Immunogenicity and Loss of Effectiveness of Biologic Therapy for Inflammatory Bowel Disease Patients Due to Anti-Drug Antibody Development
Abstract
:1. Introduction—Challenges Related to the Use of Biologic Therapy in IBD Patients
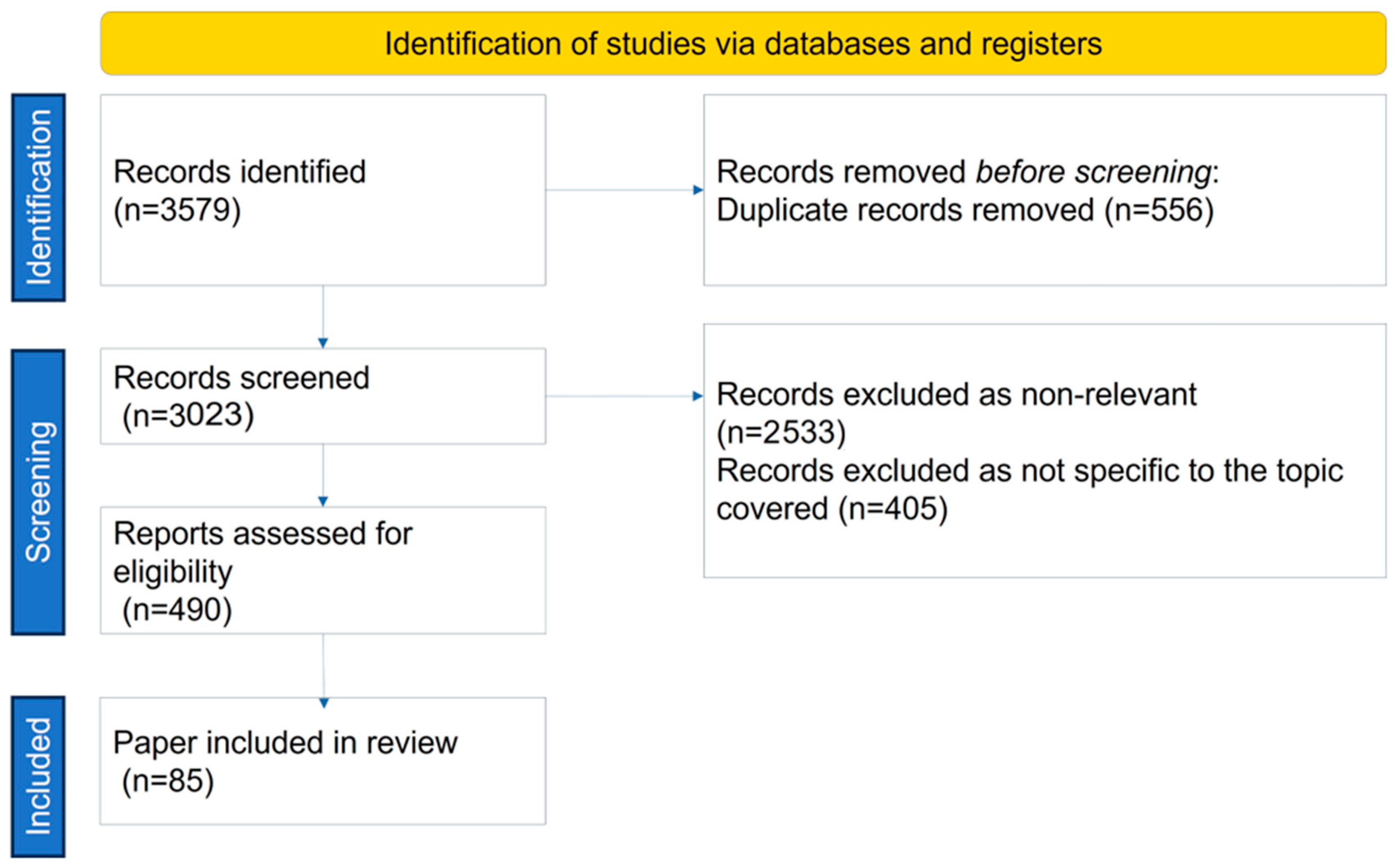
2. Search Strategy
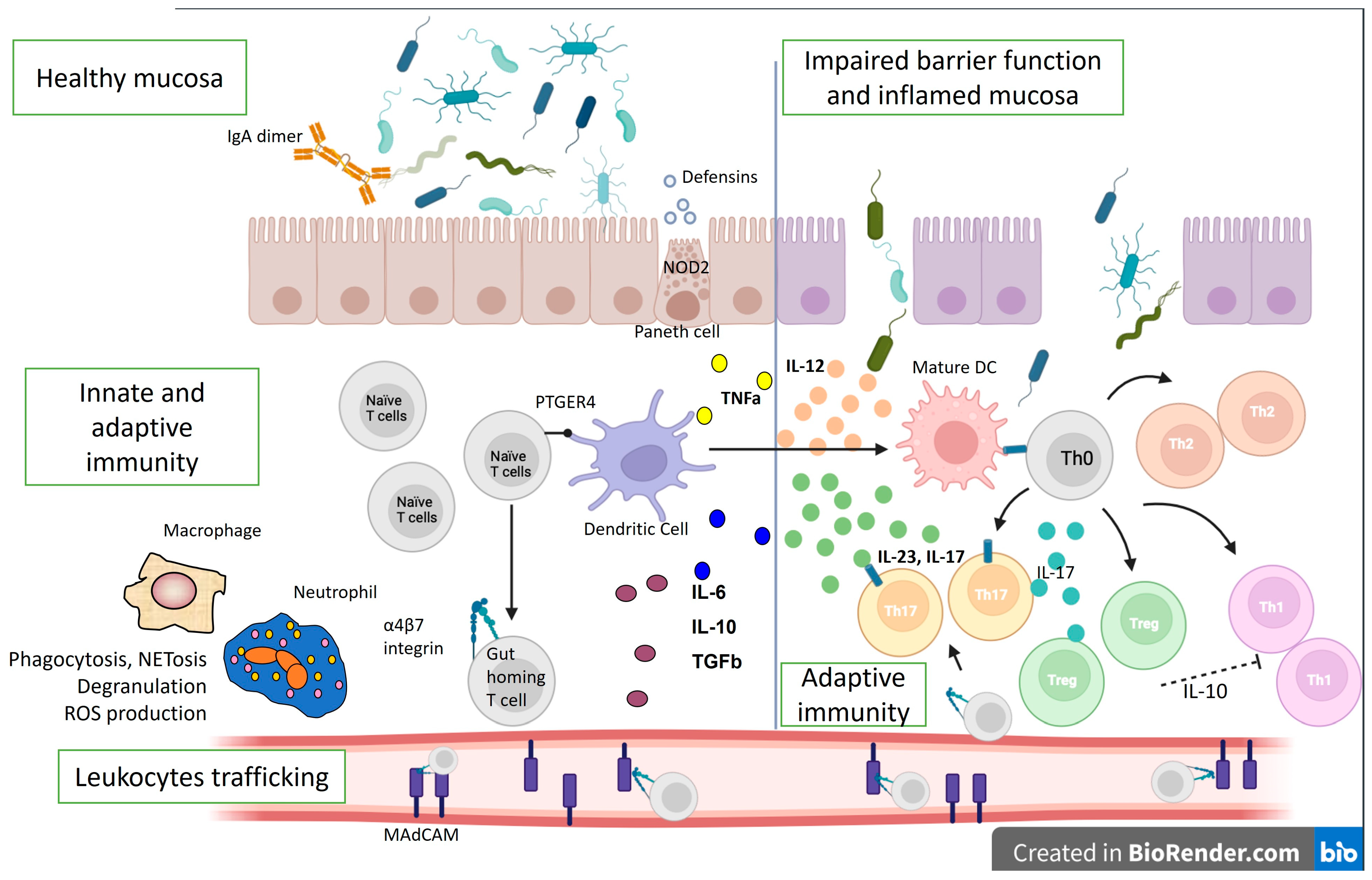
3. Biologic Therapy—Tailoring the IBD Pathogenetic Mechanisms
4. Immunological Mechanisms of Biological Therapy Failure
5. Recent Systematic Reviews and Meta-Analyses on Biologic Failure in IBD Patients
6. Strategies for Precise Detection and Preventing Immunological Failure of IBD Biologics Due to Antibody Formation
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kornbluth, A. Infliximab Approved for Use in Crohn’s Disease: A Report on the FDA GI Advisory Committee Conference. Inflamm. Bowel Dis. 1998, 4, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.M.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; Fagone, P. Past, Present and (Foreseeable) Future of Biological Anti-TNF Alpha Therapy. J. Clin. Med. 2023, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.L.; Steinlauf, A.F. Efficacy and Safety of Dual Biologic Therapy in Patients with Inflammatory Bowel Disease: A Review of the Literature. Gastroenterol. Hepatol. 2021, 17, 406–414. [Google Scholar]
- Hirten, R.P.; Iacucci, M.; Shah, S.; Ghosh, S.; Colombel, J.-F. Combining Biologics in Inflammatory Bowel Disease and Other Immune Mediated Inflammatory Disorders. Clin. Gastroenterol. Hepatol. 2018, 16, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Wheat, C.L.; Ko, C.W.; Clark-Snustad, K.; Grembowski, D.; Thornton, T.A.; Devine, B. Inflammatory Bowel Disease (IBD) pharmacotherapy and the risk of serious infection: A systematic review and network meta-analysis. BMC Gastroenterol. 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Borren, N.Z.; Ananthakrishnan, A.N. Safety of Biologic Therapy in Older Patients with Immune-Mediated Diseases: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1736–1743.e4. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients with Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397.e10. [Google Scholar] [CrossRef]
- Bressler, B. Is there an optimal sequence of biologic therapies for inflammatory bowel disease? Ther. Adv. Gastroenterol. 2023, 16, 17562848231159452. [Google Scholar] [CrossRef]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 747. [Google Scholar] [CrossRef]
- Higashiyama, M.; Hokaria, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2023, 104, 74–81, Erratum in: Digestion 2023, 104, 164. [Google Scholar] [CrossRef]
- Haider, M.; Lashner, B. Dual Targeted Therapy for the Management of Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2021, 55, 661–666. [Google Scholar] [CrossRef]
- Juillerat, P.; Grueber, M.M.; Ruetsch, R.; Santi, G.; Vuillèmoz, M.; Michetti, P. Positioning biologics in the treatment of IBD: A practical guide—Which mechanism of action for whom? Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100104. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), s1–s106. [Google Scholar] [CrossRef]
