Performance Characteristics and Limitations of the Available Assays for the Detection and Quantitation of Monoclonal Free Light Chains and New Emerging Methodologies
Abstract
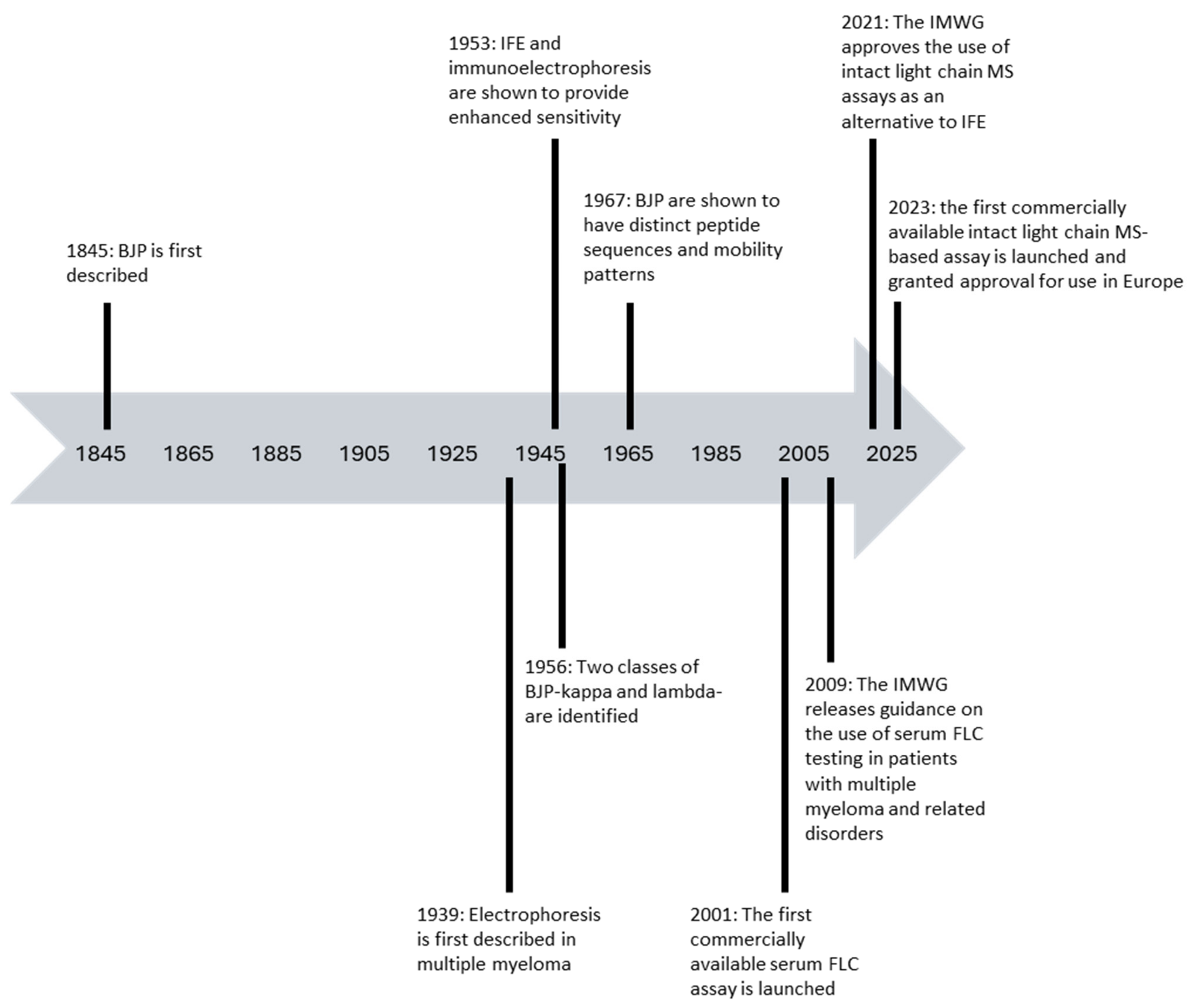
:1. Introduction
2. Laboratory Assays for the Detection and Measurement of Monoclonal FLC in Patients with Plasma Cell Dyscrasias and Their Strengths and Limitations
2.1. Urine Protein Electrophoresis and Urine Immunofixation for the Detection and Measurement of Monoclonal FLC (Bence Jones Protein) in Urine
2.2. Modifications to Enhance the Sensitivity of Serum IFE
2.3. Serum FLC Assays
The Spectrum of Commercially Available Serum FLC Assays
2.4. Mass Spectrometry-Based Assays for the Detection of Monoclonal FLC
2.5. Isoelectric Focussing
2.6. Amylite
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palladini, G.; Milani, P.; Merlini, G. Management of AL amyloidosis in 2020. Blood 2020, 136, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Sidiqi, M.H.; Aljama, M.; Kumar, S.K.; Jevremovic, D.; Buadi, F.K.; Warsame, R.; Lacy, M.Q.; Dingli, D.; Gonsalves, W.I.; Fonder, A.L.; et al. The role of bone marrow biopsy in patients with plasma cell disorders: Should all patients with a monoclonal protein be biopsied? Blood Cancer J. 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.L.; Puig, N.; Kristinsson, S.; Usmani, S.Z.; Dispenzieri, A.; Bianchi, G.; Kumar, S.; Chng, W.J.; Hajek, R.; Paiva, B.; et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: An International Myeloma Working Group Mass Spectrometry Committee Report. Blood Cancer J. 2021, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Katzmann, J.A.; Kyle, R.A.; Larson, D.R.; Melton, L.J.; Colby, C.L.; Therneau, T.M.; Clark, R.; Kumar, S.K.; Bradwell, A.; et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: A retrospective population-based cohort study. Lancet 2010, 375, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Kyle, R.; Merlini, G.; Miguel, J.S.; Ludwig, H.; Hájek, R.; Palumbo, A.; Jagannath, S.; Bladé, J.; Lonial, S.; et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia 2009, 23, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Benboubker, L.; Dimopoulos, M.A.; Dispenzieri, A.; Catalano, J.; Belch, A.R.; Cavo, M.; Pinto, A.; Weisel, K.; Ludwig, H.; Bahlis, N.; et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N. Engl. J. Med. 2014, 371, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef]
- Ravichandran, S.; Cohen, O.C.; Law, S.; Foard, D.; Fontana, M.; Martinez-Naharro, A.; Whelan, C.; Gillmore, J.D.; Lachmann, H.J.; Sachchithanantham, S.; et al. Impact of early response on outcomes in AL amyloidosis following treatment with frontline Bortezomib. Blood Cancer J. 2021, 11, 118. [Google Scholar] [CrossRef]
- Brioli, A.; Giles, H.; Pawlyn, C.; Campbell, J.P.; Kaiser, M.F.; Melchor, L.; Jackson, G.H.; Gregory, W.M.; Owen, R.G.; Child, J.A.; et al. Serum free immunoglobulin light chain evaluation as a marker of impact from intraclonal heterogeneity on myeloma outcome. Blood 2014, 123, 3414–3419. [Google Scholar] [CrossRef]
- Kühnemund, A.; Liebisch, P.; Bauchmüller, K.; zur Hausen, A.; Veelken, H.; Wäsch, R.; Engelhardt, M. ‘Light-chain escape-multiple myeloma’—An escape phenomenon from plateau phase: Report of the largest patient series using LC-monitoring. J. Cancer Res. Clin. Oncol. 2009, 135, 477–484. [Google Scholar] [CrossRef]
- Patel, U.H.; Drabick, J.J.; Malysz, J.; Talamo, G. Nonsecretory and Light Chain Escape in Patients With Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2018, 18, e515–e519. [Google Scholar] [CrossRef]
- Yadav, P.; Cockwell, P.; Cook, M.; Pinney, J.; Giles, H.; Aung, Y.S.; Cairns, D.; Owen, R.G.; Davies, F.E.; Jackson, G.H.; et al. Serum free light chain levels and renal function at diagnosis in patients with multiple myeloma. BMC Nephrol. 2018, 19, 178. [Google Scholar] [CrossRef]
- Yadav, P.; Sathick, I.J.; Leung, N.; Brown, E.E.; Cook, M.; Sanders, P.W.; Cockwell, P. Serum free light chain level at diagnosis in myeloma cast nephropathy—A multicentre study. Blood Cancer J. 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.A.; Cockwell, P.; Moroz, V.; Bradwell, A.R.; Fifer, L.; Gillmore, J.D.; Jesky, M.D.; Storr, M.; Wessels, J.; Winearls, C.G.; et al. High cutoff versus high-flux haemodialysis for myeloma cast nephropathy in patients receiving bortezomib-based chemotherapy (EuLITE): A phase 2 randomised controlled trial. Lancet Haematol. 2019, 6, e217–e228. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Palladini, G.; Minnema, M.C.; Wechalekar, A.D.; Jaccard, A.; Lee, H.C.; Sanchorawala, V.; Gibbs, S.; Mollee, P.; Venner, C.P.; et al. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N. Engl. J. Med. 2021, 385, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Rosiñol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernández, M.T.; Martínez-Martínez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2023, 390, 301–313. [Google Scholar] [CrossRef]
