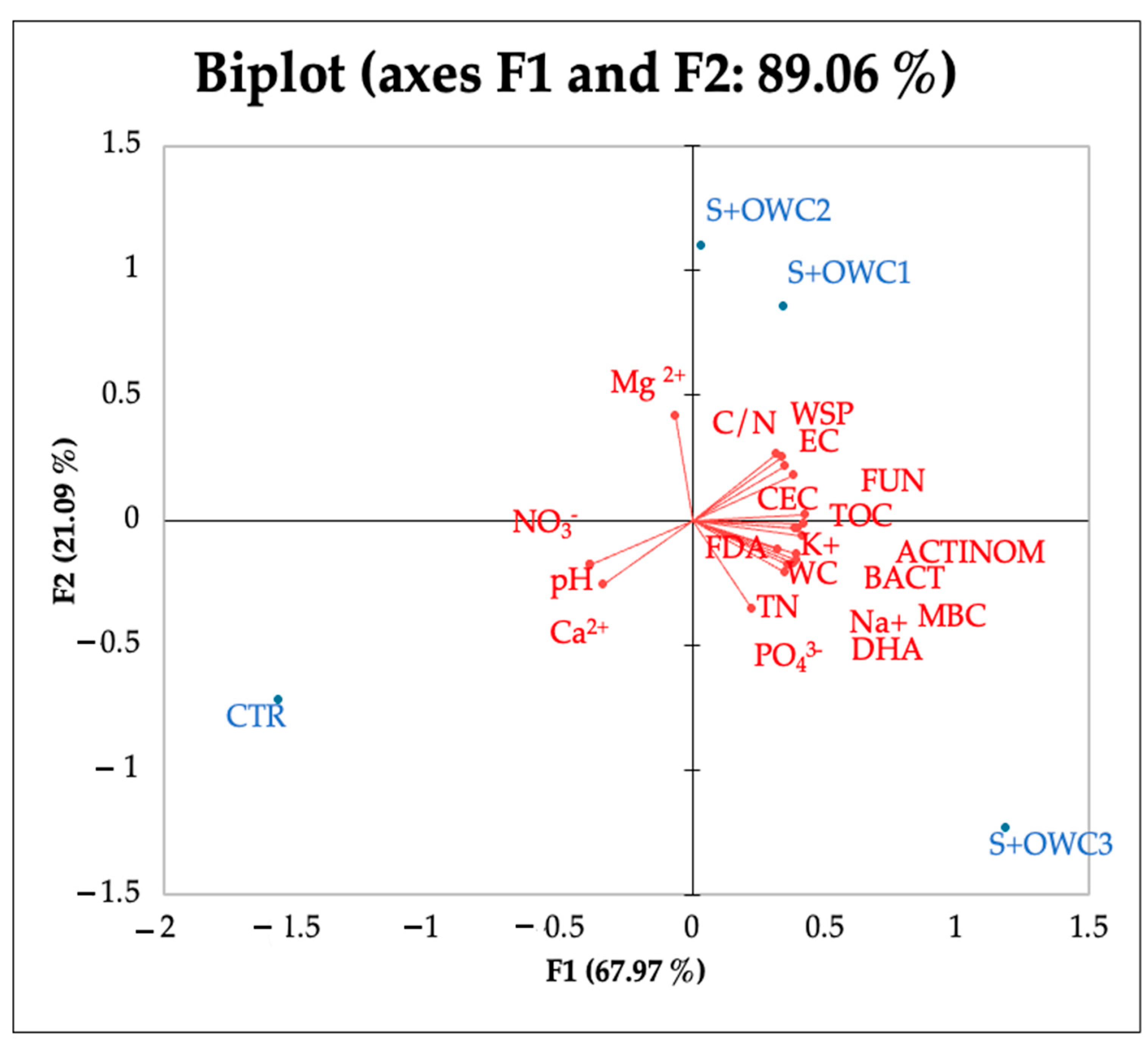
All the composts affected the soil chemical properties, lowering the pH and increasing the EC in comparison to the control. The EC values in soil were, in any case, far from the threshold values of salinization (4 dS m−1) (Table 5). The water-holding capacity significantly increased in treated soils as demonstrated by the values of water content. The greatest value of water content was detected in soil amended with OWC3. TOC and TN significantly increased in amended soils. The C/N ratio was the lowest in control soil, and was around 20 in all the other treatments (Table 5). WSP significantly increased in all the treatments and the greatest increase was observed in soil plus OWC2 (S+OWC2). Soil biological characteristics were affected by compost treatments to different extents. The MBC increased in the presence of the composts, and the greatest increment was observed in the presence of OWC3, followed by OWC1 and OWC2 (Table 6). OWC3 was the compost that increased the number of actinomycete, fungal, and bacterial colonies in respect to the control and the other treatments. FDA and DHA also increased with composts, much more so with OWC3 (Table 6). PCA analysis results (Figure 5) evidenced a good correlation between OWC3 treatment and biological soil properties. A significant increase in MBC and in the colony number of bacteria and actinomycetes, as well as in the activities of FDA and DHA enzymes, was observed, suggesting that from a microbial point of view, compost application is an environmentally friendly and rapid measure for restoring soil functionality. No significant correlations between soil amended with OWC2 and biological soil properties were detected. Soil with OWC1 correlated with fungi, TOC and WSP. Correlation matrix results (Figure 6) evidenced a significant correlation among water content, TOC, TN, C/N, MBC, fungi, bacteria, and actinomycetes, as well as FDA and DHA.

