Climate-Driven Alterations in the Mercury Cycle: Implications for Wildlife Managers Through a One Health Lens
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Effects of Increasing Global Temperatures
3.1.1. Increases in Ocean Temperatures
3.1.2. Increases in Land Temperatures
3.1.3. Increased Cryosphere Melting [28]
3.1.4. Increases in Temperatures in Freshwater Systems [28]
3.1.5. Changing Climate Oscillations
3.1.6. Sea Level Rise
3.1.7. Increase in Heavy Precipitation and Storms
3.2. Effects of Increasing Drought Conditions
3.2.1. Increase in Wildfire Intensity
3.2.2. Drying of Freshwater Systems
3.3. Other Co-Factors Influencing Mercury in Fish and Wildlife
3.3.1. Human Land Use Change
3.3.2. Overfishing
3.3.3. Other Pollutants
4. Discussion
4.1. Case Study: The National Wildlife Refuge System
4.2. Effects of Mercury on Wildlife Related to Physiology and Behavior
4.2.1. Dosing Studies
4.2.2. Immunosuppression
4.2.3. Endocrine Disruption
4.2.4. Reproduction
4.3. Changes in Mercury Exposure Related to Habitat
4.3.1. Migration
4.3.2. Range and/or Diet Shifts
4.4. Co-Exposure to Mercury and to Other Stressors
4.4.1. Other Contaminants
4.4.2. Other Stressors
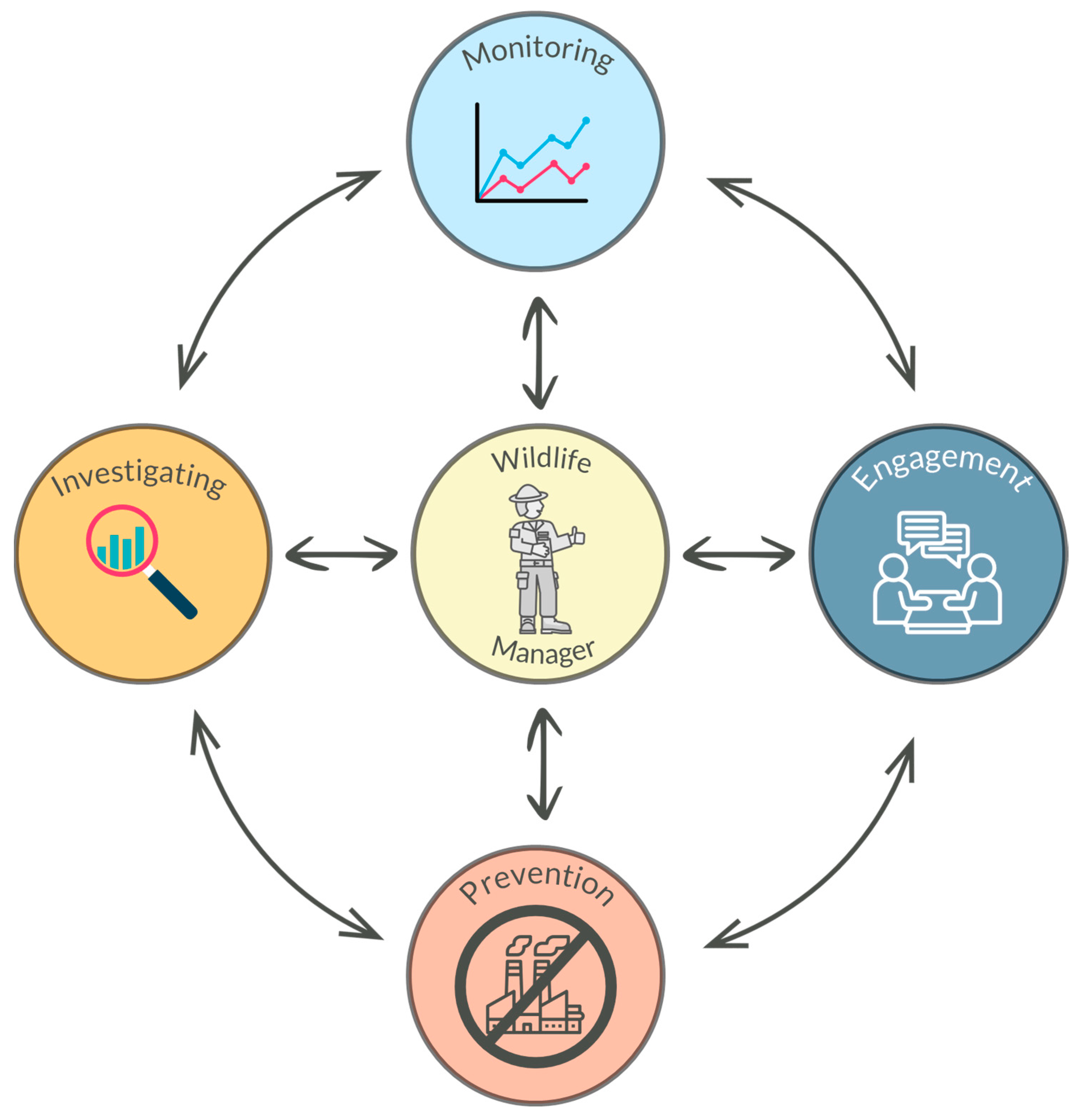
4.5. Management Actions That Could Potentially Alleviate the Effects of Mercury Exposure
4.5.1. Investigating
4.5.2. Monitoring
4.5.3. Engagement
4.5.4. Prevention
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pompeani, D.P.; Cooke, C.A.; Abbott, M.B.; Drevnick, P.E. Climate, fire, and vegetation mediate mercury delivery to midlatitude lakes over the Holocene. Environ. Sci. Technol. 2018, 52, 8157–8164. [Google Scholar] [CrossRef]
- Vo, A.T.E.; Bank, M.S.; Shine, J.P.; Edwards, S.V. Temporal increase in organic mercury in an endangered pelagic seabird assessed by century-old museum specimens. Proc. Natl. Acad. Sci. USA 2011, 108, 7466–7471. [Google Scholar] [CrossRef] [PubMed]
- Routti, H.; Atwood, T.C.; Bechshoft, T.; Boltunov, A.; Ciesielski, T.M.; Desforges, J.P.; Dietz, R.; Gabrielsen, G.W.; Jenssen, B.M.; Letcher, R.J.; et al. State of knowledge on current exposure, fate and potential health effects of contaminants in polar bears from the circumpolar Arctic. Sci. Total Environ. 2019, 664, 1063–1083. [Google Scholar] [CrossRef] [PubMed]
- Sonke, J.E.; Angot, H.; Zhang, Y.; Poulain, A.; Björn, E.; Schartup, A. Global change effects on biogeochemical mercury cycling. Ambio 2023, 52, 853–876. [Google Scholar] [CrossRef] [PubMed]
- Krabbenhoft, D.P.; Rickert, D.A. Mercury Contamination of Aquatic Ecosystems; U.S. Geological Survey: Menlo Park, CA, USA, 1995; Report 216-95; p. 4. [Google Scholar] [CrossRef]
- Edwards, G.N. Two cases of poisoning by mercuric methide. St. Bartholomew’s Hosp Rep. 1865, 1, 141–150. [Google Scholar]
- Grandjean, P.; Satoh, H.; Murata, K.; Eto, K. Adverse effects of methylmercury: Environmental health research implications. Environ. Health Perspect. 2010, 118, 1137–1145. [Google Scholar] [CrossRef]
- Choi, A.L.; Grandjean, P. Methylmercury exposure and health effects in humans. Environ. Chem. 2008, 5, 112. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, Y.; Lee, K. Methylmercury exposure and health effects. J. Prev. Med. Public Health 2012, 45, 353–363. [Google Scholar] [CrossRef]
- Karagas, M.R.; Choi, A.L.; Oken, E.; Horvat, M.; Schoeny, R.; Kamai, E.; Cowell, W.; Grandjean, P.; Korrick, S. Evidence on the human health effects of Low-Level Methylmercury exposure. Environ. Health Perspect. 2012, 120, 799–806. [Google Scholar] [CrossRef]
- Scheuhammer, A.; Braune, B.; Chan, H.M.; Frouin, H.; Krey, A.; Letcher, R.; Loseto, L.; Noël, M.; Ostertag, S.; Ross, P.; et al. Recent progress on our understanding of the biological effects of mercury in fish and wildlife in the Canadian Arctic. Sci. Total Environ. 2015, 509, 91–103. [Google Scholar] [CrossRef]
