Nutrient Resorption in Young Stands of Three Native Tree Species to Support Restoration of Degraded Tropical Peatland in Indonesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Data Collection
2.4. Data Analyses
3. Results
3.1. Chemical Properties of Soil
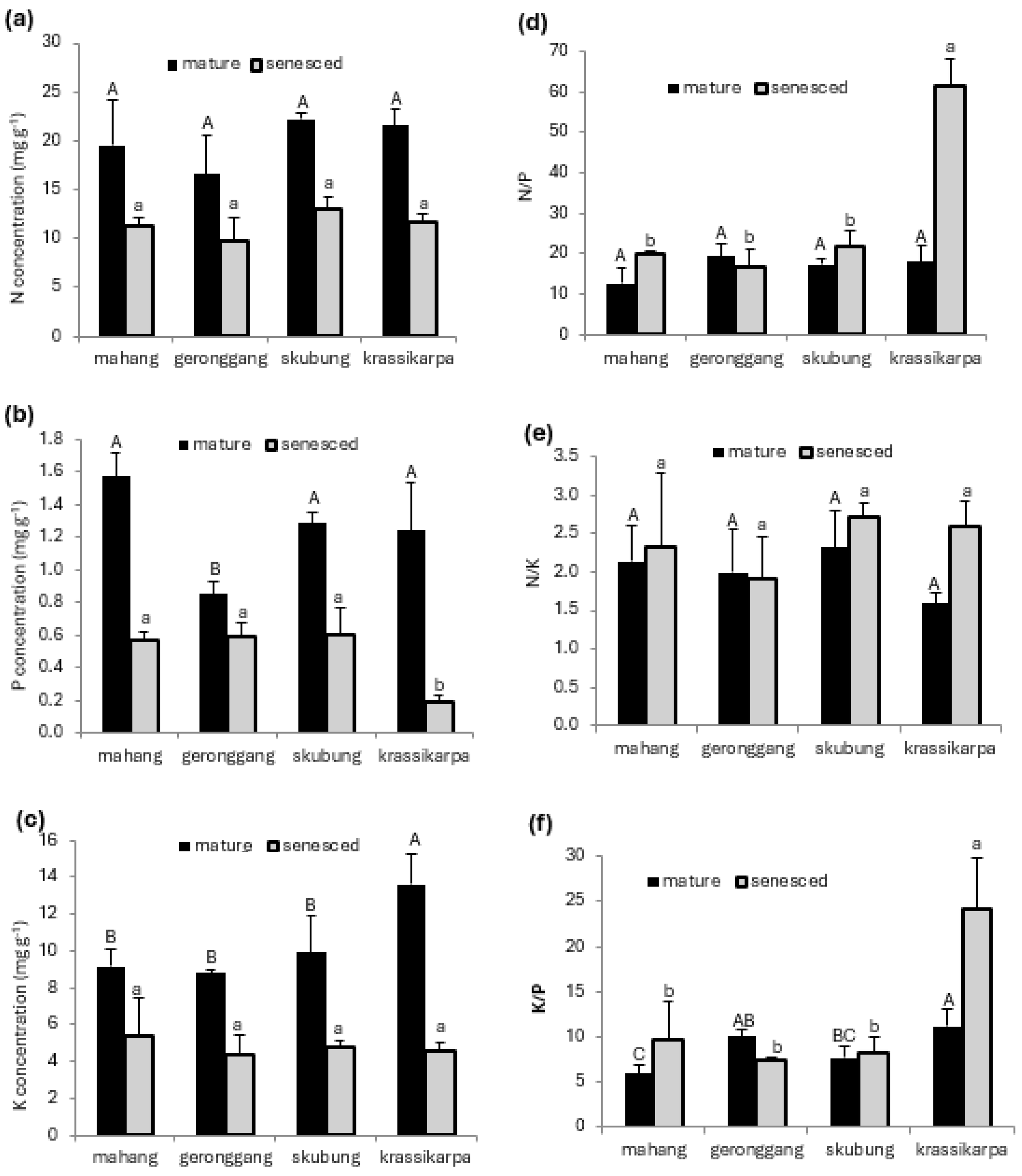
3.2. Foliar Nutrients
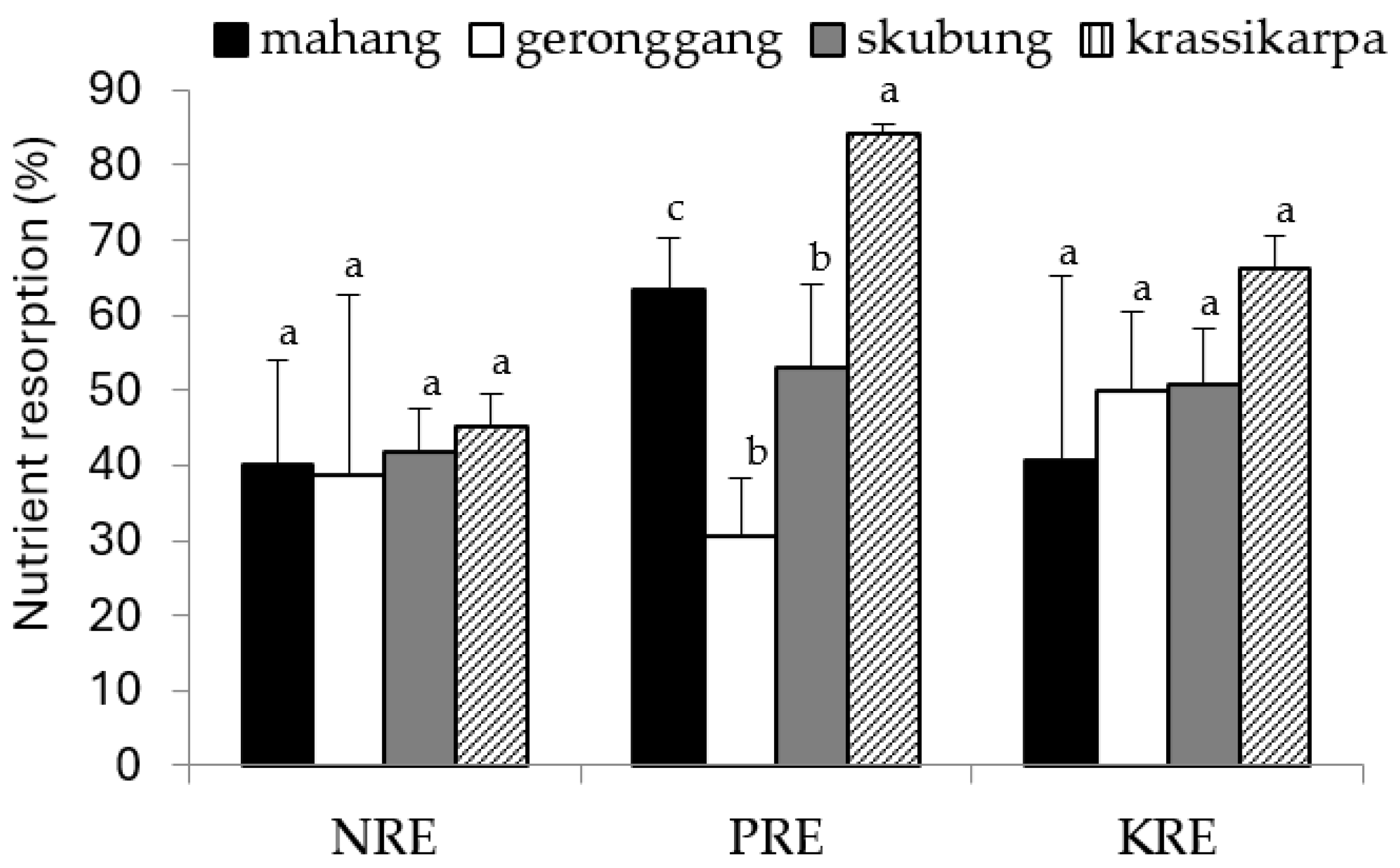
3.3. Nutrient Resorption
3.4. Relationships among Resorption, Soil Nutrients, Foliar Nutrients, and Tree Growth
4. Discussion
4.1. Site Characteristics
4.2. Foliar Nutrients and Their Stoichiometry
4.3. Nutrient Resorption
4.4. Relationships between Nutrient Resorption, Soil Nutrients and Foliar Nutrients
4.5. Nutrient Resorption and Tree Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anda, M.; Ritung, S.; Suryani, E.; Sukarman; Hikmat, M.; Yatno, E.; Mulyani, A.; Subandiono, R.E.; Suratman; Husnain. Revisiting tropical peatlands in Indonesia: Semi-detailed mapping, extent and depth distribution assessment. Geoderma 2021, 402, 115235. [Google Scholar] [CrossRef]
- Miettinen, J.; Liew, S.C. Status of peatland degradation and development in Sumatra and Kalimantan. AMBIO 2010, 39, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Dohong, A. Bolstering Peatlands restoration in Indonesia through 3Rs approach. In Workshop of Developing International Collaborations to Address Fire and Other Conservation Issues in Central Kalimantan, Indonesia; Badan Restorasi Gambut: Jakarta, Indonesia, 2017. [Google Scholar]
- Harwood, C.E.; Nambiar, E.K.S. Sustainable Plantation Forestry in South-East Asia; ACIAR: Canberra, Australia, 2014. [Google Scholar]
- Suhartati; Rahmayanto, Y.; Daeng, Y. The reducing of acacia rotation impact to sustainability of productivity, ecology and social aspect. Info Tek. Eboni 2014, 11, 103–116. [Google Scholar]
- Potensi Pulp and Paper di Indonesia. Available online: https://kemlu.go.id/maputo/id/news/11882/potensi-pulp-and-paper-di-indonesia (accessed on 6 June 2024).
- Subiakto, A.; Rachmat, H.H.; Sakai, C. Choosing native tree species for establishing man-made forest: A new perspective for sustainable forest management in changing world. Biodivers. J. Biol. Divers. 2016, 17, 620–625. [Google Scholar] [CrossRef]
