Psychiatric Symptoms in Acute and Persisting Forms of COVID-19 Associated with Neural Autoantibodies
Abstract
1. Psychiatric Disease Manifestation in COVID-19 Associated with Neural Autoantibodies
2. Methodological Approach
3. Psychiatric Symptom Spectrum in COVID-19 with Neural Autoantibodies
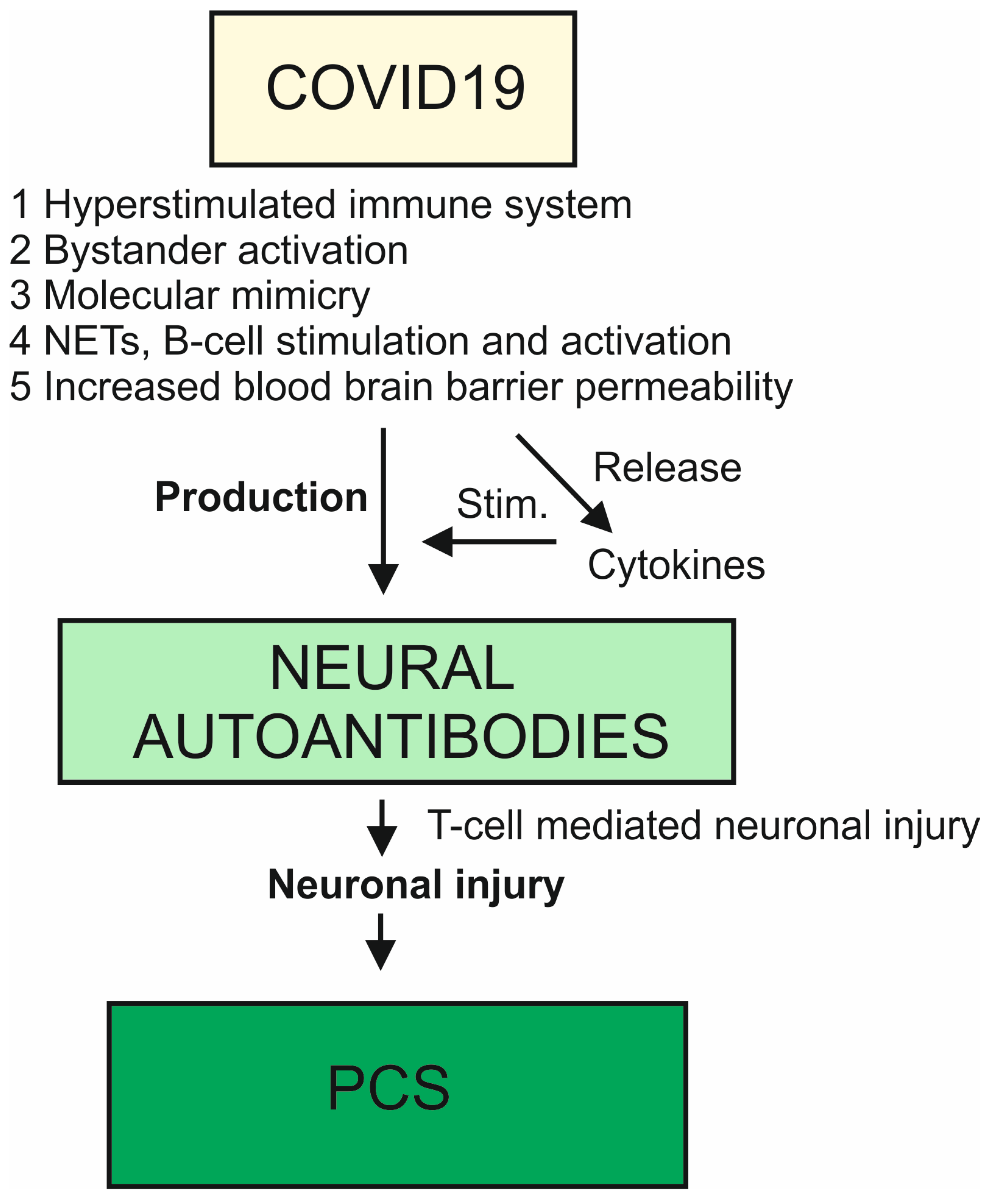
4. Pathomechanisms of Neural Autoantibody Production in COVID-19
4.1. Molecular Mimicry and Autoantibodies
4.2. Hyperstimulation of the Immune System in COVID-19
4.3. Neutrophils Extracellular Traps and Autoantibodies
4.4. A More Permeable Blood–Brain Barrier and Autoantibodies
4.5. Bystander Activation, Epitope Spreading, and Autoantibodies
5. Neural Autoantibodies in COVID-19 with Psychiatric Manifestation
6. Neural Autoantibodies in Post-COVID-19 Syndrome with a Psychiatric Manifestation
7. Potential Brain Damage in Autoantibody-Associated COVID-19 and Post-COVID-19 Syndrome with Neuropsychiatric Symptoms
8. Therapeutic and Biomarker-Supported Approach
9. Synopsis
10. Summary
- Neural autoantibodies are detected more frequently in patients presenting neuropsychiatric symptoms, COVID-19, and PCS than in controls.
