Seroprevalence of Anti-SARS-CoV-2 Antibodies in Chattogram Metropolitan Area, Bangladesh
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.1.1. Inclusion Criteria
In Case of Having Past Confirmed COVID-19 Status (by Rt PCR)
- Participants who had already passed at least 28 days after a negative Rt-PCR test;
- Participants who did not take a repeated test to ensure negativity had passed at least 42 days after the first COVID-19 test.
2.2. Baseline Blood Collection and Processing
2.3. Serological Test Examination
2.4. Data Management
2.5. Data Analysis
2.6. Ethical Approval and Informed Consent
3. Results
3.1. Seroprevalence of SARS-CoV-2 Infection
3.2. Characteristics of Study Participants
3.3. SARS-CoV-2 Antibody Titer
3.4. Risk Factor Analysis
3.4.1. Univariable Analysis (χ2 Test, Logistic Regression) to Evaluate the Association of Different Variables with the Seroprevalence of Anti-SARS-CoV-2 Antibody
3.4.2. Multivariable Analysis (Logistic Regression) to Determine the Potential Factors Associated with SARS-CoV-2 Antibody-Positive Status in the Study Area
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rana, E.A.; Chowdhury, N.S.; Islam, M.S.; Ara, J.; Nasrin, S.S.; Dutta, P.; Bristi, S.Z.; Nizami, T.A.; Chakraborty, P.; Siddiki, A.Z. Molecular detection and prevalence of SARS-CoV-2 during the early outbreak in southern Bangladesh. Int. J. One Health 2020, 6, 153–159. [Google Scholar] [CrossRef]
- Dhaka Tribune, First Coronavirus Case Confirmed in Chittagong, 6 Buildings in Lockdown. Available online: https://archive.dhakatribune.com/bangladesh/nation/2020/04/03/covid-19-first-case-confirmed-in-chittagong (accessed on 3 April 2020).
- Dhaka Tribune, Another Corona Virus Patient Dies in Chittagong. Available online: https://www.dhakatribune.com/health/coronavirus/2020/04/13/another-coronavirus-patient-dies-in-chittagong (accessed on 13 April 2020).
- Goenka, M.K.; Afzalpurkar, S.; Goenka, U.; Das, S.S.; Mukherjee, M.; Jajodia, S.; Shah, B.B.; Patil, V.U.; Rodge, G.; Khan, U.; et al. Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J. Assoc. Physicians India 2020, 68, 14–19. [Google Scholar] [CrossRef]
- Vernet, R.; Charrier, E.; Grogg, J.; Mach, N. A Quantitative ELISA Protocol for Detection of Specific Human IgG against the SARS-CoV-2 Spike Protein. Vaccines 2021, 9, 770. [Google Scholar] [CrossRef]
- Bryant, J.E.; Azman, A.S.; Ferrari, M.J.; Arnold, B.F.; Boni, M.F.; Boum, Y.; Hayford, K.; Luquero, F.J.; Mina, M.J.; Rodriguez-Barraquer, I.; et al. Serology for SARS-CoV-2: Apprehensions, opportunities, and the path forward. Sci. Immunol. 2020, 5, eabc6347. [Google Scholar] [CrossRef]
- Shakiba, M.; Nazemipour, M.; Salari, A.; Mehrabian, F.; Nazari, S.S.; Rezvani, S.M.; Ghasempour, Z.; Heidarzadeh, A.; Mansournia, M.A. Seroprevalence of SARS-CoV-2 in Guilan Province, Iran, April 2020. Emerg. Infect. Dis. 2021, 27, 636. [Google Scholar] [CrossRef]
- Bendavid, E.; Mulaney, B.; Sood, N.; Shah, S.; Bromley-Dulfano, R.; Lai, C.; Weissberg, Z.; Saavedra-Walker, R.; Tedrow, J.; Bogan, A.; et al. Covid-19 antibody seroprevalence in santa clara county, california. Int. J. Epidemiol. 2021, 50, 410–419. [Google Scholar] [CrossRef]
- Gomes, L.R.; Durans, A.M.; Napoleão-Pêgo, P.; Waterman, J.A.; Freitas, M.S.; De Sá, N.B.; Pereira, L.V.; Furtado, J.S.; Aquino, R.G.; Machado, M.C.; et al. Multiepitope Proteins for the Differential Detection of IgG Antibodies against RBD of the Spike Protein and Non-RBD Regions of SARS-CoV-2. Vaccines 2021, 9, 986. [Google Scholar] [CrossRef]
