Efficient Expression of Functionally Active Aflibercept with Designed N-glycans
Abstract
1. Introduction
2. Methods
2.1. Generation of Aflibercept Expression Vector
2.2. In Planta Expression and Purification of Aflibercept
2.3. N-Glycan Analyses
2.4. ELISA
3. Results
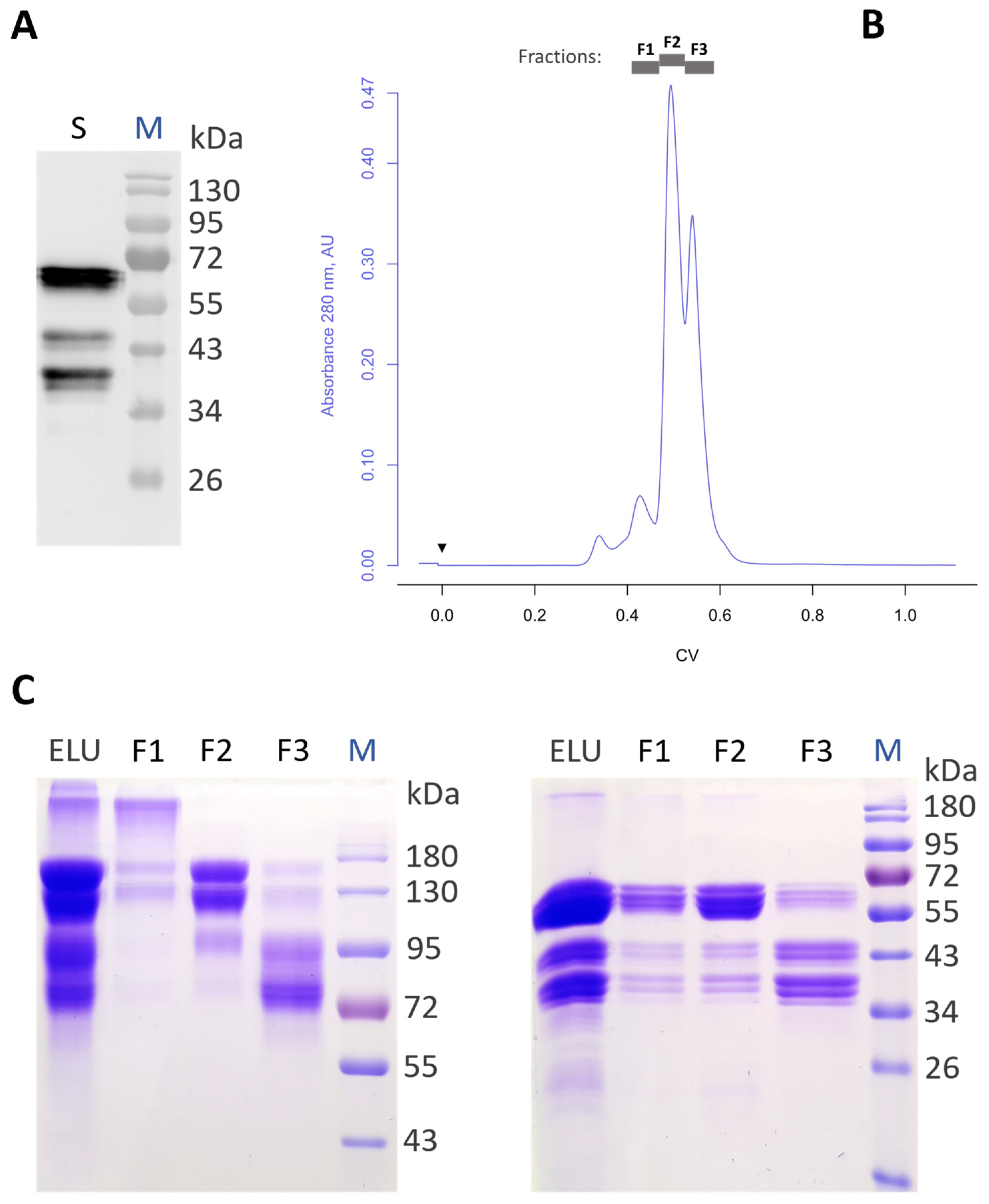
3.1. Production of Recombinant Aflibercept in Glycoengineered N. benthamiana
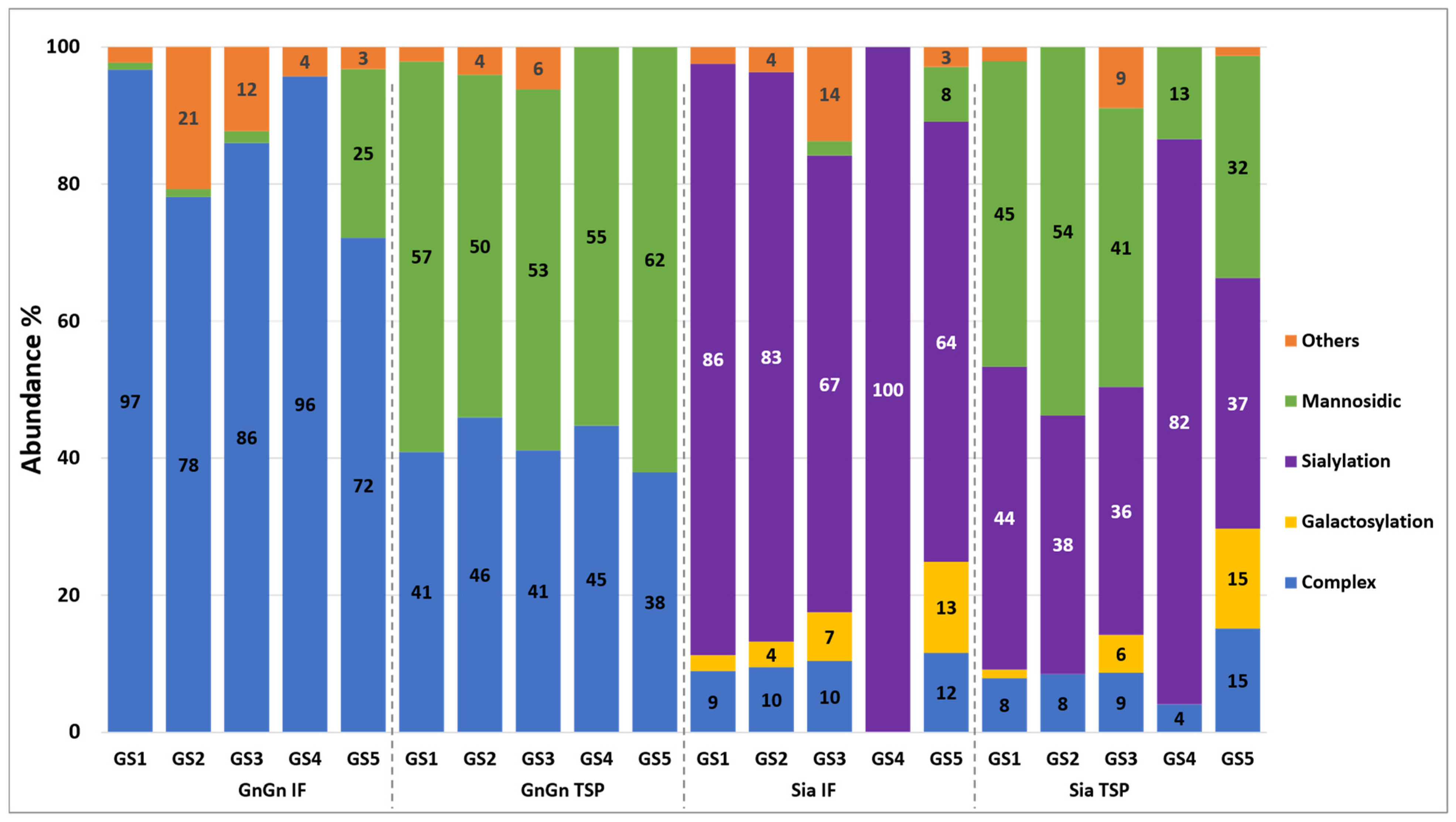
3.2. Generation of Aflibercept with Different N-Glycosylation Profiles
3.3. VEGF Binding Activity of Aflibercept Glycoforms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strohl, W.R. Current progress in innovative engineered antibodies. Protein Cell 2018, 9, 86–120. [Google Scholar] [CrossRef]
- de Oliveira Dias, J.R.; de Andrade, G.C.; Novais, E.A.; Farah, M.E.; Rodrigues, E.B. Fusion proteins for treatment of retinal diseases: Aflibercept, ziv-aflibercept, and conbercept. Int. J. Retin. Vitr. 2016, 2, 3. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Bressler, N.M.; Bressler, S.B.; Brucker, A.J.; Ferris, F.L.; Hampton, G.R.; Jhaveri, C.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology 2016, 123, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y.; McKeage, K. Aflibercept: A Review in Metastatic Colorectal Cancer. Drugs 2015, 75, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; McKeage, K. Intravitreal aflibercept (Eylea®): A review of its use in patients with macular oedema secondary to central retinal vein occlusion. Drugs Aging 2014, 31, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ciombor, K.K.; Berlin, J. Aflibercept—A decoy VEGF receptor. Curr. Oncol. Rep. 2014, 16, 368. [Google Scholar] [CrossRef] [PubMed]
- HodjatJalali, K.; Mehravaran, S.; Faghihi, H.; Hashemi, H.; Kazemi, P.; Rastad, H. Intravitreal injection of ziv-aflibercept in the treatment of choroidal and retinal vascular diseases. J. Curr. Ophthalmol. 2017, 29, 228–231. [Google Scholar] [CrossRef]
- Ge, P.; Wan, N.; Han, X.; Wang, X.; Zhang, J.; Long, X.; Wang, X.; Bian, Y. Efficacy, safety, and cost-effectiveness analysis of aflibercept in metastatic colorectal cancer: A rapid health technology assessment. Front. Pharmacol. 2022, 13, 914683. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Xu, H.; Zhang, Q.; Sha, C.; Sun, B.; Li, Q. Analytical comparability assessment on glycosylation of ziv-aflibercept and the biosimilar candidate. Int. J. Biol. Macromol. 