- The spectrum of psychiatric symptoms in patients with COVID-19 and PCS associated with neural autoantibodies ranges from cognitive impairment to psychosis.
- The autoantibodies identified in neuropsychiatric patients with COVID-19 and PCS are ARHGAP31, GAD65, acethylcholine receptor, D1/2, MOG, NMDAR, MCTP1, Yo, myelin, and Ma/Ta2.
- The pathomechanisms of possible COVID-19 and PCS disorders accompanied by psychiatric symptoms and evidence of neural autoantibodies include molecular mimicry, a hyperstimulated immune system, NETs, altered blood–brain barrier permeability, and bystander activation.
- There is indirect evidence from biomarker studies that COVID-19 and PCS with autoantibodies can lead to brain damage.
- Therapeutic approaches should follow the guidelines for known autoimmune conditions, such as autoimmune encephalitis, to provide individualized treatment.
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franke, C.; Ferse, C.; Kreye, J.; Reincke, S.M.; Sanchez-Sendin, E.; Rocco, A.; Steinbrenner, M.; Angermair, S.; Treskatsch, S.; Zickler, D.; et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 2021, 93, 415–419. [Google Scholar] [CrossRef]
- Bodansky, A.; Wang, C.Y.; Saxena, A.; Mitchell, A.; Kung, A.F.; Takahashi, S.; Anglin, K.; Huang, B.; Hoh, R.; Lu, S.; et al. Autoantigen profiling reveals a shared post-COVID signature in fully recovered and Long COVID patients. JCI Insight 2023, 8, e169515. [Google Scholar] [CrossRef] [PubMed]
- Lavi, Y.; Vojdani, A.; Halpert, G.; Sharif, K.; Ostrinski, Y.; Zyskind, I.; Lattin, M.T.; Zimmerman, J.; Silverberg, J.I.; Rosenberg, A.Z.; et al. Dysregulated Levels of Circulating Autoantibodies against Neuronal and Nervous System Autoantigens in COVID-19 Patients. Diagnostics 2023, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, L.S.; Lifland, B.; Check, J.R.; Angarita, G.A.; Ngo, T.T.; Chen, P.; Dandekar, R.; Alvarenga, B.D.; Browne, W.D.; Pleasure, S.J.; et al. Anti-SARS-CoV-2 and Autoantibody Profiling of a COVID-19 Patient With Subacute Psychosis Who Remitted After Treatment With Intravenous Immunoglobulin. Biol. Psychiatry 2023, 93, e25–e29. [Google Scholar] [CrossRef] [PubMed]
- Franke, C.; Boesl, F.; Goereci, Y.; Gerhard, A.; Schweitzer, F.; Schroeder, M.; Foverskov-Rasmussen, H.; Heine, J.; Quitschau, A.; Kandil, F.I.; et al. Association of cerebrospinal fluid brain-binding autoantibodies with cognitive impairment in post-COVID-19 syndrome. Brain Behav. Immun. 2023, 109, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Needham, E.J.; Ren, A.L.; Digby, R.J.; Norton, E.J.; Ebrahimi, S.; Outtrim, J.G.; Chatfield, D.A.; Manktelow, A.E.; Leibowitz, M.M.; Newcombe, V.F.J.; et al. Brain injury in COVID-19 is associated with dysregulated innate and adaptive immune responses. Brain 2022, 145, 4097–4107. [Google Scholar] [CrossRef] [PubMed]
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [CrossRef]
- Schild, A.K.; Goereci, Y.; Scharfenberg, D.; Klein, K.; Lülling, J.; Meiberth, D.; Schweitzer, F.; Stürmer, S.; Zeyen, P.; Sahin, D.; et al. Multidomain cognitive impairment in non-hospitalized patients with the post-COVID-19 syndrome: Results from a prospective monocentric cohort. J. Neurol. 2023, 270, 1215–1223. [Google Scholar] [CrossRef]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62,354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef]
- Ariño, H.; Heartshorne, R.; Michael, B.D.; Nicholson, T.R.; Vincent, A.; Pollak, T.A.; Vogrig, A. Neuroimmune disorders in COVID-19. J. Neurol. 2022, 269, 2827–2839. [Google Scholar] [CrossRef]
