Agricultural Landscape Composition Linked with Acoustic Measures of Avian Diversity
Abstract
1. Introduction
2. Materials and Methods
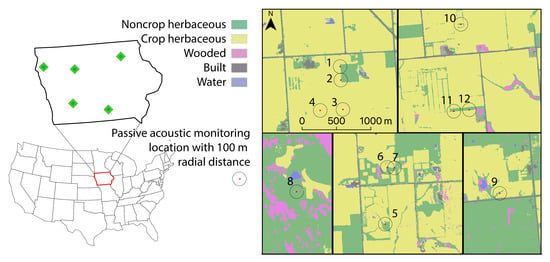
2.1. Research Sites
2.2. Acoustic Data Collection
2.3. Assessing Avian Diversity from Acoustic Recordings
2.3.1. Acoustic Detection Extent
2.3.2. Bioacoustic Index Sampling
2.3.3. Dawn Sampling
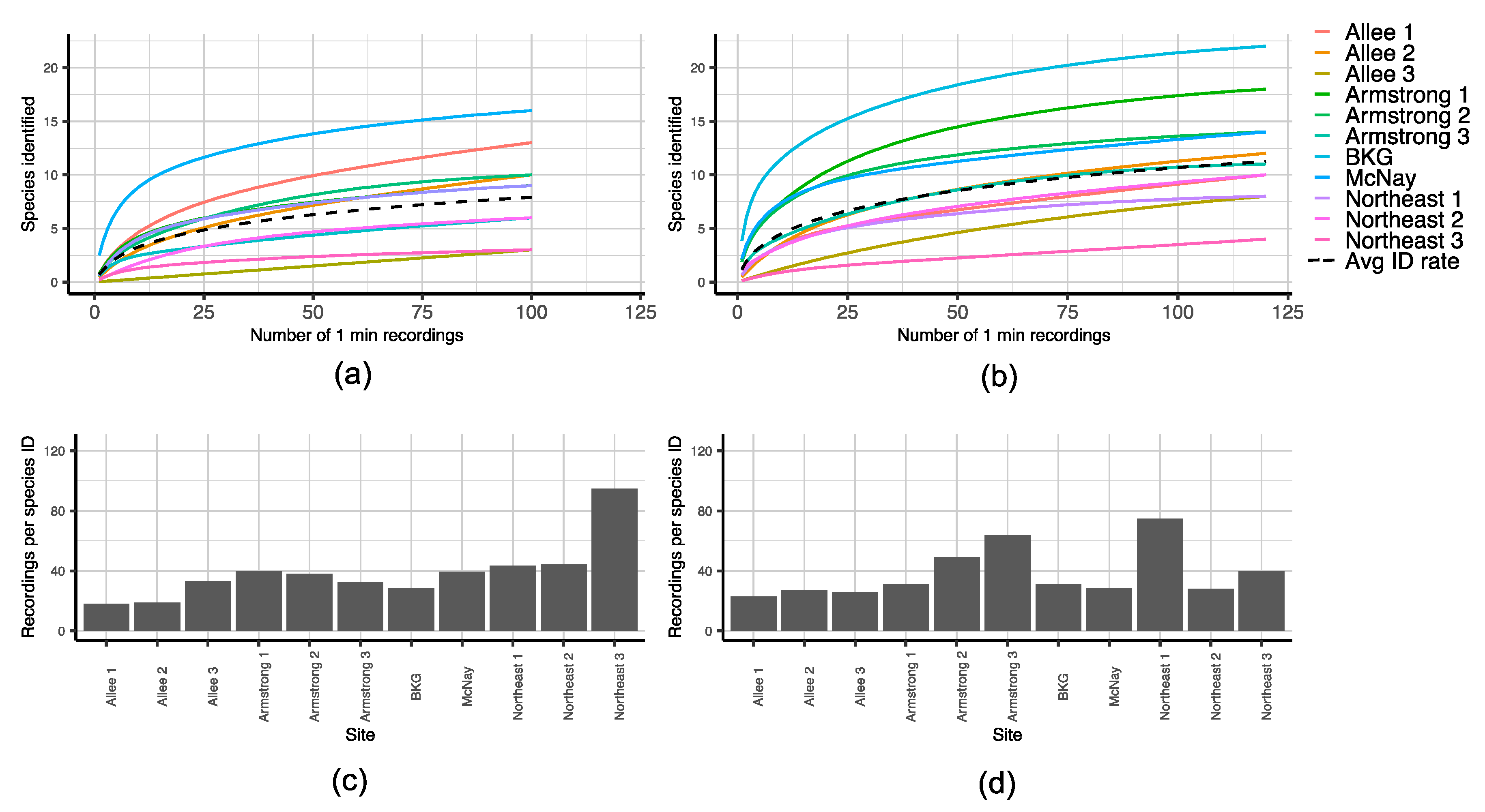
2.3.4. Comparing Sampling Approaches Using Species Accumulation Curves (SACs)
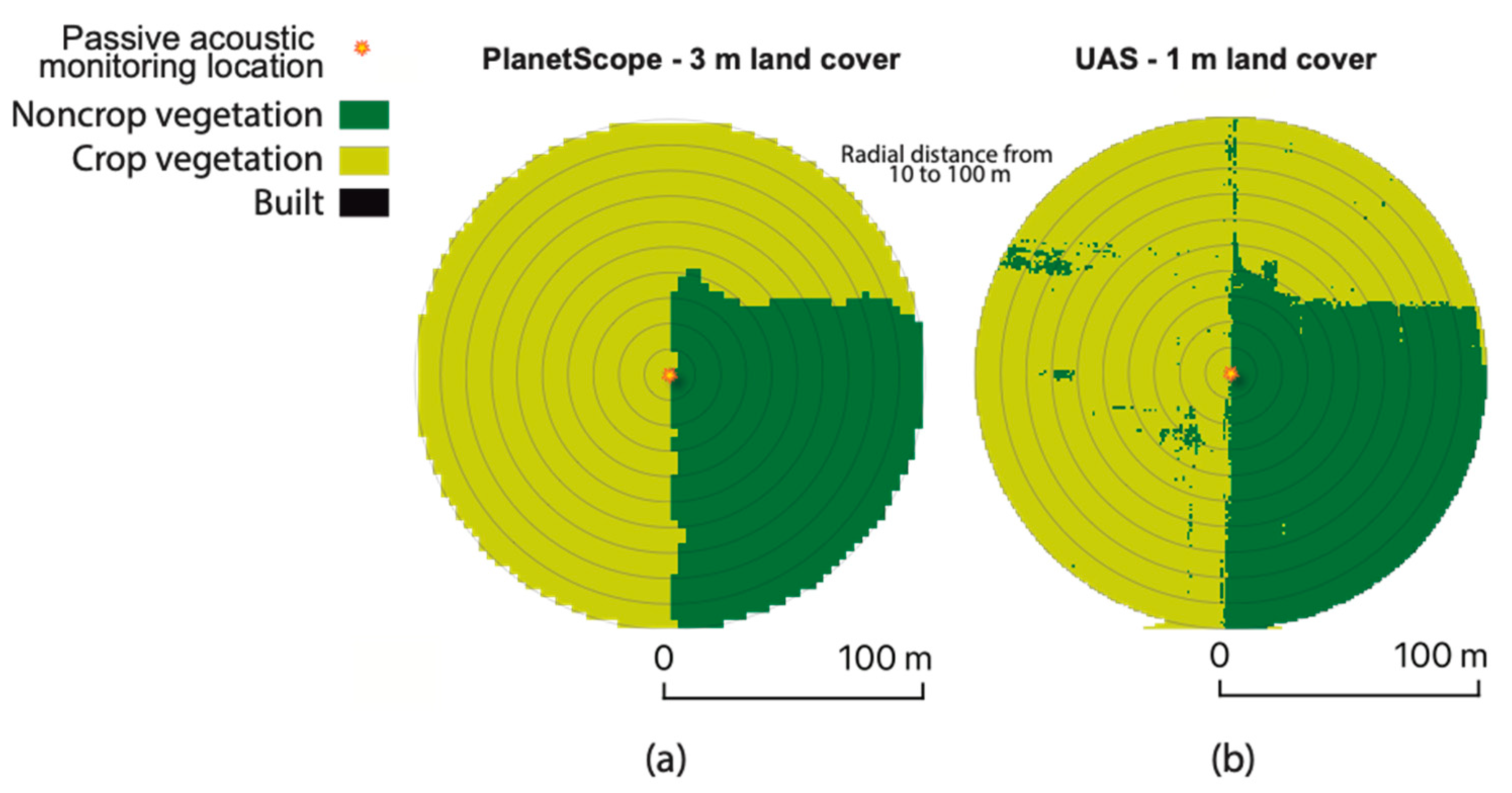
2.4. UAS and Satellite Imagery Data Collection
2.5. Land Cover Composition and Configuration
2.5.1. Configuration Metrics
2.5.2. Spatial Extents
2.6. Statistical Modeling
3. Results
3.1. Acoustic Data Analysis
3.2. Land Cover Mapping
3.3. Spatial Extents and Resolutions
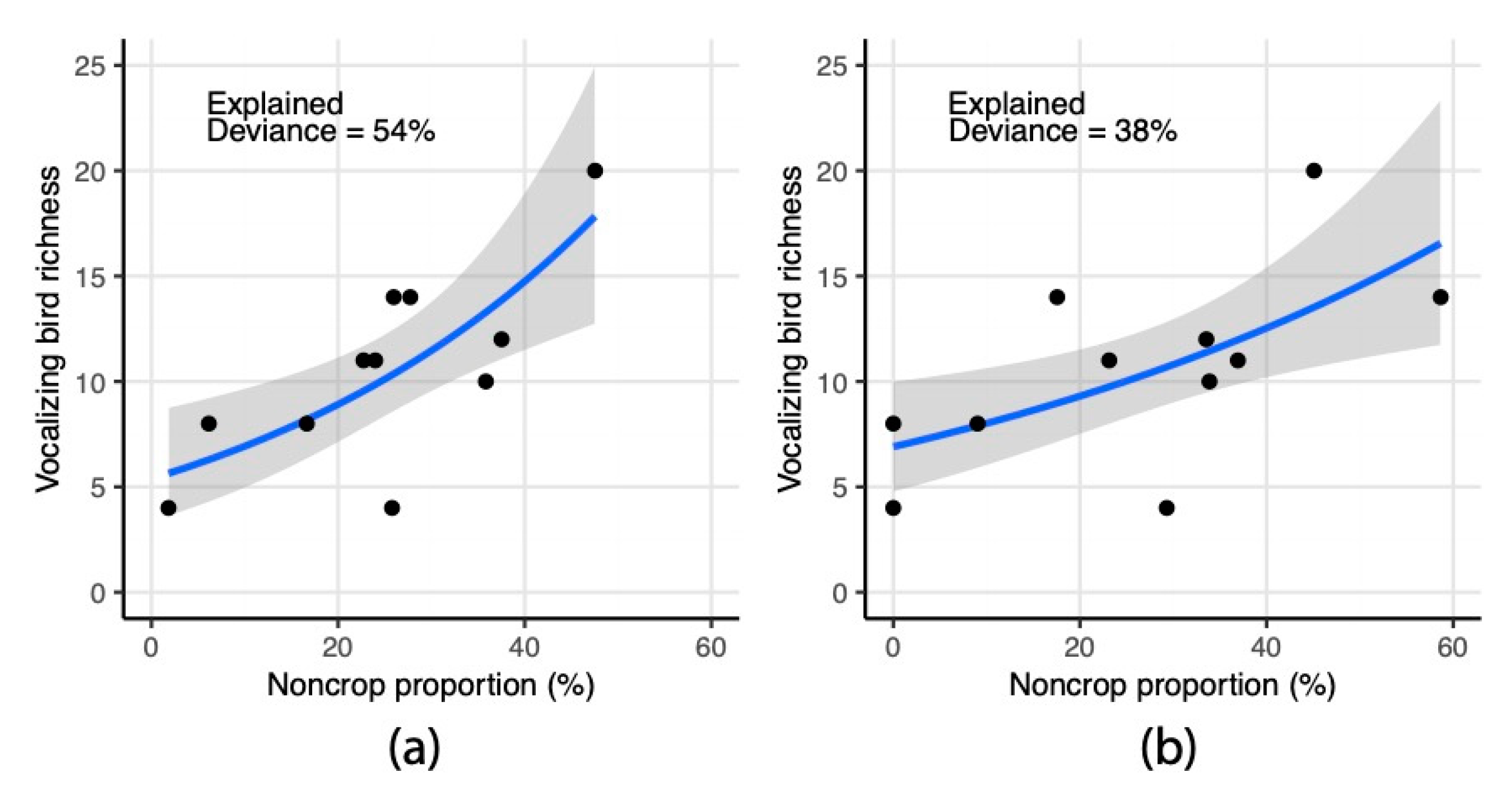
3.4. Statistical Assessment of Landscape Indicators in Relation to Vocalizing Bird Richness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramankutty, N.; Foley, J.A. Estimating historical changes in global land cover: Croplands from 1700 to 1992. Glob. Biogeochem. Cycles 1999, 13, 997–1027. [Google Scholar] [CrossRef]
- Ellis, E.C.; Ramankutty, N. Putting people in the map: Anthropogenic biomes of the world. Front. Ecol. Environ. 2008, 6, 439–447. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D. Small patches make critical contributions to biodiversity conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 717–719. [Google Scholar] [CrossRef]
