Cross-Reactivity of N6AMT1 Antibodies with Aurora Kinase A: An Example of Antibody-Specific Non-Specificity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Construction of Cell Lines
2.3. Plasmids
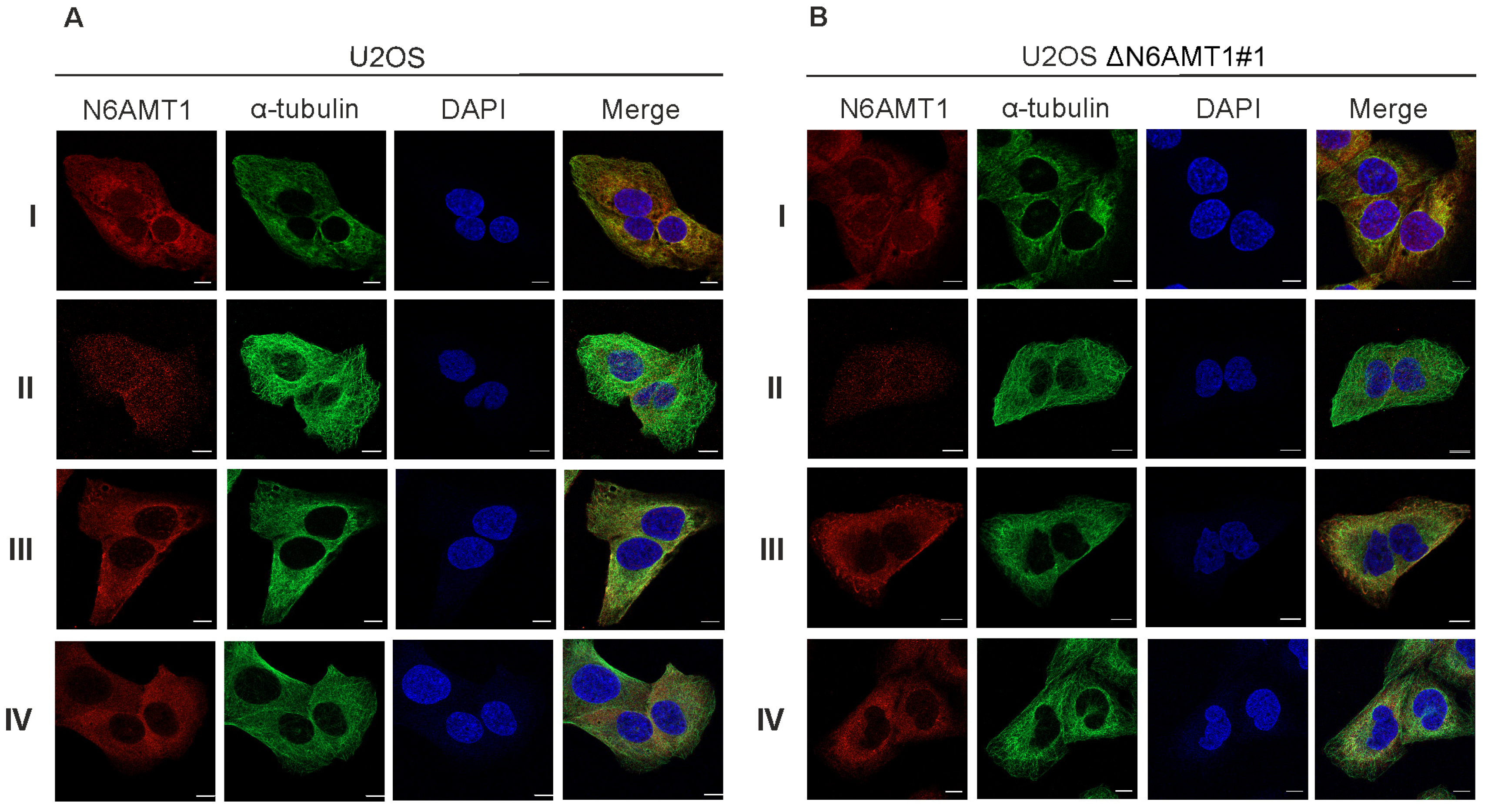
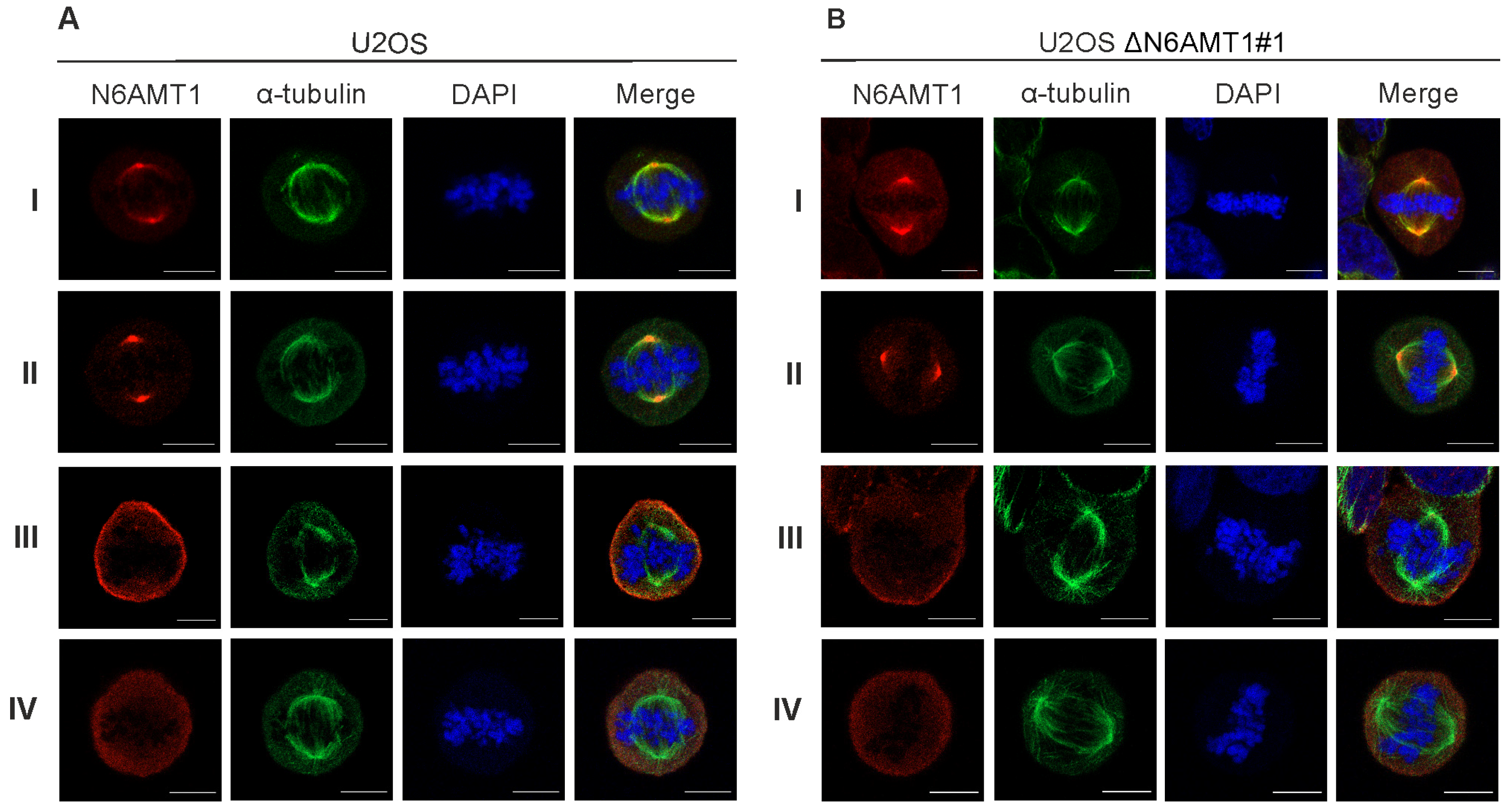
2.4. Immunofluorescence Microscopy Analysis
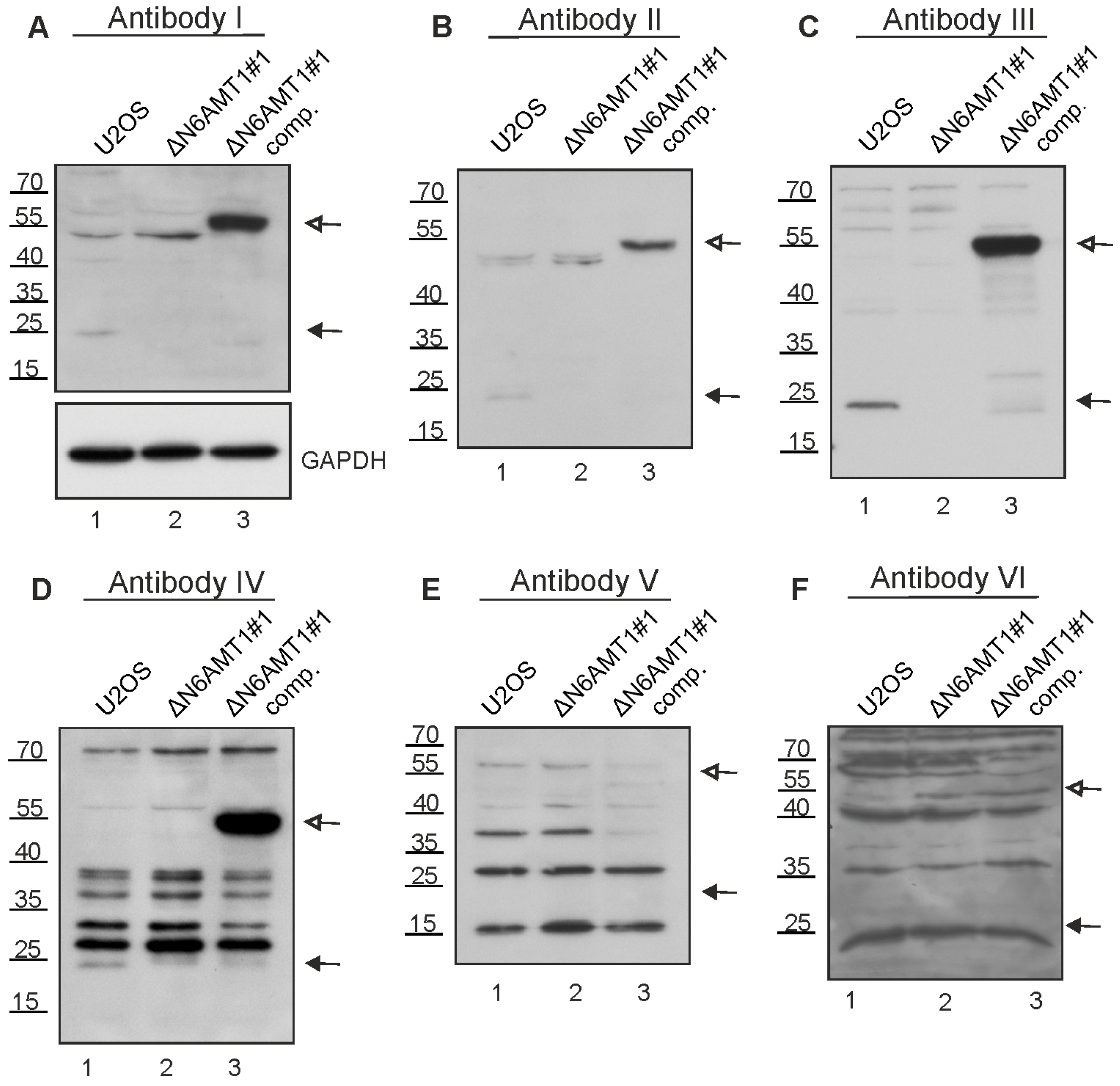
2.5. Immunoblot Analysis
2.6. Cell Cycle Analysis
2.7. Immunoprecipitation
2.8. Mass Spectrometry
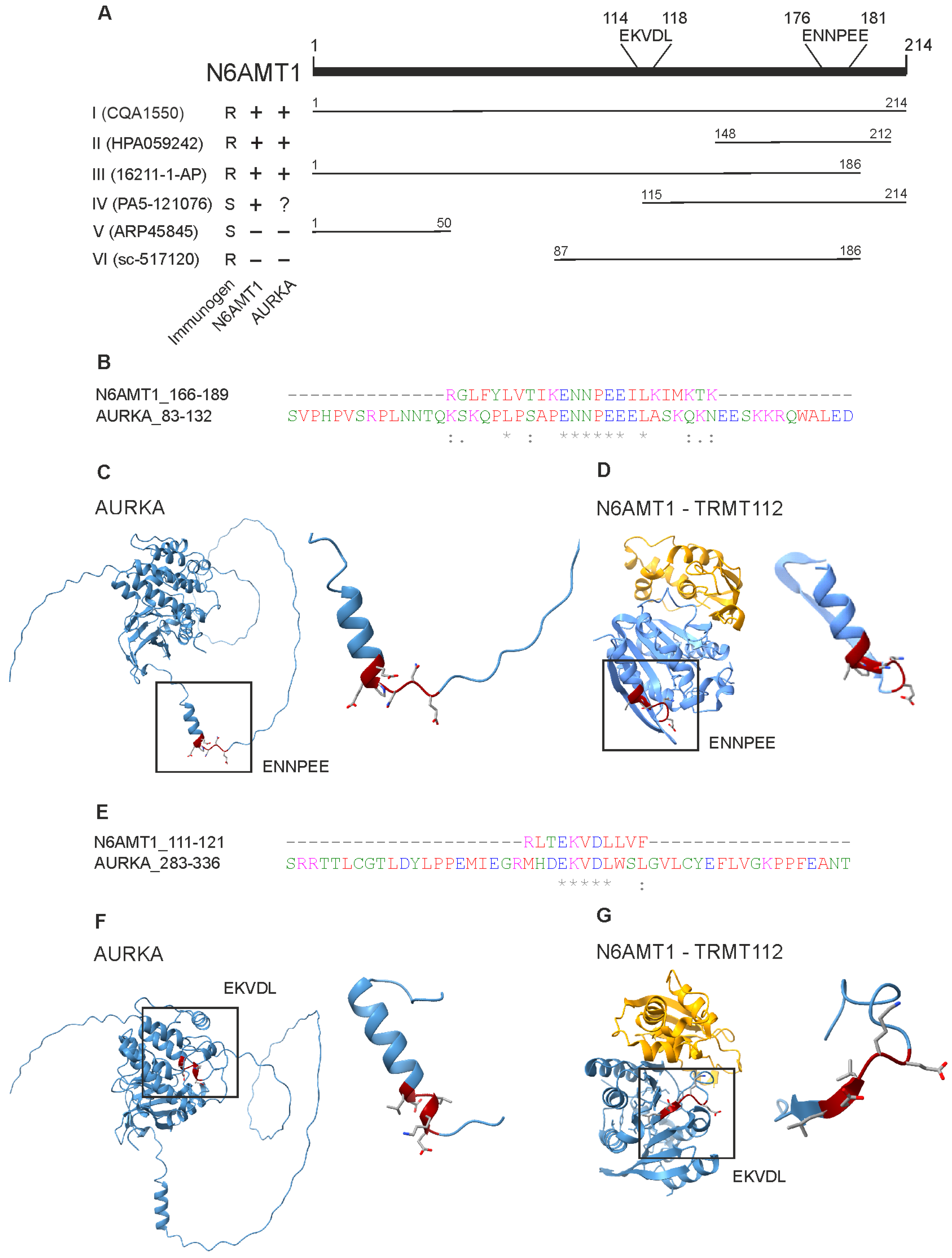
2.9. Sequence Analysis
3. Results
3.1. Antibodies against N6AMT1 Protein
3.2. N6AMT1 Antibody Recognises Protein in the Centrosomes during Mitosis
3.3. N6AMT1 Antibody Recognises Multiple Proteins in Immunoblot Analysis
3.4. N6AMT1 Antibody Recognises a Mitosis-Associated Protein in Immunoblot Analysis
3.5. N6AMT1 Antibody Immunoprecipitates Aurora Kinase A
3.6. Multiple Commercial Antibodies Raised against N6AMT1 Recognise Aurora Kinase A
3.7. N6AMT1 and Aurora Kinase A Share Similar Motifs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradbury, A.; Plückthun, A. Reproducibility: Standardize Antibodies Used in Research. Nature 2015, 518, 27–29. [Google Scholar] [CrossRef]
- Berglund, L.; Björling, E.; Oksvold, P.; Fagerberg, L.; Asplund, A.; Al-Khalili Szigyarto, C.; Persson, A.; Ottosson, J.; Wernérus, H.; Nilsson, P.; et al. A Genecentric Human Protein Atlas for Expression Profiles Based on Antibodies. Mol. Cell. Proteom. 2008, 7, 2019–2027. [Google Scholar] [CrossRef]
