Abstract
Forest Orchidaceae are important for European temperate forests, yet their distribution and abundance have so far interested limited research. In three pure or mixed silver fir stands in the Foreste Casentinesi National Park (NP) (Northern Apennines, Italy) we analysed how structural traits in mature and old-growth forests affected orchid communities in terms of abundance of the main genera, trophic strategy and rarity in the NP. We established three 20 × 60 m plots to quantify the structure of living and dead tree community, including a set of old-growth attributes connected to large trees, deadwood, and established regeneration. In each plot, we measured the abundance of all orchid species and explored their behaviour according to the trophic strategy (autotrophy/mixotrophy, obligate mycoheterotrophy), rarity within the NP, and threatened status according to the IUCN Red List. We used multivariate ordination and classification techniques to assess plot similarities according to forest structure and Orchid Community and identify the main structural factors related to orchid features. The main structural factors were used as predictors of community traits. Forest composition (i.e., the dominance/abundance of silver fir) affected the presence of the main orchid genera: Epipactis were abundant in silver fir-dominated forests, Cephalanthera in mixed beech and fir forests. Interestingly, Cephalanthera could become limited even in beech-dominated conditions if fir regeneration was abundant and established. Old-growth attributes like the density of deadwood and large tree volume were important determinants of the presence of rare and mycoheterotrophic species. Our results provided a first quantitative description of forest reference conditions to be used in the protection and restoration of threatened and rare orchid species.
1. Introduction
The Orchidaceae is one of the largest plant families globally, with approximately 25,000 to 33,000 species [1,2] distributed across diverse environments worldwide [3]. It is the second-largest plant family in terms of species count, after the Asteraceae [4]. This family includes a significant number of endangered species, particularly among terrestrial orchids [5,6]. Human activities, such as habitat destruction and direct harm to populations, represent a major threat [7,8], while climate change will affect local species distribution [9,10]. Consequently, the conservation of these species and their habitats has become a global priority [11,12].
Forest biodiversity is widely recognised as being shaped by forest structure and management practices [13,14], which are often influenced by varying degrees of human intervention in forest ecosystems [15]. The relationships between forests and orchids have increasingly drawn the attention of researchers, who have explored various ecological aspects of these interactions [16,17,18,19,20]. In the case of temperate terrestrial forest orchids, their growth is known to depend not only on climatic factors and edaphic factors, but also on biotic interactions [21,22,23]. These species depend on mutualistic mycorrhizal associations with fungi to absorb carbon from nearby trees, a process that is essential for their survival [24]. These interactions, whether obligatory or not, play a pivotal role in completing their life cycles and for some species, fungi are indispensable partners throughout all life stages [25,26]. The germination of orchid seeds, due to their lack of reserves, is entirely dependent on the establishment of mycorrhizal associations, which can range from highly specific to less [27]. Subsequently, during growth, plant carbon supplies may rely entirely on mycorrhizae (fully or obligate mycoheterotrophic species) or only partially by combining it with photosynthesis (partially mycoheterotrophic or mixotrophic species) [28]. Furthermore, the degree of mixotrophy can reduce to minimum and definitely shift towards autotrophy under certain conditions [29]. It can therefore be assumed that the more orchids rely on mycorrhizae to compensate carbon deficiencies (e.g., by establishing links with saprotrophic fungi) [30], the more they may be adapted, co-evolved, and specialised for forest ecosystems (e.g., non-photosynthetic orchid species). This underscores the intricate ecological interdependence of orchids within forest ecosystems, as well as the inherent challenges associated with studying these complex relationships.
It is well established that forest orchids are closely linked to light availability within forest ecosystems. Light is one of the primary physical factors influencing orchid distribution and abundance [3,31]. In forests, the amount of light reaching the ground can determine the presence of certain Orchidaceae species over others, depending on their ecology [32]. This phenomenon is linked to the ecological valence of each species with respect to the light factor [33,34,35]. Forest management practices often modify the canopy structure, thereby altering the amount of light that reaches the forest floor. Research indicates that human-induced disturbances, such as selective logging, can positively influence the growth of some forest orchids [36,37,38]. Conversely, studies have shown that practices like clear-cutting can negatively impact shade-tolerant species, potentially leading to their decline [39]. For strictly nemoral species, maintaining a closed canopy is essential for their survival [40]. Natural disturbance and the ecological processes connected to forest succession play a pivotal role in the survival of certain orchid species, yet these dynamics remain insufficiently explored [41,42]. Additionally, forest edge effects have been identified as significant factors influencing orchid distribution and occurrence [43]. Research on the ecology of orchids in complex habitats, such as old-growth forests, has seen some advancement in tropical regions [44,45]. However, this remains a largely underexplored area for terrestrial forest orchids in temperate climates [46].
In the forest ecosystem, other factors can be crucial. Soil characteristics play a fundamental role in influencing the abundance and distribution of Orchidaceae [3,23]. The type of geological substrate is important at both regional and local levels. Changes in the availability of soil resources (like water and nutrients) across different substrates led to variations in the richness and composition of orchid species [47,48]. Soil physical and chemical properties are essential, as well [3,49], e.g., soil moisture and pH at the microsite scale, are of great interest as they not only affect the species composition of orchids, but also their abundance [48]. Furthermore, the organic matter in the soil represents the main source of carbon and nutrients transferred to orchids through their mycorrhizal fungi [50]. It has been observed that the germination rate of orchids is positively correlated with the soil organic matter content [51]. The influence of these and other factors on population dynamics of forest orchids and their potential conservation implications has been only partially explored [21], leaving significant knowledge gaps.
Temperate orchids of open habitats have historically attracted greater attention from researchers, while forest orchids have remained relatively understudied. Genera such as Ophrys and Orchis have been extensively studied, particularly from systematic and ecological perspectives [52,53,54], even at a very fine taxonomic scale to the point of describing several possible hybrids [55,56,57]. Certain forest-specific orchids, especially those that are highly protected and threatened, like Cypripedium calceolus L. [58], have been the focus of numerous studies examining the impact of forest cover on their fitness and population dynamics [38,59,60]. However, other temperate forest orchid species, which face fewer threats, lack legal protection or fall outside designated protected areas, have generally been less studied concerning the effects of changes in forest cover. This trend partly reflects a global research bias favouring open and semi-natural ecosystems [61] and favouring “charismatic” and visually appealing flowering plants, which tend to attract more attention and funding in conservation science [62]. Some European forest orchid species exhibit a paucity of visually appealing morphological and chromatic characteristics, which often leads to their inadvertent neglect by researchers. These observations underscore the necessity for further investigation into this group of species.
In the last decade, a growing interest has focused on Mature and Old-Growth (MOG) forests to identify their potential for biodiversity conservation as well as climate change mitigation [63,64]. Besides primary forests, i.e., naturally-developing ecosystems representing undiscussed biodiversity sanctuaries [65], studying MOG naturalness-related attributes is crucial to better understand the long-term development of forests and highlight pathways to ecological restoration in the so-called proforestation approaches [66]. The old-growth forest status provides unique features in terms of large tree dominance [67] and lifespan [68], deadwood and microhabitats [69], as well as structural complexity [70]. Furthermore, the development of MOG into more advanced structural stages implies the onset of rare and complex patterns [71] and processes [72], favouring habitat conditions fundamental for the survival of highly specialised taxa [73,74]. Again, more research is still needed to fully describe and understand the casual relationships sustaining the presence of highly specialised forest species and the most advanced structural traits.
This research focused on orchid communities in pure and mixed forests with silver fir (Abies alba Mill.) at three sites within the “Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna” (National Park, NP) in the Northern Apennines (Italy). The study aimed to: (i) assess how forest structure, in particular old-growth features, influenced the prevailing traits of the Orchid Community in terms of dominant genera and trophic strategy; (ii) evaluate the ability of selected forest structure indicators to predict the selected traits of the Orchid Community; (iii) provide a first description of the potential reference values of forest structural attributes to be used in species conservation and forest restoration.
2. Materials and Methods
2.1. Study Area
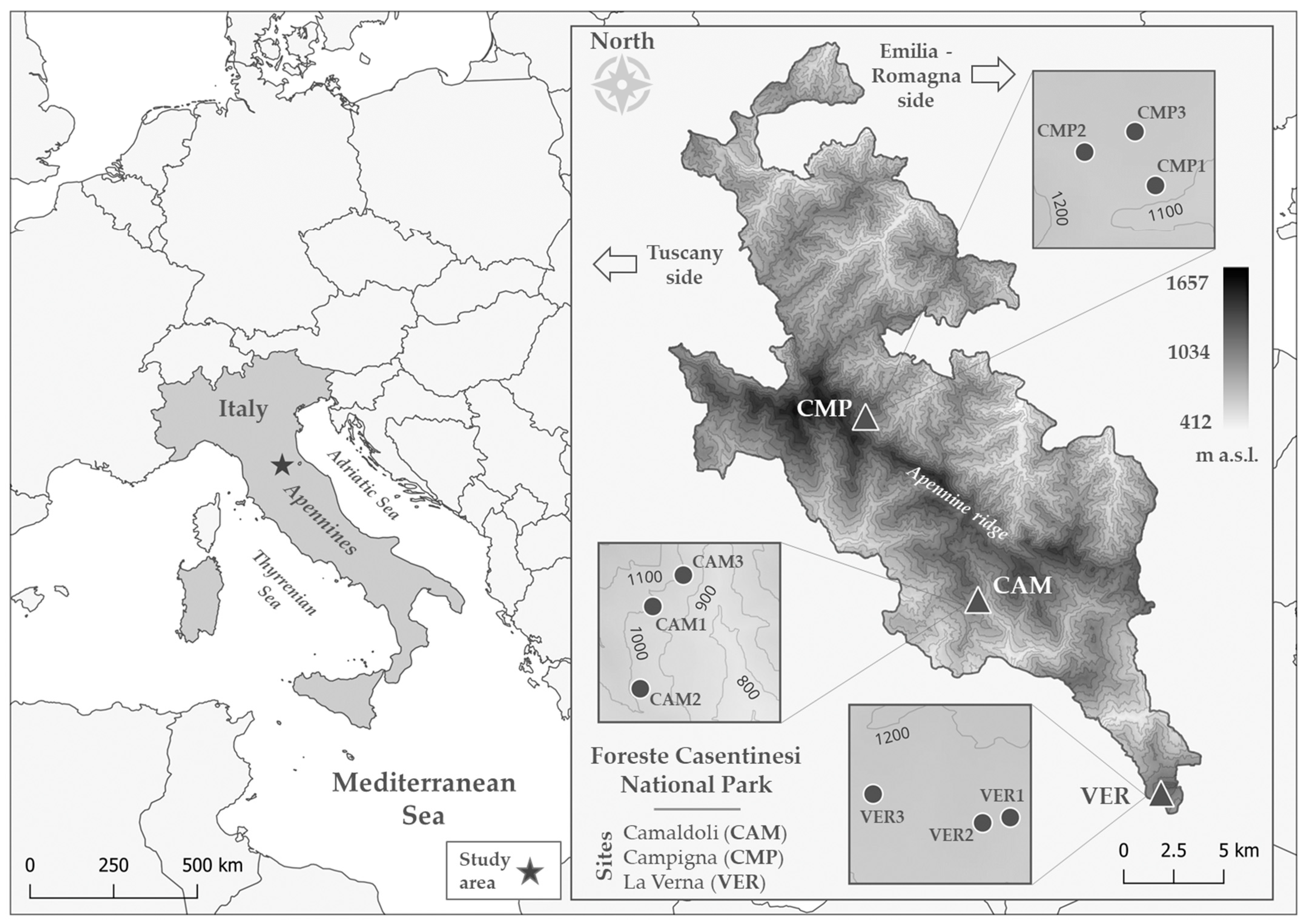
The “Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna” (hereafter referred to as the National Park or NP) is an Italian protected area located in the Northern Apennines, specifically in the “Tosco-Romagnolo” Apennine, in Central Italy (Figure 1). It spans the border between the Emilia-Romagna and Toscana regions, covering parts of the provinces of Forlì-Cesena, Arezzo, and Firenze. The NP encompasses a total area of 36,400 ha. A key feature of the NP is the Apennine ridge, which runs from northwest to southeast, dividing the landscape into two main slopes. The highest points along this ridge are “Monte Falco” Mt. (1657 m a.s.l.) and “Monte Falterona” Mt. (1654 m a.s.l.). The three study sites are situated within the boundaries of the NP (Figure 1, Table 1). The first site, called Campigna (CMP), is located within the province of Forlì-Cesena and represents the northernmost site. Moving to the south, the other two sites are Camaldoli (CAM) and La Verna (VER), in the province of Arezzo. The geology of Campigna and Camaldoli is comparable, characterised by sandstones and arenaceous marls (Middle-Lower Miocene and Palaeogene age, respectively), while La Verna can be distinguished by the presence of Middle-Lower Miocene skeletal limestones and calcarenites [75]. Soil texture and organic matter were, in general, comparable among the sites (Table S1). In all cases, soil texture included mostly sand and, only in CMP, clay content was half of the other sites (Table S1). All soils contained calcium carbonate [75] and had similar amounts of organic matter (Table S1).
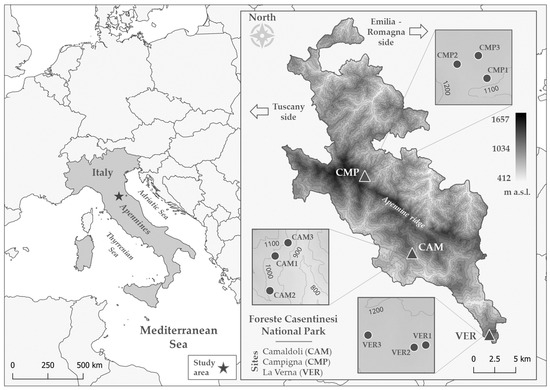
Figure 1.
The location of (left) the study area and (right) the three study sites (triangles; CAM, CMP, VER) with each plot (circles) within the boundary of the National Park.

Table 1.
Summary of site features. Coordinates refer to the WGS84 system. Mean (min–max) values of elevation, slope, and aspect were provided.
According to Pesaresi et al. [76], the areas fell within the temperate macrobioclimate and the temperate oceanic bioclimate, with a slightly semicontinental subtype. The three sites exhibit a humid ombrotype and a supratemperate upper horizon [76]. At the average plot elevation (c. 1090 m a.s.l.), the mean annual precipitation is 1625 mm, and the mean annual temperature is 8.3 °C. From an ecological perspective, Campigna is mainly characterised by a semi-natural forest of fir and beech, interspersed with small areas of conifer reforestations, Camaldoli is dominated by old coniferous reforestations undergoing spontaneous dynamics with patches of semi-natural fir or pure or mixed beech forests, and La Verna is characterised by beech and fir mixed stands [77]. A common feature of the three sites is their status as well-preserved forest which host high levels of specific diversity, comprising taxa often unique to the study areas and rarely found elsewhere in the NP [78,79,80,81,82,83,84,85]. In particular, our study sites fell within or were contiguous to habitats of interest to the Natura 2000 Network, classified under codes 9130 (Asperulo-Fagetum beech forests), 91M0 (Pannonian-Balkanic turkey oak-sessile oak forests), 9220 * (Apennine beech forests with Abies alba and beech forests with Abies nebrodensis), and 9210 * (Apennine beech forests with Taxus and Ilex) [86]. Additionally, these localities host numerous species of community interest, including the beetles Rosalia alpina Linnaeus and Osmoderma eremita Scopoli, restricted to the old-growth forest [87].
Fifty-one species of Orchidaceae, grouped into 17 genera [83,88], are included among the 1172 native taxa recorded in the NP [85]. As a result of the extensive forested area covered by woods and forests in the NP (over 80%), many of the existing orchid species are typical plants of forest environments. For example, species such as Neottia nidus-avis (L.) Rich., Cephalanthera damasonium (Mill.) Druce, and Epipactis helleborine (L.) Crantz occur in over 60% of the NP territory, divided into equal-sized 2 km cells [83]. Some species are even rarer, such as Epipogium aphyllum Sw., Epipactis placentina Bongiorni & Grünanger, and E. greuteri H. Baumann & Künkele [85].