- Martin, L.J.; Quinn, J.E.; Ellis, E.C.; Shaw, M.R.; Dorning, M.A.; Hallett, L.M.; Heller, N.E.; Hobbs, R.J.; Kraft, C.E.; Law, E.; et al. Conservation opportunities across the world’s anthromes. Divers. Distrib. 2014, 20, 745–755. [Google Scholar] [CrossRef]
- Quinn, J.E.; Johnson, R.J.; Brandle, J.R. Identifying opportunities for conservation embedded in cropland anthromes. Landsc. Ecol. 2014, 29, 1811–1819. [Google Scholar] [CrossRef]
- Dandois, J.P.; Ellis, E.C. High spatial resolution three-dimensional mapping of vegetation spectral dynamics using computer vision. Remote Sens. Environ. 2013, 136, 259–276. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Farina, A.; James, P.; Bobryk, C.; Pieretti, N.; Lattanzi, E.; McWilliam, J. Low cost (audio) recording (LCR) for advancing soundscape ecology towards the conservation of sonic complexity and biodiversity in natural and urban landscapes. Urban Ecosyst. 2014, 17, 923–944. [Google Scholar] [CrossRef]
- Dandois, J.P.; Ellis, E.C. Remote Sensing of Vegetation Structure Using Computer Vision. Remote Sens. 2010, 2, 1157–1176. [Google Scholar] [CrossRef]
- Planet Imagery Product Specification: PlanetScope & Rapideye. Available online: https://www.planet.com/products/satellite-imagery/files/1610.06_Spec%20Sheet_Combined_Imagery_Product_Letter_ENGv1.pdf (accessed on 2 May 2020).
