The Current Landscape of Immune Checkpoint Inhibitor Immunotherapy for Primary and Metastatic Brain Tumors
Abstract
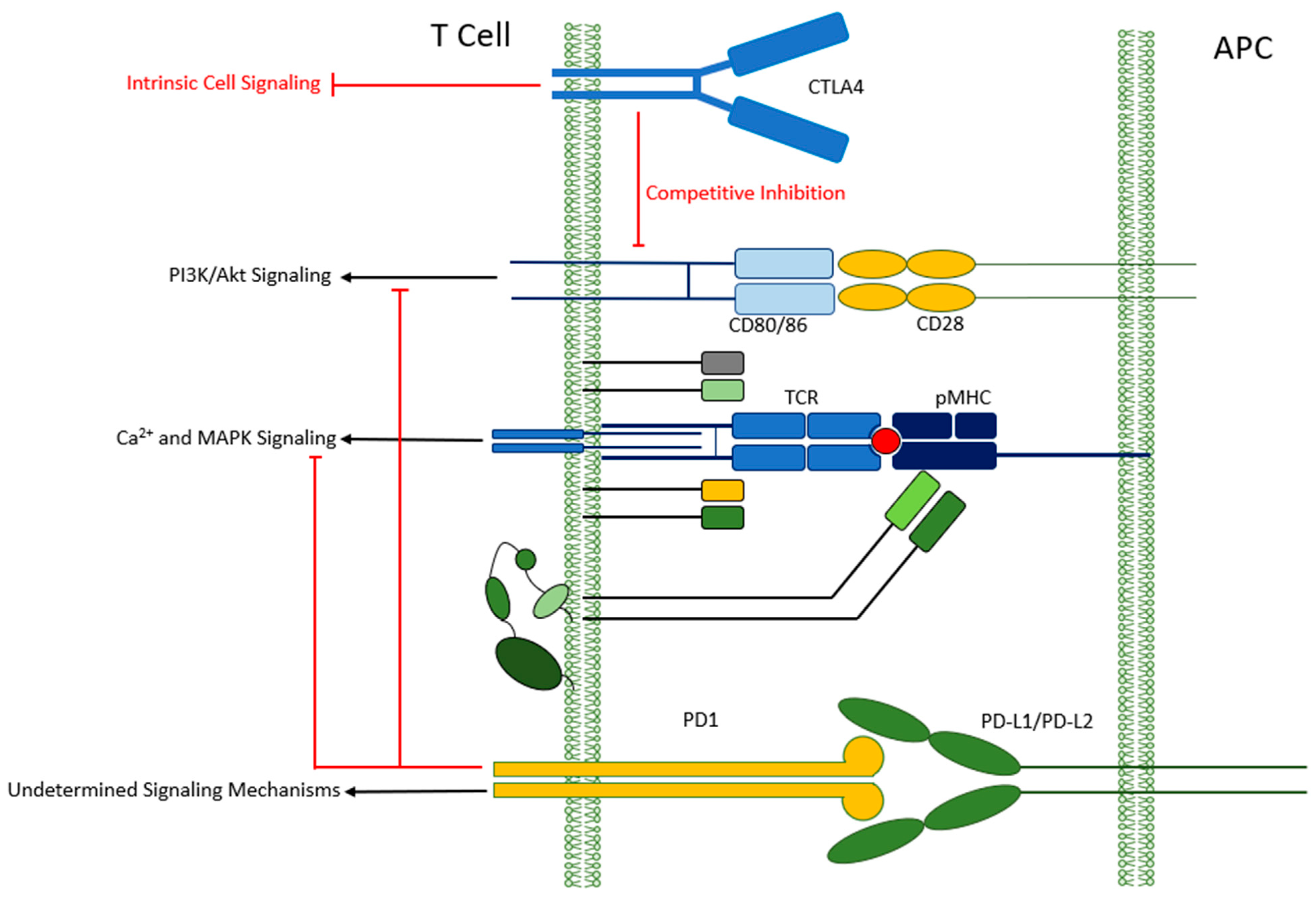
1. Introduction
2. ICI for Primary Brain Tumors
2.1. Glioblastoma
2.2. Isocitrate Dehydrogenase (IDH) Mutant Gliomas
| Author | Phase | Regimen | Treatment |
|---|---|---|---|
| NCT03557359 | II | Anti PD-1 monotherapy | Nivolumab |
| NCT03893903 | I | IDH vax + anti-PD-L1 | IDH-1 vaccine + Avelumab |
| NCT04056910 | II | Anti-PD-1 + IDH1-inhibitor | Nivolumab + Ivosidenib |
| NCT02968940 | II | Anti-PDL1 + radiation | Avelumab + HFRT |
| Author | Phase | Patients | Treatment |
|---|---|---|---|
| Bi et al., 2022 [45] | II | 25 patients with recurrent grade 2 or 3 meningioma | Nivolumab |
| Brastianos et al., 2022 [46] | II | 25 patients with grade 2 or 3 meningioma. | Pembrolizumab |
| Nidamanuri and Drappatz, 2022 [47] | Retrospective | 8 patients with meningiomas | Anti-PD1 therapy |
2.3. Meningioma
2.4. Primary Central Nervous System Lymphoma
3. ICI for Brain Metastases
3.1. Parenchymal Metastases
3.2. Melanoma
3.3. Breast Cancer
3.4. Non-Small Cell Lung Cancer
3.5. Leptomeningeal Metastases
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Rossi, E.; Schinzari, G.; Maiorano, B.A.; Indellicati, G.; Di Stefani, A.; Pagliara, M.M.; Fragomeni, S.M.; De Luca, E.V.; Sammarco, M.G.; Garganese, G.; et al. Efficacy of immune checkpoint inhibitors in different types of melanoma. Hum. Vaccin. Immunother. 2021, 17, 4–13. [Google Scholar] [CrossRef]
- Berghmans, T.; Durieux, V.; Hendriks, L.E.L.; Dingemans, A.M. Immunotherapy: From Advanced NSCLC to Early Stages, an Evolving Concept. Front. Med. 2020, 7, 90. [Google Scholar] [CrossRef]
- Tomaszewski, W.; Sanchez-Perez, L.; Gajewski, T.F.; Sampson, J.H. Brain Tumor Microenvironment and Host State: Implications for Immunotherapy. Clin. Cancer Res. 2019, 25, 4202–4210. [Google Scholar] [CrossRef]
- Medikonda, R.; Dunn, G.; Rahman, M.; Fecci, P.; Lim, M. A review of glioblastoma immunotherapy. J. Neuro-Oncol. 2021, 151, 41–53. [Google Scholar] [CrossRef]
- Sampson, J.H.; Gunn, M.D.; Fecci, P.E.; Ashley, D.M. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer 2020, 20, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Van Den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Lamborn, K.R.; Yung, W.K.A.; Chang, S.M.; Wen, P.Y.; Cloughesy, T.F.; DeAngelis, L.; Robins, H.I.; Lieberman, F.S.; Fine, H.A.; Fink, K.L.; et al. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro-Oncol. 2008, 10, 162–170. [Google Scholar] [CrossRef]
- Wu, W.; Lamborn, K.R.; Buckner, J.C.; Novotny, P.J.; Chang, S.M.; O’Fallon, J.R.; Jaeckle, K.A.; Prados, M.D. Joint NCCTG and NABTC prognostic factors analysis for high-grade recurrent glioma. Neuro-Oncol. 2009, 12, 164–172. [Google Scholar] [CrossRef]
- Clarke, J.L.; Ennis, M.M.; Yung, W.K.A.; Chang, S.M.; Wen, P.Y.; Cloughesy, T.F.; DeAngelis, L.; Robins, H.I.; Lieberman, F.S.; Fine, H.A.; et al. Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro-Oncol. 2011, 13, 1118–1124. [Google Scholar] [CrossRef]
- Reardon, D.A.; Kim, T.M.; Frenel, J.; Simonelli, M.; Lopez, J.; Subramaniam, D.S.; Siu, L.L.; Wang, H.; Krishnan, S.; Stein, K.; et al. Treatment with pembrolizumab in programmed death ligand 1–positive recurrent glioblastoma: Results from the multicohort phase 1 KEYNOTE-028 trial. Cancer 2021, 127, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wöhrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M.; et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-Oncol. 2015, 17, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Wilmotte, R.; Burkhardt, K.; Kindler, V.; Belkouch, M.-C.; Dussex, G.; De Tribolet, N.; Walker, P.R.; Dietrich, P.-Y. B7-homolog 1 expression by human glioma: A new mechanism of immune evasion. Neuroreport 2005, 16, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Song, G.; Yu, J. The prognostic significance of PD-L1 expression in patients with glioma: A meta-analysis. Sci. Rep. 2017, 7, 4231. [Google Scholar] [CrossRef]
- Nayak, L.; Molinaro, A.M.; Peters, K.B.; Clarke, J.L.; Jordan, J.T.; de Groot, J.F.; Nghiemphu, P.L.; Kaley, T.J.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef]
- Nayak, L.; Standifer, N.; Dietrich, J.; Clarke, J.L.; Dunn, G.P.; Lim, M.; Cloughesy, T.; Gan, H.K.; Flagg, E.; George, E.; et al. Circulating Immune Cell and Outcome Analysis from the Phase II Study of PD-L1 Blockade with Durvalumab for Newly Diagnosed and Recurrent Glioblastoma. Clin. Cancer Res. 2022, 28, 2567–2578. [Google Scholar] [CrossRef]
- Lukas, R.V.; Rodon, J.; Becker, K.; Wong, E.T.; Shih, K.; Touat, M.; Fassò, M.; Osborne, S.; Molinero, L.; O’Hear, C.; et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J. Neuro-Oncol. 2018, 140, 317–328. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Gelb, A.B.; Chen, C.C.; Rao, G.; A Reardon, D.; Wen, P.Y.; Bi, W.L.; Peruzzi, P.; Amidei, C.; Triggs, D.; et al. Combined immunotherapy with controlled interleukin-12 gene therapy and immune checkpoint blockade in recurrent glioblastoma: An open-label, multi-institutional phase I trial. Neuro-Oncol. 2021, 24, 951–963. [Google Scholar] [CrossRef]