- Freeman, B.; Lester, S.; Mills, L.; Rasheed, M.A.; Moye, S.; Abiona, O.; Hutchinson, G.B.; Morales-Betoulle, M.; Krapinunaya, I.; Gibbons, A.; et al. Validation of a SARS-CoV-2 spike protein ELISA for use in contact investigations and serosurveillance. bioRxiv 2020. [Google Scholar] [CrossRef]
- Brown, T.S.; Walensky, R.P. Serosurveillance and the COVID-19 epidemic in the US: Undetected, uncertain, and out of control. JAMA 2020, 324, 749–751. [Google Scholar] [CrossRef]
- Hossain, M.; Das, S.C.; Raza, M.T.; Ahmed, I.U.; Eva, I.J.; Karim, T.; Chakraborty, P.; Gupta, S.D. Immediate and post-COVID complications of symptomatic and asymptomatic COVID-19 patients in Bangladesh: A cross-sectional retrospective study. Asian J. Med. Biol. Res. 2021, 7, 191–201. [Google Scholar] [CrossRef]
- Thomas, S.N.; Altawallbeh, G.; Zaun, C.P.; Pape, K.A.; Peters, J.M.; Titcombe, P.J.; Dileepan, T.; Rapp, M.J.; Bold, T.D.; Schacker, T.W.; et al. Initial determination of COVID-19 seroprevalence among outpatients and healthcare workers in Minnesota using a novel SARS-CoV-2 total antibody ELISA. Clin. Biochem. 2021, 90, 15–22. [Google Scholar] [CrossRef]
- Lerner, A.M.; Eisinger, R.W.; Lowy, D.R.; Petersen, L.R.; Humes, R.; Hepburn, M.; Cassetti, M.C. The COVID-19 serology studies workshop: Recommendations and challenges. Immunity 2020, 53, 1–5. [Google Scholar] [CrossRef]
- Wang, H.; Wiredja, D.; Yang, L.; Bulterys, P.L.; Costales, C.; Röltgen, K.; Manalac, J.; Yee, J.; Zehnder, J.; Shi, R.Z.; et al. Case-Control Study of Individuals with Discrepant Nucleocapsid and Spike Protein SARS-CoV-2 IgG Results. Clin. Chem. 2021, 67, 977–986. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef]
- Aljazeera, Coronavirus Pandemic, Bangladesh Starts COVID Vaccination Drive. Available online: https://www.aljazeera.com/news/2021/1/28/bangladesh-starts-covid-vaccination-drive (accessed on 28 January 2021).
- Anadolu Agency, World, Asia-Pacific, Latest on CoronavirusOutbreak, Bangladesh Starts Nationwide COVID Vaccination Drive. Available online: https://www.aa.com.tr/en/asia-pacific/bangladesh-starts-nationwide-covid-vaccination-drive/2136643 (accessed on 7 February 2021).
- COVID-19 Vaccination Dashboard for Bangladesh, Health Emergency Control Center, DHIS2, Surokkha App. 2022. Available online: http://103.247.238.92/webportal/pages/covid19-vaccination-update.php (accessed on 21 December 2021).
- Dhaka Tribune, Bangladesh Begins Booster Vaccination. Available online: https://newsarchive.app/a/dhakatribune/2021/11/28/Bangladesh-begins-booster-vaccination (accessed on 28 November 2021).
- Mahmud, S.; Mohsin, M.; Khan, I.A.; Mian, A.U.; Zaman, M.A. Knowledge, beliefs, attitudes and perceived risk about COVID-19 vaccine and determinants of COVID-19 vaccine acceptance in Bangladesh. PLoS ONE 2021, 16, e0257096. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Baticulon, R.E.; Kadhum, M.; Alser, M.; Ojuka, D.K.; Badereddin, Y.; Kamath, A.; Parepalli, S.A.; Brown, G.; Iharchane, S.; et al. Infection and mortality of healthcare workers worldwide from COVID-19: A systematic review. BMJ Glob. Health 2020, 5, e003097. [Google Scholar] [CrossRef]
- Bangladesh Medical Association. District Wise Total Number of Affected Doctor, Nurse & Staff (COVID 19+) From 08/03/2020 till today on 28/09/2021. Available online: https://bma.org.bd/covid-19/Total%20Affected%20Doctor,%20Nurse%20&%20Staff.pdf (accessed on 28 September 2021).
