IL-33, IL-37, and Vitamin D Interaction Mediate Immunomodulation of Inflammation in Degenerating Cartilage †
Abstract
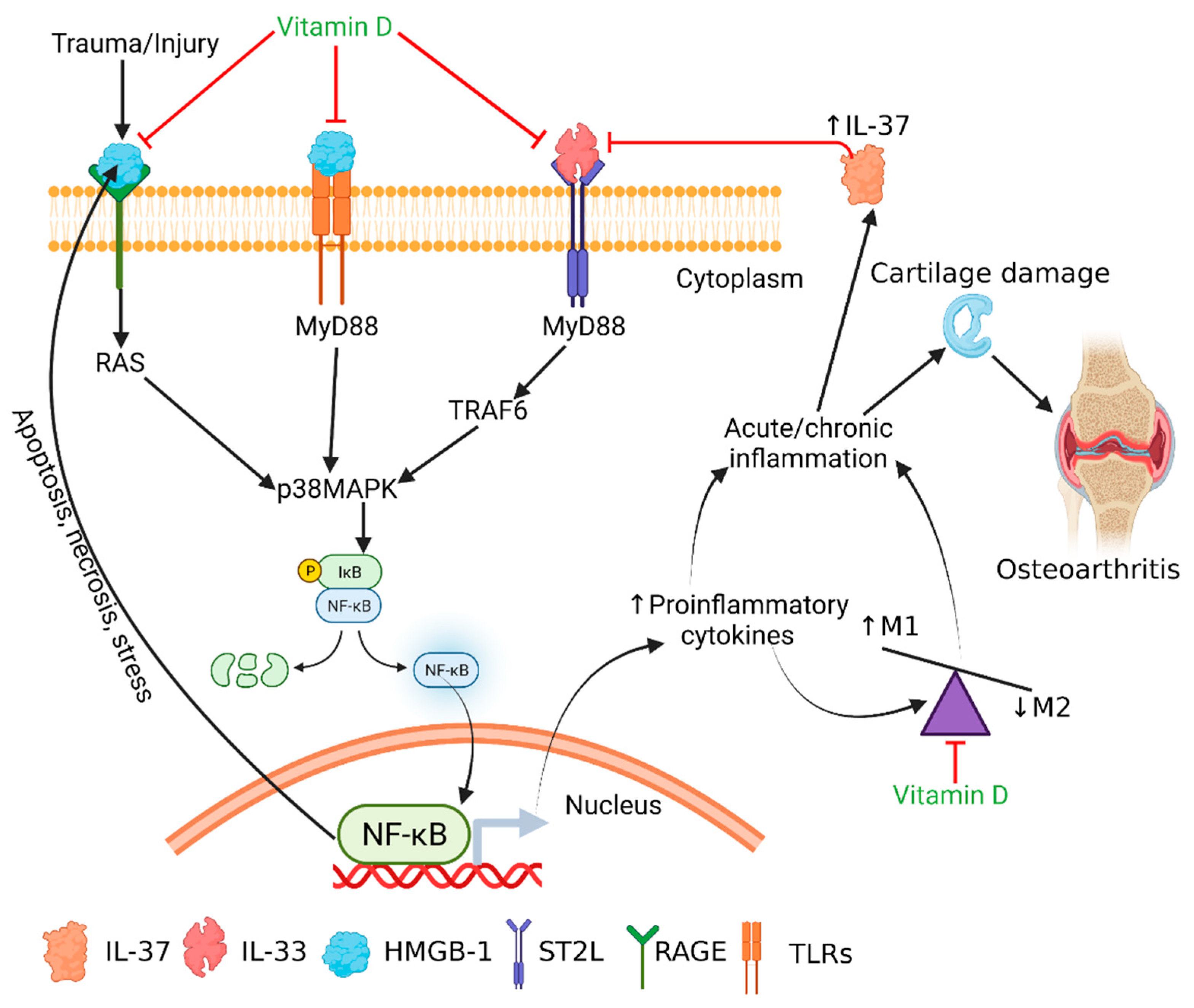
:1. Introduction
2. Materials and Methods
2.1. Porcine Model
2.2. Tissue Acquisition
2.3. Immunofluorescence Studies
2.4. Stimulation and Inhibition Studies
2.5. Cell Culture and Macrophage Polarization Studies
2.6. Statistical Analysis
3. Results
3.1. Increased Immunopositivity for IL-33 and IL-37 in Vitamin D Deficient Microswine Cartilage
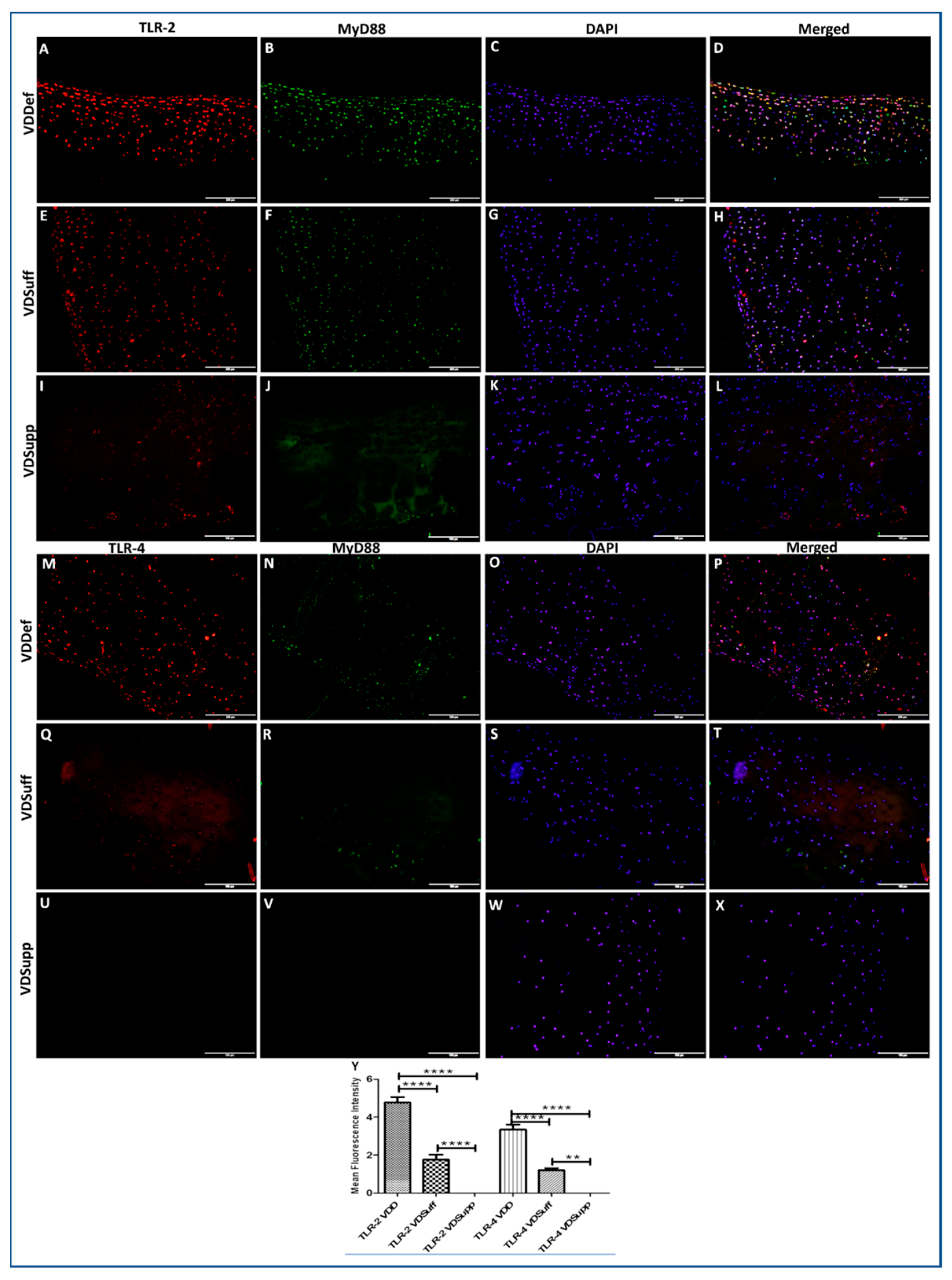
3.2. Increased Immunopositivity for TLR-2 and TLR-4 in Vitamin D-Deficient Microswine Articular Cartilage
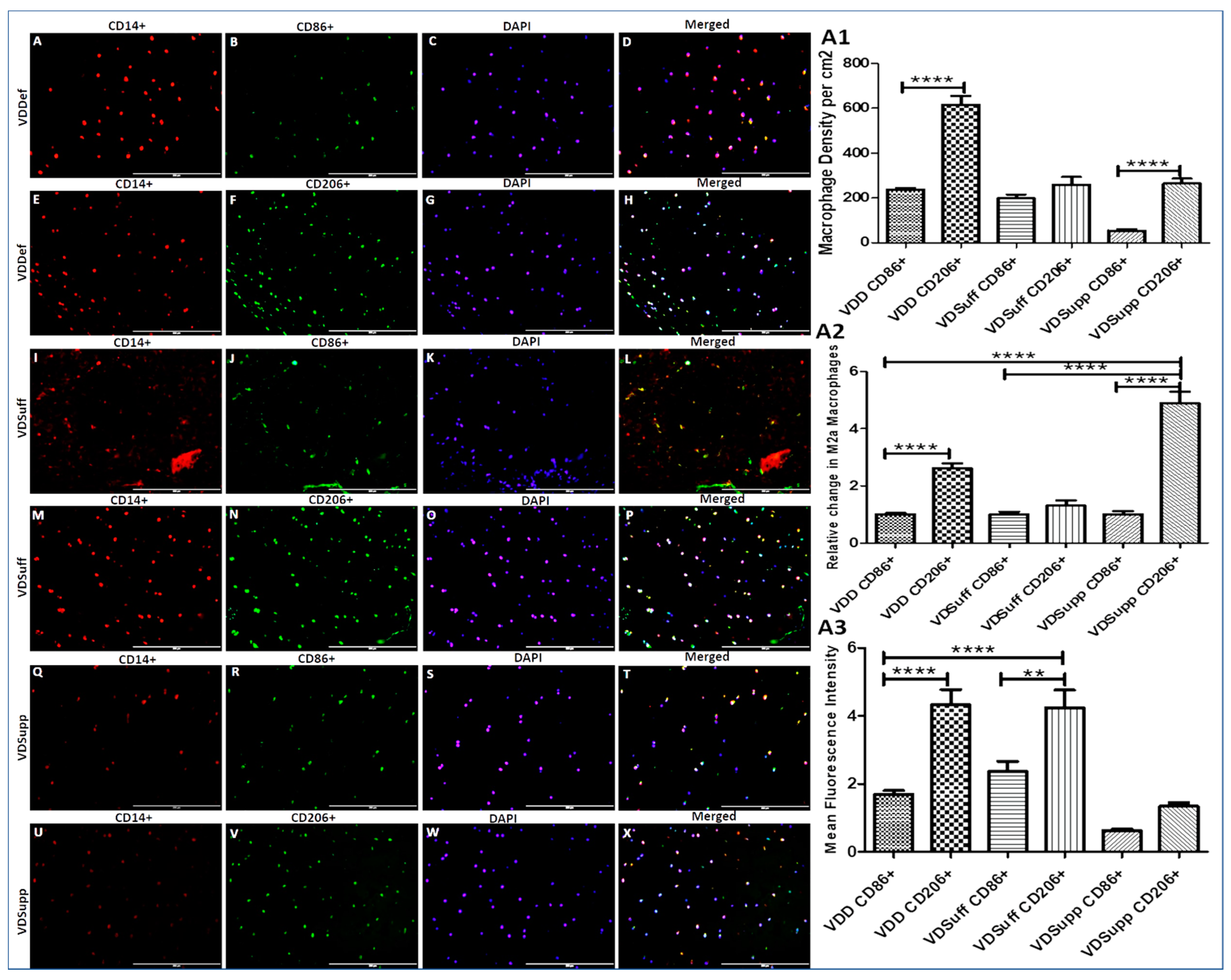
3.3. Increased Expression of M2 Macrophage in Vitamin D Deficient Microswine Cartilage
3.4. Expression of VDR, Cyp24A1, and Cyp27B1 in Swine Cartilage
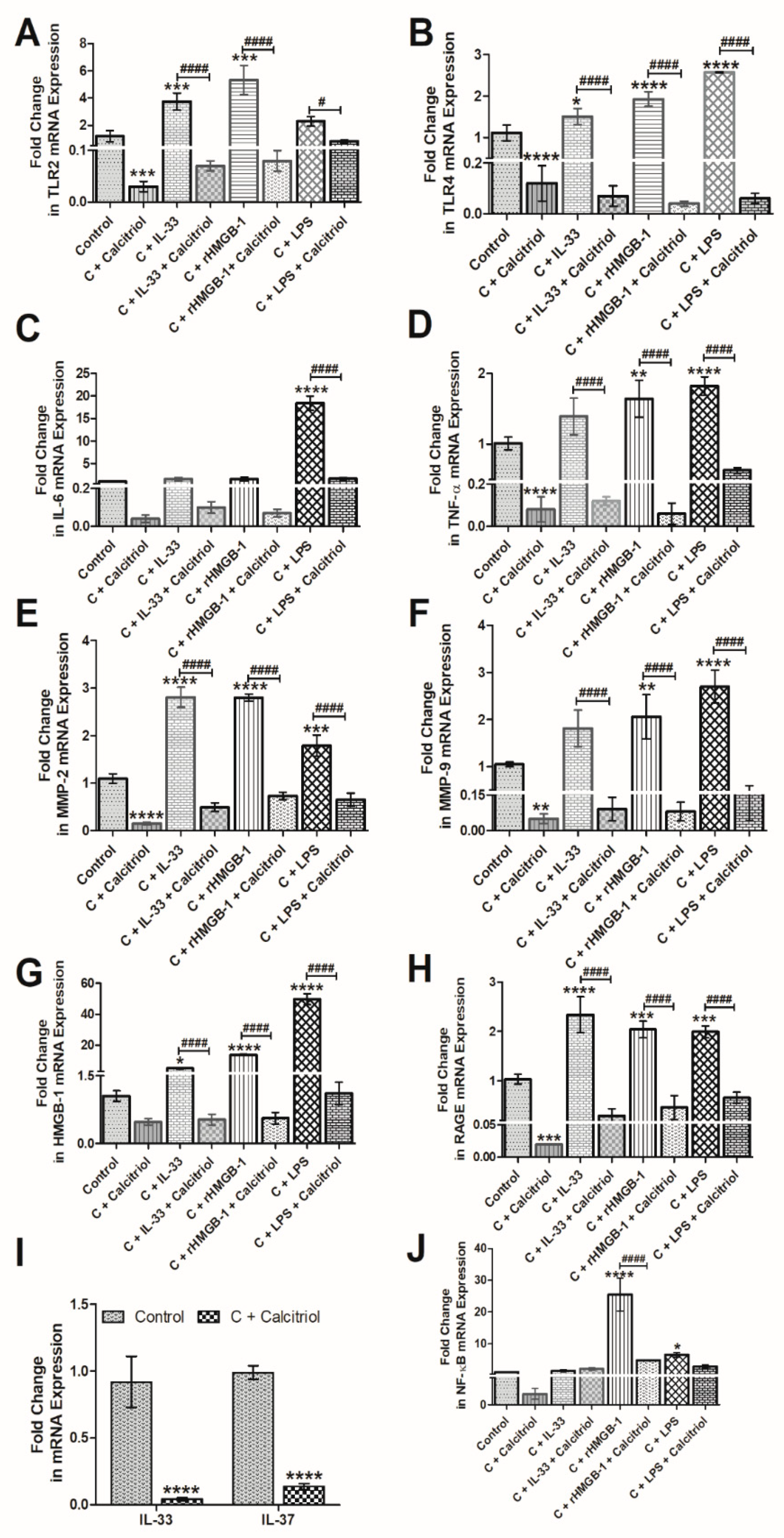