- Begley, C.G.; Ellis, L.M. Raise Standards for Preclinical Cancer Research. Nature 2012, 483, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Lacoux, C.; Wacheul, L.; Saraf, K.; Pythoud, N.; Huvelle, E.; Figaro, S.; Graille, M.; Carapito, C.; Lafontaine, D.L.J.; Heurgué-Hamard, V. The Catalytic Activity of the Translation Termination Factor Methyltransferase Mtq2-Trm112 Complex Is Required for Large Ribosomal Subunit Biogenesis. Nucleic Acids Res. 2020, 48, 12310–12325. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, Y.; Heurgué-Hamard, V.; Langerak, A.A.J.; Speijer, D.; Buckingham, R.H.; Van Der Ende, A. The N 5-Glutamine S-Adenosyl-L-Methionine-Dependent Methyltransferase PrmC/HemK in Chlamydia Trachomatis Methylates Class 1 Release Factors. J. Bacteriol. 2005, 187, 507–511. [Google Scholar] [CrossRef]
- Liu, P.; Nie, S.; Li, B.; Yang, Z.-Q.; Xu, Z.-M.; Fei, J.; Lin, C.; Zeng, R.; Xu, G.-L. Deficiency in a Glutamine-Specific Methyltransferase for Release Factor Causes Mouse Embryonic Lethality. Mol. Cell. Biol. 2010, 30, 4245–4253. [Google Scholar] [CrossRef]
- Figaro, S.; Scrima, N.; Buckingham, R.H.; Heurgué-Hamard, V. HemK2 Protein, Encoded on Human Chromosome 21, Methylates Translation Termination Factor eRF1. FEBS Lett. 2008, 582, 2352–2356. [Google Scholar] [CrossRef]
- Metzger, E.; Wang, S.; Urban, S.; Willmann, D.; Schmidt, A.; Offermann, A.; Allen, A.; Sum, M.; Obier, N.; Cottard, F.; et al. KMT9 Monomethylates Histone H4 Lysine 12 and Controls Proliferation of Prostate Cancer Cells. Nat. Struct. Mol. Biol. 2019, 26, 361–371. [Google Scholar] [CrossRef]
- Chen, J.; Zhuang, Y.; Wang, P.; Ning, J.; Liu, W.; Huang, Y.; Lin, X.; Peng, L.; Zhang, D. Reducing N6AMT1-Mediated 6mA DNA Modification Promotes Breast Tumor Progression via Transcriptional Repressing Cell Cycle Inhibitors. Cell Death Dis. 2022, 13, 216. [Google Scholar] [CrossRef]