2021, 180, 494–509. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, J.W.; Hwang, S.; Hahm, S.H.; Ahn, Y.H. Site-Specific Glycan Microheterogeneity Evaluation of Aflibercept Fusion Protein by Glycopeptide-Based LC-MSMS Mapping. Int. J. Mol. Sci. 2022, 23, 11807. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, S.; Halim, A.; Schulz, M.A.; Frodin, M.; Rahman, S.H.; Vester-Christensen, M.B.; Behrens, C.; Kristensen, C.; Vakhrushev, S.Y.; et al. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat. Biotechnol. 2015, 33, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Štor, J.; Ruckerbauer, D.E.; Széliová, D.; Zanghellini, J.; Borth, N. Towards rational glyco-engineering in CHO: From data to predictive models. Curr. Opin. Biotechnol. 2021, 71, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lobato Gómez, M.; Huang, X.; Alvarez, D.; He, W.; Baysal, C.; Zhu, C.; Armario-Najera, V.; Blanco Perera, A.; Cerda Bennasser, P.; Saba-Mayoral, A.; et al. Contributions of the international plant science community to the fight against human infectious diseases—Part 1: Epidemic and pandemic diseases. Plant Biotechnol. J. 2021, 19, 1901–1920. [Google Scholar] [CrossRef] [PubMed]
- Nosaki, S.; Hoshikawa, K.; Ezura, H.; Miura, K. Transient protein expression systems in plants and their applications. Plant Biotechnol. 2021, 38, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Montero-Morales, L.; Steinkellner, H. Advanced plant-based glycan engineering. Front. Bioeng. Biotechnol. 2018, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Jansing, J.; Sack, M.; Augustine, S.M.; Fischer, R.; Bortesi, L. CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking β-1,2-xylose and core α-1,3-fucose. Plant Biotechnol. J. 2019, 17, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Stadlmann, J.; Schähs, M.; Stiegler, G.; Quendler, H.; Mach, L.; Glössl, J.; Weterings, K.; Pabst, M.; Steinkellner, H. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 2008, 6, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.L.; Parsons, J.; Reski, R. Glyco-engineering for biopharmaceutical production in moss bioreactors. Front. Plant Sci. 2014, 5, 346. [Google Scholar] [CrossRef]
- Kallolimath, S.; Castilho, A.; Strasser, R.; Grunwald-Gruber, C.; Altmann, F.; Strubl, S.; Galuska, C.E.; Zlatina, K.; Galuska, S.P.; Werner, S.; et al. Engineering of complex protein sialylation in plants. Proc. Natl. Acad. Sci. USA 2016, 113, 9498–9503. [Google Scholar] [CrossRef]
