Soil-Specific Effects of the Bio-Growth Regulator Supporter on Seed Potato Yield and Quality Across Varieties: Unlocking Sustainable Potential in Diverse Environments
Abstract
1. Introduction
2. Materials and Methods
- −
- Barankowo (Greater Poland Voivodeship)—coordinates: 53°18′35″ N, 16°58′19″ E.
- −
- Głubczyce (Opole Voivodeship)—coordinates: 50°12′0″ N, 17°50′3″ E.
- −
- Kędrzyno (West Pomeranian Voivodeship)—coordinates: 54°3′55″ N, 15°27′1″ E.
- −
- Ryn (Wormian–Masuria Voivodeship)—coordinates: 53°56′57″ N, 21°30′54.17″ E.

2.1. Field Research
Potato Cultivation and Protection
- −
- Calypso 480 SC at 0.1 L per hectare (1 application).
- −
- Actara 25 WG at 0.08 kg per hectare (1 application).
- −
- Cyperkil Max 500 EC at 0.05 L per hectare (1 application).
- −
- Karate Zeon 050CS at 0.1 L per hectare (1 application).
- −
- Pyton Consento 450 SC at 2 L per hectare (1 application).
- −
- Infinito 687.5 S.C. at 1.6 L per hectare (1 application).
- −
- Ridomil Gold MZ 67.8 WG at 2 kg per hectare (1 application).
- −
- Acrobat MZ 69 WG at 2 kg per hectare (1 application).
2.2. Characteristics of Potato Varieties
2.3. Characteristics of the Supporter Biostimulator
2.4. Soil Analysis Methodology
2.5. Soil Conditions
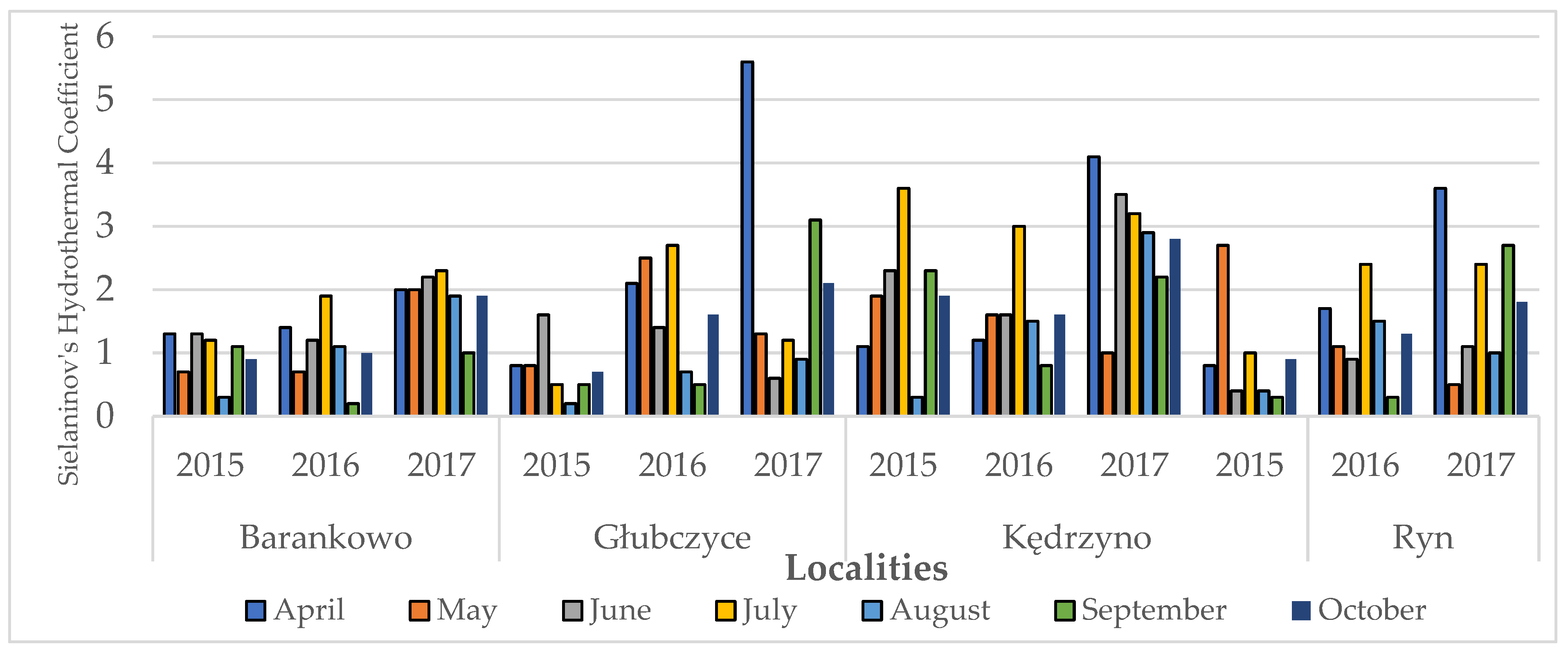
2.6. Meteorological Conditions
2.7. Statistical Calculations
3. Results
3.1. Share of Seed Potatoes
3.2. Yield of Seed Potatoes
3.3. Number of Seed Potatoes
3.4. Share of Number of Seed Potatoes
3.5. Average Seed Potato Mass
3.6. Reproduction Coefficient
3.7. Statistical Characteristics of Seed Potato Yield and Independent Variables
3.8. Correlation Analysis of Variables Influencing Seed Potato Yield
- −
- y and x1: A coefficient of −0.21 * suggests a weak, negative correlation between these variables.
- −
- y and x5: A value of 0.50 ** indicates a moderate, positive correlation.
- −
- x3 and x5: A coefficient of 0.78 ** suggests a strong, positive correlation between these variables.