- Evers, D. The Effects of Methylmercury on Wildlife: A Comprehensive Review and Approach for Interpretation. Encycl. Anthr. 2018, 5, 181–194. [Google Scholar] [CrossRef]
- Dietz, R.; Outridge, P.M.; Hobson, K.A. Anthropogenic contributions to mercury levels in present-day Arctic animals—A review. Sci. Total Environ. 2009, 407, 6120–6131. [Google Scholar] [CrossRef]
- Dietz, R.; Sonne, C.; Basu, N.; Braune, B.; O’Hara, T.; Letcher, R.J.; Scheuhammer, T.; Andersen, M.; Andreasen, C.; Andriashek, D.; et al. What are the toxicological effects of mercury in Arctic biota? Sci. Total Environ. 2013, 443, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.L.; Robertson, G.J. Mercury concentrations in multiple tissues of Arctic Iceland Gulls (Larus glaucoides) wintering in Newfoundland. Arct. Sci. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Pelletier, N.; Chételat, J.; Blarquez, O.; Vermaire, J.C. Paleolimnological assessment of wildfire-derived atmospheric deposition of trace metal(loid)s and major ions to subarctic lakes (Northwest Territories, Canada). J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005720. [Google Scholar] [CrossRef]
- Pelletier, N.; Chételat, J.; Palmer, M.J.; Vermaire, J.C. Bog and Lake Sediment Archives Reveal a Lagged Response of Subarctic Lakes to Diminishing Atmospheric Hg and Pb Deposition. Sci. Total Environ. 2021, 775, 145521. [Google Scholar] [CrossRef]
- Amos, H.M.; Jacob, D.J.; Streets, D.G.; Sunderland, E.M. Legacy impacts of all-time anthropogenic emissions on the global mercury cycle. Glob. Biogeochem. Cycles 2013, 27, 410–421. [Google Scholar] [CrossRef]
- Li, F.; Ma, C.; Zhang, P. Mercury deposition, climate change and anthropogenic activities: A review. Front. Earth Sci. 2020, 8, 316. [Google Scholar] [CrossRef]
- USGCRP. Fifth National Climate Assessment; Crimmins, A.R., Avery, C.W., Easterling, D.R., Kunkel, K.E., Stewart, B.C., Maycock, T.K., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Wilson, A.B.; Baker, J.M.; Ainsworth, E.A.; Andresen, J.; Austin, J.A.; Dukes, J.S.; Gibbons, E.; Hoppe, B.O.; LeDee, O.E.; Noel, J.; et al. Chapter 24, Midwest. In Fifth National Climate Assessment; Crimmins, A.R., Avery, C.W., Easterling, D.R., Kunkel, K.E., Stewart, B.C., Maycock, T.K., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- McPherson, R.A.; Fay, P.A.; Alvarez, S.G.; Bertrand, D.; Broadbent, T.L.; Bruno, T.; Fares, A.; McCullough, B.; Moore, G.W.; Moorhead, B.; et al. Chapter 26, Southern Great Plains. In Fifth National Climate Assessment; Crimmins, A.R., Avery, C.W., Easterling, D.R., Kunkel, K.E., Stewart, B.C., Maycock, T.K., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Eagles-Smith, C.A.; Silbergeld, E.K.; Basu, N.; Bustamante, P.; Diaz-Barriga, F.; Hopkins, W.A.; Kidd, K.A.; Nyland, J.F. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 2018, 47, 170–197. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y. Climate warming and land use change in Heilongjiang Province, Northeast China. Appl. Geogr. 2011, 31, 476–482. [Google Scholar] [CrossRef]
- Christensen, N.S.; Wood, A.W.; Voisin, N.; Lettenmaier, D.P.; Palmer, R.N. The effects of climate change on the hydrology and water resources of the Colorado River basin. Clim. Change 2004, 62, 337–363. [Google Scholar] [CrossRef]
- Olden, J.D.; Naiman, R.J. Incorporating thermal regimes into environmental flows assessments: Modifying dam operations to restore freshwater ecosystem integrity. Freshw. Biol. 2010, 55, 86–107. [Google Scholar] [CrossRef]
- Chételat, J.; Ackerman, J.T.; Eagles-Smith, C.A.; Hebert, C.E. Methylmercury exposure in wildlife: A review of the ecological and physiological processes affecting contaminant concentrations and their interpretation. Sci. Total Environ. 2020, 711, 135117. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; p. 184. Available online: https://www.ipcc.ch/report/ar6/syr/downloads/report/IPCC_AR6_SYR_FullVolume.pdf (accessed on 30 June 2024).
