-
 Anti-HMGCR-Antibody-Positive Statin-Induced Myositis: A Pilot Case Series on Treatment with Bempedoic Acid and Immunosuppressive Therapy
Anti-HMGCR-Antibody-Positive Statin-Induced Myositis: A Pilot Case Series on Treatment with Bempedoic Acid and Immunosuppressive Therapy -
 Seroprevalence of IgG and IgE Antibodies Against Anisakis in the Presumably Healthy Population of the Canary Islands
Seroprevalence of IgG and IgE Antibodies Against Anisakis in the Presumably Healthy Population of the Canary Islands -
 Generative Deep Learning Design of Single-Domain Antibodies Against Venezuelan Equine Encephalitis Virus
Generative Deep Learning Design of Single-Domain Antibodies Against Venezuelan Equine Encephalitis Virus -
 Fragment-Based Immune Cell Engager Antibodies in Treatment of Cancer, Infectious and Autoimmune Diseases: Lessons and Insights from Clinical and Translational Studies
Fragment-Based Immune Cell Engager Antibodies in Treatment of Cancer, Infectious and Autoimmune Diseases: Lessons and Insights from Clinical and Translational Studies -
 IgG to Galactose-Alpha-1,3-Galactose: Impact of Alpha-Gal IgE Sensitization, Blood Type, and Tick Bites
IgG to Galactose-Alpha-1,3-Galactose: Impact of Alpha-Gal IgE Sensitization, Blood Type, and Tick Bites
Journal Description
Antibodies
Antibodies
is an international, peer-reviewed, open access journal on immunoglobulins, published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: CiteScore - Q2 (Drug Discovery)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 22.2 days after submission; acceptance to publication is undertaken in 4.6 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
2.7 (2024);
5-Year Impact Factor:
4.7 (2024)
Latest Articles
Comparative Evaluation of Three Primary Antibody Clones for p16 Immunohistochemistry in Gynecologic Tumors
Antibodies 2025, 14(3), 77; https://doi.org/10.3390/antib14030077 - 5 Sep 2025
Abstract
Background: p16 immunohistochemistry (IHC) serves as a surrogate marker for high-risk human papillomavirus (hrHPV) and is widely used in gynecologic pathology. However, few studies have directly compared the staining performance and reproducibility of different p16 antibody clones in this context. Methods: We retrospectively
[...] Read more.
Background: p16 immunohistochemistry (IHC) serves as a surrogate marker for high-risk human papillomavirus (hrHPV) and is widely used in gynecologic pathology. However, few studies have directly compared the staining performance and reproducibility of different p16 antibody clones in this context. Methods: We retrospectively evaluated 176 gynecologic tumor specimens including 42 whole slide sections and 134 tissue microarray cores from the cervix, endometrium, vulva, and ovary using three fully automated p16 IHC assays: E6H4 (Ventana/Roche), JC8 (Agilent/Dako), and 6H12 (Leica). Two pathologists independently reviewed each case, and concordance and interobserver agreement were analyzed. Sensitivity, specificity, and Cohen’s κ statistics were calculated, with E6H4 serving as the reference. Results: All three antibody clones demonstrated excellent staining performance with preserved tissue morphology and minimal background artifacts. Concordance for p16 positivity/negativity was 100% across all clone pairings (95% CI: 97.9–100%). Interobserver reproducibility was also perfect, with a κ coefficient of 1.00 (95% CI: 0.94–1.00). Minor non-block staining patterns did not impair interpretability. Conclusions: Our findings indicate that E6H4, JC8, and 6H12 clones yield comparable staining results when used in conjunction with standardized automated protocols. These results support the practical interchangeability of these clones in clinical and research settings, particularly when cost, availability, or risk management require substitution. Laboratories should continue to perform internal validation and utilize external quality assurance programs when implementing p16 IHC.
Full article
(This article belongs to the Section Antibody-Based Diagnostics)
►
Show Figures
Open AccessReview
Infertility and Auto-Antibodies: A Review
by
Brigita Šemeklienė and Brigita Gradauskienė
Antibodies 2025, 14(3), 76; https://doi.org/10.3390/antib14030076 - 5 Sep 2025
Abstract
Infertility is a multifactorial condition with a wide range of potential causes, including anatomical, hormonal, genetic, and lifestyle-related factors. Among these, immunological mechanisms have increasingly been recognized as important contributors. The immune system plays a critical role in maintaining reproductive health, and its
[...] Read more.
Infertility is a multifactorial condition with a wide range of potential causes, including anatomical, hormonal, genetic, and lifestyle-related factors. Among these, immunological mechanisms have increasingly been recognized as important contributors. The immune system plays a critical role in maintaining reproductive health, and its dysregulation can impair fertility in both men and women. Recent scientific studies suggest that altered immune responses, particularly those involving autoimmune reactions, may negatively affect fertility by disrupting the complex immunological balance required for successful conception and pregnancy maintenance. This review focuses on the most common autoantibodies, such as antinuclear, antisperm, antiendometrial, antiovarian, antiphospholipid, and antithyroid antibodies. Treatment options, including immunomodulatory therapy, hormone replacement therapy, and lifestyle interventions, are also reviewed.
Full article
(This article belongs to the Special Issue Antibody and Autoantibody Specificities in Autoimmunity)
►▼
Show Figures
Figure 1
Open AccessArticle
Safety and Effectiveness of Dupilumab in Atopic Dermatitis Patients with Hematologic Comorbidities: A Multicenter, Retrospective Study
by
Luca Bettolini, Stefano Bighetti, Silvia Mariel Ferrucci, Angelo Valerio Marzano, Francesca Barei, Alessandra Narcisi, Matteo Bianco, Andrea Carugno, Nicola Zerbinati, Simone Ribero, Michela Ortoncelli, Elena Pezzolo, Maddalena Napolitano, Martina Maurelli, Giampiero Girolomoni, Zeno Fratton, Enzo Errichetti, Caterina Foti, Giacomo Dal Bello, Ilaria Trave, Anna Balato, Dario Didona, Niccolò Gori, Federica Veronese, Giovanni Paolino, Franco Rongioletti, Mario Bruno Guanti, Laura Calabrese, Riccardo Balestri, Manfredo Bruni and Mariateresa Rossiadd
Show full author list
remove
Hide full author list
Antibodies 2025, 14(3), 75; https://doi.org/10.3390/antib14030075 - 3 Sep 2025
Abstract
Background: Dupilumab, a monoclonal antibody targeting the interleukin-4 receptor α, is approved for moderate-to-severe atopic dermatitis (AD). However, its safety profile in patients with concomitant hematologic disorders remains unclear, as such populations were excluded from pivotal trials. Objective: To evaluate the safety and
[...] Read more.