Table 5.
Soil chemical characteristics detected 6 months after the addition of compost to the soils. Soil plus compost 1 (S+OWC1), soil plus compost 2 (S+OWC2), soil plus compost 3 (S+OWC3), and unamended soil (control, CTR).

Table 6.
Biological properties of soil plus compost 1 (S+OWC1), soil plus compost 2 (S+OWC2), soil plus compost 3 (S+OWC3), and unamended soil (control, CTR).
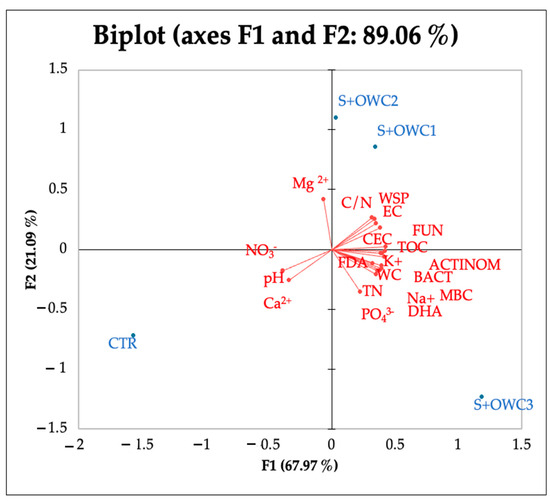
Figure 5.
PCA of chemical and biological properties and anions and cations detected in soil amended with compost 1 (S+OWC1), compost 2 (S+OWC2), compost 3 (S+OWC3), and unamended soil (control, CTR). pH, electric conductivity (EC, dS m−1), water content (WC, %), total organic carbon (TOC, %), total nitrogen (TN, %), carbon/nitrogen ratio (C/N), water-soluble phenols (WSPs, µg TAE g−1 d.s.), microbial biomass C (MBC, μg C g−1 f.s.), fluorescein di-acetate (FDA, µg fluorescein g−1 d.s.), dehydrogenase (DHA, µg TTF g−1 h−1 d.s.), bacteria (BACT, UFC g−1 d.s.), fungi (FUN, UFC g−1 d.s.), and actinomycetes (ACTINOM, UFC g−1 d.s.).
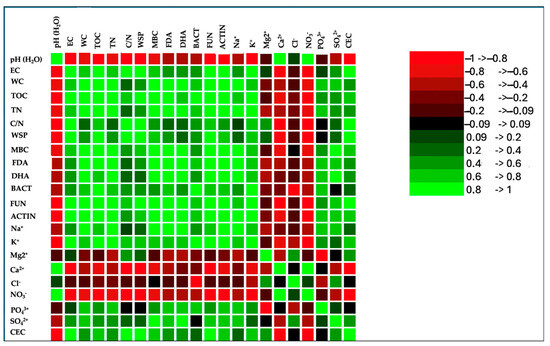
Figure 6.
Correlation matrix of chemical and biological properties of soil plus compost 1 (S+OWC1), compost 2 (S+OWC2), compost 3 (S+OWC3), and unamended soil (control, CTR). Values in bold are different from 0 with a significance level alpha = 0.05. Green color and its shades in the correlation matrix indicate a positive correlation, signifying that the variables move the same direction. On the other hand, the red color and its gradients represent an inverse correlation, suggesting that the variables move in opposite directions.
Cation and anion amounts changed after the addition of the composts. All the composts increased the amount of K+. OWC1 and OWC2 increased the amount of Ca2+ and Mg2+ in soil. PO43− increased in soil treated with OWC3, and SO42− only when OWC1 and OWC3 were used. OWC2 did not change the amount of SO42− in soil respect to the control. CEC was higher than the control in soil treated with all the composts (Table 7). These data are supported by PCA data analysis (Figure 5).

Table 7.