- Rathore, R.; Coward, R.A.; Woywodt, A. What’s in a name? Bence Jones protein. Clin. Kidney J. 2012, 5, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Korngold, L.; Lipari, R. Multiple-myeloma proteins. III. The antigenic relationship of Bence Jones proteins to normal gamma-globulin and multiple-myeloma serum proteins. Cancer 1956, 9, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Longsworth, L.G.; Shedlovsky, T.; MacInnes, D.A. Electrophoretic patterns of normal and pathological human blood serum and plasma. J. Exp. Med. 1939, 70, 399–413. [Google Scholar] [CrossRef]
- Grabar, P.; Williams, C.A. Method permitting the combined study of the electrophoretic and the immunochemical properties of protein mixtures; application to blood serum. Biochim. Biophys Acta 1953, 10, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Putnam, F.W.; Shinoda, T.; Titani, K.; Wikler, M. Immunoglobulin structure:Variation in amino acid sequence and length of human lambda light chains. Science 1967, 157, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Drayson, M.T.L.; Drew, R.; Mead, G.P.; Carr-Smith, H.; Bradwell, A.R. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory myeloma. Blood 2001, 97, 2900–2902. [Google Scholar] [CrossRef]
- Salomo, M.; Gimsing, P.; Nielsen, L.B. Simple Method for Quantification of Bence Jones Proteins. Clin. Chem. 2002, 48, 2202–2207. [Google Scholar] [CrossRef]
- Graziani, M.; Merlini, G.; Petrini, C. Guidelines for the Analysis of Bence Jones Protein. Clin. Chem. Lab. Med. 2003, 41, 338–346. [Google Scholar] [CrossRef]
- Bhole, M.V.; Sadler, R.; Ramasamy, K. Serum-free light-chain assay: Clinical utility and limitations. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2014, 51 Pt 5, 528–542. [Google Scholar] [CrossRef]
- Keren, D.F.; Alexanian, R.; A Goeken, J.; Gorevic, P.D.; A Kyle, R.; Tomar, R.H. Guidelines for clinical and laboratory evaluation patients with monoclonal gammopathies. Arch. Pathol. Lab. Med. 1999, 123, 106–107. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Comenzo, R.L.; Reece, D.; Palladini, G.; Seldin, D.; Sanchorawala, V.; Landau, H.; Falk, R.; Wells, K.; Solomon, A.; Wechalekar, A.; et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia 2012, 26, 2317–2325. [Google Scholar] [CrossRef]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.P.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Arinze, N.; Manthei, D.M.; Plapp, F.V.; Bollag, R.J. Urine Protein Immunofixation Electrophoresis: Free Light Chain Urine Immunofixation Electrophoresis Is More Sensitive than Conventional Assays for Detecting Monoclonal Light Chains and Could Serve as a Marker of Minimal Residual Disease. Lab. Med. 2023, 54, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Heaney, J.L.J.; Campbell, J.P.; Griffin, A.E.; Birtwistle, J.; Shemar, M.; Child, J.A.; Gregory, W.M.; Cairns, D.A.; Morgan, G.; Jackson, G.; et al. Diagnosis and monitoring for light chain only and oligosecretory myeloma using serum free light chain tests. Br. J. Haematol. 2017, 178, 220–230. [Google Scholar] [CrossRef]
- Vermeersch, P.; Van Hoovels, L.; Delforge, M.; Mariën, G.; Bossuyt, X. Diagnostic performance of serum free light chain measurement in patients suspected of a monoclonal B-cell disorder. Br. J. Haematol. 2008, 143, 496–502. [Google Scholar] [CrossRef]
- Singh, G.; Bollag, R. Quantification by Ultrafiltration and Immunofixation Electrophoresis Testing for Monoclonal Serum Free Light Chains. Lab. Med. 2020, 51, 592–600. [Google Scholar] [CrossRef]
- Wilhite, D.; Arfa, A.; Cotter, T.; Savage, N.M.; Bollag, R.J.; Singh, G. Multiple myeloma: Detection of free monoclonal light chains by modified immunofixation electrophoresis with antisera against free light chains. Pract. Lab. Med. 2021, 27, e00256. [Google Scholar] [CrossRef]
- Hutchison, C.A.; Plant, T.; Drayson, M.; Cockwell, P.; Kountouri, M.; Basnayake, K.; Harding, S.; Bradwell, A.R.; Mead, G. Serum free light chain measurement aids the diagnosis of myeloma in patients with severe renal failure. BMC Nephrol. 2008, 9, 11–18. [Google Scholar] [CrossRef]