- Chen, Q.; Liang, M.; Li, Y.; Guo, J.; Fei, D.; Wang, L.; He, L.I.; Sheng, C.; Cai, Y.; Li, X.; et al. Mental health care for medical staff in China during the COVID-19 outbreak. Lancet Psychiatry 2020, 7, e15–e16. [Google Scholar] [CrossRef]
- Mhango, M.; Dzobo, M.; Chitungo, I.; Dzinamarira, T. COVID-19 risk factors among health workers: A rapid review. Saf. Health Work 2020, 11, 262–265. [Google Scholar] [CrossRef]
- Iversen, K.; Bundgaard, H.; Hasselbalch, R.B.; Kristensen, J.H.; Nielsen, P.B.; Pries-Heje, M.; Knudsen, A.D.; Christensen, C.E.; Fogh, K.; Norsk, J.B.; et al. Risk of COVID-19 in health-care workers in Denmark: An observational cohort study. Lancet Infect. Dis. 2020, 20, 1401–1408. [Google Scholar] [CrossRef]
- Ehrlich, H.; McKenney, M.; Elkbuli, A. Protecting our healthcare workers during the COVID-19 pandemic. Am. J. Emerg. Med. 2020, 38, 1527–1528. [Google Scholar] [CrossRef]
- Shaw, A.; Flott, K.; Fontana, G.; Durkin, M.; Darzi, A. No patient safety without health worker safety. Lancet 2020, 396, 1541–1543. [Google Scholar] [CrossRef]
- Kontoangelos, K.; Economou, M.; Papageorgiou, C. Mental health effects of COVID-19 pandemia: A review of clinical and psychological traits. Psychiatry Investig. 2020, 17, 491. [Google Scholar] [CrossRef]
- Khan, A.N.; Ullah, M.R. Export scenario between Bangladesh and China: Opportunities of Bangladesh in RMG Sector. Eur. Sci. J. 2017, 13, 299–320. [Google Scholar] [CrossRef]
- Hosen, M.S.; Nafiujjaman, M.; Biswas, F.N. Garment employees are at higher risk than any other workers in COVID-19 pandemic in Bangladesh. Casp. J. Health Res. 2020, 5, 1–2. [Google Scholar] [CrossRef]
- Dyal, J.W. COVID-19 among workers in meat and poultry processing facilities―19 states, April 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 557–561. [Google Scholar] [CrossRef]
- Marinaccio, A.; Guerra, R.; Iavicoli, S. Work a key determinant in COVID-19 risk. Lancet Glob. Health 2020, 8, e1368. [Google Scholar] [CrossRef]
- Islam, M.S.; Hasib, F.Y.; Nath, C.; Ara, J.; Nu, M.S.; Fazal, M.A.; Chowdhury, S. Coronavirus disease 2019 and its potential animal reservoirs: A review. Int. J. One Health 2021, 7, 171–181. [Google Scholar] [CrossRef]
- World Health Organization. Population-Based Age-Stratified Seroepidemiological Investigation Protocol for Coronavirus 2019 (COVID-19) Infection. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Seroepidemiology-2020.2 (accessed on 15 May 2020).
- Bhuiyan, T.R.; Hulse, J.D.; Hegde, S.; Akhtar, M.; Islam, T.; Khan, Z.H.; Khan, I.I.; Rashid, M.; Rashid, R.; Shirin, T.; et al. SARS-CoV-2 seroprevalence in Chattogram, Bangladesh before a National Lockdown, March-April 2021. medRxiv 2021. [Google Scholar] [CrossRef]
- Icddr, B. News, Higher COVID-19 Seropositivity Observed among Residents in Dhaka and Chattogram. Available online: https://www.icddrb.org/news-and-events/news?id=878 (accessed on 22 June 2021).