- Omuro, A.; A Reardon, D.; Sampson, J.H.; Baehring, J.; Sahebjam, S.; Cloughesy, T.F.; Chalamandaris, A.-G.; Von Potter, V.; Butowski, N.; Lim, M. Nivolumab plus radiotherapy with or without temozolomide in newly diagnosed glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro-Oncol. Adv. 2022, 4, vdac025. [Google Scholar] [CrossRef]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro-Oncol. 2018, 20, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Rodriguez-Ruiz, M.E.; Diez-Valle, R.; López-Janeiro, A.; Porciuncula, A.; Idoate, M.A.; Inogés, S.; De Andrea, C.; De Cerio, A.L.-D.; Tejada, S.; et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med. 2019, 25, 470–476. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial Supplemental content. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Lim, M.; Weller, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.R.; Ansstas, G.; Baehring, J.; Taylor, J.W.; Honnorat, J.; et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro-Oncol. 2022, 24, 1935–1949. [Google Scholar] [CrossRef]
- Arjaans, M.; Oosting, S.F.; Schröder, C.P.; de Vries, E.G. Bevacizumab-Induced Vessel Normalization Hampers Tumor Uptake of Antibodies—Response. Cancer Res. 2013, 73, 7147–7148. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Sun, L.; Mochizuki, A.Y.; Reynoso, J.G.; Orpilla, J.; Chow, F.; Kienzler, J.C.; Everson, R.G.; Nathanson, D.A.; Bensinger, S.J.; et al. Neoadjuvant PD-1 blockade induces T cell and cDC1 activation but fails to overcome the immunosuppressive tumor associated macrophages in recurrent glioblastoma. Nat. Commun. 2021, 12, 6938. [Google Scholar] [CrossRef]
- Omuro, A.; A Brandes, A.; Carpentier, A.F.; Idbaih, A.; A Reardon, D.; Cloughesy, T.; Sumrall, A.; Baehring, J.; Bent, M.V.D.; Bähr, O.; et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: An international randomized phase III trial. Neuro-Oncol. 2022, 25, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Nduom, E.K.; Wei, J.; Yaghi, N.K.; Huang, N.; Kong, L.-Y.; Gabrusiewicz, K.; Ling, X.; Zhou, S.; Ivan, C.; Chen, J.Q.; et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro-Oncol. 2016, 18, 195–205. [Google Scholar] [CrossRef]
- Wang, P.; Fang, X.; Yin, T.; Tian, H.; Yu, J.; Teng, F. Efficacy and Safety of Anti-PD-1 Plus Anlotinib in Patients With Advanced Non–Small-Cell Lung Cancer After Previous Systemic Treatment Failure—A Retrospective Study. Front. Oncol. 2021, 11, 628124. [Google Scholar] [CrossRef]
- Klemm, F.; Maas, R.R.; Bowman, R.L.; Kornete, M.; Soukup, K.; Nassiri, S.; Brouland, J.-P.; Iacobuzio-Donahue, C.A.; Brennan, C.; Tabar, V.; et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell 2020, 181, 1643–1660.e17. [Google Scholar] [CrossRef]
- Phillips, J.P.; Eremin, O.; Anderson, J.R. Lymphoreticuar cells in human brain tumors and in the normal brain. Br. J. Cancer. 1982, 45, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Darmanis, S.; Sloan, S.A.; Croote, D.; Mignardi, M.; Chernikova, S.; Samghababi, P.; Zhang, Y.; Neff, N.; Kowarsky, M.; Caneda, C.; et al. Single-Cell RNA-Seq Analysis of Infiltrating Neoplastic Cells at the Migrating Front of Human Glioblastoma. Cell Rep. 2017, 21, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Gieryng, A.; Pszczolkowska, D.; Walentynowicz, K.A.; Rajan, W.D.; Kaminska, B. Immune microenvironment of gliomas. Lab. Investig. 2017, 97, 498–518. [Google Scholar] [CrossRef] [PubMed]
- Venteicher, A.S.; Tirosh, I.; Hebert, C.; Yizhak, K.; Neftel, C.; Filbin, M.G.; Hovestadt, V.; Escalante, L.E.; Shaw, M.L.; Rodman, C.; et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 2017, 355, eaai8478. [Google Scholar] [CrossRef]
- Andersen, B.M.; Akl, C.F.; Wheeler, M.A.; Chiocca, E.A.; Reardon, D.A.; Quintana, F.J. Glial and myeloid heterogeneity in the brain tumour microenvironment. Nat. Rev. Cancer 2021, 21, 786–802. [Google Scholar] [CrossRef]
- Miller, J.J.; Gonzalez Castro, L.N.; McBrayer, S.; Weller, M.; Cloughesy, T.; Portnow, J.; Andronesi, O.; Barnholtz-Sloan, J.S.; Baumert, B.G.; Berger, M.S.; et al. Isocitrate dehydrogenase (IDH) mutant gliomas: A Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro-Oncol. 2022, 25, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Bunse, L.; Pusch, S.; Bunse, T.; Sahm, F.; Sanghvi, K.; Friedrich, M.; Alansary, D.; Sonner, J.K.; Green, E.; Deumelandt, K.; et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med. 2018, 24, 1192–1203. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Liu, X.; Wang, Z.; Sun, L.; Li, G.; Liang, J.; Hu, H.; Liu, Y.; Zhang, W.; et al. Molecular and clinical characterization of PD-L1 expression at transcriptional level via 976 samples of brain glioma. Oncoimmunology 2016, 5, e1196310. [Google Scholar] [CrossRef]