2.2. Field Sampling of Forest Structure and Orchid Species Diversity
The study forests were selected as the best examples of stands with large old fir trees and mature or old-growth structure within the National Park. Once chosen, forest areas were scouted in spring–summer 2024 for the presence of orchid populations. Plot locations were randomly selected among areas within each forest hosting orchid populations with at least 2 stems. This approach was adopted because forest orchid species have a spotted and non-homogeneous distribution, often with only a few individuals. At each site, three replicated 20 m × 60 m plots (1200 m2) were established in forest ecosystems at a comparable elevation (Table 1). Three plots were set as the minimum number of replicates achievable in the three study forests. The number was deemed representative considering the patchy distribution and limited occurrence of Orchidaceae populations. Each plot was set with a short side on the forest margin and then developed with its long side into the forest, parallel to the main slope. Plot coordinate endpoints were collected using a Garmin GPSMAP 66i (Garmin Ltd., Olathe, Kansas). In each plot, we quantified living and dead tree structures. For all living trees with height > 1.3 m and Diameter at Breast Height (DBH) ≥ 2.5 cm, we recorded the species and measured DBH using a caliper. Height (H) was measured with a Haglof Vertex IV on trees sampled to represent 20 cm DBH classes. To quantify the establishment of tree regeneration, we counted the number of all trees with DBH < 12.4 cm and falling in one of the following height (H) classes: 1. <10 cm; 2. 11–50 cm; 3. 51–150 cm; 4. 151–300 cm; 5. >300 cm.
The presence and absolute abundance (i.e., number of stems) of orchid species were recorded for each plot. When it was not possible to identify a specimen at the species or subspecies level (especially in plants without flowers of Cephalanthera and Epipactis), it was assigned to the appropriate higher taxonomic group and labelled as not classified species (NC). The taxonomic nomenclature follows Bartolucci et al. [89].
2.3. Quantifying Forest and Orchid Community Traits
Site-level H-DBH curves were calculated by merging data from the three plots using the Naslund equation [90]. Tree volume was calculated using measured DBH and predicted H according to species allometries available in Tabacchi et al. [91]. The necromass components were divided into Standing Dead Trees (SDTs; DBH ≥ 10 cm) and Coarse Woody Debris (CWD; median diameter D0.5 ≥ 10 cm and length ≥ 1 m). On each SDT, we measured DBH and H, and calculated the volume using the cylinder formula. On each CWD, D0.5 and length served to calculate the volume according to the cylinder formula. Each deadwood piece was assigned to a decomposition stage on a scale from 1 (with intact shape and consistency) to 5 (advanced decomposition with loss of original shape and consistency) according to Rubino and McCarthy [92].
To quantify the degree of old-growthness of each forest plot, we calculated a set of Structural Indicators (SIs) [70,72]: stand structural complexity, measured with the DBH coefficient of variation (CVDBH); established regeneration (frequency of trees with DBH < 12.5 cm); the density of large trees (DBH > 50 cm and DBH > 70 cm), large CWD (D0.5 > 50 or 70 cm), and large SDT (DBH > 50 or 70 cm). Additionally, the mean and density-weighted decay values were calculated for both CWD and SDT.
The Index of Regeneration (IR) was calculated as the weighted sum of regeneration density multiplied by the mean height value of each class (1–5). All indicators were also calculated separately for ABAL (Abies alba), FASY (Fagus sylvatica L.), and OTBR (other broadleaves). All SI values were reported in Table S2.
The forest Orchid Community (OC) dataset comprises 119 records collected across all plots. Orchids were grouped by genus and species. Specific codes were assigned and their main features were synthesised in Table 2. Based on the existing literature, genera and species were classified into two distinct categories according to their primary trophic regime: fully mycoheterotrophic species (code MH) and autotrophic/mixotrophic species (code AUMX). The species classified as fully mycoheterotrophic include Epipogium aphyllum and Neottia nidus-avis [93,94,95]. The species belonging to the genera Cephalanthera, Epipactis, and Dactylorhiza exhibit a more complex situation. These genera include both species in which mixotrophy, to varying degrees, is well documented [26,28,96,97,98], and species that occupy a trophic continuum between autotrophy and mixotrophy, which are still under investigation [99,100,101]. From a distributional perspective, orchid species were classified as widespread (code WIDE) or narrow range and rare (code NARROW) within the study area, according to Pica and Laghi [83]. Additionally, we included the IUCN Red List category assigned to each species according to the global assessment (Epipactis greuteri, E. leptochila, E. purpurata, Neottia nidus-avis), European assessment (Cephalanthera rubra, C. longifolia, C. damasonium, Epipactis helleborine, E. microphylla, Dactylorhiza maculata s.l., Epipogium aphyllum), and Mediterranean assessment (Epipactis exilis) [102]. Finally, the Ellenberg indicator values for each species, as derived from Pignatti et al. [103] and Tichý et al. [104], were also reported (Table 2).

Table 2.
Orchidaceae species recorded in all sites, classified by genus, trophism (MH, Fully Mycoheterotrophs; AUMX, Autotrophs/Mixotrophs), distribution within the National Park (WIDE, Widespread Species; NARROW, Narrow Range and Rare Species), IUCN Red List assessment (LC, Least Concern; NT, Near Threatened; EN, Endangered), Ellenberg indicator values (L, Light; T, Temperature; K, Continentality; F, Moisture; R, Soil Reaction; N, Nutrients). Codes corresponding to the genera and species are provided.
To quantify the Orchid Community features and compare plots, we chose the following indicators: genera and species number; genera and species absolute abundance; genera and species relative abundance; trophic group relative abundance; and distribution range relative abundance. Statistical analyses were performed using the relative abundances of the taxonomic (genus), trophic, and distributional levels, indicated by the letter “p” following the variable (CEPHAp, EPIPAp, NEOTTp; MHp, AUMXp; WIDEp, NARROWp). The relative abundance was calculated as the percentage of the total absolute abundance. Species grouping was necessary due to the frequent zero or low values of some species in several plots. Scarcely represented genera (Epipogium and Dactylorhiza) were included only in trophism and distribution analysis.
2.4. The Relationships Between the Orchid Community and Forest Structure
A Principal Component Analysis (PCA) was used to identify the main Structural Indicators to describe stand/plot differences and identify the candidate predictors for the Orchid Community. The initial set of 102 variables was divided into thematic groups describing tree composition, regeneration, large trees, standing, and lying deadwood (Table S2).
To remove redundant or ineffective variables, we proceed with a two-step method [72]: (i) with the first PCA, we filtered out variables with loading <50% of the absolute maximum axis loading [105,106]; (ii) the remaining variables were passed again through a PCA, to select the best ones per each group/subgroup, i.e., those with the highest loading (satisfying the 50% threshold). In both PCAs, we retained the minimum number of axes explaining ≥2/3 of the overall variance (i.e., 2 [105,106]). The initial set of 102 Structural Indicators was first reduced to 77 and then to 32.
To further illustrate how the plots were grouped according to similarities in either structural or Orchid Community features, we classified them using Ward’s clustering algorithm and Pearson’s distance [72].
To identify potential predictors for orchids, we ran a final PCA incorporating both the selected Structural Indicators (SIs) and Orchid Community descriptors to highlight correlations between the two datasets. The best SIs, i.e., 2–3 per group (Table S2) with the highest loading, were retained as candidate predictors for modelling Orchid Community variables. The selected SIs were used to model their univariate relationship with the Orchid Community variables using linear models (LMs), second-degree polynomial models (Poly2), and nonlinear least-squares models (NLS). The lowest Akaike Information Criterion (AIC) was used for model selection. AIC was also used to explore possible improvements deriving from using a bivariate rather than a univariate model. To avoid multicollinearity among predictors, in bivariate models, we used only variables from orthogonal PCA groups. The goodness-of-fit metrics were reported for the final model, which was also checked for residual analysis [107,108]. Data were analysed and processed using the QGIS 3.32.3 software [109] and R 4.4.1 software [110].
3. Results
3.1. Analysis of Forest Structure, Living and Dead Biomass
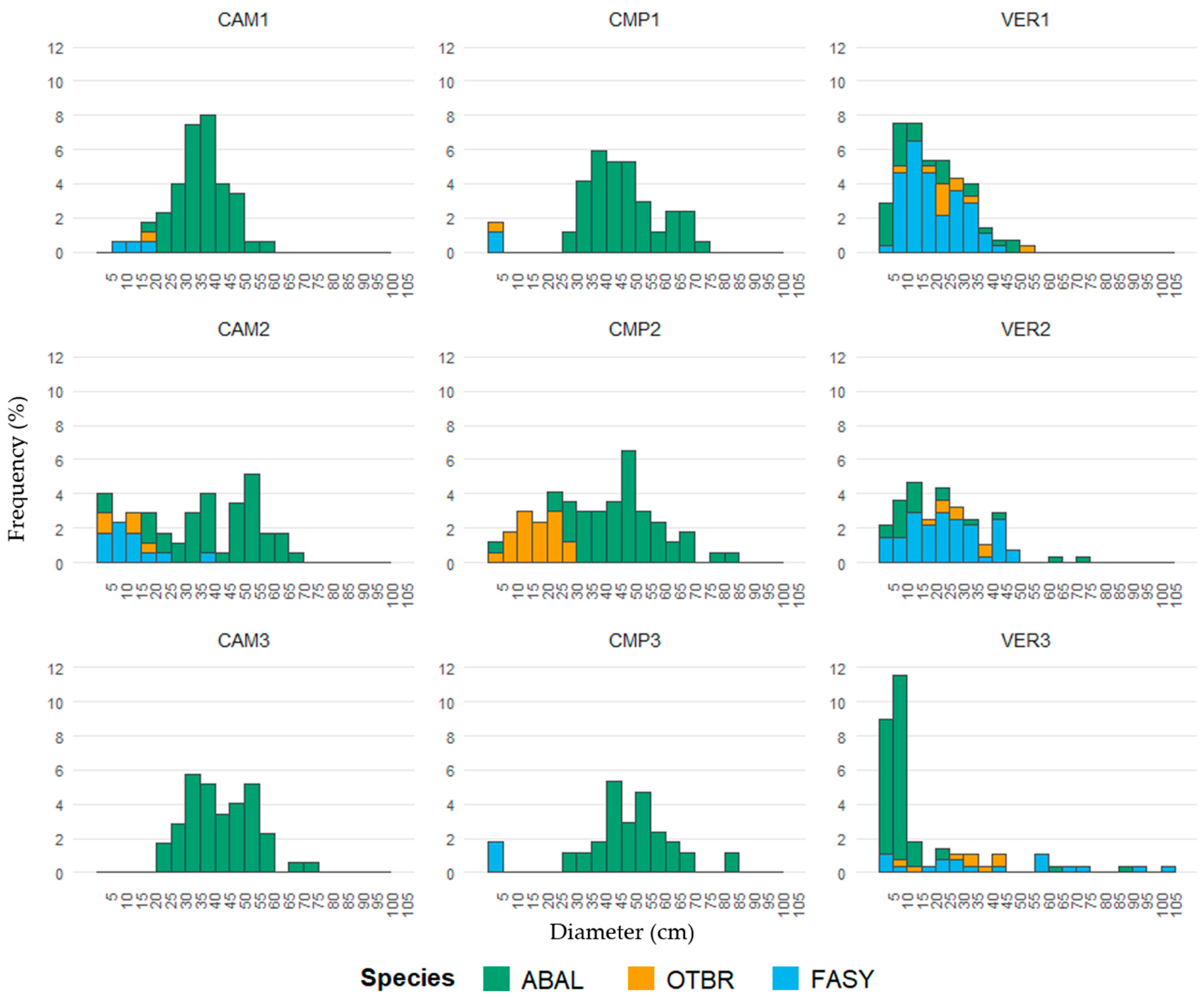
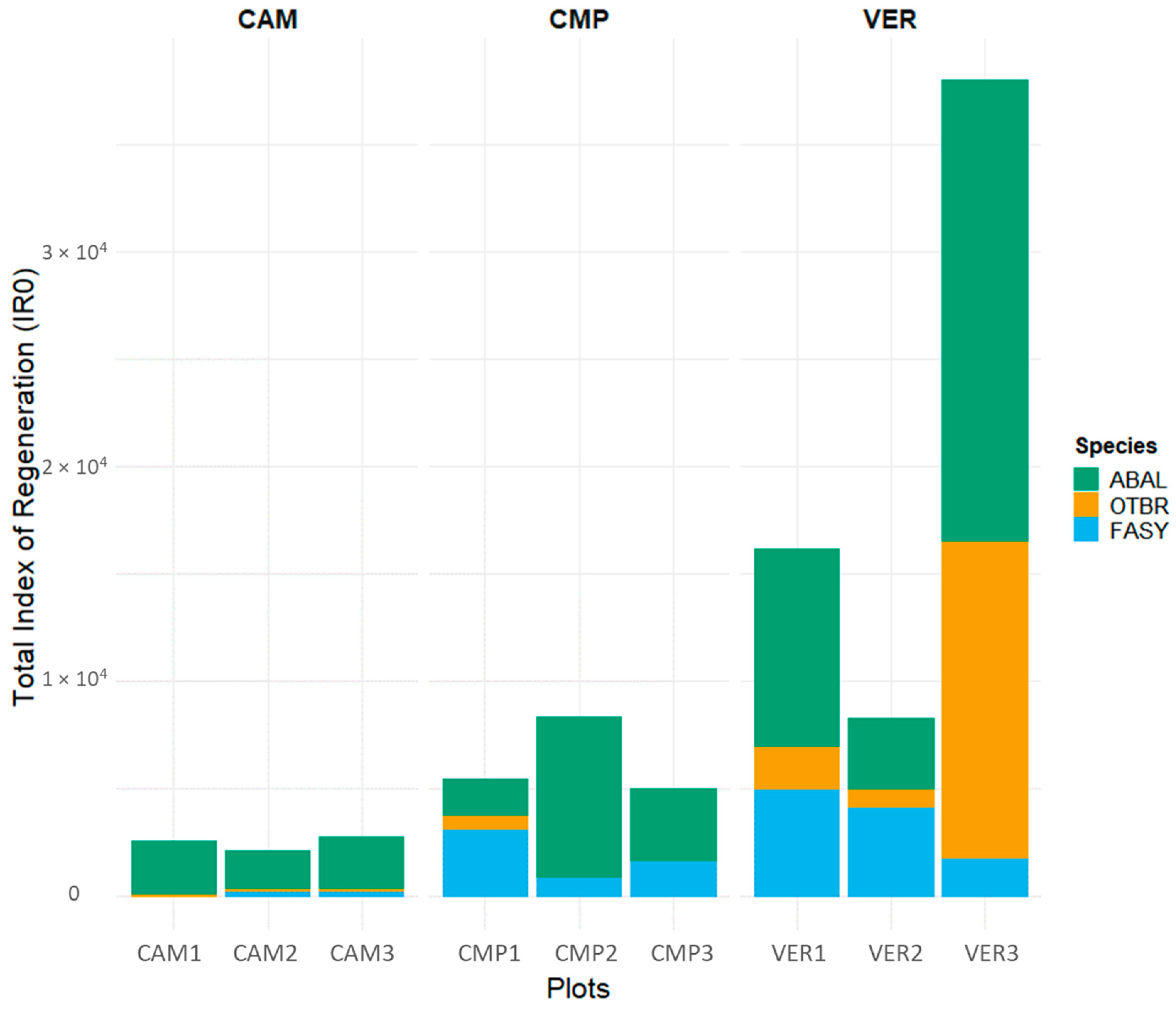
According to their DBH distributions, Campigna (CMP) and Camaldoli (CAM) hosted mainly pure even-aged silver fir stands, with angiosperms locally established in smaller size classes, while La Verna (VER) was an uneven-aged beech-dominated stand with fir and minor presence of other angiosperms (Figure 2, Table 3). Despite its lower stature (35 m compared to 40 m in CAM and CMP), VER had the largest trees (Table 3), either silver fir or beech (Figure 2). Pure forests (CAM and CMP) showed a cathedral-like canopy of silver fir, but locally (i.e., CMP2 and CAM2) plots exhibited a two-layered structure with an understory layer dominated by beech and other angiosperms. In the regeneration layer, the most established species was fir, especially at CAM, while at CMP both the establishment and the presence of beech were higher (Figure 3 and Figure S1). In VER, the multilayered structure of the mixed stand was accompanied by higher values of the regeneration index, with half of IR made of fir and the other half made of angiosperms (Figure 3 and Figure S1). VER3 had the most complex structure, with higher density of both very large and small trees, especially fir (Figure 2), and the highest IR values detected (Figure 3 and Figure S1).
