Characterization of the Charge Heterogeneity of a Monoclonal Antibody That Binds to Both Cation Exchange and Anion Exchange Columns under the Same Binding Conditions
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Ion Exchange Chromatography
2.2.1. Cation Exchange Chromatography (CEX)
2.2.2. Anion Exchange Chromatography (AEX)
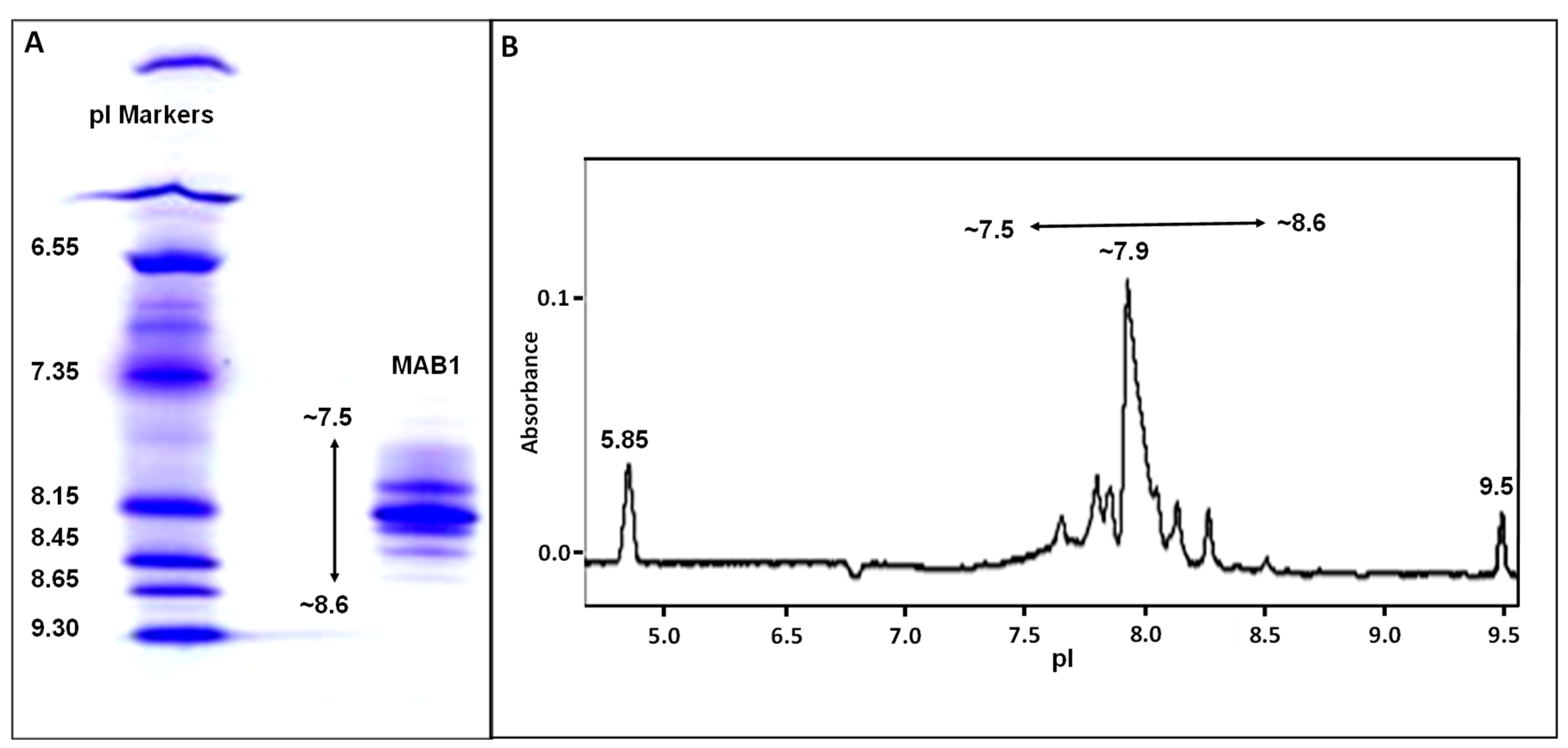
2.3. IEF Gel Electrophoresis
2.4. Imaged Capillary Isoelectric Focusing (cIEF)
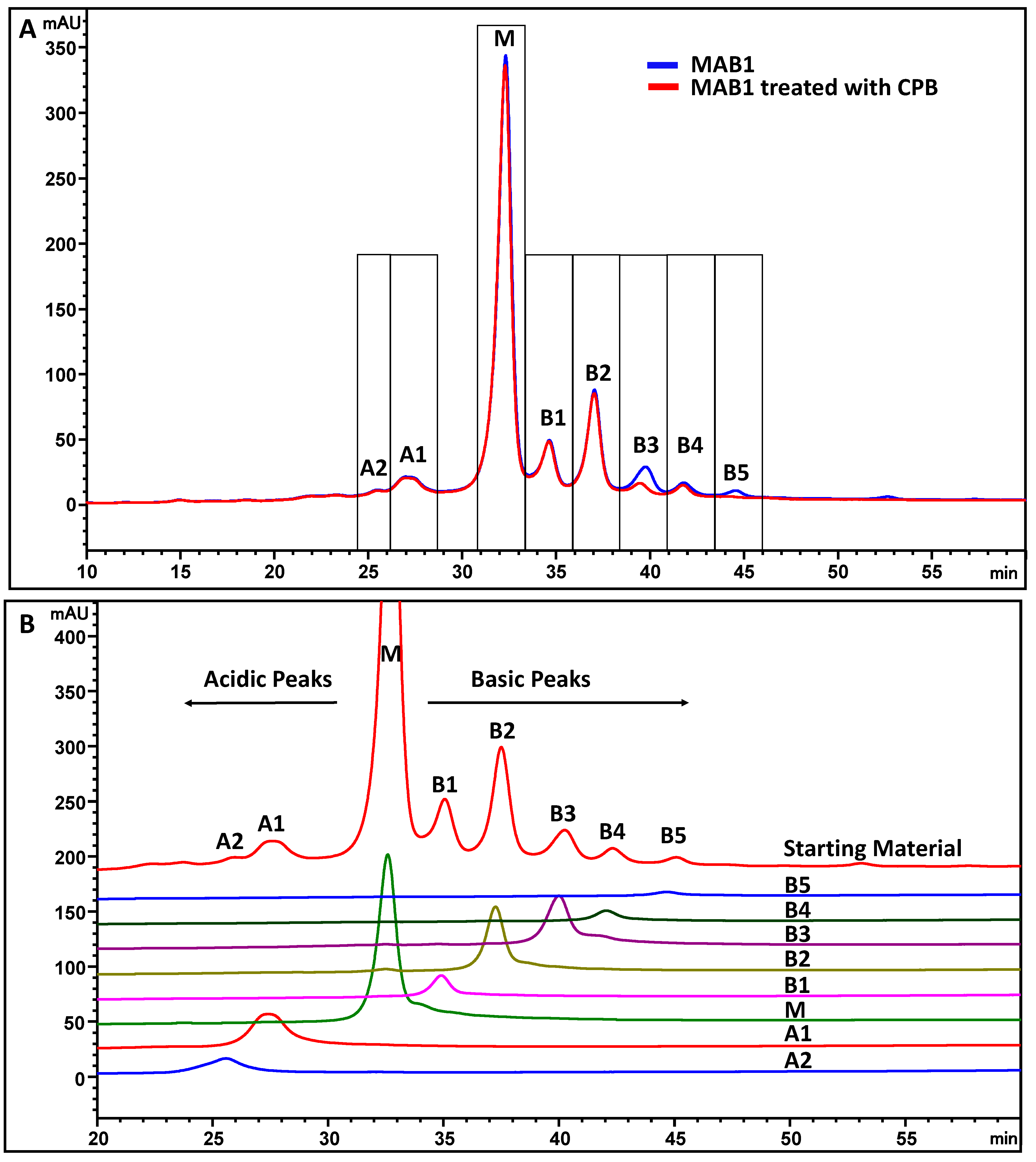
2.5. Carboxypeptidase B (CPB) Treatment
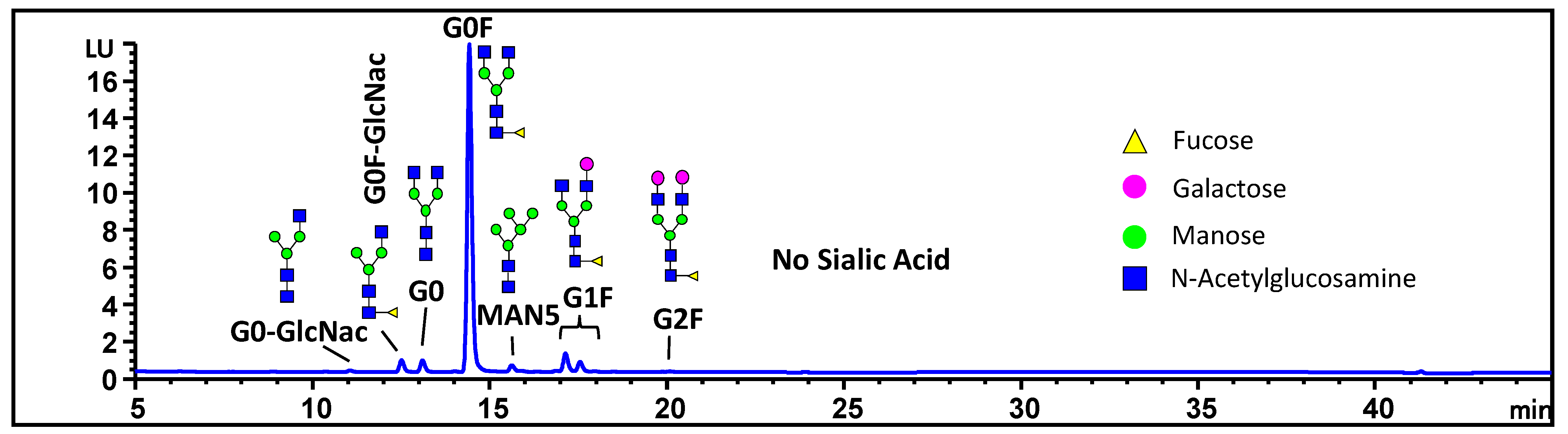
2.6. N-Linked Oligosaccharide Profiling with HILIC Analysis
