The Binding Landscape of Serum Antibodies: How Physical and Mathematical Concepts Can Advance Systems Immunology
Abstract
:1. Introduction
2. Immunological and Physical Rules of the System: B Cells as Sensors and Effectors
3. The Configuration Space of Serum Antibodies
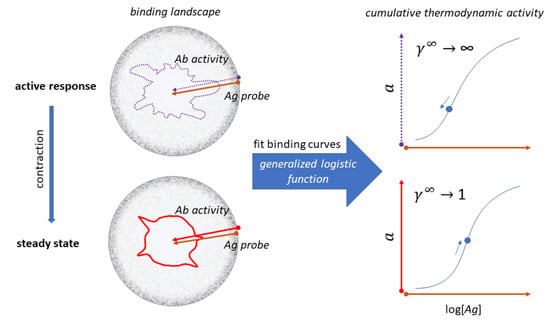
4. Probing Serum Antibody Configuration Space: Quantitative Systems Serology
5. Consolidation and Steps towards Systems Serological Mapping of Immunity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 4PL | 5PL | GL | |
|---|---|---|---|
| x = [Ag] xi = [Ag]i | |||
| x = log[Ag] xi =log[Ag]i | |||
| diff. eq. | |||
| f(x) |  |  |  |
| log f(x) |  |  |  |
References
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.D.; Alder, M.N. The evolution of adaptive immune systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, V.; de Boer, R.J.; Bonhoeffer, S.; Szathmáry, E. An evolutionary perspective on the systems of adaptive immunity. Biol. Rev. Camb. Philos. Soc. 2018, 93, 505–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannard, O.; Cyster, J.G. Germinal centers: Programmed for affinity maturation and antibody diversification. Curr. Opin. Immunol. 2017, 45, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, M.S.; Ehrenstein, M.R.; Rada, C.; Sale, J.; Batista, F.D.; Williams, G.; Milstein, C. Memory in the B-cell compartment: Antibody affinity maturation. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, S.; Zhou, Y.; Wang, Y.; Yamamura, M.; Wang, J.-Y. A model integrating tonic and antigen-triggered BCR signals to predict the survival of primary B cells. Sci. Rep. 2017, 7, 14888. [Google Scholar] [CrossRef]
- Wan, Z.; Chen, X.; Chen, H.; Ji, Q.; Chen, Y.; Wang, J.; Cao, Y.; Wang, F.; Lou, J.; Tang, Z.; et al. The activation of IgM- or isotype-switched IgG- and IgE-BCR exhibits distinct mechanical force sensitivity and threshold. eLife 2015, 4, e06925. [Google Scholar] [CrossRef]
- Prechl, J. A generalized quantitative antibody homeostasis model: Regulation of B-cell development by BCR saturation and novel insights into bone marrow function. Clin. Transl. Immunol. 2017, 6, e130. [Google Scholar] [CrossRef]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Prechl, J. A generalized quantitative antibody homeostasis model: Antigen saturation, natural antibodies and a quantitative antibody network. Clin. Transl. Immunol. 2017, 6, e131. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fc-Receptors as regulators of immunity. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 96, pp. 179–204. ISBN 9780123737090. [Google Scholar]
- DiLillo, D.J.; Ravetch, J.V. Fc-Receptor Interactions Regulate Both Cytotoxic and Immunomodulatory Therapeutic Antibody Effector Functions. Cancer Immunol. Res. 2015, 3, 704–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prechl, J. A generalized quantitative antibody homeostasis model: Maintenance of global antibody equilibrium by effector functions. Clin. Transl. Immunol. 2017, 6, e161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.H.S.; Markham, J.F.; Duffy, K.R.; Hodgkin, P.D. Stochastically timed competition between division and differentiation fates regulates the transition from B lymphoblast to plasma cell. Front. Immunol. 2018, 9, 2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.S.; Zhou, J.Q.; Han, J.; Schmitz, A.J.; Rizk, A.A.; Alsoussi, W.B.; Lei, T.; Amor, M.; McIntire, K.M.; Meade, P.; et al. Human germinal centres engage memory and naive B cells after influenza vaccination. Nature 2020, 586, 127–132. [Google Scholar] [CrossRef]
- Glaros, V.; Rauschmeier, R.; Artemov, A.V.; Reinhardt, A.; Ols, S.; Emmanouilidi, A.; Gustafsson, C.; You, Y.; Mirabello, C.; Björklund, Å.K.; et al. Limited access to antigen drives generation of early B cell memory while restraining the plasmablast response. Immunity 2021, 54, 2005–2023. [Google Scholar] [CrossRef]
- Robinson, M.J.; Webster, R.H.; Tarlinton, D.M. How intrinsic and extrinsic regulators of plasma cell survival might intersect for durable humoral immunity. Immunol. Rev. 2020, 296, 87–103. [Google Scholar] [CrossRef]
- Lightman, S.M.; Utley, A.; Lee, K.P. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Front. Immunol. 2019, 10, 965. [Google Scholar] [CrossRef]
- Khodadadi, L.; Cheng, Q.; Radbruch, A.; Hiepe, F. The maintenance of memory plasma cells. Front. Immunol. 2019, 10, 721. [Google Scholar] [CrossRef] [Green Version]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Good-Jacobson, K.L.; Shlomchik, M.J. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: The influence of germinal center interactions and dynamics. J. Immunol. 2010, 185, 3117–3125. [Google Scholar] [CrossRef]