- Sueur, J.; Pavoine, S.; Hamerlynck, O.; Duvail, S. Rapid Acoustic Survey for Biodiversity Appraisal. PLoS ONE 2008, 3, e4065. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.P.; Prince, P.; Piña Covarrubias, E.; Doncaster, C.P.; Snaddon, J.L.; Rogers, A. AudioMoth: Evaluation of a smart open acoustic device for monitoring biodiversity and the environment. Methods Ecol. Evol. 2018, 9, 1199–1211. [Google Scholar] [CrossRef]
- Towsey, M.; Wimmer, J.; Williamson, I.; Roe, P. The use of acoustic indices to determine avian species richness in audio-recordings of the environment. Ecol. Inform. 2014, 21, 110–119. [Google Scholar] [CrossRef]
- Fuller, S.; Axel, A.C.; Tucker, D.; Gage, S.H. Connecting soundscape to landscape: Which acoustic index best describes landscape configuration? Ecol. Indic. 2015, 58, 207–215. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Pasher, J.; Mitchell, S.W.; King, D.J.; Fahrig, L.; Smith, A.C.; Lindsay, K.E. Optimizing landscape selection for estimating relative effects of landscape variables on ecological responses. Landsc. Ecol. 2013, 28, 371–383. [Google Scholar] [CrossRef]
- Perović, D.; Gámez-Virués, S.; Börschig, C.; Klein, A.-M.; Krauss, J.; Steckel, J.; Rothenwöhrer, C.; Erasmi, S.; Tscharntke, T.; Westphal, C.; et al. Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J. Appl. Ecol. 2015, 52, 505–513. [Google Scholar] [CrossRef]
- Wimmer, J.; Towsey, M.; Roe, P.; Williamson, I. Sampling environmental acoustic recordings to determine bird species richness. Ecol. Appl. 2013, 23, 1419–1428. [Google Scholar] [CrossRef]