- Giesen, W. Tropical peatland restoration in Indonesia by replanting with useful indigenous peat swamp species: Paludiculture. In Tropical Peatland Eco-Management, 1st ed.; Osaki, M., Tsuji, N., Foead, N., Rielay, J., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2021; pp. 411–441. [Google Scholar]
- Junaedi, A. Growth performance of three native tree species for pulpwood plantation in drained peatland of Pelalawan District, Riau. Indones. J. For. Res. 2018, 5, 119–132. [Google Scholar] [CrossRef]
- Aprianis, Y.; Akbar, O.T.; Rizqiani. Comparison the properties of raw material and kraft pulp from Nature and Plantation of Geronggang Wood (Cratoxylon arborescen). J. Trop. Wood Sci. Technol. 2018, 16, 177–183. [Google Scholar]
- Junaedi, A.; Hardiwinoto, S.; Supriyo, H.; Mindawati, N. Litter productivity and leaf litter nutrient return of three native tree species in drained tropical peatland, Riau-Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 533, 012007. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Houlton, B.Z.; Smith, W.K.; Marklein, A.R.; Reed, S.C.; Parton, W.; Del Grosso, S.J.; Running, S.W. Patterns of new versus recycled primary production in the terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 12733–12737. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Wang, D. Response of multi-ecological component stoichiometry and tree nutrient resorption to medium-term whole-tree harvesting in secondary forests in the Qinling Mountains, China. For. Ecol. Manag. 2021, 498, 119573. [Google Scholar] [CrossRef]
- Zhang, P.; Lü, X.T.; Jin, G.; Liu, Z.; Li, M.H. Leaf nitrogen resorption is more important than litter nitrogen mineralization in mediating the diversity–productivity relationship along a nitrogen-limited temperate forest succession chronosequence. For. Ecosyst. 2023, 10, 100102. [Google Scholar] [CrossRef]
- Du, B.; Ji, H.; Liu, S.; Kang, H.; Yin, S.; Liu, C. Nutrient resorption strategies of three oak tree species in response to interannual climate variability. For. Ecosyst. 2021, 8, 70. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, Q.; Li, K.; Gong, Y.; Liu, Y.; Han, W. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2021, 199, 105100. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, S.B.; Chen, Y.J.; Zhang, Y.P.; Poorter, L. Nutrient resorption is associated with leaf vein density and growth performance of dipterocarp tree species. J. Ecol. 2015, 103, 541–549. [Google Scholar] [CrossRef]