- Capo, E.; Broman, E.; Bonaglia, S.; Bravo, A.G.; Bertilsson, S.; Soerensen, A.L.; Pinhassi, J.; Lundin, D.; Buck, M.; Hall, P.O.J.; et al. Oxygen-deficient water zones in the Baltic Sea promote uncharacterized Hg methylating microorganisms in underlying sediments. Limnol. Oceanogr. 2022, 67, 135–146. [Google Scholar] [CrossRef]
- Coppola, F.; Bessa, A.; Henriques, B.; Russo, T.; Soares, A.M.V.M.; Figueira, E.; Pereira, E.; Marques, P.; Polese, G.; Freitas, R. The Role of Temperature on the Impact of Remediated Water towards Marine Organisms. Water 2020, 12, 2148. [Google Scholar] [CrossRef]
- Schartup, A.T.; Thackray, C.P.; Qureshi, A.; Dassuncao, C.; Gillespie, K.; Hanke, A.; Sunderland, E.M. Climate change and overfishing increase neurotoxicant in marine predators. Nature 2019, 572, 648–650. [Google Scholar] [CrossRef]
- Jiskra, M.; Sonke, J.E.; Obrist, D.; Bieser, J.; Ebinghaus, R.; Myhre, C.L.; Pfaffhuber, K.A.; Wängberg, I.; Kyllönen, K.; Worthy, D.; et al. A vegetation control on seasonal variations in global atmospheric mercury concentrations. Nat. Geosci. 2018, 11, 244–250. [Google Scholar] [CrossRef]
- Luo, K.; Yuan, W.; Liu, N.; Zeng, S.; Wang, D.; Lu, Z.; Wang, X.; Feng, X. Remarkable Variation in the Process of Hg Accumulation in Timberline Forests Indicates an Aggravated Hg Burden in Alpine Forests Under Climate Warming. JGR Biogeosci. 2022, 127, e2022JG006940. [Google Scholar] [CrossRef]
- Feinberg, A.; Jiskra, M.; Borrelli, P.; Biswakarma, J.; Selin, N.E. Deforestation as an anthropogenic driver of mercury pollution. Environ. Sci. Technol. 2024, 58, 3246–3257. [Google Scholar] [CrossRef]
- Sumner, A.W.; Johnston, T.A.; Lescord, G.L.; Branfireun, B.A.; Gunn, J.M. Mercury bioaccumulation in lacustrine fish populations along a climatic gradient in Northern Ontario, Canada. Ecosystems 2020, 23, 1206–1226. [Google Scholar] [CrossRef]
- St Pierre, K.A.; Zolkos, S.; Shakil, S.; Tank, S.E.; St Louis, V.L.; Kokelj, S.V. Unprecedented increases in total and methyl mercury concentrations downstream of retrogressive thaw slumps in the western Canadian Arctic. Environ. Sci. Technol. 2018, 52, 14099–14109. [Google Scholar] [CrossRef]
- Gordon, J.; Quinton, W.; Branfireun, B.A.; Olefeldt, D. Mercury and methylmercury biogeochemistry in a thawing permafrost wetland complex, Northwest Territories, Canada. Hydrol. Process. 2016, 30, 3627–3638. [Google Scholar] [CrossRef]
- St Pierre, K.A.; St Louis, V.L.; Lehnherr, I.; Gardner, A.S.; Serbu, J.A.; Mortimer, C.A.; Muir, D.C.G.; Wiklund, J.A.; Lemire, D.; Szostek, L. Drivers of mercury cycling in the rapidly changing glacierized watershed of the high Arctic’s largest lake by volume (Lake Hazen, Nunavut, Canada). Environ. Sci. Technol. 2018, 53, 1175–1185. [Google Scholar] [CrossRef]
- Dommergue, A.; Larose, C.; Faïn, X.; Clarisse, O.; Foucher, D.; Hintelmann, H.; Schneider, D.; Ferrari, C.P. Deposition of mercury species in the Ny-Alesund area (79 N) and their transfer during snowmelt. Environ. Sci. Technol. 2010, 44, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Chételat, J.; McKinney, M.A.; Amyot, M.; Dastoor, A.; Douglas, T.A.; Heimbürger-Boavida, L.E.; Kirk, J.; Kahilainen, K.K.; Outridge, P.M.; Pelletier, N.; et al. Climate change and mercury in the Arctic: Abiotic interactions. Sci. Total Environ. 2022, 824, 153715. [Google Scholar] [CrossRef] [PubMed]
- Ramlal, P.S.; Kelly, C.A.; Rudd, J.W.M.; Furutani, A. Sites of methyl mercury production in remote Canadian shield. Can. J. Fish. Aquat. Sci. 1993, 50, 972–979. [Google Scholar] [CrossRef]
- Watras, C.J.; Bloom, N.S.; Claas, S.A.; Morrison, K.A.; Gilmour, C.C.; Craig, S.R. Methylmercury production in the anoxic hypolimnion of a dimictic seepage lake. Water Air Soil Pollut. 1995, 80, 735–745. [Google Scholar] [CrossRef]
- Rask, M.; Verta, M.; Korhonen, M.; Salo, S.; Forsius, M.; Arvola, L.; Jones, R.I.; Kiljunen, M. Does lake thermocline depth affect methyl mercury concentrations in fish? Biogeochemistry 2010, 101, 311–322. [Google Scholar] [CrossRef]
- Lescord, G.L.; Emilson, E.J.; Johnston, T.A.; Branfireun, B.A.; Gunn, J.M. Optical properties of dissolved organic matter and their relation to mercury concentrations in water and biota across a remote freshwater drainage basin. Environ. Sci. Technol. 2018, 52, 3344–3353. [Google Scholar] [CrossRef]
- Chiapella, A.M.; Eagles-Smith, C.A.; Strecker, A.L. From forests to fish: Mercury in mountain lake food webs influenced by factors at multiple scales. Limnol. Oceanogr. 2021, 66, 1021–1035. [Google Scholar] [CrossRef]
- Freitas, A.; Bernardino, M.; Guedes Soares, C. The influence of the Arctic Oscillation on North Atlantic wind and wave climate by the end of the 21st century. Ocean. Eng. 2022, 246, 110634. [Google Scholar] [CrossRef]
- Morris, A.D.; Braune, B.M.; Gamberg, M.; Stow, J.; O’Brien, J.; Letcher, R.J. Temporal change and the influence of climate and weather factors on mercury concentrations in Hudson Bay polar bears, caribou, and seabird eggs. Environ. Res. 2022, 207, 112169. [Google Scholar] [CrossRef]
- McKinney, M.A.; Chételat, J.; Burke, S.M.; Elliott, K.H.; Fernie, K.J.; Houde, M.; Kahilainen, K.K.; Letcher, R.J.; Morris, A.D.; Muir, D.C.G.; et al. Climate change and mercury in the Arctic: Biotic interactions. Sci. Total Environ. 2022, 834, 155221. [Google Scholar] [CrossRef]