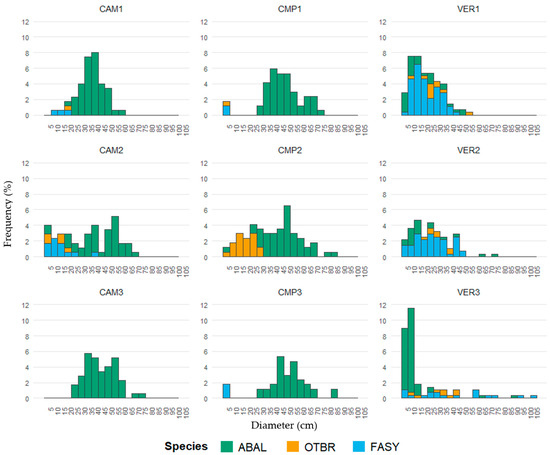
Figure 2.
DBH distribution (relative frequency) by site/plot. The bar colour represents the tree composition (ABAL, Abies alba; FASY, Fagus sylvatica; OTBR, other broadleaves). Next to each site’s code, the numbers indicate the corresponding plots.

Table 3.
Summary of the structural characteristics, Index of Regeneration, deadwood and species composition (ABAL, Abies alba; FASY, Fagus sylvatica; OTBR, other broadleaves) for each site. Values indicate the site mean and, when the parentheses are present, the plot’s minimum and maximum.
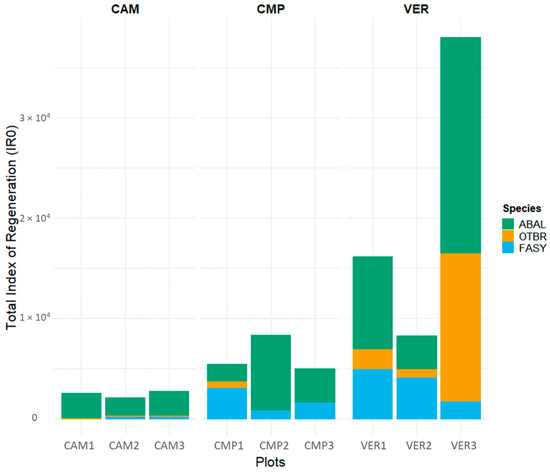
Figure 3.
Total Index of Regeneration (IR0) for different tree compositions (ABAL, Abies alba; FASY, Fagus sylvatica; OTBR, other broadleaves). IR1-5 is separately reported in Figure S1.
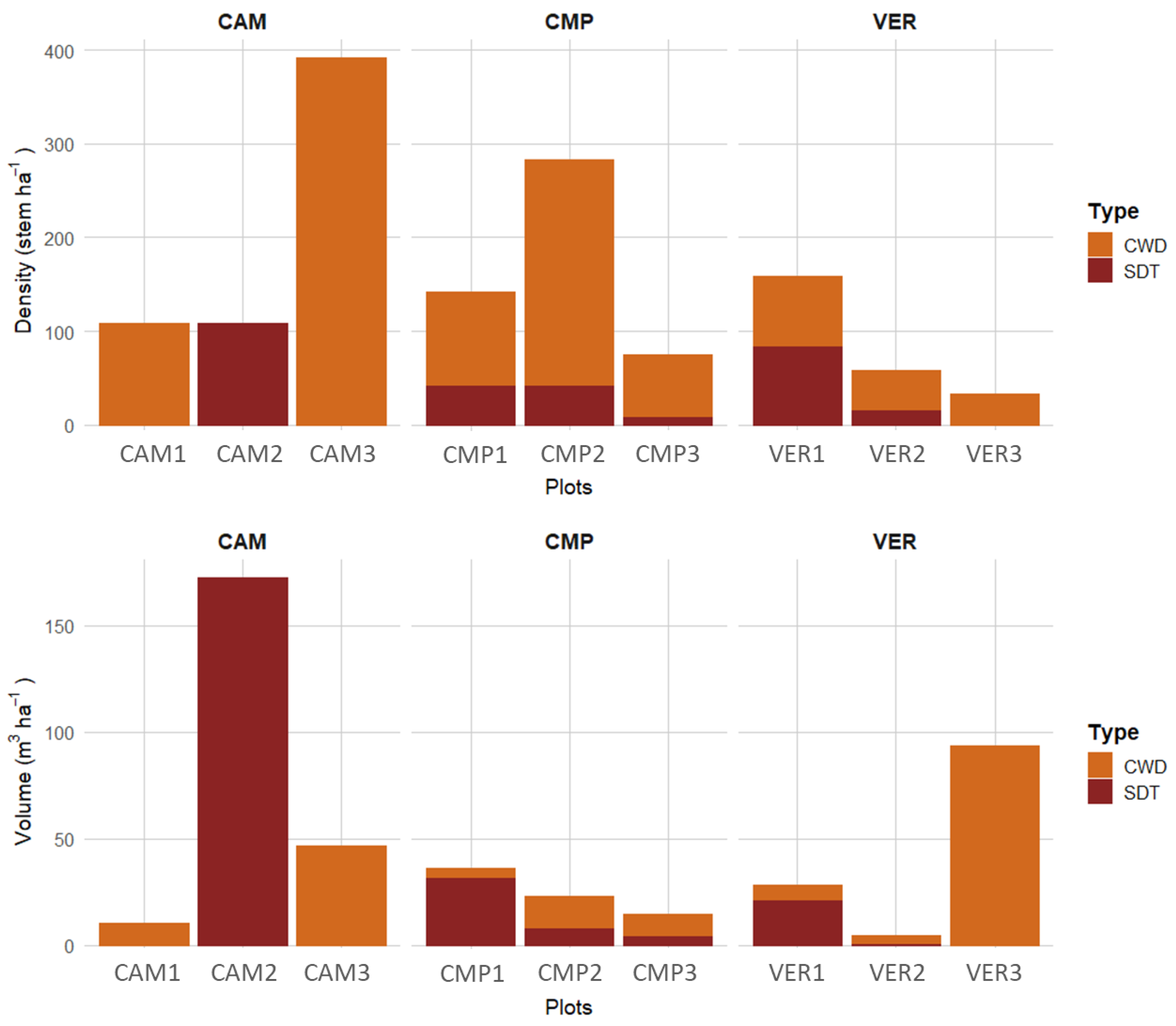
Deadwood presence was highly variable in terms of density and volume between- and within-sites (Figure 4). Deadwood density was on average around 150 elements per ha and less than 48 m3 ha−1, and originated mainly from silver fir at CAM and CMP and beech at VER. CMP showed a high density, but low volume of deadwood (particularly in CMP2), especially CWD. CAM had comparable patterns but can be distinguished for an exceptionally high SDT volume in CAM2, characterised by many large standing dead fir trees. VER3, instead, was distinguished for having a few but large CWD.

Figure 4.
Deadwood density (stem ha−1) and volume (m3 ha−1) across sites and individual plots (CWD, Coarse Woody Debris; SDTs, Standing Dead Trees).
Among all the 102 Structural Indicators (SIs) initially calculated to describe forest structure and their old-growthness, a final set of 32 SIs were selected through the PCA screening (Figure S2) to group plots in terms of similarity in structural features (Figure 5).
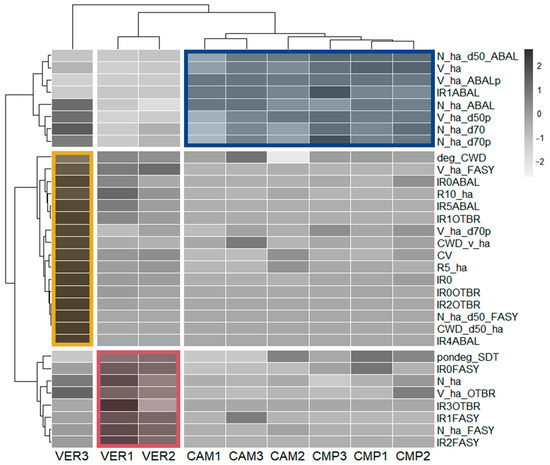
Figure 5.
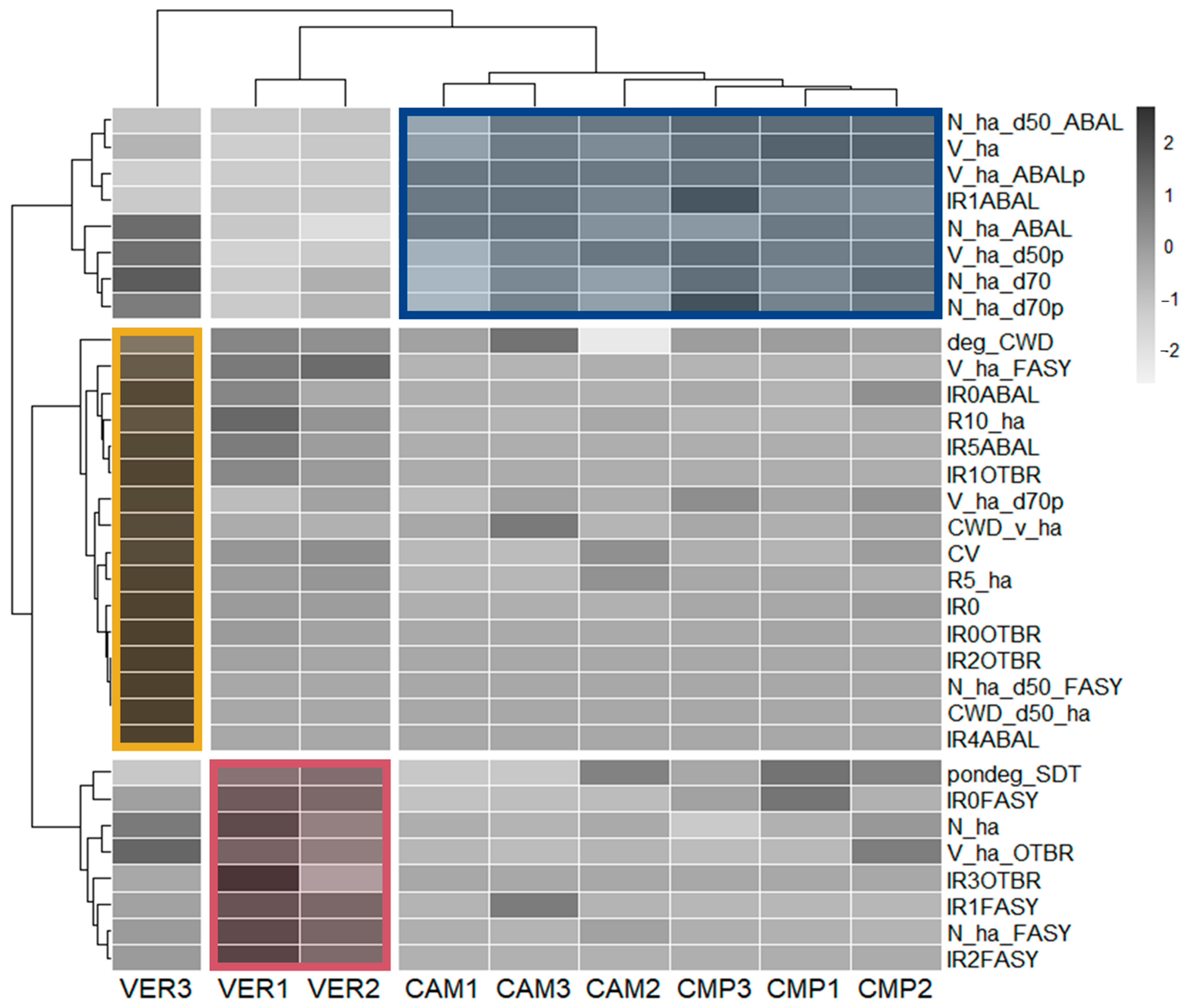
Correlation matrix ordered according to the hierarchical clustering of plots based on selected Structural Indicators (SIs). Boxes delineated the main three clusters grouping the plots: higher old-growthness (yellow; VER3); cathedral-like fir forest (blue; CAM 1-3 and CMP 1-3); mixed beech stands (red; VER 1-2).
One group (yellow), including only VER3, was characterised by beech dominance and the highest levels of structural complexity and old-growthness comprising the DBH coefficient of variation (CV); volume and density of large trees (DBH > 70 cm, FASY with DBH > 50 cm); and CWD volume with advanced decomposition stages. Furthermore, there was a substantial established regeneration of silver fir (IR4 and IR5, R5 and R10) and smaller cohorts of other broadleaf species (IR0OTBR). Plots VER1 and VER2 (red) were characterised by beech abundance (N_ha_FASY) and high biomass of other broadleaves (V_ha_OTBR), alongside younger (IR1 and IR2FASY) and intermediate (IR3FASY) regeneration often dominated by beech. Lastly (blue), all CMP and CAM plots were dominated by silver fir with a cathedral-like structure, i.e., high density of large trees, associated with small silver fir regeneration (IR1ABAL).
3.2. Analysis of Forest Orchid Community
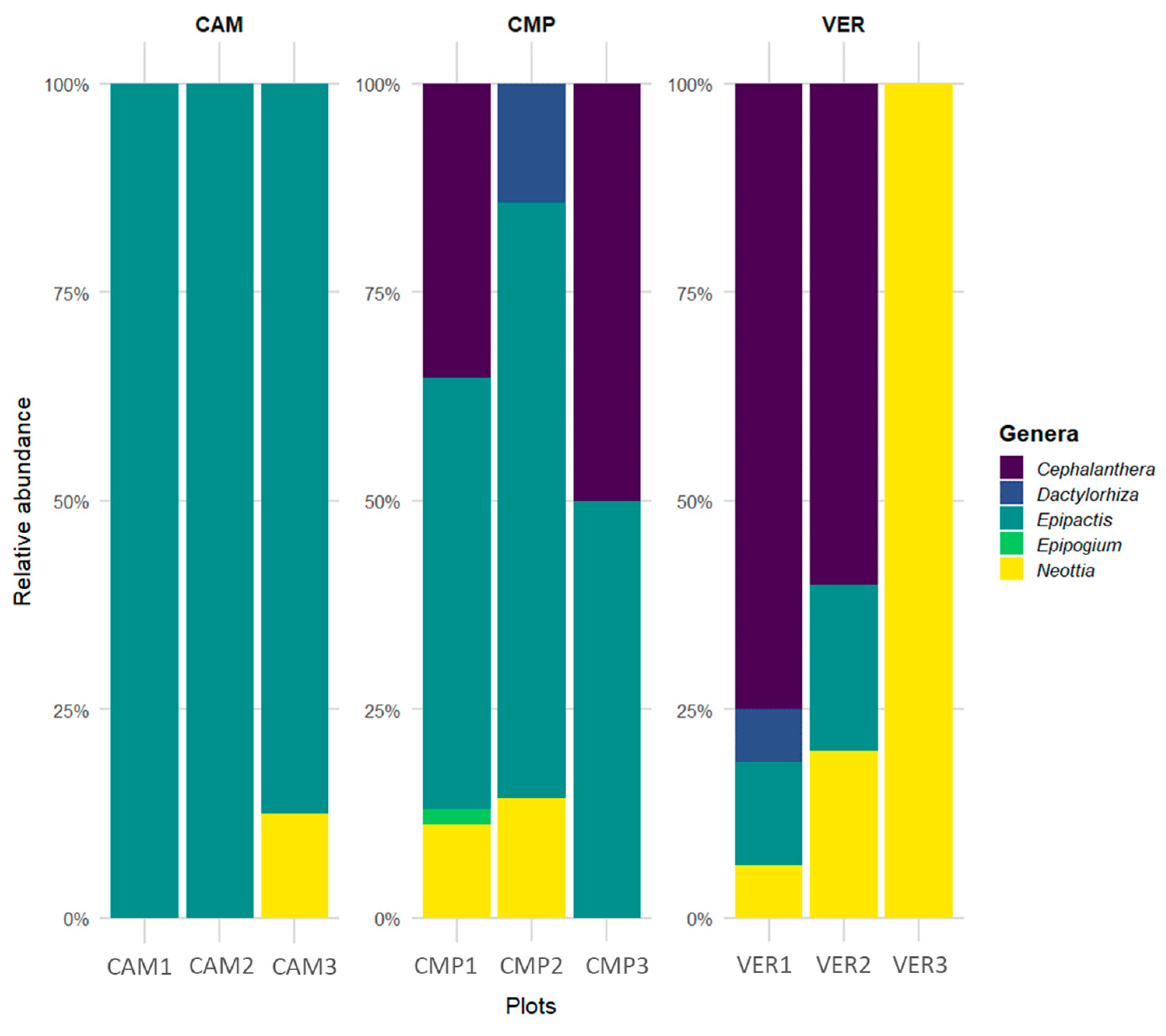
The forest Orchid Community showed that, in terms of relative abundance, the genera Epipactis and Neottia are ubiquitous and the genus Epipogium is exclusive of a single site (CMP1; Figure 6). In terms of species, some are rare and exclusively located in individual plots, i.e., Epipactis microphylla at CAM3; E. leptochila, E. purpurata, Cephalanthera damasonium, and Epipogium aphyllum at CMP1; E. exilis at VER1 (Figure S3). Each plot contained an average of three species, with CMP1 reaching a maximum of eight species. On average, there were two genera per plot, with a maximum of four genera coexisting. The average absolute abundance was around 13 individuals per plot, ranging from 54 individuals (CMP1) to 2 (CMP3).