Background: Dupilumab, a monoclonal antibody targeting the interleukin-4 receptor α, is approved for moderate-to-severe atopic dermatitis (AD). However, its safety profile in patients with concomitant hematologic disorders remains unclear, as such populations were excluded from pivotal trials. Objective: To evaluate the safety and effectiveness of dupilumab in adolescents and adults with AD and underlying hematologic comorbidities. Methods: This retrospective, multicenter study included 139 patients aged ≥15 years with moderate-to-severe AD and at least one hematologic disorder, treated with dupilumab across 21 dermatology centers. Data on disease severity, laboratory markers, and hematologic outcomes were collected over a median follow-up of 52 weeks (range 4–156). Results: The most common hematologic conditions included monoclonal gammopathies, leukemias, lymphomas, myeloproliferative neoplasms, and immune cytopenias. Clinical response to dupilumab was sustained across all endpoints, with median EASI scores decreasing from 26.0 at the baseline to 1.0 at week 52. NRS pruritus and sleep scores similarly declined to 0.0 by week 52. Serum IgE levels and eosinophil counts progressively decreased. The clinical response to dupilumab was sustained across all endpoints, with significant and progressive improvements in EASI, pruritus NRS, and sleep NRS observed up to week 52, followed by long-term stability through week 156. Serum IgE levels decreased steadily at all timepoints, while eosinophil counts declined after week 4 and stabilized beyond week 52. Hematologic conditions remained stable in 82.7% of patients, resolved in 16.5%, and progressed in only one case. Twelve patients (8.6%) received a new hematologic diagnosis during follow-up; no causal relationship could be established due to the retrospective design and absence of systematic screening, and these findings should be interpreted as descriptive associations only. Conclusions: Dupilumab appears to be safe and effective in AD patients with a broad range of hematologic comorbidities, including malignancies. These findings support its use in real-world settings, though prospective studies are warranted to further assess long-term safety in this population.
Full article
(This article belongs to the Section Antibody-Based Therapeutics)
►▼
Show Figures
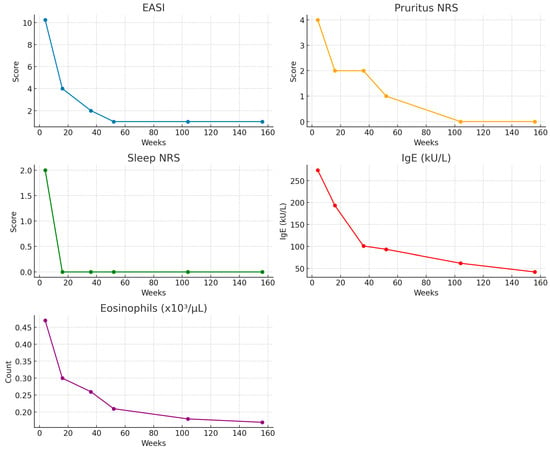
Figure 1
Open AccessArticle
Structure-Guided Stapling of Dimeric Conformations and Linker Engineering Enhance Thermostability and Fine-Tune Activity of Bispecific VHH Cytokine Agonists
by
Raphael Trenker, Deepti Rokkam, Andrew Morin, Priyanka Balasubrahmanyam, Verenice Paredes, Ivan Cheng, Rene de Waal Malefyt, Martin Oft, Patrick Lupardus and Sandro Vivona
Antibodies 2025, 14(3), 74; https://doi.org/10.3390/antib14030074 - 1 Sep 2025
Abstract
Background: Bispecific antibodies have emerged as a promising class of therapeutics, enabling simultaneous targeting of two distinct antigens. Single-domain antibodies (sdAbs) comprising camelid variable heavy chains (VHHs) provide a compact and adaptable platform for bispecific antibody design due to their small size and
[...] Read more.
Background: Bispecific antibodies have emerged as a promising class of therapeutics, enabling simultaneous targeting of two distinct antigens. Single-domain antibodies (sdAbs) comprising camelid variable heavy chains (VHHs) provide a compact and adaptable platform for bispecific antibody design due to their small size and ease of linkage. Methods: Here we investigate structure-activity relationship of VHH-based cytokine surrogates by combining cell signaling and functional assays with x-ray crystallography and other biophysical techniques. Results: We describe crystal structures of four unique bispecific VHHs that engage and activate the cytokine receptor pairs IL-18Rα/IL-18Rβ and IL-2Rβ/IL-2Rγ. These bispecific VHH molecules, referred to as surrogate cytokine agonists (SCAs), create unique cytokine signals that can be tuned by linker engineering. Our structural analysis reveals multiple dimeric conformations for these bispecific SCAs, where the two VHH domains can interact to form a compact structure. We demonstrate that the dimeric conformation can be enforced via engineering of a non-native disulfide bond between the VHH subunits, thus enhancing molecular thermostability. Conclusion: Our findings have important implications for the design and engineering of bispecific VHHs or sdAbs, offering a novel strategy for tuning their activity and increasing their stability.
Full article
(This article belongs to the Section Antibody Discovery and Engineering)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Computational Prediction of Single-Domain Immunoglobulin Aggregation Propensities Facilitates Discovery and Humanization of Recombinant Nanobodies
by
Felix Klaus Geyer, Julian Borbeck, Wiktoria Palka, Xueyuan Zhou, Jeffrey Takimoto, Brian Rabinovich, Bernd Reifenhäuser, Karlheinz Friedrich and Harald Kolmar
Antibodies 2025, 14(3), 73; https://doi.org/10.3390/antib14030073 - 28 Aug 2025
Abstract
Background/Objectives: Single-domain immunoglobulins are small protein modules with specific affinities. Among them, the variable domains of heavy chains of heavy-chain-only antibodies (VHH) as the antigen-binding fragment of heavy-chain-only antibodies (also termed nanobodies) have been widely investigated for their applicability, e.g., therapeutics and immunodiagnostics.