- de Lacerda, L.D.; Marins, R.V.; da Silva Dias, F.J. An Arctic Paradox: Response of Fluvial Hg Inputs and Bioavailability to Global Climate Change in an Extreme Coastal Environment. Front. Earth Sci. 2020, 8, 93. [Google Scholar] [CrossRef]
- Ruskin, K.J.; Herring, G.; Eagles-Smith, C.A.; Eiklor, A.B.; Elphick, C.S.; Etterson, M.A.; Field, C.R.; Longenecker, R.A.; Kovach, A.I.; Gregory Shriver, W. Mercury exposure of tidal marsh songbirds in the northeastern United States and its association with nest survival. Ecotoxicology 2022, 31, 208–220. [Google Scholar] [CrossRef]
- Hall, B.D.; Louis, V.S.; Rolfhus, K.R.; Bodaly, R.A.; Beaty, K.G.; Paterson, M.J.; Cherewyk, K.P. Impacts of reservoir creation on the biogeochemical cycling of methyl mercury and total mercury in boreal upland forests. Ecosystems 2005, 8, 248–266. [Google Scholar] [CrossRef]
- Roy, V.; Amyot, M.; Carignan, R. Seasonal methylmercury dynamics in water draining three beaver impoundments of varying age. J. Geophys. Res. Biogeosci. 2009, 114, 2–12. [Google Scholar] [CrossRef]
- Eckley, C.S.; Luxton, T.P.; McKernan, J.L.; Goetz, J.; Goulet, J. Influence of reservoir water level fluctuations on sediment methylmercury concentrations downstream of the historical Black Butte mercury mine, OR. Appl. Geochem. 2015, 61, 284–293. [Google Scholar] [CrossRef]
- Kelly, E.N.; Schindler, D.W.; St Louis, V.L.; Donald, D.B.; Vladicka, K.E. Forest fire increases mercury accumulation by fishes via food web restructuring and increased mercury inputs. Proc. Natl. Acad. Sci. USA 2006, 103, 19380–19385. [Google Scholar] [CrossRef]
- Abraham, J.; Dowling, K.; Florentine, S. Effects of prescribed fire and post-fire rainfall on mercury mobilization and subsequent contamination assessment in a legacy mine site in Victoria, Australia. Chemosphere 2018, 190, 144–153. [Google Scholar] [CrossRef]
- Pelletier, N.; Chételat, J.; Sinon, S.; Vermaire, J.C. Wildfires trigger multi-decadal increases in sedimentation rate and metal loading to subarctic montane lakes. Sci. Total Environ. 2022, 824, 153738. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Gillies, E.J.; Briner, R.; Hoover, C.A.; Sora, K.J.; Loseto, L.L.; Walters, W.J.; Cheung, W.W.L.; Giang, A. Investigating the dynamics of methylmercury bioaccumulation in the Beaufort Sea shelf food web: A modeling perspective. Environ. Sci. Process. Impacts 2022, 24, 1010–1025. [Google Scholar] [CrossRef]
- Patel, K.F.; Jakubowski, M.D.; Fernandez, I.J.; Nelson, S.J.; Gawley, W. Soil nitrogen and mercury dynamics seven decades after a fire disturbance: A case study at Acadia National Park. Water Air Soil Pollut. 2019, 230, 29. [Google Scholar] [CrossRef]
- Garcia, E.; Carignan, R. Mercury concentrations in northern pike (Esox lucius) from boreal lakes with logged, burned, or undisturbed catchments. Can. J. Fish. Aquat. Sci. 2000, 57 (Suppl. 2), 129–135. [Google Scholar] [CrossRef]
- McCullough, I.M.; Cheruvelil, K.S.; Lapierre, J.F.; Lottig, N.R.; Moritz, M.A.; Stachelek, J.; Soranno, P.A. Do lakes feel the burn? Ecological consequences of increasing exposure of lakes to fire in the continental United States. Glob. Change Biol. 2019, 25, 2841–2854. [Google Scholar] [CrossRef] [PubMed]
- Woolway, R.I.; Kraemer, B.M.; Lenters, J.D.; Merchant, C.J.; O’Reilly, C.M.; Sharma, S. Global lake responses to climate change. Nat. Rev. Earth Environ. 2020, 1, 388–403. [Google Scholar] [CrossRef]
- Hebert, C.E.; Chételat, J.; Beck, R.; Dolgova, S.; Fordy, K.; Kirby, P.; Martin, P.; Rabesca, M. Inter-annual variation of mercury in aquatic bird eggs and fish from a large subarctic lake under a warming climate. Sci. Total Environ. 2021, 766, 144614. [Google Scholar] [CrossRef]
- Zabel, N.A.; Hall, R.I.; Branfireun, B.A.; Swanson, H.K. Mercury accumulation in sediments of Lhù’ààn Mânʼ (Kluane Lake, YT): Response to past hydrological change. Arct. Antarct. Alp. Res. 2021, 53, 179–195. [Google Scholar] [CrossRef]
- Gustin, M.S.; Bank, M.S.; Bishop, K.; Bowman, K.; Branfireun, B.; Chételat, J.; Eckley, C.S.; Hammerschmidt, C.R.; Lamborg, C.; Lyman, S. Mercury biogeochemical cycling: A synthesis of recent scientific advances. Sci. Total Environ. 2020, 737, 139619. [Google Scholar] [CrossRef]
- Gerson, J.R.; Szponar, N.; Zambrano, A.A. Amazon forests capture high levels of atmospheric mercury pollution from artisanal gold mining. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Feinberg, A.; Dlamini, T.; Jiskra, M.; Shah, V.; Selin, N.E. Evaluating atmospheric mercury (Hg) uptake by vegetation in a chemistry-transport model. Environ. Sci. Process. Impacts 2022, 24, 1303–1318. [Google Scholar] [CrossRef]
- Carpi, A.; Fostier, A.H.; Orta, O.R.; dos Santos, J.C.; Gittings, M. Gaseous mercury emissions from soil following forest loss and land use changes: Field experiments in the United States and Brazil. Atmos. Environ. 2014, 96, 423–429. [Google Scholar] [CrossRef]
- Issifu, I.; Alava, J.J.; Lam, V.W.; Sumaila, U.R. Impact of ocean warming, overfishing and mercury on European fisheries: A risk assessment and policy solution framework. Front. Mar. Sci. 2022, 8, 770805. [Google Scholar] [CrossRef]
- Cusset, F.; Reynolds, S.J.; Carravieri, A.; Amouroux, D.; Asensio, O.; Dickey, R.C.; Fort, J.; Hughes, B.J.; Paiva, V.H.; Ramos, J.A. A century of mercury: Ecosystem-wide changes drive increasing contamination of a tropical seabird species in the South Atlantic Ocean. Environ. Pollut. 2023, 323, 121187. [Google Scholar] [CrossRef] [PubMed]