- Baumert, H.M.; Metzger, E.; Fahrner, M.; George, J.; Thomas, R.K.; Schilling, O.; Schüle, R. Depletion of Histone Methyltransferase KMT9 Inhibits Lung Cancer Cell Proliferation by Inducing Non-Apoptotic Cell Death. Cancer Cell Int. 2020, 20, 52. [Google Scholar] [CrossRef]
- Berlin, C.; Cottard, F.; Willmann, D.; Urban, S.; Tirier, S.M.; Marx, L.; Rippe, K.; Schmitt, M.; Petrocelli, V.; Greten, F.R.; et al. KMT9 Controls Stemness and Growth of Colorectal Cancer. Cancer Res. 2022, 82, 210–220. [Google Scholar] [CrossRef]
- Mutso, M.; Brūmele, B.; Serova, E.; Väärtnõu, F.; Suija, M.; Kurg, R. The Methyltransferase N6AMT1 Participates in the Cell Cycle by Regulating Cyclin E Levels. PLoS ONE 2024, 19, e0298884. [Google Scholar] [CrossRef]
- Xiao, C.-L.; Zhu, S.; He, M.; Chen, D.; Zhang, Q.; Chen, Y.; Yu, G.; Liu, J.; Xie, S.-Q.; Luo, F.; et al. N6-Methyladenine DNA Modification in the Human Genome. Mol. Cell 2018, 71, 306–318.e7. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Zhang, T.; Ye, J.; Ding, J. Structural Insight into Human N6amt1–Trm112 Complex Functioning as a Protein Methyltransferase. Cell Discov. 2019, 5, 51. [Google Scholar] [CrossRef]
- Woodcock, C.B.; Yu, D.; Zhang, X.; Cheng, X. Human HemK2/KMT9/N6AMT1 Is an Active Protein Methyltransferase, but Does Not Act on DNA in Vitro, in the Presence of Trm112. Cell Discov. 2019, 5, 50. [Google Scholar] [CrossRef]
- Kim, Y.; Holland, A.J.; Lan, W.; Cleveland, D.W. Aurora Kinases and Protein Phosphatase 1 Mediate Chromosome Congression through Regulation of CENP-E. Cell 2010, 142, 444–455. [Google Scholar] [CrossRef]