4. Discussion
4.1. The Role of High-Quality Seed Material and Modern Technologies in Potato Cultivation
4.2. Genetic Factors in Shaping the Quality Parameters of Seed Potatoes
4.3. Disease Resistance and Impact on Seed Potato Quality
4.4. The Role of Agrotechnics in Maximizing the Reproduction Coefficient
4.5. Seed Potato Yield Variability
Summaries
4.6. The Influence of Biostimulants on the Diversity and Functionality of Soil Microorganisms
4.6.1. The Potential of Biostimulants in Improving Sustainable Agricultural Practices
4.6.2. Interactions Between Biostimulants and Pesticides Used
4.6.3. The Influence of Biostimulants on Soil Microorganisms
4.6.4. Recommendations for Farmers and Suggestions for Further Research
5. Conclusions
- −
- Varietal Selection and Zoning: Adhering to the principle of zoning potato varieties based on their disease resistance and adaptability to specific climatic and soil conditions is essential, particularly in high-risk areas.
- −
- Agrotechnological Innovations: Continuous improvement in cultivation techniques, including optimal planting density, nutrient management, and irrigation practices, is crucial for maximizing the benefits of biostimulant application.
- −
- Adaptation to Challenging Conditions: The Supporter technology demonstrated substantial benefits in improving both the seed tuber mass and the multiplication coefficient, particularly in locations with less favorable growing conditions. Its use should be strongly promoted in such regions to ensure sustainable yields.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative effect of fertilization practices on soil microbial diversity and activity: An overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Li, Z.; Fan, F.; Chu, G.; Hou, Z.; Liang, Y. Soil biological activity and their seasonal variations in response to long-term application of organic and inorganic fertilizers. Plant Soil 2010, 326, 31–44. [Google Scholar] [CrossRef]
- Siddique, M.; Sultana, J.; Abdullah, M. Aggregate stability: An indicator of quality and resistivity of arable Soil. Asian J. Soil Sci. Plant Nutr. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Bassouny, M.; Chen, J. Effect of long-term organic and mineral fertilizer on physical properties in root zone of a clayey Ultisol. Arch. Agron. Soil Sci. 2016, 62, 819–828. [Google Scholar] [CrossRef]
- Sharma, A.; Chetani, R. A Review of the Effect of Organic and Chemical Fertilizers on Plants. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 677. [Google Scholar] [CrossRef]
- E.U. Regulation of the European Parliament and of the Council Laying Down Rules on the Making Available on the Market of EU Fertilizing Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2019:170:TOC (accessed on 10 September 2024).
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2014, 31, 1–17. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Bermejo, N.F.; Munné-Bosch, S. Biostimulants: A sufficiently effective tool for sustainable agriculture in the era of climate change? Plant Physiol. Biochem. 2024, 211, 108699. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Liu, W. The effects of biostimulants on potato yield and soil properties under varying climatic conditions. J. Soil Sci. Plant Nutr. 2023, 23, 450–462. [Google Scholar]
- Li, L.; Tong, L.; Lv, Y. Influence of bio-fertilizer type and amount jointly on microbial community composition, crop production and soil health. Agronomy 2023, 13, 1775. [Google Scholar] [CrossRef]
- Pacci, S.; Dengiz, O.; Agbor, D.T. Impact of biostimulants on soil quality. In Proceedings of the International Symposium on,,Soil Science and Plant Nutrition”, Samsun, Turkey, 8–9 December 2023. [Google Scholar]
- Wadduwage, J.; Liu, H.; Egidi, E.; Singh, B.K.; Macdonald, C.A. Effects of biostimulant application on soil biological and physicochemical properties: A field study. J. Sustain. Agric. Environ. 2023, 2, 285–300. [Google Scholar] [CrossRef]
- Wadduwage, S.; Karunaratne, A.; Wickramasinghe, W. Biostimulants for soil fertility enhancement: A meta-analysis. Front. Sustain. Agric. 2023, 5, 102–118. [Google Scholar]
- Noaema, A.H.; Sawicka, B.; Barbaś, P.; Krochmal-Marczak, B. The Effect of Supporter1 on the Reduction of Rhizoctonia Solani on Potato Tubers. In Proceedings of the 56th Scientific Session of the Institute of Plant Protection, Poznań, Poland, 11–12 February 2016; National Research Institute: Poznań, Poland, 2016; p. 103. [Google Scholar]
- Bajpai, S.; Shukla, P.S.; Prithiviraj, B.; Critchley, A.T.; Nivetha, N. Editorial: Development of next generation bio stimulants for sustainable agriculture. Front. Plant Sci. 2024, 15, 1383749. [Google Scholar] [CrossRef] [PubMed]
- Udalova, E.Y.; Zamyatin, S.A.; Maksutkin, S.A. Productivity and quality of potato tubers depending on protective measures. IOP Conf. Ser. Earth Environ. Sci. 2021, 848, 012199. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Roztropowicz, S. Methodology of Observation, Measurements and Sampling in Agricultural Experiments with Potatoes; Plant Breeding and Acclimatization Institute: Jadwisin, Poland, 1999; pp. 1–50. (In Polish) [Google Scholar]
- Lenartowicz, T.; Erlichowski, T. New potato varieties 2017. Pol. Potato 2017, 2, 4–8. Available online: https://ziemniak-bonin.pl/polish-potato-02-2017/?lang=en (accessed on 16 October 2024). (In Polish).
- Nowacki, W.; Barbaś, P.; Boguszewska-Mańkowska, D.; Jankowska, J.; Pietraszko, M.; Trawczyński, C.; Zarzyńska, K.; Michalak, K.; Urbanowicz, J. Characteristics of the National Register of Potato Cultivars, 2nd ed.; IHAR-PIB: Jadwisin, Poland, 2020; p. 45. (In Polish) [Google Scholar]
- Ustawa z dnia 10 lipca 2007 r. o nawozach i nawożeniu. Dz.U. 2007 nr 147 poz. 1033. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20071471033 (accessed on 16 October 2024). (In Polish)
- Supporter®. Agricultural Crop Yield Modulator. Instruction for Use: Supporter (PL). Available online: https://certisbelchim.pl/pdf/Label/Supporter_LABEL.pdf (accessed on 16 October 2024). (In Polish).