- Mu, L.; Long, Y.; Yang, C.; Jin, L.; Tao, H.; Ge, H.; Chang, Y.E.; Karachi, A.; Kubilis, P.S.; De Leon, G.; et al. The IDH1 Mutation-Induced Oncometabolite, 2-Hydroxyglutarate, May Affect DNA Methylation and Expression of PD-L1 in Gliomas. Front. Mol. Neurosci. 2018, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Röver, L.K.; Gevensleben, H.; Dietrich, J.; Bootz, F.; Landsberg, J.; Goltz, D.; Dietrich, D. PD-1 (PDCD1) Promoter Methylation Is a Prognostic Factor in Patients With Diffuse Lower-Grade Gliomas Harboring Isocitrate Dehydrogenase (IDH) Mutations. Ebiomedicine 2018, 28, 97–104. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Ellingson, B.M.; Touat, M.; Maher, E.; Macarena, I.; Holdhoff, M.; Cote, G.M.; Burris, H.; Janku, F.; Young, R.J.; et al. Ivosidenib in Isocitrate Dehydrogenase 1-Mutated Advanced Glioma. J. Clin. Oncol. 2020, 38, 3398. [Google Scholar] [CrossRef]
- Natsume, A.; Wakabayashi, T.; Miyakita, Y.; Narita, Y.; Mineharu, Y.; Arakawa, Y.; Yamasaki, F.; Sugiyama, K.; Hata, N.; Muragaki, Y.; et al. Phase I study of a brain penetrant mutant IDH1 inhibitor DS-1001b in patients with recurrent or progressive IDH1 mutant gliomas. J. Clin. Oncol. 2019, 37, 2004. [Google Scholar] [CrossRef]
- Bi, W.L.; Nayak, L.; Meredith, D.M.; Driver, J.; Du, Z.; Hoffman, S.; Li, Y.; Lee, E.Q.; Beroukhim, R.; Rinne, M.; et al. Activity of PD-1 blockade with nivolumab among patients with recurrent atypical/anaplastic meningioma: Phase II trial results. Neuro-Oncol. 2022, 24, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Kim, A.E.; Giobbie-Hurder, A.; Lee, E.Q.; Wang, N.; Eichler, A.F.; Chukwueke, U.; Forst, D.A.; Arrillaga-Romany, I.C.; Dietrich, J.; et al. Phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas. Nat. Commun. 2022, 13, 1325. [Google Scholar] [CrossRef]
- Nidamanuri, P.; Drappatz, J. Immune checkpoint inhibitor therapy for recurrent meningiomas: A retrospective chart review. J. Neuro-Oncol. 2022, 157, 271–276. [Google Scholar] [CrossRef]
- Kaley, T.; Barani, I.; Chamberlain, M.; McDermott, M.; Panageas, K.; Raizer, J.; Rogers, L.; Schiff, D.; Vogelbaum, M.; Weber, D.; et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: A RANO review. Neuro-Oncol. 2014, 16, 829–840. [Google Scholar] [CrossRef]
- Bi, W.L.; Greenwald, N.F.; Abedalthagafi, M.; Wala, J.; Gibson, W.J.; Agarwalla, P.K.; Horowitz, P.; Schumacher, S.E.; Esaulova, E.; Mei, Y.; et al. Genomic landscape of high-grade meningiomas. NPJ Genom. Med. 2017, 2, 15. [Google Scholar] [CrossRef]
- Huang, J.; Campian, J.L.; Gao, F.; Johanns, T.M.; Desjardins, A.; Wang-Gillam, A.; Rubin, J. A phase I/II study of nivolumab plus or minus ipilimumab in combination with multifraction stereotactic radiosurgery for recurrent high-grade radiation-relapsed meningioma. J. Clin. Oncol. 2019, 37, TPS2073. [Google Scholar] [CrossRef]
- Li, Y.D.; Veliceasa, D.; Lamano, J.B.; Lamano, J.B.; Kaur, G.; Biyashev, D.; Horbinski, C.M.; Kruser, T.J.; Bloch, O. Systemic and local immunosuppression in patients with high-grade meningiomas. Cancer Immunol. Immunother. 2019, 68, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Savardekar, A.R.; Patra, D.P.; Bir, S.; Thakur, J.D.; Mohammed, N.; Bollam, P.; Georgescu, M.-M.; Nanda, A. Differential Tumor Progression Patterns in Skull Base Versus Non–Skull Base Meningiomas: A Critical Analysis from a Long-Term Follow-Up Study and Review of Literature. World Neurosurg. 2018, 112, e74–e83. [Google Scholar] [CrossRef]
- Meling, T.R.; Da Broi, M.; Scheie, D.; Helseth, E. Meningiomas: Skull base versus non-skull base. Neurosurg. Rev. 2019, 42, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Nebot-Bral, L.; Brandao, D.; Verlingue, L.; Rouleau, E.; Caron, O.; Despras, E.; El-Dakdouki, Y.; Champiat, S.; Aoufouchi, S.; Leary, A.; et al. Hypermutated tumours in the era of immunotherapy: The paradigm of personalised medicine. Eur. J. Cancer 2017, 84, 290–303. [Google Scholar] [CrossRef]
- Du, Z.; Abedalthagafi, M.; Aizer, A.A.; McHenry, A.R.; Sun, H.H.; Bray, M.-A.; Viramontes, O.; Machaidze, R.; Brastianos, P.K.; Reardon, D.A.; et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget 2014, 6, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Ercolano, G.; Ianaro, A. The New Era of Cancer Immunotherapy: Targeting Myeloid-Derived Suppressor Cells to Overcome Immune Evasion. Front. Immunol. 2020, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Iwamoto, F.M.; LaCasce, A.; Mukundan, S.; Roemer, M.G.M.; Chapuy, B.; Armand, P.; Rodig, S.J.; Shipp, M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017, 129, 3071–3073. [Google Scholar] [CrossRef]
- Schaff, L.R.; Grommes, C. Update on Novel Therapeutics for Primary CNS Lymphoma. Cancers 2021, 13, 5372. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncol. 2020, 22 (Suppl. 2), iv1–iv96. [Google Scholar] [CrossRef]
- Zhang, N.; Zuo, Y.; Jiang, L.; Peng, Y.; Huang, X.; Zuo, L. Epstein-Barr Virus and Neurological Diseases. Front. Mol. Biosci. 2022, 8, 816098. [Google Scholar] [CrossRef]