- Stebegg, M.; Kumar, S.D.; Silva-Cayetano, A.; Fonseca, V.R.; Linterman, M.A.; Graca, L. Regulation of the germinal center response. Front. Immunol. 2018, 9, 2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golinski, M.-L.; Demeules, M.; Derambure, C.; Riou, G.; Maho-Vaillant, M.; Boyer, O.; Joly, P.; Calbo, S. Cd11c+ B cells are mainly memory cells, precursors of antibody secreting cells in healthy donors. Front. Immunol. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.P.; Baumgarth, N. Characteristics of natural antibody-secreting cells. Ann. N. Y. Acad. Sci. 2015, 1362, 132–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Gibson, A.W.; Kimberly, R.P. Human FcR polymorphism and disease. Fc Recept. 2014, 382, 275–302. [Google Scholar] [CrossRef]
- Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012, 119, 5640–5649. [Google Scholar] [CrossRef]
- Prechl, J. Network organization of antibody interactions in sequence and structure space: The RADARS model. Antibodies 2020, 9, 13. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Toellner, K.-M.; Sze, D.M.-Y.; Zhang, Y. What Are the Primary Limitations in B-Cell Affinity Maturation, and How Much Affinity Maturation Can We Drive with Vaccination? A Role for Antibody Feedback. Cold Spring Harb. Perspect. Biol. 2018, 10, a028795. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, T.R.; Jensen, A.; Haurum, J.S.; Andersen, P.S. Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J. Immunol. 2011, 187, 4229–4235. [Google Scholar] [CrossRef] [Green Version]
- Teng, G.; Papavasiliou, F.N. Immunoglobulin somatic hypermutation. Annu. Rev. Genet. 2007, 41, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Dowdle, W.R.; Orenstein, W.A. Quest for life-long protection by vaccination. Proc. Natl. Acad. Sci. USA 1994, 91, 2464–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guthmiller, J.J.; Utset, H.A.; Wilson, P.C. B Cell Responses against Influenza Viruses: Short-Lived Humoral Immunity against a Life-Long Threat. Viruses 2021, 13, 965. [Google Scholar] [CrossRef] [PubMed]
- Melchers, F. Checkpoints that control B cell development. J. Clin. Investig. 2015, 125, 2203–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prechl, J. Why current quantitative serology is not quantitative and how systems immunology could provide solutions. Biol. Futur. 2021, 72, 37–44. [Google Scholar] [CrossRef]
- Wild, D. The Immunoassay Handbook, 2nd ed.; Nature Publishing Group: London, UK, 2001; p. 944. ISBN 0-333-72306-6. [Google Scholar]
- Papp, K.; Kovács, Á.; Orosz, A.; Hérincs, Z.; Randek, J.; Liliom, K.; Pfeil, T.; Prechl, J. Absolute quantitation of serum antibody reactivity using the Richards growth model for antigen microspot titration. Sensors 2022, 22, 3962. [Google Scholar] [CrossRef]
- Eyer, K.; Doineau, R.C.L.; Castrillon, C.E.; Briseño-Roa, L.; Menrath, V.; Mottet, G.; England, P.; Godina, A.; Brient-Litzler, E.; Nizak, C.; et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. Nat. Biotechnol. 2017, 35, 977–982. [Google Scholar] [CrossRef]
- Gottschalk, P.G.; Dunn, J.R. The five-parameter logistic: A characterization and comparison with the four-parameter logistic. Anal. Biochem. 2005, 343, 54–65. [Google Scholar] [CrossRef]
- Richards, F.J. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–301. [Google Scholar] [CrossRef]
- Tjørve, E.; Tjørve, K.M.C. A unified approach to the Richards-model family for use in growth analyses: Why we need only two model forms. J. Theor. Biol. 2010, 267, 417–425. [Google Scholar] [CrossRef]
- Fuentes-Pananá, E.M.; Bannish, G.; Monroe, J.G. Basal B-cell receptor signaling in B lymphocytes: Mechanisms of regulation and role in positive selection, differentiation, and peripheral survival. Immunol. Rev. 2004, 197, 26–40. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prechl, J.; Papp, K.; Kovács, Á.; Pfeil, T. The Binding Landscape of Serum Antibodies: How Physical and Mathematical Concepts Can Advance Systems Immunology. Antibodies 2022, 11, 43. https://doi.org/10.3390/antib11030043
Prechl J, Papp K, Kovács Á, Pfeil T. The Binding Landscape of Serum Antibodies: How Physical and Mathematical Concepts Can Advance Systems Immunology. Antibodies. 2022; 11(3):43. https://doi.org/10.3390/antib11030043
Chicago/Turabian StylePrechl, József, Krisztián Papp, Ágnes Kovács, and Tamás Pfeil. 2022. "The Binding Landscape of Serum Antibodies: How Physical and Mathematical Concepts Can Advance Systems Immunology" Antibodies 11, no. 3: 43. https://doi.org/10.3390/antib11030043
APA StylePrechl, J., Papp, K., Kovács, Á., & Pfeil, T. (2022). The Binding Landscape of Serum Antibodies: How Physical and Mathematical Concepts Can Advance Systems Immunology. Antibodies, 11(3), 43. https://doi.org/10.3390/antib11030043