Cations (mg g−1 d.s.), anions (mg g−1 d.s.) and cation exchange capacity (CEC, cmol(+) Kg−1) detected 6 months after the addition of compost to the soils. Soil plus compost 1 (S+OWC1), soil plus compost 2 (S+OWC2), soil plus compost 3 (S+OWC3), and unamended soil (control, CTR).
3.3. Environmental Impact
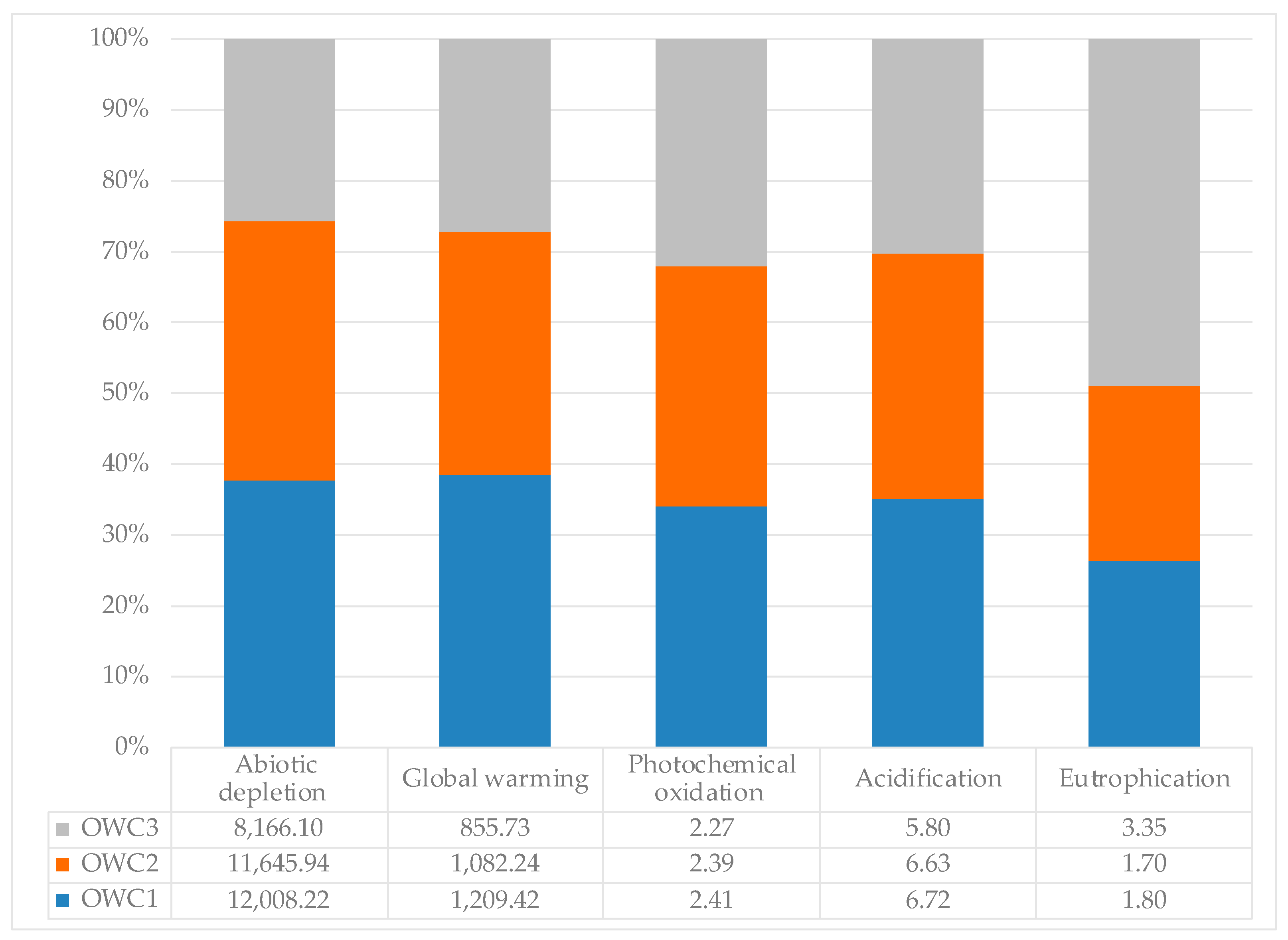
The environmental impact results, based on the functional unit (1 ton of compost), are summarized in Figure 7. OWC1 had a greater impact than the other two composts on all categories (abiotic depletion (fossil fuels), photochemical oxidation, acidification, global warming potential), except for the eutrophication impact category, where the most impactful compost was OWC3.
