The Seraph 100® Microbind Affinity Blood Filter Does Not Alter Levels of Circulating or Mucosal Antibodies in Critical COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Quantitative Binding Antibody Assays
2.3. Statistical Analysis
3. Results
3.1. Participant Demographics
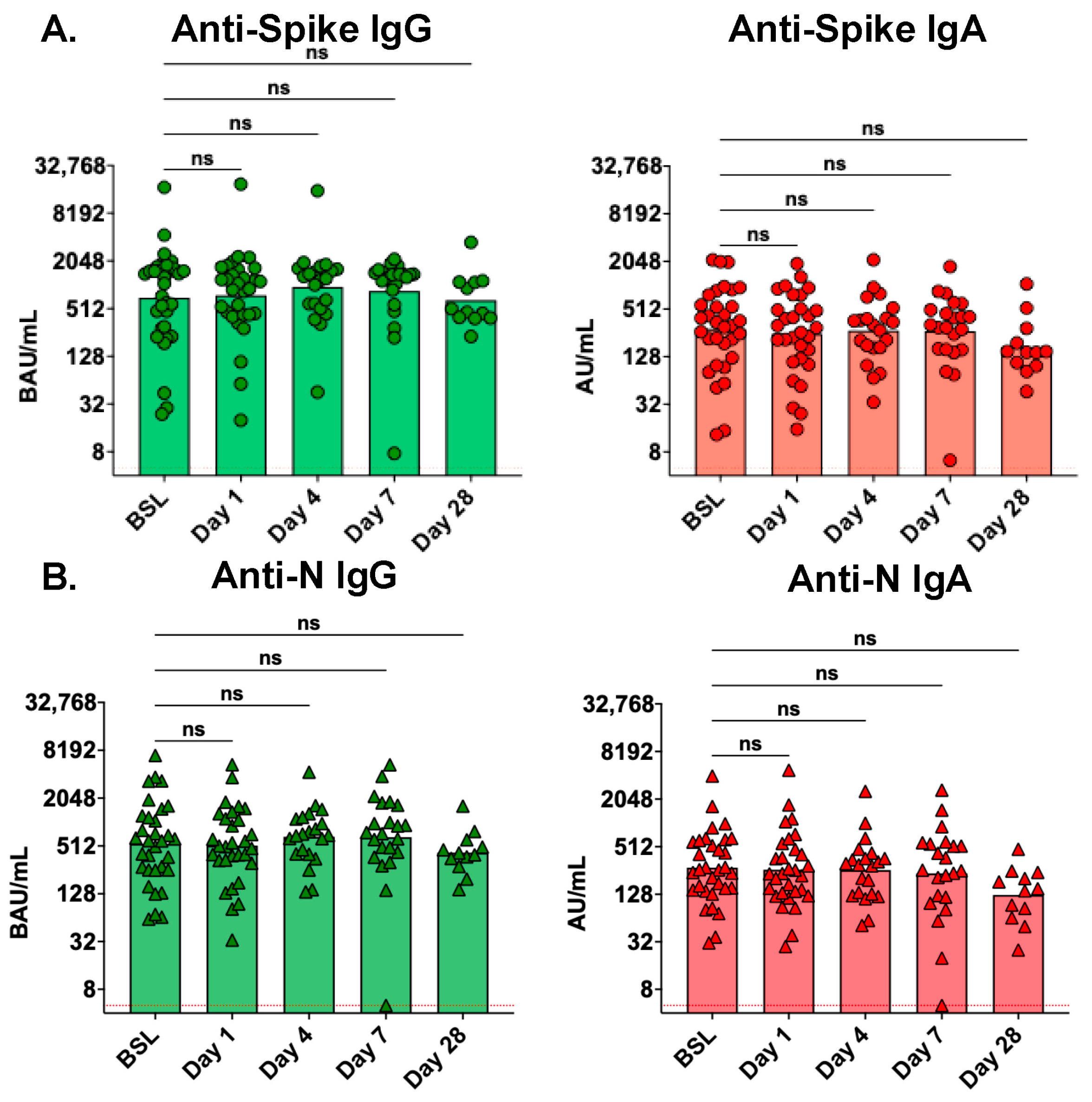
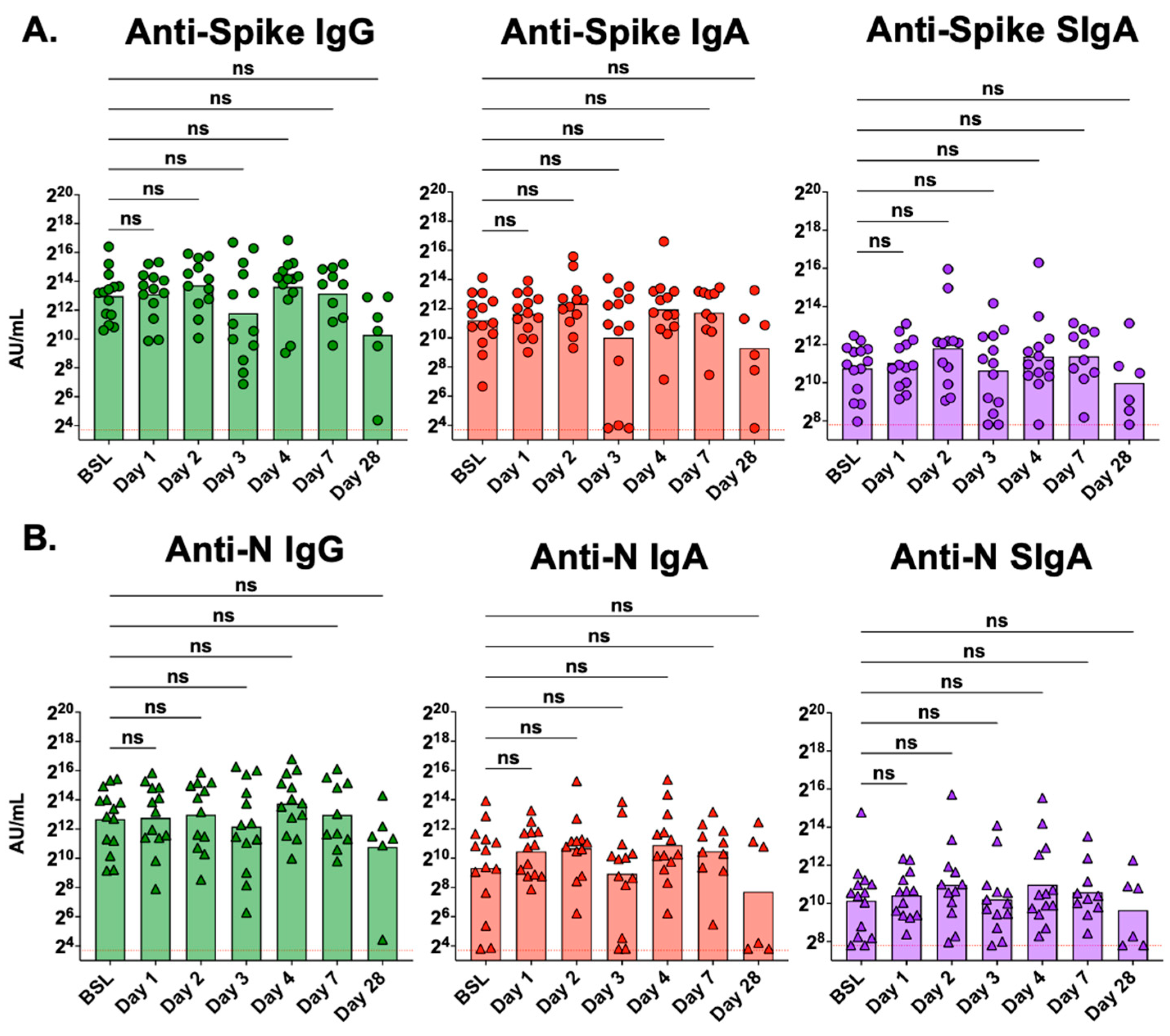
3.2. Serum and Mucosal Antibody Levels in the Days after ICU Admission
3.3. Serum Antibody Levels Are Not Affected by Treatment with the Seraph 100
3.4. Mucosal Antibody Levels Are Not Affected by Treatment with the Seraph 100
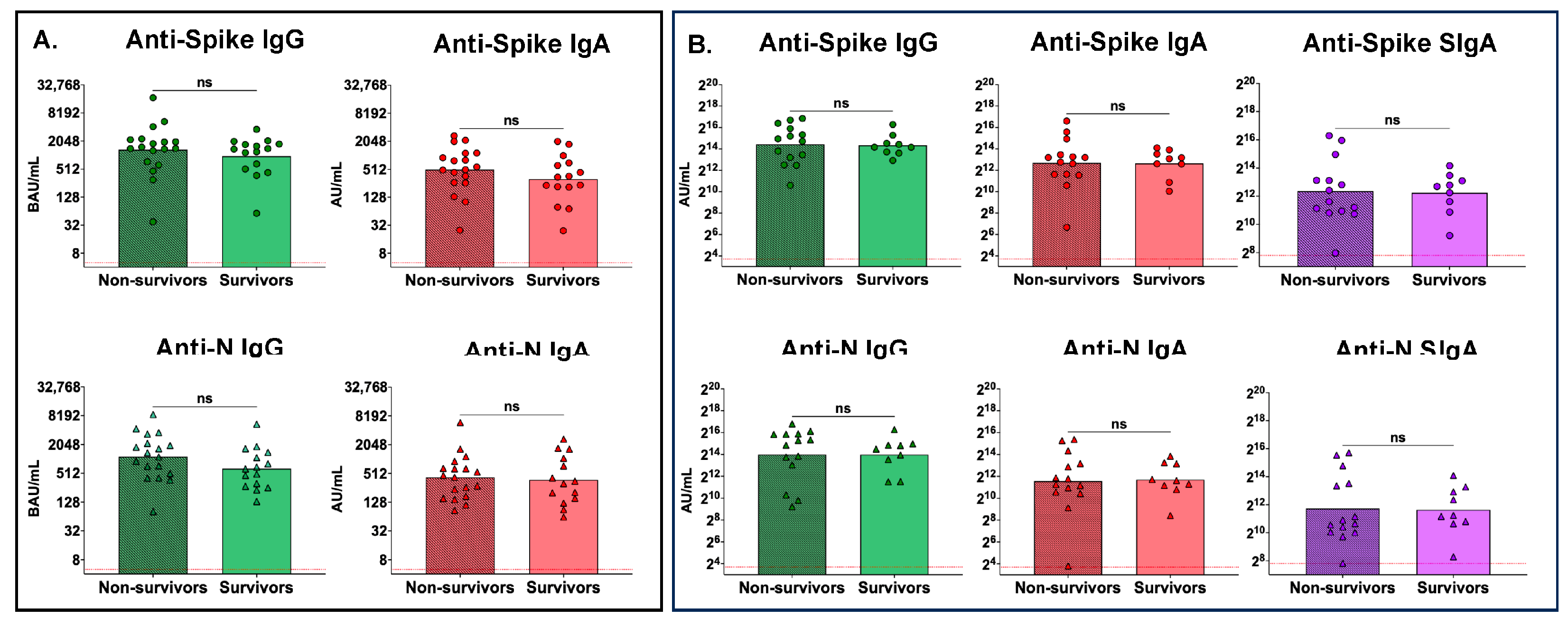
3.5. No Differences between Peak Serum or Peak Mucosal Antibody Levels between Nonsurvivors and Survivors
3.6. No Differences between Peak Serum or Peak Mucosal Antibody Levels between Males and Females
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 16 April 2024).
- Saag, M. Wonder of wonders, miracle of miracles: The unprecedented speed of COVID-19 science. Physiol. Rev. 2022, 102, 1569–1577. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Kaimakamis, E.; Voutsas, V.; Bitzani, M. An observational study on factors associated with ICU mortality in COVID-19 patients and critical review of the literature. Sci. Rep. 2023, 13, 7804. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Kane, A.D.; Cook, T.M. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia 2020, 75, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Pilishvili, T.; Gierke, R.; Fleming-Dutra, K.E.; Farrar, J.L.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C.; et al. Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. N. Engl. J. Med. 2021, 385, e90. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Emergency Use Authorization. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization (accessed on 22 April 2024).
- Food and Drug Administration. Blood Purification Devices EUAs. Available online: https://www.fda.gov/medical-devices/COVID-19-emergency-use-authorizations-medical-devices/blood-purification-devices-euas (accessed on 8 April 2024).