- Bartels, C.; Hessmann, P.; Schmidt, U.; Vogelgsang, J.; Ruhleder, M.; Kratzenberg, A.; Treptow, M.; Reh-Bergen, T.; Abdel-Hamid, M.; Heß, L.; et al. Medium-term and peri-lockdown course of psychosocial burden during the ongoing COVID-19 pandemic: A longitudinal study on patients with pre-existing mental disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 757–771. [Google Scholar] [CrossRef]
- Hansen, N.; Lipp, M.; Vogelgsang, J.; Vukovich, R.; Zindler, T.; Luedecke, D.; Gingele, S.; Malchow, B.; Frieling, H.; Kühn, S.; et al. Autoantibody-associated psychiatric symptoms and syndromes in adults: A narrative review and proposed diagnostic approach. Brain Behav. Immun. Health. 2020, 9, 100154. [Google Scholar] [CrossRef]
- Hansen, N.; Lüdecke, D.; Maier, H.; Steiner, J.; Neyazi, A.N. Psychiatrische Autoimmunenzephalitis—Diagnostik und therapeutische Ansätze. PSYCH2 Update 2023, 17, 13–28. [Google Scholar]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef]
- Knight, J.S.; Caricchio, R.; Casanova, J.L.; Combes, A.J.; Diamond, B.; Fox, S.E.; Hanauer, D.A.; James, J.A.; Kanthi, Y.; Ladd, V.; et al. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021, 131, e154886. [Google Scholar] [CrossRef]
- Taghadosi, M.; Safarzadeh, E.; Asgarzadeh, A.; Roghani, S.A.; Shamsi, A.; Jalili, C.; Assar, S.; Soufivand, P.; Pournazari, M.; Feizollahi, P.; et al. Partners in crime: Autoantibodies complicit in COVID-19 pathogenesis. Rev. Med. Virol. 2023, 33, e2412. [Google Scholar] [CrossRef]
- Vasilevska, V.; Guest, P.C.; Bernstein, H.G.; Schroeter, M.L.; Geis, C.; Steiner, J. Molecular mimicry of NMDA receptors may contribute to neuropsychiatric symptoms in severe COVID-19 cases. J. Neuroinflamm. 2021, 18, 245. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol. Res. 2020, 68, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Weaver, D.F. COVID-19 as a Trigger of Brain Autoimmunity. ACS Chem. Neurosci. 2021, 12, 2558–2561. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, L.; Nasirpour, M.H.; Masoumi, E.; Azarnaminy, A.F.; Jafari, M.; Esmaeili, S.A. SARS-CoV-2 triggering autoimmune diseases. Cytokine 2022, 154, 155873. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, L.; Lin, C. T cell response in patients with COVID-19. Blood Sci. 2020, 2, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.H.; Zhu, Y.; Mao, J.; Du, R.C. T cell immunobiology and cytokine storm of COVID-19. Scand. J. Immunol. 2021, 93, e12989. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Damoiseaux, J.; Dotan, A.; Fritzler, M.J.; Bogdanos, D.P.; Meroni, P.L.; Roggenbuck, D.; Goldman, M.; Landegren, N.; Bastard, P.; Shoenfeld, Y.; et al. Autoantibodies and SARS-CoV2 infection: The spectrum from association to clinical implication: Report of the 15th Dresden Symposium on Autoantibodies. Autoimmun. Rev. 2022, 21, 103012. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Liu, X. NETosis and Neutrophil Extracellular Traps in COVID-19, Immunothrombosis and Beyond. Front. Immunol. 2022, 13, 838011. [Google Scholar] [CrossRef]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediat. Inflamm. 2020, 2020, 8829674. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Kempuraj, D. Role of SARS-CoV-2 Spike-Protein-Induced Activation of Microglia and Mast Cells in the Pathogenesis of Neuro-COVID. Cells 2023, 12, 688. [Google Scholar] [CrossRef]
- Krasemann, S.; Haferkamp, U.; Pfefferle, S.; Woo, M.S.; Heinrich, F.; Schweizer, M.; Appelt-Menzel, A.; Cubukova, A.; Barenberg, J.; Leu, J.; et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep. 2022, 17, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Díaz, J.P.; Miranda-Narváez, C.L.; Martínez-Lazcano, J.C.; Martínez-Martínez, E. The relationship between chronic immune response and neurodegenerative damage in long COVID-19. Front. Immunol. 2022, 13, 1039427. [Google Scholar] [CrossRef]