2.7. Tryptic Peptide Mapping
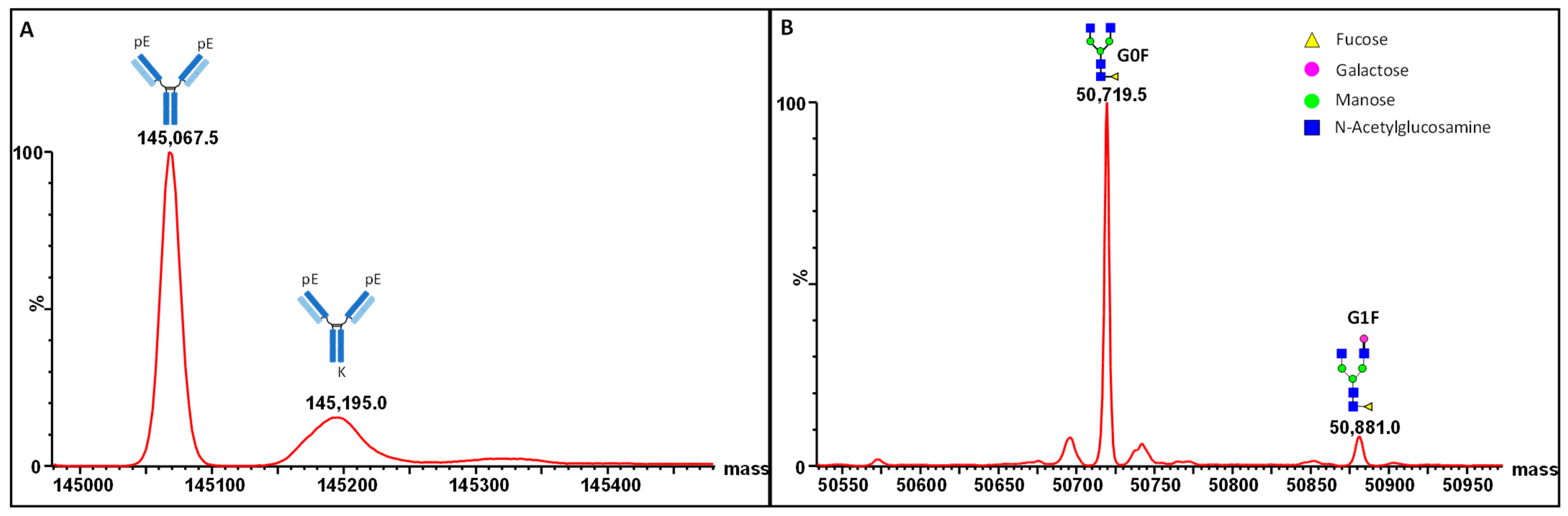
2.8. Top-Down Mass Analysis
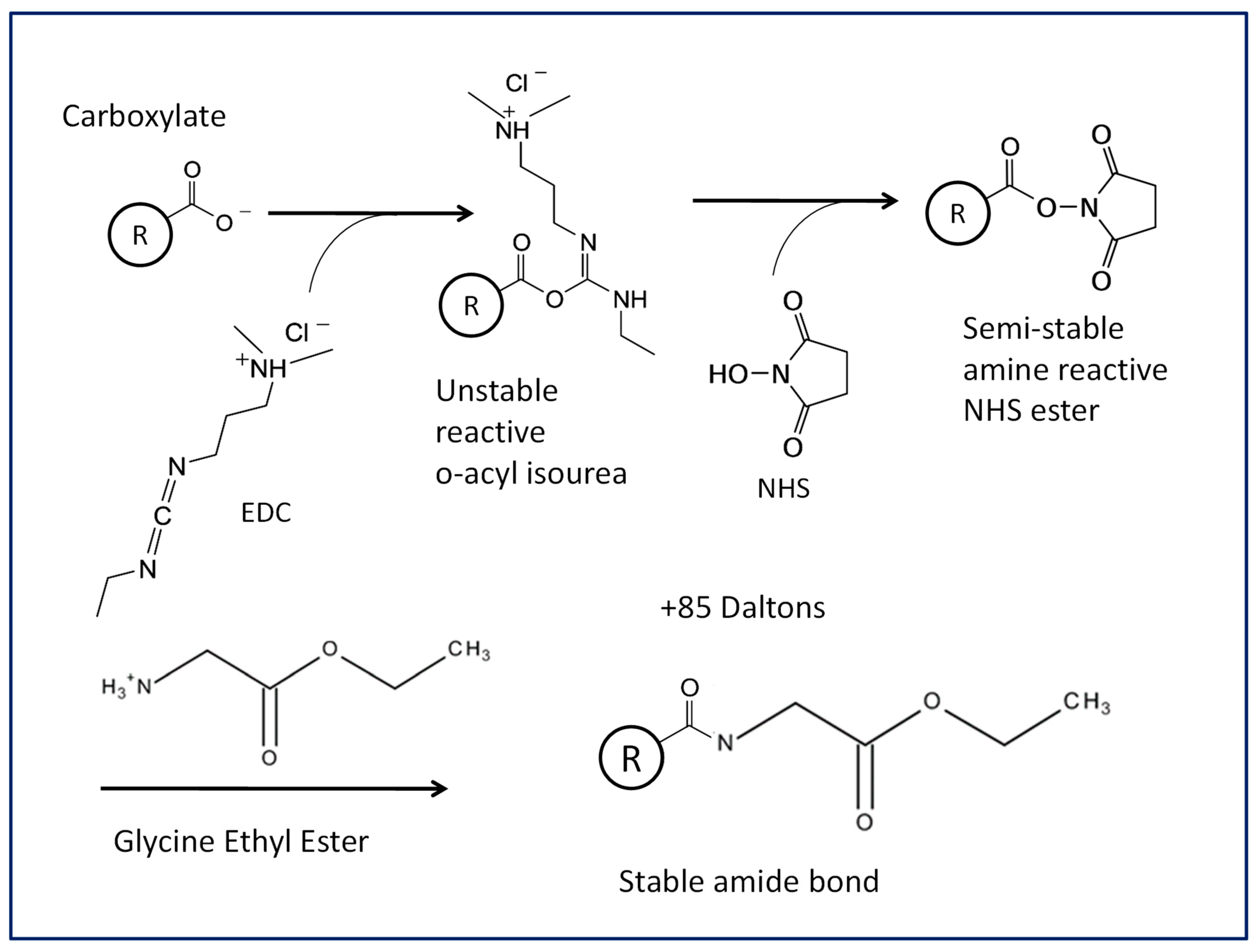
2.9. Chemical Labeling
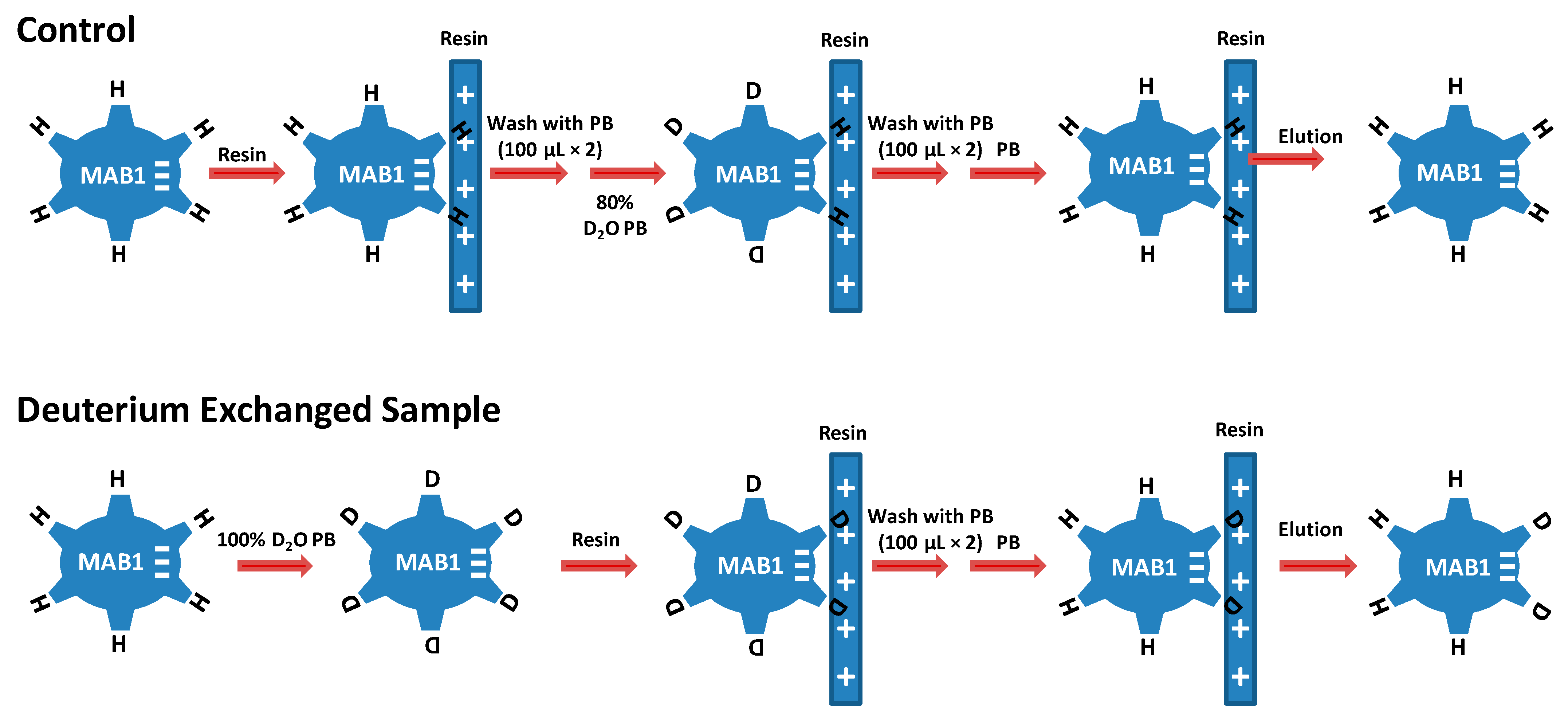
2.10. Hydrogen–Deuterium Exchange Mass Spectrometry (H/D EX MS) Analysis
2.11. H/D Exchange Data Analysis