- Durie, B.G.; Harousseau, J.; Miguel, J.S.; Blade, J.; Barlogie, B.; Anderson, K.; Rajkumar, S.V. International uniform response criteria for multiple myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Dispenzieri, A.; Gertz, M.A.; Kumar, S.; Wechalekar, A.; Hawkins, P.N.; Schönland, S.; Hegenbart, U.; Comenzo, R.; Kastritis, E.; et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: Impact on survival outcomes. J. Clin. Oncol. 2012, 30, 4541–4549. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Durie, B.G.; Rajkumar, S.V.; Landgren, O.; Blade, J.; Merlini, G.; Kroger, N.; Einsele, H.; Vesole, D.H.; Dimopoulos, M.; et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010, 24, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, A.; Rajkumar, S.V.; Buadi, F.K.; Binder, M.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Dingli, D.; Fonder, A.L.; Hayman, S.R.; et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Kumar, S.; Dimopoulos, M.A.; González-Calle, V.; Kastritis, E.; Hajek, R.; De Larrea, C.F.; Morgan, G.J.; Merlini, G.; Goldschmidt, H.; et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 2020, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Whitaker, B.M.; Wu, A.H.B.; Xu, H.; Bollag, R.J. Serum Free Light Chain Quantification Testing: Comparison of Two Methods for Disease Monitoring. J. Appl. Lab. Med. 2022, 7, 1290–1301. [Google Scholar] [CrossRef]
- Sarto, C.; Intra, J.; Fania, C.; Brivio, R.; Brambilla, P.; Leoni, V. Monoclonal free light chain detection and quantification: Performances and limits of available laboratory assays. Clin. Biochem. 2021, 95, 28–33. [Google Scholar] [CrossRef]
- Morales-García, L.J.; Rodríguez, R.M.L.; Pacheco-Delgado, M.S. Freelite and Kloneus assays in free light chain measurements in patients with renal impairment. Clin. Biochem. 2023, 118, 110610. [Google Scholar] [CrossRef]
- Caillon, H.; Avet-Loiseau, H.; Attal, M.; Moreau, P.; Decaux, O.; Dejoie, T. Comparison of Sebia Free Light Chain Assay With Freelite Assay for the Clinical Management of Diagnosis, Response, and Relapse Assessment in Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, e228–e237. [Google Scholar] [CrossRef]
- Bosmann, M.; Kößler, J.; Stolz, H.; Walter, U.; Knop, S.; Steigerwald, U. Detection of serum free light chains: The problem with antigen excess. Clin. Chem. Lab. Med. 2010, 48, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
- Murng, S.H.K.; Follows, L.; Whitfield, P.; Snowden, J.A.; Swallow, K.; Green, K.; Sargur, R.; Egner, W. Defining the impact of individual sample variability on routine immunoassay of serum free light chains (sFLC) in multiple myeloma. Clin. Exp. Immunol. 2013, 171, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Caponi, L.; Romiti, N.; Koni, E.; Di Fiore, A.; Paolicchi, A.; Franzini, M. Inter-assay variability in automated serum free light chain assays and their use in the clinical laboratory. Crit. Rev. Clin. Lab. Sci. 2020, 57, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Lutteri, L.; Aldenhoff, M.-C.; Cavalier, E. Evaluation of the new Sebia free light chain assay using the AP22 ELITE instrument. Clin. Chim. Acta 2018, 487, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.F.; Angelino, C.M.d.K.; Brouwers, H.M.; Croockewit, S.A.; Joosten, I.; van der Molen, R.G. Evaluation of a new free light chain ELISA assay: Bringing coherence with electrophoretic methods. Clin. Chem. Lab. Med. 2018, 56, 312–322. [Google Scholar] [CrossRef]
- Schieferdecker, A.; Hörber, S.; Ums, M.; Besemer, B.; Bokemeyer, C.; Peter, A.; Weisel, K. Comparison of three different serum-free light-chain assays—Implications on diagnostic and therapeutic monitoring of multiple myeloma. Blood Cancer J. 2020, 10, 2. [Google Scholar] [CrossRef]
- Fleming, C.K.; Swarttouw, T.; Angelino, C.M.d.K.; Jacobs, J.F.; Russcher, H. Method comparison of four clinically available assays for serum free light chain analysis. Clin. Chem. Lab. Med. 2019, 58, 85–94. [Google Scholar] [CrossRef]
- Mollee, P.; Tate, J.; Pretorius, C.J. Evaluation of the N Latex free light chain assay in the diagnosis and monitoring of AL amyloidosis. Clin. Chem. Lab. Med. 2013, 51, 2303–2310. [Google Scholar] [CrossRef]
- Abroud, H.; Beldi-Ferchiou, A.; Audard, V.; Lemonnier, F.; Le Bras, F.; Belhadj, K.; Moktefi, A.; Poullot, E.; El Karoui, K.; Dupuis, J.; et al. Evaluation of a new ELISA assay for monoclonal free-light chain detection in patients with cardiac amyloidosis. eJHaem 2022, 3, 828–837. [Google Scholar] [CrossRef]
- Cigliana, G.; Gulli, F.; Napodano, C.; Pocino, K.; De Santis, E.; Colacicco, L.; Cordone, I.; Conti, L.; Basile, U. Serum free light chain quantitative assays: Dilemma of a biomarker. J. Clin. Lab. Anal. 2018, 32, e22243. [Google Scholar] [CrossRef]