- PN-R-04031:1997; Analiza Chemiczno-Rolnicza Gleby. Pobieranie Próbek. Polski Komitet Normalizacyjny: Warszawa, Poland, 1997. (In Polish)
- Mocek, A. Soil Science, 1st ed.; PWN Scientific Publishing House: Warsaw, Poland, 2015; 589p, ISBN 978-83-01-18795-8. (In Polish) [Google Scholar]
- Lützenkirchen, J.; Preočanin, T.; Kovačević, D.; Tomišič, V.; Lövgren, L.; Kallay, N. Potentiometric titrations as a tool for surface charge determination. Croat. Chem. Acta 2012, 85, 391–417. [Google Scholar] [CrossRef]
- PN-R-04020:1994+AZ1:2004; Chemical and Agricultural Analysis of Soil. Polish Committee for Standardizations: Warsaw, Poland, 2004. (In Polish)
- PN-R-04023:1996; Chemical and Agricultural Analysis of Soil. Determination of Available Phosphorus Content in Mineral Soils. Polish Committee for Standardizations: Warsaw, Poland, 1996. (In Polish)
- PN-R-04022:1996+AZ1:2002; Chemical and Agricultural Analysis of Soil. Determination of Available Potassium Content in Mineral Soils. Polish Committee for Standardization: Warsaw, Poland, 2002. (In Polish)
- Wójcikowska-Kapusta, A.; Niemczuk, B. Effect of type of use on content of various magnesium and potassium forms in profiles of rendzinas. Acta Agrophys. 2006, 8, 765–771. (In Polish) [Google Scholar]
- Nawrocki, S. Fertilizer Recommendations. Part. I. Limit Numbers for Valuation of Soils in Macro- and Microelements; IUNG: Puławy, Poland, 1991; p. 44. (In Polish) [Google Scholar]
- PN-Z-15011-3:2001; Polish Version. Compost from Municipal Waste. Determination: pH, Organic Content, Organic Carbon, Nitrogen, Phosphorus and Potassium. PKN Publisher: Warsaw, Poland, 2001. (In Polish)
- Fotyma, M.; Kęsik, K.; Pietruch, C.Z. Mineral nitrogen in soils as an indicator of the fertilizer needs of plants and the condition of soil and groundwater purity. Nawozy i Nawożenie/Fertil. Fertil. 2010, 38, 5–80. (In Polish) [Google Scholar]
- WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. 2014. Available online: https://www.isric.org/explore/wrb (accessed on 12 January 2025).
- Skowera, B.; Kopcińska, J.; Kopeć, B. Changes in thermal and precipitation conditions in Poland in 1971–2010. Ann. Wars. Univ. Life Sci. Land Reclam. 2014, 46, 153–162. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT®9.2. User’s Guide; SAS Institute: Cary, NC, USA, 2008. [Google Scholar]
- Tatarczak, A. T.09. Statystyka, Akademia WSEI. Wydawnictwo Naukowe Innovatio Press, ee.202. 2021. Available online: https://wydawnictwo.wsei.eu/sklep/statystyka-tom-ix/ (accessed on 12 January 2025). (In Polish).
- Aamir, M.; Rai, K.K.; Zehra, A.; Dubey, M.K.; Kumar, S.; Shukla, V.; Upadhyay, R.S. Microbial bioformulation-based plant biostimulants: A plausible approach toward next generation of sustainable agriculture. In Microbial Endophytes; Woodhead Publishing: Sawston, Cambridge, 2020; pp. 195–225. [Google Scholar]
- Cerdà, A.; Jangid, K.; Govers, G. Soil health and agricultural sustainability: Insights from biostimulant research. Agric. Res. Technol. 2022, 11, 123–135. [Google Scholar]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. Int. Soc. Microb. Ecol. J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The application of arbuscular mycorrhizal fungi as microbial biostimulant, sustainable approaches in modern agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, G.; Ren, S.; Li, L.; Li, C.; Li, Y.; Yu, X.; Yin, Y.; Liu, T.; Liu, X. Responses of soil microbial community structure, potential ecological functions, and soil physicochemical properties to different cultivation patterns in cucumber. Geoderma 2023, 429, 116237. [Google Scholar] [CrossRef]
- Pytlarz-Kozicka, M. Porównanie produkcji nasiennej ziemniaka w systemie ekologicznym i konwencjonalnym. Biul. IHAR 2010, 255, 101–108. (In Polish) [Google Scholar] [CrossRef]
- Pytlarz-Kozicka, M.; Zagórski, P. The influence of biological potato tuber treatments on health conditions of potato plants and potato yield. Prog. Plant Prot./Post. Ochr. Roślin 2013, 53, 333–339, Instytut Ochrony Roślin—Państwowy Instytut Badawczy. Available online: https://pdfs.semanticscholar.org/b0d6/e4968e988e79b789578e287638f66bb2b211.pdf (accessed on 7 January 2025). (In Polish).