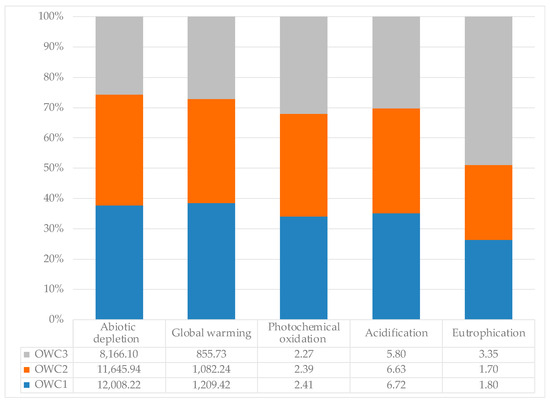
Figure 7.
Environmental impact per ton of OWC1, OWC2 and OWC3 on individual impact categories: eutrophication (kg PO4 eq), acidification (kg SO2 eq), photochemical oxidation (kg C2H4 eq), abiotic depletion (fossil fuels) (MJ), and global warming (kg CO2 eq).
From decomposition to the cradle-to-gate production processes of the three composts under study (Table 8), it was found that for all the impact categories, the most impactful processes were the upstream ones, i.e., the production process step concerning raw material procurement, including the oil mill, the collection of crop residues (olive pomace, straw and manure), and transport to the composting plant. Specifically, for the impact categories, the ranking of composts for the upstream module was abiotic depletion (fossil fuels) (OWC1 > OWC2 > OWC3), global warming (OWC1 > OWC2 > OWC3), photochemical oxidation (OWC1 = OWC2 > OWC3), acidification (OWC1 > OWC2 > OWC3), and eutrophication (OWC3 > OWC1 > OWC2). For the core processes, which refer to the composting process itself, the ranking of composts was abiotic depletion (fossil fuels) (OWC1 > OWC2 > OWC3), global warming (OWC1 = OWC2 > OWC3), photochemical oxidation (OWC1 > OWC2 > OWC3), acidification (OWC1 > OWC2 > OWC3) and eutrophication (OWC1 = OWC2 = OWC3). For the downstream processes, which encompass the transportation and application of the compost, the ranking of composts was abiotic depletion (fossil fuels) (OWC2 > OWC1 > OWC3), global warming (OWC1 = OWC2 > OWC3), photochemical oxidation (OWC1 > OWC2 > OWC3), acidification (OWC1 = OWC2 > OWC3), and eutrophication (OWC1 = OWC2 > OWC3).

Table 8.
Environmental impact per ton of compost OWC1, OWC2 and OWC3 in the entire life cycle assessment.
4. Discussion
Applying compost to agricultural land is widely recognized as an effective method of enhancing the physical properties of most soils, especially those with poor structure and low organic matter content [55]. There is growing interest in using compost to rehabilitate soils and improve their functionality [56]. Documented improvements in compost-amended soils evidenced changes in bulk density, infiltration rate, hydraulic conductivity, water content, aggregate stability, and porosity [57]. These beneficial effects are interactive and are largely due to the compost materials used and the organic matter content in the compost feedstock.
The addition of compost to the soil initiates the decomposition of organic materials by numerous soil organisms [58], collectively known as the soil food web, with a consequent increase in soil biodiversity. These organisms with their diversity are able to recycle the organic materials back into the soil, maintaining its quality and functionality. Decomposition generates a diverse array of carbon-based compounds, including simple sugars that drive biological activity, cellulose-binding agents that enhance soil structure, and nutrient release [59]. There is a cyclical process that begins with the feedstock used to produce compost, which in turn determines its composition and its effectiveness in improving soil health and fertility, and concludes with increases in productivity. Our results confirmed the importance of feedstock composition in producing compost. Even though the feedstocks used came entirely from the olive industry, their composition varied depending on the oil production system. In fact, despite using the same composting processes, the resulting composts differed in their chemical features, underscoring the significance of the chemical composition of raw materials. Considered together, the indicators examined the quality ranking of the obtained composts in terms of their effectiveness on soil, which was in the order OWC3 > OWC2 > OWC1. All the composts decreased soil pH due to their intrinsic pH, and increased EC due to the addition of salts to soil, in particular anions, and due to the stimulation of soil microorganisms that are involved in the turnover of organic matter and the release of single nutrients. Additionally, an increase in soil organic carbon, CEC, and the C/N ratio was also observed with all the composts. These results evidenced the generation of a positive feedback loop for carbon storage by maintaining a high dissolved organic C content, increasing microbial biomass and nutrients. These processes, in turn, can promote the ability of the microbial community to utilize more diverse C sources that are already present in or added to soil. These results were further supported by the increased presence of fungi, actinomycetes, and bacteria, the principal organisms involved in the transformation and decomposition of organic matter and in the mineralization process. PCA results supported these findings, evidencing a strict correlation between OWC3 and biological parameters (MBC, bacteria, actinomycetes, DHA and FDA) (Figure 5). It is well known that microbial changes accompany changes in nutrient status to drive SOM decomposition. Fungi, bacteria and actinomycetes are crucial for organic matter decomposition and nitrogen mineralization in soil, each with distinct decomposition rates and efficiencies, playing varying roles across diverse soil ecosystems [60]. In bacteria-dominated soils, bacteria accelerate organic matter decomposition and nutrient mineralization, thereby enhancing nutrient availability. Conversely, in fungus-dominated soils, fungi slow down the conversion rate of nutrients and energy, promoting organic matter storage and nutrient retention [61,62,63]. Our results showed a greater increase in microbial biomass, bacteria, fungi, actinomycetes, and enzymes in soils amended with OWC3. These results suggest intense biological activity, driving the release of nutrients that are utilizable by plants, and also evidence carbon storage due to the greatest number of fungi as well as a C/N ratio value of 20, which indicate an equilibrium state between mineralization and immobilization processes [63]. In this study, we highlighted that all the composts significantly improved soil stability, water-holding capacity, soil fertility and microbial community in respect to the control, although to different extents, and among the composts, OWC3 was the compost with the best effectiveness in soil. This highlighted the importance of the chemical composition of raw materials more than the setup parameters of composting processes in determining the stability and quality of composts.