- Marumoto, T.; Honda, S.; Hara, T.; Nitta, M.; Hirota, T.; Kohmura, E.; Saya, H. Aurora-A Kinase Maintains the Fidelity of Early and Late Mitotic Events in HeLa Cells. J. Biol. Chem. 2003, 278, 51786–51795. [Google Scholar] [CrossRef]
- Õunap, K.; Leetsi, L.; Matsoo, M.; Kurg, R. The Stability of Ribosome Biogenesis Factor WBSCR22 Is Regulated by Interaction with TRMT112 via Ubiquitin-Proteasome Pathway. PLoS ONE 2015, 10, e0133841. [Google Scholar] [CrossRef]
- Brūmele, B.; Mutso, M.; Telanne, L.; Õunap, K.; Spunde, K.; Abroi, A.; Kurg, R. Human TRMT112-Methyltransferase Network Consists of Seven Partners Interacting with a Common Co-Factor. Int. J. Mol. Sci. 2021, 22, 13593. [Google Scholar] [CrossRef]
- Leetsi, L.; Õunap, K.; Abroi, A.; Kurg, R. The Common Partner of Several Methyltransferases TRMT112 Regulates the Expression of N6AMT1 Isoforms in Mammalian Cells. Biomolecules 2019, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, Scalable Generation of High-Quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF CHIMERAX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Wu, L.; Pei, Y.; Zhu, Y.; Jiang, M.; Wang, C.; Cui, W.; Zhang, D. Association of N6-Methyladenine DNA with Plaque Progression in Atherosclerosis via Myocardial Infarction-Associated Transcripts. Cell Death Dis. 2019, 10, 909. [Google Scholar] [CrossRef]
- Garcia, B.C.B.; Horie, M.; Kojima, S.; Makino, A.; Tomonaga, K. BUD23–TRMT112 Interacts with the L Protein of Borna Disease Virus and Mediates the Chromosomal Tethering of Viral Ribonucleoproteins. Microbiol. Immunol. 2021, 65, 492–504. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, J.; Guo, Y.; Zhang, J.; Luo, J. DNA N6-Methyladenine (6mA) Modification Regulates Drug Resistance in Triple Negative Breast Cancer. Front. Oncol. 2021, 10, 616098. [Google Scholar] [CrossRef]