- Samy, M.M.; Abd El Aal AM, H.; Khalil, M.M. The impact of some treatments on improving seed potato production in the summer season. Middle East J. 2014, 3, 1065–1073.ce. [Google Scholar]
- Anikina, I.; Issayeva, K. Use of the preparation based on Solanum nigrum as a potato yield stimulator. Bulg. J. Agric. Sci. 2023, 29, 272–276. [Google Scholar]
- Pytlarz-Kozicka, M.; Słabicki, W. Wpływ zaprawiania sadzeniaków i terminu zbioru na zdrowotność roślin i plonowanie ziemniaka. Zesz. Nauk. Uniw. Przyr. We Wroclawiu Rol. 2013, 596, 97–106. (In Polish) [Google Scholar]
- Wierzejska-Bujakowska, A.; Kaczorek, S.; Gójski, B.; Goc, K.; Manikowski, Z. Wpływ warunków glebowo-klimatycznych na wydajność sadzeniaków u 15 odmian ziemniaka. Zesz. Probl. Post. Nauk. Rol. 1988, 342, 21–30. (In Polish) [Google Scholar]
- Zarzecka, K.; Gugała, M.; Sikorska, A.; Grzywacz, K.; Niewęgłowski, M. Marketable yield of potato and its quantitative parameters after application of herbicides and biostimulants. Agriculture 2020, 10, 49. [Google Scholar] [CrossRef]
- Boguszewska-Mańkowska, D.; Ruszczak, B.; Zarzyńska, K. Classification of Potato Varieties Drought Stress Tolerance Using Supervised Learning. Appl. Sci. 2022, 12, 1939. [Google Scholar] [CrossRef]
- Zarzyńska, K.; Boguszewska-Mańkowska, D. Commercial Quality of Potato Tubers of Different Varieties from Organic and Conventional Production System. Agronomy 2024, 14, 778. [Google Scholar] [CrossRef]
- Vos, J.; Haverkort, J. Chapter 16—Water Availability and Potato Crop Performance. In Potato Biology and Biotechnology; Vreugdenhil, D., Bradshaw, J., Ross, H.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 333–351. ISBN 978-0-444-51018-1. [Google Scholar] [CrossRef]
- Ostonakulov, T.E.; Lukova, I.N. Productivity, yield of seed tubers and multiplication factors of potato varieties as secondary crops, depending on planting methods. Potato Veg. 2022, 10, 24–27. (In Russian) [Google Scholar] [CrossRef]
- Struik, P.C.; Wiersema, S.G. Seed Potato Technology; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020. [Google Scholar]
- Sawicka, B. An attempt for improvement of seed potatoes quality using minitubers in the field cultivation. Ann. Univ. Mariae Curie-Skłodowska. Sect. E Agric. 2004, 59, 1233–1244. [Google Scholar]
- Sawicka, B.; Pszczółkowski, P. The effect of application of biopreparations and fungicides on the yield and selected parameters of seed value of seed potatoes. Acta Agroph. 2018, 25, 239–255. [Google Scholar] [CrossRef]
- Roztropowicz, S.; Goc, K. Wpływ terminu sadzenia na wydajność sadzeniaków w plonie ziemniaków uprawianych w różnych warunkach glebowych i klimatycznych. Zesz. Probl. Post. Nauk. Rol. 1988, 59, 63–74. (In Polish) [Google Scholar]
- Gójski, B. Wpływ wielkości bulw matecznych na udział sadzeniaków w plonie odmian wczesnych. Zesz. Probl. Post. Nauk Rol. 1988, 342, 41–47. (In Polish) [Google Scholar]
- Gójski, B.; Manikowski, Z. Wpływ terminu zbioru na współczynnik rozmnażania u 17 odmian ziemniaka. Zesz. Probl. Post. Nauk Rol. 1988, 342, 75–84. (In Polish) [Google Scholar]
- Kumar, M.K.; Tiwari, R.K.; Jaiswal, A.K.; Kumar, A.; Dutt, S.; Kumar, R.; Kumar, A.; Dutt, S.; Kumar, R.; Kumar, D.; et al. Post-harvest management and value addition in potato: Emerging technologies in preserving quality and sustainability in potato processing. Indian J. Agron. 2023, 68, 241–261. [Google Scholar]
- Ewing, E.E.; Struik, P.C. Physiology of tuber growth and development in potato. J. Agron. Crop Sci. 2022, 208, 245–258. [Google Scholar]
- Haverkort, A.J.; Struik, P.C. Yield levels of potato crops: Recent achievements and future prospects. Field Crops Res. 2015, 182, 76–85. [Google Scholar] [CrossRef]
- Van Es, H.M.; Karlen, D.L. Revisiting the relationship between soil quality and potato yields. Soil Sci. Soc. Am. J. 2019, 83, 1562–1575. [Google Scholar]
- Haverkort, A.J. On Processing Potato. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Hellequin, E.; Monard, C.; Chorin, M.; Le Bris, N.; Daburon, V.; Klarzynski, O.; Binet, F. Responses of active soil microorganisms facing to a soil biostimulant input compared to plant legacy effects. Sci. Rep. 2020, 10, 13727. [Google Scholar] [CrossRef]