- Miyatani, Y.; Kobayashi, T. Evidence-Based Approach to the Discontinuation of Immunomodulators or Biologics in Inflammatory Bowel Disease. Digestion 2023, 104, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Laredo, V.; Gargallo-Puyuelo, C.J.; Gomollón, F. How to Choose the Biologic Therapy in a Bio-naïve Patient with Inflammatory Bowel Disease. J. Clin. Med. 2022, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.R.; Colombel, J.-F.; Ungaro, R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Ben-Horin, S.; Novack, L.; Mao, R.; Guo, J.; Zhao, Y.; Sergienko, R.; Zhang, J.; Kobayashi, T.; Hibi, T.; Chowers, Y.; et al. Efficacy of Biologic Drugs in Short-Duration Versus Long-Duration Inflammatory Bowel Disease: A Systematic Review and an Individual-Patient Data Meta-Analysis of Randomized Controlled Trials. Gastroenterology 2022, 162, 482–494. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Blackmore, H.; Kitas, G.D. Writing a narrative biomedical review: Considerations for authors, peer reviewers, and editors. Rheumatol. Int. 2011, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.O.; Fernández-Tomé, S.; Abalo, R. Biological Treatments in Inflammatory Bowel Disease: A Complex Mix of Mechanisms and Actions. Biologics 2021, 1, 189–210. [Google Scholar] [CrossRef]
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD—Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jeldres, T.; Tyler, C.J.; Boyer, J.D.; Karuppuchamy, T.; Bamias, G.; Dulai, P.S.; Boland, B.S.; Sandborn, W.J.; Patel, D.R.; Rivera-Nieves, J. Cell Trafficking Interference in Inflammatory Bowel Disease: Therapeutic Interventions Based on Basic Pathogenesis Concepts. Inflamm. Bowel Dis. 2019, 25, 270–282. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, Q.; Pan, L.-L.; Sun, J.; Dong, X.; Li, J.; Liu, H.; Ren, Z.; Li, B. Macrophage immunometabolism in inflammatory bowel diseases: From pathogenesis to therapy. Pharmacol. Ther. 2022, 238, 108176. [Google Scholar] [CrossRef]
- Velikova, T.; Kyurkchiev, D.; Ivanova-Todorova, E.; Spassova, Z.; Stanilova, S.; Altankova, I. Cytokines in Inflamed Mucosa of IBD Patients; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Illig, D.; Kotlarz, D. Dysregulated inflammasome activity in intestinal inflammation—Insights from patients with very early onset IBD. Front. Immunol. 2022, 13, 1027289. [Google Scholar] [CrossRef]
- Zundler, S.; Becker, E.; Weidinger, C.; Siegmund, B. Anti-Adhesion Therapies in Inflammatory Bowel Disease—Molecular and Clinical Aspects. Front. Immunol. 2017, 8, 891. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Huang, Y.-H.; Jiang, M. Combination therapy in inflammatory bowel disease: Current evidence and perspectives. Int. Immunopharmacol. 2023, 114, 109545. [Google Scholar] [CrossRef] [PubMed]
- Jani, M.; Barton, A.; Warren, R.B.; Griffiths, C.E.M.; Chinoy, H. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology 2013, 53, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Garcês, S.; Demengeot, J.; Benito-Garcia, E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: A systematic review of the literature with a meta-analysis. Ann. Rheum. Dis. 2012, 72, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, R.; Ghosh, S. Optimal use of biologics in the management of Crohn’s disease. Ther. Adv. Gastroenterol. 2010, 3, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Jeen, Y.T. Current and Emerging Biologics for Ulcerative Colitis. Gut Liver 2015, 9, 18–27. [Google Scholar] [CrossRef] [PubMed]