- Katzmann, J.A.; Clark, R.J.; Abraham, R.S.; Bryant, S.; Lymp, J.F.; Bradwell, A.R.; Kyle, R.A. Serum Reference Intervals and Diagnostic Ranges for Free κ and Free λ Immunoglobulin Light Chains: Relative Sensitivity for Detection of Monoclonal Light Chains. Clin. Chem. 2002, 48, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Augustijn, D.; Jacobs, J.F.M.; Russcher, H. Method comparison of three serum free light chain assays on the Roche Cobas 6000 c501 chemistry analyzer. Clin. Chem. Lab. Med. 2022, 60, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Kyle, R.A.; Therneau, T.M.; Melton, L.J., 3rd; Bradwell, A.R.; Clark, R.J.; Larson, D.R.; Plevak, M.F.; Dispenzieri, A.; Katzmann, J.A. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005, 106, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, T.; Maclachlan, K.; Korde, N.; Mailankody, S.; Lesokhin, A.; Hassoun, H.; Lu, S.X.; Patel, D.; Shah, U.; Tan, C.; et al. S177: Evaluating serum free light chain ratio as a biomarker for multiple myeloma. HemaSphere 2022, 6, 78–79. [Google Scholar] [CrossRef]
- Visram, A.; Rajkumar, S.V.; Kapoor, P.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Hayman, S.R.; Dingli, D.; Kourelis, T.; et al. Monoclonal proteinuria predicts progression risk in asymptomatic multiple myeloma with a free light chain ratio ≥100. Leukemia 2022, 36, 1429–1431. [Google Scholar] [CrossRef]
- Ludwig, H.; Kainz, S.; Schreder, M.; Zojer, N.; Hinke, A. SLiM CRAB criteria revisited: Temporal trends in prognosis of patients with smoldering multiple myeloma who meet the definition of ‘biomarker-defined early multiple myeloma’—A systematic review with meta-analysis. EClinicalMedicine 2023, 58, 101910. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Terpos, E.; Moulopoulos, L.; Spyropoulou-Vlachou, M.S.; Kanellias, N.; Eleftherakis-Papaiakovou, E.; Gkotzamanidou, M.; Migkou, M.; Gavriatopoulou, M.; Roussou, M.; et al. Extensive bone marrow infiltration and abnormal free light chain ratio identifies patients with asymptomatic myeloma at high risk for progression to symptomatic disease. Leukemia 2013, 27, 947–953. [Google Scholar] [CrossRef]
- Larsen, J.T.; Kumar, S.K.; Dispenzieri, A.; Kyle, R.A.; Katzmann, J.A.; Rajkumar, S.V. Serum free light chain ratio as a biomarker for high-risk smoldering multiple myeloma. Leukemia 2013, 27, 941–946. [Google Scholar] [CrossRef]
- Chantry, A.; Kazmi, M.; Barrington, S.; Goh, V.; Mulholland, N.; Streetly, M.; Lai, M.; Pratt, G.; Guidelines, T.B.S.F.H. Guidelines for the use of imaging in the management of patients with myeloma. Br. J. Haematol. 2017, 178, 380–393. [Google Scholar] [CrossRef]
- Molina-Andújar, A.; Robles, P.; Cibeira, M.T.; Montagud-Marrahi, E.; Guillen, E.; Xipell, M.; Blasco, M.; Poch, E.; Rosiñol, L.; Bladé, J.; et al. The renal range of the κ/λ sFLC ratio: Best strategy to evaluate multiple myeloma in patients with chronic kidney disease. BMC Nephrol. 2020, 21, 111. [Google Scholar] [CrossRef]
- Long, T.E.; Indridason, O.S.; Palsson, R.; Rognvaldsson, S.; Love, T.J.; Thorsteinsdottir, S.; Sverrisdottir, I.S.; Vidarsson, B.; Onundarson, P.T.; Agnarsson, B.A.; et al. Defining new reference intervals for serum free light chains in individuals with chronic kidney disease: Results of the iStopMM study. Blood Cancer J. 2022, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Milani, P.; Foli, A.; Basset, M.; Russo, F.; Bosoni, T.; Pirolini, L.; Valentini, V.; Ferraro, G.; Lavatelli, F.; et al. The impact of renal function on the clinical performance of FLC measurement in AL amyloidosis. Clin. Chem. Lab Med. 2016, 54, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Zeman, D.; Štork, M.; Švancarová, L.; Borský, M.; Pospíšilová, M.; Adam, Z.; Beňovská, M.; Pour, L. Isoelectric focusing followed by affinity immunoblotting to detect monoclonal free light chains in monoclonal gammopathies: Comparison with immunofixation electrophoresis and free light chain ratio. Ann. Clin. Biochem. 2023, 45632231221439. [Google Scholar] [CrossRef] [PubMed]
- Abbi, K.K.S.; Silverman, M.; Farooq, U.; Tricot, A.; Dozeman, L.; Nadiminti, K.; Krasowski, M.D.; Tricot, G.J. Potential pitfalls of serum free light chain analysis to assess treatment response for multiple myeloma. Br. J. Haematol. 2016, 174, 536–540. [Google Scholar] [CrossRef]