- Castilho, A.; Strasser, R.; Stadlmann, J.; Grass, J.; Jez, J.; Gattinger, P.; Kunert, R.; Quendler, H.; Pabst, M.; Leonard, R.; et al. In planta protein sialylation through overexpression of the respective mammalian pathway. J. Biol. Chem. 2010, 285, 15923–15930. [Google Scholar] [CrossRef] [PubMed]
- Kallolimath, S.; Hackl, T.; Gahn, R.; Grünwald-Gruber, C.; Zich, W.; Kogelmann, B.; Lux, A.; Nimmerjahn, F.; Steinkellner, H. Expression Profiling and Glycan Engineering of IgG Subclass 1-4 in Nicotiana benthamiana. Front. Bioeng. Biotechnol. 2020, 8, 825. [Google Scholar] [CrossRef] [PubMed]
- Marillonnet, S.; Thoeringer, C.; Kandzia, R.; Klimyuk, V.; Gleba, Y. Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat. Biotechnol. 2005, 23, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Kogelmann, B.; Melnik, S.; Bogner, M.; Kallolimath, S.; Stoger, E.; Sun, L.; Strasser, R.; D’Aoust, M.A.; Lavoie, P.O.; Saxena, P.; et al. A genome-edited N. benthamiana line for industrial-scale production of recombinant glycoproteins with targeted N-glycosylation. Biotechnol. J. 2023, 19, e2300323. [Google Scholar] [CrossRef] [PubMed]
- Kallolimath, S.; Sun, L.; Palt, R.; Stiasny, K.; Mayrhofer, P.; Gruber, C.; Kogelmann, B.; Chen, Q.; Steinkellner, H. Highly active engineered IgG3 antibodies against SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021, 118, e2107249118. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Kallolimath, S.; Palt, R.; Stiasny, K.; Mayrhofer, P.; Maresch, D.; Eidenberger, L.; Steinkellner, H. Increased in vitro neutralizing activity of SARS-CoV-2 IgA1 dimers compared to monomers and IgG. Proc. Natl. Acad. Sci. USA 2021, 118, e2107148118. [Google Scholar] [CrossRef]
- Consortium for Functional Glycomics. Available online: http://www.functionalglycomics.org (accessed on 13 December 2023).
- Proglycan. Available online: https://homepage.boku.ac.at/jstadlmann/Proglycan_nomenclature_2023.pdf (accessed on 13 December 2023).
- Gattinger, P.; Izadi, S.; Grünwald-Gruber, C.; Kallolimath, S.; Castilho, A. The Instability of Dimeric Fc-Fusions Expressed in Plants Can Be Solved by Monomeric Fc Technology. Front. Plant Sci. 2021, 12, 671728. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Hirano, H.; Lane, N.E.; Nandi, S.; McDonald, K.A. Plant-based production and characterization of a promising Fc-fusion protein against microgravity-induced bone density loss. Front. Bioeng. Biotechnol. 2022, 10, 962292. [Google Scholar] [CrossRef] [PubMed]