- Seffer, M.T.; Cottam, D.; Forni, L.G.; Kielstein, J.T. Heparin 2.0: A New Approach to the Infection Crisis. Blood Purif. 2021, 50, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pushpakumar, S.; Zheng, Y.; Smolenkova, I.; Akinterinwa, O.E.; Luulay, B.; Tyagi, S.C. Novel mechanism of the COVID-19 associated coagulopathy (CAC) and vascular thromboembolism. NPJ Viruses 2023, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.K.; Spiller, R.E.; Mount, C.A.; Colombo, C.; Khayat, M.I. Novel Use of Seraph-100™ Blood Purification Therapy in Heat Stroke. Mil. Med. 2022, 188, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Scourfield, D.O.; Reed, S.G.; Quastel, M.; Alderson, J.; Bart, V.M.T.; Teijeira Crespo, A.; Jones, R.; Pring, E.; Richter, F.C.; Consortium, T.O.-C.C.-L.; et al. The role and uses of antibodies in COVID-19 infections: A living review. Oxf. Open Immunol. 2021, 2, iqab003. [Google Scholar] [CrossRef] [PubMed]
- Andermatt, R.; Bloemberg, G.V.; Ganter, C.C.; Mueller, N.J.; Mueller, A.M.S.; Muellhaupt, B.; Kielstein, J.T.; David, S. Elimination of Herpes Simplex Virus-2 and Epstein-Barr Virus with Seraph 100 Microbind Affinity Blood Filter and Therapeutic Plasma Exchange: An Explorative Study in a Patient with Acute Liver Failure. Crit. Care Explor. 2022, 4, e0745. [Google Scholar] [CrossRef] [PubMed]
- Chitty, S.A.; Mobbs, S.; Rifkin, B.S.; Stogner, S.W.; Lewis, M.S.; Betancourt, J.; DellaVolpe, J.; Abouzahr, F.; Wilhelm, A.M.; Szerlip, H.M.; et al. A Multicenter Evaluation of the Seraph 100 Microbind Affinity Blood Filter for the Treatment of Severe COVID-19. Crit. Care Explor. 2022, 4, e0662. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Borchina, D.N.; Fühner, T.; Hwang, S.; Mattoon, D.; Ball, A.J. Hemofiltration with the Seraph(®) 100 Microbind(®) Affinity filter decreases SARS-CoV-2 nucleocapsid protein in critically ill COVID-19 patients. Crit. Care 2021, 25, 190. [Google Scholar] [CrossRef]
- Pape, A.; Kielstein, J.T.; Krüger, T.; Fühner, T.; Brunkhorst, R. Treatment of a Critically Ill COVID-19 Patient with the Seraph 100 Microbind Affinity Filter. TH Open 2021, 5, e134–e138. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, S.; Boster, J.; Jarrett, Z.; Rosas, M.; Kalra, A.; Nugyen, M.; Morris, M.; Walter, R. Single-Center Experience with the Seraph-100® Microbind® Affinity Blood Filter in Patients with SARS-CoV-2 Infection and Septic Shock at a Military Treatment Facility. Mil. Med. 2023, 188, e2670–e2674. [Google Scholar] [CrossRef] [PubMed]
- Merrill, K.A.; Krallman, K.A.; Loeb, D.; Standage, S.W.; Mattoon, D.; Shan, D.; Goldstein, S.L.; Schuh, M.P. First-Time Use of the Seraph® 100 Microbind® Affinity Blood Filter in an Adolescent Patient with Severe COVID-19 Disease: A Case Report. Case Rep. Nephrol. Dial. 2023, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Eden, G.; Schmidt, J.J.; Büttner, S.; Kümpers, P.; Hafer, C.; Rovas, A.; Koch, B.F.; Schmidt, B.M.W.; Kielstein, J.T. Safety and efficacy of the Seraph® 100 Microbind® Affinity Blood Filter to remove bacteria from the blood stream: Results of the first in human study. Crit. Care 2022, 26, 181. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007, 25, 5467–5484. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.; Talwar, S.; Cross, A.; Willett, B.J.; Scott, S.; Logan, N.; Siggins, M.K.; Swieboda, D.; Sidhu, J.K.; Efstathiou, C.; et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. eBioMedicine 2023, 87, 104402. [Google Scholar] [CrossRef] [PubMed]