- Jahnke, K.; Thiel, E.; Martus, P.; Herrlinger, U.; Weller, M.; Fischer, L.; Korfel, A.; on behalf of the German Primary Central Nervous System Lymphoma Study Group (G-PCNSL-SG). Relapse of primary central nervous system lymphoma: Clinical features, outcome and prognostic factors. J. Neuro-Oncol. 2006, 80, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Wang, X.; Tian, X. Relapsed Primary Central Nervous System Lymphoma: Current Advances. Front. Oncol. 2021, 11, 649789. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Song, Y.; Zhu, J. Immune checkpoint inhibitors in malignant lymphoma: Advances and perspectives. Chin. J. Cancer Res. 2020, 32, 303–318. [Google Scholar] [CrossRef]
- Chapuy, B.; Roemer, M.G.M.; Stewart, C.; Tan, Y.; Abo, R.P.; Zhang, L.; Dunford, A.J.; Meredith, D.M.; Thorner, A.R.; Jordanova, E.S.; et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016, 127, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Nayak, L.; Tun, H.W.; Batchelor, T.T. Introduction of novel agents in the treatment of primary CNS lymphoma. Neuro-Oncol. 2018, 21, 306–313. [Google Scholar] [CrossRef]
- Ou, A.; Sumrall, A.; Phuphanich, S.; Spetzler, D.; Gatalica, Z.; Xiu, J.; Michelhaugh, S.; Brenner, A.; Pandey, M.; Kesari, S.; et al. Primary CNS lymphoma commonly expresses immune response biomarkers. Neuro-Oncol. Adv. 2020, 2, vdaa018. [Google Scholar] [CrossRef]
- Monabati, A.; Nematollahi, P.; Dehghanian, A.; Safaei, A.; Sadeghipour, A.; Movahedinia, S.; Mokhtari, M. Immune Checkpoint Molecules in Primary Diffuse Large B-Cell Lymphoma of the Central Nervous System. Basic Clin. Neurosci. J. 2020, 11, 491–498. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Ricken, G.; Widhalm, G.; Rajky, O.; Hainfellner, J.A.; Birner, P.; Raderer, M.; Preusser, M. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clin. Neuropathol. 2014, 33, 42–49. [Google Scholar] [CrossRef]
- Furuse, M.; Nonoguchi, N.; Omura, N.; Shirahata, M.; Iwasaki, K.; Inui, T.; Kuroiwa, T.; Kuwabara, H.; Miyatake, S.-I. Immunotherapy of Nivolumab with Dendritic Cell Vaccination Is Effective against Intractable Recurrent Primary Central Nervous System Lymphoma: A Case Report. Neurol. Med.-Chir. 2017, 57, 191–197. [Google Scholar] [CrossRef]
- Panjwani, P.K.; Charu, V.; DeLisser, M.; Molina-Kirsch, H.; Natkunam, Y.; Zhao, S. Programmed death-1 ligands PD-L1 and PD-L2 show distinctive and restricted patterns of expression in lymphoma subtypes. Hum. Pathol. 2018, 71, 91–99. [Google Scholar] [CrossRef]
- Miyasato, Y.; Takashima, Y.; Takeya, H.; Yano, H.; Hayano, A.; Nakagawa, T.; Makino, K.; Takeya, M.; Yamanaka, R.; Komohara, Y. The expression of PD-1 ligands and IDO1 by macrophage/microglia in primary central nervous system lymphoma. J. Clin. Exp. Hematop. 2018, 58, 95–101. [Google Scholar] [CrossRef]
- Alame, M.; Pirel, M.; Costes-Martineau, V.; Bauchet, L.; Fabbro, M.; Tourneret, A.; De Oliveira, L.; Durand, L.; Roger, P.; Gonzalez, S.; et al. Characterisation of tumour microenvironment and immune checkpoints in primary central nervous system diffuse large B cell lymphomas. Virchows Arch. 2020, 476, 891–902. [Google Scholar] [CrossRef] [PubMed]
- El-Tawab, R.; Hamada, A.; Elhagracy, R.; Pinto, K.; Alshemmari, S. Promising effect of PDL1 inhibitors in the front-line management of primary aggressive central nervous system lymphoma: A case report. Hematol. Oncol. Stem Cell Ther. 2020, 20. [Google Scholar] [CrossRef]
- Graber, J.J.; Plato, B.; Mawad, R.; Moore, D.J. Pembrolizumab immunotherapy for relapsed CNS Lymphoma. Leuk. Lymphoma 2020, 61, 1766–1768. [Google Scholar] [CrossRef]
- Ambady, P.; Szidonya, L.; Firkins, J.; James, J.; Johansson, K.; White, T.; Jezierski, C.; Doolittle, N.D.; Neuwelt, E.A. Combination immunotherapy as a non-chemotherapy alternative for refractory or recurrent CNS lymphoma. Leuk. Lymphoma 2019, 60, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Gavrilenko, A.N.; Volkov, N.P.; Shmidt, D.I.; Polushin, A.Y.; Kondakova, E.; Lepik, K.V.; Zalaylov, Y.R.; Popova, M.O.; Kulagin, A.D.; Afanasyev, B.V.; et al. Nivolumab in Primary CNS Lymphoma and Primary Testicular Lymphoma with CNS Involvement: Single Center Experience. Blood 2020, 136, 4. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, K. Epidemiology and prognosis of brain metastases. Surg. Neurol. Int. 2013, 4, S192–S202. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef]
- Orozco, J.I.J.; Knijnenburg, T.A.; Manughian-Peter, A.O.; Salomon, M.P.; Barkhoudarian, G.; Jalas, J.R.; Wilmott, J.S.; Hothi, P.; Wang, X.; Takasumi, Y.; et al. Epigenetic profiling for the molecular classification of metastatic brain tumors. Nat. Commun. 2018, 9, 4627. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Ren, H.; Sutor, S.; Sarangi, V.; Nair, A.; Davila, J.; Elsbernd, L.R.; Udell, J.B.; Dronca, R.S.; Park, S.; et al. Contraction of T cell richness in lung cancer brain metastases. Sci. Rep. 2018, 8, 2171. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.M.; Jalali, A.; Kircher, D.A.; Lee, W.-C.; McQuade, J.L.; Haydu, L.E.; Joon, A.Y.; Reuben, A.; de Macedo, M.P.; Carapeto, F.C.L.; et al. Molecular Profiling Reveals Unique Immune and Metabolic Features of Melanoma Brain Metastases. Cancer Discov. 2019, 9, 628–645. [Google Scholar] [CrossRef]