- Ward, H.; Cooke, G.; Whitaker, M.; Redd, R.; Eales, O.; Brown, J.C.; Collet, K.; Cooper, E.; Daunt, A.; Jones, K.; et al. REACT-2 Round 5: Increasing prevalence of SARS-CoV-2 antibodies demonstrate impact of the second wave and of vaccine roll-out in England. medRxiv 2021. [Google Scholar] [CrossRef]
- Tripathi, R.; Alqahtani, S.S.; Albarraq, A.A.; Meraya, A.M.; Tripathi, P.; Banji, D.; Alshahrani, S.; Ahsan, W.; Alnakhli, F.M. Awareness and preparedness of COVID-19 outbreak among healthcare workers and other residents of South-West Saudi Arabia: A cross-sectional survey. Front. Public Health 2020, 8, 482. [Google Scholar] [CrossRef]
- Maheshwari, U.; Sahai, J.; Hebbar, V. Serosurveillance of Anti SARS-Cov-2 Antibodies among Essential Workers in Navi Mumbai–A Single Centre Study. Int. J. Health Sci. Res. 2021, 11, 99–104. [Google Scholar] [CrossRef]
- Bayram, A.; Demirbakan, H.; Karadeniz, P.G.; Erdoğan, M.; Koçer, I. Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in health care workers. J. Med. Virol. 2021, 93, 5560–5567. [Google Scholar] [CrossRef]
- Neumann, F.; Rose, R.; Römpke, J.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Development of SARS-CoV-2 specific IgG and virus-neutralizing antibodies after infection with variants of concern or vaccination. Vaccines 2021, 9, 700. [Google Scholar] [CrossRef]
- Callow, K.A.; Parry, H.F.; Sergeant, M.; Tyrrell, D.A. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990, 105, 435–446. [Google Scholar] [CrossRef]
- Amanat, F.; Krammer, F. SARS-CoV-2 vaccines: Status report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Kundu, R.; Bhaduri, R.; Ray, D.; Beesley, L.J.; Salvatore, M.; Mukherjee, B. Incorporating false negative tests in epidemiological models for SARS-CoV-2 transmission and reconciling with seroprevalence estimates. Sci. Rep. 2021, 11, 9748. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, Y.; Yuan, J.; Ye, H.; Wei, L.; Liao, X.; Wang, H.; Qian, S.; Wang, Z.; Liu, L.; et al. Early viral clearance and antibody kinetics of COVID-19 among asymptomatic carriers. Front. Med. 2021, 8, 595773. [Google Scholar] [CrossRef]
- Alkurt, G.; Murt, A.; Aydin, Z.; Tatli, O.; Agaoglu, N.B.; Irvem, A.; Aydin, M.; Karaali, R.; Gunes, M.; Yesilyurt, B.; et al. Seroprevalence of coronavirus disease 2019 (COVID-19) among health care workers from three pandemic hospitals of Turkey. PLoS ONE 2021, 16, e0247865. [Google Scholar] [CrossRef]
- Mukhtar, A.; Alfishawy, M.; Alkhatib, E.; Hosny, M.; Ollaek, M.; Elsayed, A.; Salem, M.R.; Ghaith, D. Asymptomatic SARS-CoV-2 infection among healthcare workers in a non-COVID-19 teaching university hospital. J. Public Health Res. 2021, 10, 2102. [Google Scholar] [CrossRef]
- Selvaraju, S.; Kumar, M.S.; Thangaraj, J.W.; Bhatnagar, T.; Saravanakumar, V.; Kumar, C.P.; Sekar, K.; Ilayaperumal, E.; Sabarinathan, R.; Jagadeesan, M.; et al. Population-based serosurvey for severe acute respiratory syndrome coronavirus 2 transmission, Chennai, India. Emerg. Infect. Dis. 2021, 27, 586. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Dhaka Tribune, Bangladesh Lowers COVID-19 Vaccine Age Limit to 18 Years. Available online: https://www.dhakatribune.com/bangladesh/2021/10/20/bangladesh-lowers-covid-19-vaccine-age-limit-to-18-years (accessed on 20 October 2021).
- Dhaka Tribune, COVID Vaccination Campaign for School Students. Available online: https://www.dhakatribune.com/bangladesh/2021/11/01/covid-vaccination-campaign-for-school-students-kicks-off (accessed on 1 November 2021).