- Roche, D.; Rickson, J.R.; Pawlett, M. Moving towards a mechanistic understanding of biostimulant impacts on soil properties and processes: A semi-systematic review. Front. Agron. 2024, 6, 1271672. [Google Scholar] [CrossRef]
- Hellequin, E.; Monard, C.; Quaiser, A.; Henriot, M.; Klarzynski, O.; Binet, F. Specific recruitment of soil bacteria and fungi decomposers following a biostimulant increased crop residues mineralization. PLoS ONE 2018, 13, e0209089. [Google Scholar] [CrossRef]
- García-Martínez, A.M.; Díaz, A.; Tejada, M.; Bautista, J.; Rodríguez, B.; Santa María, C.; Revilla, C.; Parrado, J. Enzymatic production of an organic soil biostimulant from wheat-condensed distiller solubles: Effects on soil biochemistry and biodiversity. Process Biochem. 2010, 45, 1127–1133. [Google Scholar] [CrossRef]
- Carillo, P.; Avice, J.C.; Vasconcelos, M.W.; du Jardin, P.; Brown, P.H. Biostimulants in Agriculture. Physiol. Plant. 2025, 177, 1–4. [Google Scholar] [CrossRef]
- Tarantino, E.; Disciglio, G.; Frabboni, L.; Libutti, A.; Gatta, G.; Gagliaridi, A.; Tarantino, A. Effect of biostimulants application on quali-quantitative characteristics of cauliflower, pepper, and fennel crops under organic and conventional fertilization. Int. J. Agric. Biosyst. Eng. 2015, 9, 734–738. [Google Scholar]
- Disciglio, G.; Frabboni, L.; Tarantino, A.; Tarantino, E. Applying natural fertiliers to herbaceous crops. J. Life Sci. 2014, 8, 504–510. [Google Scholar]
- Raza, Q.-U.-A.; Bashir, M.A.; Rehim, A.; Geng, Y.; Raza HM, A.; Hussain, S.; Ahmad, I.; Wasif, M. Identifying the Role of Biostimulants in Turnip (Brassica rapa L.) Production Compared with Chemical Fertilization. Sustainability 2023, 15, 11851. [Google Scholar] [CrossRef]
- Tejada, M.; García-Martínez, A.M.; Gómez, I.; Parrado, J. Application of MCPA Herbicide on Soils Amended with Biostimulants: Short-Time Effects on Soil Biological Properties. Chemosphere 2010, 80, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Botella, M.; Gómez, I.; Paneque, P.; Caballero, P.; Parrado, J.; Vera, A.; Bastida, F.; García, C.; Tejada, M. Use of Biostimulants Obtained from Okara in the Bioremediation of Soils Polluted by Imazamox. Bioremediat. J. 2022, 26, 53–63. [Google Scholar] [CrossRef]
- Deng, X. Current advances in the action mechanisms of safeners. Agronomy 2022, 12, 2824. [Google Scholar] [CrossRef]
- Barros-Rodríguez, A.; Rangseekaew, P.; Lasudee, K.; Pathomaree, W.; Manzanera, M. Regulatory risks associated with bacteria as biostimulants and biofertilizers in the frame of the European Regulation (EU) 2019/1009. Sci. Total Environ. 2020, 740, 140239. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Rai, N.; Rai, S.P.; Sarma, B.K. Prospects for abiotic stress tolerance in crops utilizing phyto- and bio-stimulants. Front. Sustain. Food Syst. 2021, 5, 455. [Google Scholar] [CrossRef]
- Bitterlich, M.; Rouphael, Y.; Graefe, J.; Franken, P. Arbuscular mycorrhizas: A promising component of plant production systems provided favorable conditions for their growth. Front. Plant Sci. 2018, 9, 1329. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress—A meta-analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef]


| Varieties | Breeder | Peel Color | Flesh Color | Culinary Type | Starch Content (%) | Yield of Tubers (t·ha−1) |
|---|---|---|---|---|---|---|
| Early varieties | ||||||
| Innovator | HZPC Holland, BV | Yellow | Creamy | B | 14.8 | 44.2 |
| Lilly | Solana Polska | Yellow | Yellow | BC | 14.0–14.5 | 40.0 |
| Lady Claire | HZPC Holland, BV | Yellow | Light yellow | BC | 16.7 | 38.4 |
| Moderate variety | ||||||
| Verdi | Solana Poland | Yellow | Yellow | BC | 19–20 | 36.4 |
| Location | Year | Available Phosphorus (P2O5) | Available Potassium (K2O) | Available Magnesium (Mg) | Soil pH (KCl) | Humus Content (g·kg−1) |
|---|---|---|---|---|---|---|
| Kędrzyno | 2015 | 24.8 | 9.0 | 3.4 | 5.6 | 1.01 |
| 2016 | 18.8 | 18.0 | 3.9 | 6.0 | 1.06 | |
| 2017 | 17.0 | 15.0 | 6.2 | 6.4 | 1.12 | |
| Barankowo | 2015 | 31.9 | 14.8 | 3.3 | 5.8 | 1.2 |
| 2016 | 25.0 | 12.7 | 3.2 | 6.0 | 1.4 | |
| 2017 | 30.4 | 12.4 | 2.9 | 5.9 | 1.3 | |
| Ryn | 2015 | 14.5 | 16.0 | 3.6 | 6.1 | 1.1 |