- Fukumura, K.; Malgulwar, P.B.; Fischer, G.M.; Hu, X.; Mao, X.; Song, X.; Hernandez, S.D.; Zhang, X.H.-F.; Zhang, J.; Parra, E.R.; et al. Multi-omic molecular profiling reveals potentially targetable abnormalities shared across multiple histologies of brain metastasis. Acta Neuropathol. 2021, 141, 303–321. [Google Scholar] [CrossRef]
- Alvarez-Breckenridge, C.; Remon, J.; Piña, Y.; Nieblas-Bedolla, E.; Forsyth, P.; Hendriks, L.; Brastianos, P.K. Emerging Systemic Treatment Perspectives on Brain Metastases: Moving Toward a Better Outlook for Patients. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 147–165. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- A Tawbi, H.; A Forsyth, P.; Hodi, F.S.; Lao, C.D.; Moschos, S.J.; Hamid, O.; Atkins, M.B.; Lewis, K.; Thomas, R.P.; A Glaspy, J.; et al. Safety and efficacy of the combination of nivolumab plus ipilimumab in patients with melanoma and asymptomatic or symptomatic brain metastases (CheckMate 204). Neuro-Oncol. 2021, 23, 1961–1973. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef] [PubMed]
- A Tawbi, H.; A Forsyth, P.; Hodi, F.S.; Algazi, A.P.; Hamid, O.; Lao, C.D.; Moschos, S.J.; Atkins, M.B.; Lewis, K.; A Postow, M.; et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): Final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 1692–1704. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; A Ascierto, P.; Pilla, L.; Santinami, M.; Ferrucci, P.F.; Giannarelli, D.; Marasco, A.; Rivoltini, L.; Simeone, E.; Nicoletti, S.V.; et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): An open-label, single-arm phase 2 trial. Lancet Oncol. 2012, 13, 879–886. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; Chiarion-Sileni, V.; Del Vecchio, M.; Ferrucci, P.F.; Guida, M.; Quaglino, P.; Guidoboni, M.; Marchetti, P.; Cutaia, O.; Amato, G.; et al. Primary Analysis and 4-Year Follow-Up of the Phase III NIBIT-M2 Trial in Melanoma Patients With Brain Metastases. Clin. Cancer Res. 2021, 27, 4737–4745. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 976–983. [Google Scholar] [CrossRef]
- Kluger, H.M.; Chiang, V.; Mahajan, A.; Zito, C.R.; Sznol, M.; Tran, T.; Weiss, S.A.; Cohen, J.V.; Yu, J.; Hegde, U.; et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J. Clin. Oncol. 2018, 37, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Del Vecchio, M.; Robert, C.; Mackiewicz, A.; Chiarion-Sileni, V.; Arance, A.; Lebbé, C.; Bastholt, L.; Hamid, O.; Rutkowski, P.; et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017, 18, 611–622. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Mackiewicz, A.; Robert, C.; Chiarion-Sileni, V.; Arance, A.; Lebbé, C.; Svane, I.M.; McNeil, C.; Rutkowski, P.; et al. Overall survival at 5 years of follow-up in a phase III trial comparing ipilimumab 10 mg/kg with 3 mg/kg in patients with advanced melanoma. J. Immunother. Cancer 2020, 8, e000391. [Google Scholar] [CrossRef] [PubMed]
- Pelster, M.S.; Amaria, R.N. Combined targeted therapy and immunotherapy in melanoma: A review of the impact on the tumor microenvironment and outcomes of early clinical trials. Ther. Adv. Med Oncol. 2019, 11, 1758835919830826. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.; Xu, W.; Sahgal, A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 2012, 118, 2486–2493. [Google Scholar] [CrossRef]
- Tallet, A.V.; Azria, D.; Barlesi, F.; Spano, J.-P.; Carpentier, A.F.; Gonçalves, A.; Metellus, P. Neurocognitive function impairment after whole brain radiotherapy for brain metastases: Actual assessment. Radiat. Oncol. 2012, 7, 77. [Google Scholar] [CrossRef]
- Krummel, D.A.P.; Nasti, T.H.; Izar, B.; Press, R.H.; Xu, M.; Lowder, L.; Kallay, L.; Rupji, M.; Rosen, H.; Su, J.; et al. Impact of Sequencing Radiation Therapy and Immune Checkpoint Inhibitors in the Treatment of Melanoma Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 157–163. [Google Scholar] [CrossRef]
- Weil, R.J.; Palmieri, D.C.; Bronder, J.L.; Stark, A.M.; Steeg, P.S. Breast Cancer Metastasis to the Central Nervous System. Am. J. Pathol. 2005, 167, 913–920. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Chao, S.T.; Shanley, R.; Luo, X.; Sneed, P.K.; Suh, J.; Weil, R.J.; Jensen, A.W.; et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J. Neuro-Oncol. 2013, 112, 467–472. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Fuchs, E.; Ricken, G.; Mlecnik, B.; Bindea, G.; Spanberger, T.; Hackl, M.; Widhalm, G.; Dieckmann, K.; Prayer, D.; et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 2016, 5, e1057388. [Google Scholar] [CrossRef]
- Waqar, S.N.; Samson, P.P.; Robinson, C.G.; Bradley, J.; Devarakonda, S.; Du, L.; Govindan, R.; Gao, F.; Puri, V.; Morgensztern, D. Non–small-cell Lung Cancer With Brain Metastasis at Presentation. Clin. Lung Cancer 2018, 19, e373–e379. [Google Scholar] [CrossRef]