- de Silva, P.S.; Nguyen, D.D.; Sauk, J.; Korzenik, J.; Yajnik, V.; Ananthakrishnan, A.N. Long-term outcome of a third anti-TNF monoclonal antibody after the failure of two prior anti-TNFs in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2012, 36, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.B.; Morand, E.F.; Murphy, K.; Mackay, F.; Mariette, X.; Marcelli, C. Anti-drug antibodies (ADAb) to tumour necrosis factor (TNF)-specific ptimization agents in chronic inflammatory diseases: A real issue, a clinical perspective. Ann. Rheum. Dis. 2013, 72, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Bendtzen, K.; Ainsworth, M.; Steenholdt, C.; Thomsen, O.Ø.; Brynskov, J. Individual medicine in inflammatory bowel disease: Monitoring bioavailability, pharmacokinetics and immunogenicity of anti-tumour necrosis factor-alpha antibodies. Scand. J. Gastroenterol. 2009, 44, 774–781. [Google Scholar] [CrossRef]
- Guerra, I.; Chaparro, M.; Bermejo, F.; Gisbert, J.P. Utility of Measuring Serum Concentrations of Anti-TNF Agents and Anti-Drug Antibodies in Inflammatory Bowel Disease. Curr. Drug Metab. 2011, 12, 594–598. [Google Scholar] [CrossRef]
- Bloem, K.; van Leeuwen, A.; Verbeek, G.; Nurmohamed, M.T.; Wolbink, G.J.; van der Kleij, D.; Rispens, T. Systematic comparison of drug-tolerant assays for anti-drug antibodies in a cohort of adalimumab-treated rheumatoid arthritis patients. J. Immunol. Methods 2015, 418, 29–38. [Google Scholar] [CrossRef]
- Hart, M.H.; de Vrieze, H.; Wouters, D.; Wolbink, G.-J.; Killestein, J.; de Groot, E.R.; Aarden, L.A.; Rispens, T. Differential effect of drug interference in immunogenicity assays. J. Immunol. Methods 2011, 372, 196–203. [Google Scholar] [CrossRef]
- Steenholdt, C.; Ainsworth, M.A.; Tovey, M.; Klausen, T.W.; Thomsen, O.; Brynskov, J.; Bendtzen, K. Comparison of Techniques for Monitoring Infliximab and Antibodies Against Infliximab in Crohn’s Disease. Ther. Drug Monit. 2013, 35, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Gils, A.; Accossato, P.; Lula, S.; Marren, A. Immunogenicity of biologics in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2018, 11, 1756283X17750355. [Google Scholar] [CrossRef] [PubMed]
- Bots, S.J.; Parker, C.E.; Brandse, J.F.; Löwenberg, M.; Feagan, B.G.; Sandborn, W.J.; Jairath, V.; D’haens, G.; Casteele, N.V. Anti-Drug Antibody Formation Against Biologic Agents in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. BioDrugs 2021, 35, 715–733. [Google Scholar] [CrossRef]
- Sazonovs, A.; Kennedy, N.A.; Moutsianas, L.; Heap, G.A.; Rice, D.L.; Reppell, M.; Bewshea, C.M.; Chanchlani, N.; Walker, G.J.; Perry, M.H.; et al. HLA-DQA1*05 Carriage Associated with Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients with Crohn’s Disease. Gastroenterology 2020, 158, 189–199. [Google Scholar] [CrossRef]
- Karmiris, K.; Paintaud, G.; Noman, M.; Magdelaine–Beuzelin, C.; Ferrante, M.; Degenne, D.; Claes, K.; Coopman, T.; Van Schuerbeek, N.; Van Assche, G.; et al. Influence of Trough Serum Levels and Immunogenicity on Long-term Outcome of Adalimumab Therapy in Crohn’s Disease. Gastroenterology 2009, 137, 1628–1640. [Google Scholar] [CrossRef]