[...] Read more.
Background/Objectives: Single-domain immunoglobulins are small protein modules with specific affinities. Among them, the variable domains of heavy chains of heavy-chain-only antibodies (VHH) as the antigen-binding fragment of heavy-chain-only antibodies (also termed nanobodies) have been widely investigated for their applicability, e.g., therapeutics and immunodiagnostics. However, despite their advantageous biochemical and biophysical characteristics, protein aggregation throughout recombinant synthesis is a serious drawback in the development of nanobodies with application perspectives. Therefore, we aimed to develop a computational method to predict the aggregation propensity of VHH antibodies for the selection of promising candidates in early discovery. Methods: We employed a deep learning-based structure prediction for VHHs and derived from it likely biophysical and biochemical properties of the framework region 2 with relevance for aggregation. A total of 106 nanobody variants were produced by recombinant expression and characterized for their aggregation behavior using size exclusion chromatography (SEC). Results: Quantitative characteristics of framework region 2 patches were combined into a function that defines an aggregation score (AS) predicting the aggregation propensities of VHH variants. AS was evaluated for its capability to forecast recombinant VHH aggregation by experimentally studying VHH Fc-fusion proteins for their aggregation. We observed a clear correlation between the calculated aggregation score and the actual aggregation propensities of biochemically characterized VHHs Fc-fusion proteins. Moreover, we implemented an easily accessible pipeline of software modules to design nanobodies with desired solubility properties. Conclusions: AI-based prediction of VHH structures, followed by analysis of framework region 2 properties, can be used to predict the aggregation propensities of VHHs, providing a convenient and efficient tool for selecting stable recombinant nanobodies.
Full article
(This article belongs to the Collection Computational Antibody and Antigen Design)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Cost-Effective Method for Full-Length Sequencing of Monoclonal Antibodies from Hybridoma Cells
by
Sarah Döring, Georg Tscheuschner, Sabine Flemig, Michael G. Weller and Zoltán Konthur
Antibodies 2025, 14(3), 72; https://doi.org/10.3390/antib14030072 - 22 Aug 2025
Abstract
►▼
Show Figures
Background: Monoclonal antibodies play an important role in therapeutic and analytical applications. For recombinant expression, the coding sequences of the variable regions of the heavy and light chains are required. In addition, cloning antibody sequences, including constant regions, reduces the impact of hybridoma
[...] Read more.
Background: Monoclonal antibodies play an important role in therapeutic and analytical applications. For recombinant expression, the coding sequences of the variable regions of the heavy and light chains are required. In addition, cloning antibody sequences, including constant regions, reduces the impact of hybridoma cell loss and ensures preservation of the naturally occurring full antibody sequence. Method: We combined amplification of IgG antibody variable regions from hybridoma mRNA with an advanced method for full-length cloning of monoclonal antibodies in a simple two-step workflow. Following Sanger sequencing and evaluation of consensus sequences, the best matching variable, diversity, and joining (V-(D-)J) gene segments were identified according to identity scores from IgBLAST reference sequences. Simultaneously, the mouse IgG subclass was determined at the DNA level based on isotype-specific sequence patterns in the CH1 domain. Knowing the DNA sequence of V-(D-)J recombination responsible for the complementary determining region 3 (CDR 3), variable region-specific primers were designed and used to amplify the corresponding antibody constant regions. Results: To verify the approach, we applied it to the hybridoma clone BAM-CCMV-29-81 and obtained identical full-length antibody sequences as with RNA Illumina sequencing. Further validation at the protein level using an established MALDI-TOF MS-fingerprinting protocol showed that five out of six genetically encoded CDR domains of the monoclonal antibody BAM-CCMV-29-81 could be efficiently correlated. Conclusion: This simple, streamlined method enables the cost-effective determination of the full-length sequence of monoclonal antibodies from hybridoma cell lines, with the added benefit of obtaining the DNA sequence of the antibody ready for recombinant expression.
Full article

Figure 1
Open AccessArticle
Guidelines in the Preparation of Fully Synthetic, Human Single-Domain Antibody Phage Display Libraries
by
Mark A. Tornetta, Brian P. Whitaker, Olivia M. Cantwell, Peter N. Haytko, Eileen D. Pisors, Fulai Zhou and Mark L. Chiu
Antibodies 2025, 14(3), 71; https://doi.org/10.3390/antib14030071 - 15 Aug 2025
Abstract
Background/Objectives: The complexity of diseases such as cancer and auto-immune disorders drives the need for unique, target-driven therapeutics. A broader arsenal to generate better biologics-based therapeutics is needed to provide more efficient and effective antibody generation technologies. The critical parameter for antibody generation
[...] Read more.
Background/Objectives: The complexity of diseases such as cancer and auto-immune disorders drives the need for unique, target-driven therapeutics. A broader arsenal to generate better biologics-based therapeutics is needed to provide more efficient and effective antibody generation technologies. The critical parameter for antibody generation is to generate as much candidate diversity to each target as possible. Method/Results: We present guidelines for having an efficient process using a fully synthetic human single-domain antibody (sdAb) phage display library. Critical milestones for success focused on library quality control (QC) assessments, evaluation of specific biopanning outputs, and construct designs that enabled efficient transition to mammalian expression. The synthetic VHO libraries produced epitope diversity better than an immunized sourced library with candidates possessing nM potencies and monodispersity > 90% via SEC. Conclusions: Synthetic human scaffold sdAb phage display libraries was constructed, biopanned, and selected candidates that could be directly transitioned for mammalian expression. The diverse VHO sets of candidates produced from many targets easily provided opportunities to make a multi-specific biological compound. Both synthetic and immunized phage selection campaign results suggested that these technologies complemented each other to generate therapeutic candidates. Finally, we demonstrated how diverse data produced from a process that used VHO synthetic libraries could accelerate drug discovery.