3.5. Calcitriol Attenuates the mRNA Expression of IL-33, TLR-2, TLR-4, NF-κB, IL-6, TNF-α, HMGB-1, RAGE, MMP-2, and MMP-9 in Normal Human Chondrocytes
3.6. Calcitriol Downregulates the Effect of Recombinant(r) IL-33, rHMGB-1, and LPS on TLR-2, TLR-4, NF-κB, IL-6, TNF-α, MMP-2, MMP-9, HMGB-1, and RAGE in NHAC
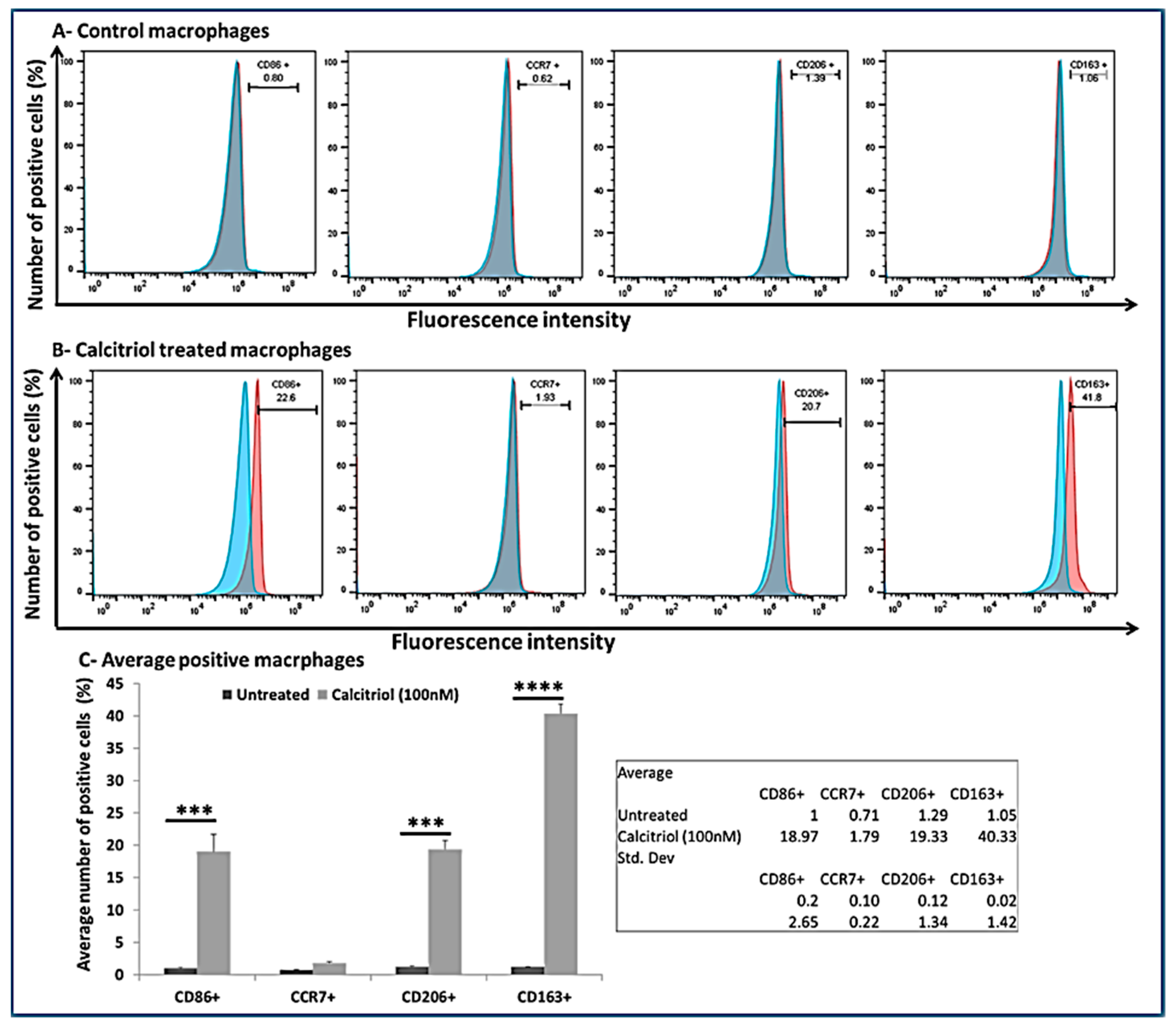
3.7. Calcitriol Favors M2 Macrophage Polarization
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, V.; Dietz, N.E.; Dilisio, M.F.; Radwan, M.M.; Agrawal, D.K. Vitamin D attenuates inflammation, fatty infiltration, and cartilage loss in the knee of hyperlipidemic microswine. Arthritis Res. Ther. 2016, 18, 203. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.H.; Rai, V.; Dilisio, M.F.; Agrawal, D.K. Damage-associated molecular patterns in the pathogenesis of osteoarthritis: Potentially novel therapeutic targets. Mol. Cell. Biochem. 2017, 434, 171–179. [Google Scholar] [CrossRef]