- Baert, F.; Noman, M.; Vermeire, S.; Van Assche, G.; Haens, G.D.; Carbonez, A.; Rutgeerts, P. Influence of Immunogenicity on the Long-Term Efficacy of Infliximab in Crohn’s Disease. N. Engl. J. Med. 2003, 348, 601–608. [Google Scholar] [CrossRef] [PubMed]
- 43. Casanova, M.J.; Chaparro, M.; Garcia-Sanchez, V.; Nantes, O.; Leo, E.; Rojas-Feria, M.; Jauregui-Amezaga, A.; García-López, S.; Huguet, J.M.; Arguelles-Arias, F.; et al. Evolution After Anti-TNF Discontinuation in Patients with Inflammatory Bowel Disease: A Multicenter Long-Term Follow-Up Study. Am. J. Gastroenterol. 2017, 112, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Boyapati, R.K.; Kennedy, N.A.; Louis, E.; Colombel, J.-F.; Satsangi, J. Systematic Review of Effects of Withdrawal of Immunomodulators or Biologic Agents from Patients with Inflammatory Bowel Disease. Gastroenterology 2015, 149, 1716–1730. [Google Scholar] [CrossRef]
- Farrell, R.J.; Alsahli, M.; Jeen, Y.-T.; Falchuk, K.R.; Peppercorn, M.A.; Michetti, P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: A randomized controlled trial. Gastroenterology 2003, 124, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Casteele, N.V.; Ferrante, M.; Van Assche, G.; Ballet, V.; Compernolle, G.; Van Steen, K.; Simoens, S.; Rutgeerts, P.; Gils, A.; Vermeire, S. Trough Concentrations of Infliximab Guide Dosing for Patients with Inflammatory Bowel Disease. Gastroenterology 2015, 148, 1320–1329.e3. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Wolf, D.C.; Kosutic, G.; Parker, G.; Schreiber, S.; Lee, S.D.; Abraham, B.; Afazali, A.; Arsenescu, R.I.; Gutierrez, A. Effects of Transient and Persistent Anti-drug Antibodies to Certolizumab Pegol: Longitudinal Data from a 7-Year Study in Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 1047–1056. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mould, D.; Smeekes, O.; Ashruf, Y.; Kuin, S.; Strik, A.; Brink, G.R.v.D.; Dʼhaens, G.R. A Real-life Population Pharmacokinetic Study Reveals Factors Associated with Clearance and Immunogenicity of Infliximab in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 650–660. [Google Scholar] [CrossRef]
- Papamichael, K.; Chachu, K.A.; Vajravelu, R.K.; Vaughn, B.P.; Ni, J.; Osterman, M.T.; Cheifetz, A.S. Improved Long-term Outcomes of Patients with Inflammatory Bowel Disease Receiving Proactive Compared with Reactive Monitoring of Serum Concentrations of Infliximab. Clin. Gastroenterol. Hepatol. 2017, 15, 1580–1588.e3. [Google Scholar] [CrossRef]
- Roblin, X.; Verot, C.; Paul, S.; Duru, G.; Williet, N.; Boschetti, G.; Del Tedesco, E.; Peyrin-Biroulet, L.; Phelip, J.M.; Nancey, S.; et al. Is the Pharmacokinetic Profile of a First Anti-TNF Predictive of the Clinical Outcome and Pharmacokinetics of a Second Anti-TNF? Inflamm. Bowel Dis. 2018, 24, 2078–2085. [Google Scholar] [CrossRef]
- Bartelds, G.M.; Wijbrandts, C.A.; Nurmohamed, M.T.; Stapel, S.; Lems, W.F.; Aarden, L.; Dijkmans, B.A.C.; Tak, P.P.; Wolbink, G.J. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: A cohort study. Ann. Rheum. Dis. 2009, 69, 817–821. [Google Scholar] [CrossRef]