- Koll, F.J.; Metzger, E.; Hamann, J.; Ramos-Triguero, A.; Bankov, K.; Köllermann, J.; Döring, C.; Chun, F.K.H.; Schüle, R.; Wild, P.J.; et al. Overexpression of KMT9α Is Associated with Aggressive Basal-like Muscle-Invasive Bladder Cancer. Cells 2023, 12, 589. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Q.; Wei, W.; Lin, Q.; Magnan, C.; Emami, M.R.; da Silva, L.E.W.; Viola, T.W.; Marshall, P.R.; Yin, J.; et al. The DNA Modification N6-Methyl-2’-Deoxyadenosine (m6dA) Drives Activity-Induced Gene Expression and Is Required for Fear Extinction. Nat. Neurosci. 2019, 22, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a Target for Anticancer Drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Doxsey, S.J.; Stein, P.; Evans, L.; Calarco, P.D.; Kirschner, M. Pericentrin, a Highly Conserved Centrosome Protein Involved in Microtubule Organization. Cell 1994, 76, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.D.; Brooks, L.; Zhang, X.; Mahoney, J.M.; Martyanov, V.; Wood, T.A.; Sherlock, G.; Cheng, C.; Whitfield, M.L. Identification of Cell Cycle–Regulated Genes Periodically Expressed in U2OS Cells and Their Regulation by FOXM1 and E2F Transcription Factors. Mol. Biol. Cell 2013, 24, 3634–3650. [Google Scholar] [CrossRef] [PubMed]