- Rosenberg, J.H.; Rai, V.; Dilisio, M.F.; Sekundiak, T.D.; Agrawal, D.K. Increased expression of damage-associated molecular patterns (DAMPs) in osteoarthritis of human knee joint compared to hip joint. Mol. Cell. Biochem. 2017, 436, 59–69. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Paris, G.; Pozharskaya, T.; Asempa, T.; Lane, A.P. Damage-associated molecular patterns stimulate interleukin-33 expression in nasal polyp epithelial cells. Int. Forum Allergy Rhinol. 2014, 4, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Xia, L.; Shen, H.; Lu, J. Elevated serum and synovial fluid levels of interleukin-37 in patients with rheumatoid arthritis: Attenuated the production of inflammatory cytokines. Cytokine 2015, 76, 553–557. [Google Scholar] [CrossRef]
- Imamura, M.; Ezquerro, F.; Marcon Alfieri, F.; Vilas Boas, L.; Tozetto-Mendoza, T.R.; Chen, J.; Ozcakar, L.; Arendt-Nielsen, L.; Rizzo Battistella, L. Serum levels of proinflammatory cytokines in painful knee osteoarthritis and sensitization. Int. J. Inflam. 2015, 2015, 329792. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 2016, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurowska-Stolarska, M.; Stolarski, B.; Kewin, P.; Murphy, G.; Corrigan, C.J.; Ying, S.; Pitman, N.; Mirchandani, A.; Rana, B.; van Rooijen, N.; et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 2009, 183, 6469–6477. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Gao, C.; Chi, X.; Hu, Y.W.; Zheng, L.; Zeng, T.; Wang, Q. IL-37 Expression is Upregulated in Patients with Tuberculosis and Induces Macrophages Towards an M2-like Phenotype. Scand. J. Immunol. 2015, 82, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Rai, V. Immunomodulation of Inflammatory Response in Osteoarthritis: Therapeutic Potential of Blocking Il-33/ST2 Receptor. Creighton University, 2017. Available online: https://dspace2.creighton.edu/xmlui/handle/10504/114071 (accessed on 11 July 2020).