- Husnain, H.; Sipahutar, I.A.; Purnomo, J.; Widyanto, H.; Nurhayati, N. CO2 Emissions from Tropical Peat Soil Affected by Fertilization. J. Trop. Soils 2017, 22, 1–9. [Google Scholar] [CrossRef]
- Lu, J.; Liu, M.; Yang, M.; Xie, J.; Yang, H.; Li, L. Leaf resorption and stoichiometry of N and P of 1, 2 and 3 year-old lucerne under one-time P fertilization. Soil Tillage Res. 2020, 197, 104481. [Google Scholar] [CrossRef]
- Junaedi, A.; Mindawati, N.; Pribadi, A.; Hardiwinoto, S. Leaf litter decomposition and nutrient release of three native tree species in a drained tropical peatland in Riau, Indonesia. HAYATI J. Biosci. 2022, 29, 182–191. [Google Scholar] [CrossRef]
- Pan, X.; Ping, Y.; Cui, L.; Zhang, X.; Li, W.; Hu, Y.; Cornelissen, J.H.C. Nutrient resorption from leaves of wetland plants in a constructed wetland depends on green leaf nutrient content and life form. Wetlands 2020, 40, 983–991. [Google Scholar] [CrossRef]
- Eviati, S. Analisis Kimia Tanah, Tanaman, Air, dan Pupuk, Petunjuk Teknis, 2nd ed.; Balai Penelitian Tanah: Bogor, Indonesia, 2009. [Google Scholar]
- Gao, S.; Song, Y.; Song, C.; Wang, X.; Gong, C.; Ma, X.; Gao, J.; Cheng, X.; Du, Y. Long-term nitrogen addition alters peatland plant community structure and nutrient resorption efficiency. Sci. Total Environ. 2020, 844, 157176. [Google Scholar] [CrossRef] [PubMed]
- Kučerová, A.; Adamec, L. Foliar resorption efficiency does not change along an elevational gradient in two dominant peatbog plant species. Folia Geobot. 2022, 57, 247–257. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2024, 164, 243–266. [Google Scholar] [CrossRef]
- Tsujii, Y.; Fan, B.; Atwell, B.J.; Lambers, H.; Lei, Z.; Wright, I.J. A survey of leaf phosphorus fractions and leaf economic traits among 12 co-occurring woody species on phosphorus-impoverished soils. Plant Soil 2023, 489, 107–124. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.; Ma, S.; Ding, Y.; Luo, Y.; Chen, Y.; Du, E.; Han, W.; Kovacs, E.D.; Shen, H.; et al. Family-level leaf nitrogen and phosphorus stoichiometry of global terrestrial plants. Sci. China Life Sci. 2019, 62, 1047–1057. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Mohamed, N.; Mohan, S.; Ameen, M.; Ibrahim, S.; Adam, I.; Yahayu, M.; Zeenelabdin, L.; Elhag, O. α-Mangostin from Cratoxylum arborescens: An in vitro and in vivo toxicological evaluation. Arab. J. Chem. 2015, 8, 129–137. [Google Scholar] [CrossRef]
