Abstract
The Mediterranean region is one of the 36 hotspots of the world that will be most affected by climate change, with river ecosystems being among the most sensitive to these effects. Therefore, it is necessary to understand and monitor the effects that are occurring through the use of aquatic macroinvertebrates as bioindicators of climate change. To study the use of macroinvertebrates as bioindicators, a systematic literature review was conducted using the PRISMA method. The obtained bibliography was analyzed alongside other known studies to determine the response of these organisms to temperature increases and decreases and alterations in precipitation, as well as their reaction to extreme drought and flood events. The results show that different taxa of macroinvertebrates respond differently to the effects of climate change, always leading to a community alteration with changes in the abundance, richness, phenology, and composition. Therefore, aquatic macroinvertebrates are good bioindicators of the changes caused by climate change, as they respond clearly to the alterations induced by climate change.
1. Introduction
Climate change is one of the greatest challenges that the environment must face. Depending on the study region, the effects of climate change will be more or less pronounced and will produce different consequences, making some regions more vulnerable than others. Specifically, the Mediterranean region has characteristics that make it more vulnerable to climate change, as it is a transition zone between the arid climate found in north Africa and the temperate climate of central Europe [1]. In fact, the Mediterranean region is considered one of the most sensitive areas to climate change, or a hotspot, according to the Regional Climate Change Index (RCCI). This index is based on the regional average precipitation, changes in the mean surface air temperature, and the interannual variability of precipitation and the temperature [2]. The most significant effects of climate change in the Mediterranean region are a decrease in precipitation, especially during the warm season, pronounced warming, and an increase in interannual variability [1].
Over the last century, the Mediterranean region has increased in temperature by 1.5 °C above the global average [3], and it is expected that the average temperature in the Mediterranean region will increase by the end of the century [1,4], increasing the likelihood of species extinctions [5]. All of this will negatively impact the conservation potential of the Mediterranean biome, which is a biodiversity hotspot and a priority for conservation [6]. This temperature increase may cause critical temperature thresholds in aquatic ecosystems to be exceeded, severely degrading the habitat [7].
Regarding precipitation, it is expected to decrease across the entire Mediterranean region. Some authors indicate that, based on observations from different climate models and various emission scenarios, by the end of the 21st century, precipitation could decrease by up to 25–30% compared to the period of 1961–1990 [1]. These reductions will be more pronounced in specific seasons depending on the area within the Mediterranean region. In some areas, increases in precipitation are expected during certain times of the year, leading to greater interannual variability in precipitation and an increase in extreme events such as heat waves and a rise in consecutive dry days [4,8], as well as other extreme events like severe hailstorms [9] and torrential rains causing floods [10].
These effects of climate change in the Mediterranean region will negatively impact global climatic heterogeneity, significantly reducing it with a pronounced latitudinal trend, being more strongly influenced by temperature increases than by precipitation decreases [11]. Suitable areas for aquatic macroinvertebrate species are expected to shift 4.7–6.6° northward and 3.9–5.4° eastward in Europe [12]. This rise in temperature and decrease in precipitation will lead to the losses of various Mediterranean ecosystems and their associated biodiversity [13,14,15], as well as changes in the potential species richness [16] and the occurrence of species extinctions [5].
Different modeling studies have shown that climate change will contribute to a reduction in climatically suitable areas, potentially leading to a 57–59% decline in riverine macroinvertebrate species in Europe, with endemic species being the most affected by the loss of significant portions of their suitable climatic areas [12]. All of this will negatively impact the conservation potential of the Mediterranean biome, which is a biodiversity hotspot and a priority for conservation [6].
The effects of climate change in the Mediterranean region pose a major threat to biodiversity, making it one of the most vulnerable areas in terms of conservation [17]. Some of the ecosystems in the Mediterranean region that will be most affected are riparian ecosystems [18], with a high probability of some aquatic ecosystems disappearing or transitioning from permanent to seasonal [19] due to the influences of climate change, such as rising temperatures [20].
Additionally, Mediterranean rivers have a high degree of endemism and a long history of anthropogenic impacts, which limits their ability to cope with future stressors [21]. Water availability in the Mediterranean region will be reduced due to rising temperatures and changes in precipitation patterns (greater variability and decreased precipitation) [22], potentially leading to a 10–30% reduction in river discharge, decreasing the freshwater availability [23,24]. This has already been observed in the Douro River in the Iberian Peninsula, where winter and spring flows have decreased due to reduced precipitation in both rain and snow forms, which are essential for runoff generation [25].
Furthermore, several rivers and streams will be affected as rising temperatures lead to lower snow accumulation in mountainous regions, altering seasonal flow patterns by increasing the discharge in winter and reducing it in spring [5]. Other impacts include reduced hydrological connectivity [26], an increased pollutant concentration during drought periods, and changes in biological communities [5], as well as a decline in water quality, the mobility and dilution of pollutants, eutrophication, and changes in sediment transport, severely affecting habitats [27].
All these effects will alter biological communities in river ecosystems, leading to changes due to reduced resilience in rivers and streams under dry-year stress [28]. Stream and river communities will become more homogeneous and shift to higher latitudes and elevations as aquatic organisms respond to climate change impacts [29,30]. This shift in species distribution due to climate change is evident in many aquatic insect species, with headwater-dwelling macroinvertebrates—adapted to cold conditions—experiencing significant habitat loss [31]. These species will be gradually replaced by organisms from mid- and lower river reaches or by more generalist species [31,32].
Additionally, the available habitats will be reduced, with the most fragile habitats being those with lower response and adaptation capacities [17], leading to decreased habitat suitability [33]. This will have drastic effects on biodiversity, particularly impacting species with low dispersal ranges or those occupying highly specific habitats [17].
Currently, aquatic macroinvertebrates are used as bioindicators to assess the conservation status and quality of rivers. Numerous indices, such as the IBMWP index, are based on the tolerance of these aquatic organisms to environmental disturbances [34,35] and are widely used in research and public administration. These aquatic macroinvertebrates reflect changes occurring in the ecosystems they inhabit, as they are highly sensitive to different disturbances due to the presence of specialized lower taxonomic groups. This makes them excellent bioindicators in both the medium and long term [36,37], as well as allowing for the tracking of environmental variations over temporal scales of up to several months due to their diverse life cycles [38].
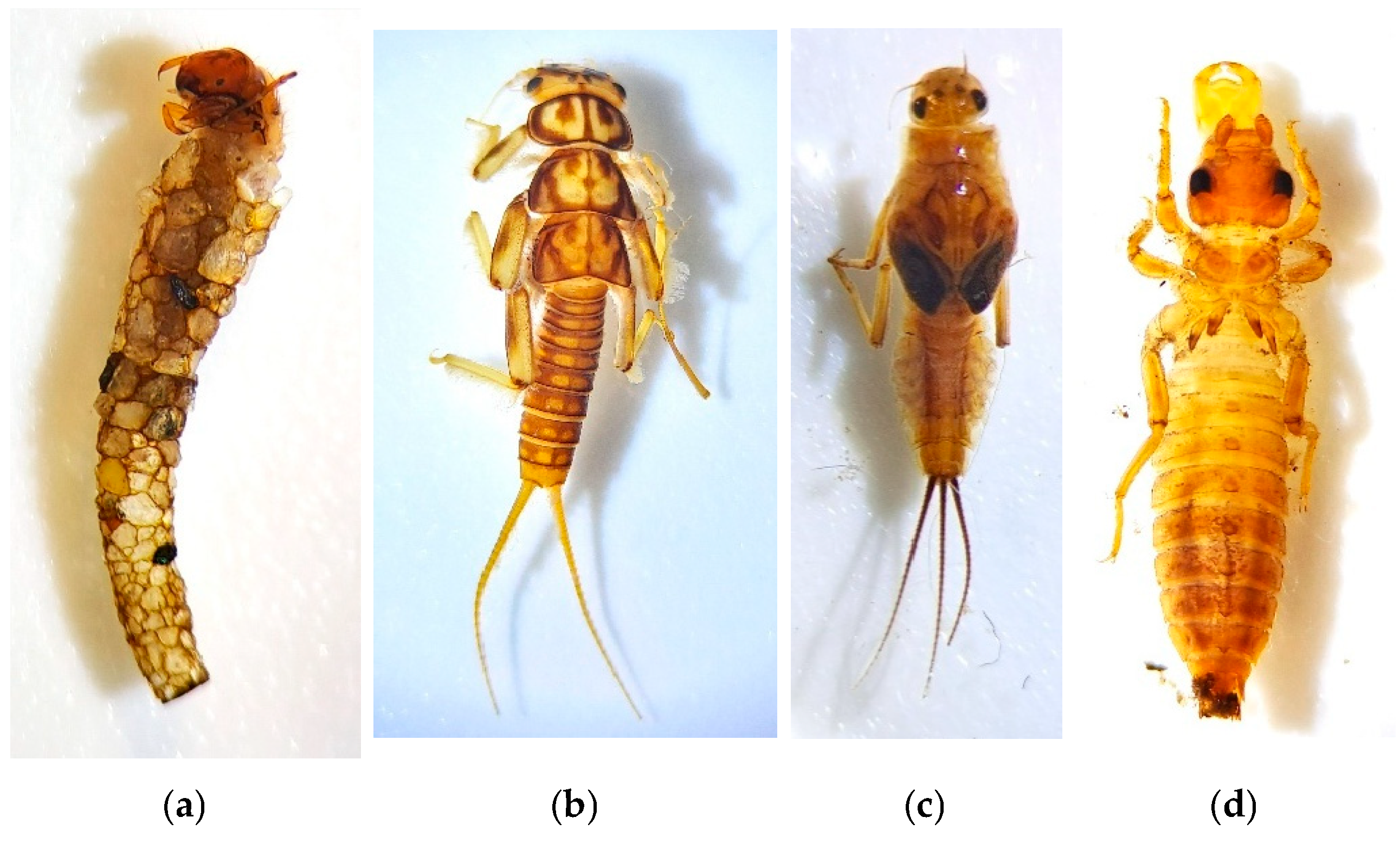
Among the most sensitive groups to pollution and ecosystem alterations are aquatic larvae belonging to the orders Trichoptera, Ephemeroptera, Plecoptera, and Coleoptera [36]. The most specialized aquatic macroinvertebrates with lower tolerance to pollution—such as stoneflies (Plecoptera), caddisflies (Trichoptera), mayflies (Ephemeroptera), and dragonflies (Odonata)—are the most affected, potentially experiencing significant episodes of rarity and local extinctions [39]. Conversely, other groups, such as certain species of Diptera, Oligochaeta, and Mollusca, are more resistant to these alterations [40]. Figure 1 shows two pictures of riparian habitats in the Mediterranean region, and Figure 2 shows photos of aquatic macroinvertebrates of the orders Trichoptera, Plecoptera, Ephemeroptera, and Odonata.

Figure 1.
(a) River Corneja in the province of Ávila, Spain. (b) River Voltoya in the province of Avila, Spain.