- Mohammadi-Kordkhayli, M.; Ahangar-Parvin, R.; Azizi, S.V.; Nemati, M.; Shamsizadeh, A.; Khaksari, M.; Moazzeni, S.M.; Jafarzadeh, A. Vitamin D Modulates the Expression of IL-27 and IL-33 in the Central Nervous System in Experimental Autoimmune Encephalomyelitis (EAE). Iran J. Immunol. 2015, 12, 35–49. [Google Scholar]
- Paplinska-Goryca, M.; Nejman-Gryz, P.; Proboszcz, M.; Krenke, R. The effect of 1,25-dihydroxyvitamin D3 on TSLP, IL-33 and IL-25 expression in respiratory epithelium. Eur. Cytokine Netw. 2016, 27, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Islam, V.I.; Babu, N.P.; Pandikumar, P.; Thirugnanasambantham, K.; Chellappandian, M.; Raj, C.S.; Paulraj, M.G.; Ignacimuthu, S. Swertiamarin attenuates inflammation mediators via modulating NF-kappaB/I kappaB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur. J. Pharm. Sci. 2014, 56, 70–86. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Cherry, W.B.; Yoon, J.; Bartemes, K.R.; Iijima, K.; Kita, H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 2008, 121, 1484–1490. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Song, Y.; Yi, Y.; Qiu, F.; Wang, J.; Li, J.; Jin, Q.; Sacitharan, P.K. Blockade of IL-33 signalling attenuates osteoarthritis. Clin. Transl. Immunol. 2020, 9, e1185. [Google Scholar] [CrossRef]
- Bergink, A.P.; Zillikens, M.C.; Van Leeuwen, J.P.; Hofman, A.; Uitterlinden, A.G.; van Meurs, J.B. 25-Hydroxyvitamin D and osteoarthritis: A meta-analysis including new data. Semin. Arthritis Rheum. 2016, 45, 539–546. [Google Scholar] [CrossRef]
- Goula, T.; Kouskoukis, A.; Drosos, G.; Tselepis, A.S.; Ververidis, A.; Valkanis, C.; Zisimopoulos, A.; Kazakos, K. Vitamin D status in patients with knee or hip osteoarthritis in a Mediterranean country. J. Orthop. Traumatol. 2015, 16, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanghi, D.; Mishra, A.; Sharma, A.C.; Singh, A.; Natu, S.M.; Agarwal, S.; Srivastava, R.N. Does vitamin D improve osteoarthritis of the knee: A randomized controlled pilot trial. Clin. Orthop. Relat. Res. 2013, 471, 3556–3562. [Google Scholar] [CrossRef] [Green Version]
- Al-Jarallah, K.F.; Shehab, D.; Al-Awadhi, A.; Nahar, I.; Haider, M.Z.; Moussa, M.A. Are 25(OH)D levels related to the severity of knee osteoarthritis and function? Med. Princ. Pract. 2012, 21, 74–78. [Google Scholar] [CrossRef]
- Heidari, B.; Heidari, P.; Hajian-Tilaki, K. Association between serum vitamin D deficiency and knee osteoarthritis. Int. Orthop. 2011, 35, 1627–1631. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.F.; Driban, J.B.; Lo, G.H.; Price, L.L.; Booth, S.; Eaton, C.B.; Lu, B.; Nevitt, M.; Jackson, B.; Garganta, C.; et al. Vitamin D deficiency is associated with progression of knee osteoarthritis. J. Nutr. 2014, 144, 2002–2008. [Google Scholar] [CrossRef] [Green Version]
- Konstari, S.; Kaila-Kangas, L.; Jaaskelainen, T.; Heliovaara, M.; Rissanen, H.; Marniemi, J.; Knekt, P.; Arokoski, J.; Karppinen, J. Serum 25-hydroxyvitamin D and the risk of knee and hip osteoarthritis leading to hospitalization: A cohort study of 5274 Finns. Rheumatology (Oxford) 2014, 53, 1778–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstari, S.; Paananen, M.; Heliovaara, M.; Knekt, P.; Marniemi, J.; Impivaara, O.; Arokoski, J.; Karppinen, J. Association of 25-hydroxyvitamin D with the incidence of knee and hip osteoarthritis: A 22-year follow-up study. Scand. J. Rheumatol. 2012, 41, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Cauci, S.; Lombardi, G.; Lanteri, P.; Croiset, S.; Brayda-Bruno, M.; Banfi, G. Relationship between vitamin D receptor gene (VDR) polymorphisms, vitamin D status, osteoarthritis and intervertebral disc degeneration. J. Steroid. Biochem. Mol. Biol. 2013, 138, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Tetlow, L.C.; Woolley, D.E. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthr. Cartil. 2001, 9, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinans, H.; Siebelt, M.; Agricola, R.; Botter, S.M.; Piscaer, T.M.; Waarsing, J.H. Pathophysiology of peri-articular bone changes in osteoarthritis. Bone 2012, 51, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ushiyama, T.; Inoue, K.; Kawasaki, T.; Hukuda, S. Vitamin D receptor gene polymorphisms and osteoarthritis of the hand, hip, and knee: Acase-control study in Japan. Rheumatology (Oxford) 2000, 39, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, T.; Henriksen, V.T.; Rogers, V.E.; Aguirre, D.; Trawick, R.H.; Lynn Rasmussen, G.; Momberger, N.G. Vitamin D deficiency associates with gamma-tocopherol and quadriceps weakness but not inflammatory cytokines in subjects with knee osteoarthritis. Redox Biol. 2014, 2, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Felson, D.T. CORR Insights (R): Does vitamin D improve osteoarthritis of the knee: A randomized controlled pilot trial. Clin. Orthop. Relat. Res. 2013, 471, 3563–3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, N.; Muraki, S.; Oka, H.; Nakamura, K.; Kawaguchi, H.; Tanaka, S.; Akune, T. Serum levels of 25-hydroxyvitamin D and the occurrence of musculoskeletal diseases: A 3-year follow-up to the road study. Osteoporos Int. 2015, 26, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Winzenberg, T.; Nguo, K.; Lin, J.; Jones, G.; Ding, C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: A systematic review. Rheumatology (Oxford) 2013, 52, 1323–1334. [Google Scholar] [CrossRef] [Green Version]