- Steenholdt, C.; Brynskov, J.; Thomsen, O.Ø.; Munck, L.K.; Fallingborg, J.; Christensen, L.A.; Ainsworth, M.A. Individualised therapy is more cost-effective than dose intensification in patients with ’rohn’s disease who lose response to anti-TNF treatmen ptimizatimised, controlled trial. Gut 2014, 63, 919–927. [Google Scholar] [CrossRef]
- van Schie, K.A.; Hart, M.H.; de Groot, E.R.; Kruithof, S.; Aarden, L.A.; Wolbink, G.J.; Rispens, T. The antibody response against human and chimeric anti-TNF therapeutic antibodies primarily targets the TNF binding region. Ann. Rheum. Dis. 2015, 74, 311–314. [Google Scholar] [CrossRef]
- Van der Laken, C.J.; Voskuyl, A.E.; Roos, J.C.; Van Walsum, M.S.; De Groot, E.R.; Wolbink, G.; Aarden, L.A. Imaging and serum analysis of immune complex formation of radiolabelled infliximab and anti-infliximab in responders and non-responders to therapy for rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 253–256. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Gils, A.; Singh, S.; Ohrmund, L.; Hauenstein, S.; Rutgeerts, P.; Vermeire, S. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am. J. Gastroenterol. 2013, 108, 962–971. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Cuypers, L.; Singh, S.; Hauensteins, S. Transient versus sustained antibodies to infliximab: Possibility to overcome low titer antibody responses by ptimizationation. J. Crohns Colitis 2012, 6, S110. [Google Scholar] [CrossRef]
- Casteele, N.V.; Cuypers, L.; Singh, S.; Ohrmund, L.; Hauenstein, S.; Van Assche, G.; Rutgeerts, P.J.; Gils, A.; Vermeire, S. 563 Antibodies to Infliximab Can Either Be Persistent or Transient: A Retrospective Case-Control Study in IBD Patients Treated with Infliximab Maintenance Therapy. Gastroenterology 2012, 142, S-114. [Google Scholar] [CrossRef]
- Ungar, B.; Chowers, Y.; Yavzori, M.; Picard, O.; Fudim, E.; Har-Noy, O.; Ben-Horin, S. The temporal evolution of anti-drug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014, 63, 1258–1264. [Google Scholar] [CrossRef]
- Roblin, X.; Marotte, H.; Leclerc, M.; Del Tedesco, E.; Phelip, J.; Peyrin-Biroulet, L.; Paul, S. Combination of C-reactive Protein, Infliximab Trough Levels, and Stable but Not Transient Antibodies to Infliximab Are Associated with Loss of Response to Infliximab in Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 9, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Marín, A.C.; McNicholl, A.G.; Chaparro, M. Systematic review with meta-analysis: The efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment. Pharmacol. Ther. 2015, 41, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Attauabi, M.; Madsen, G.R.; Bendtsen, F.; Seidelin, J.B.; Burisch, J. Vedolizumab as the first line of biologic therapy for ulcerative colitis and Crohn’s disease—A systematic review with meta-analysis. Dig. Liver Dis. 2022, 54, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V.; Sankoh, S.; Fox, I.; et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef]
- Gubatan, J.; Keyashian, K.; Rubin, S.J.; Wang, J.; Buckman, C.; Sinha, S. Anti-Integrins for the Treatment of Inflammatory Bowel Disease: Current Evidence and Perspectives. Clin. Exp. Gastroenterol. 2021, 14, 333–342. [Google Scholar] [CrossRef]