- Krammer, F.; Simon, V. Serology assays to manage COVID-19. Science 2020, 368, 1060–1061. [Google Scholar] [CrossRef]
- Rana, E.A.; Dutta, P.; Islam, M.S.; Nizami, T.A.; Das, T.; Chowdhury, S.; Das, G.B. Severity assessment of single dose Oxford–AstraZeneca vaccinated individuals infected with SARS CoV-2 in the Southeast Bangladesh. Int. J. One Health 2021, 7, 220–226. [Google Scholar] [CrossRef]
| Anti-SARS-CoV-2 Antibody | Total Population | Unadjusted Seroprevalence, % (95% CI) | Test Performance Adjusted Seroprevalence % (95% CI) | Known Positives (RT-qPCR Positive) (%) |
|---|---|---|---|---|
| Present | 498 | 66.58 (63.1–70.0) | 66.99 (63.40–70.40) | 91 (80.53) |
| Absent | 250 | 33.42 (30.1–36.9) | 32.60 (29.20–36.19) | 22 (19.47) |
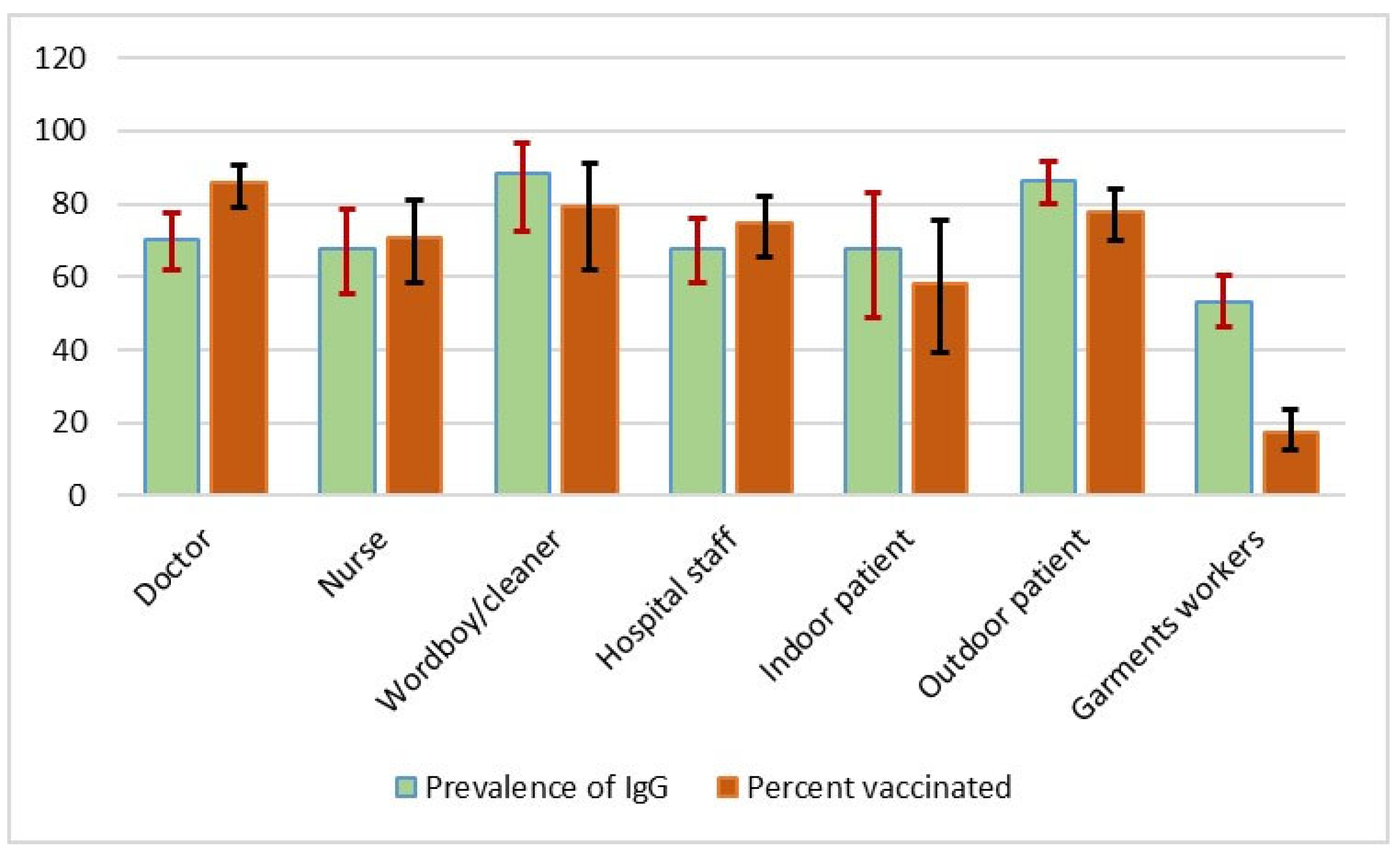
| Variables | Level | Total Population | Known Positives (RT-qPCR Positive) | Asymptomatic |
|---|---|---|---|---|
| Donor type | Doctor | 145 (19.44) | 40 (35.40) | 85 (16.13) |
| Nurse | 67 (8.98) | 19 (16.81) | 43 (8.16) | |
| Hospital staff | 150 (20.11) | 27 (23.89) | 109 (20.68) | |
| Indoor patient | 31 (4.16) | 2 (1.77) | 26 (4.93) | |
| Outdoor patient | 148 (19.84) | 21 (18.58) | 109 (20.68) | |
| Garments worker | 205 (27.48) | 4 (3.54) | 155 (29.41) | |
| Gender | Male | 507 (67.96) | 73 (65.18) | 362 (68.69) |
| Female | 239 (32.04) | 39 (34.82) | 165 (31.31) | |
| Age (year) | 19 to 29 | 201 (26.91) | 15 (13.27) | 149 (28.27) |
| 30 to 35 | 184 (24.63) | 30 (26.55) | 123 (23.34) | |
| 36 to 44 | 180 (24.10) | 34 (30.09) | 123 (23.34) | |
| 45 to 84 | 182 (24.36) | 34 (30.09) | 132 (25.05) | |
| Vaccination | No | 292 (39.14) | 11 (9.82) | 222 (42.13) |
| Only 1st dose | 223 (29.89) | 38 (33.93) | 153 (29.03) | |
| Both doses | 231 (30.97) | 63 (56.25) | 152 (28.84) | |
| Days passed after first dose of vaccine | 14 to 30 days | 45 (24.06) | 8 (25.81) | 30 (23.08; 16.1–31.3) |