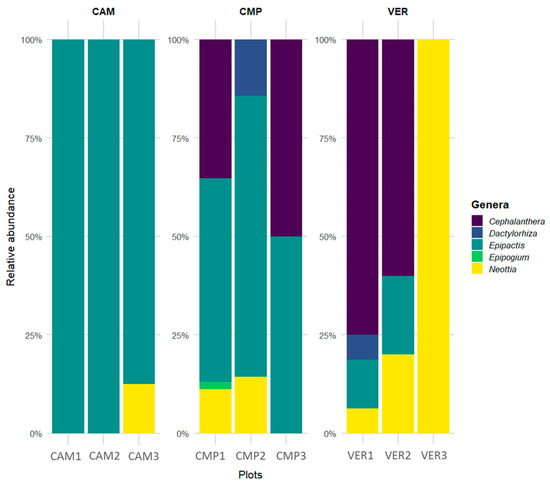
Figure 6.
Relative abundance and distribution of Orchidaceae genera across sites and plots.
The analysis of the Orchid Community in terms of trophic strategy (Figure S4) or distribution range (Figure S5) revealed that the most abundant species were mixotrophic and with a wide range. Mycoheterotrophic and narrow distribution species were present at all sites, often with lower abundance (except for VER3). A correlation matrix, ordered according to hierarchical clustering, was conducted to better understand the grouping of the Orchid Community and plots (Figure 7).
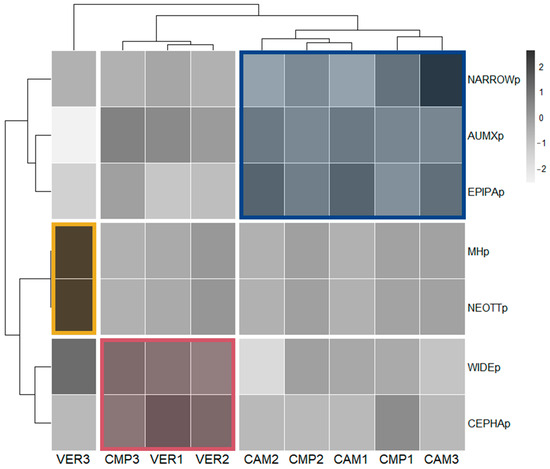
Figure 7.
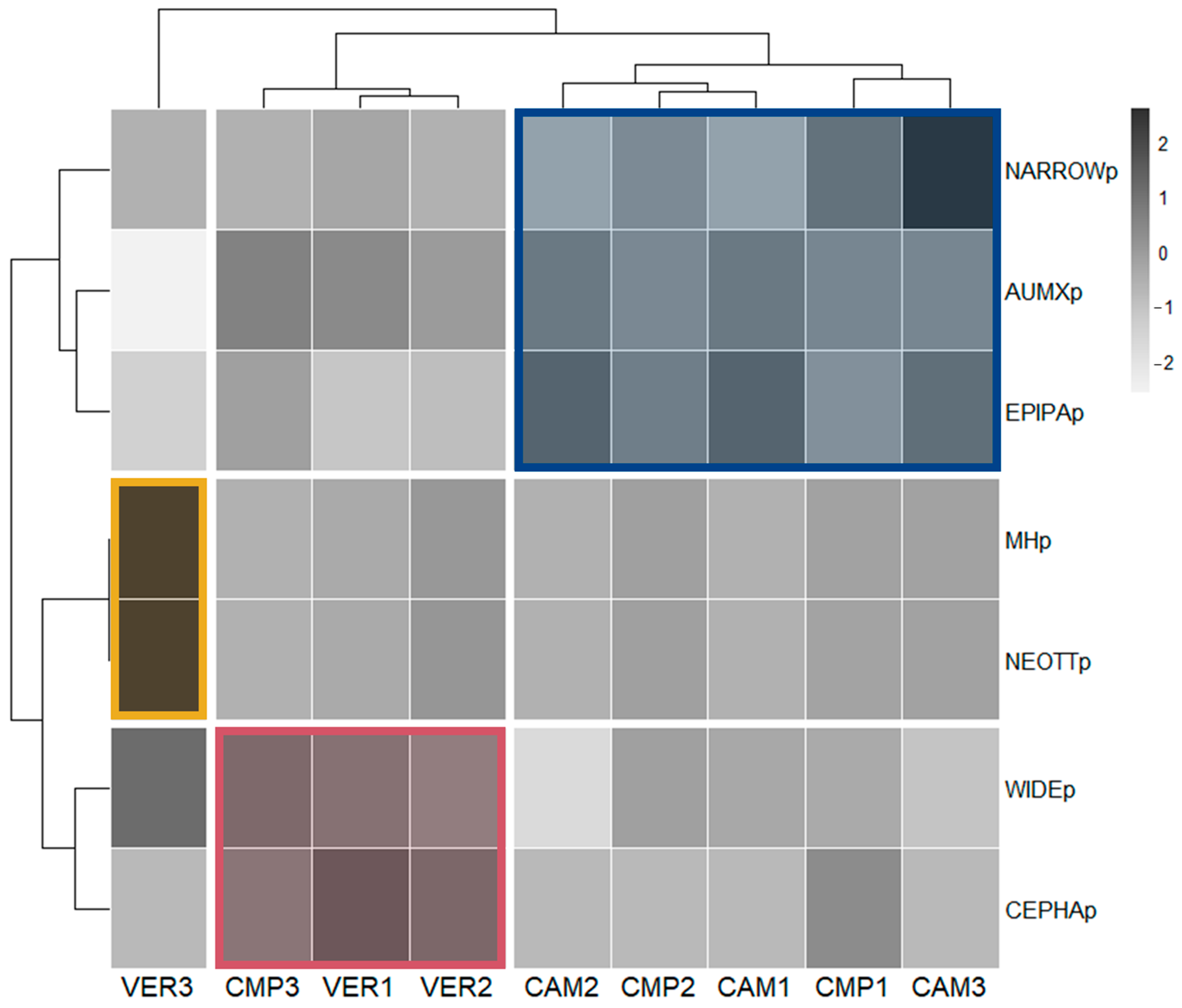
Correlation matrix ordered according to the hierarchical clustering of plots based on the Orchid Community. All orchid variables were calculated as percentage p-values.
Interestingly, plot clustering according to similarity in the Orchid Community well reflected the classification made with the Structural Indicators (SIs; Figure 5). The cluster with VER3 (yellow; old-growthness, beech dominance) had a higher presence of mycoheterotrophs (MHps), all represented by the genus Neottia (NEOTTp). Most plots (blue; CMP 1-2 and CAM 1-3), including cathedral-like silver fir stands, were characterised by a high abundance of the genus Epipactis (EPIPAp) and, only in some plots (especially CAM3), by a higher percentage of narrow range species (NARROWp). In contrast to the SI classification, Orchid Community clustering associated CMP3 (cathedral-like silver fir stands) to VER 1-2 (red; mixed forests with high beech density and regeneration), due to the presence of widely distributed orchids (WIDEp) and the abundance of the genus Cephalanthera (CEPHAp). The last two clusters shared also the highest frequency of autotrophic/mixotrophic species (AUMXp).
3.3. Forest Structure and Orchid Community Relations
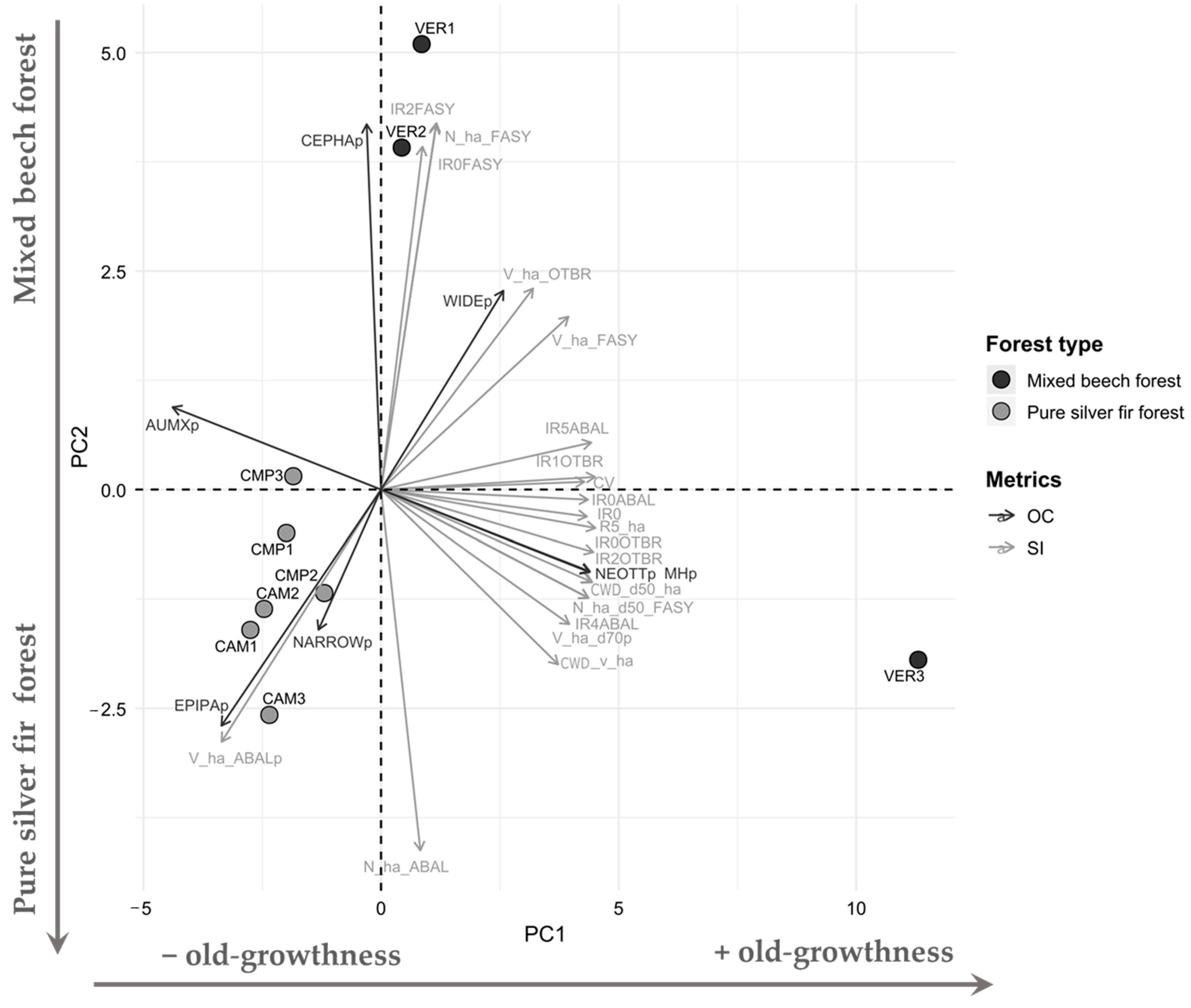
The joint PCA analysis (Figure 8) made on selected Structural Indicators and Orchid Community metrics arranged plots primarily along an increasing gradient of old-growthness (PC1, 64.5% total variance) and then along species composition (PC2, 23.8% total variance). The composition varied from mixed forests dominated by beech to pure silver fir forests. Along the PC1 axis, positive PC1 loadings (VER3) featured higher values of structural complexity and old-growth attributes (e.g., coefficient of variation, deadwood volume, large tree volume) where mycoheterotrophic species (MH), i.e., Neottia (NEOTTp), prevailed. Under these conditions, high broadleaved regeneration was accompanied by advanced silver fir regeneration (Figure 2 and Figure 3), whose shadow depressed the establishment of autotrophic-mixotrophic species (AUMXp). Negative PC1 loadings characterised even-aged, biostatic forests with low regeneration (CMP and CAM plots). On the PC2, these plots with negative loadings were dominated by silver fir with a higher frequency of Epipactis (EPIPAp) and narrow range species (NARROWp). These were juxtaposed to mixed forests with abundant beech and high beech regeneration (positive loadings, VER 1-2), where the genus Cephalanthera (CEPHAp) is widely distributed (WIDEp).
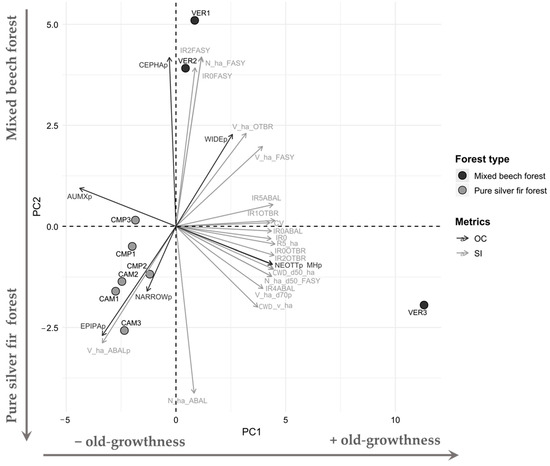
Figure 8.
PCA loadings biplot illustrating the relationship between Structural Indicators (SIs, grey arrows) and Orchid Community (OC, black arrows) metrics. Dots indicate the plots coloured according to the forest type. The PCA explained 88.3% of total variance.
Modelling the Orchid Community According to Forest Features
Among the Orchid Community descriptors, we focused the modelling on those showing good correlations: (1) plot abundance of genus Epipactis (six species; four with narrow distribution; one endangered and one nearly-threatened species); (2) plot abundance of genus Cephalanthera (three species); (3) plot abundance of mycoheterotrophs (two species, one with narrow distribution); (4) plot abundance of narrow distributed species (five species). Widespread and autotrophic/mixotrophic species showed low correlation and were therefore not considered.
The best models between Orchid Community features and forest structure were reported in Table 4, alongside with their goodness-of-fit metrics, while the analysis of regression residuals was reported in Table S3. To predict the relative abundance of Epipactis sp.pl. (Table 4, Model 1) and Cephalanthera sp.pl. (Table 4, Model 2), the best model based on the AIC value was the linear model (LM); to predict the relative abundance of mycoheterotrophic species (Table 4, Model 3), the best model was the nonlinear least-squares regression (NLS); to predict the relative abundance of narrow range species (Table 4, Model 4), the best model was the second-degree polynomial (Poly2). All univariate models performed better than bivariate ones in terms of AIC values, avoiding collinearity among predictors (Table 4).

Table 4.
AIC values of the univariate and multivariate models. Selected models (bold) are linear model (LM), second-degree polynomial model (Poly2), and nonlinear least-squares model (NLS) with the lowest AIC. NA indicates the lack of fit for NLS.