- Triadiati; Tjitrosemito, S.; Guhardja, E.; Sudarsono; Qayim, I.; Leuschner, C. Nitrogen resorption and nitrogen use efficiency in cacao agroforestry systems managed differently in Central Sulawesi. HAYATI J. Biosci. 2007, 14, 127–132. [Google Scholar] [CrossRef]
- Erwiyono, R.; Prawoto, A.A.; Murdiyati, A. Efisiensi resorpsi hara pada tanaman kakao di dataran rendah pada tanah Aluvial. Pelita Perkeb. 2012, 28, 32–44. [Google Scholar]
- Susanto, D.; Hayatudin; Setiawan, A.; Purnomo, H.; Ruhiyat, D.; Amirta, R. Characterizing nutrient status and growth of Macaranga gigantea in tropical rainforest gaps after selective logging in East Kalimantan, Indonesia. Biodiversitas 2017, 18, 996–1003. [Google Scholar] [CrossRef]
- Machado, M.R.; Sampaio, P.D.T.B.; Ferraz, J.; Camara, R.; Pereira, M.G. Retranslocação de nutrientes em espécies florestais na Amazônia Brasileira. Acta Sci.-Agron. 2016, 38, 93–101. [Google Scholar] [CrossRef]
- van der Colff, D.; Dreyer, L.L.; Valentine, A.; Roets, F. Comparison of nutrient cycling abilities between the invasive Acacia mearnsii and the native Virgilia divaricata trees growing sympatrically in forest margins in South Africa. S. Afr. J. Bot. 2017, 111, 358–364. [Google Scholar] [CrossRef]
- O’Brien, P.L.; Thomas, A.L.; Sauer, T.J.; Brauer, D.K. Foliar nutrient concentrations of three economically important tree species in an alley-cropping system. J. Plant Nutr. 2020, 43, 2557–2568. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, H.; He, B.; Chen, H.; Zhou, Z. Nitrogen and phosphorus stoichiometry of Schima superba under nitrogen deposition. Sci. Rep. 2018, 8, 13669. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Ferreira, N.R.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- You, C.; Wu, F.; Yang, W.; Xu, Z.; Tan, B.; Zhang, L.; Yue, K.; Ni, X.; Li, H.; Chang, C.; et al. Does foliar nutrient resorption regulate the coupled relationship between nitrogen and phosphorus in plant leaves in response to nitrogen deposition? Sci. Total Environ. 2018, 645, 733–742. [Google Scholar] [CrossRef]