Full article
(This article belongs to the Special Issue Therapeutic Antibodies: New Trends in Discovery, Developability and Characterization)
►▼
Show Figures

Figure 1
Open AccessArticle
Comparative Molecular Dynamics Study of 19 Bovine Antibodies with Ultralong CDR H3
by
Olena Denysenko, Anselm H. C. Horn and Heinrich Sticht
Antibodies 2025, 14(3), 70; https://doi.org/10.3390/antib14030070 - 13 Aug 2025
Abstract
►▼
Show Figures
Background/Objectives: Cows produce antibodies with ultralong CDRH3 segments (ulCABs) that contain a disulfide-stabilized knob domain. This domain is connected to the globular core of the antibody by a β-strand stalk. In the crystal structures, the stalk protrudes from the core in an
[...] Read more.
Background/Objectives: Cows produce antibodies with ultralong CDRH3 segments (ulCABs) that contain a disulfide-stabilized knob domain. This domain is connected to the globular core of the antibody by a β-strand stalk. In the crystal structures, the stalk protrudes from the core in an extended conformation and presents the knob at its distal end. However, the rigidity of this topology has been questioned due to the extensive crystal packing present in most ulCAB crystal structures. To gain more insight into the dynamics of ultralong CDRH3s, we performed a comparative molecular dynamics (MD) study of 19 unique ulCABs. Methods: For all 19 systems, one-microsecond MD simulations were performed in explicit solvent. The analyses included an investigation of the systems’ conformational stability and the dynamics of the knob domain as well as an energetic analysis of the intramolecular knob interactions. Results: The simulations show that the extended stalk–knob conformation observed in the crystal structures is not preserved in solution. There are significant differences in the degree of knob dynamics, the orientations of the knobs, the number of flexible stalk residues, and the frequency of the motions. Furthermore, interactions between the knob and the light chain (LC) of the ulCABs were observed in about half of the systems. Conclusions: The study reveals that pronounced knob dynamics is a general feature of ulCABs rather than an exception. The magnitude of knob motions depends on the system, thus reflecting the high sequence diversity of the CDRH3s in ulCABs. The observed knob–LC interactions might play a role in stabilizing distinct knob orientations. The MD simulations of ulCABs could also help to identify suitable knob fragments as mini-antibodies by suggesting appropriate truncation points based on flexible sites in the stalks.
Full article

Graphical abstract
Open AccessArticle
Conversion Factors to Compare Serum Concentrations of Anti-HBs, Anti-SARS-CoV-2 and Anti-Tetanus Toxin IgG
by
Aurelia Knispel and Christian Jassoy
Antibodies 2025, 14(3), 69; https://doi.org/10.3390/antib14030069 - 13 Aug 2025
Abstract
Background: The concentration of antigen-specific antibodies in serum is usually measured in international units/mL. Therefore, the actual concentration of virus-specific antibodies in sera is unknown. Objectives: The aim of the study was to determine conversion factors for concentrations of IgG against
[...] Read more.
Background: The concentration of antigen-specific antibodies in serum is usually measured in international units/mL. Therefore, the actual concentration of virus-specific antibodies in sera is unknown. Objectives: The aim of the study was to determine conversion factors for concentrations of IgG against hepatitis B surface antigen (HBs), SARS-CoV-2 receptor binding domain (RBD) and nucleoprotein (NP) as well as tetanus toxin (Ttx) in serum and to compare antigen-specific IgG concentrations in serum samples. Methods: Absorption equivalence ELISAs were used to determine conversion factors for international units (IU) for anti-HBs, anti-SARS-CoV-2-RBD and NP and for anti-Ttx immunoglobulin G. The antigen-specific IgG concentrations in serum samples were then measured in units/mL and the ratio of IgG concentrations in the sera was determined using the conversion factors. Results: One IU of anti-HBs IgG corresponded to 24.4 BAU of anti-CoV-2 RBD IgG, 6.87 BAU of anti-CoV-2 NP and 14 mIU of anti-Ttx IgG. One BAU anti-SARS-CoV-2 NP-specific IgG is equivalent to 3.5 BAU SARS-CoV-2 RBD-specific IgG. Conversion of international units showed that median serum anti-Ttx-IgG concentrations were 50 times higher and anti-CoV-2-RBD-IgG concentrations were 390 times higher than median anti-HBs-IgG concentrations. In addition, after SARS-CoV-2 infection, the concentration of NP-specific IgG in serum was generally higher than that of RBD-specific IgG. Conclusions: The study provides conversion factors for serum concentrations of IgG against HBs, SARS-CoV-2 RBD and NP, as well as Ttx-IgG. This offers new insights into serum IgG concentrations and allows conclusions to be drawn about plasma cell pools.
Full article
(This article belongs to the Section Humoral Immunity)
►▼
Show Figures

Figure 1
Open AccessArticle
A 3D Surface Plot for the Effective Visualization of Specific Serum Antibody Binding Properties
by
József Prechl, Ágnes Kovács, Krisztián Papp, Zoltán Hérincs and Tamás Pfeil
Antibodies 2025, 14(3), 68; https://doi.org/10.3390/antib14030068 - 13 Aug 2025
Abstract
Background: When an antigen molecule is exposed to serum, many different kinds of antibodies bind to it. The complexity of these binding events is only poorly characterized by assays that generate a single variable, generally reflecting the fractional saturation of the antigen, as
[...] Read more.
Background: When an antigen molecule is exposed to serum, many different kinds of antibodies bind to it. The complexity of these binding events is only poorly characterized by assays that generate a single variable, generally reflecting the fractional saturation of the antigen, as the readout. Methods: We have previously devised an assay that delivers the essential biochemical variables to determine fractional saturation as the output: an equilibrium dissociation constant for affinity, the ratio of antibody concentration to the equilibrium constant and the concentration of bound antibodies under reference conditions. Here we propose a visualization method for the practical and informative display of these variables. Results: Using total antigen concentration and free and bound antibody concentration as coordinates in a three-dimensional space, a surface plot can depict the behavior of serum antibodies in the measurement range and identify the values of the key variables of binding activity. This surface display (antibody binding in 3-concentration display, Ab3cD) was used for the characterization of antibody binding to the SARS-CoV-2 spike protein in seronegative and seropositive sera. We demonstrate that this visualization scheme is suitable for presenting both individual and group differences and that epitope density changes, not commonly measured by immunoassays, are also revealed by the method. Conclusions: We recommend the use of 3D visualization whenever detailed, informative and characteristic differences in serum antibody reactivity are studied.