- Coleman Wasik, J.K.; Mitchell, C.P.; Engstrom, D.R.; Swain, E.B.; Monson, B.A.; Balogh, S.J.; Jeremiason, J.D.; Branfireun, B.A.; Eggert, S.L.; Kolka, R.K.; et al. Methylmercury declines in a boreal peatland when experimental sulfate deposition decreases. Environ. Sci. Technol. 2012, 46, 6663–6671. [Google Scholar] [CrossRef]
- Peraza, I.; Chételat, J.; Richardson, M.; Jung, T.S.; Awan, M.; Baryluk, S.; Dastoor, A.; Harrower, W.; Kukka, P.M.; McClelland, C.; et al. Diet and landscape characteristics drive spatial patterns of mercury accumulation in a high-latitude terrestrial carnivore. PLoS ONE 2023, 18, e0285826. [Google Scholar] [CrossRef] [PubMed]
- Barst, B.D.; Chételat, J.; Basu, N. Toxicological risk of mercury for fish and invertebrate prey in the Arctic. Sci. Total Environ. 2022, 836, 155702. [Google Scholar] [CrossRef]
- Eagles-Smith, C.A.; Willacker, J.J.; Nelson, S.J.; Flanagan Pritz, C.M.; Krabbenhoft, D.P.; Chen, C.Y.; Ackerman, J.T.; Grant, E.H.C.; Pilliod, D.S. A national-scale assessment of mercury bioaccumulation in United States national parks using dragonfly larvae as biosentinels through a citizen-science framework. Environ. Sci. Technol. 2020, 54, 8779–8790. [Google Scholar] [CrossRef]
- Eagles-Smith, C.A.; Wiener, J.G.; Eckley, C.S.; Willacker, J.J.; Evers, D.C.; Marvin-DiPasquale, M.; Obrist, D.; Fleck, J.A.; Aiken, G.R.; Lepak, J.M.; et al. Mercury in western North America: A synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci. Total Environ. 2016, 568, 1213–1226. [Google Scholar] [CrossRef]
- Congressional Research Service (CRS). National Wildlife Refuge System (MWRS): Overview and Issues for Congress. 2025. R48381. Available online: https://crsreports.congress.gov (accessed on 13 February 2025).
- Rosa, L.; Malcom, J. Finding refuge: Imperiled species and the National Wildlife Refuge System. Def. Wildl. 2020, 8, 1–8. [Google Scholar]
- Custer, T.W.; Custer, C.M.; Johnson, K.M.; Hoffman, D.J. Mercury and other element exposure to tree swallows (Tachycineta bicolor) nesting on Lostwood National Wildlife Refuge, North Dakota. Environ. Pollut. 2008, 155, 217–226. [Google Scholar] [CrossRef]
- Sando, S.K.; Krabbenhoft, D.P.; Johnson, K.M.; Lundgren, R.F.; Emerson, D.G. Mercury and Methylmercury in Water and Bottom Sediments of Wetlands at Lostwood National Wildlife Refuge, North Dakota, 2003–04; U.S. Geological Survey: Reston, VA, USA, 2007; Volume 5219. [Google Scholar]
- Cowdery, T.K.; Brigham, M.E. Mercury in Wetlands at the Glacial Ridge National Wildlife Refuge, Northwestern Minnesota, 2007–9; U.S. Geological Survey: Reston, VA, USA, 2013; Volume 5086. [Google Scholar]
- Nilsen, F.M.; Dorsey, J.E.; Lowers, R.H.; Guillette, L.J., Jr.; Long, S.E.; Bowden, J.A.; Schock, T.B. Evaluating mercury concentrations and body condition in American alligators (Alligator mississippiensis) at Merritt Island National Wildlife Refuge (MINWR), Florida. Sci. Total Environ. 2017, 607, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, S.; Gerstenberger, S. Mercury concentrations in largemouth bass (Micropterus salmoides) collected from ash meadows National Wildlife Refuge, Nye County, Nevada. Arch. Environ. Contam. Toxicol. 2011, 60, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Lane, O.; Adams, E.M.; Pau, N.; O'Brien, K.M.; Regan, K.; Farina, M.; Schneider-Moran, T.; Zarudsky, J. Long-term monitoring of mercury in adult saltmarsh sparrows breeding in Maine, Massachusetts and New York, USA 2000–2017. Ecotoxicology 2020, 29, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Raftovich, B.; Fleming, K.K.; Chandler, S.C.; Cain, C. Migratory Bird Hunting Activity and Harvest During the 2021–22 and 2022–23 Hunting Seasons; U.S. Fish and Wildlife Service: Laurel, MD, USA, 2023. [Google Scholar]
- Hoskins, B.B.; Hupp, E.W. Methylmercury effects in rat, hamster, and squirrel monkey: Lethality, symptoms, brain mercury, and amino acids. Environ. Res. 1978, 15, 5–19. [Google Scholar] [CrossRef]
- Rimmer, C.C.; McFarland, K.P.; Evers, D.C.; Miller, E.K.; Aubry, Y.; Busby, D.; Taylor, R.J. Mercury concentrations in Bicknell’s thrush and other insectivorous passerines in montane forests of northeastern North America. Ecotoxicology 2005, 14, 223–240. [Google Scholar] [CrossRef]
- Bank, M.S.; Crocker, J.B.; Davis, S.; Brotherton, D.K.; Cook, R.; Behler, J.; Connery, B. Population decline of northern dusky salamanders at Acadia National Park, Maine, USA. Biol. Conserv. 2006, 130, 230–238. [Google Scholar] [CrossRef]
- Dietz, R.; Letcher, R.J.; Aars, J.; Andersen, M.; Boltunov, A.; Born, E.W.; Ciesielski, T.M.; Das, K.; Dastnai, S.; Derocher, A.E.; et al. A risk assessment review of mercury exposure in Arctic marine and terrestrial mammals. Sci. Total Environ. 2022, 829, 154445. [Google Scholar] [CrossRef]
- Sonne, C.; Siebert, U.; Gonnsen, K.; Desforges, J.P.; Eulaers, I.; Persson, S.; Roos, A.; Bäcklin, B.-M.; Kauhala, K.; Olsen, M.T.; et al. Health effects from contaminant exposure in Baltic Sea birds and marine mammals: A review. Environ. Int. 2020, 139, 105725. [Google Scholar] [CrossRef]
- Chastel, O.; Fort, J.; Ackerman, J.T.; Albert, C.; Angelier, F.; Basu, N.; Blévin, P.; Brault-Favrou, M.; Bustnes, J.O.; Bustamante, P.; et al. Mercury contamination and potential health risks to Arctic seabirds and shorebirds. Sci. Total Environ. 2022, 844, 156944. [Google Scholar] [CrossRef]