| 31 to 60 days | 142 (75.94) | 23 (74.19) | 100 (76.92) | |
| Days passed after second dose vaccine | 14 to 60 days | 19 (8.26) | 6 (9.38) | 12 (8.00) |
| 61 to 120 days | 86 (37.39) | 20 (31.25) | 60 (40.00) | |
| 120 to 180 days | 125 (54.35) | 37 (59.38) | 78 (52.00) | |
| Days between PCR test and antibody test | 21 to 60 days | - | 17 (15.60) | - |
| 61 to 120 days | - | 16 (14.68) | - | |
| 121 to 180 days months | - | 23 (21.10) | - | |
| >180 days | - | 53 (48.62) | - | |
| Contact with confirmed case | Yes | 342 (47.17) | 79 (71.17) | 230 (45.19) |
| No | 307 (42.34) | 17 (15.32) | 232 (45.58) | |
| Don’t know | 76 (10.48) | 15 (13.51) | 47 (9.23) | |
| Family member | 1 to 3 | 186 (26.23) | 31 (29.52) | 130 (25.79) |
| 4 to 6 | 443 (62.48) | 64 (60.95) | 321 (63.69) | |
| ≥7 | 80 (11.28) | 10 (9.52) | 53 (10.52) | |
| Taking immunosuppressive drugs | Yes | 15 (2.13) | 7 (6.42) | 8 (1.63) |
| No | 688 (97.87) | 102 (93.58) | 484 (98.37) | |
| Comorbidities | Yes | 197 (32.35) | 38 (37.25) | 291 (68.79) |
| No | 412 (67.65) | 64 (62.75) | 132 (31.29) |
| Variable | Level | Mean Titer of IgG (DU/mL) | SD | p-Value |
|---|---|---|---|---|
| Doner type | Health worker | 163.30 | 153.54 | <0.001 |
| In/outpatient | 197.18 | 147.04 | ||
| Garment worker | 77.05 | 115.63 | ||
| Gender | Female | 140.09 | 151.36 | 0.31 |
| Male | 151.83 | 148.38 | ||
| Age (year) | 19 to 29 | 106.90 | 132.23 | <0.001 |
| 30 to 35 | 151.16 | 157.71 | ||
| 36 to 44 | 160.85 | 143.08 | ||
| 45 to 84 | 176.95 | 155.92 | ||
| Vaccination | No | 53.71 | 91.16 | <0.001 |
| Only first dose | 159.08 | 161.05 | ||
| Both doses | 255.46 | 117.04 | ||
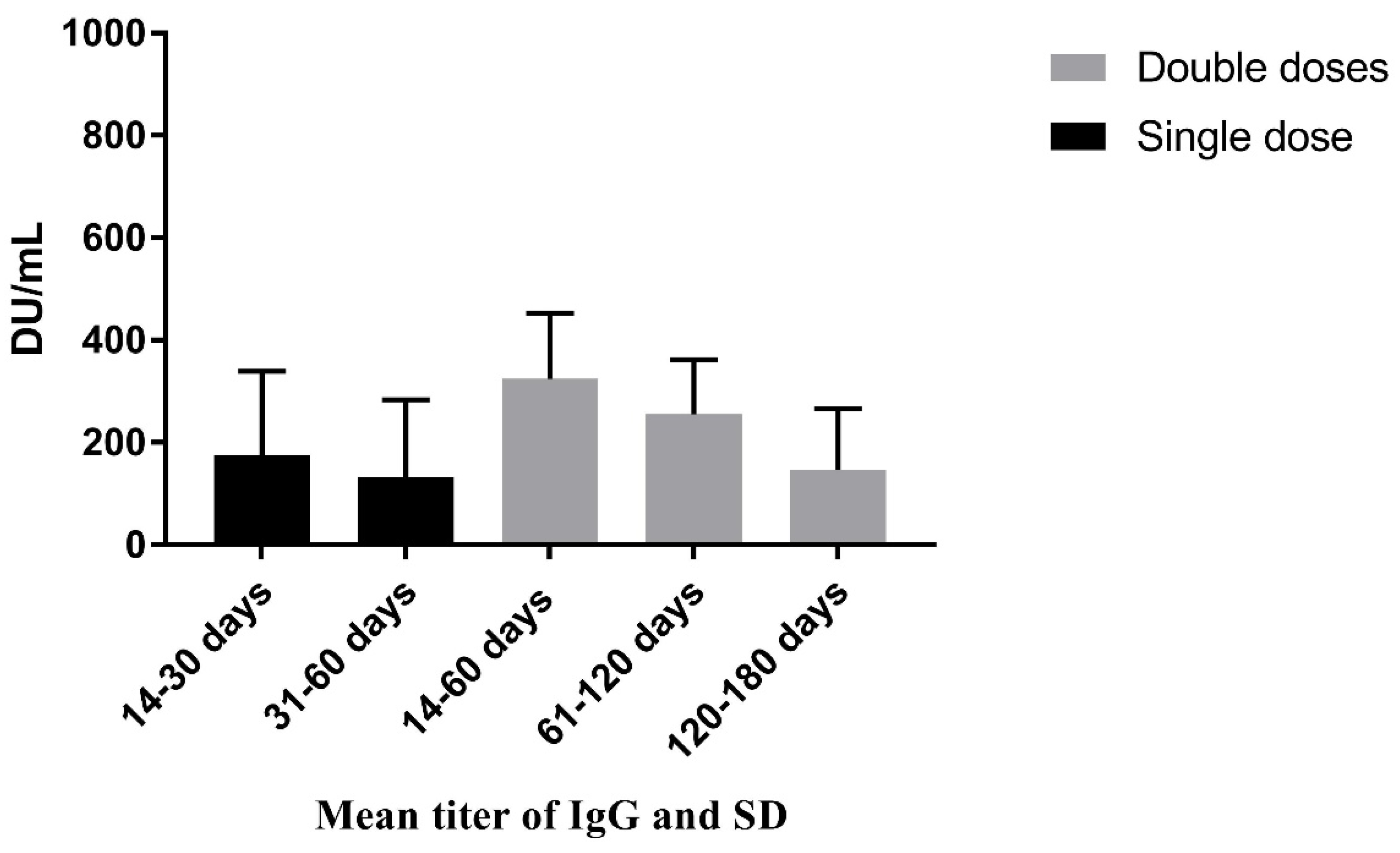
| Days passed after first dose of vaccine | 31 to 60 days | 131.39 | 152.08 | 0.10 |
| 14 to 30 days | 175.10 | 164.09 | ||
| Days passed after second dose vaccine | 120 to 180 days | 147.09 | 119.29 | 0.02 |
| 61 to 120 days | 255.82 | 106.00 | ||