- Green, T.R.; Kipka, H.; David, O.; McMaster, G.S. Where is the USA Corn Belt, and how is it changing? Sci. Total Environ. 2018, 618, 1613–1618. [Google Scholar] [CrossRef]
- Boryan, C.; Yang, Z.; Mueller, R.; Craig, M. Monitoring US agriculture: The US Department of Agriculture, National Agricultural Statistics Service, Cropland Data Layer Program. Geocarto Int. 2011, 26, 341–358. [Google Scholar] [CrossRef]
- Hutto, R.L.; Pletschet, S.M.; Hendricks, P. A Fixed-radius Point Count Method for Nonbreeding and Breeding Season Use. Auk 1986, 103, 593–602. [Google Scholar] [CrossRef]
- Searcy, W.A. Sound-pressure levels and song preferences in female red-winged blackbirds (Agelaius phoeniceus) (Aves, Emberizidae). Ethology 1996, 102, 187–196. [Google Scholar] [CrossRef]
- Results for ‘Red-Winged Blackbird’ :: Page 1 :: Xeno-Canto. Available online: https://www.xeno-canto.org/explore?query=red-winged%20blackbird (accessed on 15 December 2019).
- Sueur, J. Sound Analysis and Synthesis with R; Springer: Culemborg, The Netherlands, 2018; ISBN 3-319-77645-2. [Google Scholar]
- Boelman, N.T.; Asner, G.P.; Hart, P.J.; Martin, R.E. Multi-trophic invasion resistance in Hawaii: Bioacoustics, field surveys, and airborne remote sensing. Ecol. Appl. 2007, 17, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Commun. Ecol. Package 2007, 10, 631–637. [Google Scholar]