- Teitelbaum, C.S.; Ackerman, J.T.; Hill, M.A.; Satter, J.M.; Casazza, M.L.; De La Cruz, S.E.W.; Boyce, W.M.; Buck, E.J.; Eadie, J.M.; Herzog, M.P.; et al. Avian Influenza Antibody Prevalence Increases with Mercury Contamination in Wild Waterfowl. Proc. R. Soc. B 2022, 289, 20221312. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.H.; Ackerman, J.T.; Holser, R.R.; McDonald, B.I.; Costa, D.P.; Crocker, D.E. Mercury Bioaccumulation and Cortisol Interact to Influence Endocrine and Immune Biomarkers in a Free-Ranging Marine Mammal. Environ. Sci. Technol. 2023, 57, 5678–5692. [Google Scholar] [CrossRef]
- Das, K.; Siebert, U.; Gillet, A.; Dupont, A.; Di-Poï, C.; Fonfara, S.; Mazzucchelli, G.; De Pauw, E.; De Pauw-Gillet, M. Mercury immune toxicity in harbour seals: Links to in vitro toxicity. Environ. Health 2008, 7, 1–17. [Google Scholar] [CrossRef]
- Haskins, D.L.; Brown, M.K.; Meichner, K.; Tuberville, T.D.; Gogal, R.M. Mercury Immunotoxicity in the Brown Watersnake (Nerodia taxispilota): An in Vitro Study. J. Appl. Toxicol. 2022, 42, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Van Den Berg, H.; Loonen, M.J.J.E.; Mateo, R.; Van Den Brink, N.W. Mercury-Modulated Immune Responses in Arctic Barnacle Goslings (Branta leucopsis) upon a Viral-Like Immune Challenge. Environ. Sci. Technol. 2023, 57, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- King, M.D.; Su, G.; Crump, D.; Farhat, A.; Marlatt, V.; Lee, S.L.; Williams, T.D.; Elliott, J.E. Contaminant biomonitoring augmented with a qPCR array indicates hepatic mRNA gene expression effects in wild-collected seabird embryos. Sci. Total Environ. 2023, 904, 166784. [Google Scholar] [CrossRef]
- Wada, H.; Cristol, D.A.; McNabb, F.A.; Hopkins, W.A. Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ. Sci. Technol. 2009, 43, 6031–6038. [Google Scholar] [CrossRef]
- Bleau, H.; Daniel, C.; Chevalier, G.; Van Tra, H.; Hontela, A. Effects of acute exposure to mercury chloride and methylmercury on plasma cortisol, T3, T4, glucose and liver glycogen in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 1996, 34, 221–235. [Google Scholar] [CrossRef]
- Pinheiro, M.D.O.; Simmons, D.B.D.; Villella, M.; Tetreault, G.R.; Muir, D.C.G.; McMaster, M.E.; Hewitt, L.M.; Parrott, J.L.; Park, B.J.; Brown, S.B.; et al. Brown bullhead at the St. Lawrence River (Cornwall) Area of Concern: Health and endocrine status in the context of tissue concentrations of PCBs and mercury. Environ. Monit. Assess. 2020, 192, 1–23. [Google Scholar] [CrossRef]
- Eccles, K.M.; Thomas, P.J.; Chan, H.M. Spatial Patterns of the Exposure-Response Relationship between Mercury and Cortisol in the Fur of River Otter (Lontra canadensis). Chemosphere 2021, 263, 127992. [Google Scholar] [CrossRef]
- Esparza, I.; Elliott, K.H.; Choy, E.S.; Braune, B.M.; Letcher, R.J.; Patterson, A.; Fernie, K.J. Mercury, legacy and emerging POPs, and endocrine-behavioural linkages: Implications of Arctic change in a diving seabird. Environ. Res. 2022, 212, 113190. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Fort, J.; Legagneux, P.; Chastel, O.; Mallory, M.L.; Bustamante, P.; Danielsen, J.; Hanssen, S.A.; Jónsson, J.E.; Magnúsdóttir, E.; et al. Do Foraging Ecology and Contaminants Interactively Predict Parenting Hormone Levels in Common Eider? Gen. Comp. Endocrinol. 2023, 337, 114261. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, K.; Tozer, D.C.; Alvo, R.; Bhavsar, S.P.; Mallory, M.L. Drivers of Declines in Common Loon (Gavia immer) Productivity in Ontario, Canada. Sci. Total Environ. 2020, 738, 139724. [Google Scholar] [CrossRef]
- Heddle, C.; Elliott, J.E.; Brown, T.M.; Eng, M.L.; Perkins, M.; Basu, N. Continuous Exposure to Mercury during Embryogenesis and Chick Development Affects Later Survival and Reproduction of Zebra Finch (Taeniopygia guttata). Ecotoxicology 2020, 29, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Naidu, A.S.; Kelley, J.J.; Jewett, S.C.; Dasher, D.; Duffy, L.K. Baseline concentrations of total mercury and methylmercury in salmon returning via the Bering Sea (1999–2000). Mar. Pollut. Bull. 2001, 42, 993–997. [Google Scholar] [CrossRef]
- Baker, M.R.; Schindler, D.E.; Holtgrieve, G.W.; St Louis, V.L. Bioaccumulation and transport of contaminants: Migrating sockeye salmon as vectors of mercury. Environ. Sci. Technol. 2009, 43, 8840–8846. [Google Scholar] [CrossRef]
- Pratte, I.; Noble, D.G.; Mallory, M.L.; Braune, B.M.; Provencher, J.F. The influence of migration patterns on exposure to contaminants in Nearctic shorebirds: A historical study. Environ. Monit. Assess. 2020, 192, 256. [Google Scholar] [CrossRef]
- McIntyre, J.A.; O’Driscoll, N.J.; Spooner, I.; Robertson, G.J.; Smol, J.P.; Mallory, M.L. Scavenging gulls are biovectors of mercury from industrial wastes in Nova Scotia, Canada. Chemosphere 2022, 304, 135279. [Google Scholar] [CrossRef]
- Lacombe, R.M.; Martigny, P.; Pelletier, D.; Barst, B.D.; Guillemette, M.; Amyot, M.; Elliott, K.H.; Lavoie, R.A. Exploring the spatial variation of mercury in the Gulf of St. Lawrence using northern gannets as fish samplers. Sci. Total Environ. 2024, 927, 172152. [Google Scholar] [CrossRef]
- Albert, C.; Helgason, H.H.; Brault-Favrou, M.; Robertson, G.J.; Descamps, S.; Amélineau, F.; Danielsen, J.; Dietz, R.; Elliott, K.; Erikstad, K.E.; et al. Seasonal variation of mercury contamination in Arctic seabirds: A pan-Arctic assessment. Sci. Total Environ. 2021, 750, 142201. [Google Scholar] [CrossRef]