- Sohaei, D.; Ulndreaj, A.; Mathew, A.; Campbell, C.; Stengelin, M.; Sigal, G.; Joe, J.; Romero, D.; Padmanabhan, N.; Ren, A.; et al. Sensitive Serology Measurements in the Saliva of Individuals with COVID-19 Symptoms Using a Multiplexed Immunoassay. J. Appl. Lab. Med. 2022, 7, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Goguet, E.; Olsen, C.H.; Meyer, W.A.; Ansari, S.; Powers, J.H.; Conner, T.L.; Coggins, S.A.A.; Wang, W.; Wang, R.; Illinik, L.; et al. Immune and behavioral correlates of protection against symptomatic post-vaccination SARS-CoV-2 infection. Front. Immunol. 2024, 15, 1287504. [Google Scholar] [CrossRef]
- Laing, E.D.; Weiss, C.D.; Samuels, E.C.; Coggins, S.A.; Wang, W.; Wang, R.; Vassell, R.; Sterling, S.L.; Tso, M.S.; Conner, T.; et al. Durability of Antibody Response and Frequency of SARS-CoV-2 Infection 6 Months after COVID-19 Vaccination in Healthcare Workers. Emerg. Infect. Dis. 2022, 28, 828–832. [Google Scholar] [CrossRef]
- Conner, T.L.; Goguet, E.; Haines-Hull, H.; Segard, A.; Darcey, E.S.; Kobi, P.; Balogun, B.; Olsen, C.; Esposito, D.; Jones, M.; et al. Subclinical SARS-CoV-2 Infections and Endemic Human Coronavirus Immunity Shape SARS-CoV-2 Saliva Antibody Responses. medRxiv 2024. [Google Scholar] [CrossRef]
- Seffer, M.-T.; Weinert, M.; Molinari, G.; Rohde, M.; Gröbe, L.; Kielstein, J.T.; Engelmann, S. Staphylococcus aureus binding to Seraph® 100 Microbind® Affinity Filter: Effects of surface protein expression and treatment duration. PLoS ONE 2023, 18, e0283304. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Smeriglio, A.; Campo, S.; La Camera, E.; Lanteri, G.; Giunta, E.; Monardo, P.; Trombetta, D. In Vitro Simulated Hemoperfusion on Seraph®-100 as a Promising Strategy to Counteract Sepsis. Biomedicines 2024, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, J.P.; Selig, D.J.; Vir, P.; Vuong, C.V.; Della-Volpe, J.; Rivera, I.M.; Park, C.; Levi, B.; Pratt, K.P.; Stewart, I.J. Seraph 100 Microbind Affinity Blood Filter Does Not Clear Antibiotics: An Analysis of Antibiotic Concentration Data from PURIFY-OBS. Blood Purif. 2024, 53, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.W.; Oliver, J.D.; Collen, J.; Bunin, J.; Gleeson, T.D.; Foster, B.E.; Simmons, M.P.; Chen, H.W.; Ficke, J.B.; Brown, T.E.; et al. Treatment for Severe Coronavirus Disease 2019 with the Seraph-100 Microbind Affinity Blood Filter. Crit. Care Explor. 2020, 2, e0180. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Longchamp, J.; Croxatto, A.; Greub, G.; Sanchez, B.; Delaloye, J.; Whiting, L.; Jeanneret, S.; Coste, A.T.; Dumoulin, A.; et al. Serum antibody response in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Hastak, P.S.; Andersen, C.R.; Kelleher, A.D.; Sasson, S.C. Frontline workers: Mediators of mucosal immunity in community acquired pneumonia and COVID-19. Front. Immunol. 2022, 13, 983550. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Q.; Lin, Q.; Fang, J.; Wang, H.; Kwok, H.; Tang, H.; Nishiura, K.; Peng, J.; Tan, Z.; et al. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019, 4, e123158. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Siracusano, G.; Cottignies-Calamarte, A.; Tudor, D.; Real, F.; Zhu, A.; Pastori, C.; Capron, C.; Rosenberg, A.R.; Temperton, N.; et al. Persistent but dysfunctional mucosal SARS-CoV-2-specific IgA and low lung IL-1β associate with COVID-19 fatal outcome: A cross-sectional analysis. Front. Immunol. 2022, 13, 842468. [Google Scholar] [CrossRef]