- Jarius, S.; Pache, F.; Körtvelyessy, P.; Jelčić, I.; Stettner, M.; Franciotta, D.; Keller, E.; Neumann, B.; Ringelstein, M.; Senel, M.; et al. Cerebrospinal fluid findings in COVID-19, a multicenter study of 150 lumbar punctures in 127 patients. J. Neuroinflamm. 2022, 19, 19. [Google Scholar] [CrossRef]
- Gonen, M.S.; De Bellis, A.; Durcan, E.; Bellastella, G.; Cirillo, P.; Scappaticcio, L.; Longo, M.; Bircan, B.E.; Sahin, S.; Sulu, C.; et al. Assessment of Neuroendrocrine Changes and Hypthalamo-Pituitary Autoimmunity in patients with COVID-19. Horm. Metab. Res. 2022, 54, 153–161. [Google Scholar] [CrossRef]
- Visvabharathy, L.; Zhu, C.; Orban, Z.S.; Yarnoff, K.; Palacio, N.; Jimenez, M.; Lim, P.H.; Penaloza-MacMaster, P.; Koralnik, I.J. Autoantibody production is enhanced after mild SARS-CoV-2 infection despite vaccination in individuals with and without long COVID. medRxiv 2023. medRxiv: 04.07.23288243. [Google Scholar] [CrossRef]
- Thomasson, M.; Voruz, P.; Cionca, A.; Jacot de Alcântara, I.; Nuber-Champier, A.; Allali, G.; Benzakour, L.; Lalive, P.H.; Lövblad, K.O.; Braillard, O.; et al. Markers of limbic system damage following SARS-CoV-2 infection. Brain Commun. 2023, 13, fcad177. [Google Scholar] [CrossRef]
- Chang, L.; Ryan, M.C.; Liang, H.; Zhang, X.; Cunningham, E.; Wang, J.; Wilson, E.; Herskovits, E.H.; Kottilil, S.; Ernst, T.M. Changes in Brain Activation Patterns During Working Memory Tasks in People With Post-COVID Condition and Persistent Neuropsychiatric Symptoms. Neurology 2023, 100, e2409–e2423. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Waliszewska-Prosół, M.; Budrewicz, S. The unusual course of a migraine attack during COVID-19 infection—Case studies of three patients. J. Infect. Public Health 2021, 14, 903–905. [Google Scholar] [CrossRef]
- Saeedi, N.; Gohari, N.S.F.; Ghalibaf, A.A.M.; Dehghan, A.; Owlia, M.B. COVID-19 infection: A possible induction factor for development of autoimmune diseases? Immunol. Res. 2023; ahead of print. [Google Scholar] [CrossRef]
- Ariño, H.; Ruiz García, R.; Rioseras, B.; Naranjo, L.; Martinez-Hernandez, E.; Saiz, A.; Graus, F.; Dalmau, J. Frequency and Referral Patterns of Neural Antibody Studies During the COVID-19 Pandemic: Experience From an Autoimmune Neurology Center. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200129. [Google Scholar] [CrossRef]
- Abboud, H.; Probasco, J.C.; Irani, S.; Ances, B.; Benavides, D.R.; Bradshaw, M.; Christo, P.P.; Dale, R.C.; Fernandez-Fournier, M.; Flanagan, E.P.; et al. Autoimmune Encephalitis Alliance Clinicians Network. Autoimmune encephalitis: Proposed best practice recommendations for diagnosis and acute management. J. Neurol. Neurosurg. Psychiatry 2021, 92, 757–768. [Google Scholar] [CrossRef]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. Autoantibody-associated psychiatric symptoms and syndromes in adults: A narrative review and proposed diagnostic approach. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Guest, P.C.; Neyazi, A.; Braun-Dullaeus, R.C.; Müller, P.; Schreiber, J.; Haghikia, A.; Vasilevska, V.; Steiner, J. A Molecular Biomarker-Based Triage Approach for Targeted Treatment of Post-COVID-19 Syndrome Patients with Persistent Neurological or Neuropsychiatric Symptoms. Adv. Exp. Med. Biol. 2023, 1412, 97–115. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Pelazza, C.; Bertolotti, M.; Agatea, L.; De Gaspari, P.; Tamiazzo, S.; Ielo, D.; Stobbione, P.; Grappiolo, M.; Bolgeo, T.; et al. The onset of de novo autoantibodies in healthcare workers after mRNA based anti-SARS-CoV-2 vaccines: A single centre prospective follow-up study. Autoimmunity 2023, 56, 2229072. [Google Scholar] [CrossRef]