- Ronken, S.; Arnold, M.P.; Ardura Garcia, H.; Jeger, A.; Daniels, A.U.; Wirz, D. A comparison of healthy human and swine articular cartilage dynamic indentation mechanics. Biomech. Model. Mechanobiol. 2012, 11, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.R.; Szczodry, M.; Bruno, S. Animal models for cartilage regeneration and repair. Tissue Eng. Part B Rev. 2010, 16, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.K.; Agrawal, T.; DelCore, M.G.; Mohiuddin, S.M.; Agrawal, D.K. Vitamin D deficiency induces cardiac hypertrophy and inflammation in epicardial adipose tissue in hypercholesterolemic swine. Exp. Mol. Pathol. 2012, 93, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, V.; Rao, V.H.; Shao, Z.; Agrawal, D.K. Dendritic cells expressing triggering receptor expressed on myeloid cells-1 correlate with plaque stability in symptomatic and asymptomatic patients with carotid stenosis. PLoS ONE 2016, 11, e0154802. [Google Scholar] [CrossRef]
- Kypriotou, M.; Fossard-Demoor, M.; Chadjichristos, C.; Ghayor, C.; de Crombrugghe, B.; Pujol, J.P.; Galera, P. SOX9 exerts a bifunctional effect on type II collagen gene (COL2A1) expression in chondrocytes depending on the differentiation state. DNA Cell Biol. 2003, 22, 119–129. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Ballak, D.B.; van Diepen, J.A.; Moschen, A.R.; Jansen, H.J.; Hijmans, A.; Groenhof, G.J.; Leenders, F.; Bufler, P.; Boekschoten, M.V.; Muller, M.; et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat. Commun. 2014, 5, 4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boraschi, D.; Lucchesi, D.; Hainzl, S.; Leitner, M.; Maier, E.; Mangelberger, D.; Oostingh, G.J.; Pfaller, T.; Pixner, C.; Posselt, G.; et al. IL-37: A new anti-inflammatory cytokine of the IL-1 family. Eur. Cytokine Netw. 2011, 22, 127–147. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.A.; Haddad, F.S. High prevalence of vitamin D deficiency in elderly patients with advanced osteoarthritis scheduled for total knee replacement associated with poorer preoperative functional state. Ann. R. Coll. Surg. Engl. 2013, 95, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, J.; Wainwright, S.D.; Lauder, S.; Amos, N.; Hughes, C.E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 2006, 8, R187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Liu, F.; Tang, M.; Yuan, M.; Hu, A.; Zhan, Z.; Li, Z.; Li, J.; Ding, X.; Lu, L. Macrophage polarization in experimental and clinical choroidal neovascularization. Sci. Rep. 2016, 6, 30933. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, M.; Guo, Y.; Song, Z.; Liu, B. 1,25-Dihydroxyvitamin D(3) Promotes High Glucose-Induced M1 Macrophage Switching to M2 via the VDR-PPARgamma Signaling Pathway. Biomed. Res. Int. 2015, 2015, 157834. [Google Scholar]
- Koschmieder, S.; Agrawal, S.; Radomska, H.S.; Huettner, C.S.; Tenen, D.G.; Ottmann, O.G.; Berdel, W.E.; Serve, H.L.; Muller-Tidow, C. Decitabine and vitamin D3 differentially affect hematopoietic transcription factors to induce monocytic differentiation. Int. J. Oncol. 2007, 30, 349–355. [Google Scholar] [PubMed]
- Li, D.; Guabiraba, R.; Besnard, A.-G.; Komai-Koma, M.; Jabir, M.S.; Zhang, L.; Graham, G.J.; Kurowska-Stolarska, M.; Liew, F.Y.; McSharry, C. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 2014, 134, 1422–1432.e11. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.D.; Oak, S.R.; Hartigan, A.J.; Finn, W.G.; Kunkel, S.L.; Duffy, K.E.; Das, A.; Hogaboam, C.M. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010, 11, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearley, J.; Silver, J.S.; Sanden, C.; Liu, Z.; Berlin, A.A.; White, N.; Mori, M.; Pham, T.H.; Ward, C.K.; Criner, G.J.; et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity 2015, 42, 566–579. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Neff, C.P.; Barber, K.; Hong, J.; Luo, Y.; Azam, T.; Palmer, B.E.; Fujita, M.; Garlanda, C.; Mantovani, A.; et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc. Natl. Acad. Sci. USA 2015, 112, 2497–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaganti, R.K.; Parimi, N.; Cawthon, P.; Dam, T.L.; Nevitt, M.C.; Lane, N.E. Association of 25-hydroxyvitamin D with prevalent osteoarthritis of the hip in elderly men: The osteoporotic fractures in men study. Arthritis Rheum. 2010, 62, 511–514. [Google Scholar] [CrossRef] [Green Version]
- Palmer, G.; Talabot-Ayer, D.; Lamacchia, C.; Toy, D.; Seemayer, C.A.; Viatte, S.; Finckh, A.; Smith, D.E.; Gabay, C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009, 60, 738–749. [Google Scholar] [CrossRef]
- Blom, A.B.; van Lent, P.L.; Holthuysen, A.E.; van der Kraan, P.M.; Roth, J.; van Rooijen, N.; van den Berg, W.B. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 2004, 12, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Riek, A.E.; Darwech, I.; Funai, K.; Shao, J.; Chin, K.; Sierra, O.L.; Carmeliet, G.; Ostlund, R.E., Jr.; Bernal-Mizrachi, C. Deletion of macrophage Vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep. 2015, 10, 1872–1886. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Harvey, W.F.; Price, L.L.; Paulus, J.K.; Dawson-Hughes, B.; McAlindon, T.E. Relationship of bone mineral density to progression of knee osteoarthritis. Arthritis Rheum. 2013, 65, 1541–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-Sánchez, L.; López Casimiro, K.; Winzenberg, T.M.; Tugwell, P.; Clark, P. Calcium and Vitamin D for Increasing Bone Mineral Density in Premenopausal Women. The Cochrane Library 2017. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012664/full (accessed on 11 July 2020).