- Carmena, M.; Earnshaw, W.C. The Cellular Geography of Aurora Kinases. Nat. Rev. Mol. Cell Biol. 2003, 4, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, D.; Majkowska-Skrobek, G.; Roszkowiak, J.; Dorotkiewicz-Jach, A. Defensive and Offensive Cross-Reactive Antibodies Elicited by Pathogens: The Good, the Bad and the Ugly. CMC 2017, 24, 4002–4037. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.G. Quality Issues of Research Antibodies. Anal. Chem. Insights 2016, 11, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, I.; Glasziou, P. Avoidable Waste in the Production and Reporting of Research Evidence. Lancet 2009, 374, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Why Most Published Research Findings Are False. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef]
- Macleod, M.R.; Michie, S.; Roberts, I.; Dirnagl, U.; Chalmers, I.; Ioannidis, J.P.A.; Salman, R.A.-S.; Chan, A.-W.; Glasziou, P. Biomedical Research: Increasing Value, Reducing Waste. Lancet 2014, 383, 101–104. [Google Scholar] [CrossRef]


| Nr | Anti-N6AMT1 | Host | Clonality | Immunogen | References |
|---|---|---|---|---|---|
| I | CQA1550; ABIN2966707; orb341253 | r | Poly | 1–214, R | [12] |
| II | HPA059242; PA5-66242 | r | Poly | 148–212, R | [19] |
| III | 16211-1-AP | r | poly | 1–186, R | [25,26] |
| IV | PA5-121076 | r | poly | 115–214, S | [9] |
| V * | PA5-42782; ARP45845_P050; orb579590; LS-C111069 ABIN2782381 | r | poly | 1–50, S | [27] |
| VI | sc-517120; ABIN565408 | m | mono | 87–186, R | n/a |
| VII | #27630 | n/a | n/a | n/a | [8,10,11,28] |
| VIII | Discontinued—sc-83304 | r | poly | n/a | [29] |
| VIV * | A7201 | r | n/a | 1–186, S | n/a |
| X * | STJ29281; NBP3-03312; ABIN6144314 | r | poly | 1–186, R | n/a |
| XI | ab238897; orb352915; ABIN7154967 | r | poly | 1–186, R | n/a |