| 14 to 60 days | 324.42 | 128.42 | ||
| Asymptomatic | No | 190.01 | 161.93 | <0.001 |
| Yes | 130.03 | 140.19 | ||
| Had COVID-19 confirmed status | No | 191.69 | 142.70 | 0.005 |
| Yes | 244.87 | 159.74 | ||
| Contact with confirmed case | No | 116.45 | 135.21 | <0.001 |
| Yes | 170.89 | 154.19 | ||
| Don’t know | 160.05 | 158.98 | ||
| Taking immunosuppressive drugs | No | 143.02 | 150.09 | 0.32 |
| Yes | 181.38 | 152.08 |
| Variable | Level (n) | Presence of IgG | TP (95% CI of TP) ** | OR | p-Value |
|---|---|---|---|---|---|
| Donor type | Health worker (362) | 248 | 68.99 (63.8–73.7) | Ref. | <0.001 |
| Indoor/outdoor patient (179) | 144 | 81.37 (74.7–86.7) | 1.8 | ||
| Garments worker (205) | 104 | 50.56 (43.5–57.5) | 0.47 | ||
| Gender | Female (239) | 151 | 63.47 (56.9–69.5) | Ref. | 0.15 |
| Male (507) | 347 | 68.92 (64.6–72.9) | 1.26 | ||
| Age (year) | 19 to 29 (201) | 114 | 56.76 (49.5–63.6) | Ref. | 0.002 |
| 30 to 35 (184) | 119 | 65.01 (57.6–71.8) | 1.39 | ||
| 36 to 44 (180) | 132 | 73.99 (66.8–80.1) | 2.09 | ||
| 45 to 84 (182) | 133 | 73.73 (66.6–79.8) | 2.07 | ||
| Vaccination | No (292) | 131 | 44.47 (38.6–50.4) | Ref. | <0.001 |
| Only first dose (223) | 137 | 61.66 (54.8–68.0) | 1.95 | ||
| Both doses (231) | 229 | 100 (98.4–100.0) | 140.72 | ||
| Days passed after first dose of vaccine | 31 to 60 days (142) | 79 | 55.64 (47.1–63.8) | Ref. | 0.29 |
| 14 to 30 days (45) | 29 | 64.78 (49.6–77.5) | 1.44 | ||
| Days passed after second dose vaccine | 120 to 180 days (125) | 123 | 99.9 (95.7–100) | - | - |
| 61 to 120 days (86) | 86 | 100 (97.2–100) | - | ||
| 14 to 60 days (19) | 19 | 100 (84.2–100) | - | ||
| Asymptomatic | No (220) | 160 | 73.36 (66.9–79.03) | Ref. | 0.13 |
| Yes (528) | 355 | 67.66 (63.4–71.68) | 0.76 | ||
| Had COVID-19 confirmed status | No (144) | 119 | 83.65 (76.3–89.1) | Ref. | 0.66 |
| Yes (113) | 91 | 81.46 (72.9–87.9) | 0.86 | ||