- Amiot, A.; Grimaud, J.-C.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. Effectiveness and Safety of Vedolizumab Induction Therapy for Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 1593–1601.e2. [Google Scholar] [CrossRef]
- Amiot, A.; Serrero, M.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: A prospective multicentre cohort study. Aliment. Pharmacol. Ther. 2017, 46, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Danese, S.; Argollo, M.; Pouillon, L.; Peppas, S.; Gonzalez-Lorenzo, M.; Lytras, T.; Bonovas, S. Loss of Response to Vedolizumab and Ability of Dose Intensification to Restore Response in Patients with Crohn’s Disease or Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 17, 838–846.e2. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Odorici, G.; Pacifico, A.; Morrone, A.; Conic, R.R.Z.; Davidson, T.; Watad, A.; Pigatto, P.D.M.; Colombo, D.; Malagoli, P.; et al. Secukinumab Loss of Efficacy Is Perfectly Counteracted by the Introduction of Combination Therapy (Rescue Therapy): Data from a Multicenter Real-Life Study in a Cohort of Italian Psoriatic Patients That Avoided Secukinumab Switching. Pharmaceuticals 2022, 15, 95. [Google Scholar] [CrossRef]
- Augustin, M.; Thaci, D.; Eyerich, K.; Pinter, A.; Radtke, M.; Lauffer, F.; Mrowietz, U.; Gerdes, S.; Pariser, D.; Lebwohl, M.; et al. Continued treatment with secukinumab is associated with high retention or regain of response. Br. J. Dermatol. 2020, 182, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, S.; Wu, C.; Wang, C. IL-17 inhibitor-associated inflammatory bowel disease: A study based on literature and database analysis. Front. Pharmacol. 2023, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.D.; Dyer, E.C.; Rubin, D.T. IL-23 Monoclonal Antibodies for IBD: So Many, So Different? J. Crohn’s Colitis 2022, 16 (Suppl. 2), ii42–ii53. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Sandborn, W.J.; Feagan, B.G.; Gasink, C.; Jacobstein, D.; Zou, B.; Ghosh, S. IM-UNITI: Three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J. Crohns Colitis 2019, 14, 23–32. [Google Scholar] [CrossRef]
- Hu, A.; Kotze, P.G.; Burgevin, A.; Tan, W.; Jess, A.; Li, P.-S.; Kroeker, K.; Halloran, B.; Panaccione, R.; Peyrin-Biroulet, L.; et al. Combination Therapy Does Not Improve Rate of Clinical or Endoscopic Remission in Patients with Inflammatory Bowel Diseases Treated with Vedolizumab or Ustekinumab. Clin. Gastroenterol. Hepatol. 2021, 19, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Gasink, C.; Chan, D.; Lang, Y.; Pollack, P.; Colombel, J.-F.; Wolf, D.C.; Jacobstein, D.; Johanns, J.; Szapary, P.; et al. Efficacy of Ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology 2018, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- State, M.; Negreanu, L. Defining the Failure of Medical Therapy for Inflammatory Bowel Disease in the Era of Advanced Therapies: A Systematic Review. Biomedicines 2023, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, R.; Ghosh, S.; Middleton, S.; Marquez, J.R.; Scott, B.B.; Flint, L.; Rutgeerts, P. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014, 146, 392–400.e3. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Hanauer, S.B. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004, 126, 402–413. [Google Scholar] [CrossRef]