- Hu, R.; Liu, T.; Zhang, Y.; Zheng, R.; Guo, J. Leaf nutrient resorption of two life-form tree species in urban gardens and their response to soil nutrient availability. PeerJ 2023, 11, e15738. [Google Scholar] [CrossRef]
- Urbina, I.; Grau, O.; Sardans, J.; Margalef, O.; Peguero, G.; Asensio, D.; LLusià, J.; Ogaya, R.; Gargallo-Garriga, A.; Van Langenhove, L.; et al. High foliar K and P resorption efficiencies in old-growth tropical forests growing on nutrient-poor soils. Ecol. Evol. 2021, 11, 8969–8982. [Google Scholar] [CrossRef]
- Violita. Dinamika Nitrogen pada Sistem Transformasi Hutan Alam Menjadi Perkebunan Kelapa Sawit di Sumatera, Indonesia. Ph.D. Thesis, Institut Pertanian Bogor, Bogor, Indonesia, 2015. [Google Scholar]
- Yuan, Z.; Shi, X.; Jiao, F.; Han, F. N and P resorption as functions of the needle age class in two conifer trees. J. Plant Ecol. 2018, 11, 780–788. [Google Scholar] [CrossRef]
- Jiang, D.; Geng, Q.; Li, Q.; Luo, Y.; Vogel, J.; Shi, Z.; Ruan, H.; Xu, X. Nitrogen and phosphorus resorption in planted forests worldwide. Forests 2019, 10, 201. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Chen, B.; Ming, A.; Pang, L. Nutrient resorption and C:N:P stoichiometry responses of a Pinus massoniana plantation to various thinning intensities in Southern China. Forests 2020, 13, 1699. [Google Scholar] [CrossRef]
- Tully, K.L.; Wood, T.E.; Schwantes, A.M.; Lawrence, D. Soil nutrient availability and reproductive effort drive patterns in nutrient resorption in Pentaclethra macroloba. Ecology 2013, 94, 930–940. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.; Liu, Y. Nursery fertilization affected field performance and nutrient resorption of Populus tomentosa Carr. ploidy levels. iForest 2022, 15, 16–23. [Google Scholar] [CrossRef]
- Gerdol, R.; Iacumin, P.; Brancaleoni, L. Differential effects of soil chemistry on the foliar resorption of nitrogen and phosphorus across altitudinal gradients. Funct. Ecol. 2019, 33, 1351–1361. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Gonzales, K.E.; Yanai, R.D.; Fahey, T.J.; Fisk, M.C. Evidence for P limitation in eight northern hardwood stands: Foliar concentrations and resorption by three tree species in a factorial N by P addition experiment. For. Ecol. Manag. 2023, 529, 120696. [Google Scholar] [CrossRef]