- Jugler, C.; Grill, F.J.; Eidenberger, L.; Karr, T.L.; Grys, T.E.; Steinkellner, H.; Lake, D.F.; Chen, Q. Humanization and expression of IgG and IgM antibodies in plants as potential diagnostic reagents for Valley Fever. Front. Plant Sci. 2022, 13, 925008. [Google Scholar] [CrossRef]
- Stelter, S.; Paul, M.J.; Teh, A.Y.-H.; Grandits, M.; Altmann, F.; Vanier, J.; Bardor, M.; Castilho, A.; Allen, R.L.; Ma, J.K.-C. Engineering the interactions between a plant-produced HIV antibody and human Fc receptors. Plant Biotechnol. J. 2020, 18, 402–414. [Google Scholar] [CrossRef]
- Zeitlin, L.; Pettitt, J.; Scully, C.; Bohorova, N.; Kim, D.; Pauly, M.; Hiatt, A.; Ngo, L.; Steinkellner, H.; Whaley, K.J. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc. Natl. Acad. Sci. USA 2011, 108, 20690–20694. [Google Scholar] [CrossRef]
- Izadi, S.; Kunnummel, V.; Steinkellner, H.; Werner, S.; Castilho, A. Assessment of transient expression strategies to sialylate recombinant proteins in N. benthamiana. J. Biotechnol. 2023, 365, 48–53. [Google Scholar] [CrossRef]
- MacDonald, D.A.; Martin, J.; Muthusamy, K.K.; Luo, J.K.; Pyles, E.; Rafique, A.; Huang, T.; Potocky, T.; Liu, Y.; Cao, J.; et al. Aflibercept exhibits VEGF binding stoichiometry distinct from bevacizumab and does not support formation of immune-like complexes. Angiogenesis 2016, 19, 389–406. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef]
- Chen, Q. Development of plant-made monoclonal antibodies against viral infections. Curr. Opin. Virol. 2022, 52, 148–160. [Google Scholar] [CrossRef]
- Bendandi, M.; Marillonnet, S.; Kandzia, R.; Thieme, F.; Nickstadt, A.; Herz, S.; Fröde, R.; Inoges, S.; De Cerio, A.L.-D.; Soria, E. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Ann. Oncol. 2010, 21, 2420–2427. [Google Scholar] [CrossRef]
- Xiong, Y.; Karuppanan, K.; Bernardi, A.; Li, Q.; Kommineni, V.; Dandekar, A.M.; Lebrilla, C.B.; Faller, R.; McDonald, K.A.; Nandi, S. Effects of N-glycosylation on the structure, function, and stability of a plant-made Fc-fusion anthrax decoy protein. Front. Plant Sci. 2019, 10, 768. [Google Scholar] [CrossRef]
- Eidenberger, L.; Kogelmann, B.; Steinkellner, H. Plant-based biopharmaceutical engineering. Nat. Rev. Bioeng. 2023, 1, 426–439. [Google Scholar] [CrossRef]