- Pavlidis, G.; Kampolis, C.F.; Perlepe, G.; Pagonis, A.; Maniotis, C.; Koullias, E.; Kranidioti, H.; Kyritsis, A.; Pavlou, E.; Sinis, S.; et al. Do the Kinetics of Antibody Responses Predict Clinical Outcome in Hospitalized Patients with Moderate-to-Severe COVID-19? Vivo 2022, 36, 1944–1948. [Google Scholar] [CrossRef]
- Phipps, W.S.; SoRelle, J.A.; Li, Q.-Z.; Mahimainathan, L.; Araj, E.; Markantonis, J.; Lacelle, C.; Balani, J.; Parikh, H.; Solow, E.B.; et al. SARS-CoV-2 Antibody Responses Do Not Predict COVID-19 Disease Severity. Am. J. Clin. Pathol. 2020, 154, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef]
- Cervia, C.; Nilsson, J.; Zurbuchen, Y.; Valaperti, A.; Schreiner, J.; Wolfensberger, A.; Raeber, M.E.; Adamo, S.; Weigang, S.; Emmenegger, M.; et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2021, 147, 545–557.e549. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.C.; Ramonell, R.P.; Nguyen, D.C.; Cashman, K.S.; Saini, A.S.; Haddad, N.S.; Ley, A.M.; Kyu, S.; Howell, J.C.; Ozturk, T.; et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020, 21, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Oh, J.E.; Lee, H.K. Single Cell Transcriptomic Re-analysis of Immune Cells in Bronchoalveolar Lavage Fluids Reveals the Correlation of B Cell Characteristics and Disease Severity of Patients with SARS-CoV-2 Infection. Immune Netw. 2021, 21, e10. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; Sancilio, A.; Velez, M.E.; Ryan, D.T.; Pesce, L.; Saber, R.; Vaught, L.A.; Reiser, N.L.; Hsieh, R.R.; D’Aquila, R.T.; et al. COVID-19 mRNA Vaccination Generates Greater Immunoglobulin G Levels in Women Compared to Men. J. Infect. Dis. 2021, 224, 793–797. [Google Scholar] [CrossRef]


| All | Survivors | Non-Survivors | |
|---|---|---|---|
| Total study cohort | 33/33 (100%) | 15/33 (45.5%) | 18/33 (54.5%) |
| Sex | |||
| Male | 21/33 (63.6%) | 11/15 (73.3%) | 10/18 (55.6%) |
| Female | 12/33 (36.4%) | 4/15 (26.7%) | 8/18 (44.4%) |
| Race | |||
| Asian | 1/33 (3.0%) | 1/15 (6.7%) | 0/18 (0.0%) |
| Black | 2/33 (6.1%) | 0/15 (0.0%) | 2/18 (11.1%) |
| Latin American | 1/33 (3.0%) | 1/15 (6.7%) | 0/18 (0.0%) |
| Other | 4/33 (12.1%) | 1/15 (6.7%) | 3/18 (16.7%) |
| Unknown | 3/33 (9.1%) | 0/15 (0.0%) | 3/18 (16.7%) |
| White | 22/33 (66.7%) | 12/15 (80.0%) | 10/18 (55.6%) |
| Ethnicity | |||
| Hispanic | 14/33 (42.4%) | 5/15 (33.3%) | 9/18 (50.0%) |
| Non-Hispanic | 13/33 (39.4%) | 6/15 (40.0%) | 7/18 (38.9%) |
| Unknown | 6/33 (18.2%) | 4/15 (26.7%) | 2/18 (11.1%) |
| Age, median (IQR) | 45.0 (32.0, 52.0) | 45.0 (32.0, 58.0) | 45.5 (30.5, 52.3) |
| Body Mass Index, median (IQR) | 35.5 (29.4, 43.1) | 32.0 (28.9, 43.9) | 36.3 (31.6, 43.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conner, T.L.; Vir, P.; Laing, E.D.; Stewart, I.J.; Mitre, E.; Pratt, K.P. The Seraph 100® Microbind Affinity Blood Filter Does Not Alter Levels of Circulating or Mucosal Antibodies in Critical COVID-19 Patients. Antibodies 2024, 13, 65. https://doi.org/10.3390/antib13030065
Conner TL, Vir P, Laing ED, Stewart IJ, Mitre E, Pratt KP. The Seraph 100® Microbind Affinity Blood Filter Does Not Alter Levels of Circulating or Mucosal Antibodies in Critical COVID-19 Patients. Antibodies. 2024; 13(3):65. https://doi.org/10.3390/antib13030065
Chicago/Turabian StyleConner, Tonia L., Pooja Vir, Eric D. Laing, Ian J. Stewart, Edward Mitre, and Kathleen P. Pratt. 2024. "The Seraph 100® Microbind Affinity Blood Filter Does Not Alter Levels of Circulating or Mucosal Antibodies in Critical COVID-19 Patients" Antibodies 13, no. 3: 65. https://doi.org/10.3390/antib13030065