| 2016 | 14.7 | 17.5 | 3.8 | 6.3 | 1.3 | |
| 2017 | 15.0 | 19.0 | 4.0 | 6.4 | 1.4 | |
| Głubczyce | 2015 | 27.8 | 22.6 | 11.8 | 6.6 | 1.8 |
| 2016 | 25.2 | 21.5 | 12.8 | 6.5 | 1.7 | |
| 2017 | 21.8 | 19.1 | 12.0 | 6.4 | 1.6 |
| Location | Year | Total Rainfall [mm] | Average Air Temperature [°C] |
|---|---|---|---|
| Barankowo | 2015 | 237.1 | 13.4 |
| 2016 | 306.9 | 15.3 | |
| 2017 | 508.9 | 14.1 | |
| Głubczyce | 2015 | 218.4 | 15.9 |
| 2016 | 472.3 | 15.7 | |
| 2017 | 465.9 | 15.2 | |
| Kędrzyno | 2015 | 383.9 | 12.2 |
| 2016 | 476.1 | 14.9 | |
| 2017 | 712.8 | 14.0 | |
| Ryn | 2015 | 241.3 | 14.5 |
| 2016 | 350.0 | 13.8 | |
| 2017 | 451.6 | 14.1 |
| Variety | Technology | 2015 | 2016 | 2017 | Mean |
|---|---|---|---|---|---|
| Verdi | Traditional | 80.69 | 82.46 | 68.39 | 77.18 |
| With Supporter | 94.48 | 73.72 | 86.88 | 85.03 | |
| Mean | 82.97 | 87.26 | 79.99 | 83.40 | |
| Innovator | Traditional | 89.13 | 70.17 | 79.78 | 79.69 |
| With Supporter | 86.81 | 78.40 | 78.76 | 81.33 | |
| Mean | 80.52 | 83.73 | 74.08 | 79.45 | |
| Lilly | Traditional | 94.68 | 77.79 | 83.99 | 85.49 |
| With Supporter | 88.15 | 81.18 | 87.89 | 85.74 | |
| Mean | 92.01 | 78.75 | 75.83 | 82.20 | |
| Lady Claire | Traditional | 88.84 | 80.36 | 80.45 | 83.22 |
| With Supporter | 80.60 | 83.10 | 71.24 | 78.31 | |
| Mean | 94.58 | 75.76 | 85.43 | 85.26 | |
| Overall Mean | Traditional | 85.56 | 84.22 | 83.94 | 84.57 |
| With Supporter | 90.57 | 74.46 | 77.81 | 80.95 | |
| Years Mean | 87.83 | 79.38 | 79.60 | 82.27 |
| Technology | Varieties | Years | Mean | ||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | |||
| Traditional | Verdi | 80.69 | 82.46 | 68.39 | 77.18 |
| Innovator | 94.48 | 73.72 | 86.88 | 85.03 | |
| Lilly | 82.97 | 87.26 | 79.99 | 83.40 | |
| Lady Claire | 89.13 | 70.17 | 79.78 | 79.69 | |
| Mean | 86.81 | 78.40 | 78.76 | 81.33 | |
| With Supporter | Verdi | 80.52 | 83.73 | 74.08 | 79.45 |
| Innovator | 94.68 | 77.79 | 83.99 | 85.49 | |
| Lilly | 88.15 | 81.18 | 87.89 | 85.74 | |
| Lady Claire | 92.01 | 78.75 | 75.83 | 82.20 | |
| Mean | 88.84 | 80.36 | 80.45 | 83.22 | |
| Overall Mean | Verdi | 80.60 | 83.10 | 71.24 | 78.31 |
| Innovator | 94.58 | 75.76 | 85.43 | 85.26 | |
| Lilly | 85.56 | 84.22 | 83.94 | 84.57 | |
| Lady Claire | 90.57 | 74.46 | 77.81 | 80.95 | |
| Mean | 87.83 | 79.38 | 79.60 | 82.27 | |
| Technology | Varieties | Years | Mean | ||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | |||
| Traditional | Verdi | 26.89 | 35.81 | 26.13 | 29.61 |
| Innovator | 19.72 | 22.46 | 24.80 | 22.33 | |
| Lilly | 39.47 | 36.22 | 35.03 | 36.91 | |
| Lady Claire | 28.93 | 34.11 | 36.04 | 33.03 | |
| Mean | 28.75 | 32.15 | 30.50 | 30.47 | |
| With Supporter | Verdi | 25.42 | 43.22 | 30.03 | 32.89 |
| Innovator | 34.30 | 21.99 | 30.08 | 28.79 | |
| Lilly | 53.32 | 39.13 | 40.13 | 44.19 | |
| Lady Claire | 28.79 | 40.29 | 40.81 | 36.63 | |
| Mean | 35.46 | 36.16 | 35.26 | 35.63 | |
| Overall Mean | Verdi | 26.15 | 39.52 | 28.08 | 31.25 |
| Innovator | 27.01 | 22.23 | 27.44 | 25.56 | |
| Lilly | 46.39 | 37.67 | 37.58 | 40.55 | |
| Lady Claire | 28.86 | 37.20 | 38.43 | 34.83 | |
| Mean | 32.11 | 34.15 | 32.88 | 34.43 | |
| Localities | Technology | 2015 | 2016 | 2017 | Mean |
|---|---|---|---|---|---|
| Kędrzyno | Traditional | 31.89 | 39.88 | 26.13 | 32.63 |
| With Supporter | 29.58 | 45.95 | 30.03 | 35.19 | |
| Mean | 30.74 | 42.91 | 28.08 | 33.91 | |
| Barankowo | Traditional | 20.66 | 24.33 | 24.80 | 23.26 |
| With Supporter | 36.05 | 26.48 | 30.08 | 30.87 | |
| Mean | 28.36 | 25.41 | 27.44 | 27.07 | |
| Ryn | Traditional | 41.65 | 40.53 | 35.03 | 39.07 |
| With Supporter | 56.11 | 46.12 | 40.13 | 47.45 | |
| Mean | 48.88 | 43.33 | 37.58 | 43.26 | |
| Głubczyce | Traditional | 31.23 | 28.28 | 36.04 | 31.85 |
| With Supporter | 30.18 | 46.12 | 40.81 | 39.04 | |
| Mean | 30.71 | 43.33 | 38.43 | 37.49 | |
| Overall Mean | Traditional | 31.36 | 33.25 | 30.50 | 31.70 |
| With Supporter | 37.98 | 38.24 | 38.43 | 38.22 | |
| Mean | 34.67 | 35.75 | 32.88 | 34.43 |
| Technology | Varieties | Years | Mean | ||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | |||
| Traditional | Verdi | 514.80 | 526.09 | 436.33 | 492.41 |
| Innovator | 602.78 | 470.33 | 554.29 | 542.47 | |