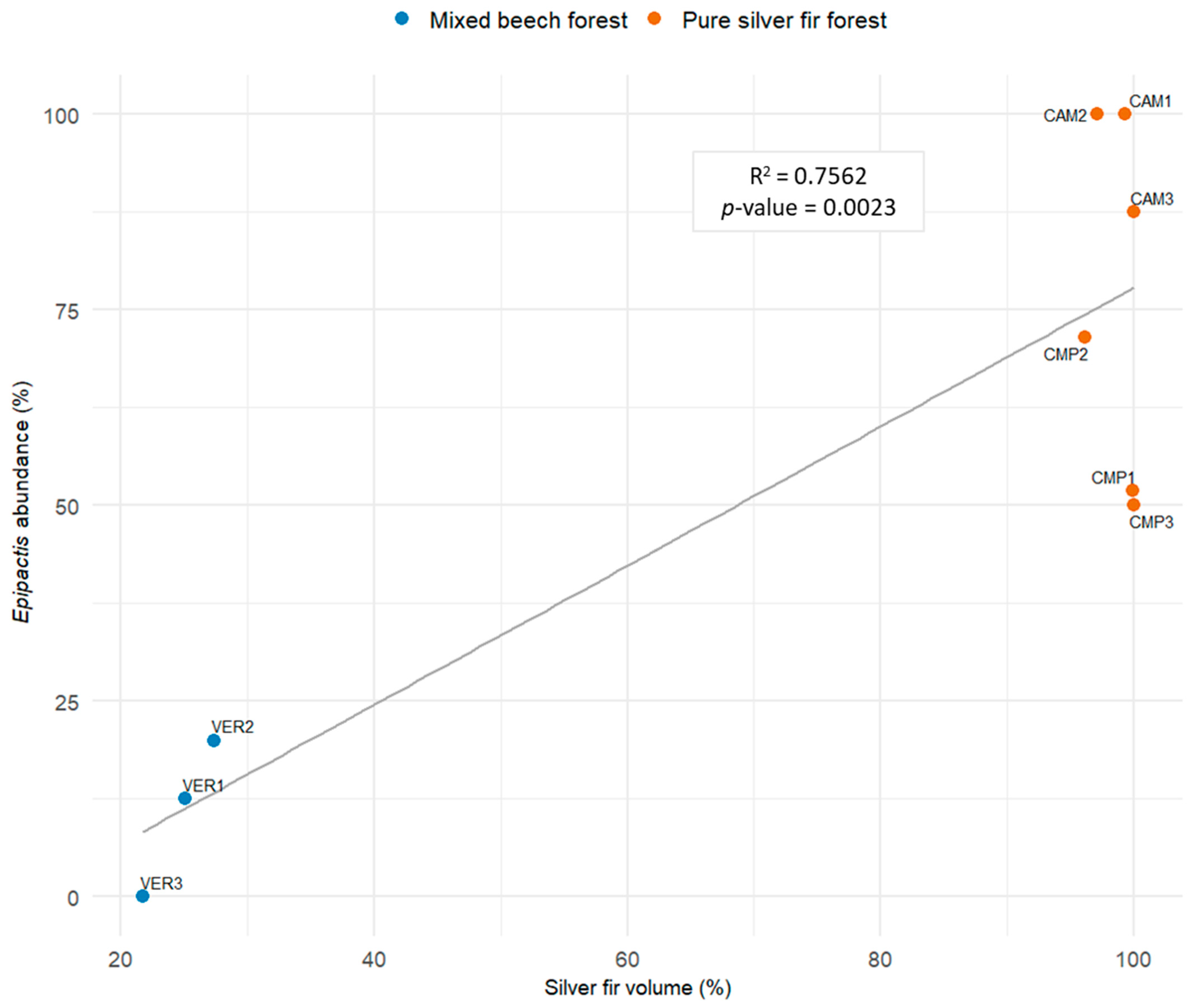
The relative abundance of Epipactis was mainly predicted by silver fir dominance (Table 4, Model 1; Figure 9), confirming the strong dependency of the species of this genus on the conditions found in pure and mature fir stands, where their average abundance was 75% compared to 12% in mixed stands, with less than 30% biomass made of fir (t-test: p-value = 0.002).
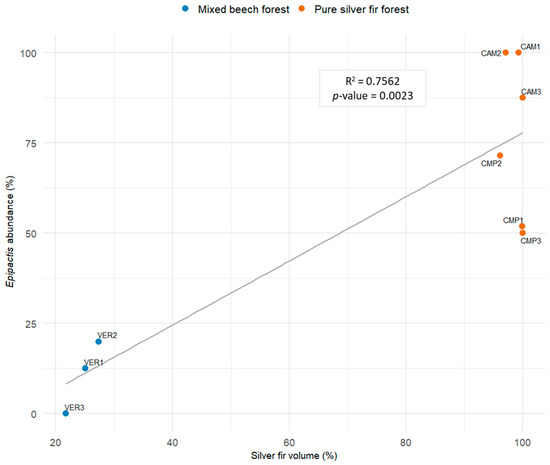
Figure 9.
Linear regression (LM) modelling the relative abundance of the genus Epipactis with silver fir volume at the plot level (Model 1, Table 4).
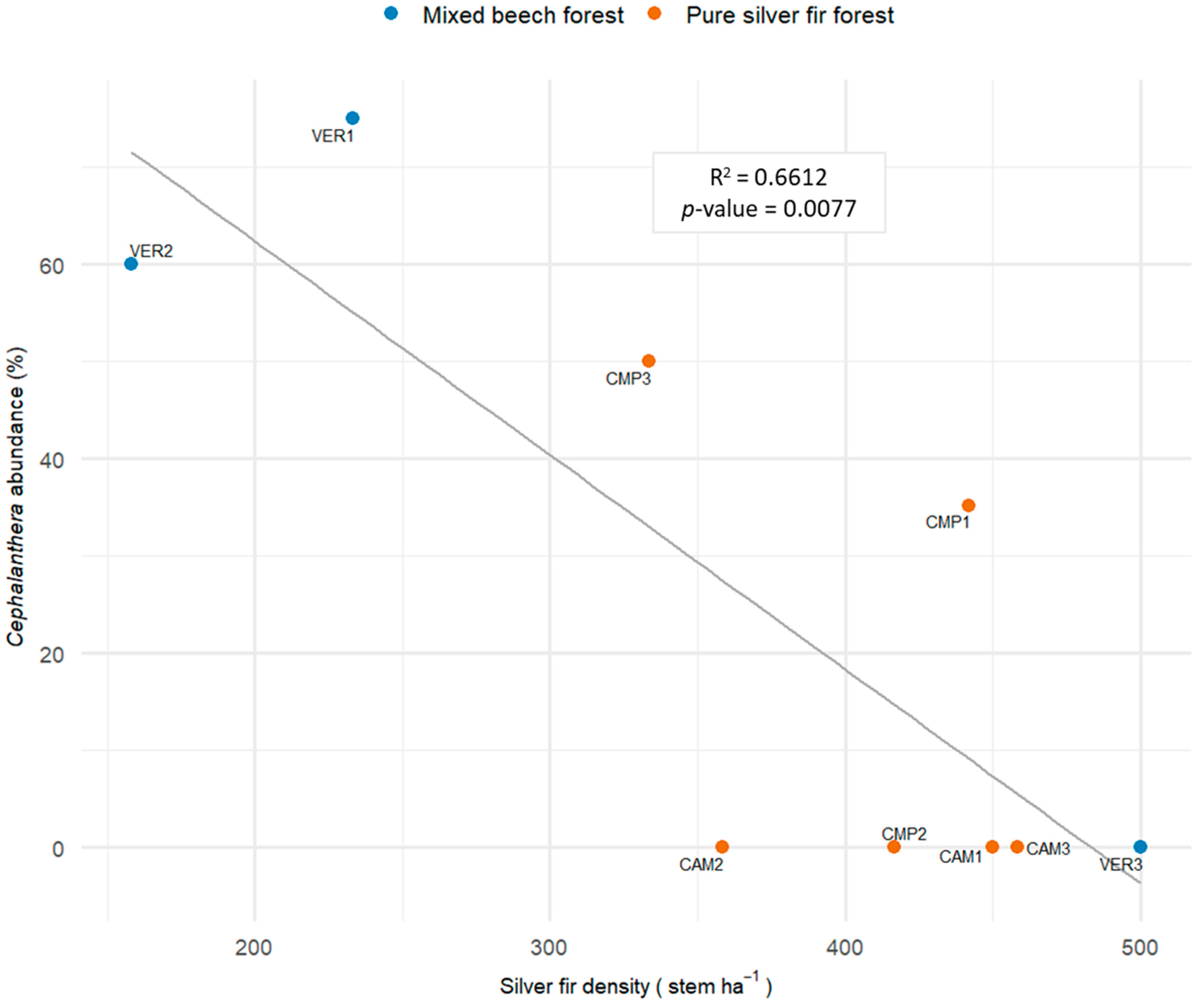
Conversely, the genus Cephalanthera was negatively affected by the density of silver fir (Figure 10). Fir density below 250 stem ha−1 provided increases in Cephalanthera up to 50% of the overall diversity, while values exceeding 350 stem ha−1 Cephalanthera declined below 30%. This pattern held both for mixed forests (VER 1-2) and low-density fir stands (CMP3). Among the mixed forest plot, VER3 was the only one without Cephalanthera; this plot had the highest density of established silver fir regeneration (Figure 2 and Figure 3).

Figure 10.
Linear regression (LM) modelling the relative abundance of genus Cephalanthera and the density of silver fir at the plot level (Model 2, Table 4).
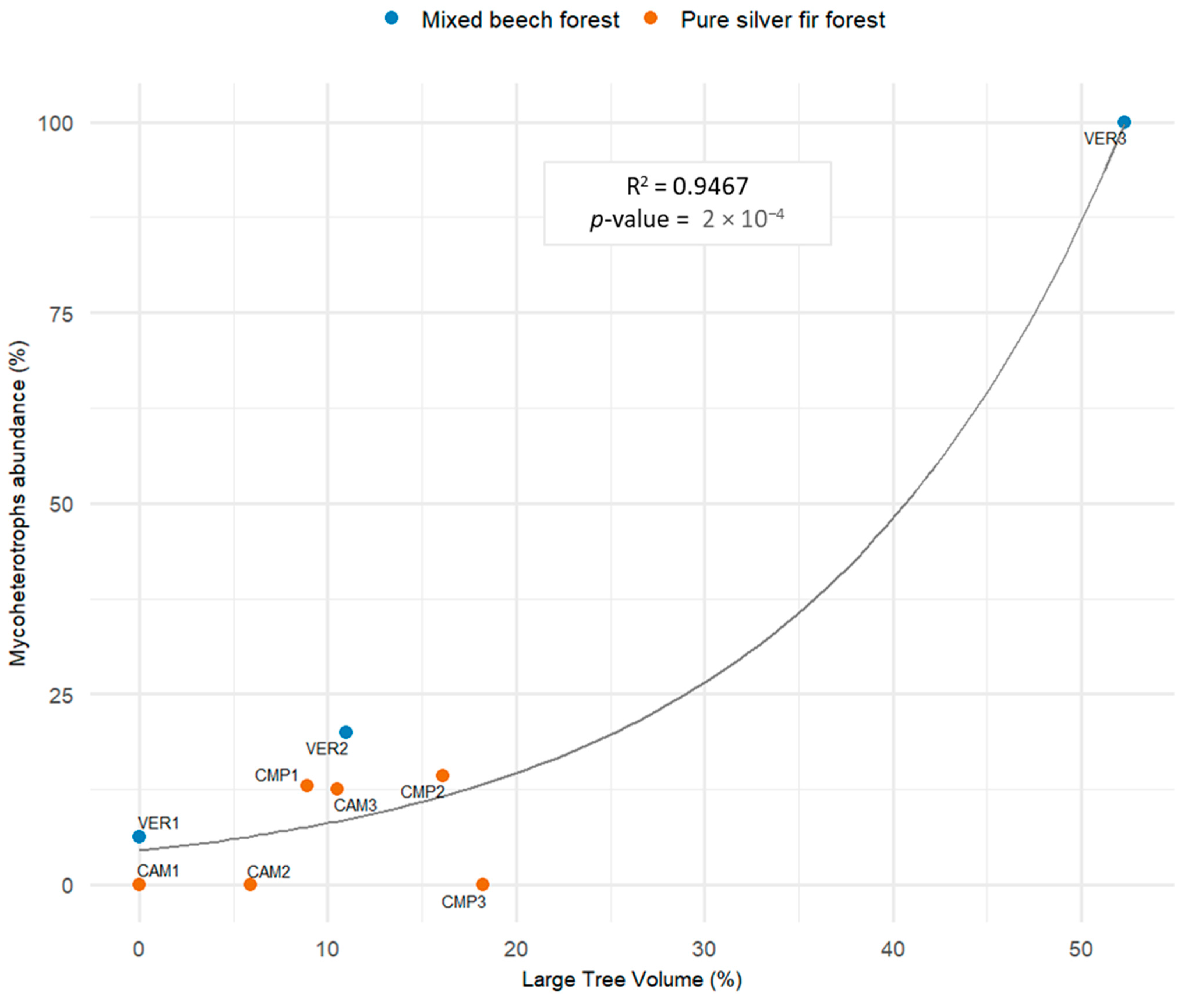
The abundance of fully mycoheterotrophic species (Neottia nidus-avis and Epipogium aphyllum, MH) was directly predicted by the percentage of volume in large trees (DBH > 70 cm) (Figure 11). Mycoheterotrophic species accounted for 0 to 15% of the Orchid Community if the large tree volume was less than 20%, and increased to 100% in the case with the largest shares (50%). Furthermore, their relative abundance is directly related to the coefficient of variation (CV) and the CWD volume (Figure S6).

Figure 11.
Nonlinear least-squares regression (NLS) modelling the relative abundance of fully mycoheterotrophic species (Neottia nidus-avis and Epipogium aphyllum) and the volume of large trees (DBH > 70 cm) (Model 3, Table 4).
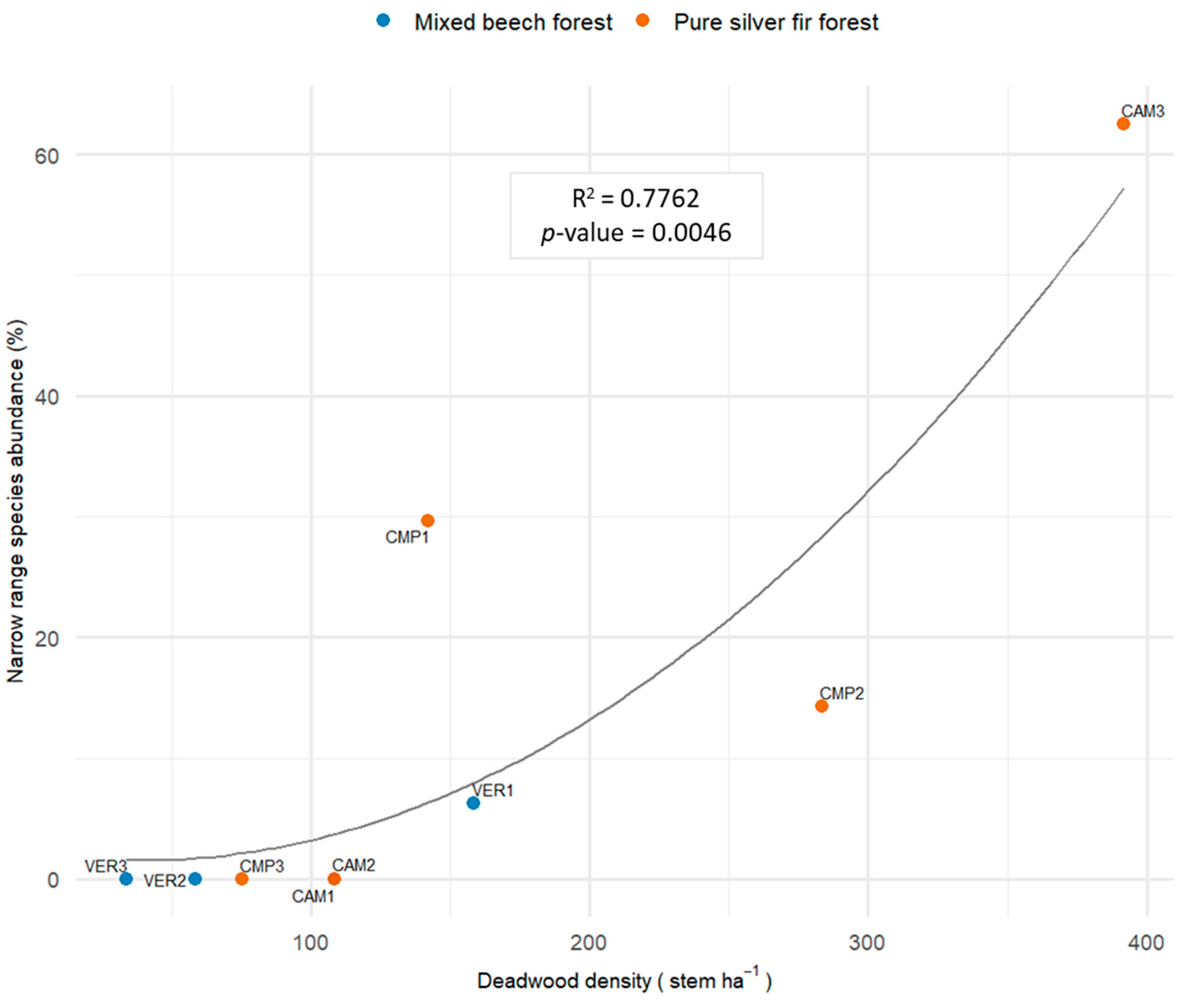
Orchid species with limited distribution (Epipactis exilis, E. greuteri, E. leptochila, E. purpurata, and Epipogium aphyllum) were directly related to the cumulative deadwood density (CWD + SDT; Figure 12). The highest values were reached in pure silver fir forest plots, with the maximum in CAM3.