- de Larrea, C.F.; Cibeira, M.T.; Elena, M.; Arostegui, J.I.; Rosiñol, L.; Rovira, M.; Filella, X.; Yagüe, J.; Bladé, J. Abnormal serum free light chain ratio in patients with multiple myeloma in complete remission has strong association with the presence of oligoclonal bands: Implications for stringent complete remission definition. Blood 2009, 114, 4954–4956. [Google Scholar] [CrossRef]
- García de Veas Silva, J.L.; Bermudo Guitarte, C.; Menéndez Valladares, P.; Rojas Noboa, J.C.; Kestler, K.; Duro Millán, R. Prognostic Value of Serum Free Light Chains Measurements in Multiple Myeloma Patients. PLoS ONE 2016, 11, e0166841. [Google Scholar] [CrossRef]
- Kapoor, P.; Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Buadi, F.; Dingli, D.; Russell, S.J.; Hayman, S.R.; Witzig, T.E.; Lust, J.A.; et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J. Clin. Oncol. 2013, 31, 4529–4535. [Google Scholar] [CrossRef]
- Cedena, M.-T.; Martin-Clavero, E.; Wong, S.; Shah, N.; Bahri, N.; Alonso, R.; Barcenas, C.; Valeri, A.; Tabares, J.S.; Sanchez-Pina, J.; et al. The clinical significance of stringent complete response in multiple myeloma is surpassed by minimal residual disease measurements. PLoS ONE 2020, 15, e0237155. [Google Scholar] [CrossRef]
- Long, T.E.; Rögnvaldsson, S.; Thorsteinsdottir, S.; Sverrisdottir, I.; Eythorsson, E.; Indridason, O.; Palsson, R.; Aspelund, T.; Vidarsson, B.; Pall, T.; et al. Revised Definition of Free Light Chains in Serum and Light Chain Monoclonal Gammopathy of Undetermined Significance: Results of the Istopmm Study. Blood 2023, 142 Suppl. S1, 535. [Google Scholar] [CrossRef]
- Liang, Y.-F.; Chen, W.-M.; Wang, Q.-T.; Zhai, Y.-H.; Yang, Y.-J.; Liu, J.-Y.; Levoguer, A.; Sun, W.-H. Establishment and validation of serum free light chain reference intervals in an ethnic Chinese population. Clin. Lab. 2014, 60, 193–198. [Google Scholar] [CrossRef]
- Mohammed, N.; Chandran, P.A.; Kandregula, M.; Mattaparthi, R.D.; Gundeti, S.; Volturi, J.; Darapuneni, R.; Raju, S.B.; Dattatreya, P.S. Robust Reference Intervals for Serum Kappa and Lambda Free Light Chains from a Multi Centre Study Population from Hyderabad, India: Myeloma Diagnostic Implications. Asian. Pac. J. Cancer Prev. 2016, 17, 2605–2610. [Google Scholar] [PubMed]
- Liyasova, M.; McDonald, Z.; Taylor, P.; Gorospe, K.; Xu, X.; Yao, C.; Liu, Q.; Yang, L.; Atenafu, E.G.; Piza, G.; et al. A Personalized Mass Spectrometry–Based Assay to Monitor M-Protein in Patients with Multiple Myeloma (EasyM). Clin. Cancer Res. 2021, 27, 5028–5037. [Google Scholar] [CrossRef] [PubMed]
- Langerhorst, P.; Brinkman, A.B.; VanDuijn, M.M.; Wessels, H.J.C.T.; A Groenen, P.J.T.; Joosten, I.; van Gool, A.J.; Gloerich, J.; Scheijen, B.; Jacobs, J.F.M. Clonotypic Features of Rearranged Immunoglobulin Genes Yield Personalized Biomarkers for Minimal Residual Disease Monitoring in Multiple Myeloma. Clin. Chem. 2021, 67, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Bergen, H.R., III; Dasari, S.; Dispenzieri, A.; Mills, J.R.; Ramirez-Alvarado, M.; Tschumper, R.C.; Jelinek, D.F.; Barnidge, D.R.; Murray, D.L. Clonotypic Light Chain Peptides Identified for Monitoring Minimal Residual Disease in Multiple Myeloma without Bone Marrow Aspiration. Clin Chem. 2016, 62, 243–251. [Google Scholar] [CrossRef]
- McDonald, Z.; Taylor, P.; Liyasova, M.; Liu, Q.; Ma, B. Mass Spectrometry Provides a Highly Sensitive Noninvasive Means of Sequencing and Tracking M-Protein in the Blood of Multiple Myeloma Patients. J. Proteome Res. 2021, 20, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.O.; Huet, S.; Yi, S.S.; Ritorto, M.S.; Landgren, O.; Dogan, A.; Chapman, J.R. Mass Spectrometry–Based Method Targeting Ig Variable Regions for Assessment of Minimal Residual Disease in Multiple Myeloma. J. Mol. Diagn. 2020, 22, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.R.; Barnidge, D.R.; Dispenzieri, A.; Murray, D.L. High sensitivity blood-based M-protein detection in sCR patients with multiple myeloma. Blood Cancer J. 2017, 7, e590. [Google Scholar] [CrossRef]
- Murray, D.; Kumar, S.K.; Kyle, R.A.; Dispenzieri, A.; Dasari, S.; Larson, D.R.; Vachon, C.; Cerhan, J.R.; Rajkumar, S.V. Detection and prevalence of monoclonal gammopathy of undetermined significance: A study utilizing mass spectrometry-based monoclonal immunoglobulin rapid accurate mass measurement. Blood Cancer J. 2019, 9, 102. [Google Scholar] [CrossRef]
- Santockyte, R.; Puig, O.; Zheng, N.; Ouyang, Z.; Titsch, C.; Zhang, Y.J.; Pillutla, R.; Zeng, J. High-Throughput Therapeutic Antibody Interference-Free High-Resolution Mass Spectrometry Assay for Monitoring M-Proteins in Multiple Myeloma. Anal. Chem. 2021, 93, 834–842. [Google Scholar] [CrossRef]