| Lilly | 529.35 | 556.72 | 510.34 | 532.13 | |
| Lady Claire | 568.65 | 447.68 | 509.00 | 508.44 | |
| Mean | 553.90 | 500.21 | 502.49 | 518.86 | |
| With Supporter | Verdi | 515.33 | 535.87 | 474.11 | 508.44 |
| Innovator | 606.90 | 497.86 | 537.54 | 547.43 | |
| Lilly | 564.16 | 519.55 | 562.50 | 548.74 | |
| Lady Claire | 588.86 | 503.61 | 486.07 | 526.18 | |
| Mean | 568.81 | 514.22 | 515.05 | 532.70 | |
| Overall varieties | Verdi | 515.07 | 530.98 | 455.22 | 500.42 |
| Innovator | 604.84 | 484.09 | 545.92 | 544.95 | |
| Lilly | 546.75 | 538.14 | 536.42 | 540.44 | |
| Lady Claire | 578.76 | 475.65 | 497.53 | 517.31 | |
| Mean | 561.35 | 507.21 | 508.77 | 525.78 | |
| Localities | Technology | 2015 | 2016 | 2017 | Mean |
|---|---|---|---|---|---|
| Kędrzyno | Traditional | 718.40 | 701.20 | 740.00 | 719.87 |
| With Supporter | 582.40 | 812.00 | 585.20 | 659.87 | |
| Mean | 650.40 | 756.60 | 662.60 | 689.87 | |
| Barankowo | Traditional | 302.40 | 301.20 | 281.20 | 294.93 |
| With Supporter | 348.00 | 354.40 | 325.20 | 342.53 | |
| Mean | 325.20 | 327.80 | 303.20 | 318.73 | |
| Ryn | Traditional | 505.20 | 530.40 | 484.00 | 506.53 |
| With Supporter | 621.20 | 530.40 | 585.20 | 578.93 | |
| Mean | 563,20 | 530.40 | 534.60 | 542.73 | |
| Głubczyce | Traditional | 481.20 | 566.40 | 693.20 | 580.27 |
| With Supporter | 462.40 | 570.40 | 537.20 | 523.33 | |
| Mean | 471.80 | 568.40 | 615.20 | 551.80 | |
| Overall localities | Traditional | 501.80 | 524.80 | 549.60 | 525.40 |
| With Supporter | 503.50 | 566.80 | 508.20 | 526.17 | |
| Mean | 502.65 | 545.80 | 528.90 | 525.78 | |
| Technology | Varieties | Years | Mean | ||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | |||
| Traditional | Verdi | 80.69 | 82.46 | 68.39 | 77.18 |
| Innovator | 94.48 | 73.72 | 86.88 | 85.03 | |
| Lilly | 82.97 | 87.26 | 79.99 | 83.40 | |
| Lady Claire | 89.13 | 70.17 | 79.78 | 79.69 | |
| Mean | 86.81 | 78.40 | 78.76 | 81.33 | |
| With Supporter | Verdi | 80.52 | 83.73 | 74.08 | 79.45 |
| Innovator | 94.68 | 77.79 | 83.99 | 85.49 | |
| Lilly | 88.15 | 81.18 | 87.89 | 85.74 | |
| Lady Claire | 92.01 | 78.75 | 75.83 | 82.20 | |
| Mean | 88.84 | 80.36 | 80.45 | 83.22 | |
| Overall varieties | Verdi | 80.60 | 83.10 | 71.24 | 78.31 |
| Innovator | 94.58 | 75.76 | 85.43 | 85.26 | |
| Lilly | 85.56 | 84.22 | 83.94 | 84.57 | |
| Lady Claire | 90.57 | 74.46 | 77.81 | 80.95 | |
| Mean | 87.83 | 79.38 | 79.60 | 82.27 | |
| Experimental Factors | Years | Mean | |||
|---|---|---|---|---|---|
| Localities | Technology | 2015 | 2016 | 2017 | |
| Kędrzyno | Traditional | 95.97 | 91.79 | 94.20 | 93.92 |
| With Supporter | 93.82 | 88.95 | 91.38 | 91.38 | |
| Mean | 94.90 | 90.37 | 92.79 | 92.65 | |
| Barankowo | Traditional | 99.14 | 80.94 | 88.03 | 89.37 |
| With Supporter | 99.48 | 94.98 | 90.56 | 95.01 | |
| Mean | 99.31 | 87.96 | 89.30 | 92.19 | |
| Ryn | Traditional | 88.24 | 96.79 | 84.56 | 89.86 |
| With Supporter | 93.01 | 95.50 | 92.41 | 93.64 | |
| Mean | 90.63 | 96.15 | 88.49 | 91.80 | |
| Głubczyce | Traditional | 96.22 | 58.31 | 82.01 | 78.85 |
| With Supporter | 96.62 | 67.24 | 69.45 | 77.77 | |
| Mean | 96.42 | 62.78 | 75.73 | 78.31 | |
| Technology | Varieties | Years | Mean | ||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | |||
| Traditional | Verdi | 52.23 | 68.07 | 59.89 | 60.06 |
| Innovator | 32.72 | 47.75 | 44.74 | 41.74 | |
| Lilly | 74.56 | 65.06 | 68.64 | 69.42 | |
| Lady Claire | 50.87 | 76.19 | 70.81 | 65.96 | |
| Mean | 52.60 | 64.27 | 61.02 | 59.29 | |
| With Supporter | Verdi | 49.33 | 80.65 | 63.91 | 64.63 |
| Innovator | 56.52 | 44.17 | 55.96 | 52.21 | |
| Lilly | 94.51 | 75.32 | 71.34 | 80.39 | |
| Lady Claire | 48.89 | 80.00 | 83.96 | 70.95 | |
| Mean | 62.31 | 70.04 | 68.79 | 67.05 | |
| Overall Varieties | Verdi | 50.78 | 74.36 | 61.90 | 62.35 |
| Innovator | 44.62 | 45.96 | 50.35 | 46.98 | |
| Lilly | 84.54 | 70.19 | 69.99 | 74.91 | |
| Lady Claire | 49.88 | 78.10 | 77.38 | 68.45 | |
| Mean | 57.45 | 67.15 | 64.91 | 63.17 | |
| Localities | Technology | 2015 | 2016 | 2017 | Mean |
|---|---|---|---|---|---|
| Kędrzyno | Traditional | 44.39 | 56.87 | 35.31 | 45.53 |
| With Supporter | 50.79 | 56.59 | 51.32 | 52.90 | |