Figure 12.
Second-degree polynomial (Poly2) regression modelling the relative abundance of narrow range species and the density of total deadwood (CWD + SDT) (Model 4, Table 4).
4. Discussion
Forest structure could be related to the presence of orchid species in various ways, which must be considered individually. The structure of the stands can be broken down and analysed into two main components: species composition and old-growthness. The relations between forest orchids and plots should be examined by comparing structural metrics with the groups of species associated with them.
For soil aspects, although partly comparable among the study sites, local differences in lithology and soil properties may additionally affect the presence of orchids either directly (e.g., pH, Cation Exchange Capacity, nutrients, etc.) [23] or indirectly (e.g., tree height, forest composition) [3]. However, we acknowledge that the lack of enough replications in our study does not allow for fully disentangling the relative effects of tree community vs. soil features on the presence of orchids.
4.1. Tree Composition and Orchid Diversity
Orchid species diversity was highly variable among the plots (one to eight species) but reached high values both in pure and mixed silver fir forests. In particular, one pure silver fir plot (CMP1) hosted the highest number of orchid species found (8). In general, beech forests host most of the European forest orchid species [3,111], but a significant number can also be present in conifer forests [3]. It has been observed that, in contexts dominated by broadleaf trees, forest orchids avoid conifer-dominated zones [112], attributing their absence to substrate acidification caused by needle litter [112,113]. The high widespread presence especially of Epipactis sp.pl. in our silver fir-dominated sites is therefore noteworthy.
The abundance of the main genera of our Orchid Community (Epipactis and Cephalanthera, 9/12 species found) was anticorrelated and highly dependent on tree composition and light availability. Epipactis was the main genus in silver fir-dominated cathedral-like forests, reaching 100% when fir exceeded 90% dominance and preferring even-aged, biostatic tree communities without established regeneration and relatively scarce light conditions. Similar to other forest orchid species, Epipactis responds differently to light conditions [3]. Canopy closure has been observed to reduce the reproductive success of E. helleborine [114], even if it is possible that shaded conditions promote the production of heavier seeds, and therefore higher seedling survival rates [115]. Epipactis species are also associated with a wide range of fungal species [26,29,98,116,117,118]. The initial germination process of Epipactis does not necessarily depend on the presence of specific symbionts, and Epipactis species with different ecological requirements share similar mycorrhizal partners during germination [119]. A high presence of ectomycorrhizal symbionts associated with silver fir has been confirmed, even in very small areas [120]. Furthermore, large, mature plants of different species can promote high diversity in fungal communities associated with their roots [121], even if simply co-occurring in nearby habitats [119].
Cephalanthera species responded positively to conditions characterised by greater light availability associated to structural heterogeneity and the dominance of beech both in the dominant and regeneration layer, with scarce fir regeneration. In southeastern England, C. damasonium was able to colonize rapidly young beech plantations [122]. C. longifolia has high photosynthetic efficiency and can benefit from higher light intensity conditions [123]. In C. damasonium, carbon gain can increase by 52% when transitioning from a more illuminated forest habitat (open pine forest) to a darker one (dark beech forest) [124,125,126]. Increased light exposure can lead Cephalanthera species (C. damasonium and C. longifolia) to transition from partially mycoheterotrophy to autotrophy, thereby balancing the use of naturally available carbon [127]. Light response can also affect physiological and reproductive efficiency [3], i.e., reductions in dormancy and the number of flowers due to low light conditions (C. longifolia [128,129]) or the reduced fruit set rates caused by dense tree and shrub cover (C. rubra [130]).
4.2. Trophic Strategies in Orchids and Old-Growth Attributes
Fully mycoheterotrophic species increased with old-growth features, i.e., higher dominance of large trees (DBH > 70 cm), deadwood (CWD) volume, and structural complexity. One of the best predictive factors was the amount of downed deadwood, confirming that these orchids depend on ground deadwood for carbon [30,117,131], differing in their life cycle from orchids associated with leaf-litter-decaying fungi [132]. Mycoheterotrophs were favoured by deadwood in the form of large logs with advanced decomposition stages (Table 5), which confirmed their preference for habitat integrity [133]. Their presence in several pure fir plots and their prevalence in multilayered forests with established fir regeneration and lower light availability at the ground (i.e., VER3) confirmed the shade tolerance of mycoheterotrophic species [134]. VER3 differed in many aspects from all other plots. However, we primarily interpreted its features to the structural variability associated to old-growth forests and their mosaic-like, multilayered structure and, therefore, we did not consider it as an outlier to be excluded from the analyses.

Table 5.
Reference values of forest attributes (rounded) related to the occurrence and relative abundance of the studied Orchid Community.
Rare species within the NP responded positively to the overall deadwood density (CWD + SDT), with substantial increases when the density was >200 elements per ha. The total deadwood counted per ha includes both downed deadwood (CWD) and standing deadwood (SDT) at various decay stages. Contrarily to mycoheterotrophs, rare species were more related to medium/small size deadwood (no need of large, decomposed logs). Almost all rare orchids were autotrophic/mixotrophic Epipactis, but included also the mycoheterotroph Epipogium aphyllum (only at CMP1). Overall, rare species are present in cathedral-like even-aged silver fir forests (promoting Epipactis) with prevailing self-thinning/self-pruning producing small deadwood (promoting Epipogium aphyllum). Mycorrhizal formation within specific microhabitats may be difficult and influence orchid abundance [135] and the rarity of terrestrial orchids is both the cause and consequence of high mycorrhizal specialisation [136]. Nevertheless, the biological differences of our rare taxa demonstrated the need for further research to investigate separately and with more detail the ecology of rare species.
According to the variety of the ecology and the trophic strategies possessed by orchid species in forests with silver fir in the National Park, an ensemble of approaches must be implemented to maximize the conservation and promote the restoration of orchid diversity. Specifically, maintaining or restoring tree communities with mixed rather than pure silver fir, with even-aged or complex structure, and with different degrees of old-growth attributes may lead to the presence and abundance of some taxa rather than others (Table 5).
The presence of mycoheterotrophs (MHs) may be promoted by allowing for the development of old-growth conditions that generate large trees (>stems ha−1), a tree community with high coefficient of variation (>) and, especially, substantial deadwood accumulation (>m3 ha−1) (Table 5). Rare species (localised distribution within the NP) are mainly represented by Epipactis sp.pl. and are, therefore, favoured in cathedral-like pure silver fir forests (770–1140 m3 ha−1, 90–100% silver fir). These conditions were also associated to a high density of small deadwood (medium/small size CWD and SDT, c. 200–400 per ha), a factor probably favouring the mycoheterotroph Epipogium aphyllum. Such conditions also allowed for hosting the only two globally threatened (e.g., Epipactis microphylla, E. greuteri) orchids in our dataset, stressing once again the importance of conserving pure mature fir stands.
As a preferential habitat for Epipactis sp.pl., which comprised 50% of the orchid species found in this study, pure silver fir mature forests conservation is key in the area. The other main genus, Cephalanthera, needed instead opposite conditions, i.e., lower silver fir density (<350 stems ha−1 focus on composition), as well as a significant proportion of beech in the canopy and in the regeneration layer. Forests with a more complex, multilayered structure with sufficient light availability would promote the presence of Cephalanthera, especially in areas where Cephalanthera species are threatened [137,138].
4.3. Recommendations
To maximize the overall biodiversity of the Orchid Community, both in terms of abundance and dominance, the forest structure should include a mosaic of different structural phases. In fact, fir-dominated mature patches favour Epipactis, self-thinning forest patches with small deadwood favour Epipogium, uneven-aged patches dominated by angiosperms favour Cephalanthera and, finally, degradation patches with dominant tree mortality and large deadwood favour Neottia. Such a structure characterizes natural temperate forests with a mixed disturbance regime characterised by gap-dynamics, that could ideally benefit the entire ecological spectrum of the orchid diversity described [139]. In any case, the conservation of orchid communities is closely tied to natural forest processes and the preservation of older forest areas, and the attributes associated to forest age both in mature and old-growth tree communities, such as self-thinning or snag/log abundance [41].
In summary, localised changes in the vertical structure and texture of forests can profoundly impact the presence of certain orchid species. Implementing targeted management practices may facilitate the abundance of specific forest orchid taxa, depending on conservation goals. This highlights valuable opportunities for future research that could refine forest management practices, emphasising the preservation of species that are critically threatened at both European and global scales.
However, it must be considered that the study of forest orchids is highly limited by their rarity and spotty distribution. Moreover, forests left to regenerate naturally are increasingly threatened by human intervention, making it difficult to understand their dynamics [61]. Further in-depth case studies from different environments are highly required to adequately document the effects of forest structure on orchid communities [38,39]. The availability of larger samples exploring wider environmental gradients will help in addressing with more confidence the interplay among forest structure and site factors (such as soil, temperature, light, etc.) [21,23] and providing our results with more generality. In particular, the use of quantitative results in ecological restoration projects as the one reported here must carefully assess possible alterations by silvicultural activities to the forest microclimate and soil and, consequently, to the Orchid Community. Providing recommendations in these cases is challenging because they must be based on the most objective criteria. Also, the risks connected to future climate change impacts must be monitored in situ to determine the long-term efficacy of any action [17]. In this context, given their unique trophic role within ecosystems and their sensitivity to drought, orchid taxa may represent important indicators of climate change impacts on forest ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/land14030579/s1. Figure S1: Indices of Regeneration (IR1-5); Figure S2: PCA of Structural Indicators (SIs); Figure S3: Orchid species abundance; Figure S4: Trophic regime abundance; Figure S5: NP distribution abundance; Figure S6: Mycoheterotrophs model regressions; Table S1: Soil texture and carbon content; Table S2: List of Structural Indicators (SIs); Table S3: Residual validation tests.
Author Contributions
Conceptualization, A.D.F. and A.P.; methodology, A.D.F. and A.P.; formal analysis, A.P.; writing—original draft preparation, A.D.F. and A.P.; writing—review and editing, A.D.F., A.P., S.M., K.C., B.S. and P.L.; supervision, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
A.D.F. was funded by the Agritech National Research Centre (European Union Next-GenerationEU PNRR M4C2-I1.4 Code: CN00000022) and the Ministry of University and Research (MUR) “Departments of Excellence” (Law 232/2016) Project 2023–2027 “Digital, Intelligent, Green and Sustainable (D.I.Ver.So).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Foreste Casentinesi, Monte Falterona e Campigna National Park Authority for their logistical and technical support and the Raggruppamento Carabinieri Biodiversità—Reparto Biodiversità of Pratovecchio, as the managing and authorizing entity Camaldoli and Campigna Biogenetic Natural Reserves. The authors would also like to express their gratitude to all the surveyors who contributed to data collection, listed in alphabetical order: Capodicasa, G.; Doria, A.; Lupoletti, J.; Milandri, M.; Pica, Al.; Ricci, R.; Salvaneschi, P.; Sayssa, T.; Vela, D. The research activities were conducted as part of the first author (A.P.) Ph.D. project (supervisors: S.M. and B.S.; co-supervisor: K.C.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- GBIF Secretariat. Orchidaceae. GBIF Backbone Taxonomy. Checklist Dataset. Available online: https://www.gbif.org/species/7689 (accessed on 16 January 2024).