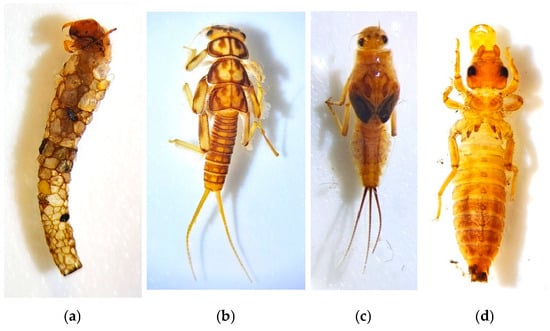
Figure 2.
(a) Photo of an aquatic macroinvertebrate belonging to the order Tricoptera. (b) Photo of an aquatic macroinvertebrate belonging to the order Plecoptera. (c) Photo of an aquatic macroinvertebrate belonging to the order Ephemeroptera. (d) Photo of an aquatic macroinvertebrate belonging to the order Odonata. These photos were financed by the Gran Duque de Alba Institution belonging to the Provincial Council of Ávila, from the call for grants for research on Avila issues, 2019.
Thus, the variability in tolerance to environmental disturbances leads to the disappearance of or reduction in the abundance of the most sensitive species while allowing more tolerant species to persist or even increase in abundance [36]. In conclusion, changes in the thermal regimes and freshwater ecosystem flow will affect the species phenology and distribution ranges, as well as the population composition and dynamics, as observed in numerous studies [41,42].
Due to their known taxonomy, their role in characterizing water quality in different riparian ecosystems, and their effectiveness as bioindicators, aquatic macroinvertebrates can be used as bioindicators of climate change in the riparian ecosystems of the Mediterranean region. They serve as a fundamental tool for the biological monitoring of aquatic ecosystems, helping to detect changes caused by environmental impacts [43].
2. Materials and Methods
A systematic search was conducted using the PRISMA methodology [44] in the Web of Science, Scopus, and Google Scholar databases. In all three databases, the search focused on aquatic macroinvertebrates and climate change, using different Boolean operators to refine the search for each database.
In Web of Science, the following search expression was used: (TS = (‘Macroinvertebrates’) AND TS = (‘climate change’) AND (TS = (‘bioindicators’) OR (TS = (‘bioindicator’)). The publication dates ranged from 1 January 2014 to 31 December 2024, yielding a total of 42 results.
In the Google Scholar database, the same search query as in Web of Science was used, resulting in a total of 1760 results.
From this initial search, a more specific filter was applied to focus only on articles related to the Mediterranean region and aquatic macroinvertebrates, as most of the initial results were found to be irrelevant. The refined search expression used was: (TS = (“macroinvertebrados acuáticos” OR “aquatic macroinvertebrates”) AND TS = (“Mediterráneo” OR “Mediterranean” OR “Mediterranean region”) AND TS = (“cambio climático” OR “climate change”) AND (TS = (“bioindicadores” OR “bioindicators” OR “biological indicators”)). This search yielded a total of 126 results.
In Scopus, the search was conducted using the following Boolean operator: Title-Abs-Key (“macroinvertebrados acuáticos” OR “aquatic macroinvertebrates” OR “macroinvertebrates”) AND All (“bioindicadores” OR “bioindicators” OR “biological indicators”) AND All (“cambio climático” OR “climate change”) AND All (“Mediterránea” OR “Mediterranean” OR “Mediterranean region”), resulting in a total of 297 results.
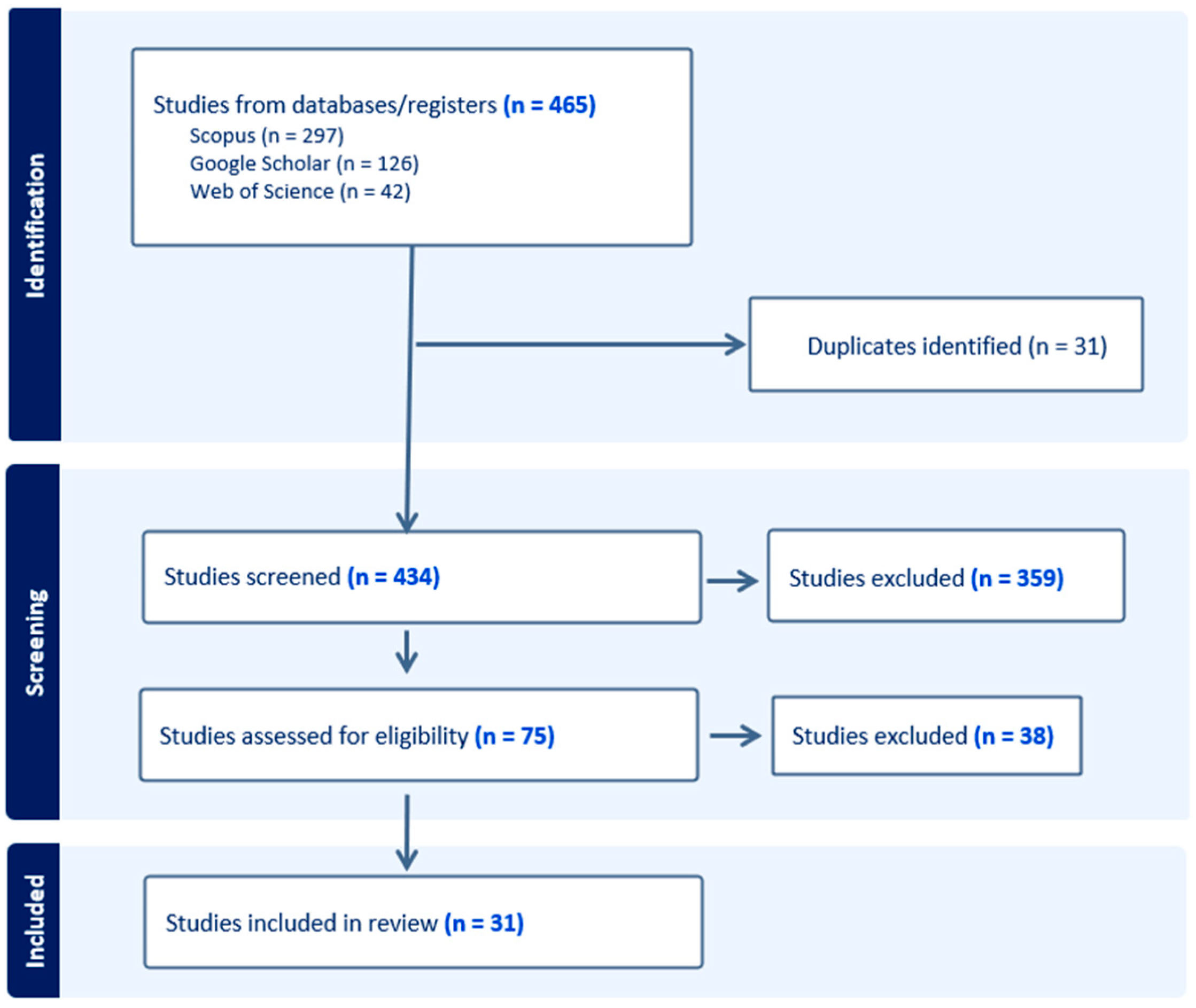
Across the three search engines, a total of 465 articles were obtained, of which 31 were identified as duplicates. Subsequently, the titles and abstracts of the remaining 434 articles were analyzed, leading to the elimination of 359 articles. These studies were discarded mainly because they focused on macroinvertebrates in coastal and/or estuarine environments, referred to regions other than the Mediterranean (while we kept general articles that did not focus on a specific region), concentrated on the effects of pollution on aquatic macroinvertebrates, or were purely case studies characterizing the quality of a single river.
Finally, the full texts of the remaining 75 articles were reviewed, with an additional 44 articles being excluded. These articles were deemed irrelevant because they did not contribute to the use of macroinvertebrates as indicators of climate change, focused on local effects unrelated to climate change, or examined study areas that were not truly representative of the Mediterranean region despite mentioning it (Figure 3).
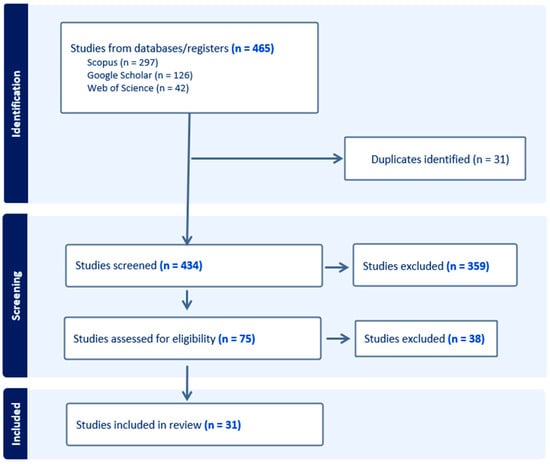
Figure 3.
Flowchart of the methodology used.
In the end, a total of 31 articles were found to be relevant to the study objective.
Upon analyzing the full texts of the selected articles, it was found that some relevant studies were published before the initially chosen date range. Consequently, these articles were reviewed and included in the literature review to achieve the study’s objective, given the limited information available on the effects of climate change on aquatic macroinvertebrate communities in the Mediterranean region. This analysis highlighted a significant gap in research on this topic.
Additionally, more general articles were sought to understand the effects of extreme events on aquatic ecosystems, as no studies specifically addressing these events in the Mediterranean region were found within the selected time period.
Ultimately, this study was conducted using the articles obtained from the systematic search, as well as additional relevant articles identified within the selected studies. These included articles that fell outside the original date range or were not detected by the initial search formulation.
3. Results and Discussion
Analyzing the selected articles, it was observed that macroinvertebrate communities will be affected differently depending on increases in temperature and changes in precipitation patterns, including both decreases and variations. This was evident in a study conducted over 42 years in the Breitenbach stream (Germany), where effects such as decreased abundance, altered richness, increased evenness, and changes in the trophic structure and phenology were observed, among other impacts [45].
Currently, there are few studies in the Mediterranean region dedicated to examining the effects of climate change on aquatic macroinvertebrates [29]. The majority of existing studies—although still limited—have been conducted in different regions of the world, mainly focusing on specific localities where long-term data on these communities are available. Since understanding the climate change effects on these communities requires relatively long-term studies, the number of available studies remains low [45].
Additionally, research has been observed to focus primarily on three main orders: Plecoptera, Trichoptera, and Ephemeroptera [46,47,48,49]. The temporal range of these studies varies greatly, indicating that the effects of climate change on these communities have been recognized for many years.
The following section outlines the effects of temperature increases, changes in hydrological regimes, and extreme drought and flood events on macroinvertebrate communities and how aquatic macroinvertebrates in the Mediterranean region respond to climate change, with Table 1 showing a summary of all these effects.

Table 1.
Effects of climate change on aquatic macroinvertebrates.