- Mohammed, N.Z.; Ghazi, A.; Mustafa, H.E. Positional accuracy testing of Google Earth. Int. J. Multidiscip. Sci. Eng. 2013, 4, 6–9. [Google Scholar]
- Samapriya Roy. Available online: https://samapriya.github.io/ (accessed on 19 July 2019).
- Satellite Imagery and Archive. Available online: https://planet.com/products/planet-imagery/ (accessed on 19 July 2019).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. Available online: http://qgis.osgeo.org (accessed on 9 May 2020).
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Soft. 2008, 28. [Google Scholar] [CrossRef]
- Hijmans, R.J.; van Etten, J. Raster: Geographic analysis and modeling with raster data. Found. Stat. Comput. 2012. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 17 December 2018).
- Schuster, C.; Schmidt, T.; Conrad, C.; Kleinschmit, B.; Förster, M. Grassland habitat mapping by intra-annual time series analysis -Comparison of RapidEye and TerraSAR-X satellite data. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 25–34. [Google Scholar] [CrossRef]
- Olimb, S.K.; Dixon, A.P.; Dolfi, E.; Engstrom, R.; Anderson, K. Prairie or planted? Using time-series NDVI to determine grassland characteristics in Montana. GeoJournal 2017. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Ene, E. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps.; Computer software Program Produced by the Authors at the University of Massachusetts, Amherst. Available online: http://www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 2 May 2020).