- Rejmánková, E. Nutrient resorption in wetland macrophytes: Comparison across several regions of different nutrient status. New Phytol. 2005, 167, 471–482. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Liesche, J. How regulation of phloem transport could link potassium fertilization to increased growth. Tree Physiol. 2015, 36, 1–5. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, F.; Yang, Y.; Lu, G.; Yang, X. Arbuscular mycorrhizal fungi alter plant N and P resorption of dominant species in a degraded grassland of northern China. Ecol. Indic. 2023, 150, 110195. [Google Scholar] [CrossRef]
- Hofmann, K.; Heuck, C.; Spohn, M. Phosphorus resorption by young beech trees and soil phosphatase activity as dependent on phosphorus availability. Oecologia 2016, 181, 369–379. [Google Scholar] [CrossRef]
- Ji, H.; Wen, J.; Du, B.; Sun, N.; Berg, B.; Liu, C. Comparison of the nutrient resorption stoichiometry of Quercus variabilis Blume growing in two sites contrasting in soil phosphorus content. Ann. For. Sci. 2018, 75, 59. [Google Scholar] [CrossRef]
- Munford, K.E.; Casamatta, M.; Basiliko, N.; Glasauer, S.; Mykytczuk, N.C.S.; Watmough, S.A. Paper Birch (Betula papyrifera) Nutrient Resorption Rates on Nutrient-Poor Metal-Contaminated Soils and Mine Tailings. Water Air Soil Pollut. 2021, 232, 33. [Google Scholar] [CrossRef]
- Tawaraya, K.; Takaya, Y.; Turjaman, M.; Tuah, S.J.; Limin, S.H.; Tamai, Y.; Cha, J.Y.; Wagatsuma, T.; Osaki, M. Arbuscular mycorrhizal colonization of tree species grown in peat swamp forests of Central Kalimantan, Indonesia. For. Ecol. Manag. 2003, 182, 381–386. [Google Scholar] [CrossRef]
- Brant, A.N.; Chen, H.Y.H. Patterns and Mechanisms of nutrient resorption in llants. Crit. Rev. Plant Sci. 2015, 34, 471–486. [Google Scholar] [CrossRef]
- Veneklaas, E.J. Phosphorus resorption and tissue longevity of roots and leaves—Importance for phosphorus use efficiency and ecosystem phosphorus cycles. Plant Soil 2022, 476, 627–637. [Google Scholar] [CrossRef]
- Deng, J.; Wang, S.; Ren, C.; Zhang, W.; Zhao, F.; Li, X.; Zhang, D.; Han, X.; Yang, G. Nitrogen and phosphorus resorption in relation to nutrition limitation along the chronosequence of black locust (Robinia pseudoacacia L.) plantation. Forests 2019, 10, 261. [Google Scholar] [CrossRef]