3.1. Effects of Temperature Increase on Aquatic Macroinvertebrates
The thermal regime in fluvial ecosystems is one of the most influential abiotic factors affecting the processes occurring in these environments [50]. It influences various biological aspects of organisms, including growth, feeding, emergence, fecundity, and survival [50], as well as the habitat distribution and the abundance of thermophobic taxa, such as the families Blephariceridae and Notonemouridae [54].
Additionally, rising temperatures will negatively impact the water quality by reducing the water volume, increasing pollutant concentrations, accelerating organic matter decomposition, and speeding up nutrient cycling while decreasing critical components such as dissolved oxygen [56]. The dissolved oxygen concentration is positively correlated with the taxonomic richness [85], thereby affecting the population dynamics of organisms [86].
The increase in river and stream temperatures will impact aquatic macroinvertebrate communities, potentially eliminating rarer taxonomic groups and reducing the overall abundance [57], particularly in spring populations within high mountain river ecosystems [51]. The decline in abundance due to rising temperatures will be more pronounced when streams are isolated from other water sources [67]. The extinction of certain taxonomic groups and rising temperatures may also facilitate the invasion of exotic species or species from lower river sections, altering the composition of macroinvertebrate communities [51].
These temperature increases may have more severe effects during certain seasons, such as spring, leading to disruptions in the emergence phenology and energy flow [51]. The expected emergence timing of some aquatic macroinvertebrate species may shift earlier [87], and certain groups, such as shredding insects, may decline due to their dependence on leaf litter input [88].
Other studies have shown that organisms may exhibit reduced body size as an indirect consequence of rising temperatures, along with further degradation of the water quality and a decrease in the water volume [52]. These climate change effects have been observed in various regions worldwide, with macroinvertebrate responses to temperature increases being consistent across different locations [53]. The most common biological responses include changes in the growth rates, fecundity, and emergence timing and duration, while the most frequent ecological responses involve alterations in the species composition, richness, and distribution [53].
Research also indicates that ectothermic invertebrates are potentially vulnerable to climate change if water temperature increases exceed their thermal threshold [89]. However, not all watersheds will be equally affected by warming, as the impact will depend on the pre-existing habitat conditions before the temperature rise occurs [90].
Another effect of rising temperatures is the extension of the snowmelt season in high mountain streams, which may create colder conditions during certain parts of the year, posing adaptation challenges for species that inhabit these streams and depend on temperature stability [91]. However, this increased snowmelt due to rising temperatures can negatively impact species that rely on the presence of permanent ice or snow, such as certain species of the genus Diamesa. The melting of ice and snow will lead to higher water temperatures, potentially causing these species to disappear from cold mountain streams [92].
These changes in the abundance and composition of macroinvertebrate communities were observed in Llyn Brianne, where future climate projections with increased temperatures indicated a net loss of four to ten taxa [51]. This loss of taxonomic richness has also been observed in other studies, such as research conducted on the Saône River in France [58]. This phenomenon could be even more pronounced in the Mediterranean region, as more drastic changes in the community composition are expected due to climate change [48].
Ultimately, species adapted to low water temperatures will be the most vulnerable to rising temperatures due to climate change. Their genetic adaptation to cold and stable temperatures will make them much less capable of adjusting their phenotype to increasing temperatures. Additionally, many of these species may already be at the upper limit of their thermal tolerance range. Some species may shift their distribution to areas with lower temperatures, but others will be unable to migrate, while warming is expected to be more pronounced at higher latitudes [55].
Cold-adapted species will also experience habitat loss at high latitudes and elevations. Likewise, heat-adapted species found at lower latitudes, especially those with narrow ecological niches and specialized traits, will also be affected by rising temperatures [12].
Therefore, certain families of macroinvertebrates characteristic of the Mediterranean region could be used as bioindicators of temperature increases, depending on their sensitivity to rising temperatures. This has been demonstrated in a study conducted in the rivers of the southwestern Cape, South Africa, where four thermosensitive families and five moderately sensitive families were identified [50]. Similar findings have been reported in numerous studies focusing on specific macroinvertebrate groups, such as decapods [93], caddisflies (Trichoptera) [94], stoneflies (Plecoptera) [95] and dragonflies (Odonata) [96].
3.2. Effects of Decreased Precipitation and Altered Hydrological Regime on Aquatic Macroinvertebrates
According to various authors, hydrological variability is one of the most important factors determining the structure and composition of communities [97,98,99], with the community composition being significantly different in perennial and temporary river ecosystems [100]. This hydrological variation is of the utmost importance in regions that experience river intermittency, such as the Mediterranean region [101]. Moreover, studies show that the distribution of intermittent rivers and ephemeral streams is expanding due to climate change [102]. These intermittent rivers are crucial for biodiversity conservation, demonstrating that a greater prevalence of intermittent rivers and streams will lead to a decline in biodiversity [59].
Additionally, macroinvertebrate communities are adapted according to the temporary or permanent nature of the water, with wetter streams being dominated by taxa with slower growth, lacking adaptations for desiccation resistance or strong dispersal [99], making these taxa threatened by the loss of such permanent flow. Therefore, hydrological changes, particularly extreme variations, influence the structuring of macroinvertebrate communities [63,64], as they are a group that reflects river level fluctuations. The resistance and resilience traits of macroinvertebrates determine the persistence of communities in intermittent rivers [103].
Naturally, macroinvertebrate communities fluctuate between wet and dry seasons, with the richness and abundance being determined by changes in the flow permanence [104]. These macroinvertebrates exhibit adaptations to such oscillations [68,105,106], but climate change will cause extreme river level fluctuations, altering macroinvertebrate communities by reducing their density or even leading to the disappearance of several taxa [68,69]. This will result in the predominance of tolerant and generalist taxa [65], but groups such as Trichoptera and mayflies are sensitive to river level variations, significantly declining when these variations are extreme or abrupt [68], potentially leading to biotic homogenization [68].
Other organism groups sensitive to drought periods include the family Leuctridae (Plecoptera) and the family Athericidae (Diptera) [101], as well as the order Ephemeroptera [67]. In contrast, the species of Plecoptera and Diptera (families of Simuliidae, Tipulidae, Stratiomyidae, Athericeridae, and Dixidae) are adapted to waterless periods, allowing them to survive without disappearing, although their abundance would be negatively affected [67]. Therefore, within the Mediterranean ecosystem, macroinvertebrate communities in perennial streams and rivers will be more affected, as these organisms are less adapted to water scarcity and droughts [101].
Due to climate change, changes in precipitation patterns are expected, as more consecutive days without rainfall and/or more concentrated rainfall in specific periods of the year are anticipated, leading to a spatial alteration of precipitation, which will affect aquatic macroinvertebrate communities [107,108]. Intense rainfall and dry events will impact the spatial and temporal distribution of diversity and habitat stability [70].
These more concentrated rainfalls will enhance the flow of river ecosystems, causing the macroinvertebrate community to be increasingly characterized by taxa typical of lotic environments during these periods [109], reducing the species richness and altering the proportion of filtering and gathering macroinvertebrates, as well as the presence of species characteristic of lotic and lentic environments [60].
At the same time, longer periods of low or no precipitation will alter communities, which will be characterized by species tolerant to low flows, with an increase in predatory, opportunistic organisms and those that preferentially feed on algae [65]. These high or low flow indices (including the absence of flow) in winter and spring may determine the composition of the macroinvertebrate community found at the end of summer, making flow variability a crucial factor in understanding the structure of communities within river channels [66].
Additionally, this decrease in precipitation will lead to an increase in the water temperature, as well as a rise in metal and metalloid concentrations [71].
Another effect of climate change is an increase in the duration of dry periods, as the hydrological regime will be affected. This is one of the main factors determining the aquatic response, leading to a decrease in the density and richness of aquatic taxa as the duration of the dry period increases [61]. This richness declines linearly in many streams and rivers as the flow permanence decreases [67].
Taxa with a low dispersal capacity that depend on the presence of water flow for movement will become isolated [68], leading to increased competition among them for space and food, potentially excluding other taxa [62]. If changes in the hydrological regime occur more rapidly than the evolutionary capacity of species to adapt, mass extinctions will occur [29].
As precipitation decreases, along with other effects of climate change, regions are expected to become more arid, leading to the loss of competitive taxa with low environmental tolerance, such as some species belonging to the Trichoptera and Ephemeroptera groups [110]. Additionally, the water quality of rivers and streams deteriorates during drought periods, as the reduced flow decreases the capacity to dilute pollution [72].
The response of biodiversity to the hydrology will depend on several factors, such as the habitat, as a decrease in its suitability is expected due to climate change [33]. Additionally, the spatial scale of the study is crucial, as at smaller scales, hydrological connectivity becomes highly important. Therefore, adopting a multi-scale perspective is essential to understanding biodiversity responses to hydrological regimes [111].
A study conducted in California streams revealed that ecological connectivity in streams is fundamental for maintaining the diversity index in macroinvertebrate communities in temporary streams. It was observed that macroinvertebrates were more abundant in temporary streams closer to perennial reaches compared to more remote temporary sections [67].
3.3. Extreme Weather Events and Aquatic Macroinvertebrate Communities
Due to climate change, extreme events such as heat waves, droughts, and floods will have a greater probability of occurring. These events will negatively affect aquatic macroinvertebrates, modifying the community composition as a reflection of their impact on these organisms, since extreme fluctuations such as floods and droughts are associated with a decrease in the richness, abundance, and diversity of macroinvertebrates, with tolerant taxa predominating [68,73] and regulating the richness according to the magnitude and frequency of these extreme events [112]. Furthermore, an alteration in the occurrence of extreme events, such as a reduction in floods and an increase in drought events, may cause an imbalance that would lead to changes in the community composition, favoring species resilient to dry periods but compromising species adapted to floods [112].
Mediterranean ecosystems are adapted to drought periods that occur during the summer season, meaning that habitats and diversity have adapted to these natural droughts, which cause a natural variability in flow [113]. The frequency and intensity of heat waves and droughts are increased by climate change, leading to extreme drought events, which are phenomena without a marked seasonality that alter the natural flow of rivers, preventing organisms from adapting [114].
This will directly affect aquatic macroinvertebrate communities, impacting certain taxa that lack physiological and behavioral adaptations to promote resistance and resilience during the drying of riverbed ecosystems and depend on free water, thereby altering the richness and abundance [74,75]. For example, in the Saône River region (France), it is expected that half of the mollusk species will be threatened with extinction due to the increased frequency of very hot summers [58].
Droughts will negatively impact invertebrate communities in lotic environments, excluding taxa that depend on flowing waters [74,75]. Additionally, species that require a long aquatic phase will be negatively affected, as this phase may coincide with drought, preventing the completion of their life cycle [100], leading to the homogenization of these communities if several extreme droughts occur relatively consecutively [76]. Other taxonomic groups, such as dipterans, will modify functional traits during extreme drought periods, such as their body size and feeding strategy [65].