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic data analysis and modeling. R Package Version 2019, 517, 2–12. [Google Scholar]
- Pebesma, E. Simple Features for R: Standardized Support for Spatial Vector Data. R J. 2018, 10, 439. [Google Scholar] [CrossRef]
- Lechner, A.M.; Langford, W.T.; Jones, S.D.; Bekessy, S.A.; Gordon, A. Investigating species–environment relationships at multiple scales: Differentiating between intrinsic scale and the modifiable areal unit problem. Ecol. Complex. 2012, 11, 91–102. [Google Scholar] [CrossRef]
- Jackson, H.B.; Fahrig, L. Are ecologists conducting research at the optimal scale? Glob. Ecol. Biogeogr. 2015, 24, 52–63. [Google Scholar] [CrossRef]
- Flick, T.; Feagan, S.; Fahrig, L. Effects of landscape structure on butterfly species richness and abundance in agricultural landscapes in eastern Ontario, Canada. Agric. Ecosyst. Environ. 2012, 156, 123–133. [Google Scholar] [CrossRef]
- Fahrig, L. Rethinking patch size and isolation effects: The habitat amount hypothesis. J. Biogeogr. 2013, 40, 1649–1663. [Google Scholar] [CrossRef]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batáry, P.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F.; et al. Landscape moderation of biodiversity patterns and processes—Eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 0-387-87458-5. [Google Scholar]
- Evans, T.; Mahoney, M.; Cashatt, E.; de Snoo, G.; Musters, C.J.M. Enhancement of Linear Agricultural Areas to Provide Invertebrates as Potential Food for Breeding Birds. Land 2016, 5, 26. [Google Scholar] [CrossRef]
- Bennett, A.F.; Radford, J.Q.; Haslem, A. Properties of land mosaics: Implications for nature conservation in agricultural environments. Biol. Conserv. 2006, 133, 250–264. [Google Scholar] [CrossRef]
- Vasseur, C.; Joannon, A.; Aviron, S.; Burel, F.; Meynard, J.-M.; Baudry, J. The cropping systems mosaic: How does the hidden heterogeneity of agricultural landscapes drive arthropod populations? Agric. Ecosyst. Environ. 2013, 166, 3–14. [Google Scholar] [CrossRef]
- Mitchell, M.S.; Rutzmoser, S.H.; Wigley, T.B.; Loehle, C.; Gerwin, J.A.; Keyser, P.D.; Lancia, R.A.; Perry, R.W.; Reynolds, C.J.; Thill, R.E.; et al. Relationships between avian richness and landscape structure at multiple scales using multiple landscapes. For. Ecol. Manag. 2006, 221, 155–169. [Google Scholar] [CrossRef]
- Rosenberg, K.V.; Blancher, P.J.; Stanton, J.C.; Panjabi, A.O. Use of North American Breeding Bird Survey data in avian conservation assessments. Condor 2017, 119, 594–606. [Google Scholar] [CrossRef]
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D.; Kelling, S. eBird: A citizen-based bird observation network in the biological sciences. Biol. Conserv. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- Klingbeil, B.T.; Willig, M.R. Bird biodiversity assessments in temperate forest: The value of point count versus acoustic monitoring protocols. PeerJ 2015, 3, e973. [Google Scholar] [CrossRef]
- Cyr, A.; Lepage, D.; Freemark, K. Evaluating Point Count Efficiency Relative to Territory Mapping in Cropland Birds. In Monitoring Bird Populations by Point Counts; US Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 1995. [Google Scholar]
- Zhao, Z.; Zhang, S.; Xu, Z.; Bellisario, K.; Dai, N.; Omrani, H.; Pijanowski, B.C. Automated bird acoustic event detection and robust species classification. Ecol. Inform. 2017, 39, 99–108. [Google Scholar] [CrossRef]
- Acevedo, M.A.; Corrada-Bravo, C.J.; Corrada-Bravo, H.; Villanueva-Rivera, L.J.; Aide, T.M. Automated classification of bird and amphibian calls using machine learning: A comparison of methods. Ecol. Inform. 2009, 4, 206–214. [Google Scholar] [CrossRef]
- Balantic, C.; Donovan, T. Dynamic wildlife occupancy models using automated acoustic monitoring data. Ecol. Appl. 2019, 29, e01854. [Google Scholar] [CrossRef] [PubMed]
- Ovaskainen, O.; Moliterno de Camargo, U.; Somervuo, P. Animal Sound Identifier (ASI): Software for automated identification of vocal animals. Ecol. Lett. 2018, 21, 1244–1254. [Google Scholar] [CrossRef]