- Guo, Q.; Li, H.; Sun, X.; An, Z.; Ding, G. Patterns of Needle Nutrient Resorption and Ecological Stoichiometry Homeostasis along a Chronosequence of Pinus massoniana Plantations. Forests 2023, 14, 607. [Google Scholar] [CrossRef]
| Species | Height (cm) | Root Collar Diameter (cm) | Diameter at Breast Height (cm) | |||
|---|---|---|---|---|---|---|
| 3.5 Years | 4.5 Years | 3.5 Years | 4.5 Years | 3.5 Years | 4.5 Years | |
| Mahang | 8.9 ± 0.6 | 11.5 ± 0.5 | 11.6 ± 0.7 | 13.5 ± 1.2 | 9.9 ± 0.5 | 12.1 ± 1.3 |
| Geronggang | 7.7 ± 0.2 | 10.0 ± 0.5 | 10.2 ± 0.3 | 11.7 ± 0.3 | 8.4 ± 0.4 | 10.2 ± 0.3 |
| Skubung | 5.9 ± 1.4 | 7.5 ± 1.2 | 9.9 ± 1.5 | 12.0 ± 1.0 | 7.5 ± 1.0 | 9.5 ± 0.4 |
| Krassikarpa | 18.0 ± 1.5 | 18.9 ± 1.0 | 21.9 ± 2.5 | 27.1 ± 2.6 | 18.3 ± 1.4 | 23.0 ± 2.3 |
| Variables | Mahang | Geronggang | Skubung | Krassikarpa |
|---|---|---|---|---|
| pH H2O ns | 3.2 ± 0.00 (extremely acid) | 3.2 ± 0.00 (extremely acid) | 3.1 ± 0.06 (extremely acid) | 3.2 ± 0.06 (extremely acid) |
| C (%) ns | 43.1 ± 2.02 (very high) | 43.6 ± 1.27 (very high) | 42.6 ± 0.72 (very high) | 42.4 ± 1.93 (very high) |
| Total N (%) ns | 1.3 ± 0.19 (very high) | 1.3 ± 0.03 (very high) | 1.4 ± 0.08 (very high) | 1.4 ± 0.16 (very high) |
| C/N ns | 34.9 ± 6.79 (very high) | 34.0 ± 1.29 (very high) | 30.3 ± 1.32 (very high) | 31.2 ± 4.85 (very high) |
| Available P (ppm) | 106.2 ± 17.03 b (very high) | 89. 7 ± 13.5 b (very high) | 65.8 ± 15.68 a (very high) | 91.4 ± 25.61 b (very high) |
| Available Ca (me/100 g) ns | 10.2 ± 4.8 (high) | 12.8 ± 4.06 (high) | 9.1 ± 2.70 (moderate) | 10.1 ± 5.43 (high) |
| Available Mg (me/100 g) | 2.0 ± 0.12 b (moderate) | 2.7 ± 0.62 a (high) | 1.6 ± 0.30 b (moderate) | 2.8 ± 0.71 a (high) |
| Available K (me/100 g) ns | 0.48 ± 0.19 (moderate) | 0.46 ± 0.143 (moderate) | 0.29 ± 0.07 (low) | 0.38 ± 0.051 (moderate) |
| Available Na (me/100 g) ns | 0.003 ± 0.006 (very low) | 0.02 ± 0.02 (very low) | 0.01 ± 0.005 (very low) | 0.02 ± 0.006 (very low) |
| CEC (me/100 g) ns | 143.3 ± 5.03 (extremely high) | 142. 7 ± 11.67 (extremely high) | 135.7 ± 6.45 (extremely high) | 153 ± 14.18 (extremely high) |
| Base saturation (%) ns | 8.9 ± 3.69 (extremely low) | 11.4 ± 3.56 (extremely low) | 8.1 ± 2.02 (extremely low) | 9.0 ± 4.96 (extremely low) |