Nevertheless, extreme events like droughts can exceed the tolerance threshold, reducing the recovery capacity, causing local extinctions, triggering ecosystem changes, and facilitating the invasion of exotic species [77]. Low flows and droughts will increase sedimentation, eliminate habitat connectivity, and modify the composition of aquatic vegetation, which in turn will affect the water chemistry [75].
The recolonization of a stream or river after a drought will depend on the timing, intensity, and duration of the dry phase, affecting some taxa more negatively than others [75]. All these effects of extreme drought can lead to permanent alterations in river ecosystems [65].
According to several studies, aquatic macroinvertebrate communities will experience a reduction in abundance and richness due to floods, as the water quality will deteriorate following such an extreme event, exhibiting characteristics similar to those of polluted waters. However, these communities may recover several months after the flood [78,79]. This decline in biodiversity may be attributed to the changes that occur in streams and rivers during flooding, such as the sudden increase in water flow, the displacement of surface substrates, the washing away of the riverbed substrate, and the removal of vegetation [80,115,116].
These environmental modifications will affect the composition of aquatic macroinvertebrate communities, altering their distribution and abundance, which decreases after the flood and modifies the community composition even years later [74,82,83,84]. The recovery of macroinvertebrate communities may take between three and five years, while crustaceans may require up to ten years for full recovery [81].
The impact on macroinvertebrates will depend on whether they follow a K-strategy or an r-strategy, as some taxa, such as Chironomidae, are less affected by floods due to their r-strategist nature, whereas larger taxa with a K-strategy are the most affected [81].
For example, a study conducted in the Jizera Mountains Beech Forests National Nature Reserve (Czech Republic) found that after a severe summer flood, the species and taxa richness, as well as the overall biodiversity, decreased by approximately 50%, while the abundance of the surviving taxa declined by about 10%. Two years after the flood, the community had not yet recovered [80]. Another study in the Glenfinish River (Ireland) showed that the taxon richness decreased by 70% following a flood [81].
In addition to the biodiversity loss caused by flooding, the subsequent displacement of organic matter and plants negatively impacts species that inhabit soft substrates or rely on plants such as mosses, making it extremely difficult for them to reestablish themselves after the flood. In some cases, they may completely disappear in the years following the event [80].
These effects of the increased flow caused by floods can be mitigated if local refuges exist to help aquatic macroinvertebrates escape the rise in the water flow [83]. Streams with more homogeneous channels are more affected by floods than those with heterogeneous channels, as the latter provide more refuges, resulting in a shorter recovery time for communities [117].
Therefore, in streams and rivers with recurrent flooding, macroinvertebrate communities will be severely affected, as the riverbed will be altered, eliminating refuges for these organisms and thus extending the time required for community recovery. In some cases, the return interval of floods may be shorter than the time needed for macroinvertebrate communities to recover.
3.4. Aquatic Macroinvertebrates as Bioindicators of Climate Change in the Mediterranean Region
Aquatic macroinvertebrate communities exhibit different responses to the effects of climate change, being primarily affected by rising temperatures, decreased precipitation, alterations in the hydrological regime, and the increased occurrence and frequency of extreme events (droughts and floods). Understanding these changes in communities (modifications in the richness, composition, and abundance) allows for the quantification and monitoring of climate change effects on river ecosystems in the Mediterranean region.
The loss of species and decline in abundance in Mediterranean streams, such as a temporary Mediterranean stream in southern Portugal, are more strongly associated with years of lower precipitation. In these cases, chironomid and simuliid species dominate, while the abundance of plecopterans and odonates decreases [118].
Alterations in the hydrological regime, specifically the transition from permanent to intermittent flow in a Mediterranean-climate stream, will impact shredder species that are sensitive to desiccation, modifying the community composition. In such cases, trichopteran and plecopteran species will be replaced by dipterans [107].
Additionally, climate change effects influence the habitat quality, promoting the accumulation of pollutants in the sediments of intermittent streams, which in turn negatively affects macroinvertebrate communities [119].
It is essential to study and identify groups and/or species that are sensitive to climate change. More thermophobic species, which require cooler environments, will shift towards higher latitudes and move northward, whereas species that thrive in warmer conditions may expand their distribution range [120].
In Europe, research has demonstrated a decreasing south–north gradient of aquatic macroinvertebrates’ vulnerability to climate change, positioning the Mediterranean region as the area expected to experience the most significant potential impacts [47]. One of the groups expected to be most sensitive to climate change is caddisflies (Trichoptera). Studies suggest that up to 30% of this order could face extinction in southern Europe, mainly due to the restricted distribution of several species, with the most sensitive taxa in the Mediterranean region potentially migrating northward [48].
Another taxonomic group at high risk is stoneflies (Plecoptera), as they have narrow ecological niches, particularly those inhabiting high-altitude environments [47]. In the river basins of Castilla-La Mancha (Spain), it was demonstrated that rising spring temperatures due to climate change will negatively affect sensitive orders such as Ephemeroptera, Plecoptera, Megaloptera, Trichoptera, Coleoptera, and Diptera. Some taxa, including Ephemeroptera, Trichoptera, Hemiptera, and Coleoptera, exhibit negative correlations with the minimum winter temperature, maximum spring temperature, and precipitation levels [121].
These more temperature-sensitive taxa could face extinction if they do not have access to cooler water habitats upstream or in nearby stream headwaters [122]. Another study conducted in Andean Mediterranean streams observed that certain taxa are more sensitive to the temperature than others. For example, the families Gripopterygidae (Plecoptera) and Austroperlidae (Plecoptera) were more abundant at low water temperatures, whereas families such as Notonemouridae (Plecoptera), Perlidae (Plecoptera), Hydroptilidae (Trichoptera), Hidrobiosidae (Trichoptera), and Elmidae (Diptera) had an optimal abundance at higher water temperatures [123].
All of this evidence indicates that changes in macroinvertebrate communities will occur, ultimately altering the functioning of river ecosystems [119,121].
Aquatic macroinvertebrates in the Mediterranean region will shift towards higher elevations and latitudes, altering the community composition and leading to a homogenization of these communities. However, some will exhibit resilience and resistance, allowing them to adapt to new climatic conditions [29].
This change in distribution is evident in the species Trithemis annulata (Palisot de Beauvois, 1805) (Odonata), which expanded from Africa into southern Spain due to rising temperatures and warmer summers [124]. It was first recorded in Cuenca (Spain) in 2011 [125] and later that same year in Navarra (northern Spain) [126], gradually expanding its range to Italy and Greece in subsequent years [127]. The expansion of this species and other Mediterranean species can be attributed to increasing temperatures [128]. Another odonate species that has extended its distribution from Africa into southern Spain is Trithemis kirbyi (Selys, 1841) [129].
Nevertheless, taxa with limited dispersal abilities or without access to suitable nearby habitats for survival will face local extinction. One such example is the hemipteran Aphelocheirus aestivalus (Fabricius, 1794), which lacks functional wings and relies on the water flow for dispersal [121].
Other species will experience alterations in their life cycle, as seen in the mayfly Ephoron virgo (Olivier, 1791) (Polymitarcyidae) in the Ebro River (Catalonia, Spain). This species has accelerated larval development, leading to an earlier emergence and changes in the sex ratio [49].
Due to the high number of temporary streams in the Mediterranean region, it is essential to identify species resistant to desiccation, as they can serve as valuable bioindicators of anthropogenic impacts and climate change. For instance, Nemoura is known for its desiccation resistance, while other groups such as Calopteryx are sensitive to desiccation, and some, like Lepidostoma, are partially tolerant [130].
Additionally, European stonefly species (Plecoptera) with broad thermal tolerance ranges and high drought resilience will be the least vulnerable, leading to a decline in European taxa of this order and a decrease in the overall diversity [122]. Therefore, macroinvertebrate species that exhibit adaptations such as egg resistance to desiccation, aerial dispersal, and drift dispersal in currents will be more resistant and resilient to prolonged droughts, as they are less dependent on drought refuges [131].
However, despite these adaptations to hydric stress, these species remain vulnerable to further reductions in the water availability due to climate change [119].
The effects of climate change in the Mediterranean region are expected to be particularly severe. A study conducted on the Iberian Peninsula using MEDPACS (MEDiterranean Prediction And Classification Systems) predicted drastic changes in the taxon composition in Mediterranean mountains and southern river basins, with species that have restricted distributions and narrow ecological requirements being especially affected [132]. Families such as Ephemeridae, Perlidae, Sericotomatidae, and Brachycentridae will be unable to adapt to various climate change scenarios [132]. The presence area of taxa such as Coleoptera, Hirudinea, Neuroptera, Diptera, Plecoptera, Trichoptera, and Turbellaria will shrink due to climate change [132]. Other taxa, including Heteroptera, Mollusca, Ephemeroptera, Diptera, Crustacea, Trichoptera, Odonata, Plecoptera, Coleoptera, and Turbellaria, will shift their altitudinal distribution, moving upstream from lower elevations [132]. According to the study in the Iberian Peninsula, most taxa (65.96%) will see a reduction in their presence area and move to higher altitudes to colonize new sites, although some families will expand their presence by shifting to lower altitude areas [132].
Currently, there are few studies on how aquatic macroinvertebrates in the Mediterranean region respond to extreme events such as floods and droughts. Regarding droughts, research has shown that macroinvertebrate responses to river drying occur in stages, with drastic community structure changes when the water flow ceases. Initially, the densities increase in isolated pools, but they then drop sharply due to physicochemical changes caused by river fragmentation. The community present before the drying event differs significantly from the one after rehydration when the flow resumes [133]. Additionally, the extended duration of drought periods, and consequently longer river drying periods, will reduce the abundance and richness, impoverishing communities in pre-Alpine rivers, as observed in the Po River (Italy), where an increase in collector–gatherers and a decline in scrapers and shredders were recorded as the drought duration increased [134]. The habitat composition also influences macroinvertebrates’ resistance to floods, with inorganic substrate stability being more influential than that of woody substrates [135].
Floods also cause decreases in the richness and density, as observed in a study on the Matarranya River (Spain), where the taxon presence after flooding was reduced to 31.9% and 40.5%, depending on the sampling station. The community composition changed, with chironomids becoming dominant at both sites, although recovery times varied [136]. Flooding leads to macroinvertebrate displacement and relocation, altering competition, predation, and the spatial organization of resources, as observed in the Tagliamento River (Italy) [137].
In summary, aquatic macroinvertebrates will exhibit specific responses to climate change, such as reductions in abundance and richness, latitudinal and altitudinal shifts in taxa, and local extinctions. Because of these responses, they can serve as effective indicators of climate change, as demonstrated in a study conducted in the Albaida Valley (Spain). This study found that the IBMWP index (a biological index using macroinvertebrates as indicators of water quality) declined under various climate change scenarios, with the reduction being most significant under the most extreme projections [138].