Regarding environmental impact assessment, OWC1 and OWC2 had higher values of abiotic depletion (MJ) and global warming (Kg CO2 eq) than OWC3, mainly due to the high consumption of electricity and diesel in the crushing, harvesting, and composting stages. OWC3, despite using more resources such as straw and buffalo manure, had a lower impact due to lower overall energy consumption.
Emissions of VOCs and acid gasses (SO2 and NOx) were higher in OWC1 and OWC2, adversely affecting photochemical oxidation (kg C2H4 eq) and acidification (kg SO2 eq), which are related to higher diesel use. OWC3 had a significantly greater impact on eutrophication (kg PO4 eq) due to the high nutrient content in the buffalo manure used. Reductions in the electricity and diesel consumption at all stages can have multiple benefits on different categories of environmental impact. Optimizing nutrient management and transport logistics is crucial to minimize eutrophication and other harmful emissions.
In summary, improving energy efficiency and resource management during the various stages of the compost life cycle can significantly reduce overall environmental impacts.
Furthermore, when OWC1, OWC2, and OWC3 composts were applied to the soil, they exhibited reduced environmental impacts compared to the impacts reported by other studies for NPK synthetic fertilizers—in which the global warming is 2107 kg CO2 eq per ton, the acidification is 2001 Kg of SO2 eq per ton, and the eutrophication is 2.93 kg of PO4 eq per ton [64]—and compost derived from food wastes [65], in which the global warming is 6190 kg of CO2 eq per ton and acidification is 4820 Kg of SO2 eq per ton. Furthermore, among the composts used, OWC3 showed a lower impact on the soil than the other two, possibly due to its lower phenol content and higher C/N ratio. The lower phenol content may stem from the fact that the compost was prepared using a lower percentage of olive waste, which was replaced by manure. The higher C/N ratio, also a result of the initial material percentages, suggests a higher stable fraction of organic matter, which is reflected in the slow release of nutrients.
The production of compost from recalcitrant wastes of the olive food processing industry can be, in any case, an opportunity for the soil, the environment, and the economy, with a significant improvement in soil fertility and a great reduction in greenhouse gas emissions [66] and waste disposal costs [67,68].
5. Conclusions
In conclusion, this study underscores the substantial advantages of repurposing olive oil extraction wastes into compost for sustainable agricultural practices. This research demonstrates that both the proportion of olive oil waste and the olive oil extraction methods significantly impact the compost quality and its environmental footprint. Incorporating organic waste into soil not only enhances soil’s chemical and biochemical attributes by enriching it with essential nutrients but also bolsters its long-term sustainability. Furthermore, this approach presents an effective strategy for olive waste management, offering notable environmental benefits and positively influencing the olive oil supply chain’s economy. Moving forward, embracing composting techniques could transform waste management within the olive industry, fostering a more robust and environmentally conscious agricultural sector. Agriculture is the biggest market for compost; trials have shown that quality compost can significantly improve the long-term health of the soil. But it can also benefit the farming economy.
Future investigations could delve into novel waste material combinations and refine composting methodologies to amplify soil advantages while mitigating environmental repercussions. Such advancements will not only fortify sector sustainability but also advance a circular economy paradigm, where waste evolves into valuable resources for agricultural cultivation.