- Castilho, A.; Beihammer, G.; Pfeiffer, C.; Göritzer, K.; Montero-Morales, L.; Vavra, U.; Maresch, D.; Grünwald-Gruber, C.; Altmann, F.; Steinkellner, H. An oligosaccharyltransferase from Leishmania major increases the N-glycan occupancy on recombinant glycoproteins produced in Nicotiana benthamiana. Plant Biotechnol. J. 2018, 16, 1700–1709. [Google Scholar] [CrossRef]
- Beihammer, G.; König-Beihammer, J.; Kogelmann, B.; Ruocco, V.; Grünwald-Gruber, C.; D’Aoust, M.-A.; Lavoie, P.-O.; Saxena, P.; Gach, J.S.; Steinkellner, H.; et al. An oligosaccharyltransferase from Leishmania donovani increases the N-glycan occupancy on plant-produced IgG1. Front. Plant Sci. 2023, 14, 1233666. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.; Mayer, K.L.; Verjan Garcia, N.; Guo, H.; Kajiura, H.; Fujiyama, K.; Matoba, N. Impact of glycoengineering and antidrug antibodies on the anticancer activity of a plant-made lectin-Fc fusion protein. Plant Biotechnol. J. 2022, 20, 2217–2230. [Google Scholar] [CrossRef]
- Hahm, Y.H.; Lee, J.Y.; Ahn, Y.H. Investigation of Site-Specific Differences in Glycan Microheterogeneity by N-Glycopeptide Mapping of VEGFR-IgG Fusion Protein. Molecules 2019, 24, 3924. [Google Scholar] [CrossRef]
- Liu, L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J. Pharm. Sci. 2015, 104, 1866–1884. [Google Scholar] [CrossRef]
- Ko, K.; Tekoah, Y.; Rudd, P.M.; Harvey, D.J.; Dwek, R.A.; Spitsin, S.; Hanlon, C.A.; Rupprecht, C.; Dietzschold, B.; Golovkin, M.; et al. Function and glycosylation of plant-derived antiviral monoclonal antibody. Proc. Natl. Acad. Sci. USA 2003, 100, 8013–8018. [Google Scholar] [CrossRef]
- Chandler, K.B.; Leon, D.R.; Kuang, J.; Meyer, R.D.; Rahimi, N.; Costello, C.E. N-Glycosylation regulates ligand-dependent activation and signaling of vascular endothelial growth factor receptor 2 (VEGFR2). J. Biol. Chem. 2019, 294, 13117–13130. [Google Scholar] [CrossRef]
- Castilho, A.; Schwestka, J.; Kienzl, N.F.; Vavra, U.; Grünwald-Gruber, C.; Izadi, S.; Hiremath, C.; Niederhöfer, J.; Laurent, E.; Monteil, V. Generation of enzymatically competent SARS-CoV-2 decoy receptor ACE2-Fc in glycoengineered Nicotiana benthamiana. Biotechnol. J. 2021, 16, 2000566. [Google Scholar] [CrossRef]
- Swope, K.; Morton, J.; Pogue, G.P.; Burden, L.; Partain, N.; Hume, S.; Shepherd, J.; Simpson, C.A.; Brennan, M.B.; Furman, T.C. Reproducibility and flexibility of monoclonal antibody production with Nicotiana benthamiana. MAbs 2022, 14, 2013594. [Google Scholar] [CrossRef]