Monitoring these ecosystems is essential to mitigating the effects of climate change impacts through the conservation, protection, and restoration of freshwater ecosystems. Current research aims to identify sentinel families for global warming in aquatic ecosystems. A study in the Júcar River basin (Spain) demonstrated that the robust optimum method can be used to estimate taxon distributions by determining ecological optima, optimal ranges, and tolerance ranges, allowing for the identification of sentinel taxa [139]. Other studies focus on identifying indicator taxa for intermittent rivers and ephemeral streams. In the Mediterranean region, five families have been identified as indicative of these water bodies: Dytiscidae (Coleoptera), Lestidae (Odonata), Notonectidae (Hymenoptera), Sphaeriidae (Veneroida), and Libellulidae (Odonata) [140].
4. Conclusions
The increase in temperature, decrease in precipitation, alteration of the hydrological regime, and the occurrence of extreme events such as droughts and floods will determine the richness, abundance, composition, and phenology of aquatic macroinvertebrates in river ecosystems. Each taxon will respond differently to these effects, with some being more sensitive than others.
In the Mediterranean region, macroinvertebrate communities are expected to shift both latitudinally and altitudinally, with specialized and endemic taxa disappearing and being replaced by more tolerant and generalist species. Additionally, the introduction of exotic species may occur, leading to homogenization and a loss of biodiversity. Therefore, generalist taxa such as Diptera and Mollusca are expected to thrive in warmer and drier conditions, and there will be an expansion in the distribution of species that are favored by the effects of climate change, such as the odonate Trithemis annulata. This will negatively affect endemic species because they will have to compete for resources and habitats with more species. These changes could aggravate biotic homogenization and alter ecosystem functions. On the other hand, species that are not very tolerant to changes in their habitats, such as an increase in temperature in species that are adapted to low water temperatures, will be observed to move to colder areas and disappear from these habitats, and endemic species with these characteristics may become extinct. For this reason, aquatic macroinvertebrates are an excellent tool for assessing the effects of climate change on river ecosystems, as they exhibit specific and measurable responses to these environmental changes.
Macroinvertebrates are closely linked to the ecosystems they inhabit, serving as key components and indicators of the ecosystem health and conservation status. It is necessary to carry out the long-term monitoring of aquatic macroinvertebrates in different riparian habitats of the Mediterranean region to understand the changes that are occurring in riparian ecosystems and thus be able to react to these alterations. Furthermore, these studies are the basis for deciding the conservation measures that should be taken to conserve both aquatic macroinvertebrates and riparian habitats, paying more attention to endemic macroinvertebrates since they will be the most negatively affected by climate change. In turn, climate change can favor the invasion of exotic species that negatively affect endemic species. The Mediterranean region is one of the first to suffer the negative effects of climate change, so it is necessary to know these effects in order to extrapolate them to other regions where similar problems will occur, although with a delay of years, and thus be able to apply appropriate conservation and management measures in these other regions before observing these effects.
Author Contributions
Conceptualization, S.E.-H. and J.V.; methodology, S.E.-H. and J.V.; validation, G.P.-A., K.Ç. and V.R.; formal analysis, S.E.-H.; investigation, S.E.-H.; resources, V.R.; data curation, G.P.-A. and K.Ç.; writing—original draft preparation, S.E.-H.; writing—review and editing, S.E.-H., J.V., G.P.-A., K.Ç. and V.R.; supervision, J.V. and G.P.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
Authors Samanta Espinar-Herranz and Víctor Rincón were employed by the company Tragsatec. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Change 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Giorgi, F. Climate Change Hot-Spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The Relation between Climate Change in the Mediterranean Region and Global Warming. Reg. Environ. Change 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Todaro, V.; D’Oria, M.; Secci, D.; Zanini, A.; Tanda, M.G. Climate Change over the Mediterranean Region: Local Temperature and Precipitation Variations at Five Pilot Sites. Water 2022, 14, 2499. [Google Scholar] [CrossRef]
- Balzan, M.V.; Hassoun, A.E.R.; Aroua, N.; Baldy, V.; Dagher, M.B.; Branquinho, C.; Dutay, J.-C.; Bour, M.E.; Médail, F.; Mojtahid, M.; et al. First Mediterranean Assessment Report—Chapter 4: Ecosystems. In Climate and Environmental Change in the Mediterranean Basin-Current Situation and Risks for the Future; Union for the Mediterranean, Plan Bleu: Marseille, France, 2020; pp. 323–468. ISBN 978-2-9577416-0-1. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Khamis, K.; Hannah, D.M.; Brown, L.E.; Tiberti, R.; Milner, A.M. The Use of Invertebrates as Indicators of Environmental Change in Alpine Rivers and Lakes. Sci. Total Environ. 2014, 493, 1242–1254. [Google Scholar] [CrossRef]
- Campo, R.; Hervella, B.; Luna, M.Y. Efectos Observados y Previstos Del Cambio Climático En España. Es Momento de Actuar. Ambient. Rev. Minist. Medio Ambiente 2021, 130, 16–25. [Google Scholar]
- Martín, M.L.; Calvo-Sancho, C.; Taszarek, M.; González-Alemán, J.J.; Montoro-Mendoza, A.; Díaz-Fernández, J.; Bolgiani, P.; Sastre, M.; Martín, Y. Major Role of Marine Heatwave and Anthropogenic Climate Change on a Giant Hail Event in Spain. Geophys. Res. Lett. 2024, 51, e2023GL107632. [Google Scholar] [CrossRef]
- Olcina, J. Cambio Climático y Riesgos Climáticos En España. Investig. Geográficas 2009, 49, 197–220. [Google Scholar] [CrossRef]
- Guan, Y.; Lu, H.; Jiang, Y.; Tian, P.; Qiu, L.; Pellikka, P.; Heiskanen, J. Changes in Global Climate Heterogeneity Under the 21st Century Global Warming. Ecol. Indic. 2021, 130, 108075. [Google Scholar] [CrossRef]
- Domisch, S.; Araújo, M.B.; Bonada, N.; Pauls, S.U.; Jähnig, S.C.; Haase, P. Modelling Distribution in European Stream Macroinvertebrates under Future Climates. Glob. Change Biol. 2013, 19, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Cramer, W. Climate Change: The 2015 Paris Agreement Thresholds and Mediterranean Basin Ecosystems. Science 2016, 354, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Aurelle, D.; Thomas, S.; Albert, C.; Bally, M.; Bondeau, A.; Boudouresque, C.-F.; Cahill, A.E.; Carlotti, F.; Chenuil, A.; Cramer, W.; et al. Biodiversity, Climate Change, and Adaptation in the Mediterranean. Ecosphere 2022, 13, e3915. [Google Scholar] [CrossRef]
- Klausmeyer, K.R.; Shaw, M.R. Climate Change, Habitat Loss, Protected Areas and the Climate Adaptation Potential of Species in Mediterranean Ecosystems Worldwide. PLoS ONE 2009, 4, e6392. [Google Scholar] [CrossRef]
- Maiorano, L.; Falcucci, A.; Zimmermann, N.E.; Psomas, A.; Pottier, J.; Baisero, D.; Rondinini, C.; Guisan, A.; Boitani, L. The Future of Terrestrial Mammals in the Mediterranean Basin Under Climate Change. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2681–2692. [Google Scholar] [CrossRef]
- Arribas, P.; Abellán, P.; Velasco, J.; Bilton, D.T.; Lobo, J.M.; Millán, A.; Sánchez-Fernández, D. La Vulnerabilidad de Las Especies Frente al Cambio Climático, Un Reto Urgente Para La Conservación de La Biodiversidad. Ecosistemas 2012, 21, 78–84. [Google Scholar] [CrossRef]
- Camarero, J.J. Sed En El Río: Cómo El Calentamiento Climático y Los Cambios En La Dinámica Fluvial Contribuyen al Declive de Los Bosques de Ribera (SED-IBER); Instituto Pirenaico de Ecología (CSIC): Zaragoza, Spain, 2016. [Google Scholar]
- Moreno, J.M.; Álvarez Cobelas, M.; Benito, G.; Catalán, J.; Ramos, M.Á.; de la Rosa, D.; Valladares Ros, F.; Zazo, C. Principales Conclusiones de La Evaluación Preliminar de Los Impactos En España Por Efecto Del Cambio Climático; Ministerio de Medio Ambiente (España): Madrid, Spain, 2005. [Google Scholar]
- Wanders, N.; van Vliet, M.T.H.; Wada, Y.; Bierkens, M.F.P.; van Beek, L.P.H. High-Resolution Global Water Temperature Modeling. Water Resour. Res. 2019, 55, 2760–2778. [Google Scholar] [CrossRef]
- Colin, N.; Porte, C.; Fernandes, D.; Barata, C.; Padrós, F.; Carrassón, M.; Monroy, M.; Cano-Rocabayera, O.; de Sostoa, A.; Piña, B.; et al. Ecological Relevance of Biomarkers in Monitoring Studies of Macro-Invertebrates and Fish in Mediterranean Rivers. Sci. Total Environ. 2016, 540, 307–323. [Google Scholar] [CrossRef]
- Calbó, J. Possible Climate Change Scenarios with Specific Reference to Mediterranean Regions. In Water Scarcity in the Mediterranean: Perspectives Under Global Change; Sabater, S., Barceló, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–13. ISBN 978-3-642-03971-3. [Google Scholar]