- McAlindon, T.; LaValley, M.; Schneider, E.; Nuite, M.; Lee, J.Y.; Price, L.L.; Lo, G.; Dawson-Hughes, B. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: A randomized controlled trial. JAMA 2013, 309, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, W.E., Jr.; Balakir, R.; Precht, P.; Liang, C.T. 1,25-Dihydroxyvitamin D3 down-regulates aggrecan proteoglycan expression in immortalized rat chondrocytes through a post-transcriptional mechanism. J. Biol. Chem. 1991, 266, 24804–24808. [Google Scholar] [CrossRef]
- Miller, A.M.; Asquith, D.L.; Hueber, A.J.; Anderson, L.A.; Holmes, W.M.; McKenzie, A.N.; Xu, D.; Sattar, N.; McInnes, I.B.; Liew, F.Y. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ. Res. 2010, 107, 650–658. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Chen, Y.H.; Woszczek, G.; Matthews, N.C.; Chevretton, E.; Gupta, A.; Saglani, S.; Bush, A.; Corrigan, C.; Cousins, D.J.; et al. Vitamin D enhances production of soluble ST2, inhibiting the action of IL-33. J. Allergy Clin. Immunol. 2015, 135, 824–827.e823. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Zheng, X.F.; Qian, B.H.; Fang, H.; Wang, J.J.; Zhang, L.L.; Pang, Y.F.; Zhang, J.; Wei, X.Q.; Xia, Z.F.; et al. Plasma Interleukin-37 Is Elevated in Patients with Rheumatoid Arthritis: Its Correlation with Disease Activity and Th1/Th2/Th17-Related Cytokines. Dis. Markers 2015, 2015, 795043. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Jiang, B.; Deng, J.; Du, J.; Xiong, W.; Guan, Y.; Wen, Z.; Huang, K.; Huang, Z. IL-37 Alleviates Rheumatoid Arthritis by Suppressing IL-17 and IL-17-Triggering Cytokine Production and Limiting Th17 Cell Proliferation. J. Immunol. 2015, 194, 5110–5119. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Van Sweringen, H.L.; Belizaire, R.M.; Quillin, R.C.; Schuster, R.; Blanchard, J.; Burns, J.M.; Tevar, A.D.; Edwards, M.J.; Lentsch, A.B. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J. Gastroenterol. Hepatol. 2012, 27, 1609–1616. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Wang, Y.; Zhang, W.; Luan, J. A novel therapeutic target of spontaneous bacterial peritonitis: Skewing M2 polarization through vitamin D-VDR-IL-37 pathway. Liver Int. 2016, 36, 313. [Google Scholar] [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Tete, S.; Tripodi, D.; Rosati, M.; Conti, F.; Maccauro, G.; Saggini, A.; Cianchetti, E.; Caraffa, A.; Antinolfi, P.; Toniato, E.; et al. IL-37 (IL-1F7) the newest anti-inflammatory cytokine which suppresses immune responses and inflammation. Int. J. Immunopathol. Pharmacol. 2012, 25, 31–38. [Google Scholar] [CrossRef]
- Bulau, A.M.; Nold, M.F.; Li, S.; Nold-Petry, C.A.; Fink, M.; Mansell, A.; Schwerd, T.; Hong, J.; Rubartelli, A.; Dinarello, C.A.; et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc. Natl. Acad. Sci. USA 2014, 111, 2650–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulk, S.E.; Liao, K.C.; Muruve, D.A.; Li, Y.; Beck, P.L.; MacDonald, J.A. Vitamin D(3) metabolites enhance the NLRP3-dependent secretion of IL-1beta from human THP-1 monocytic cells. J. Cell. Biochem. 2015, 116, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Schelbergen, R.F.; Blom, A.B.; van den Bosch, M.H.; Sloetjes, A.; Abdollahi-Roodsaz, S.; Schreurs, B.W.; Mort, J.S.; Vogl, T.; Roth, J.; van den Berg, W.B.; et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012, 64, 1477–1487. [Google Scholar] [CrossRef]
- Sillat, T.; Barreto, G.; Clarijs, P.; Soininen, A.; Ainola, M.; Pajarinen, J.; Korhonen, M.; Konttinen, Y.T.; Sakalyte, R.; Hukkanen, M.; et al. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop. 2013, 84, 585–592. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.; Ding, A.; Golenbock, D.T.; Latz, E.; Czura, C.J.; Fenton, M.J.; Tracey, K.J.; Yang, H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 2006, 26, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Do, J.E.; Kwon, S.Y.; Park, S.; Lee, E.S. Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet’s disease. Rheumatology (Oxford) 2008, 47, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Wang, H.; Wu, F.; Li, M.; Chen, W.; Lv, L. Vitamin D3 alters Toll-like receptor 4 signaling in monocytes of pregnant women at risk for preeclampsia. Int. J. Clin. Exp. Med. 2015, 8, 18041–18049. [Google Scholar]