Full article
(This article belongs to the Section Humoral Immunity)
►▼
Show Figures

Graphical abstract
Open AccessArticle
A Cancer-Specific Anti-Podocalyxin Monoclonal Antibody (humPcMab-60) Demonstrated Antitumor Efficacy in Pancreatic and Colorectal Cancer Xenograft Models
by
Hiroyuki Suzuki, Tomokazu Ohishi, Takuro Nakamura, Miyuki Yanaka, Saori Handa, Tomohiro Tanaka, Mika K. Kaneko and Yukinari Kato
Antibodies 2025, 14(3), 67; https://doi.org/10.3390/antib14030067 - 11 Aug 2025
Abstract
Background: Podocalyxin (PODXL) has been identified as a promising therapeutic target and a potential diagnostic biomarker in various tumors. Despite the therapeutic potential of anti-PODXL monoclonal antibodies (mAbs), their further development has been limited by concerns regarding potential on-target off-tumor toxicities. To
[...] Read more.
Background: Podocalyxin (PODXL) has been identified as a promising therapeutic target and a potential diagnostic biomarker in various tumors. Despite the therapeutic potential of anti-PODXL monoclonal antibodies (mAbs), their further development has been limited by concerns regarding potential on-target off-tumor toxicities. To minimize adverse effects on normal tissues, developing a cancer-specific mAb (CasMab) against PODXL is essential. Methods: Our group established a cancer-specific anti-PODXL mAb, PcMab-60 (IgM, κ), through the screening of over one hundred hybridoma clones. In this study, PcMab-60 was engineered into a humanized IgG1-type mAb (humPcMab-60), and its antitumor activity was examined using mouse xenograft models of pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer. Results: HumPcMab-60 retains cancer-specific reactivity; humPcMab-60 reacted to PDAC cell lines (PK-45H and MIA PaCa-2) and the colorectal cancer cell line (Caco-2), but not to a normal lymphatic endothelial cell line in flow cytometry. Furthermore, humPcMab-60 exerted antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity against PODXL-expressing cell lines and showed antitumor effects against the tumor xenografts. Conclusions: A humanized anti-PODXL CasMab, humPcMab-60, could be a promising mAb-based tumor therapy.
Full article
(This article belongs to the Special Issue A Festschrift Celebrating Dr. Dimiter Stanchev Dimitrov: Antibodies, Innovation, and Impact on Infectious Disease and Cancer Research)
►▼
Show Figures

Figure 1
Open AccessArticle
Antibodies to Laminin β4 in Pemphigoid Diseases: Clinical–Laboratory Experience of a Single Central European Reference Centre
by
Maciej Marek Spałek, Magdalena Jałowska, Natalia Welc, Monika Bowszyc-Dmochowska, Takashi Hashimoto, Justyna Gornowicz-Porowska and Marian Dmochowski
Antibodies 2025, 14(3), 66; https://doi.org/10.3390/antib14030066 - 1 Aug 2025
Abstract
Background/Objectives: Anti-p200 pemphigoid is a rare and likely underdiagnosed autoimmune blistering disorder. Laminin γ1 and laminin β4 have been implicated as potential target antigens in its pathogenesis. Recently, a novel indirect immunofluorescence assay targeting anti-laminin β4 antibodies has been developed, demonstrating high sensitivity
[...] Read more.
Background/Objectives: Anti-p200 pemphigoid is a rare and likely underdiagnosed autoimmune blistering disorder. Laminin γ1 and laminin β4 have been implicated as potential target antigens in its pathogenesis. Recently, a novel indirect immunofluorescence assay targeting anti-laminin β4 antibodies has been developed, demonstrating high sensitivity and specificity, and offering a valuable tool for improved diagnosis. Methods: Of the 451 patients, 21 were selected for further laboratory analysis based on medical records. Sera from 10 patients, which showed a positive direct immunofluorescence (DIF) result and negative results in multiplex enzyme-linked immunosorbent assays (ELISAs) and/or mosaic six-parameter indirect immunofluorescence (IIF) for various autoimmune bullous diseases, were tested for the presence of anti-laminin β4 antibodies. Additionally, sera from 11 patients with positive DIF and positive ELISA for antibodies against BP180 and/or BP230 were analyzed. Results: Among the 10 patients with positive DIF and negative ELISA and/or mosaic six-parameter IIF, 6 sera were positive for anti-laminin β4 antibodies. These patients presented with atypical clinical features. In contrast, all 11 sera from patients with both positive DIF and positive ELISA for BP180 and/or BP230 were negative for anti-laminin β4 antibodies. Conclusions: In patients with a positive DIF result but negative ELISA and/or mosaic six-parameter IIF findings, testing for anti-laminin β4 antibodies should be considered. Furthermore, in cases presenting with atypical clinical features—such as acral distribution of lesions, intense pruritus, or erythematous–edematous plaques—the possibility of anti-p200 pemphigoid should be included in the differential diagnosis.
Full article
(This article belongs to the Section Antibody-Based Diagnostics)
►▼
Show Figures

Figure 1
Open AccessReview
FcRn Blockade as a Targeted Therapeutic Strategy in Antibody-Mediated Autoimmune Diseases: A Focus on Warm Autoimmune Hemolytic Anemia
by
Michael Sandhu and Irina Murakhovskaya
Antibodies 2025, 14(3), 65; https://doi.org/10.3390/antib14030065 - 1 Aug 2025
Abstract
Antibody-mediated autoimmune diseases are common, can involve any organ system, and pose a large burden for patients and healthcare systems. Most antibody-mediated diseases are mediated by IgG antibodies. Selective targeting of pathogenic antibodies is an attractive treatment option which has already proven to
[...] Read more.
Antibody-mediated autoimmune diseases are common, can involve any organ system, and pose a large burden for patients and healthcare systems. Most antibody-mediated diseases are mediated by IgG antibodies. Selective targeting of pathogenic antibodies is an attractive treatment option which has already proven to be effective in antibody-positive generalized myasthenia gravis, maternal-fetal alloimmune cytopenias, and immune thrombocytopenic purpura. Warm autoimmune hemolytic anemia (wAIHA) is an autoimmune disorder mediated by pathogenic antibodies mainly of the IgG class with no approved therapy. Current treatment includes non-specific immunosuppression with corticosteroids, rituximab, and other immunosuppressive agents. With most therapies, time to response can be delayed and transfusions may be needed. Neonatal Fc receptor (FcRN) therapies provide rapid and sustained reduction of pathogenic IgG levels providing potential for fast, effective therapy in antibody-mediated autoimmune diseases including warm autoimmune hemolytic anemia. This review focuses on the emerging role of FcRn inhibition in autoimmune hematologic diseases, and their therapeutic potential in wAIHA.