2.12. In Vitro Binding Assay
2.13. Molecular Modeling
3. Results and Discussion
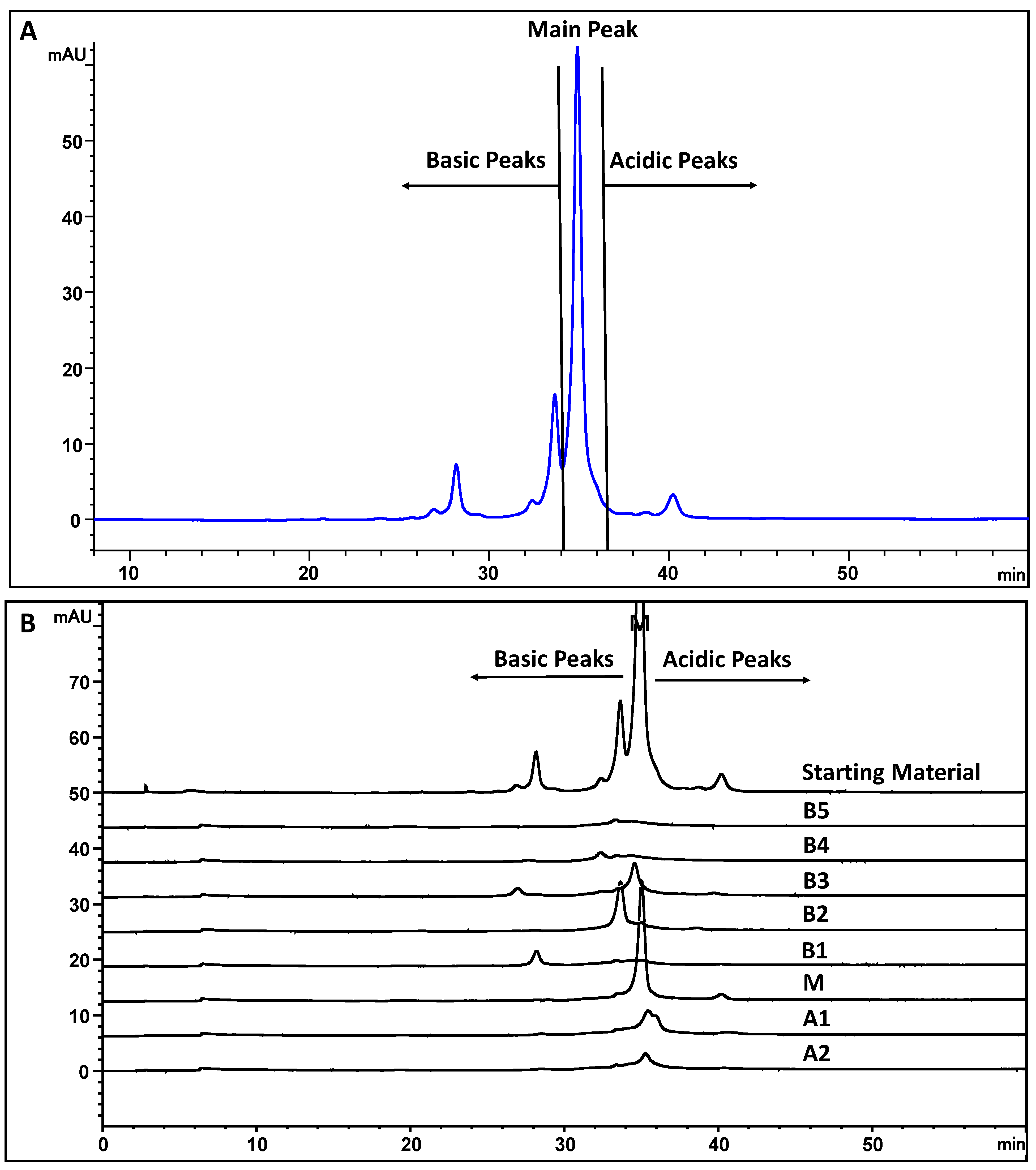
3.1. Characterization of MAB1 Charge Heterogeneity
3.2. Monoclonal Antibody MAB1 Binds to Anion Exchange and Cation Exchange Resins
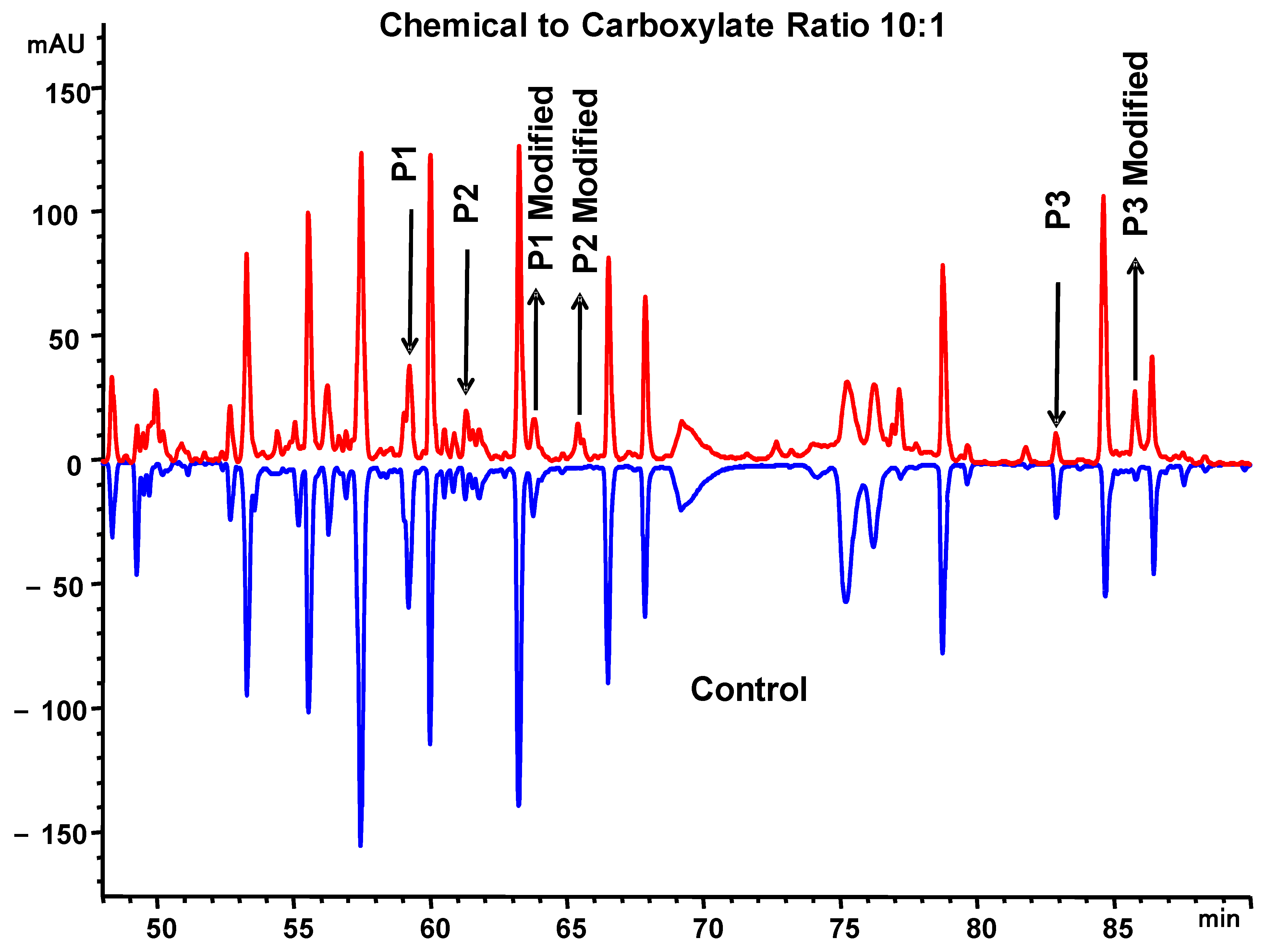