| XII | abx005435; orb247851; GTX32649 | r | poly | 1–186, R | n/a |
| XIII | abx030019; orb165187; ABIN1538845 | r | poly | 1–30, S | n/a |
| XIV | abx235532; SH-A13857 | r | poly | 1–214, R | n/a |
| XV | ABIN1713839 | r | poly | 1–100, S | n/a |
| XVI | ABIN949232 | r | poly | 1–186, R | n/a |
| XVII | ABIN2752353 | m | poly | 1–186, R | n/a |
| XVIII | ABIN7116668 | r | poly | 1–214, R | n/a |
| XIX | abx320643 | r | poly | 1–186, R | n/a |
| XX | abx112962 | r | poly | 1–214, R | n/a |
| XXI | orb649232 | r | poly | 1–214, R | n/a |
| XXII | A10854 | r | poly | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brūmele, B.; Serova, E.; Lupp, A.; Suija, M.; Mutso, M.; Kurg, R. Cross-Reactivity of N6AMT1 Antibodies with Aurora Kinase A: An Example of Antibody-Specific Non-Specificity. Antibodies 2024, 13, 33. https://doi.org/10.3390/antib13020033
Brūmele B, Serova E, Lupp A, Suija M, Mutso M, Kurg R. Cross-Reactivity of N6AMT1 Antibodies with Aurora Kinase A: An Example of Antibody-Specific Non-Specificity. Antibodies. 2024; 13(2):33. https://doi.org/10.3390/antib13020033
Chicago/Turabian StyleBrūmele, Baiba, Evgeniia Serova, Aleksandra Lupp, Mihkel Suija, Margit Mutso, and Reet Kurg. 2024. "Cross-Reactivity of N6AMT1 Antibodies with Aurora Kinase A: An Example of Antibody-Specific Non-Specificity" Antibodies 13, no. 2: 33. https://doi.org/10.3390/antib13020033
APA StyleBrūmele, B., Serova, E., Lupp, A., Suija, M., Mutso, M., & Kurg, R. (2024). Cross-Reactivity of N6AMT1 Antibodies with Aurora Kinase A: An Example of Antibody-Specific Non-Specificity. Antibodies, 13(2), 33. https://doi.org/10.3390/antib13020033