- Puig, N.; Sanfeliciano, T.C.; Paiva, B.; Cedena, M.T.; Rosinol, L.; Garcia-Sanz, R.; Martínez-López, J.; Oriol, A.; Blanchard, M.J.; Rios, R.; et al. Assessment of Treatment Response By Ife, Next Generation Flow Cytometry and Mass Spectrometry Coupled with Liquid Chromatography in the GEM2012MENOS65 Clinical Trial. Blood 2021, 138 (Suppl. S1), 544. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Arendt, B.; Dasari, S.; Kohlhagen, M.; Kourelis, T.; Kumar, S.K.; Leung, N.; Muchtar, E.; Buadi, F.K.; Warsame, R.; et al. Blood mass spectrometry detects residual disease better than standard techniques in light-chain amyloidosis. Blood Cancer J. 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Giles, H.V.; Wechalekar, A.; Pratt, G. The potential role of mass spectrometry for the identification and monitoring of patients with plasma cell disorders: Where are we now and which questions remain unanswered? Br. J. Haematol. 2022, 198, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Kohlhagen, M.; Dasari, S.; Willrich, M.; Hetrick, M.; Netzel, B.; Dispenzieri, A.; Murray, D.L. Automation and validation of a MALDI-TOF MS (Mass-Fix) replacement of immunofixation electrophoresis in the clinical lab. Clin. Chem. Lab. Med. 2020, 59, 155–163. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Krishnan, A.Y.; Arendt, B.; Dasari, S.; Efebera, Y.A.; Geller, N.; Giralt, S.; Hahn, T.; Kohlhagen, M.C.; Landau, H.J.; et al. MASS-FIX versus standard methods to predict for PFS and OS among patients participating on the STAMINA trial. J. Clin. Oncol. 2021, 39, 8009. [Google Scholar] [CrossRef]
- Abeykoon, J.P.; Murray, D.L.; Murray, I.; Jevremovic, D.; Otteson, G.E.; Dispenzieri, A.; Arendt, B.K.; Dasari, S.; Gertz, M.; Gonsalves, W.I.; et al. Implications of detecting serum monoclonal protein by MASS-fix following stem cell transplantation in multiple myeloma. Br. J. Haematol. 2021, 193, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Mellors, P.W.; Dasari, S.; Kohlhagen, M.C.; Kourelis, T.; Go, R.S.; Muchtar, E.; Gertz, M.A.; Kumar, S.K.; Buadi, F.K.; Willrich, M.A.; et al. MASS-FIX for the detection of monoclonal proteins and light chain N-glycosylation in routine clinical practice: A cross-sectional study of 6315 patients. Blood Cancer J. 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Moonen, D.H.; Kohlhagen, M.; Dasari, S.; A Willrich, M.; Kourelis, T.; Dispenzieri, A.; Murray, D.L. Utilizing Mass Spectrometry to Detect and Isotype Monoclonal Proteins in Urine: Comparison to Electrophoretic Methods. Clin. Chem. 2023, 69, 746–753. [Google Scholar] [CrossRef]
- Sakrikar, D.; Marrot, N.; North, S.; McEntee, D.; Stanton, R.; Matters, D.; Ouverson, L.; Berlanga, O.; Montgomery, H.; Barnidge, D.; et al. Multi-Site Verification of the Automated EXENT MALDI-TOF-MS System and Immunoglobulin Isotypes Assay for the Identification and Quantification of Monoclonal Immunoglobulins 2021. Available online: https://meeting.myadlm.org/-/media/Files/Meetings-and-Events/Annual-Meeting/2021/AACC21_AbstractBook_Final.pdf (accessed on 1 March 2024).
- Mills, J.R.; Kohlhagen, M.C.; Dasari, S.; Vanderboom, P.M.; Kyle, R.A.; Katzmann, J.A.; Willrich, M.A.; Barnidge, D.R.; Dispenzieri, A.; Murray, D.L. Comprehensive Assessment of M-Proteins Using Nanobody Enrichment Coupled to MALDI-TOF Mass Spectrometry. Clin Chem. 2016, 62, 1334–1344. [Google Scholar] [CrossRef]
- Perry, M.C.; Kyle, R.A. The clinical significance of Bence Jones proteinuria. Mayo Clin Proc. 1975, 50, 234–238. [Google Scholar]
- Giles, H.V.; Drayson, M.T.; Wright, N.; Cook, G.; Davies, F.E.; Morgan, G.J.; de Tute, R.M.; Owen, R.G.; Cairns, D.; Hockaday, A.; et al. Residual Monoclonal Free Light Chain Positivity By Mass Spectrometry Identifies Patients at Increased Risk of Early Relapse Following First-Line Anti-Myeloma Treatment. Blood 2021, 138 (Suppl. S1), 820. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Merlini, G.; Bridoux, F.; Leung, N.; Mikhael, J.; Harrison, S.J.; Kastritis, E.; Garderet, L.; Gozzetti, A.; van de Donk, N.W.; et al. Management of multiple myeloma-related renal impairment: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2023, 24, e293–e311. [Google Scholar] [CrossRef] [PubMed]
- Giles, H.; Wright, N.; Pasha, S.; North, S.; Booth, S.; Berlanga, O. On-Bead Deglycosylation Coupled with MALDI-TOF Mass Spectrometry Provides a Simple Method for Confirming Light Chain Glycosylation and Provides a Sensitive Method for Residual Disease Detection. Available online: https://www.isaamyloidosis.org/assets/docs/ISA22_Abstract%20Book_final.pdf (accessed on 31 January 2024).