- Shi, A.A.; Digumarthy, S.R.; Temel, J.S.; Halpern, E.F.; Kuester, L.B.; Aquino, S.L. Does Initial Staging or Tumor Histology Better Identify Asymptomatic Brain Metastases in Patients with Non–small Cell Lung Cancer? J. Thorac. Oncol. 2006, 1, 205–210. [Google Scholar] [CrossRef]
- Sorensen, J.B.; Hansen, H.H.; Hansen, M.; Dombernowsky, P. Brain Metastases in Adenocarcinoma of the Lung: Frequency, Risk Groups, and Prognosis. J. Clin. Oncol. 1988, 6, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.; A Bunn, P. Is it time to reevaluate our approach to the treatment of brain metastases in patients with non-small cell lung cancer? Lung Cancer 1998, 20, 85–91. [Google Scholar] [CrossRef]
- Patil, T.; Smith, D.E.; Bunn, P.A.; Aisner, D.L.; Le, A.T.; Hancock, M.; Purcell, W.T.; Bowles, D.W.; Camidge, D.R.; Doebele, R.C. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non–Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J. Thorac. Oncol. 2018, 13, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Rangachari, D.; Yamaguchi, N.; VanderLaan, P.A.; Folch, E.; Mahadevan, A.; Floyd, S.R.; Uhlmann, E.J.; Wong, E.T.; Dahlberg, S.E.; Huberman, M.S.; et al. Brain metastases in patients with EGFR -mutated or ALK -rearranged non-small-cell lung cancers. Lung Cancer 2015, 88, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef]
- Urbanska, E.M.; Santoni-Rugiu, E.; Melchior, L.C.; Carlsen, J.F.; Sørensen, J.B. Intracranial Response of ALK+ Non-Small-cell Lung Cancer to Second-line Dose-escalated Brigatinib After Alectinib Discontinuation Due to Drug-induced Hepatitis and Relapse After Whole Brain Radiotherapy Followed by Stereotactic Radiosurgery. Clin. Lung Cancer 2020, 22, e528–e532. [Google Scholar] [CrossRef]
- Lin, J.J.; Jiang, G.Y.; Joshipura, N.; Ackil, J.; Digumarthy, S.R.; Rincon, S.P.; Yeap, B.Y.; Gainor, J.F.; Shaw, A.T. Efficacy of Alectinib in Patients with ALK-Positive NSCLC and Symptomatic or Large CNS Metastases. J. Thorac. Oncol. 2019, 14, 683–690. [Google Scholar] [CrossRef]
- Facchinetti, F.; Levy, A.; Ammari, S.; Naltet, C.; Lavaud, P.; Aldea, M.; Vasseur, D.; Planchard, D.; Besse, B. Meningeal “Lazarus Response” to Lorlatinib in a ROS1-Positive NSCLC Patient Progressing to Entrectinib. Cancer Manag. Res. 2021, 13, 2805–2810. [Google Scholar] [CrossRef]
- Hochmair, M.; Weinlinger, C.; Prosch, H. Intracranial remission with brigatinib rechallenge as fifth-line ALK inhibition therapy in a lung cancer patient. Anti-Cancer Drugs 2019, 30, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Koba, T.; Kijima, T.; Takimoto, T.; Hirata, H.; Naito, Y.; Hamaguchi, M.; Otsuka, T.; Kuroyama, M.; Nagatomo, I.; Takeda, Y.; et al. Rapid intracranial response to osimertinib, without radiotherapy, in nonsmall cell lung cancer patients harboring the EGFR T790M mutation two case reports. Medicine 2017, 96, e6087. [Google Scholar] [CrossRef] [PubMed]
- Molinier, O.; Besse, B.; Barlesi, F.; Audigier-Valette, C.; Friard, S.; Monnet, I.; Jeannin, G.; Mazières, J.; Cadranel, J.; Hureaux, J.; et al. IFCT-1502 CLINIVO: Real-world evidence of long-term survival with nivolumab in a nationwide cohort of patients with advanced non-small-cell lung cancer. ESMO Open 2022, 7, 100353. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Herbst, R.S.; de Castro, G.; Hui, R.; Peled, N.; Kim, D.-W.; Novello, S.; Satouchi, M.; Wu, Y.-L.; Garon, E.B.; et al. Outcomes With Pembrolizumab Monotherapy in Patients With Programmed Death-Ligand 1–Positive NSCLC With Brain Metastases: Pooled Analysis of KEYNOTE-001, 010, 024, and 042. JTO Clin. Res. Rep. 2021, 2, 100205. [Google Scholar] [CrossRef]
- Chen, H.; Feng, Y.; Zhou, Y.; Tao, Y.; Tang, L.; Shi, Y. Brain metastases and immune checkpoint inhibitors in non-small cell lung cancer: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2022, 71, 3071–3085. [Google Scholar] [CrossRef]
- Camidge, D.R.; Lee, E.Q.; Lin, N.U.; Margolin, K.; Ahluwalia, M.S.; Bendszus, M.; Chang, S.M.; Dancey, J.; E de Vries, E.G.; Harris, G.J.; et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: A guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018, 19, e20–e32. [Google Scholar] [CrossRef]
- Schoenmaekers, J.J.A.O.; Dursun, S.; Biesmans, C.; De Ruysscher, D.K.M.; Broen, M.P.G.; Remon, J.; Dingemans, A.-M.C.; Hendriks, L.E.L. Dynamics of eligibility criteria for central nervous system metastases in non-small cell lung cancer randomized clinical trials over time: A systematic review. Crit. Rev. Oncol./Hematol. 2021, 166, 103460. [Google Scholar] [CrossRef]
- El Rassy, E.; Botticella, A.; Kattan, J.; Le Péchoux, C.; Besse, B.; Hendriks, L. Non-small cell lung cancer brain metastases and the immune system: From brain metastases development to treatment. Cancer Treat. Rev. 2018, 68, 69–79. [Google Scholar] [CrossRef]
- Vilariño, N.; Bruna, J.; Bosch-Barrera, J.; Valiente, M.; Nadal, E. Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat. Rev. 2020, 89, 102067. [Google Scholar] [CrossRef] [PubMed]