- Vitt, P.; Taylor, A.; Rakosy, D.; Kreft, H.; Meyer, A.; Weigelt, P.; Knight, T.M. Global Conservation Prioritization for the Orchidaceae. Sci. Rep. 2023, 13, 6718. [Google Scholar] [CrossRef] [PubMed]
- Djordjević, V.; Tsiftsis, S. The Role of Ecological Factors in Distribution and Abundance of Terrestrial Orchids. In Orchids Phytochemistry, Biology and Horticulture: Fundamentals and Applications; Mérillon, J.-M., Kodja, H., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–72. ISBN 978-3-030-38392-3. [Google Scholar]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; van den Berg, C.; Schuiteman, A. An Updated Classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial Orchid Conservation in the Age of Extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef]
- Kumar, J.; Katoch, D.; Thakur, A.; Pathania, A.; Anand, A.; Choudhary, K.; Shelja, A. Comprehensive Review on Threats and Conservation Status of Orchids. J. Appl. Biol. Biotechnol. 2024, 12, 43–47. [Google Scholar] [CrossRef]
- Wraith, J.; Pickering, C. Quantifying Anthropogenic Threats to Orchids Using the IUCN Red List. Ambio 2018, 47, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jacquemyn, H.; He, X.; Chen, W.; Huang, Y.; Yu, S.; Lu, Y.; Zhang, Y. The Impact of Human Pressure and Climate Change on the Habitat Availability and Protection of Cypripedium (Orchidaceae) in Northeast China. Plants 2021, 10, 84. [Google Scholar] [CrossRef]
- Kolanowska, M.; Michalska, E. The Effect of Global Warming on the Australian Endemic Orchid Cryptostylis leptochila and Its Pollinator. PLoS ONE 2023, 18, e0280922. [Google Scholar] [CrossRef]
- Pica, A.; Vela, D.; Magrini, S. Forest Orchids under Future Climate Scenarios: Habitat Suitability Modelling to Inform Conservation Strategies. Plants 2024, 13, 1810. [Google Scholar] [CrossRef]
- Gale, S.W.; Fischer, G.A.; Cribb, P.J.; Fay, M.F. Orchid Conservation: Bridging the Gap between Science and Practice. Bot. J. Linn. Soc. 2018, 186, 425–434. [Google Scholar] [CrossRef]
- Li, S.; Liang, C.; Deng, S.; Chen, C.; Yuan, L.; Liu, Z.; Wu, S.; Lan, S.; Tang, Z.; Liu, Z.; et al. Comparison of Orchid Conservation Between China and Other Countries. Diversity 2024, 16, 692. [Google Scholar] [CrossRef]
- Lelli, C.; Bruun, H.H.; Chiarucci, A.; Donati, D.; Frascaroli, F.; Fritz, Ö.; Goldberg, I.; Nascimbene, J.; Tøttrup, A.P.; Rahbek, C.; et al. Biodiversity Response to Forest Structure and Management: Comparing Species Richness, Conservation Relevant Species and Functional Diversity as Metrics in Forest Conservation. For. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Bricca, A.; Bonari, G.; Padullés Cubino, J.; Cutini, M. Effect of Forest Structure and Management on the Functional Diversity and Composition of Understorey Plant Communities. Appl. Veg. Sci. 2023, 26, e12710. [Google Scholar] [CrossRef]
- Ledig, F.T. Human Impacts on Genetic Diversity in Forest Ecosystems. Oikos 1992, 63, 87–108. [Google Scholar] [CrossRef]
- Migenis, L.E.; Ackerman, J.D. Orchid—Phorophyte Relationships in a Forest Watershed in Puerto Rico. J. Trop. Ecol. 1993, 9, 231–240. [Google Scholar] [CrossRef]
- Besi, E.E.; Mustafa, M.; Yong, C.S.Y.; Go, R. Deforestation Impacts on Diversity of Orchids with Inference on the Conservation Initiatives: Malaysia Case Study. Bot. Rev. 2023, 89, 386–420. [Google Scholar] [CrossRef]
- Besi, E.E.; Mustafa, M.; Yong, C.S.Y.; Go, R. Habitat Ecology, Structure Influence Diversity, and Host-Species Associations of Wild Orchids in Undisturbed and Disturbed Forests in Peninsular Malaysia. Forests 2023, 14, 544. [Google Scholar] [CrossRef]
- Mirioba, J.N.; Emitaro, W.; Obwanga, B.; Gaya, H.; Leley, N.; Otuoma, J.; Maina, J.M.; Kawaka, F. Orchid Species Diversity across a Forest Disturbance Gradient in West Mau Forest, Kenya. PLoS ONE 2024, 19, e0307887. [Google Scholar] [CrossRef]
- Awasthi, M.; Thapa, S.; Awasthi, B.; Lim, C.R.; You, Y.H.; Chung, K.W. Diversity Patterns of Epiphytic Orchids Along Elevation in the Mountains of Western Nepal. Plants 2024, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Djordjević, V.; Tsiftsis, S. Patterns of Orchid Species Richness and Composition in Relation to Geological Substrates. Wulfenia 2019, 26, 1–21. [Google Scholar]
- Hemrová, L.; Kotilínek, M.; Konečná, M.; Paulič, R.; Jersáková, J.; Těšitelová, T.; Knappová, J.; Münzbergová, Z. Identification of Drivers of Landscape Distribution of Forest Orchids Using Germination Experiment and Species Distribution Models. Oecologia 2019, 190, 411–423. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Jakovljević, K.; Stevanović, V. Patterns of Distribution, Abundance and Composition of Forest Terrestrial Orchids. Biodivers. Conserv. 2020, 29, 4111–4134. [Google Scholar] [CrossRef]
- Bidartondo, M.I.; Read, D.J. Fungal Specificity Bottlenecks during Orchid Germination and Development. Mol. Ecol. 2008, 17, 3707–3716. [Google Scholar] [CrossRef]
- Selosse, M.A.; Weiß, M.; Jany, J.L.; Tillier, A. Communities and Populations of Sebacinoid Basidiomycetes Associated with the Achlorophyllous Orchid Neottia Nidus-Avis (L.) L.C.M. Rich. and Neighbouring Tree Ectomycorrhizae. Mol. Ecol. 2002, 11, 1831–1844. [Google Scholar] [CrossRef]
- Selosse, M.A.; Faccio, A.; Scappaticci, G.; Bonfante, P. Chlorophyllous and Achlorophyllous Specimens of Epipactis microphylla (Neottieae, Orchidaceae) Are Associated with Ectomycorrhizal Septomycetes, Including Truffles. Microb. Ecol. 2004, 47, 416–426. [Google Scholar] [CrossRef]
- Waud, M.; Busschaert, P.; Lievens, B.; Jacquemyn, H. Specificity and Localised Distribution of Mycorrhizal Fungi in the Soil May Contribute to Co-Existence of Orchid Species. Fungal Ecol. 2016, 20, 155–165. [Google Scholar] [CrossRef]
- Abadie, J.-C.; Püttsepp, Ü.; Gebauer, G.; Faccio, A.; Bonfante, P.; Selosse, M.-A. Cephalanthera longifolia (Neottieae, Orchidaceae) Is Mixotrophic: A Comparative Study between Green and Nonphotosynthetic Individuals. Can. J. Bot. 2006, 84, 1462–1477. [Google Scholar] [CrossRef]
- May, M.; Jąkalski, M.; Novotná, A.; Dietel, J.; Ayasse, M.; Lallemand, F.; Figura, T.; Minasiewicz, J.; Selosse, M.-A. Three-Year Pot Culture of Epipactis helleborine Reveals Autotrophic Survival, without Mycorrhizal Networks, in a Mixotrophic Species. Mycorrhiza 2020, 30, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, K.; Matsubayashi, J.; Tayasu, I. Some Mycoheterotrophic Orchids Depend on Carbon from Dead Wood: Novel Evidence from a Radiocarbon Approach. New Phytol. 2020, 227, 1519–1529. [Google Scholar] [CrossRef]
- Abernethy, A. Light Regimes as a Control of Terrestrial Orchid Distribution in New Zealand; University of Canterbury: Christchurch, New Zealand, 2002. [Google Scholar]
- Su, X.; Wang, M.; Huang, Z.; Fu, S.; Chen, H.Y.H. Forest Understorey Vegetation: Colonization and the Availability and Heterogeneity of Resources. Forests 2019, 10, 944. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Hu, H.; Xu, K.; Li, Z.-R.; Yang, Y.-P. Flexible and Reversible Responses to Different Irradiance Levels during Photosynthetic Acclimation of Cypripedium guttatum. J. Plant Physiol. 2007, 164, 611–620. [Google Scholar] [CrossRef]
- Zheng, B.-Q.; Zou, L.-H.; Li, K.; Wan, X.; Wang, Y. Photosynthetic, Morphological, and Reproductive Variations in Cypripedium tibeticum in Relation to Different Light Regimes in a Subalpine Forest. PLoS ONE 2017, 12, e0181274. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Li, Y.; Zhang, Y.; Bai, Y.; Cong, H.; Liu, W.; Zhou, Y. Comparative Study of Cypripedium Plant Photosynthetic Characteristics from Changbai Mountain. Horticulturae 2023, 9, 358. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Honnay, O.; Hermy, M. Effects of Coppicing on Demographic Structure, Fruit and Seed Set in Orchis Mascula. Basic Appl. Ecol. 2008, 9, 392–400. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Adriaens, D.; Honnay, O.; Roldán-Ruiz, I. Effects of Population Size and Forest Management on Genetic Diversity and Structure of the Tuberous Orchid Orchis Mascula. Conserv. Genet. 2009, 10, 161–168. [Google Scholar] [CrossRef]
- Foremnik, K.; Krawczyk, W.; Surmacz, B.; Malicki, M.; Suchan, T.; Gazda, A.; Pielech, R. Effects of Forest Stand Structure on Population of Endangered Orchid Species Cypripedium calceolus L. J. Nat. Conserv. 2021, 64, 126089. [Google Scholar] [CrossRef]
- Lõhmus, A.; Kull, T. Orchid Abundance in Hemiboreal Forests: Stand-Scale Effects of Clear-Cutting, Green-Tree Retention, and Artificial Drainage. Can. J. For. Res. 2011, 41, 1352–1358. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I.; Papaioannou, A. Ecology of the Orchid Goodyera repens in Its Southern Distribution Limits. Plant Biosyst. 2012, 146, 857–866. [Google Scholar] [CrossRef]
- Cruz-Fernández, Q.T.; Alquicira-Arteaga, M.L.; Flores-Palacios, A. Is Orchid Species Richness and Abundance Related to the Conservation Status of Oak Forest? Plant Ecol. 2011, 212, 1091–1099. [Google Scholar] [CrossRef]
- Bayu, B.; Delforterie, W.; Mokria, M.; Petterson, M.; Laarhoven, K.; De Wrachien, D.; Alfonso Parra Sanchez, E. Effects of Forest Succession on the Occurrence of Orchid Species. J. Agric. Ecol. Res. Int. 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Parra Sánchez, E.; Armenteras, D.; Retana, J. Edge Influence on Diversity of Orchids in Andean Cloud Forests. Forests 2016, 7, 63. [Google Scholar] [CrossRef]
- Liu, G.-F.; Zang, R.-G.; Ding, Y.; Wang, W.-Y.; Li, R.-C.; Chen, S.-W.; Zhou, Z.-L. Diversity and Distribution of Epiphytic Orchids in Different Types of Old-Growth Tropical Forests in Bawangling National Nature Reserve, Hainan Island, China. Chin. J. Plant Ecol. 2010, 34, 396. [Google Scholar]
- Hsu, R.C.-C.; Chen, Y.-C.; Lin, C. The Impact of Changing Climate on an Endangered Epiphytic Orchid (Pleione formosana) in a Montane Cloud Forest and the Conservation Challenge Ahead. Plants 2024, 13, 2414. [Google Scholar] [CrossRef]
- Akhalkatsi, M.; Arabuli, G.; Lorenz, R. Orchids as Indicator Species of Forest Disturbances on Limestone Quarry in Georgia (South Caucasus). J. Eur. Orchid. 2014, 46, 123–160. [Google Scholar]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Stevanović, V. Niche Analysis of Orchids of Serpentine and Non-Serpentine Areas: Implications for Conservation. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016, 150, 710–719. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Stevanović, V. Factors Affecting the Distribution and Abundance of Orchids in Grasslands and Herbaceous Wetlands. Syst. Biodivers. 2016, 14, 355–370. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I.; Karagiannakidou, V.; Alifragis, D. Niche Analysis and Conservation of the Orchids of East Macedonia (NE Greece). Acta Oecologica 2008, 33, 27–35. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Terrestrial Orchids: From Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995; ISBN 978-0-521-45165-9. [Google Scholar]
- Batty, A.L.; Dixon, K.W.; Brundrett, M.; Sivasithamparam, K. Constraints to Symbiotic Germination of Terrestrial Orchid Seed in a Mediterranean Bushland. New Phytol. 2001, 152, 511–520. [Google Scholar] [CrossRef]
- Bateman, R.M.; Rudall, P.J. Morphological Continua Make Poor Species: Genus-Wide Morphometric Survey of the European Bee Orchids (Ophrys L.). Biology 2023, 12, 136. [Google Scholar] [CrossRef]
- Devey, D.S.; Bateman, R.M.; Fay, M.F.; Hawkins, J.A. Genetic Structure and Systematic Relationships within the Ophrys fuciflora Aggregate (Orchidaceae: Orchidinae): High Diversity in Kent and a Wind-Induced Discontinuity Bisecting the Adriatic. Ann. Bot. 2009, 104, 483–495. [Google Scholar] [CrossRef]
- Calevo, J.; Viruel, J.; Adamo, M.; Bersweden, L.; Gargiulo, R.; Cowan, R.S.; Fay, M.F. Estimation of Divergence Time and Comparative Plastid Genomics of Orchis Species (Orchidaceae). Bot. J. Linn. Soc. 2024, 20, 1–10. [Google Scholar] [CrossRef]
- Pellegrino, G.; Bellusci, F.; Musacchio, A. Morphological and Molecular Investigation of the Parentage of Ophrys × circlarium (O. lutea × O. tarentina), a New Hybrid Orchid from Italy. Ann. Bot. Fenn. 2008, 45, 61–67. [Google Scholar] [CrossRef]
- Buono, S.; Rempicci, M.; Gransinigh, E.; Grassetti, N.; Chicote, X.; Haile, G.; Fonck, M.; Onofri, S.; Magrini, S. Ophrys ×grottagliensis P. & C. Delforge (Ophrys bertolonii subsp. bertolonii × Ophrys garganica), a New Hybrid Population in Central Italy. J. Eur. Orchid. 2013, 45, 25–30. [Google Scholar]
- Soca, R. Description of Ten New Ophrys-Hybrids (Orchidaceae) of the Abruzzo (Italy). J. Eur. Orchid. 2014, 46, 661–678. [Google Scholar]
- Jakubska-Busse, A.; Tsiftsis, S.; Śliwiński, M.; Křenová, Z.; Djordjević, V.; Steiu, C.; Kolanowska, M.; Efimov, P.; Hennigs, S.; Lustyk, P.; et al. How to Protect Natural Habitats of Rare Terrestrial Orchids Effectively: A Comparative Case Study of Cypripedium calceolus in Different Geographical Regions of Europe. Plants 2021, 10, 404. [Google Scholar] [CrossRef]
- Kirillova, I.; Kirillov, D. Effect of Lighting Conditions on the Reproductive Success of Cypripedium calceolus L. (Orchidaceae, Liliopsida). Biol. Bull. 2019, 46, 1317–1324. [Google Scholar] [CrossRef]
- Křenová, Z.; Lustyk, P.; Kindlmann, P.; Vosmíková, A. Forest Disturbances Threatening Cypripedium calceolus Populations Can Improve Its Habitat Conditions. Diversity 2023, 15, 319. [Google Scholar] [CrossRef]
- Cianfaglione, K. An Editorial to Introduce the New Journal Wild: Issues, Approaches, Ideas and Proposals. Wild 2024, 1, 30–38. [Google Scholar] [CrossRef]
- Adamo, M.; Chialva, M.; Calevo, J.; Bertoni, F.; Dixon, K.; Mammola, S. Plant Scientists’ Research Attention Is Skewed towards Colourful, Conspicuous and Broadly Distributed Flowers. Nat. Plants 2021, 7, 574–578. [Google Scholar] [CrossRef]
- DellaSala, D.A.; Mackey, B.; Norman, P.; Campbell, C.; Comer, P.J.; Kormos, C.F.; Keith, H.; Rogers, B. Mature and Old-Growth Forests Contribute to Large-Scale Conservation Targets in the Conterminous United States. Front. For. Glob. Change 2022, 5, 979528. [Google Scholar] [CrossRef]
- Keith, H.; Kun, Z.; Hugh, S.; Svoboda, M.; Mikoláš, M.; Adam, D.; Bernatski, D.; Blujdea, V.; Bohn, F.; Camarero, J.J.; et al. Carbon Carrying Capacity in Primary Forests Shows Potential for Mitigation Achieving the European Green Deal 2030 Target. Commun. Earth Environ. 2024, 5, 1–13. [Google Scholar] [CrossRef]
- IUCN Approval of a Policy Statement on the Importance of the Conservation of Primary Forests 2020. Available online: https://iucn.org/sites/default/files/2022-05/iucn-policy-statement-for-primary-forests.pdf (accessed on 2 January 2025).
- Moomaw, W.R.; Masino, S.A.; Faison, E.K. Intact Forests in the United States: Proforestation Mitigates Climate Change and Serves the Greatest Good. Front. For. Glob. Change 2019, 2, 449206. [Google Scholar] [CrossRef]
- Mildrexler, D.J.; Berner, L.T.; Law, B.E.; Birdsey, R.A.; Moomaw, W.R. Large Trees Dominate Carbon Storage in Forests East of the Cascade Crest in the United States Pacific Northwest. Front. For. Glob. Change 2020, 3, 594274. [Google Scholar] [CrossRef]
- Di Filippo, A.; Pederson, N.; Baliva, M.; Brunetti, M.; Dinella, A.; Kitamura, K.; Knapp, H.D.; Schirone, B.; Piovesan, G. The Longevity of Broadleaf Deciduous Trees in Northern Hemisphere Temperate Forests: Insights from Tree-Ring Series. Front. Ecol. Evol. 2015, 3, 46. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.; Regnery, B.; Vandekerkhove, K. Tree Related Microhabitats in Temperate and Mediterranean European Forests: A Hierarchical Typology for Inventory Standardization. Ecol. Indic. 2017, 84, 194–207. [Google Scholar] [CrossRef]
- Hirschmugl, M.; Sobe, C.; Di Filippo, A.; Berger, V.; Kirchmeir, H.; Vandekerkhove, K. Review on the Possibilities of Mapping Old-Growth Temperate Forests by Remote Sensing in Europe. Environ. Model. Assess. 2023, 28, 761–785. [Google Scholar] [CrossRef]
- Pavlin, J.; Nagel, T.A.; Svitok, M.; Di Filippo, A.; Mikac, S.; Keren, S.; Dikku, A.; Toromani, E.; Panayotov, M.; Zlatanov, T.; et al. Pathways and Drivers of Canopy Accession across Primary Temperate Forests of Europe. Sci. Total Environ. 2024, 906, 167593. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, A.; Biondi, F.; Piovesan, G.; Ziaco, E. Tree Ring-Based Metrics for Assessing Old-Growth Forest Naturalness. J. Appl. Ecol. 2017, 54, 737–749. [Google Scholar] [CrossRef]
- Ódor, P.; Heilmann-Clausen, J.; Christensen, M.; Aude, E.; van Dort, K.W.; Piltaver, A.; Siller, I.; Veerkamp, M.T.; Walleyn, R.; Standovár, T.; et al. Diversity of Dead Wood Inhabiting Fungi and Bryophytes in Semi-Natural Beech Forests in Europe. Biol. Conserv. 2006, 131, 58–71. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Barron, E.S.; Boddy, L.; Dahlberg, A.; Griffith, G.W.; Nordén, J.; Ovaskainen, O.; Perini, C.; Senn-Irlet, B.; Halme, P. A Fungal Perspective on Conservation Biology. Conserv. Biol. J. Soc. Conserv. Biol. 2015, 29, 61–68. [Google Scholar] [CrossRef]
- Geoportale Nazionale. Available online: http://www.pcn.minambiente.it/viewer/ (accessed on 17 October 2024).