- Caviglia, R.; Boskoski, I.; Cicala, M. Maintenance treatment with infliximab for the management of Crohn’s disease in adults. Biologics 2009, 3, 39–49. [Google Scholar]
- Takeuchi, T.; Yamamoto, K.; Yamanaka, H.; Ishiguro, N.; Tanaka, Y.; Eguchi, K.; Watanabe, A.; Origasa, H.; Kobayashi, M.; Shoji, T. Post-hoc analysis showing better clinical response with the loading dose of certolizumab pegol in Japanese patients with active rheumatoid arthritis. Mod. Rheumatol. 2016, 26, 473–480. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Waterman, M.; Kopylov, U.; Yavzori, M.; Picard, O.; Fudim, E.; Awadie, H.; Weiss, B.; Chowers, Y. Addition of an immunomodulator to infliximab therapy eliminates anti-drug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2013, 11, 444–447. [Google Scholar] [CrossRef]
- Strik, A.S.; van den Brink, G.R.; Ponsioen, C.; Mathot, R.; Lowenberg, M.; D’Haens, G.R. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 1128–1134. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Assche, G.W.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, S.; Nanda, K.S.; Moss, A.C. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease: A systematic review and meta-analysis. Inflamm. Bowel Dis. 2014, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Steenholdt, C.; Svenson, M.; Bendtzen, K.; Thomsen, O.O.; Brynskov, J.; Ainsworth, M.A. Severe infusion reactions to infliximab: Aetiology, immunogenicity and risk factors in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2011, 34, 51–58. [Google Scholar] [CrossRef]
- West, R.; Woude, C.; Hansen, B.; Felt-Bersma, R.; Tilburg, A.; Drapers, J.; Kuipers, E.J. Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn’s disease with infliximab: A double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2004, 20, 1329–1336. [Google Scholar] [CrossRef]
- Schreiber, S.L.J.; Dudkowiak, R.; Gawdis Lahat, A.; Wojnarska, B.P.A.; Horynski, M.; Farkas, K.; Kierkus, J.; Kowalski, M.; Ben-Horin, S.Y.B.D.; Lee, S.J.; et al. Noninferiority of novel subcutaneous infliximab (ct-p13) to intravenous infliximab (ct-p13) in patients with active crohn’s disease and ulcerative colitis: Week 30 results from a multicentre, randomised controlled pivotal trial. Unit Eur. Gastroenterol. J. 2019, 7, 1412. [Google Scholar]
- Adedokun, O.J.; Gunn, G.R.; Leu, J.H.; Gargano, C.; Xu, Z.; Sandborn, W.J.; Rutgeerts, P.; Shankaret, G. Immunogenicity of Golimumab and its Clinical Relevance in Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 25, 1532–1540. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Wagner, C.L.; Bala, M.; Mayer, L.; Travers, S.; Diamond, R.H.; Olson, A.; Bao, W.; Rutgeerts, P. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2004, 2, 542–553. [Google Scholar] [CrossRef]
- Kothari, M.M.; Nguyen, D.L.; Parekh, N.K. Strategies for overcoming anti-tumor necrosis factor drug antibodies in inflammatory bowel disease: Case series and review of literature. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 155–161. [Google Scholar] [CrossRef]
- Ordás, I.; Feagan, B.G.; Sandborn, W.J. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2012, 10, 1079–1087. [Google Scholar] [CrossRef]
- Moss, A.C. Approach to Treatment Failure in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2022, 18, 360–363. [Google Scholar]