Author Contributions
Conceptualization, A.M. (Adele Muscolo) and G.C.; writing—original draft preparation, A.M. (Adele Muscolo) and A.M. (Angela Maffia); writing—review and editing, A.M. (Angela Maffia), M.I.H. and A.M. (Adele Muscolo); supervision, A.M. (Adele Muscolo) and G.C.; project administration, A.M. (Adele Muscolo); software, A.M. (Angela Maffia) and F.M.; formal analysis, C.M. and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry for University and Research (MUR), Project CN_00000022, “National Research Centre for Agricultural Technologies-Agritech” and “Solutions for soil quality assessment and protection” 3.2.1.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Farnia, A.; Hassanpour, K. Comparison between effect of chemical and biological fertilizers on yield and yield components of wheat (Triticum aestivum L.) Pishtaz cultivar. Indian J. Sci. 2015, 5, 7792–7808. [Google Scholar]
- Mariyam, S.; Upadhyay, S.K.; Chakraborty, K.; Verma, K.K.; Duhan, J.S.; Muneer, S.; Meena, M.; Sharma, R.K.; Ghodake, G.; Seth, C.S. Nanotechnology, a frontier in agricultural science, a novel approach in abiotic stress management and convergence with new age medicine-A review. Sci. Total Environ. 2024, 912, 169097. [Google Scholar] [CrossRef]
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Hedlund, K.; Jackson, L.E.; Kätterer, T.; Lugato, E.; Thomsen, I.K.; Jørgensen, H.B.; Söderström, B. What are the effects of agricultural management on soil organic carbon in boreo-temperate systems? Environ. Evid. 2015, 4, 23. [Google Scholar] [CrossRef]
- Bisht, N.; Chauhan, P.S. Excessive and disproportionate use of chemicals cause soil contamination and nutritional stress. In IntechOpen eBooks; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Alessandri, A.; De Felice, M.; Zeng, N.; Mariotti, A.; Pan, Y.; Cherchi, A.; Lee, J.-Y.; Wang, B.; Ha, K.-J.; Ruti, P.; et al. Robust assessment of the expansion and retreat of Mediterranean climate in the 21st century. Sci. Rep. 2014, 4, 7211. [Google Scholar] [CrossRef] [PubMed]
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The Future Challenges of Food and Agriculture: An Integrated analysis of Trends and solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, G.; Rani, N.; Rajput, V.D.; Seth, C.S.; Dwivedi, P.; Minkina, T.; Wong, M.H.; Show, P.L.; Khoo, K.S. Transforming bio-waste into value-added products mediated microbes for enhancing soil health and crop production: Perspective views on circular economy. Environ. Technol. Innov. 2024, 34, 103573. [Google Scholar] [CrossRef]
- Istat, Censimento Agricoltura 2020. Available online: https://esploradati.istat.it/databrowser/#/it/censimentoagricoltura (accessed on 10 April 2024).
- Donner, M.; Radić, I. Innovative circular business models in the olive oil sector for sustainable Mediterranean agrifood systems. Sustainability 2021, 13, 2588. [Google Scholar] [CrossRef]
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive mill wastes: From wastes to resources. Environ. Sci. Pollut. Res. Int. 2024, 31, 20853–20880. [Google Scholar] [CrossRef]
- Markou, G.; Georgakakis, D.; Plagou, K.; Salakou, G.; Christopoulou, N. Balanced waste management of 2-and 3-phase olive oil mills in relation to the seed oil extraction plant. Terr. Aquat. Environ. Toxicol. 2010, 4, 109–112. [Google Scholar]
- Directive, E.C. Directive 2008/98/EC of The European Parliament and of The Council on Waste and Repealing Certain Directives. Off. J. Eur. Union 2008, 312, 3–30. [Google Scholar]
- Brunetti, G.; Plaza, C.; Senesi, N. Olive pomace amendment in Mediterranean conditions: Effect on soil and humic acid properties and wheat (Triticum turgidum L.) yield. J. Agric. Food Chem. 2005, 53, 6730–6736. [Google Scholar] [CrossRef] [PubMed]
- Roig, A.; Cayuela, M.L.; Sànchez-Monedero, M.A. An overview on olive mill wastes and their valorization methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Composting of a solid olive-mill by-product (“alperujo”) and the potential of the resulting compost for cultivating pepper under commercial conditions. Waste Manag. 2006, 26, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Jeske-Kaczanowska, A. Are raw materials or composting conditions and time that most influence the maturity and/or quality of composts? Comparison of obtained composts on soil properties. J. Clean. Prod. 2018, 195, 93–101. [Google Scholar] [CrossRef]
- Vignozzi, N.; Andrenelli, M.C.; Agnelli, A.E.; Fiore, A.; Pellegrini, S. Short-Term Effect of Different Inputs of Organic Amendments from Olive Oil Industry By-Products on Soil Organic Carbon and Physical Properties. Land 2023, 12, 1628. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Bernal, M.P.; Roig, A. Composting olive mill waste and sheep manure for orchard use. Compost. Sci. Util. 2004, 12, 130–136. [Google Scholar] [CrossRef]