3.3. Anion Exchange Binding Site Identification by Chemical Labeling, Peptide Mapping Coupled with Mass Spectrometry
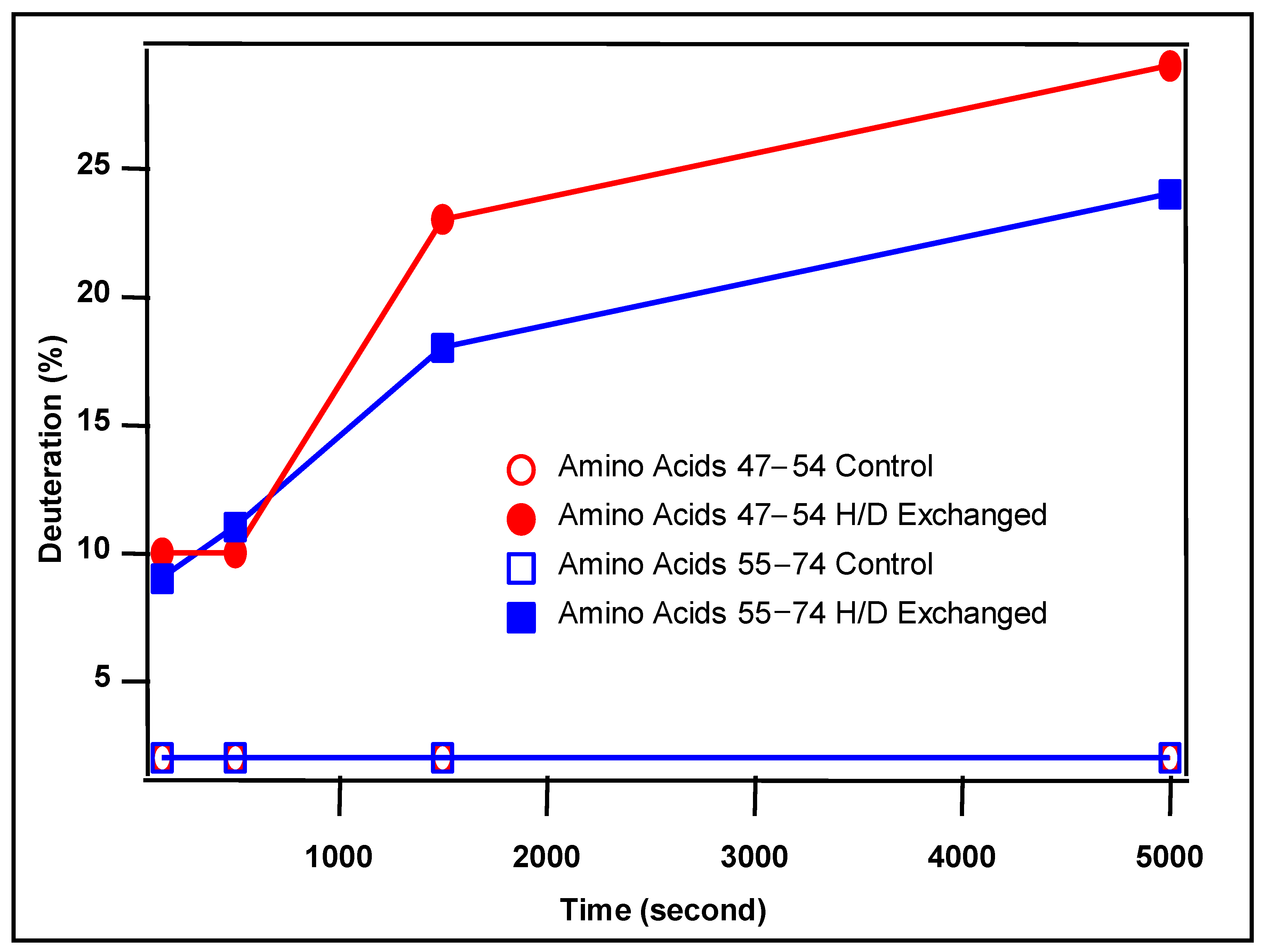
3.4. Anion Exchange Binding Site Identification by H/D Exchange Experiment
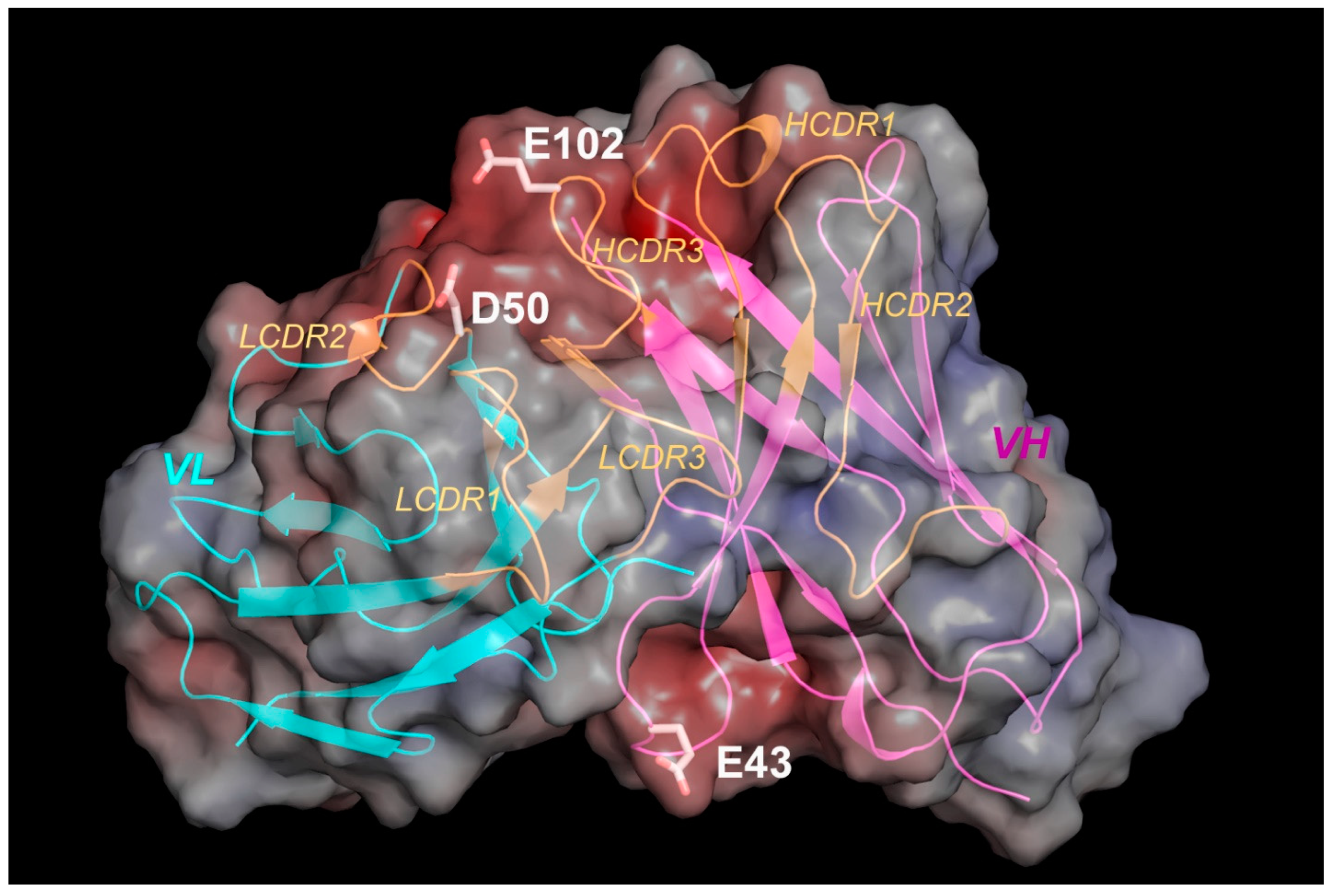
3.5. Predicted Structural Model Explains Residues Involved in Anion Exchange Column Binding
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Antibody Society. Available online: https://www.antibodysociety.org/ (accessed on 24 June 2024).
- U.S. Food & Drug Administration. Available online: https://www.fda.gov/drugs/biosimilars/biosimilar-product-information (accessed on 24 June 2024).