| Available Fe (ppm) ns | 39.5 ± 2.00 (extremely high) | 39.9 ± 2.00 (extremely high) | 38.7 ± 2.75 (extremely high) | 40.3 ± 2.16 (extremely high) |
| NRE | PRE | KRE | |
|---|---|---|---|
| N Soil | −0.024 | 0.185 | −0.109 |
| P Soil | −0.002 | 0.101 | −0.399 |
| K soil | 0.281 | −0.372 | −0.163 |
| N mature leaves | 0.70 *** | 0.34 | 0.46 |
| P mature leaves | −0.02 | 0.54 | −0.32 |
| Ratio of N mature leaves/Pmature laeaves | 0.38 | −0.3 | 0.44 |
| K mature leaves | 0.07 | 0.74 *** | 0.63 *** |
| N senesced leaves | −0.4 | 0.22 | 0.13 |
| P senesced leaves | −0.13 | −0.81 *** | −0.55 |
| K senesced leaves | −0.48 | 0.07 | −0.56 |
| dBHh3.5 | dBH4.5 | h3.5 | h4.5 | |
|---|---|---|---|---|
| Nm | 0.07 | −0.16 | 0.13 | −0.03 |
| Pm | 0.08 | 0.07 | 0.009 | 0.045 |
| Km | 0.72 *** | 0.73 *** | 0.41 | 0.74 *** |
| Nm/Pm | −0.02 | −0.43 | 0.10 | −0.01 |
| h3.5 | dBH3.5 | h4.5 | dBH4.5 | |
|---|---|---|---|---|
| NRE | 0.32 | 0.34 | 0.14 | 0.10 |
| PRE | 0.73 *** | 0.78 *** | 0.73 *** | 0.77 *** |
| KRE | 0.52 | 0.47 | 0.46 | 0.49 |
| NRP | 0.001 | 0.04 | −0.05 | 0.05 |
| PRP | 0.89 *** | 0.89 *** | 0.90 *** | 0.89 *** |
| KRP | −0.13 | −0.11 | −0.08 | −0.12 |
| NRP/PRP | 0.92 *** | 0.93 *** | 0.90 *** | 0.93 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junaedi, A.; Pribadi, A.; Mindawati, N.; Dharmawan, I.W.S.; Octavia, D.; Kurniawan, H.; Fauzi, R.; Siahaan, H.; Premono, B.T.; Nugroho, A.W.; et al. Nutrient Resorption in Young Stands of Three Native Tree Species to Support Restoration of Degraded Tropical Peatland in Indonesia. Land 2024, 13, 1169. https://doi.org/10.3390/land13081169
Junaedi A, Pribadi A, Mindawati N, Dharmawan IWS, Octavia D, Kurniawan H, Fauzi R, Siahaan H, Premono BT, Nugroho AW, et al. Nutrient Resorption in Young Stands of Three Native Tree Species to Support Restoration of Degraded Tropical Peatland in Indonesia. Land. 2024; 13(8):1169. https://doi.org/10.3390/land13081169
Chicago/Turabian StyleJunaedi, Ahmad, Avry Pribadi, Nina Mindawati, I Wayan Susi Dharmawan, Dona Octavia, Hery Kurniawan, Ridwan Fauzi, Hengki Siahaan, Bambang Tejo Premono, Ardiyanto Wahyu Nugroho, and et al. 2024. "Nutrient Resorption in Young Stands of Three Native Tree Species to Support Restoration of Degraded Tropical Peatland in Indonesia" Land 13, no. 8: 1169. https://doi.org/10.3390/land13081169