- Pesaresi, S.; Galdenzi, D.; Biondi, E.; Casavecchia, S. Bioclimate of Italy: Application of the Worldwide Bioclimatic Classification System. J. Maps 2014, 10, 538–553. [Google Scholar] [CrossRef]
- Viciani, D.; Agostini, N. La carta della vegetazione del Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna (Appennino Tosco-Romagnolo): Note illustrative. Quad. Studi Nat. Romagna 2009, 27, 97–134. [Google Scholar]
- Agostini, N.; Senni, L.; Benvenuto, C. (Eds.) Atlante della Biodiversità del Parco Nazionale delle Foreste Casentinesi; Graphic Vit: San Giustino, Italy, 2005. [Google Scholar]
- Viciani, D.; Geri, F.; Agostini, N.; Gonnelli, V.; Lastrucci, L. Role of a Geodatabase to Assess the Distribution of Plants of Conservation Interest in a Large Protected Area: A Case Study for a Major National Park in Italy. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 152, 631–641. [Google Scholar] [CrossRef]
- Ceccarelli, P.P.; Gellini, S.; Londi, G.; Agostini, N. (Eds.) Atlante Degli Uccelli Nidificanti nel Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna (2012–2017); Ente Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna: Santa Sofia, Italy, 2019. [Google Scholar]
- Viciani, D.; Agostini, N. Check-List Aggiornata della Flora Vascolare del “Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna” (Appennino Settentrionale); Ente Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna: Santa Sofia, Italy, 2020. [Google Scholar]
- Piazzini, S.; Alberti, D. (Eds.) Atlante Degli Anfibi e Rettili del Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna (2010–2022); Ente, P.N. delle Foreste Casentinesi, Monte Falterona e Campigna: Santa Sofia, Italy, 2022. [Google Scholar]
- Pica, A.; Laghi, P. Atlante delle Orchidee del Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna: Guida Alle Specie e Chiavi di Riconoscimento; Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna, Premiato Stabilimento Tipografico dei Comuni: Santa Sofia, Italy, 2023; ISBN 978-88-95719-04-7. [Google Scholar]
- Ente Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna L’Arca Della Biodiversità. Available online: https://biodiversita.parcoforestecasentinesi.it/it/#/it/maps (accessed on 17 October 2024).
- Viciani, D.; Alberti, D. The Vascular Flora of the Foreste Casentinesi, Monte Falterona, Campigna National Park (Northern Apennines, Italy): Updating the Checklist. Nat. Conserv. Res. 2024, 9, 66–79. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C.; Burrascano, S.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Galdenzi, D.; Gigante, D.; Lasen, C.; Spampinato, G.; et al. Manuale Italiano di Interpretazione degli Habitat della Direttiva 92/43/CEE; Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2009. [Google Scholar]
- Ente Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna Species of Community Interest of the Park. Available online: https://www.parcoforestecasentinesi.it/en/nature/biodiversity/natura-2000-network/species-of-community-interest-of-the-park (accessed on 17 October 2024).
- Laghi, P.; Pica, A. Una Nuova Orchidea per il Parco: Himantoglossum robertianum (Loisel.) P. Delforge. Available online: https://www.parcoforestecasentinesi.it/it/news/una-nuova-orchidea-il-parco-0 (accessed on 8 January 2025).
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A Second Update to the Checklist of the Vascular Flora Native to Italy. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Mehtatalo, L.; Kansanen, K. Lmfor: Functions for Forest Biometrics, version 1.6; 2022. Available online: https://cran.r-project.org/web/packages/lmfor/lmfor.pdf (accessed on 2 January 2025).
- Tabacchi, G.; Di Cosmo, L.; Gasparini, P.; Morelli, S. Stima del Volume e della Fitomassa delle Principali Specie Forestali Italiane; Unità di Ricerca per il Monitoraggio e la Pianificazione Forestale: Trento, Italy, 2011. [Google Scholar]
- Rubino, D.L.; McCarthy, B.C. Evaluation of Coarse Woody Debris and Forest Vegetation across Topographic Gradients in a Southern Ohio Forest. For. Ecol. Manag. 2003, 183, 221–238. [Google Scholar] [CrossRef]
- McKendrick, S.L.; Leake, J.R.; Taylor, D.L.; Read, D.J. Symbiotic Germination and Development of the Myco-Heterotrophic Orchid Neottia nidus-avis in Nature and Its Requirement for Locally Distributed Sebacina spp. New Phytol. 2002, 154, 233–247. [Google Scholar] [CrossRef]
- Schelkunov, M.I.; Shtratnikova, V.Y.; Nuraliev, M.S.; Selosse, M.-A.; Penin, A.A.; Logacheva, M.D. Exploring the Limits for Reduction of Plastid Genomes: A Case Study of the Mycoheterotrophic Orchids Epipogium aphyllum and Epipogium roseum. Genome Biol. Evol. 2015, 7, 1179–1191. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Zhai, J.-W.; Liu, Z.-J.; Li, M.-H. Organelle Genomes of Epipogium roseum Provide Insight into the Evolution of Mycoheterotrophic Orchids. Int. J. Mol. Sci. 2024, 25, 1578. [Google Scholar] [CrossRef]
- Gebauer, G.; Meyer, M. 15N and 13C Natural Abundance of Autotrophic and Myco-Heterotrophic Orchids Provides Insight into Nitrogen and Carbon Gain from Fungal Association. New Phytol. 2003, 160, 209–223. [Google Scholar] [CrossRef]
- Julou, T.; Burghardt, B.; Gebauer, G.; Berveiller, D.; Damesin, C.; Selosse, M.-A. Mixotrophy in Orchids: Insights from a Comparative Study of Green Individuals and Nonphotosynthetic Individuals of Cephalanthera damasonium. New Phytol. 2005, 166, 639–653. [Google Scholar] [CrossRef]
- Ogórek, R.; Kurczaba, K.; Łobas, Z.; Żołubak, E.; Jakubska-Busse, A. Species Diversity of Micromycetes Associated with Epipactis helleborine and Epipactis purpurata (Orchidaceae, Neottieae) in Southwestern Poland. Diversity 2020, 12, 182. [Google Scholar] [CrossRef]
- Gebauer, G.; Preiss, K.; Gebauer, A.C. Partial Mycoheterotrophy Is More Widespread among Orchids than Previously Assumed. New Phytol. 2016, 211, 11–15. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Waud, M.; Brys, R.; Lallemand, F.; Courty, P.-E.; Robionek, A.; Selosse, M.-A. Mycorrhizal Associations and Trophic Modes in Coexisting Orchids: An Ecological Continuum between Auto- and Mixotrophy. Front. Plant Sci. 2017, 8, 1497. [Google Scholar] [CrossRef]
- Schiebold, J.M.-I.; Bidartondo, M.I.; Lenhard, F.; Makiola, A.; Gebauer, G. Exploiting Mycorrhizas in Broad Daylight: Partial Mycoheterotrophy Is a Common Nutritional Strategy in Meadow Orchids. J. Ecol. 2018, 106, 168–178. [Google Scholar] [CrossRef]
- IUCN The IUCN Red List of Threatened Species. Version 2024-2. Available online: https://www.iucnredlist.org/en (accessed on 10 December 2024).
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia; Agricole di New Business Media: Bologna, Italy, 2017; ISBN 978-88-506-5242-6. [Google Scholar]
- Tichý, L.; Axmanová, I.; Dengler, J.; Guarino, R.; Jansen, F.; Midolo, G.; Nobis, M.P.; Van Meerbeek, K.; Aćić, S.; Attorre, F.; et al. Ellenberg-Type Indicator Values for European Vascular Plant Species. J. Veg. Sci. 2023, 34, e13168. [Google Scholar] [CrossRef]
- Jackson, J.E. A User’s Guide to Principal Components; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 978-0-471-72532-9. [Google Scholar]
- Peres-Neto, P.R.; Jackson, D.A.; Somers, K.M. How Many Principal Components? Stopping Rules for Determining the Number of Non-Trivial Axes Revisited. Comput. Stat. Data Anal. 2005, 49, 974–997. [Google Scholar] [CrossRef]
- Kolmogorov, A.N. Sulla Determinazione Emperica Delle Leggi Di Probabilita. G. Ist. Ital. Attuari 1933, 4, 89–91. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Statistics for Biology and Health: Springer: New York, NY, USA, 2007; ISBN 978-0-387-45967-7. [Google Scholar]
- QGIS Geographic Information System. QGIS Association. Available online: https://www.qgis.org/it/site/ (accessed on 20 December 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://Www.R-Project.Org/ (accessed on 14 July 2024).
- Delforge, P. Orchidées d’Europe, d’Afrique du Nord et du Proche-Orient, 4th ed.; Delachaux & Nistlé: Paris, France, 2016. [Google Scholar]
- Procházka, A.; Mikita, T.; Jelínek, P. The Relationship between Some Forest Stand Properties and the Occurrence of Orchids in the Central Part of the Moravian Karst Protected Landscape Area. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 919–931. [Google Scholar] [CrossRef]
- Dykyjová, D. Ekologie Středoevropských Orchidejí; KOPP: České Budějovice, Czech Republic, 2003. [Google Scholar]
- Kirillova, I.A.; Kirillov, D.V. Effect of Illumination Conditions on the Reproductive Success of Epipactis helleborine (L.) Crantz (Orchidaceae). Russ. J. Ecol. 2020, 51, 389–393. [Google Scholar] [CrossRef]
- Rewicz, A.; Kolodziejek, J.; Jakubska-Busse, A. The Role of Anthropogenic Habitats as Substitutes for Natural Habitats: A Case Study on Epipactis helleborine (L.) Crantz (Orchidaceae, Neottieae). Variations in Size and Nutrient Composition of Seeds. Turk. J. Bot. 2016, 40, 258–268. [Google Scholar] [CrossRef]
- Salmia, A. Endomycorrhizal Fungus in Chlorophyll-Free and Green Forms of the Terrestrial Orchid Epipactis helleborine. Karstenia 1988, 28, 3–18. [Google Scholar] [CrossRef]
- Ogura-Tsujita, Y.; Yukawa, T. Epipactis Helleborine Shows Strong Mycorrhizal Preference towards Ectomycorrhizal Fungi with Contrasting Geographic Distributions in Japan. Mycorrhiza 2008, 18, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Waud, M.; Lievens, B.; Brys, R. Differences in Mycorrhizal Communities between Epipactis palustris, E. helleborine and Its Presumed Sister Species E. neerlandica. Ann. Bot. 2016, 118, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tĕšitelová, T.; Tĕšitel, J.; Jersáková, J.; RÍhová, G.; Selosse, M.-A. Symbiotic Germination Capability of Four Epipactis Species (Orchidaceae) Is Broader than Expected from Adult Ecology. Am. J. Bot. 2012, 99, 1020–1032. [Google Scholar] [CrossRef]
- Pacioni, G.; Comandini, O.; Rinaldi, A.C. An Assessment of Below-Ground Ectomycorrhizal Diversity of Abies Alba Miller in Central Italy. Plant Biosyst.–Int. J. Deal. Asp. Plant Biol. 2001, 135, 337–350. [Google Scholar] [CrossRef]
- McCormick, M.K.; Jacquemyn, H. What Constrains the Distribution of Orchid Populations? New Phytol. 2014, 202, 392–400. [Google Scholar] [CrossRef]
- Stroh, P.A.; Humphrey, T.A.; Burkmar, R.J.; Pescott, O.L.; Roy, D.B.; Walker, K.J. Cephalanthera damasonium (Mill.) Druce in BSBI Online Plant Atlas 2020. 2023. Available online: https://plantatlas2020.org/atlas/2cd4p9h.ygt (accessed on 8 January 2025).
- Serafini, D.; Brilli, F.; Pinelli, P.; Delfine, S.; Loreto, F. Photosynthetic Properties of an Orchid Community in Central Italy. J. Plant Interact. 2007, 2, 253–261. [Google Scholar] [CrossRef]
- Gebauer, G. Partnertausch im Dunklen Wald—Stabile Isotope Geben Neue Einblicke in das Ernährungsverhalten von Orchideen; Pfeil: München, Germany, 2005; Volume 30, pp. 55–67. ISBN 978-3-89937-060-7. [Google Scholar]
- Hynson, N.A.; Madsen, T.P.; Selosse, M.-A.; Adam, I.K.U.; Ogura-Tsujita, Y.; Roy, M.; Gebauer, G. The Physiological Ecology of Mycoheterotrophy. In Mycoheterotrophy: The Biology of Plants Living on Fungi; Merckx, V., Ed.; Springer: New York, NY, USA, 2013; pp. 297–342. ISBN 978-1-4614-5209-6. [Google Scholar]
- Schiebold, J.M.-I.; Bidartondo, M.I.; Karasch, P.; Gravendeel, B.; Gebauer, G. You Are What You Get from Your Fungi: Nitrogen Stable Isotope Patterns in Epipactis Species. Ann. Bot. 2017, 119, 1085–1095. [Google Scholar] [CrossRef]
- Preiss, K.; Adam, I.K.U.; Gebauer, G. Irradiance Governs Exploitation of Fungi: Fine-Tuning of Carbon Gain by Two Partially Myco-Heterotrophic Orchids. Proc. R. Soc. B Biol. Sci. 2010, 277, 1333–1336. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Kull, T.; Tali, K. Adult Whole-Plant Dormancy Induced by Stress in Long-Lived Orchids. Ecology 2005, 86, 3099–3104. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Kull, T.; Tali, K. Demographic Response to Shading and Defoliation in Two Woodland Orchids. Folia Geobot. 2006, 41, 95–106. [Google Scholar] [CrossRef]
- Taura, L.; Gudžinskas, Z. What Factors Determine the Natural Fruit Set of Cephalanthera longifolia and Cephalanthera rubra? Diversity 2024, 16, 333. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Whigham, D.F. Importance of Woody Debris in Seed Germination of Tipularia discolor (Orchidaceae). Am. J. Bot. 1998, 85, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Ogura-Tsujita, Y.; Tetsuka, K.; Tagane, S.; Kubota, M.; Anan, S.; Yamashita, Y.; Tone, K.; Yukawa, T. Differing Life-History Strategies of Two Mycoheterotrophic Orchid Species Associated with Leaf Litter- and Wood-Decaying Fungi. Diversity 2021, 13, 161. [Google Scholar] [CrossRef]
- Brujić, S. Contribution to the Knowledge of the Distribution of the Rare Orchid Epipogium aphyllum Swartz in Bosnia and Herzegovina. Glas. Šumar. Fak. Univ. Banjoj Luci 2022, 32, 71–76. [Google Scholar] [CrossRef]
- Jersáková, J.; Minasiewicz, J.; Selosse, M.-A. Biological Flora of Britain and Ireland: Neottia nidus-avis. J. Ecol. 2022, 110, 2246–2263. [Google Scholar] [CrossRef]
- Phillips, R.D.; Barrett, M.D.; Dixon, K.W.; Hopper, S.D. Do Mycorrhizal Symbioses Cause Rarity in Orchids? J. Ecol. 2011, 99, 858–869. [Google Scholar] [CrossRef]
- Swarts, N.D.; Sinclair, E.A.; Francis, A.; Dixon, K.W. Ecological Specialization in Mycorrhizal Symbiosis Leads to Rarity in an Endangered Orchid. Mol. Ecol. 2010, 19, 3226–3242. [Google Scholar] [CrossRef]
- Sajkiewicz, R.; Gabka, M.; Owsianny, P.M. Cephalanthera rubra (L.) Rich. (Orchidaceae) in Mid-West Poland (Wielkopolska)—Distribution, Endangerment and Conservation Status. Rocz. Akad. Rol. W Pozn. Bot.-Steciana 2009, 13, 15–21. [Google Scholar]
- Jakubska-Busse, A.; Pielech, R.; Szczęśniak, E. The Extinction of Terrestrial Orchids in Europe: Does Disappearance of Cephalanthera Rich., 1817 (Orchidaceae, Neottieae) Species Show Pattern Consistent with the Elevation Gradient? Life Sci. J. 2014, 11, 140–144. [Google Scholar]
- Attiwill, P.M. The Disturbance of Forest Ecosystems: The Ecological Basis for Conservative Management. For. Ecol. Manag. 1994, 63, 247–300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).