Full article
(This article belongs to the Special Issue Antibody and Autoantibody Specificities in Autoimmunity)
►▼
Show Figures

Figure 1
Open AccessBrief Report
Determination of the Epitopes of Alpha-Glucosidase Anti-Drug Antibodies in Pompe Disease Patient Plasma Samples
by
Evgeniy V. Petrotchenko, Andreas Hahn and Christoph H. Borchers
Antibodies 2025, 14(3), 64; https://doi.org/10.3390/antib14030064 - 28 Jul 2025
Abstract
Pompe disease is a rare autosomal-recessive neuromuscular disorder caused by a deficiency of the lysosomal enzyme acid alpha-glucosidase (GAA), leading to the pathological accumulation of glycogen and impaired autophagy. Enzyme replacement therapy (ERT) with recombinant human alpha-glucosidase (rhGAA) has been available since 2006,
[...] Read more.
Pompe disease is a rare autosomal-recessive neuromuscular disorder caused by a deficiency of the lysosomal enzyme acid alpha-glucosidase (GAA), leading to the pathological accumulation of glycogen and impaired autophagy. Enzyme replacement therapy (ERT) with recombinant human alpha-glucosidase (rhGAA) has been available since 2006, but may lead to the formation of anti-drug antibodies (ADAs) against the recombinant human enzyme, which, in turn, may adversely affect the response to ERT. Knowledge of the antigenic determinants of rhGAA involved in interaction with ADAs may facilitate the development of strategies to attenuate the anti-drug immune response in patients. Here, we determined the rhGAA ADA epitopes in the plasma of Pompe disease patients using a series of affinity purifications combined with epitope extraction and label free quantitation LC-MS.
Full article
(This article belongs to the Section Humoral Immunity)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Anti-HMGCR-Antibody-Positive Statin-Induced Myositis: A Pilot Case Series on Treatment with Bempedoic Acid and Immunosuppressive Therapy
by
Maurizio Benucci, Riccardo Terenzi, Francesca Li Gobbi, Emanuele Antonio Maria Cassarà, Tommaso Picchioni, Edda Russo, Barbara Lari, Mariangela Manfredi and Maria Infantino
Antibodies 2025, 14(3), 63; https://doi.org/10.3390/antib14030063 - 23 Jul 2025
Abstract
Background/Objectives: Immune-mediated necrotizing myopathy (IMNM) is a severe inflammatory myopathy marked by proximal muscle weakness, elevated creatine kinase (CK), and the presence of anti-HMGCR antibodies. Statin exposure is a recognized trigger for anti-HMGCR-positive IMNM, which may persist despite statin withdrawal. This pilot case
[...] Read more.
Background/Objectives: Immune-mediated necrotizing myopathy (IMNM) is a severe inflammatory myopathy marked by proximal muscle weakness, elevated creatine kinase (CK), and the presence of anti-HMGCR antibodies. Statin exposure is a recognized trigger for anti-HMGCR-positive IMNM, which may persist despite statin withdrawal. This pilot case series explores, for the first time, the use of bempedoic acid—a liver-specific lipid-lowering agent with minimal muscle toxicity—as an alternative to statins in these patients. Methods: We report 10 anti-HMGCR-antibody-positive IMNM patients (6 females, 4 males) previously on statins for primary prevention (8 on atorvastatin, 2 on simvastatin) without prior cardiovascular events. Statins were discontinued at myositis onset. All patients received prednisone and immunosuppressants (methotrexate in 7, mycophenolate in 3), plus bempedoic acid. Anti-HMGCR antibodies were measured using a chemiluminescence method. Results: Their mean anti-HMGCR antibody levels decreased significantly from 390.93 ± 275.22 to 220.89 ± 113.37 CU/L (p = 0.027) after 6 months of treatment. Their CK levels dropped from 1278.9 ± 769.39 to 315.1 ± 157.72 IU/L (p = 0.001), and aldolase dropped from 11.63 ± 2.18 to 6.61 ± 1.22 U/L (p = 0.0001). The mean LDL-C value was 96.1 ± 8.16 mg/dL. No disease recurrence was observed. Autoimmune panels were negative for other myositis-associated and/or -specific antibodies. Conclusions: Bempedoic acid appears to be a safe, effective, and cost-efficient lipid-lowering alternative in statin-intolerant IMNM patients. Larger studies are warranted to confirm its efficacy across different subgroups and to optimize dyslipidemia management in this setting.
Full article
(This article belongs to the Section Antibody-Based Diagnostics)
Open AccessArticle
Immunogenicity Risk Assessment of Biotherapeutics Using an Ex Vivo B Cell Assay
by
Kevin M. Budge, Ross Blankenship, Patricia Brown-Augsburger and Lukasz K. Chlewicki
Antibodies 2025, 14(3), 62; https://doi.org/10.3390/antib14030062 - 22 Jul 2025
Abstract
Background/Objectives: Anti-drug antibody (ADA) formation can impact the safety, pharmacokinetics, and/or efficacy of biotherapeutics, including monoclonal antibodies (mAbs). Current strategies for ADA/immunogenicity risk prediction of mAbs include in silico algorithms, T cell proliferation assays, MHC-associated peptide proteomics assays (MAPPs), and dendritic cell internalization
[...] Read more.