- Crinò, L.; Bronte, G.; Bidoli, P.; Cravero, P.; Minenza, E.; Cortesi, E.; Garassino, M.C.; Proto, C.; Cappuzzo, F.; Grossi, F.; et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2019, 129, 35–40. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Schalper, K.A.; Gettinger, S.N.; Mahajan, A.; Herbst, R.S.; Chiang, A.C.; Lilenbaum, R.; Wilson, F.H.; Omay, S.B.; Yu, J.B.; et al. Pembrolizumab for management of patients with NSCLC and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 655–663. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Strickland, M.R.; Lee, E.Q.; Wang, N.; Cohen, J.V.; Chukwueke, U.; Forst, D.A.; Eichler, A.; Overmoyer, B.; Lin, N.U.; et al. Phase II study of ipilimumab and nivolumab in leptomeningeal carcinomatosis. Nat. Commun. 2021, 12, 5954. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Lee, E.Q.; Cohen, J.V.; Tolaney, S.M.; Lin, N.U.; Wang, N.; Chukwueke, U.; White, M.D.; Nayyar, N.; Kim, A.; et al. Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat. Med. 2020, 26, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Krabak, M.J. Leptomeningeal Carcinomatsis. Cancer Treat Rev. 1999, 25, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Preusser, M.; Bent, M.V.D.; Andratschke, N.; Weller, M. How we treat patients with leptomeningeal metastases. ESMO Open 2019, 4, e000507. [Google Scholar] [CrossRef] [PubMed]
- Beauchesne, P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010, 11, 871–879. [Google Scholar] [CrossRef]
- Iorgulescu, J.B.; Gokhale, P.C.; Speranza, M.C.; Eschle, B.K.; Poitras, M.J.; Wilkens, M.K.; Soroko, K.M.; Chhoeu, C.; Knott, A.; Gao, Y.; et al. Concurrent Dexamethasone Limits the Clinical Benefit of Immune Checkpoint Blockade in Glioblastoma. Clin. Cancer Res. 2021, 27, 276–287. [Google Scholar] [CrossRef]
| Author | Phase | Patients | Trial Arms | Outcomes |
|---|---|---|---|---|
| KeyNote-028 [13] | Ib | 26 patients with PD-L1-positive recurrent GBM | Pembrolizumab | Investigation-assessed ORR (by RECIST v1.1): 8%. DOR: 8.3 and 22.8 months in 2 patients, respectively; mPFS: 2.8 months; PFS6 rate: 37.7% mOS: 13.1 months; OS rate at 12 months: 58% |
| Nayak L et al., 2021 [17] | II | 80 patients with recurrent GBM | A: Pembrolizumab + Bevacizumab B: Pembrolizumab | A: 20% ORR, 26% PFS-6 rate;mOS: 8.8 months; B: 0% ORR |
| Nayak et al., 2022 [18] | II | 137 patients with newly diagnosed and recurrent GBM | A: Durvalumab + RT B: Durvalumab B2: Durvalumab + Bevacizumab B3 and C: Durvalumab + low-dose Bevacizumab | Primary endpoints: A: OS-12 (not met) B, B2, B3: PFS-6 (not met) C: OS-6 (not met) |
| Lukas R et al., 2018 [19] | Ia | 16 patients with recurrent GBM | Atezolizumab | Treatment safe and tolerated. 6% ORR; mPFS: 1.2 months; mOS: 4.2 months; |
| Chiocca EA et al. 2021 [20] | I | 21 patients with recurrent GBM | A: Nivolumab 1 mg/kg + 10 mg VDX; B: Nivolumab 3 mg/kg + 10 mg VDX C: Nivolumab 3 mg/kg + 20 mg VDX | Treatment safe and tolerated. A and B: mOS: 16.9 months; C: mOS: 8.5 months |
| Omuro et al., 2022 (CheckMate-143) [21] | I | 136 patients with newly diagnosed GBM | A: Nivolumab + TMZ + RT B: Nivolumab + RT | Primary endpoints: safety and tolerability (met; more frequent lymphopenia in cohort A); 2ry endpoint: OS (similar between A and B, different according to MGMT methylation status); Exploratory endpoints: PFS |
| CheckMate 498 [22] | III | 560 patients with newly diagnosed MGMTunm GBM | A. Nivolumab + RT B: TMZ + RT | Treatment safe and tolerated. A: mOS: 13.4 months; PFS: 6 months; grade 3/4 AEs: 21.9%; serious AEs: 17.3%; B: mOS: 14.9 months; PFS: 6.2 months; grade 3/4 AEs; 25.1%; serious AEs: 7.6% |
| Schalper K et al., 2019 [23] | II | 30 patients with resectable GBM (twenty-seven recurrent and three newly diagnosed) | Neoadjuvant Nivolumab | Safe and tolerable treatment. mPFS: 4.1 months; mOS: 7.3 months |
| Cloughesy T et al., 2019 [24] | NA | 32 patients with recurrent GBM (53% MGMTm, 34% MGMTunm, and 13% unknown) | A: Neoadjuvant + adjuvant Pembrolizumab B. adjuvant Pembrolizumab | Treatment safe and tolerated. mOS: 13.7 vs. 7.5 months (A vs. B); mPFS: 3.3 vs. 2.4 months (A vs. B) |
| Reardon et al., 2020 [25] | III | 369 with recurrent GBM (23.4% MGMTm, 22.7% MGMTunm, and 36.2% unknown) | A: Nivolumab B: Bevacizumab | Primary endpoint not met. Nivolumab safe and tolerated. A: mOS: 9.8 months; ORR: 7.8%; B: mOS: 10 months; ORR: 23.1%; 1-yr OS: 42% for both groups;grade 3/4 AEs similar in A and B |
| CheckMate 548 [26] | III | 716 patients with newly diagnosed MGMTm GBM | A: Nivolumab + TMZ + RT B: PBO + TMZ +RT | Treatment safe and tolerated. mPFS: 10.6 vs. 10.3 months (A vs. B); mOS: 28.9 vs. 32.1 months (A vs. B); with basal corticosteroids, mOS: 31.3 vs. 33 months (A vs. B) |
| Checkate-143 [22] | I | 40 patients with recurrent GBM | A: Nivolumab 3mg/kg B. Ipilimumab 3 mg/kg + Nivolumab 1 mg/kg C: Ipilimumab 1mg/kg+ Nivolumab 3 mg/kg | Nivolumab monotherapy better tolerated than Ipilimumab-nivolumab combo; mPFS: 1.9 vs. 1.5 vs. 2.1 months (A vs. B vs. C); mOS: 10.4 vs. 9.2 vs. 7.3 months (A vs. B vs. C) |
| Author or Trial Name | Phase | Drug | Cohort | Results |
|---|---|---|---|---|