- Garcia-Gomez, A. Growth of ornamental plants in two composts prepared from agroindustrial wastes. Bioresour. Technol. 2002, 83, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Alfano, G.; Lustrato, G.; Lima, G.; Vitullo, D.; Ranalli, G. Characterization of composted olive mill wastes to predict potential plant disease suppressiveness. Biol. Control 2011, 58, 199–207. [Google Scholar] [CrossRef]
- López-Piñeiro, A.A.; Albarrán, J.M.; Rato Nunes, C. Barreto, Short and medium-term effects of two-phase olive mill waste application on olive grove production and soil properties under semiarid mediterranean conditions. Bioresour. Technol. 2008, 99, 7982–7987, ISSN 0960-8524. [Google Scholar] [CrossRef]
- Liang, C.; Das, K.C.; McClendon, R.W. The Influence of Temperature and Moisture Contents Regimes on the Aerobic Microbial Activity of a Biosolids Composting Blend. Bioresour. Technol. 2003, 86, 131–137. [Google Scholar] [CrossRef] [PubMed]
- ANPA (National Agency for Environmental Protection). National Agency for Environmental Protection Guidelines. “Methods of Compost Analysis”, Manuals and Guidelines 3/2001; 6334 Manuali 3; SPED S.r.l.: Rome, Italy, 2001; ISBN 88-448-0258-9. [Google Scholar]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity usingfluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Perucci, P. Enzyme activity and microbial biomass in a field soil amended with municipal 460 refuse. Biol. Fertil. Soils 1992, 14, 54–60. [Google Scholar] [CrossRef]
- Von Mersi, W.; Schinner, F. An improved and accurate method for determining the dehydrogenase activity of soils withiodonitrotetrazolium chloride. Biol. Fertil. Soils 1991, 11, 216–220. [Google Scholar] [CrossRef]
- Box, J.D. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Muscolo, A.; Papalia, T.; Settineri, G.; Romeo, F.; Mallamaci, C. Three different methods for turning olive pomace in resource: Benefits of the end products for agricultural purpose. Sci. Total Environ. 2019, 662, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mehlich, A. Rapid Determination of Cation and Anion Exchange Properties and pHe of Soils. J. Assoc. Off. Agric. Chem. 1953, 36, 445–457. [Google Scholar] [CrossRef][Green Version]
- Gariglio, N.; Buyatti, M.; Pilatti, R.A.; Russia, D.E.G.; Acosta, M.R. Use of a germination bioassay to test compost maturity ofwillow (Salix sp.) sawdust. N. Z. J. Crop Hortic. Sci. 2002, 30, 135–139. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y. Elimination of phytotoxicity during co-composting of spent pig-manure sawdust litter and pig sludge. Bioresour. Technol. 1998, 65, 43–49. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; De Bertoldi, M. Evaluating toxicity of immature compost. BioCycle 1981, 22, 54–57. [Google Scholar]
- Association of Official Analytical Chemist. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 1990. [Google Scholar]
- Da Silva, E.F.; Melo, M.F.; Sombra, K.E.S.; Silva, T.S.; De Freitas, D.F.; Da Costa, M.E.; Da Silva Santos, E.P.; Da Silva, L.F.; Serra, A.P.; De Morais Cavalcante Neitzke, P.R. Organic nitrogen in agricultural systems. In IntechOpen eBooks; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- FAO. Methods of Analysis for Soils of Arid and Semi-Arid Regions; Food and Agricultural Organization: Rome, Italy, 2007; p. 57. [Google Scholar]
- Klute, A. Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; Agronomy Monograph 9; ASA-SSSA: Madison, WI, USA, 1986. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Papalia, T.; Attinà, E.; Basile, C.; Panuccio, M.R. (Anaerobic co-digestion of recalcitrant agricultural wastes: Characterizing of biochemical parameters of digestate and its impacts on soil ecosystem. Sci. Total Environ. 2017, 586, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modificationof the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoff in organishen Kopern. Anal. Chem. 1883, 22, 354–358. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Insam, H.; Goberna, M. Section 4 Update: Use of Biolog® for the Community Level Physiological Profiling (CLPP) of Environmental Samples; Springer eBooks: Berlin/Heidelberg, Germany, 2008; pp. 2755–2762. [Google Scholar]
- Waksman, S.A. Soil Microbiology; John Wiley & Sons: New York, NY, USA, 1952. [Google Scholar]
- Johnson, L.F.; Curl, E.A. Methods for the Research on Ecology of Soil-Borne Plant Pathogens; Burgess Publishing Co.: Minneapolis, MN, USA, 1972. [Google Scholar]
- UNI EN ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- UNI EN ISO 14044:2006; Environmental Management, Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- Pergola, M.; Persiani, A.; Pastore, V.; Palese, A.M.; D’Adamo, C.; De Falco, E.; Celano, G. Sustainability Assessment of the Green Compost Production Chain from Agricultural Waste: A Case Study in Southern Italy. Agronomy 2020, 10, 230. [Google Scholar] [CrossRef]
- Ecoinvent Version 3. 2013. Available online: http://www.ecoinvent.org/database/database.html (accessed on 4 April 2024).