Background/Objectives: Anti-drug antibody (ADA) formation can impact the safety, pharmacokinetics, and/or efficacy of biotherapeutics, including monoclonal antibodies (mAbs). Current strategies for ADA/immunogenicity risk prediction of mAbs include in silico algorithms, T cell proliferation assays, MHC-associated peptide proteomics assays (MAPPs), and dendritic cell internalization assays. However, B cell-mediated responses are not assessed in these assays. B cells are professional antigen-presenting cells (APCs) and secrete antibodies toward immunogenic mAbs. Therefore, methods to determine B cell responses would be beneficial for immunogenicity risk prediction and may provide a more comprehensive assessment of risk. Methods: We used a PBMC culture method with the addition of IL-4, IL-21, B cell activating factor (BAFF), and an anti-CD40 agonist mAb to support B cell survival and activation. Results: B cells in this assay format become activated, proliferate, and secrete IgG. A panel of 51 antibodies with varying clinical immunogenicity rates were screened in this assay with IgG secretion used as a readout for immunogenicity risk. IgG secretion differed among test articles but did not correlate with the clinical immunogenicity rating. Conclusions: This dataset highlights the challenges of developing a B cell assay for immunogenicity risk prediction and provides a framework for further refinement of a B cell-based assay for immunogenicity risk prediction of mAbs.
Full article
(This article belongs to the Special Issue Unravelling Effector Functions of B cells in Infectious Diseases and Cancer)
►▼
Show Figures

Graphical abstract
Open AccessBrief Report
Mepolizumab-Related Blood Eosinophil Decreases Are Associated with Clinical Remission in Severe Asthmatic Patients: A Real-World Study
by
Matteo Bonato, Francesca Savoia, Enrico Orzes, Elisabetta Favero, Gianenrico Senna and Micaela Romagnoli
Antibodies 2025, 14(3), 61; https://doi.org/10.3390/antib14030061 - 22 Jul 2025
Abstract
Background: Mepolizumab is an effective treatment for severe eosinophilic asthma, leading to a depletion of blood eosinophil levels, the clinical relevance of which remains unclear. Objective: The aim of this study was to assess the relationship between mepolizumab-induced blood eosinophil reduction
[...] Read more.
Background: Mepolizumab is an effective treatment for severe eosinophilic asthma, leading to a depletion of blood eosinophil levels, the clinical relevance of which remains unclear. Objective: The aim of this study was to assess the relationship between mepolizumab-induced blood eosinophil reduction and clinical outcome in patients with severe eosinophilic asthma, in particular, whether the magnitude of blood eosinophil reduction was associated with clinical remission. Methods: We conducted a real-world retrospective analysis of 58 adult patients with severe eosinophilic asthma treated with mepolizumab. Clinical and respiratory functional parameters were evaluated at the start of mepolizumab treatment (T0) and after two years of treatment (T2; mean follow-up: 22.8 ± 7.5 months). Blood eosinophil counts were recorded at T0 and during the first year of treatment (T1; mean follow-up: 7.7 ± 4.1 months). Results: After two years of mepolizumab treatment, 58 severe asthmatic patients showed significant improvements in ACT score, FVC, and FEV1 and a reduction in acute exacerbations and the use of maintenance therapies. Clinical remission was achieved in 55.1% of patients. Lower blood eosinophil counts during the first year (T1) were associated with greater improvements in lung function and fewer exacerbations. A greater relative decrease in eosinophils from baseline to T1 (ΔEOS%) was significantly associated with remission, reductions in exacerbations, and no maintenance OCS use. ΔEOS% was the only independent predictor of remission in the multivariate analysis. A ≥90% reduction predicted remission with 80% specificity (AUC = 0.726). Conclusions: Monitoring blood eosinophils after mepolizumab initiation could be a useful tool for predicting long-term response to treatment. In particular, a reduction by over 90% of peripheral blood eosinophils during the first year of mepolizumab treatment predicts clinical remission with a specificity of 80%. Considering the accessibility and the low cost of this biomarker, it may help to optimize long-term asthma management.
Full article
(This article belongs to the Section Antibody-Based Therapeutics)
►▼
Show Figures

Figure 1
Open AccessArticle
Seroprevalence of IgG and IgE Antibodies Against Anisakis in the Presumably Healthy Population of the Canary Islands
by
Eligia González-Rodríguez, Marta Rodero, J. Alberto Montoya-Alonso, Kevin M. Santana-Hernández, Myriam R. Ventura, Carmen Cuéllar and Eligia Rodríguez-Ponce
Antibodies 2025, 14(3), 60; https://doi.org/10.3390/antib14030060 - 17 Jul 2025
Abstract
Food-borne zoonoses, particularly anisakiosis caused by Anisakis spp., are an increasing public health concern due to the rising consumption of raw fish. Anisakiosis results from the ingestion of third-stage larvae of Anisakidae nematodes, with the genus Anisakis re-sponsible for approximately 97% of human
[...] Read more.
Food-borne zoonoses, particularly anisakiosis caused by Anisakis spp., are an increasing public health concern due to the rising consumption of raw fish. Anisakiosis results from the ingestion of third-stage larvae of Anisakidae nematodes, with the genus Anisakis re-sponsible for approximately 97% of human cases. While regulatory protocols exist to minimize infection risk in commercial settings, domestic food preparation often lacks such safeguards, creating a gap in public health protection. In the Canary Islands, a major Spanish aquaculture region, farmed fish exhibit a low Anisakis prevalence, suggesting minimal risk from aquaculture products. In contrast, wild-caught fish demonstrate varia-ble parasitism, with recent studies reporting a 25% prevalence among commercial species. Methods: This study assessed Anisakis exposure in the Canary Islands by measuring specific IgG and IgE antibodies in 1043 serum samples collected from all seven islands between March 2014 and October 2015. ELISA assays detected anti-Anisakis antibodies, and the results were analyzed by age, sex, island, and isoclimatic zone. Results: Overall, 16.9% of samples were IgG-positive and 6.8% were IgE-positive. Seroprevalence was significantly higher in indi-viduals aged 60 years and above. Geographic heterogeneity was notable: La Palma had the highest IgG seroprevalence (35.3%), while El Hierro showed the highest IgE prevalence (16.3%). Temperate isoclimatic zones exhibited higher antibody prevalence than dry zones. These findings indicate variable Anisakis exposure across the Canary Islands, likely influenced by environmental and behavioral factors. Conclusions: The results highlight the need for targeted public health interventions to reduce the anisakiosis risk, particularly in regions and populations with elevated exposure.