- Qiao, J.; Song, L.; Zhang, Y.; Luan, B. HMGB1/TLR4/NF-κB signaling pathway and role of vitamin D in asthmatic mice. Chin. J. Contemp. Pediatrics 2017, 19, 95–103. [Google Scholar]
- Qiao, J.; Luan, B.; Gu, H.; Zhang, Y. Effect of different 1, 25-(OH)2D3 doses on high mobility group box1 and toll-like receptors 4 expression in lung tissue of asthmatic mice. Int. J. Clin. Exp. Med. 2015, 8, 4016–4023. [Google Scholar]
- Dean, D.D.; Schwartz, Z.; Schmitz, J.; Muniz, O.E.; Lu, Y.; Calderon, F.; Howell, D.S.; Boyan, B.D. Vitamin D regulation of metalloproteinase activity in matrix vesicles. Connect Tissue Res. 1996, 35, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Tetlow, L.C.; Woolley, D.E. The effects of 1 alpha,25-dihydroxyvitamin D(3) on matrix metalloproteinase and prostaglandin E(2) production by cells of the rheumatoid lesion. Arthritis Res. 1999, 1, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J. IL-33 is a potential new target in OA. Nat. Rev. Rheumatol. 2021, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Van Geffen, E.; van Caam, A.; Schreurs, W.; van de Loo, F.; van Lent, P.; Koenders, M.; Thudium, C.S.; Bay-Jensen, A.C.; Davidson, E.B.; van der Kraan, P.M. IL-37 diminishes proteoglycan loss in human OA cartilage: Donor-specific link between IL-37 and MMP-3. Osteoarthr. Cartil. 2019, 27, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Geffen, E.W.; van Caam, A.P.M.; Vitters, E.L.; van Beuningen, H.M.; van de Loo, F.A.; van Lent, P.L.E.M.; Koenders, M.I.; van der Kraan, P.M. Interleukin-37 protects stem cell-based cartilage formation in an inflammatory osteoarthritis-like microenvironment. Tissue Eng. Part A 2019, 25, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Compton, R.A.; Lonergan, A.R.; Tsillioni, I.; Conti, P.; Ronconi, G.; Lauritano, D.; Rebeiz, E.E.; Theoharides, T.C. Neurohormonal markers in chronic rhinosinusitis. J. Biol. Regul. Homeost. Agents 2021, 35, 901–908. [Google Scholar] [PubMed]
- Conti, P.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Ronconi, G. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: A promising inhibitory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 1971–1975. [Google Scholar] [PubMed]

| Gene of Interest | Forward Primer | Reverse Primer |
|---|---|---|
| HMGB-1 | 5′-AAG CAC CCA GAT GCT TCA GT-3′ | 5′-TCC GCT TTT GCC ATA TCT TC-3′ |
| IL-33 | 5′-GTGACGGTGTTGATGGTAAGA -3′ | 5′-CTCCACAGAGTGTTCCTTGTT -3′ |
| IL-37 | 5′-GCACCTCCTGCAATTGTAATG -3′ | 5′-CAGTTTCCTAATCGCTGACCT -3′ |
| IL-6 | 5′-ATA GGA CTG GAG ATG TCT GAG G-3′ | 5′-GCT TGT GGA GAA GGA GTT CAT AG-3′ |
| MMP-2 | 5′-TGA TGG TGT CTG CTG GAA AG-3′ | 5′-CTA CAG GAC AGA GGG ACT AGA G-3′ |
| MMP-9 | 5′-ACA AGC TCT TCG GCT TCT G -3′ | 5′-GGT ACA GGT CGA GTA CTC CTT A-3′ |
| NF-κB | 5′-GAC TAC GAC CTG AAT GCT GTG-3′ | 5′-GTC AAA GAT GGG ATG AGG AAG G-3′ |
| RAGE | 5′- CCT GCA GGG ACT CTT AGC TG-3′ | 5′- CTC CGA CTG CAG TGT GAA GA-3′ |
| TLR-2 | 5′-CTG GAG AAA GCC TTG AAC TCT AT-3′ | 5′-GAC ACT CGG TCT CTA GCA ATT T-3′ |
| TLR-4 | 5′-TCA AAG AGC TGG TGC GAA A-3′ | 5′-CAG CTG CTT GTC TGC ATT TG-3′ |
| TNF-α | 5′-ACC CTC AAC CTC TTC TGG CTC AA-3′ | 5′-AAT CCC AGG TTT CGA AGT GGT GGT-3′ |
| GAPDH | 5′-GGT GAA GGT CGG AGT CAA CGG ATT TGG TCG-3′ | 5′-GGA TCT CGC TCC TGG AAG ATG GTG ATG GG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, V.; Radwan, M.M.; Agrawal, D.K. IL-33, IL-37, and Vitamin D Interaction Mediate Immunomodulation of Inflammation in Degenerating Cartilage. Antibodies 2021, 10, 41. https://doi.org/10.3390/antib10040041
Rai V, Radwan MM, Agrawal DK. IL-33, IL-37, and Vitamin D Interaction Mediate Immunomodulation of Inflammation in Degenerating Cartilage. Antibodies. 2021; 10(4):41. https://doi.org/10.3390/antib10040041
Chicago/Turabian StyleRai, Vikrant, Mohamed M. Radwan, and Devendra K. Agrawal. 2021. "IL-33, IL-37, and Vitamin D Interaction Mediate Immunomodulation of Inflammation in Degenerating Cartilage" Antibodies 10, no. 4: 41. https://doi.org/10.3390/antib10040041
APA StyleRai, V., Radwan, M. M., & Agrawal, D. K. (2021). IL-33, IL-37, and Vitamin D Interaction Mediate Immunomodulation of Inflammation in Degenerating Cartilage. Antibodies, 10(4), 41. https://doi.org/10.3390/antib10040041