| Mean | 47.59 | 56.73 | 43.31 | 49.21 | |
| Barankowo | Traditional | 68.32 | 76.07 | 79.10 | 74.50 |
| With Supporter | 83.61 | 74.72 | 85.83 | 81.39 | |
| Mean | 75.97 | 75.39 | 82.47 | 77.94 | |
| Ryn | Traditional | 76.98 | 76.41 | 72.38 | 75.26 |
| With Supporter | 81.85 | 79.13 | 68.57 | 76.52 | |
| Mean | 79.42 | 77.77 | 70.48 | 75.89 | |
| Głubczyce | Traditional | 52.51 | 49.93 | 51.99 | 51.48 |
| With Supporter | 65.27 | 70.35 | 71.76 | 69.13 | |
| Mean | 58.89 | 60.14 | 61.88 | 60.30 | |
| Technology | Varieties | Years | Mean | ||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | |||
| Traditional | Verdi | 3.51 | 6.09 | 3.91 | 4.51 |
| Innovator | 4.04 | 3.17 | 3.30 | 3.50 | |
| Lilly | 7.36 | 5.89 | 6.01 | 6.42 | |
| Lady Claire | 3.68 | 6.50 | 6.38 | 5.52 | |
| Mean | 4.65 | 5.41 | 4.90 | 4.99 | |
| With Supporter | Verdi | 3.13 | 8.71 | 4.84 | 5.56 |
| Innovator | 4.85 | 3.83 | 4.21 | 4.29 | |
| Lilly | 12.60 | 7.37 | 7.16 | 9.04 | |
| Lady Claire | 6.70 | 8.06 | 8.57 | 7.77 | |
| Mean | 6.82 | 6.99 | 6.19 | 6.67 | |
| Overall Varieties | Verdi | 3.32 | 7.40 | 4.38 | 5.03 |
| Innovator | 4.44 | 3.50 | 3.75 | 3.90 | |
| Lilly | 9.98 | 6.63 | 6.58 | 7.73 | |
| Lady Claire | 5.19 | 7.28 | 7.47 | 6.65 | |
| Mean | 5.73 | 6.20 | 5.55 | 5.83 | |
| Localities | Technology | 2015 | 2016 | 2017 | Mean |
|---|---|---|---|---|---|
| Kędrzyno | Traditional | 3.54 | 5.67 | 2.31 | 3.84 |
| With Supporter | 3.76 | 6.50 | 3.85 | 4.70 | |
| Mean | 3.65 | 6.09 | 3.08 | 4.27 | |
| Barankowo | Traditional | 3.53 | 4.63 | 4.90 | 4.35 |
| With Supporter | 7.54 | 4.95 | 6.45 | 6.31 | |
| Mean | 5.53 | 4.79 | 5.68 | 5.33 | |
| Ryn | Traditional | 7.99 | 7.74 | 6.34 | 7.36 |
| With Supporter | 11.48 | 9.12 | 6.88 | 9.16 | |
| Mean | 9.73 | 8.43 | 6.61 | 8.26 | |
| Głubczyce | Traditional | 4.10 | 3.53 | 4.68 | 4.10 |
| With Supporter | 4.92 | 8.11 | 7.32 | 6.79 | |
| Mean | 4.51 | 5.82 | 6.00 | 5.45 | |
| Overall localities | Traditional | 4.79 | 5.39 | 4.56 | 4.91 |
| With Supporter | 6.92 | 7.17 | 6.13 | 6.74 | |
| Mean | 5.86 | 6.28 | 5.34 | 5.83 | |
| Specification | y | x1 | x2 | x3 | x4 | x5 |
|---|---|---|---|---|---|---|
| Mean | 34.43 | 82.38 | 133.39 | 525.78 | 63.17 | 5.83 |
| Median | 32.38 | 83.01 | 131.00 | 533.80 | 63.45 | 6.11 |
| Standard deviation | 9.17 | 7.78 | 43.08 | 147.72 | 9.04 | 1.27 |
| Kurtosis | −0.05 | −0.34 | −0.99 | −0.80 | −0.41 | −0.30 |
| Skewness | 0.39 | −0.33 | 0.03 | −0.06 | −0.07 | −0.36 |
| Coefficient of variation V (%) | 26.63 | 9.45 | 32.30 | 28.10 | 14.31 | 21.78 |
| Specification | y | x1 | x2 | x3 | x4 | x5 |
|---|---|---|---|---|---|---|
| y | 1.00 | |||||
| x1 | −0.21 * | 1.00 | ||||
| x2 | 0.01 | 0.04 | 1.00 | |||
| x3 | −0.01 | 0.15 * | −0.15 * | 1.00 | ||
| x4 | 0.17 * | −0.10 | 0.10 | −0.15 * | 1.00 | |
| x5 | 0.50 ** | 0.14 * | −0.13 * | 0.78 ** | 0.07 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbaś, P.; Pszczółkowski, P.; Krochmal-Marczak, B.; Hameed, T.S.; Sawicka, B. Soil-Specific Effects of the Bio-Growth Regulator Supporter on Seed Potato Yield and Quality Across Varieties: Unlocking Sustainable Potential in Diverse Environments. Land 2025, 14, 595. https://doi.org/10.3390/land14030595
Barbaś P, Pszczółkowski P, Krochmal-Marczak B, Hameed TS, Sawicka B. Soil-Specific Effects of the Bio-Growth Regulator Supporter on Seed Potato Yield and Quality Across Varieties: Unlocking Sustainable Potential in Diverse Environments. Land. 2025; 14(3):595. https://doi.org/10.3390/land14030595
Chicago/Turabian StyleBarbaś, Piotr, Piotr Pszczółkowski, Barbara Krochmal-Marczak, Talal Saeed Hameed, and Barbara Sawicka. 2025. "Soil-Specific Effects of the Bio-Growth Regulator Supporter on Seed Potato Yield and Quality Across Varieties: Unlocking Sustainable Potential in Diverse Environments" Land 14, no. 3: 595. https://doi.org/10.3390/land14030595
APA StyleBarbaś, P., Pszczółkowski, P., Krochmal-Marczak, B., Hameed, T. S., & Sawicka, B. (2025). Soil-Specific Effects of the Bio-Growth Regulator Supporter on Seed Potato Yield and Quality Across Varieties: Unlocking Sustainable Potential in Diverse Environments. Land, 14(3), 595. https://doi.org/10.3390/land14030595