- Best, L.B.; Freemark, K.E.; Dinsmore, J.J.; Camp, M. A Review and Synthesis of Habitat Use by Breeding Birds in Agricultural Landscapes of Iowa. Am. Midl. Nat. 1995, 134, 1. [Google Scholar] [CrossRef]
- Best, L.B. Bird Use of Fencerows: Implications of Contemporary Fencerow Management Practices. Wildl. Soc. Bull. 1983, 11, 6. [Google Scholar]
- Best, L.B.; Whitmore, R.C.; Booth, G.M. Use of Cornfields by Birds during the Breeding Season: The Importance of Edge Habitat. Am. Midl. Nat. 1990, 123, 84. [Google Scholar] [CrossRef]
- Lindsay, K.E.; Kirk, D.A.; Bergin, T.M.; Best, L.B.; Sifneos, J.C.; Smith, J. Farmland Heterogeneity Benefits Birds in American Mid-west Watersheds. Am. Midl. Nat. 2013, 170, 121–143. [Google Scholar] [CrossRef]
- Cunningham, M.A.; Johnson, D.H. Seeking parsimony in landscape metrics. J. Wildl. Manag. 2011, 75, 692–701. [Google Scholar] [CrossRef]
- Watling, J.I.; Arroyo-Rodríguez, V.; Pfeifer, M.; Baeten, L.; Banks-Leite, C.; Cisneros, L.M.; Fang, R.; Hamel-Leigue, A.C.; Lachat, T.; Leal, I.R.; et al. Support for the habitat amount hypothesis from a global synthesis of species density studies. Ecol. Lett. 2020, 23, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.H.; Milne, B.T.; Turnei, M.G.; O’Neill, R.V. Neutral models for the analysis of broad-scale landscape pattern. Landsc. Ecol. 1987, 1, 19–28. [Google Scholar] [CrossRef]
- Shen, W.; Darrel Jenerette, G.; Wu, J.H.; Gardner, R. Evaluating empirical scaling relations of pattern metrics with simulated landscapes. Ecography 2004, 27, 459–469. [Google Scholar] [CrossRef]
- Corry, R.C. Characterizing Fine-scale Patterns of Alternative Agricultural Landscapes with Landscape Pattern Indices. Landsc. Ecol. 2005, 20, 591–608. [Google Scholar] [CrossRef]
- Corry, R.C.; Lafortezza, R. Sensitivity of landscape measurements to changing grain size for fine-scale design and management. Landsc. Ecol. Eng. 2007, 3, 47–53. [Google Scholar] [CrossRef]
- Buxton, R.; McKenna, M.F.; Clapp, M.; Meyer, E.; Stabenau, E.; Angeloni, L.M.; Crooks, K.; Wittemyer, G. Efficacy of extracting indices from large-scale acoustic recordings to monitor biodiversity. Conserv. Biol. 2018. [Google Scholar] [CrossRef]






| Noncrop vegetation | Potential habitat area, as proportion (%) of herbaceous noncrop and woody vegetation (composition) |
| Shannon’s Diversity Index (SDI) | An index value measuring relative proportional representativeness of all land cover types (configuration) |
| Contagion | An index value measuring patch isolation and proximity of all land cover types (configuration) |
| Perimeter Area Ratio (PAR) | Ratio of total perimeter of noncrop vegetation patches divided by the total area (configuration) |
| CIRCLE | Circumscribing circle index to measure linearity of noncrop vegetation patches using area and radius (configuration) |
| UAS | PlanetScope | |||
|---|---|---|---|---|
| (Intercept) | 1.68(0.24) | *** | 1.93(0.19) | *** |
| UAS Noncrop | 0.03(0.01) | *** | ||
| PS Noncrop | 0.01(0.01) | ** | ||
| N | 11 | 11 | ||
| AICc | 60.47 | 63.82 | ||
| Explained Dev. | 53.59 | 37.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixon, A.P.; Baker, M.E.; Ellis, E.C. Agricultural Landscape Composition Linked with Acoustic Measures of Avian Diversity. Land 2020, 9, 145. https://doi.org/10.3390/land9050145
Dixon AP, Baker ME, Ellis EC. Agricultural Landscape Composition Linked with Acoustic Measures of Avian Diversity. Land. 2020; 9(5):145. https://doi.org/10.3390/land9050145
Chicago/Turabian StyleDixon, Adam P., Matthew E. Baker, and Erle C. Ellis. 2020. "Agricultural Landscape Composition Linked with Acoustic Measures of Avian Diversity" Land 9, no. 5: 145. https://doi.org/10.3390/land9050145
APA StyleDixon, A. P., Baker, M. E., & Ellis, E. C. (2020). Agricultural Landscape Composition Linked with Acoustic Measures of Avian Diversity. Land, 9(5), 145. https://doi.org/10.3390/land9050145