- Global Aflibercept Market. Available online: https://www.databridgemarketresearch.com/reports/global-aflibercept-market (accessed on 13 December 2023).
- Hutton, D.W.; Glassman, A.R.; Liu, D.; Sun, J.K.; Network, D.R.; Sneath, M.; Chen, M.; Jelemensky, P.A.; Miller, R.; Basham, S.R. Cost-effectiveness of aflibercept monotherapy vs bevacizumab first followed by aflibercept if needed for diabetic macular edema. JAMA Ophthalmol. 2023, 141, 268–274. [Google Scholar] [CrossRef]
- Pennington, B.; Alshreef, A.; Flight, L.; Metry, A.; Poku, E.; Hykin, P.; Sivaprasad, S.; Prevost, A.T.; Vasconcelos, J.C.; Murphy, C. Cost Effectiveness of Ranibizumab vs Aflibercept vs Bevacizumab for the Treatment of Macular Oedema Due to Central Retinal Vein Occlusion: The LEAVO Study. PharmacoEconomics 2021, 39, 913–927. [Google Scholar] [CrossRef]
- McNulty, M.J.; Nandi, S.; McDonald, K.A. Technoeconomic Modeling and Simulation for Plant-Based Manufacturing of Recombinant Proteins. Methods Mol. Biol. 2022, 2480, 159–189. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshvari, T.; Melnik, S.; Sun, L.; Niazi, A.; Aram, F.; Moghadam, A.; Kogelmann, B.; Wozniak-Knopp, G.; Kallolimath, S.; Ramezani, A.; et al. Efficient Expression of Functionally Active Aflibercept with Designed N-glycans. Antibodies 2024, 13, 29. https://doi.org/10.3390/antib13020029
Keshvari T, Melnik S, Sun L, Niazi A, Aram F, Moghadam A, Kogelmann B, Wozniak-Knopp G, Kallolimath S, Ramezani A, et al. Efficient Expression of Functionally Active Aflibercept with Designed N-glycans. Antibodies. 2024; 13(2):29. https://doi.org/10.3390/antib13020029
Chicago/Turabian StyleKeshvari, Tahereh, Stanislav Melnik, Lin Sun, Ali Niazi, Farzaneh Aram, Ali Moghadam, Benjamin Kogelmann, Gordana Wozniak-Knopp, Somanath Kallolimath, Amin Ramezani, and et al. 2024. "Efficient Expression of Functionally Active Aflibercept with Designed N-glycans" Antibodies 13, no. 2: 29. https://doi.org/10.3390/antib13020029
APA StyleKeshvari, T., Melnik, S., Sun, L., Niazi, A., Aram, F., Moghadam, A., Kogelmann, B., Wozniak-Knopp, G., Kallolimath, S., Ramezani, A., & Steinkellner, H. (2024). Efficient Expression of Functionally Active Aflibercept with Designed N-glycans. Antibodies, 13(2), 29. https://doi.org/10.3390/antib13020029