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2021. MAbs 2021, 13, 1860476. [Google Scholar] [CrossRef] [PubMed]
- Bloomberg.com. Monoclonal Antibodies Market to Hit USD 425 Billion by 2028, Says Global Market Insights Inc. [News]. 2022. Available online: https://www.bloomberg.com/press-releases/2022-05-17/monoclonal-antibodies-market-to-hit-usd-425-billion-by-2028-says-global-market-insights-inc (accessed on 17 May 2022).
- Chu, L.; Robinson, D.K. Industrial choices for protein production by large-scale cell culture. Curr. Opin. Biotechnol. 2001, 12, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Chadd, H.E.; Chamow, S.M. Therapeutic antibody expression technology. Curr. Opin. Biotechnol. 2001, 12, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Bleckwenn, N.A.; Shiloach, J. Large-scale cell culture. Curr. Protoc. Immunol. 2004, 59, A-1U.1–A.1U.44. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J. Heterogeneity of recombinant antibodies: Linking structure to function. Dev. Biol. 2005, 122, 117–127. [Google Scholar]
- Liu, H.; Gaza-Bulseco, G.; Faldu, D.; Chumsae, C.; Sun, J. Heterogeneity of monoclonal antibodies. J. Pharm. Sci. 2008, 97, 2426–2447. [Google Scholar] [CrossRef] [PubMed]
- Kelley, B. Industrialization of mAb production technology: The bioprocessing industry at a crossroads. MAbs 2009, 1, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.A.; Thommes, J. Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol. 2010, 28, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Strauss, D.M.; Lute, S.; Tebaykina, Z.; Frey, D.D.; Ho, C.; Blank, G.S.; Brorson, K.; Chen, Q.; Yang, B. Understanding the mechanism of virus removal by Q sepharose fast flow chromatography during the purification of CHO-cell derived biotherapeutics. Biotechnol. Bioeng. 2009, 104, 371–380. [Google Scholar] [CrossRef]
- Yigzaw, Y.; Hinckley, P.; Hewig, A.; Vedantham, G. Ion exchange chromatography of proteins and clearance of aggregates. Curr. Pharm. Biotechnol. 2009, 10, 421–426. [Google Scholar] [CrossRef]
- Beck, A.; Liu, H. Macro- and Micro-Heterogeneity of Natural and Recombinant IgG Antibodies. Antibodies 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Grodzki, A.C.; Berenstein, E. Antibody purification: Ion-exchange chromatography. Methods Mol. Biol. 2010, 588, 27–32. [Google Scholar] [PubMed]
- Regnier, F.E. The role of protein structure in chromatographic behavior. Science 1987, 238, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Vlasak, J.; Ionescu, R. Heterogeneity of monoclonal antibodies revealed by charge-sensitive methods. Curr. Pharm. Biotechnol. 2008, 9, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Yuce, M.; Sert, F.; Torabfam, M.; Parlar, A.; Gurel, B.; Cakir, N.; Daglikoca, D.E.; Khan, M.A.; Capan, Y. Fractionated charge variants of biosimilars: A review of separation methods, structural and functional analysis. Anal. Chim. Acta 2021, 1152, 238189. [Google Scholar] [CrossRef] [PubMed]
- Khawli, L.A.; Goswami, S.; Hutchinson, R.; Kwong, Z.W.; Yang, J.; Wang, X.; Yao, Z.; Sreedhara, A.; Cano, T.; Tesar, D.; et al. Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs 2010, 2, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Chumsae, C.; Gifford, K.; Lian, W.; Liu, H.; Radziejewski, C.H.; Zhou, Z.S. Arginine modifications by methylglyoxal: Discovery in a recombinant monoclonal antibody and contribution to acidic species. Anal. Chem. 2013, 85, 11401–11409. [Google Scholar] [CrossRef]
- Miao, S.; Xie, P.; Zou, M.; Fan, L.; Liu, X.; Zhou, Y.; Zhao, L.; Ding, D.; Wang, H.; Tan, W.S. Identification of multiple sources of the acidic charge variants in an IgG1 monoclonal antibody. Appl. Microbiol. Biotechnol. 2017, 101, 5627–5638. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Walsh, A.; Ehrick, R.; Xu, W.; May, K.; Liu, H. Chromatographic analysis of the acidic and basic species of recombinant monoclonal antibodies. MAbs 2012, 4, 578–585. [Google Scholar] [CrossRef]
- Ponniah, G.; Kita, A.; Nowak, C.; Neill, A.; Kori, Y.; Rajendran, S.; Liu, H. Characterization of the acidic species of a monoclonal antibody using weak cation exchange chromatography and LC-MS. Anal. Chem. 2015, 87, 9084–9092. [Google Scholar] [CrossRef]
- Liu, Z.; Valente, J.; Lin, S.; Chennamsetty, N.; Qiu, D.; Bolgar, M. Cyclization of N-Terminal Glutamic Acid to pyro-Glutamic Acid Impacts Monoclonal Antibody Charge Heterogeneity Despite Its Appearance as a Neutral Transformation. J. Pharm. Sci. 2019, 108, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Tu, J.; Kuo, T.T. A new protein subunit k for RNA polymerase from Xanthomonas campestris pv. oryzae. J. Biol. Chem. 1989, 264, 4362–4366. [Google Scholar] [CrossRef] [PubMed]
- Dismer, F.; Hubbuch, J. A novel approach to characterize the binding orientation of lysozyme on ion-exchange resins. J. Chromatogr. A 2007, 1149, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lilyestrom, W.; Li, C.; Scherer, T.; van Reis, R.; Zhang, B. Revealing a positive charge patch on a recombinant monoclonal antibody by chemical labeling and mass spectrometry. Anal. Chem. 2011, 83, 8501–8508. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Tomechko, S.E.; Kiselar, J.; Shi, W.; Deperalta, G.; Wecksler, A.T.; Gokulrangan, G.; Ling, V.; Chance, M.R. Characterizing monoclonal antibody structure by carboxyl group footprinting. MAbs 2015, 7, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Houde, D.; Arndt, J.; Domeier, W.; Berkowitz, S.; Engen, J.R. Characterization of IgG1 conformation and conformational dynamics by hydrogen/deuterium exchange mass spectrometry. Anal. Chem. 2009, 81, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Houde, D.; Peng, Y.; Berkowitz, S.A.; Engen, J.R. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol. Cell. Proteom. 2010, 9, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Sellers, B.D.; Nilmeier, J.P.; Jacobson, M.P. Antibodies as a model system for comparative model refinement. Proteins 2010, 78, 2490–2505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Smith, D.L. Determination of amide hydrogen exchange by mass spectrometry: A new tool for protein structure elucidation. Protein Sci. 1993, 2, 522–531. [Google Scholar] [CrossRef]
- Hamuro, Y.; Molnar, K.S.; Coales, S.J.; OuYang, B.; Simorellis, A.K.; Pochapsky, T.C. Hydrogen-deuterium exchange mass spectrometry for investigation of backbone dynamics of oxidized and reduced cytochrome P450cam. J. Inorg. Biochem. 2008, 102, 364–370. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.; Park, J. DaliLite workbench for protein structure comparison. Bioinformatics 2000, 16, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66 Pt 4, 486–501. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL Molecular Graphics System on World Wide Web. 2002. Available online: http://www.pymol.org (accessed on 30 May 2024).