- Pollet, I.L.; Provencher, J.F.; Tranquilla, L.M.; Burgess, N.M.; Mallory, M.L. Mercury levels in North Atlantic seabirds: A synthesis. Mar. Pollut. Bull. 2022, 181, 113884. [Google Scholar] [CrossRef]
- Smith, R.A.; Yurkowski, D.J.; Parkinson, K.J.; Fort, J.; Hennin, H.L.; Gilchrist, H.G.; Hobson, K.A.; Mallory, M.L.; Danielsen, J.; Garbus, S.E.; et al. Environmental and life-history factors influence inter-colony multidimensional niche metrics of a breeding Arctic marine bird. Sci. Total Environ. 2021, 796, 148935. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.S.; Blight, L.K.; Elliott, J.E.; Hobson, K.A.; Zanuttig, M.; Elliott, K.H. Stable mercury trends support a long-term diet shift away from marine foraging in Salish Sea glaucous-winged gulls over the last century. Environ. Sci. Technol. 2022, 56, 12097–12105. [Google Scholar] [CrossRef] [PubMed]
- Millard, G.; Driscoll, C.; Montesdeoca, M.; Yang, Y.; Taylor, M.; Boucher, S.; Shaw, A.; Richter, W.; Paul, E.; Parker, C.; et al. Patterns and trends of fish mercury in New York State. Ecotoxicology 2020, 29, 1709–1720. [Google Scholar] [CrossRef]
- Lepak, R.F.; Hoffman, J.C.; Janssen, S.E.; Krabbenhoft, D.P.; Ogorek, J.M.; DeWild, J.F.; Tate, M.T.; Babiarz, C.L.; Yin, R.; Murphy, E.W.; et al. Mercury source changes and food web shifts alter contamination signatures of predatory fish from Lake Michigan. Proc. Natl. Acad. Sci. USA 2019, 116, 23600–23608. [Google Scholar] [CrossRef]
- Steevens, J.A.; Benson, W.H. Toxicokinetic interactions and survival of Hyalella azteca exposed to binary mixtures of chlorpyrifos, dieldrin, and methyl mercury. Aquat. Toxicol. 2001, 51, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Leonzio, C.; Fossi, M.C.; Casini, S. Porphyrins as biomarkers of methylmercury and PCB exposure in experimental quail. Bull. Environ. Contam. Toxicol. 1996, 56, 244–250. [Google Scholar] [CrossRef]
- Bemis, J.C.; Seegal, R.F. Polychlorinated biphenyls and methylmercury act synergistically to reduce rat brain dopamine content in vitro. Environ. Health Perspect. 1999, 107, 879–885. [Google Scholar] [CrossRef]
- Manceau, A.; Bourdineaud, J.P.; Oliveira, R.B.; Sarrazin, S.L.F.; Krabbenhoft, D.P.; Eagles-Smith, C.A.; Ackerman, J.T.; Stewart, A.R.; Ward-Deitrich, C.; Del Castillo Busto, M.E.; et al. Demethylation of methylmercury in bird, fish, and earthworm. Environ. Sci. Technol. 2021, 55, 1527–1534. [Google Scholar] [CrossRef]
- Cruz-Flores, M.; Lemaire, J.; Brault-Favrou, M.; Christensen-Dalsgaard, S.; Churlaud, C.; Descamps, S.; Elliott, K.; Erikstad, K.E.; Ezhov, A.; Gavrilo, M.; et al. Spatial Distribution of Selenium-Mercury in Arctic Seabirds. Environ. Pollut. 2024, 343, 123110. [Google Scholar] [CrossRef]
- Charapata, P.; Clark, C.T.; Miller, N.; Kienle, S.S.; Costa, D.P.; Goebel, M.E.; Gunn, H.; Sperou, E.S.; Kanatous, S.B.; Crocker, D.E. Whiskers provide time-series of toxic and essential trace elements, Se: Hg molar ratios, and stable isotope values of an apex Antarctic predator, the leopard seal. Sci. Total Environ. 2023, 854, 158651. [Google Scholar] [CrossRef] [PubMed]
- Ponton, D.E.; Ruelas-Inzunza, J.; Lavoie, R.A.; Lescord, G.L.; Johnston, T.A.; Graydon, J.A.; Reichert, M.; Donadt, C.; Poesch, M.; Gunn, J.M.; et al. Mercury, Selenium and Arsenic Concentrations in Canadian Freshwater Fish and a Perspective on Human Consumption Intake and Risk. J. Hazard. Mater. Adv. 2022, 6, 100060. [Google Scholar] [CrossRef]
- Calvert, A.M.; Gutowsky, S.E.; Fifield, D.A.; Burgess, N.M.; Bryant, R.; Fraser, G.S.; Gjerdrum, C.; Hedd, A.; Jones, P.L.; Mauck, R.A.; et al. Inter-colony variation in predation, mercury burden and adult survival in a declining seabird. Sci. Total Environ. 2024, 911, 168549. [Google Scholar] [CrossRef] [PubMed]
- Dietz, R.; Desforges, J.-P.; Rigét, F.F.; Aubail, A.; Garde, E.; Ambus, P.; Drimmie, R.; Heide-Jørgensen, M.P.; Sonne, C. Analysis of Narwhal Tusks Reveals Lifelong Feeding Ecology and Mercury Exposure. Curr. Biol. 2021, 31, 2012–2019.e2. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.E.; Drever, M.C.; Studholme, K.R.; Silverthorn, V.; Miller, A.A.; Elliott, K.H.; Lee, S.L.; Drouillard, K.G.; Porter, E.; Idrissi, A.M.; et al. Exposure to Persistent Organic Pollutants Is Linked to Over-Wintering Latitude in a Pacific Seabird, the Rhinoceros Auklet, Cerorhinca Monocerata. Environ. Pollut. 2021, 279, 116928. [Google Scholar] [CrossRef]
- Smith, R.A.; Albonaimi, S.S.; Hennin, H.L.; Gilchrist, H.G.; Fort, J.; Parkinson, K.J.L.; Provencher, J.F.; Love, O.P. Exposure to cumulative stressors affects the laying phenology and incubation behaviour of an Arctic-breeding marine bird. Sci. Total Environ. 2022, 807, 150882. [Google Scholar] [CrossRef]
- Buttke, D.E.; Decker, D.J.; Wild, M.A. The role of one health in wildlife conservation: A challenge and opportunity. J. Wildl. Dis. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Stephen, C.; Wilcox, A.; Sine, S.; Provencher, J. A reimagined One Health framework for wildlife conservation. Res. Dir. One Health 2023, 1, e12. [Google Scholar] [CrossRef]
- Fusillo, R.; Romanucci, M.; Marcelli, M.; Massimini, M.; Della Salda, L. Health and mortality monitoring in threatened mammals: A first post mortem study of otters (Lutra lutra L.) Italy. Animals 2022, 12, 609. [Google Scholar] [CrossRef]
- Kim, G.; Ahn, S.; Lee, S.J.; Choi, S.Y.; Cho, H.S.; Oh, Y. Mercury poisoning in Eurasian river otter (Lutra lutra). J. Ecol. Environ. 2023, 47, 5. [Google Scholar] [CrossRef]