| Furuse et al., 2017 [69] | Case report | Nivolumab + DC vaccination | 1 patient | CR maintained for 10 months |
| Nayak et al., 2017 [57] | Case series | Nivolumab | 5 patietns (4 with PCNSL and 1 with PTL) | 4 patients with CR and 1 with PR; |
| Graber J et al., 2020 [74] | Case series | Pembrolizumab | 5 patients (PCNSL and SCNSL) | Prolonged remission in 3 out of 5 patients |
| Ambady et al., 2019 [75] | Retrospective study | Nivolumab/Pembrolizumab and Rituximab | 6 patients (three with PCNSL and three SCNSL) | 3 out of 6 patients with CR |
| Gavrilenko A et al., 2020 [75,76]; | Case series | Nivolumab | 8 patients with PCNSL and one with PTL | 2-year OS: 44%; mOS: 12 months; 2-year PFS: 26%; mPFS: 12 months; |
| Study/Studies | Phase | Therapies | Patient Cohort(s) | Results (ORR, PFS, OS) |
|---|---|---|---|---|
| Margolin et al., 2012 [86] | II | Ipilimumab | Cohort A: asymptomatic MBM (51); Cohort B: symptomatic MBM on (21) | iDCR: 24 vs. 10% (A vs. B); mPFS: 1.5–1.9 vs. 1.2 months (A vs. B); mOS: 7 vs. 3.7 months (A vs. B) |
| NIBIT M1 [90]; | II | Ipilimumab + Fotemustine | 20 patients with asymptomatic brain metastases out of a cohort of eight-six patients with advanced melanoma | In MBM patients: Brain-PFS: 3 months |
| NIBIT-M2 [91] | III | Ipilimumab, Nivolumab, Fotemustine | 80 patients with MBM Arm A: Fotemustine (27) Arm B: Ipilimumab + Fotemustine (26) Arm C: Ipilimumab + Nivolumab (27) | mOS: 8.5 vs. 8.2 vs. 29.2 months (A vs. B vs. C); mPFS: 3 vs. 3 vs. 8.7 months (A vs. B vs. C); ICR: 0 vs. 19.2 vs. 44.4% (A vs. B vs. C) |
| Goldberg et al., 2016 [92] | II | Pembrolizumab | 18 patients with MBM in a cohort of 52 patients with brain metastases | ORR: 22% mOS: not reached |
| Kluger et al., 2018; [93] | II | Pembrolizumab | 23 patients with MBM | RR: 26% mPFS: 2 months; mOS: 17 months |
| Ascierto et al., 2017,2020 [94,95] | III | Ipilimumab A: 10 mg/kg; B: 3 mg/kg; | 127 patients with MBMs in a cohort of 727 patients with advanced melanoma | In MBM patients: mOS: 7 months vs. 5.7 months (A vs. B) |
| Checkmate204 [89] | II | Nivolumab + Ipilimumab | 119 patients with MBM Cohort A: asymptomatic (101); Cohort B: symptomatic (18) | iORR: 53.5% vs. 16.7% (A vs. B); 36-month iPFS: 54.1% vs. 18.9% (A vs. B); 36-month OS: 71.9% vs. 36.6% (A vs. B) |
| ABC study [88] | II | Nivolumab + Ipilimumab vs. Nivolumab | 79 patients with MBM; Cohort A: Nivolumab + Ipilimumab (36) Cohort B: Nivolumab (27); Cohort C: Nivolumab prior Tx, symptomatic, or with LM (16); | ICR 46% vs. 20% vs. 6% (A vs. B vs. C); ICCR 17% vs. 12% vs. 0% (A vs. B vs. C); iPFS: not reached vs. 2.5 vs. 2.3 months (A vs. B vs. C); OS: not reached vs. 18.5 vs. 5.1 months (A vs. B vs. C) |
| Study | Phase | Therapy | Patient Cohort | Patients with BM | Results |
|---|---|---|---|---|---|
| Goldberg et al., 2020 [126] | II | Pembrolizumab | 42 asymptomatic patients with untreated BM from NSCLC; Cohort A: PD-L1 expression ≥1%; Cohort B: PD-L1 expression <1% or unevaluable; | 100% | Cohort A: 29.7% BM response rate; Cohort 2: no response |
| Keynote-189 study [128,129] | III | ICI Arm (A): Pembrolizumab + Pemetrexed + a Pt-based CT; Control Arm (B): Pemetrexed + a Pt-based CT; | 108 patents among a cohort of six hundred and sixteen patients with metastatic n-sq-NSCLC | 17.53% | For BM patients: mOS: 19.2 vs. 7.5 months (A vs. B); HR for OS (A vs. B): 0.41 |
| Crinò et al., 2019 [125] | EAP | Nivolumab | 409 patients with asymptomatic or controlled BM in a cohort of 1588 patients with advanced n-sq-NSCLC | 26% | BM patients: mOS: 8.6 months; mPFS: 3 months; iDCR: 40%; ORR:17% |
| OAK trial [130] | III | ICI Arm (A): Atezolizumab; Control Arm (B): docetaxel | 85 patients with BM in a cohort of 850 patients with previously treated stage IIIB/IV NSCLC; | 10% | For BM patients: mOS: 20.1 vs. 11.9 months (A vs. B); OS HR for Atezolizumab: 0.54 |
| Author | Phase | Patients | Treatment |
|---|---|---|---|
| Brastianos et al., 2021 [131] | II | 18 patients with LM | Ipilimumab–Nivolumab |
| Brastianos et al., 2020 [132] | II | 20 patients with pretreated LM | Pembrolizumab monotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimonti, P.; Gonzalez Castro, L.N. The Current Landscape of Immune Checkpoint Inhibitor Immunotherapy for Primary and Metastatic Brain Tumors. Antibodies 2023, 12, 27. https://doi.org/10.3390/antib12020027
Alimonti P, Gonzalez Castro LN. The Current Landscape of Immune Checkpoint Inhibitor Immunotherapy for Primary and Metastatic Brain Tumors. Antibodies. 2023; 12(2):27. https://doi.org/10.3390/antib12020027
Chicago/Turabian StyleAlimonti, Paolo, and L. Nicolas Gonzalez Castro. 2023. "The Current Landscape of Immune Checkpoint Inhibitor Immunotherapy for Primary and Metastatic Brain Tumors" Antibodies 12, no. 2: 27. https://doi.org/10.3390/antib12020027
APA StyleAlimonti, P., & Gonzalez Castro, L. N. (2023). The Current Landscape of Immune Checkpoint Inhibitor Immunotherapy for Primary and Metastatic Brain Tumors. Antibodies, 12(2), 27. https://doi.org/10.3390/antib12020027