- Allen, M.R.; Ingram, W.J. Constraints on Future Changes in Climate and the Hydrologic Cycle. Nature 2002, 419, 224–232. [Google Scholar] [CrossRef]
- Lelieveld, J.; Hadjinicolaou, P.; Kostopoulou, E.; Chenoweth, J.; El Maayar, M.; Giannakopoulos, C.; Hannides, C.; Lange, M.A.; Tanarhte, M.; Tyrlis, E.; et al. Climate Change and Impacts in the Eastern Mediterranean and the Middle East. Clim. Change 2012, 114, 667–687. [Google Scholar] [CrossRef]
- Morán-Tejeda, E.; Ceballos-Barbancho, A.; Llorente-Pinto, J.M. Hydrological Response of Mediterranean Headwaters to Climate Oscillations and Land-Cover Changes: The Mountains of Duero River Basin (Central Spain). Glob. Planet. Change 2010, 72, 39–49. [Google Scholar] [CrossRef]
- Rincón, V.; Velázquez, J.; Pascual, Á.; Herráez, F.; Gómez, I.; Gutiérrez, J.; Sánchez, B.; Hernando, A.; Santamaría, T.; Sánchez-Mata, D. Connectivity of Natura 2000 Potential Natural Riparian Habitats Under Climate Change in the Northwest Iberian Peninsula: Implications for Their Conservation. Biodivers. Conserv. 2022, 31, 585–612. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Wilby, R.L.; Battarbee, R.W.; Kernan, M.; Wade, A.J. A Review of the Potential Impacts of Climate Change on Surface Water Quality. Hydrol. Sci. J. 2009, 54, 101–123. [Google Scholar] [CrossRef]
- Magalhaes, M.F.; Beja, P.; Schlosser, I.J.; Collares-Pereira, M.J. Effects of Multi-Year Droughts on Fish Assemblages of Seasonally Drying Mediterranean Streams. Freshw. Biol. 2007, 52, 1494–1510. [Google Scholar] [CrossRef]
- Filipe, A.F.; Lawrence, J.E.; Bonada, N. Vulnerability of Stream Biota to Climate Change in Mediterranean Climate Regions: A Synthesis of Ecological Responses and Conservation Challenges. Hydrobiologia 2013, 719, 331–351. [Google Scholar] [CrossRef]
- Jol, A.; Raes, F.; Menne, B. Impacts of Europe’s Changing Climate—2008 Indicator Based Assessment. IOP Conf. Ser. Earth Environ. Sci. 2009, 6, 292042. [Google Scholar] [CrossRef]
- Domisch, S.; Jähnig, S.C.; Haase, P. Climate-Change Winners and Losers: Stream Macroinvertebrates of a Submontane Region in Central Europe. Freshw. Biol. 2011, 56, 2009–2020. [Google Scholar] [CrossRef]
- Daufresne, M.; Bady, P.; Fruget, J.-F. Impacts of Global Changes and Extreme Hydroclimatic Events on Macroinvertebrate Community Structures in the French Rhône River. Oecologia 2007, 151, 544–559. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Szałkiewicz, E.; Marcinkowski, P.; Mirosław-Świątek, D.; Piniewski, M. Assessment of Climate Change Effect on Environmental Flows for Macroinvertebrates Using an Integrated Hydrological-Hydraulic-Habitat Modelling. J. Hydrol. Reg. Stud. 2024, 56, 101982. [Google Scholar] [CrossRef]
- Alba-Tercedor, J.; Jáimez-Cuéllar, P.; Álvarez, M.; Avilés, J.; Bonada, N.; Casas, J.; Mellado, A.; Ortega, M.; Pardo, I.; Prat, N.; et al. Caracterización Del Estado Ecológico de Ríos Mediterráneos Ibéricos Mediante El Índice IBMWP (Antes BMWP’). Limnetica 2002, 21, 175–185. [Google Scholar] [CrossRef]
- Alba-Tercedor, J.; Sánchez-Ortega, A. Un Método Rápido y Simple Para Evaluar La Calidad Biológica de Las Aguas Corrientes Basado En El de Hellawell (1978). Limnetica 1988, 4, 51–56. [Google Scholar] [CrossRef]
- Alonso, A.; Camargo, J.A. Estado Actual y Perspectivas En El Empleo de La Comunidad de Macroinvertebrados Bentónicos Como Indicadora Del Estado Ecológico de Los Ecosistemas Fluviales Españoles. Ecosistemas 2005, 14, 87–99. [Google Scholar]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés d’eau Douce. Systematique, Biologie, Écologie; CNRS Editions: París, France, 2003. [Google Scholar]
- Muñoz, I.; Sabater, S.; Barata, C. Evaluating Ecological Integrity in Multistressed Rivers: From the Currently Used Biotic Indices to Newly Developed Approaches Using Biofilms and Invertebrates. In Emerging and Priority Pollutants in Rivers: Bringing Science into River Management Plans; Guasch, H., Ginebreda, A., Geiszinger, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 219–241. ISBN 978-3-642-25722-3. [Google Scholar]
- McNeely, J.A. Monitoring Climate Change with Dragonflies: Foreword. BioRisk 2010, 5, 1–2. [Google Scholar] [CrossRef][Green Version]
- Camargo, J.A.; Alonso, A.; De La Puente, M. Multimetric Assessment of Nutrient Enrichment in Impounded Rivers Based on Benthic Macroinvertebrates. Environ. Monit. Assess. 2004, 96, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Sáinz-Bariáin, M.; Zamora-Muñoz, C.; Soler, J.J.; Bonada, N.; Sáinz-Cantero, C.E.; Alba-Tercedor, J. Changes in Mediterranean High Mountain Trichoptera Communities After a 20-Year Period. Aquat. Sci. 2016, 78, 669–682. [Google Scholar] [CrossRef]
- Parmesan, C.; Root, T.L.; Willig, M.R. Impacts of Extreme Weather and Climate on Terrestrial Biota. Bull. Am. Meteorol. Soc. 2000, 81, 443–450. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Likens, G.E.; Andersen, A.; Bowman, D.; Bull, C.M.; Burns, E.; Dickman, C.R.; Hoffmann, A.A.; Keith, D.A.; Liddell, M.J.; et al. Value of Long-Term Ecological Studies. Austral Ecol. 2012, 37, 745–757. [Google Scholar] [CrossRef]
- Sarkis-Onofre, R.; Catalá-López, F.; Aromataris, E.; Lockwood, C. How to Properly Use the PRISMA Statement. Syst. Rev. 2021, 10, 117. [Google Scholar] [CrossRef]
- Baranov, V.; Jourdan, J.; Pilotto, F.; Wagner, R.; Haase, P. Complex and Nonlinear Climate-Driven Changes in Freshwater Insect Communities over 42 Years. Conserv. Biol. 2020, 34, 1241–1251. [Google Scholar] [CrossRef]
- Souza, N.F.; Leal, J.S.; Tourinho, L.; Farjalla, V.F.; Rocha, D.S.B.; Vale, M.M. Bioindicator Aquatic Insects at Risk from Climate Change in a Biodiversity Hotspot. Sci. Total Environ. 2024, 948, 174824. [Google Scholar] [CrossRef]
- Conti, L.; Schmidt-Kloiber, A.; Grenouillet, G.; Graf, W. A Trait-Based Approach to Assess the Vulnerability of European Aquatic Insects to Climate Change. Hydrobiologia 2014, 721, 297–315. [Google Scholar] [CrossRef]
- Hering, D.; Schmidt-Kloiber, A.; Murphy, J.; Lücke, S.; Zamora-Muñoz, C.; López-Rodríguez, M.J.; Huber, T.; Graf, W. Potential Impact of Climate Change on Aquatic Insects: A Sensitivity Analysis for European Caddisflies (Trichoptera) Based on Distribution Patterns and Ecological Preferences. Aquat. Sci. 2009, 71, 3–14. [Google Scholar] [CrossRef]
- Cid, N.; Ibáñez, C.; Prat, N. Life History and Production of the Burrowing Mayfly Ephoron virgo (Olivier, 1791) (Ephemeroptera: Polymitarcyidae) in the Lower Ebro River: A Comparison after 18 Years. Aquat. Insects 2008, 30, 163–178. [Google Scholar] [CrossRef]
- Dallas, H.; Rivers-Moore, N.A. Critical Thermal Maxima of Aquatic Macroinvertebrates: Towards Identifying Bioindicators of Thermal Alteration. Hydrobiologia 2012, 679, 61–76. [Google Scholar] [CrossRef]
- Durance, I.; Ormerod, S.J. Climate Change Effects on Upland Stream Macroinvertebrates over a 25-Year Period. Glob. Change Biol. 2007, 13, 942–957. [Google Scholar] [CrossRef]
- De Melo, R.R.; Rameh Barbosa, I.M.; Ferreira, A.A.; Lee Barbosa Firmo, A.; Da Silva, S.R.; Cirilo, J.A.; De Aquino, R.R. Influence of Extreme Strength in Water Quality of the Jucazinho Reservoir, Northeastern Brazil, PE. Water 2017, 9, 955. [Google Scholar] [CrossRef]
- Bonacina, L.; Fasano, F.; Mezzanotte, V.; Fornaroli, R. Effects of Water Temperature on Freshwater Macroinvertebrates: A Systematic Review. Biol. Rev. 2023, 98, 191–221. [Google Scholar] [CrossRef]
- Ramulifho, P.A.; Rivers-Moore, N.A.; Dallas, H.F.; Foord, S.H. A Conceptual Framework towards More Holistic Freshwater Conservation Planning through Incorporation of Stream Connectivity and Thermal Vulnerability. J. Hydrol. 2018, 556, 173–181. [Google Scholar] [CrossRef]
- Johnson, M.F.; Albertson, L.K.; Algar, A.C.; Dugdale, S.J.; Edwards, P.; England, J.; Gibbins, C.; Kazama, S.; Komori, D.; MacColl, A.D.C.; et al. Rising Water Temperature in Rivers: Ecological Impacts and Future Resilience. WIREs Water 2024, 11, e1724. [Google Scholar] [CrossRef]
- Hamilton, S.K. Biogeochemical Implications of Climate Change for Tropical Rivers and Floodplains. Hydrobiologia 2010, 657, 19–35. [Google Scholar] [CrossRef]
- Burgmer, T.; Hillebrand, H.; Pfenninger, M. Effects of Climate-Driven Temperature Changes on the Diversity of Freshwater Macroinvertebrates. Oecologia 2007, 151, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Mouthon, J.; Daufresne, M. Effects of the 2003 Heatwave and Climatic Warming on Mollusc Communities of the Saône: A Large Lowland River and of Its Two Main Tributaries (France). Glob. Change Biol. 2006, 12, 441–449. [Google Scholar] [CrossRef]
- Soria, M.; Leigh, C.; Datry, T.; Bini, L.M.; Bonada, N. Biodiversity in Perennial and Intermittent Rivers: A Meta-Analysis. Oikos 2017, 126, 1078–1089. [Google Scholar] [CrossRef]
- Mérigoux, S.; Dolédec, S. Hydraulic Requirements of Stream Communities: A Case Study on Invertebrates. Freshw. Biol. 2004, 49, 600–613. [Google Scholar] [CrossRef]