- Nevone, A.; Girelli, M.; Mangiacavalli, S.; Paiva, B.; Milani, P.; Cascino, P.; Piscitelli, M.; Speranzini, V.; Cartia, C.S.; Benvenuti, P.; et al. An N-glycosylation hotspot in immunoglobulin κ light chains is associated with AL amyloidosis. Leukemia 2022, 36, 2076–2085. [Google Scholar] [CrossRef]
- Kumar, S.; Murray, D.; Dasari, S.; Milani, P.; Barnidge, D.; Madden, B.; Kourelis, T.; Arendt, B.; Merlini, G.; Ramirez-Alvarado, M.; et al. Assay to rapidly screen for immunoglobulin light chain glycosylation: A potential path to earlier AL diagnosis for a subset of patients. Leukemia 2019, 33, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Omtvedt, L.A.; Bailey, D.; Renouf, D.V.; Davies, M.J.; Paramonov, N.A.; Haavik, S.; Husby, G.; Sletten, K.; Hounsell, E.F. Glycosylation of immunoglobulin light chains associated with amyloidosis. Amyloid 2000, 7, 227–244. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Larson, D.R.; Rajkumar, S.V.; Kyle, R.A.; Kumar, S.K.; Kourelis, T.; Arendt, B.; Willrcih, M.; Dasari, S.; Murray, D. N-glycosylation of monoclonal light chains on routine MASS-FIX testing is a risk factor for MGUS progression. Leukemia 2020, 34, 2749–2753. [Google Scholar] [CrossRef] [PubMed]
- Giles, H.V.K.B.; Drayson, M.T.; Wright, N.; Cook, G.; Kaiser, M.; de Tute, R.; Morgan, G.; Jackson, G.; Pratt, G. A Comparison of the Clinical Characteristics, Treatment Response and Survival of Patients with and without Light Chain Glycosylation on their Monoclonal Proteinin Patients Treated with Carfilzomib, Cyclophosphamide, Lenalidomide and Dexamethasone in the Myeloma XI Trial. Br. J. Haematol. 2023, 201 (Suppl. S1), 4–185. [Google Scholar]
- Giles, H.V.; Cook, M.A.; Drayson, M.T.; Cook, G.; Wright, N.J.; North, S.J.; Harding, S.; Cairns, D.A.; Hockaday, A.; Kaiser, M.F.; et al. Redefining nonmeasurable multiple myeloma using mass spectrometry. Blood 2022, 139, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, F.A.G.H.; Manwani, R.; Mahmood, S.; Sachchithanantham, S.; Lachmann, H.J.; Gillmore, J.D.; Whelan, C.J.; Hawkins, P.N.; Wechalekar, A.D. Quantitative Immunoprecipitation Free Light Chain Mass Spectrometry (QIP-FLC-MS) Simplifies Monoclonal Protein Assessment and Provides Added Clinical Value in Systemic AL Amyloidosis. Blood 2019, 134 (Suppl. S1), 4375. [Google Scholar] [CrossRef]
- Spencer, A.K.T.; Yuen, F.; Giles, H.V.; Gorniak, M.; Quach, H.; Horvath, N.; Kerridge, I.H.; Lee, E.S.-H.; Bergin, K.; Sridesai, S.; et al. A Longitudinal Evaluation of Euroflow and Combined Quantitative Immunoprecipitation (QIP) and Free Light Chain (FLC) Mass Spectometry (MS) in Functional High Risk Multiple Myeloma. Blood 2019, 134 (Suppl. S1), 3090. [Google Scholar] [CrossRef]
- Puig, N.; Contreras, M.-T.; Agulló, C.; Martínez-López, J.; Oriol, A.; Blanchard, M.-J.; Ríos, R.; Martín, J.; Iñigo, M.-B.; Sureda, A.; et al. Mass spectrometry vs immunofixation for treatment monitoring in multiple myeloma. Blood Adv. 2022, 6, 3234–3239. [Google Scholar] [CrossRef]
- Puíg, N.; Contreras, T.; Paiva, B.; Cedena, M.T.; Martinez-Lopez, J.; Oriol, A.; Gutiérrez, N.; Ríos-Tamayo, R.; Rosiñol, L.; Calasanz, M.J.; et al. Analysis of treatment efficacy in the GEM-CESAR trial for high-risk smoldering multiple myeloma patients: Comparison between the standard and IMWG MRD criteria and QIP-MS including FLC (QIP-FLC-MS). J. Clin. Oncol. 2020, 38 (Suppl. S15), 8512. [Google Scholar] [CrossRef]
- Mai, E.K.; Huhn, S.; Miah, K.; Poos, A.M.; Scheid, C.; Weisel, K.C.; Bertsch, U.; Munder, M.; Berlanga, O.; Hose, D.; et al. Implications and prognostic impact of mass spectrometry in patients with newly-diagnosed multiple myeloma. Blood Cancer J. 2023, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, F.A.; Manwani, R.; Mahmood, S.; Sachchithanantham, S.; Lachmann, H.J.; Gillmore, J.D.; Whelan, C.J.; Fontana, M.; Hawkins, P.N.; Wechalekar, A.D. A novel mass spectrometry method to identify the serum monoclonal light chain component in systemic light chain amyloidosis. Blood Cancer J. 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Bomsztyk, J.; Ravichandran, S.; Giles, H.V.; Wright, N.J.; Berlanga, O.; Khwaja, J.; Mahmood, S.; Wisniowski, B.; Cohen, O.C.; Foard, D.; et al. Complete Responses in AL Amyloidosis Are Unequal—The Impact of Free Light Chain Mass Spectrometry in AL Amyloidosis. Blood 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.; Parrott, D.M.; Stott, D.I. Quantitation of monoclonal immunoglobulins by immuno-isoelectric focusing and its application for monitoring secretory B cell neoplasia. J. Immunol. Methods 1986, 90, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.; Kumararatne, D.S.; Stott, D. Detection and identification of serum monoclonal immunoglobulin by immunoisoelectric focusing. Limits of sensitivity and use during relapse of multiple myeloma. J. Clin. Pathol. 1984, 37, 255–262. [Google Scholar] [CrossRef]
- Norden, A.G.; Fulcher, L.M.; Flynn, F.V. Detection of Bence-Jones protein by isoelectric focussing of unconcentrated urine followed by nitrocellulose blotting and immunoperoxidase staining. Clin. Chim. Acta 1985, 153, 149–156. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Li, Y.C.; Jackman, A.B.; Flaherty, H.; Rogers, I.; Muchtar, E.; Dispenzieri, A.; Spencer, B.H.; Sanchorawala, V.; et al. Blood-Based Diagnostic Assay for λ Light Chain Amyloidosis through Quantification of an Amyloidogenicity-Indicating Neo-Epitope. Blood 2023, 142 (Suppl. S1), 536. [Google Scholar] [CrossRef]
- Dejoie, T.; Attal, M.; Moreau, P.; Harousseau, J.L.; Avet-Loiseau, H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica 2016, 101, 356–362. [Google Scholar] [CrossRef]
- Migkou, M.; Avivi, I.; Gavriatopoulou, M.; Cohen, Y.C.; Fotiou, D.; Kanellias, N.; Ziogas, D.; Eleutherakis-Papaiakovou, E.; Terpos, E.; Roussou, M.; et al. Clinical characteristics and outcomes of oligosecretory and non-secretory multiple myeloma. Ann. Hematol. 2020, 99, 1251–1255. [Google Scholar] [CrossRef]