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Gupta, S.; Jiskoot, W.; Schoneich, C.; Rathore, A.S. Oxidation and Deamidation of Monoclonal Antibody Products: Potential Impact on Stability, Biological Activity, and Efficacy. J. Pharm. Sci. 2021, 111, 903–918. [Google Scholar] [CrossRef]
- Karlsson, E.; Ryden, L.; Brewer, J. Ion Exchange Chromatography. In Protein Purification: Principles, High-Resolution Methods, and Applications, 2nd ed.; Janson, J.-C., Ryden, L., Eds.; WILEY-VCH: New York, NY, USA, 1998; pp. 145–205. [Google Scholar]
- Creighton, T.E. Proteins. Structures and Molecular Properties, 2nd ed.; W. H. Freeman and Company: New York, NY, USA, 1993; p. 507. [Google Scholar]


| Sequence | Modification | Modified AAs | Position | CEXA2 | CEXA1 | CEXM | CEXB1 | CEXB2 | CEXB3 | CEXB4 | CEXB5 | CRTL1 | CRTL2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | ||||
| QDQLVESGGGVVQPGR | 1.6 | 1.5 | 0.4 | 1.5 | 36.9 | 13.7 | 51.4 | 33.2 | 8.8 | 8.9 | |||
| pyro-Glu | Q | H1 | 98.4 | 98.5 | 99.6 | 98.5 | 63.1 | 86.1 | 48.5 | 66.7 | 91.1 | 91.1 | |
| SLSLSPG | Des-K | 99.0 | 99.4 | 100 | 98.6 | 99.1 | 62.3 | 86.4 | 60.7 | 95.3 | 95.4 | ||
| SLSLSPGK | 0.9 | 0.5 | 0 | 1.3 | 0.8 | 36.8 | 13.1 | 38.4 | 4.5 | 4.4 | |||
| SLSLSP | Amidation | P | H448 | 0.1 | 0.1 | 0 | 0.1 | 0.1 | 0.9 | 0.5 | 0.9 | 0.2 | 0.2 |
| GDYEVDYGMDVWGQGTTVTV ASASTK | Succinimide | D | H100 | 1.0 | 0.4 | 0.1 | 10.7 | 0.8 | 2.6 | 2.7 | 2.2 | 1.4 | 1.3 |
| GFYPSDIAVEWESNGQPENNYK | Succinimide | N | H387 | 1.4 | 1.2 | 1.2 | 1.4 | 1.4 | 1.5 | 1.4 | 1.6 | 1.6 | 1.5 |
| Deamidated | N | H387 | 1.8 | 7.6 | 0.7 | 1.0 | 0.9 | 0.8 | 0.8 | 0.8 | 1.4 | 1.3 | |
| Deamidated | N | H392 | 2.3 | 6.6 | 0.4 | 0.4 | 0.6 | 0.5 | 0.3 | 0.4 | 0.9 | 0.7 |
| Peak | Fraction | MassTheo | Massobs | 1 Modified Massobs | Position | Sequence |
|---|---|---|---|---|---|---|
| P1 | LC_D50 | 1705.90 | 1705.84 | 1790.92 | 46–61 | LLIYDASSLESGVPSR |
| P2 2 | HC_E102/D104 | 2736.22 | 2736.00 | 2821.12 | 99–124 | GDYEVDYGMDVWGQGTTVTV ASASTK |
| P3 | HC_E43 | 2218.07 | 2217.84 | 2302.92 | 39–58 | QAPGEGLEWVAVIWYDGSNK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, M.-C.; Zhang, J.; Tang, L.; Huang, C.-Y.; Shen, Y.; Matathia, A.; Qian, J.; Parekh, B.S. Characterization of the Charge Heterogeneity of a Monoclonal Antibody That Binds to Both Cation Exchange and Anion Exchange Columns under the Same Binding Conditions. Antibodies 2024, 13, 52. https://doi.org/10.3390/antib13030052
Hsieh M-C, Zhang J, Tang L, Huang C-Y, Shen Y, Matathia A, Qian J, Parekh BS. Characterization of the Charge Heterogeneity of a Monoclonal Antibody That Binds to Both Cation Exchange and Anion Exchange Columns under the Same Binding Conditions. Antibodies. 2024; 13(3):52. https://doi.org/10.3390/antib13030052
Chicago/Turabian StyleHsieh, Ming-Ching, Jingming Zhang, Liangjie Tang, Cheng-Yen Huang, Yang Shen, Alice Matathia, Jun Qian, and Babita Saxena Parekh. 2024. "Characterization of the Charge Heterogeneity of a Monoclonal Antibody That Binds to Both Cation Exchange and Anion Exchange Columns under the Same Binding Conditions" Antibodies 13, no. 3: 52. https://doi.org/10.3390/antib13030052
APA StyleHsieh, M.-C., Zhang, J., Tang, L., Huang, C.-Y., Shen, Y., Matathia, A., Qian, J., & Parekh, B. S. (2024). Characterization of the Charge Heterogeneity of a Monoclonal Antibody That Binds to Both Cation Exchange and Anion Exchange Columns under the Same Binding Conditions. Antibodies, 13(3), 52. https://doi.org/10.3390/antib13030052