Full article
(This article belongs to the Section Antibody-Based Diagnostics)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Loss of IgA and IgM Compromises Broad Neutralization of Structurally Divergent SARS-CoV-2 Variants
by
Yalcin Pisil, Tomoyuki Miura, Kiyoki Ito and Yoshihiro Watanabe
Antibodies 2025, 14(3), 59; https://doi.org/10.3390/antib14030059 - 12 Jul 2025
Abstract
Objectives: The durability and breadth of neutralizing antibodies following SARS-CoV-2 mRNA vaccination remain incompletely understood. This study aimed to investigate how longitudinal changes in antibody isotype composition impact neutralization against structurally diverse SARS-CoV-2 variants. Methods: After screening a broader cohort of mRNA-vaccinated sera,
[...] Read more.
Objectives: The durability and breadth of neutralizing antibodies following SARS-CoV-2 mRNA vaccination remain incompletely understood. This study aimed to investigate how longitudinal changes in antibody isotype composition impact neutralization against structurally diverse SARS-CoV-2 variants. Methods: After screening a broader cohort of mRNA-vaccinated sera, time-matched samples collected one month (1 mpv) and three months post-vaccination (3 mpv) were selected for detailed analysis. Neutralization assays against live virus variants, enzyme-linked immunosorbent assays (ELISA), and immunogold electron microscopy were performed to assess antibody titers, isotype levels, and virion morphology. Results: Neutralization titers declined markedly at 3 mpv, particularly against immune-evasive variants. Notably, the Lambda variant showed disproportionately high sensitivity to early-phase sera despite its divergence from the vaccine strain. Antibody isotyping showed that IgA and IgM decreased over time, while IgG levels were relatively more sustained. Electron microscopy revealed broader virion size heterogeneity in Lambda (50–200 nm) compared to Wuhan (80–120 nm), potentially enhancing multivalent antibody engagement. Consistently, ELISA under reduced spike density conditions showed that IgA and IgM retained stronger binding than IgG. Conclusions: These findings indicate that the decline of IgA and IgM compromises neutralization breadth, especially against structurally divergent variants such as Lambda. Sustaining dynamic multivalent isotype responses that adapt to diverse spike morphologies may be critical for broad cross-variant immunity.
Full article
(This article belongs to the Special Issue Antiviral Antibody Immune Responses in the Context of Vaccination and Infection)
►▼
Show Figures

Graphical abstract
Open AccessReview
Immunotherapy in GI Cancers: Lessons from Key Trials and Future Clinical Applications
by
Supriya Peshin, Faizan Bashir, Naga Anvesh Kodali, Adit Dharia, Sajida Zaiter, Sakshi Singal and Nagaishwarya Moka
Antibodies 2025, 14(3), 58; https://doi.org/10.3390/antib14030058 - 11 Jul 2025
Cited by 2
Abstract
Immunotherapy has emerged as a transformative approach in gastrointestinal (GI) cancers, addressing historically poor survival rates in advanced-stage disease. Immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 axis demonstrate remarkable efficacy in colorectal cancer with deficient mismatch repair (dMMR) or high microsatellite instability (MSI-H),
[...] Read more.
Immunotherapy has emerged as a transformative approach in gastrointestinal (GI) cancers, addressing historically poor survival rates in advanced-stage disease. Immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 axis demonstrate remarkable efficacy in colorectal cancer with deficient mismatch repair (dMMR) or high microsatellite instability (MSI-H), exemplified by trials like NICHE-2 achieving exceptional pathological response rates. However, significant limitations persist, including resistance in some dMMR/MSI-H tumors, minimal efficacy in proficient mismatch repair (pMMR) tumors, and low overall response rates across most GI malignancies due to tumor heterogeneity and immune evasion mechanisms. Predictive biomarkers such as tumor mutational burden (TMB) and PD-L1 expression are crucial for optimizing patient selection, while hypermutated pMMR tumors with POLE mutations represent emerging therapeutic opportunities. In pancreatic adenocarcinoma, where survival remains dismal, combination strategies with chemotherapy and novel approaches like cancer vaccines show promise but lack transformative breakthroughs. Esophagogastric cancers benefit from ICIs combined with chemotherapy, particularly in MSI-H and HER2-positive tumors, while hepatocellular carcinoma has achieved significant progress with combinations like atezolizumab–bevacizumab and durvalumab–tremelimumab surpassing traditional therapies. Biliary tract cancers show modest improvements with durvalumab–chemotherapy combinations. Despite these advances, immunotherapy faces substantial challenges including immune-related adverse events, acquired resistance through cancer immunoediting, and the need for biomarker-driven approaches to overcome tumor microenvironment barriers. This review discusses key clinical trials, therapeutic progress, and emerging modalities including CAR T-cell therapies and combination strategies, emphasizing the critical need to address resistance mechanisms and refine precision medicine approaches to fully realize immunotherapy’s potential in GI malignancies.
Full article
(This article belongs to the Section Antibody-Based Therapeutics)
►▼
Show Figures

Figure 1

Journal Menu
► ▼ Journal Menu-
- Antibodies Home
- Aims & Scope
- Editorial Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Cancers, CIMB, Current Oncology, Sci. Pharm., Antibodies, IJMS, IJTM
Antibody-Mediated Therapy and Other Emerging Therapies in Cancer Treatment
Topic Editors: Won Sup Lee, Yaewon Yang, Seil GoDeadline: 31 July 2026

Conferences
Special Issues
Special Issue in
Antibodies
Emerging Antibody Engineering Strategies and Applications for Immunotherapy of Cancer
Guest Editors: Christian Klein, Sophia Karagiannis, Ann WhiteDeadline: 20 September 2025
Special Issue in
Antibodies
Antibody-Mediated Rejection in Kidney Transplantation
Guest Editors: Kazuhiro Iwadoh, Hiroto EgawaDeadline: 31 October 2025
Special Issue in
Antibodies
Therapeutic Antibodies: New Trends in Discovery, Developability and Characterization
Guest Editors: Anne Zeck, David J VanceDeadline: 15 December 2025
Special Issue in
Antibodies
Antiviral Antibody Immune Responses in the Context of Vaccination and Infection
Guest Editors: Jagadeesh Bayry, Guglielmo LuccheseDeadline: 15 December 2025
Topical Collections
Topical Collection in
Antibodies
Computational Antibody and Antigen Design
Collection Editor: Buyong Ma