- Chawla, S.S.; Kumar, S.K.; Dispenzieri, A.; Greenberg, A.J.; Larson, D.R.; Kyle, R.A.; Lacy, M.Q.; Gertz, M.A.; Rajkumar, S.V. Clinical course and prognosis of non-secretory multiple myeloma. Eur. J. Haematol. 2015, 95, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Rosenberg, A.; Mendelson, L.M.; Comenzo, R.L.; Varga, C.; Sanchorawala, V. Outcomes of patients with AL amyloidosis and low serum free light chain levels at diagnosis. Amyloid 2018, 25, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, J.; Paiva, B.; López-Anglada, L.; Mateos, M.V.; Cedena, T.; Vidríales, M.B.; Sáez-Gómez, M.A.; Contreras, T.; Oriol, A.; Rapado, I.; et al. Critical analysis of the stringent complete response in multiple myeloma: Contribution of sFLC and bone marrow clonality. Blood 2015, 126, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Ubieto, A.J.; Paiva, B.; Puig, N.; Cedena, M.T.; Martinez-Lopez, J.; Oriol, A.; Blanchard, M.J.; Tamayo, R.R.; Sánchez, J.M.; Martinez, R.; et al. Validation of the IMWG standard response criteria in the PETHEMA/GEM2012MENOS65 study: Are these times of change? Blood 2021, 138, 1901–1905. [Google Scholar] [CrossRef]


| Assay Name | Antisera | Testing Methodology | FLC Reference Ranges (mg/L) | FLC Ratio Reference Range |
|---|---|---|---|---|
| Freelite [61,62] | Polyclonal | Turbidimetry/ nephelometry | Kappa 3.3–19.4 | 0.26–1.65 |
| Lambda 5.7–26.3 | ||||
| Sebia FLC [55] | Polyclonal | ELISA | Kappa 6.4–17.4 | 0.46–1.51 |
| Lambda 8.4–21.8 | ||||
| Diazyme [47,62] | Polyclonal | Turbidimetry | Kappa 2.37–20.73 | 0.22–1.74 |
| Lambda 4.23–27.69 | ||||
| Kloneus Free Light Chain [62] | Polyclonal | Turbidimetry/nephelometry | Kappa 3.3–19.4 | 0.26–1.65 |
| Lambda 5.7–26.3 | ||||
| Seralite [57] | Monoclonal | Competitive inhibition | Kappa 5.3–22.7 | 0.5–2.5 |
| immunochromatography | Lambda 4.0–25.1 | |||
| N Latex FLC [57] | Monoclonal | Nephelometry | Kappa 6.7–22.4 | 0.31–1.56 |
| Lambda 8.3–27.0 |
| Method | Approved Applications | Advantages | Disadvantages |
|---|---|---|---|
| SPE and sIFE | Monitoring patients with MGUS, multiple myeloma, and AL amyloidosis [31,33] | Widely available | Low sensitivity compared to FLC-specific assays [26] |
| Relatively low cost | Not used for quantitative assessment of FLC monoclonal proteins | ||
| uPE and uIFE | Response assessment in patients with multiple myeloma and AL amyloidosis [31,33] | Widely available | False positives for BJP in patients with chronic kidney disease [100] |
| ≥500 mg/24 h BJP differentiates myeloma from MGUS in the IMWG diagnostic criteria [31] | Relatively low cost | Requires a 24 h urine collection | |
| Serum FLC assays | Response assessment in patients with multiple myeloma and AL amyloidosis [5,31,33] | Widely available | No international consensus on the most appropriate reference range to use in patients with varying degrees of renal impairment |
| Risk stratification of patients with MGUS, SMM and AL amyloidosis [43,44,45] | Provides additional sensitivity for the detection of low-level monoclonal FLC in patients with FLC myeloma and AL amyloidosis [5,26,36] | Rely on the ratio between the uninvolved and involved FLC as an indirect indicator of clonality, which can lead to false positives in the presence of oligoclonal immune reconstitution and/or treatment related immune suppression [101] | |
| The level of the involved FLC helps identify patients with myeloma and renal impairment who are likely to have renal impairment due to cast nephropathy [12,13,102] | Automated sample processing and result generation | ||
| FLC ratio ≥100 is incorporated into the SLiM CRAB criteria for the identification of patients with symptomatic multiple myeloma [30] | Convenient -analysis can be performed on the same sample used for SPE and sIFE | ||
| Intact light chain MS assays | In lieu of immunofixation in patients with multiple myeloma and related disorders [3] | Greater sensitivity compared to sIFE [91,94,103] | More expensive than the electrophoretic assays and serum FLC assays [92] |
| Tracking the monoclonal protein using its isotype and mass-to-charge ratio enables more reliable differentiation between oligoclonal peaks and residual low-level monoclonal protein monoclonal protein | The availability of the intact light chain assays is limited: EXENT is currently only approved for use in Europe; MASS-FIX is only available for use in Europe; and FLC-MS is not currently approved for clinical use | ||
| High sample throughput possible due to automated sample processing and semi- or fully automated result interpretation depending on the assay used [93] | Risk of missing low-level FLC-only monoclonal protein if sFLC and uIFE are not used alongside MS assays that only include total light chain-specific reagents [96] | ||
| Able to identify post-translational modifications such as N-linked glycosylation [92,96,103] | MASS-FIX and EXENT only provide a qualitative assessment about the presence absence of monoclonal light chains (FLC-MS could provide quantitative assessments but is currently only a research tool) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giles, H.V.; Karunanithi, K. Performance Characteristics and Limitations of the Available Assays for the Detection and Quantitation of Monoclonal Free Light Chains and New Emerging Methodologies. Antibodies 2024, 13, 19. https://doi.org/10.3390/antib13010019
Giles HV, Karunanithi K. Performance Characteristics and Limitations of the Available Assays for the Detection and Quantitation of Monoclonal Free Light Chains and New Emerging Methodologies. Antibodies. 2024; 13(1):19. https://doi.org/10.3390/antib13010019
Chicago/Turabian StyleGiles, Hannah V., and Kamaraj Karunanithi. 2024. "Performance Characteristics and Limitations of the Available Assays for the Detection and Quantitation of Monoclonal Free Light Chains and New Emerging Methodologies" Antibodies 13, no. 1: 19. https://doi.org/10.3390/antib13010019
APA StyleGiles, H. V., & Karunanithi, K. (2024). Performance Characteristics and Limitations of the Available Assays for the Detection and Quantitation of Monoclonal Free Light Chains and New Emerging Methodologies. Antibodies, 13(1), 19. https://doi.org/10.3390/antib13010019