- Cadena, E.; Colón, J.; Artola, A.; Sánchez, A.; Font, X. Environmental impact of two aerobic composting technologies using life cycle assessment. Int. J. Life Cycle Assess. 2009, 14, 401–410. [Google Scholar] [CrossRef]
- Banar, M.; Cokaygil, Z.; Ozkan, A. Life cycle assessment of solid waste management options for Eskisehir, Turkey. Waste Manag. 2009, 29, 54–62. [Google Scholar] [CrossRef]
- Blengini, G.A. Using LCA to evaluate impacts and resources conservation potential of composting: A case study of the Asti District in Italy. Resour. Conserv. Recycl. 2008, 52, 1373–1381. [Google Scholar] [CrossRef]
- Emery, A.; Davies, A.; Griffiths, A.; Williams, K. Environmental and economic modelling: A case study of municipal solid waste management in Wales. Resour. Conserv. Recycl. 2007, 49, 244–263. [Google Scholar] [CrossRef]
- Eriksson, O.; Reich, M.; Frostell, B.; Björklund, A.; Assefa, G.; Sundquvist, J.; Granath, J.; Baky, A.; Thyselius, L. Municipal solid waste management from a systems perspective. J. Clean. Prod. 2005, 13, 241–252. [Google Scholar] [CrossRef]
- Matisic, M.; Dugan, I.; Bogunovic, I. Challenges in Sustainable Agriculture—The Role of Organic Amendments. Agriculture 2024, 14, 643. [Google Scholar] [CrossRef]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Kranz, C.N.; McLaughlin, R.A.; Johnson, A.; Miller, G.; Heitman, J.L. The effects of compost incorporation on soil physical properties in urban soils—A concise review. J. Environ Manag. 2020, 261, 110209. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Cheruiyot, N.K.; Bui, X.-T.; Ngo, H.H. Composting and its application in bioremediation of organic contaminants. Bioengineered 2022, 13, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Hoffland, E.; Kuyper, T.W.; Comans, R.N.J.; Creamer, R.E. Eco-functionality of organic matter in soils. Plant Soil 2020, 455, 1–22. [Google Scholar] [CrossRef]
- Maron, P.-A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High microbial diversity promotes soil ecosystem functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal:bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: Insights from meta-analysis and theoretical models. Ecol. Lett. 2013, 16, 887–894. [Google Scholar] [CrossRef]
- El Chami, D.; Santagata, R.; Moretti, S.; Moreschi, L.; Del Borghi, A.; Gallo, M. A Life Cycle Assessment to Evaluate the Environmental Benefits of Applying the Circular Economy Model to the Fertiliser Sector. Sustainability 2023, 15, 15468. [Google Scholar] [CrossRef]
- Saer, A.; Lansing, S.; Davitt, N.H.; Graves, R.E. Life cycle assessment of a food waste composting system: Environmental impact hotspot. J. Clean. Prod. 2012, 52, 234–244. [Google Scholar] [CrossRef]
- Bong, C.P.-C.; Goh, R.K.Y.; Lim, J.-S.; Ho, W.S.; Lee, C.-T.; Hashim, H.; Mansor, N.N.A.; Ho, C.S.; Ramli, A.R.; Takeshi, F. Towards low carbon society in Iskandar Malaysia: Implementation and feasibility of community organic waste composting. J. Environ. Manag. 2017, 203, 679–687. [Google Scholar] [CrossRef]
- Zaman, A.U. A comprehensive study of the environmental and economic benefits of resource recovery from global waste management systems. J. Clean. Prod. 2016, 124, 41–50. [Google Scholar] [CrossRef]
- Pergola, M.; Piccolo, A.; Palese, A.M.; Ingrao, C.; Di Meo, V.; Celano, G. A combined assessment of the energy, economic and environmental issues associated with on-farm manure composting processes: Two case studies in South of Italy. J. Clean. Prod. 2018, 172, 3969–3981. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).