- Daguano Gastaldi, V.; Bh Wilke, J.; Weidinger, C.A.; Walter, C.; Barnkothe, N.; Teegen, B.; Luessi, F.; Stöcker, W.; Lühder, F.; Begemann, M.; et al. Factors predisposing to humoral autoimmunity against brain-antigens in health and disease: Analysis of 49 autoantibodies in over 7000 subjects. Brain Behav. Immun. 2023, 108, 135–147. [Google Scholar] [CrossRef]
- Muri, J.; Cecchinato, V.; Cavalli, A.; Shanbhag, A.A.; Matkovic, M.; Biggiogero, M.; Maida, P.A.; Moritz, J.; Toscano, C.; Ghovehoud, E.; et al. Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course. Nat. Immunol. 2023, 24, 604–611. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
| DISEASE | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | PCS |
|---|---|---|---|---|---|---|
| PATIENT NUMBERS | N = 64 with prior COVID-19 vs. N = 57 pre-COVID-19 controls | N = 121 with long COVID-19 vs. N = 64 with prior COVID-19 and full recovery | N = 169 with COVID-19 vs. N = 77 controls | N = 1 with COVID-19 | N = 11 with COVID-19 | N = 50 with PCS |
| NEURAL AUTOANTIBODY SPECTRUM | Most prominent autoreactivity: ARHGAP31 | Most prominent autoreactivity: ARHGAP31 | GAD65, acetylcholine receptor, D1/D2 receptor, Myelin Basic Protein, MOG, NMDAR | MCTP1 | NMDAR, Yo, myelin, unknown target antigen | Yo; Ma/Ta2, GAD65, NMDAR, undetermined epitopes of antigens on brain sections |
| CSF | − | − | − | + | + | |
| BLOOD | + | + | + | + | + | |
| RESULTS | Autoreactive signature in patients with prior COVID-19 vs. pre-COVID-19 controls | No autoreactive signature in patients with prior COVID-19 vs. pre-COVID-19 controls detected | Levels of IgA autoantibodies against acetylcholine receptors, D2 receptors, and myelin basic protein elevated in COVID-19 patients vs. controls regardless of the disease severity. Levels of IgG autoantibodies against NMDAR, GAD65, D1R, and MOG elevated in patients with severe form of COVID-19 and the need for oxygen. Levels of NMDAR IgA lower in COVID-19 patients than in controls | Enriched MCTP1 significantly higher than in combined 3408 healthy CSF and sera and 808 negative controls | A total of 4 in 11 (36%) COVID-19 patients positive for neural autoantibodies in CSF All patients had anti-neural autoantibodies, but some were unspecific | Strong correlation between dysfunctional cognitive performance on the MoCA test and presence of antineuronal antibodies detected in 52% of 50 patients |
| REFERENCE | [2] | [2] | [3] | [4] | [1] | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, N. Psychiatric Symptoms in Acute and Persisting Forms of COVID-19 Associated with Neural Autoantibodies. Antibodies 2023, 12, 49. https://doi.org/10.3390/antib12030049
Hansen N. Psychiatric Symptoms in Acute and Persisting Forms of COVID-19 Associated with Neural Autoantibodies. Antibodies. 2023; 12(3):49. https://doi.org/10.3390/antib12030049
Chicago/Turabian StyleHansen, Niels. 2023. "Psychiatric Symptoms in Acute and Persisting Forms of COVID-19 Associated with Neural Autoantibodies" Antibodies 12, no. 3: 49. https://doi.org/10.3390/antib12030049
APA StyleHansen, N. (2023). Psychiatric Symptoms in Acute and Persisting Forms of COVID-19 Associated with Neural Autoantibodies. Antibodies, 12(3), 49. https://doi.org/10.3390/antib12030049