| Contact with confirmed case | No (307) | 187 | 61.11 (55.3–66.6) | Ref. | 0.01 |
| Yes (342) | 244 | 71.93 (66.7–76.6) | 1.59 | ||
| Don’t know (76) | 49 | 64.81 (53.1–75.0) | 1.16 | ||
| Taking immunosuppressive drugs | No (688) | 447 | 65.32 (61.5–68.9) | Ref. | 0.20 |
| Yes (15) | 12 | 80.91 (54.7–94.3) | 2.15 |
| Variable | Level | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Doner type | Health worker | Ref. | ||
| Indoor/outdoor patient | 2.22 | 1.33–3.68 | 0.002 | |
| Garment worker | 1.69 | 1.09–2.62 | 0.01 | |
| Vaccination | No | Ref. | ||
| Only first dose | 2.34 | 1.56–3.50 | <0.001 | |
| Both doses | 174.02 | 41.46–730.40 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ara, J.; Islam, M.S.; Quader, M.T.U.; Das, A.; Hasib, F.M.Y.; Islam, M.S.; Rahman, T.; Das, S.; Chowdhury, M.A.H.; Das, G.B.; et al. Seroprevalence of Anti-SARS-CoV-2 Antibodies in Chattogram Metropolitan Area, Bangladesh. Antibodies 2022, 11, 69. https://doi.org/10.3390/antib11040069
Ara J, Islam MS, Quader MTU, Das A, Hasib FMY, Islam MS, Rahman T, Das S, Chowdhury MAH, Das GB, et al. Seroprevalence of Anti-SARS-CoV-2 Antibodies in Chattogram Metropolitan Area, Bangladesh. Antibodies. 2022; 11(4):69. https://doi.org/10.3390/antib11040069
Chicago/Turabian StyleAra, Jahan, Md. Sirazul Islam, Md. Tarek Ul Quader, Anan Das, F. M. Yasir Hasib, Mohammad Saiful Islam, Tazrina Rahman, Seemanta Das, M. A. Hassan Chowdhury, Goutam Buddha Das, and et al. 2022. "Seroprevalence of Anti-SARS-CoV-2 Antibodies in Chattogram Metropolitan Area, Bangladesh" Antibodies 11, no. 4: 69. https://doi.org/10.3390/antib11040069
APA StyleAra, J., Islam, M. S., Quader, M. T. U., Das, A., Hasib, F. M. Y., Islam, M. S., Rahman, T., Das, S., Chowdhury, M. A. H., Das, G. B., & Chowdhury, S. (2022). Seroprevalence of Anti-SARS-CoV-2 Antibodies in Chattogram Metropolitan Area, Bangladesh. Antibodies, 11(4), 69. https://doi.org/10.3390/antib11040069