- Datry, T.; Corti, R.; Philippe, M. Spatial and Temporal Aquatic–Terrestrial Transitions in the Temporary Albarine River, France: Responses of Invertebrates to Experimental Rewetting. Freshw. Biol. 2012, 57, 716–727. [Google Scholar] [CrossRef]
- Matías, L.; Godoy, O.; Gómez-Aparicio, L.; Pérez-Ramos, I.M. An Experimental Extreme Drought Reduces the Likelihood of Species to Coexist Despite Increasing Intransitivity in Competitive Networks. J. Ecol. 2018, 106, 826–837. [Google Scholar] [CrossRef]
- Datry, T.; Larned, S.T.; Scarsbrook, M.R. Responses of Hyporheic Invertebrate Assemblages to Large-Scale Variation in Flow Permanence and Surface–Subsurface Exchange. Freshw. Biol. 2007, 52, 1452–1462. [Google Scholar] [CrossRef]
- Smith, H.; Wood, P.J.; Gunn, J. The Influence of Habitat Structure and Flow Permanence on Invertebrate Communities in Karst Spring Systems. Hydrobiologia 2003, 510, 53–66. [Google Scholar] [CrossRef]
- Jovem-Azevêdo, D.; Bezerra-Neto, J.F.; Azevêdo, E.L.; Gomes, W.I.A.; Molozzi, J.; Feio, M.J. Dipteran Assemblages as Functional Indicators of Extreme Droughts. J. Arid Environ. 2019, 164, 12–22. [Google Scholar] [CrossRef]
- Wood, P.J.; Agnew, M.D.; Petts, G.E. Flow Variations and Macroinvertebrate Community Responses in a Small Groundwater-Dominated Stream in South-East England. Hydrol. Process. 2000, 14, 3133–3147. [Google Scholar] [CrossRef]
- Twardochleb, L.A. Climate Effects on Freshwater Ecological Communities: From Local Species Interactions to Continental Biodiversity Patterns. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2020. [Google Scholar]
- Moi, D.A.; Ernandes-Silva, J.; Baumgartner, M.T.; Mormul, R.P. The Effects of River-Level Oscillations on the Macroinvertebrate Community in a River–Floodplain System. Limnology 2020, 21, 219–232. [Google Scholar] [CrossRef]
- Townsend, C.R.; Hildrew, A.G. Species Traits in Relation to a Habitat Templet for River Systems. Freshw. Biol. 1994, 31, 265–275. [Google Scholar] [CrossRef]
- Bae, M.J.; Park, Y.S. Evaluation of Precipitation Impacts on Benthic Macroinvertebrate Communities at Three Different Stream Types. Ecol. Indic. 2019, 102, 446–456. [Google Scholar] [CrossRef]
- López-de Sancha, A.; Roig, R.; Jiménez, I.; Guasch, H. Impacts of Damming and Climate Change on the Ecosystem Structure of Headwater Streams: A Case Study from the Pyrenees. Inland Waters 2022, 12, 434–450. [Google Scholar] [CrossRef]
- Stubbington, R.; Bogan, M.T.; Bonada, N.; Boulton, A.J.; Datry, T.; Leigh, C.; Vander Vorste, R. Chapter 4.3—The Biota of Intermittent Rivers and Ephemeral Streams: Aquatic Invertebrates. In Intermittent Rivers and Ephemeral Streams; Datry, T., Bonada, N., Boulton, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 217–243. ISBN 978-0-12-803835-2. [Google Scholar]
- Piniewski, M.; Prudhomme, C.; Acreman, M.C.; Tylec, L.; Oglęcki, P.; Okruszko, T. Responses of Fish and Invertebrates to Floods and Droughts in Europe. Ecohydrology 2017, 10, e1793. [Google Scholar] [CrossRef]
- Stubbington, R.; Greenwood, A.M.; Wood, P.J.; Armitage, P.D.; Gunn, J.; Robertson, A.L. The Response of Perennial and Temporary Headwater Stream Invertebrate Communities to Hydrological Extremes. Hydrobiologia 2009, 630, 299–312. [Google Scholar] [CrossRef]
- Boulton, A.J. Parallels and Contrasts in the Effects of Drought on Stream Macroinvertebrate Assemblages. Freshw. Biol. 2003, 48, 1173–1185. [Google Scholar] [CrossRef]
- Chase, J.M. Drought Mediates the Importance of Stochastic Community Assembly. Proc. Natl. Acad. Sci. USA 2007, 104, 17430–17434. [Google Scholar] [CrossRef]
- Chessman, B.C. Relationships between Lotic Macroinvertebrate Traits and Responses to Extreme Drought. Freshw. Biol. 2015, 60, 50–63. [Google Scholar] [CrossRef]
- Gholizadeh, M. Effects of Floods on Macroinvertebrate Communities in the Zarin Gol River of Northern Iran: Implications for Water Quality Monitoring and Biological Assessment. Ecol. Process. 2021, 10, 46. [Google Scholar] [CrossRef]
- Smith, A.J.; Baldigo, B.P.; Duffy, B.T.; George, S.D.; Dresser, B. Resilience of Benthic Macroinvertebrates to Extreme Floods in a Catskill Mountain River, New York, USA: Implications for Water Quality Monitoring and Assessment. Ecol. Indic. 2019, 104, 107–115. [Google Scholar] [CrossRef]
- Pažourková, E.; Křeček, J.; Bitušík, P.; Chvojka, P.; Kamasová, L.; Senoo, T.; Špaček, J.; Stuchlík, E. Impacts of an Extreme Flood on the Ecosystem of a Headwater Stream. J. Limnol. 2021, 80, 1998. [Google Scholar] [CrossRef]
- Woodward, G.; Bonada, N.; Feeley, H.B.; Giller, P.S. Resilience of a Stream Community to Extreme Climatic Events and Long-Term Recovery from a Catastrophic Flood. Freshw. Biol. 2015, 60, 2497–2510. [Google Scholar] [CrossRef]
- Foster, A.D.; Claeson, S.M.; Bisson, P.A.; Heimburg, J. Aquatic and Riparian Ecosystem Recovery from Debris Flows in Two Western Washington Streams, USA. Ecol. Evol. 2020, 10, 2749–2777. [Google Scholar] [CrossRef]
- Bond, N.R.; Downes, B.J. The Independent and Interactive Effects of Fine Sediment and Flow on Benthic Invertebrate Communities Characteristic of Small Upland Streams. Freshw. Biol. 2003, 48, 455–465. [Google Scholar] [CrossRef]
- Snyder, C.D.; Johnson, Z.B. Macroinvertebrate Assemblage Recovery Following a Catastrophic Flood and Debris Flows in an Appalachian Mountain Stream. J. N. Am. Benthol. Soc. 2006, 25, 825–840. [Google Scholar] [CrossRef]
- Croijmans, L.; de Jong, J.F.; Prins, H.H.T. Oxygen Is a Better Predictor of Macroinvertebrate Richness than Temperature—A Systematic Review. Environ. Res. Lett. 2021, 16, 023002. [Google Scholar] [CrossRef]
- Pineda-Pineda, J.J.; Muñoz-Rojas, J.; Morales-García, Y.E.; Hernández-Gómez, J.C.; Sigarreta, J.M. Biomathematical Model for Water Quality Assessment: Macroinvertebrate Population Dynamics and Dissolved Oxygen. Water 2022, 14, 2902. [Google Scholar] [CrossRef]
- Briers, R.A.; Gee, J.H.R.; Geoghegan, R. Effects of the North Atlantic Oscillation on Growth and Phenology of Stream Insects. Ecography 2004, 27, 811–817. [Google Scholar] [CrossRef]
- Dobson, M.; Hildrew, A.G.; Orton, S.; Ormerod, S.J. APPLIED ISSUES Increasing Litter Retention in Moorland Streams: Ecological and Management Aspects of a Field Experiment. Freshw. Biol. 1995, 33, 325–337. [Google Scholar] [CrossRef]
- Cox, B.; Reichelt-Brushett, A.; Taffs, K.; Smith, R. Plasticity of Upper Thermal Limits of Australian Paratya spp. (Decapoda, Atyidae) and Considerations of Climate-Change Adaptation. Mar. Freshw. Res. 2023, 74, 491–499. [Google Scholar] [CrossRef]
- Theodoropoulos, C.; Karaouzas, I.; Stamou, A. Environmental Flows as a Proactive Tool to Mitigate the Impacts of Climate Warming on Freshwater Macroinvertebrates. Water 2021, 13, 2586. [Google Scholar] [CrossRef]
- Jacobus, L.M.; Macadam, C.R.; Sartori, M. Mayflies (Ephemeroptera) and Their Contributions to Ecosystem Services. Insects 2019, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Dočkalová, K.; Stuchlík, E.; Hamerlík, L.; Bitušík, P.; Turek, J.; Svitok, M.; Novikmec, M.; Lackner, R.; Martin, D.; Kopáček, J.; et al. Cold Mountain Stream Chironomids (Diptera) of the Genus Diamesa Indicate Both Historical and Recent Climate Change. Environ. Entomol. 2024, 53, 604–618. [Google Scholar] [CrossRef]
- Manush, S.M.; Pal, A.K.; Chatterjee, N.; Das, T.; Mukherjee, S.C. Thermal Tolerance and Oxygen Consumption of Macrobrachium rosenbergii Acclimated to Three Temperatures. J. Therm. Biol. 2004, 29, 15–19. [Google Scholar] [CrossRef]
- Moulton, S.R., II; Beitinger, T.L.; Stewart, K.W.; Currie, R.J. Upper Temperature Tolerance of Four Species of Caddisflies (Insecta: Trichoptera). J. Freshw. Ecol. 1993, 8, 193–198. [Google Scholar] [CrossRef]
- Ernst, M.R.; Beitinger, T.L.; Stewart, K.W. Critical Thermal Maxima of Nymphs of Three Plecoptera Species from an Ozark Foothill Stream. Freshw. Invertebr. Biol. 1984, 3, 80–85. [Google Scholar] [CrossRef]
- Garten, C.T.; Gentry, J.B. Thermal Tolerance of Dragonfly Nymphs. II. Comparison of Nymphs from Control and Thermally Altered Environments. Physiol. Zool. 1976, 49, 206–213. [Google Scholar] [CrossRef]
- Monk, W.A.; Wood, P.J.; Hannah, D.M.; Wilson, D.A. Macroinvertebrate Community Response to Inter-Annual and Regional River Flow Regime Dynamics. River Res. Appl. 2008, 24, 988–1001. [Google Scholar] [CrossRef]
- Nava, D.; Restello, R.; Hepp, L. Intra- and Inter-Annual Variations in Chironomidae (Insecta: Diptera) Communities in Subtropical Streams. Zoologia 2015, 32, 207–214. [Google Scholar] [CrossRef]
- Carvallo, F.R.; Strickland, B.A.; Kinard, S.K.; Reese, B.K.; Hogan, J.D.; Patrick, C.J. Structure and Functional Composition of Macroinvertebrate Communities in Coastal Plain Streams across a Precipitation Gradient. Freshw. Biol. 2022, 67, 1725–1738. [Google Scholar] [CrossRef]
- Smith, H.; Wood, P.J. Flow Permanence and Macroinvertebrate Community Variability in Limestone Spring Systems. Hydrobiologia 2002, 487, 45–58. [Google Scholar] [CrossRef]
- Santos, J.I.; Silva, C.; Gonçalves, F.J.M.; Pereira, J.L.; Castro, B.B. Macroinvertebrate Community Structure and Ecological Status in Portuguese Streams Across Climatic and Water Scarcity Gradients. Hydrobiologia 2023, 850, 967–984. [Google Scholar] [CrossRef]