- Canham, R.; González-Prieto, A.M.; Elliott, J.E. Mercury exposure and toxicological consequences in fish and fish-eating wildlife from anthropogenic activity in Latin America. Integr. Environ. Assess. Manag. 2020, 17, 13–26. [Google Scholar] [CrossRef]
- Jędruch, A.; Falkowska, L.; Saniewska, D.; Grajewska, A.; Bełdowska, M.; Meissner, W.; Kalisińska, E.; Duzinkiewicz, K.; Pacyna, J.M. Mercury in the Polish part of the Baltic Sea: A response to decreased atmospheric deposition and changing environment. Mar. Pollut. Bull. 2023, 186, 114426. [Google Scholar] [CrossRef] [PubMed]
- Eccles, K.M.; Pauli, B.; Chan, H.M. Geospatial analysis of the patterns of chemical exposures among biota in the Canadian Oil Sands Region. PLoS ONE 2020, 15, e0239086. [Google Scholar] [CrossRef]
- Morgado, F.; Santos, R.M.; Sampaio, D.; de Lacerda, L.D.; Soares, A.M.; Vieira, H.C.; Abreu, S. Chronological trends and mercury bioaccumulation in an aquatic semiarid ecosystem under a global climate change scenario in the northeastern coast of Brazil. Animals 2021, 11, 2402. [Google Scholar] [CrossRef]
- Morris, A.D.; Wilson, S.J.; Fryer, R.J.; Thomas, P.J.; Hudelson, K.; Andreasen, B.; Blévin, P.; Bustamante, P.; Chastel, O.; Christensen, G.; et al. Temporal trends of mercury in Arctic biota: 10 more years of progress in Arctic monitoring. Sci. Total Environ. 2022, 839, 155803. [Google Scholar] [CrossRef] [PubMed]
- Duffy, L.K.; Vertigan, T.; Dainowski, B.; Dunlap, K.; Hirons, A. Climate Change, One Health and Mercury. Adv. Clin. Toxicol. 2017, 2, 000114. [Google Scholar]
- Houde, M.; Krümmel, E.M.; Mustonen, T.; Brammer, J.; Brown, T.M.; Chételat, J.; Dahl, P.E.; Dietz, R.; Evans, M.; Gamberg, M.; et al. Contributions and perspectives of Indigenous Peoples to the study of mercury in the Arctic. Sci. Total Environ. 2022, 841, 156566. [Google Scholar] [CrossRef]
- Park, S.; Johnson, M.A. Awareness of fish advisories and mercury exposure in women of childbearing age. Nutr. Rev. 2006, 64, 250–256. [Google Scholar] [CrossRef]
- Shimshack, J.P.; Ward, M.B.; Beatty, T.K. Mercury advisories: Information, education, and fish consumption. J. Environ. Econ. Manag. 2007, 53, 158–179. [Google Scholar] [CrossRef]
- Lauber, T.B.; Connelly, N.A.; Niederdeppe, J.; Knuth, B.A. What We Know About Fish Consumption Advisories: Insights from the Experts and the Literature; Cornell University: Ithaca, NY, USA, 2013; p. 121. [Google Scholar]
- Manfredo, M.J.; Sullivan, L.; Don Carlos, A.W.; Dietsch, A.M.; Teel, T.; Bright, A.D.; Bruskotter, J. America’s Wildlife Values: The Social Context of Wildlife Management in the U.S.; Association of Fish and Wildlife Agencies: Washington, DC, USA, 2018. [Google Scholar]
- Richards, J.C.; Miller, Z.D.; Norvell, R.; Smith, J.W. Integrating moral norms and stewardship identity into the theory of planned behavior to understand altruistic conservation behavior among hunters in southwestern Utah (USA). Hum. Dimens. Wildl. 2024, 29, 670–690. [Google Scholar] [CrossRef]
- Charvát, P.; Klimeš, L.; Pospíšil, J.; Klemeš, J.J.; Varbanov, P.S. An overview of mercury emissions in the energy industry—A step to mercury footprint assessment. J. Clean. Prod. 2020, 267, 122087. [Google Scholar] [CrossRef]



| Northeast | Southeast | Caribbean | Midwest | Northern Great Plains | Southern Great Plains | Northwest | Southwest | Alaska | Hawaii and US-Affiliated Pacific Islands | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effects of increasing global temperatures | Increases in ocean temperatures | X | X | X | X | X | X | ||||
| Increases in land temperatures | X | X | X | X | X | X | X | X | X | X | |
| Increased cryosphere melting | X | X | X | ||||||||
| Increases in temperatures in freshwater systems | X | X | X | X | X | ||||||
| Changing climate oscillations | X | X | X | X | X | X | |||||
| Sea level rise | X | X | X | X | X | X | X | X | |||
| Increase in heavy precipitation and storms | X | X | X | X | X | X | X | X | X | X | |
| Effects of increasing drought conditions | Increase in wildfire intensity | X | X | X | X | X | X | X | X | ||
| Drying of freshwater systems | X | X | X | X | X | X | X | X | X | ||
| Other co-factors influencing mercury in wildlife | Human land use change | X | X | X | X | X | |||||
| Overfishing | X | X | X | X | |||||||
| Other pollutants | X | X | X | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkening, J.L.; Kurthen, A.L.; Guilbeau, K.; Libera, D.A.; Nelson, S.J.; Ming, J. Climate-Driven Alterations in the Mercury Cycle: Implications for Wildlife Managers Through a One Health Lens. Land 2025, 14, 856. https://doi.org/10.3390/land14040856
Wilkening JL, Kurthen AL, Guilbeau K, Libera DA, Nelson SJ, Ming J. Climate-Driven Alterations in the Mercury Cycle: Implications for Wildlife Managers Through a One Health Lens. Land. 2025; 14(4):856. https://doi.org/10.3390/land14040856
Chicago/Turabian StyleWilkening, Jennifer L., Angelika L. Kurthen, Kelly Guilbeau, Dominic A. Libera, Sarah J. Nelson, and Jaron Ming. 2025. "Climate-Driven Alterations in the Mercury Cycle: Implications for Wildlife Managers Through a One Health Lens" Land 14, no. 4: 856. https://doi.org/10.3390/land14040856
APA StyleWilkening, J. L., Kurthen, A. L., Guilbeau, K., Libera, D. A., Nelson, S. J., & Ming, J. (2025). Climate-Driven Alterations in the Mercury Cycle: Implications for Wildlife Managers Through a One Health Lens. Land, 14(4), 856. https://doi.org/10.3390/land14040856