- Van Stappen, T.; Vande Casteele, N.; Van Assche, G.; Ferrante, M.; Vermeire, S.; Gils, A. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: Post hoc analysis of the TAXIT trial. Gut 2018, 67, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznaric, Z.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kishalvi, K. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 562–572.e12. [Google Scholar] [CrossRef] [PubMed]
- Steenholdt, C.; Bendtzen, K.; Brynskov, J.; Thomsen, O.O.; Ainsworth, M.A. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: Post hoc analysis of a randomized controlled trial. Am. J. Gastroenterol. 2014, 109, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- García Beloso, N.; Altabás González, I.; Samartín Ucha, M.; Gayoso Rey, M.; De Castro Parga, M.L.; Salgado Barreira, Á.; Martínez-López de Castro, N. Switching between reference adalimumab and biosimilars in chronic immune-mediated inflammatory disease: A systematic literature review. Br. J. Clin. Pharmacol. 2021, 88, 1529–1550. [Google Scholar] [CrossRef]
- Albshesh, A.; Ben-Horin, S. CT-P13: A review on a biosimilar to infliximab in the treatment of inflammatory bowel disease. Expert Opin. Biol. Ther. 2019, 19, 971–978. [Google Scholar] [CrossRef]
- Tovey, M.G.; Legrand, J.; Lallemand, C. Overcoming immunogenicity associated with the use of biopharmaceuticals. Expert Rev. Clin. Pharmacol. 2011, 4, 623–631. [Google Scholar] [CrossRef]

| Aspects | Consequences | References |
|---|---|---|
| Treatment efficacy |
| Vermeire et al. [38]; Karmiris et al. [41] |
| Treatment safety |
| Vermeire et al. [38]; Bots et al. [39]; Baert et al. [42]; Casanova et al. [43]; Torres et al. [44]; Farrell et al. [45]; Vande Casteele et al. [46]; Sandborn et al. [47]; |
| Treatment pharmacokinetics |
| Vermeire et al. [38]; Brandse et al. [48]; Papamichael et al. [49]; Roblin et al. [50]; Bartelds et al. [51] |
| Timing of immunogenicity |
| Vermeire et al. [38]; |
| Pharmacoeconomics |
| Vermeire et al. [38]; Steenholdt et al. [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velikova, T.; Sekulovski, M.; Peshevska-Sekulovska, M. Immunogenicity and Loss of Effectiveness of Biologic Therapy for Inflammatory Bowel Disease Patients Due to Anti-Drug Antibody Development. Antibodies 2024, 13, 16. https://doi.org/10.3390/antib13010016
Velikova T, Sekulovski M, Peshevska-Sekulovska M. Immunogenicity and Loss of Effectiveness of Biologic Therapy for Inflammatory Bowel Disease Patients Due to Anti-Drug Antibody Development. Antibodies. 2024; 13(1):16. https://doi.org/10.3390/antib13010016
Chicago/Turabian StyleVelikova, Tsvetelina, Metodija Sekulovski, and Monika Peshevska-Sekulovska. 2024. "Immunogenicity and Loss of Effectiveness of Biologic Therapy for Inflammatory Bowel Disease Patients Due to Anti-Drug Antibody Development" Antibodies 13, no. 1: 16. https://doi.org/10.3390/antib13010016
APA StyleVelikova, T., Sekulovski, M., & Peshevska-Sekulovska, M. (2024). Immunogenicity and Loss of Effectiveness of Biologic Therapy for Inflammatory Bowel Disease Patients Due to Anti-Drug Antibody Development. Antibodies, 13(1), 16. https://doi.org/10.3390/antib13010016