- Döll, P.; Schmied, H.M. How Is the Impact of Climate Change on River Flow Regimes Related to the Impact on Mean Annual Runoff? A Global-Scale Analysis. Environ. Res. Lett. 2012, 7, 014037. [Google Scholar] [CrossRef]
- Leigh, C.; Bonada, N.; Boulton, A.J.; Hugueny, B.; Larned, S.T.; Vander Vorste, R.; Datry, T. Invertebrate Assemblage Responses and the Dual Roles of Resistance and Resilience to Drying in Intermittent Rivers. Aquat. Sci. 2016, 78, 291–301. [Google Scholar] [CrossRef]
- Chacón López, L. Variability in Stream Macroinvertebrate Community Composition Along Climate and Flow Permanence Gradients in California. Bachelor’s Thesis, California State University Stanislaus, Turlock, CA, USA, 2021. [Google Scholar]
- Chessman, B.C.; Jones, H.A.; Searle, N.K.; Growns, I.O.; Pearson, M.R. Assessing Effects of Flow Alteration on Macroinvertebrate Assemblages in Australian Dryland Rivers. Freshw. Biol. 2010, 55, 1780–1800. [Google Scholar] [CrossRef]
- Bonada, N.; Dolédec, S.; Statzner, B. Taxonomic and Biological Trait Differences of Stream Macroinvertebrate Communities Between Mediterranean and Temperate Regions: Implications for Future Climatic Scenarios. Glob. Change Biol. 2007, 13, 1658–1671. [Google Scholar] [CrossRef]
- Carey, N.; Chester, E.T.; Robson, B.J. Flow Regime Change Alters Shredder Identity but Not Leaf Litter Decomposition in Headwater Streams Affected by Severe, Permanent Drying. Freshw. Biol. 2021, 66, 1813–1830. [Google Scholar] [CrossRef]
- Vaughn, C.C. Biodiversity Losses and Ecosystem Function in Freshwaters: Emerging Conclusions and Research Directions. BioScience 2010, 60, 25–35. [Google Scholar] [CrossRef]
- Pinna, B.; Laini, A.; Negro, G.; Burgazzi, G.; Viaroli, P.; Vezza, P. Physical Habitat Modeling for River Macroinvertebrate Communities. J. Environ. Manag. 2024, 358, 120919. [Google Scholar] [CrossRef]
- Kinard, S.; Patrick, C.J.; Carvallo, F. Effects of a Natural Precipitation Gradient on Fish and Macroinvertebrate Assemblages in Coastal Streams. PeerJ 2021, 9, e12137. [Google Scholar] [CrossRef] [PubMed]
- Rolls, R.J.; Heino, J.; Ryder, D.S.; Chessman, B.C.; Growns, I.O.; Thompson, R.M.; Gido, K.B. Scaling Biodiversity Responses to Hydrological Regimes. Biol. Rev. 2018, 93, 971–995. [Google Scholar] [CrossRef] [PubMed]
- Haubrock, P.J. Site Characteristics Determine the Prevalence of Extreme Weather Events Affecting Freshwater Macroinvertebrate Communities. Sci. Total Environ. 2024, 950, 175436. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.; Sheldon, F.; Thoms, M.; Stanley, E.H. Problems and Constraints in Managing Rivers with Variable Flow Regimes. In Global Perspectives on River Conservation: Science, Policy and Practice; Boon, P.J., Davies, B.R., Petts, G.E., Eds.; Wiley: Chichester, UK, 2000; pp. 415–430. [Google Scholar]
- Lytle, D.A.; Poff, N.L. Adaptation to Natural Flow Regimes. Trends Ecol. Evol. 2004, 19, 94–100. [Google Scholar] [CrossRef]
- Gibbins, C.N.; Scott, E.; Soulsby, C.; Mcewan, I. The Relationship between Sediment Mobilisation and the Entry of Baetis Mayflies into the Water Column in a Laboratory Flume. Hydrobiologia 2005, 533, 115–122. [Google Scholar] [CrossRef]
- Olsen, D.A.; Townsend, C.R. Flood Effects on Invertebrates, Sediments and Particulate Organic Matter in the Hyporheic Zone of a Gravel-Bed Stream. Freshw. Biol. 2005, 50, 839–853. [Google Scholar] [CrossRef]
- Fisher, S.G.; Gray, L.J.; Grimm, N.B.; Busch, D.E. Temporal Succession in a Desert Stream Ecosystem Following Flash Flooding. Ecol. Monogr. 1982, 52, 93–110. [Google Scholar] [CrossRef]
- Feio, M.J.; Coimbra, C.N.; Graça, M.A.S.; Nichols, S.J.; Norris, R.H. The Influence of Extreme Climatic Events and Human Disturbance on Macroinvertebrate Community Patterns of a Mediterranean Stream over 15 y. J. N. Am. Benthol. Soc. 2010, 29, 1397–1409. [Google Scholar] [CrossRef]
- Smeti, E.; von Schiller, D.; Karaouzas, I.; Laschou, S.; Vardakas, L.; Sabater, S.; Tornés, E.; Monllor-Alcaraz, L.S.; Guillem-Argiles, N.; Martinez, E.; et al. Multiple Stressor Effects on Biodiversity and Ecosystem Functioning in a Mediterranean Temporary River. Sci. Total Environ. 2019, 647, 1179–1187. [Google Scholar] [CrossRef]
- Hannah, L. (Ed.) Climate Change Biology; Academic Press: Boston, MA, USA, 2015; ISBN 978-0-12-420218-4. [Google Scholar]
- Kroll, S.A.; Ringler, N.H.; Costa, M.L.C.C.; Ibanez, J.D.L.H. Macroinvertebrates on the Front Lines: Projected Community Response to Temperature and Precipitation Changes in Mediterranean Streams. J. Freshw. Ecol. 2017, 32, 513–528. [Google Scholar] [CrossRef]
- Figueroa, J.; López-Rodríguez, M.J.; Lorenz, A.; Wolfram, G.; Schmidt-Kloiber, A.; Hering, D. Vulnerable Taxa of European Plecoptera (Insecta) in the Context of Climate Change. Biodivers. Conserv. 2010, 19, 1269–1277. [Google Scholar] [CrossRef]
- Pedreros, P.; Guevara-Mora, M.; Stehr, A.; Araneda, A.; Urrutia, R. Response of Macroinvertebrate Communities to Thermal Regime in Small Mediterranean Streams (Southern South America): Implications of Global Warming. Limnologica 2020, 81, 125763. [Google Scholar] [CrossRef]
- Ferreras-Romero, M. Un Odonato Nuevo Para La Fauna Ibérica, Trithemis annulata (Palisot de Beauvais, 1805) (Anisoptera, Libellulidae). Bol. Asoc. Esp. Entomol. 1981, 4, 191–193. [Google Scholar]
- Ayllon, E.; Ayres, C.; Sastre, P. Primera Cita de Trithemis annulata (Palisot de Beauvois, 1805) (Odonata, Libellulidae) Para Cuenca (Este de España). Bol. Soc. Entomológica Aragon. 2013, 52, 276. [Google Scholar]
- Mezquita Aranburu, I.; Torralba-Burrial, A. Primera Cita de Trithemis annulata (Palisot de Beauvois, 1805) (Odonata, Libellulidae) Para Navarra (Norte de España). Bol. Soc. Entomológica Aragon. 2011, 49, 360. [Google Scholar]
- Betoret, B. Expansion de Trithemis annulata En Europa En Los Años 80 y 90 (Odonata). Bol. Soc. Entomológica Aragon. 2000, 27, 85–86. [Google Scholar]
- Ott, J. Dragonflies and Climatic Change—Recent Trends in Germany and Europe. BioRisk 2010, 5, 253–286. [Google Scholar] [CrossRef]
- Márquez-Rodríguez, J. Trithemis kirbyi ardens (Gerstaecker, 1891) (Odonata: Libellulidae); Datos de Campo Sobre Su Ecología En El Sur de España y Primeros Registros Para La Provincia de Sevilla (España). Métod. Ecol. Sist. 2011, 6, 10–20. [Google Scholar]
- Arias-Real, R.; Gutiérrez-Cánovas, C.; Menéndez, M.; Muñoz, I. Drying Niches of Aquatic Macroinvertebrates Identify Potential Biomonitoring Indicators in Intermittent and Ephemeral Streams. Ecol. Indic. 2022, 142, 109263. [Google Scholar] [CrossRef]
- López-Rodríguez, M.J.; Figueroa, J.; Alba-Tercedor, J. The Life History of Serratella ignita (Poda, 1761) (Insecta: Ephemeroptera) in a Temporary and Permanent Mediterranean Stream. Aquat. Sci. 2009, 71, 179–188. [Google Scholar] [CrossRef]
- Alba-Tercedor, J.; Sainz, M.; Poquet, J.; Rodríguez-López, R. Predicting River Macroinvertebrate Communities Distributional Shifts under Future Global Change Scenarios in the Spanish Mediterranean Area. PLoS ONE 2017, 12, e0167904. [Google Scholar] [CrossRef] [PubMed]
- Acuña, V.; Muñoz, I.; Giorgi, A.; Omella, M.; Sabater, F.; Sabater, S. Drought and Postdrought Recovery Cycles in an Intermittent Mediterranean Stream: Structural and Functional Aspects. J. N. Am. Benthol. Soc. 2005, 24, 919–933. [Google Scholar] [CrossRef]
- Fenoglio, S.; Bo, T.; Cucco, M.; Malacarne, G. Response of Benthic Invertebrate Assemblages to Varying Drought Conditions in the Po River (NW Italy). Ital. J. Zool. 2007, 74, 191–201. [Google Scholar] [CrossRef]
- Bosco Imbert, J.; Gonzalez, J.M.; Basaguren, A.; Pozo, J. Influence of Inorganic Substrata Size, Leaf Litter and Woody Debris Removal on Benthic Invertebrates Resistance to Floods in Two Contrasting Headwater Streams. Int. Rev. Hydrobiol. 2005, 90, 51–70. [Google Scholar] [CrossRef]
- Argerich, A.; Puig, M.; Pupilli, E. Effect of Floods of Different Magnitude on the Macroinvertebrate Communities of Matarranya Stream (Ebro River Basin, NE Spain). Limnetica 2004, 23, 283–294. [Google Scholar] [CrossRef]
- Arscott, D.; Tockner, K.; Ward, J.V. Spatio-Temporal Patterns of Benthic Invertebrates along the Continuum of a Braided Alpine River. Arch. Hydrobiol. 2003, 158, 431–460. [Google Scholar] [CrossRef]
- Vagheei, H.; Laini, A.; Vezza, P.; Palau-Salvador, G.; Boano, F. Climate Change Impact on the Ecological Status of Rivers: The Case of Albaida Valley (SE Spain). Sci. Total Environ. 2023, 893, 164645. [Google Scholar] [CrossRef]
- Cristóbal, E.; Ayuso, S.V.; Justel, A.; Toro, M. Robust Optima and Tolerance Ranges of Biological Indicators: A New Method to Identify Sentinels of Global Warming. Ecol. Res. 2014, 29, 55–68. [Google Scholar] [CrossRef]
- Miliša, M.; Stubbington, R.; Datry, T.; Cid, N.; Bonada, N.; Šumanović, M.; Milošević, D. Taxon-Specific Sensitivities to Flow Intermittence Reveal Macroinvertebrates as Potential Bioindicators of Intermittent Rivers and Streams. Sci. Total Environ. 2022, 804, 150022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).