Novel Monoclonal Antibody Specific toward Amyloid-β Binds to a Unique Epitope within the N-Terminal Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Novel Anti-Aβ Mouse Monoclonal Antibody
2.2. Antibodies
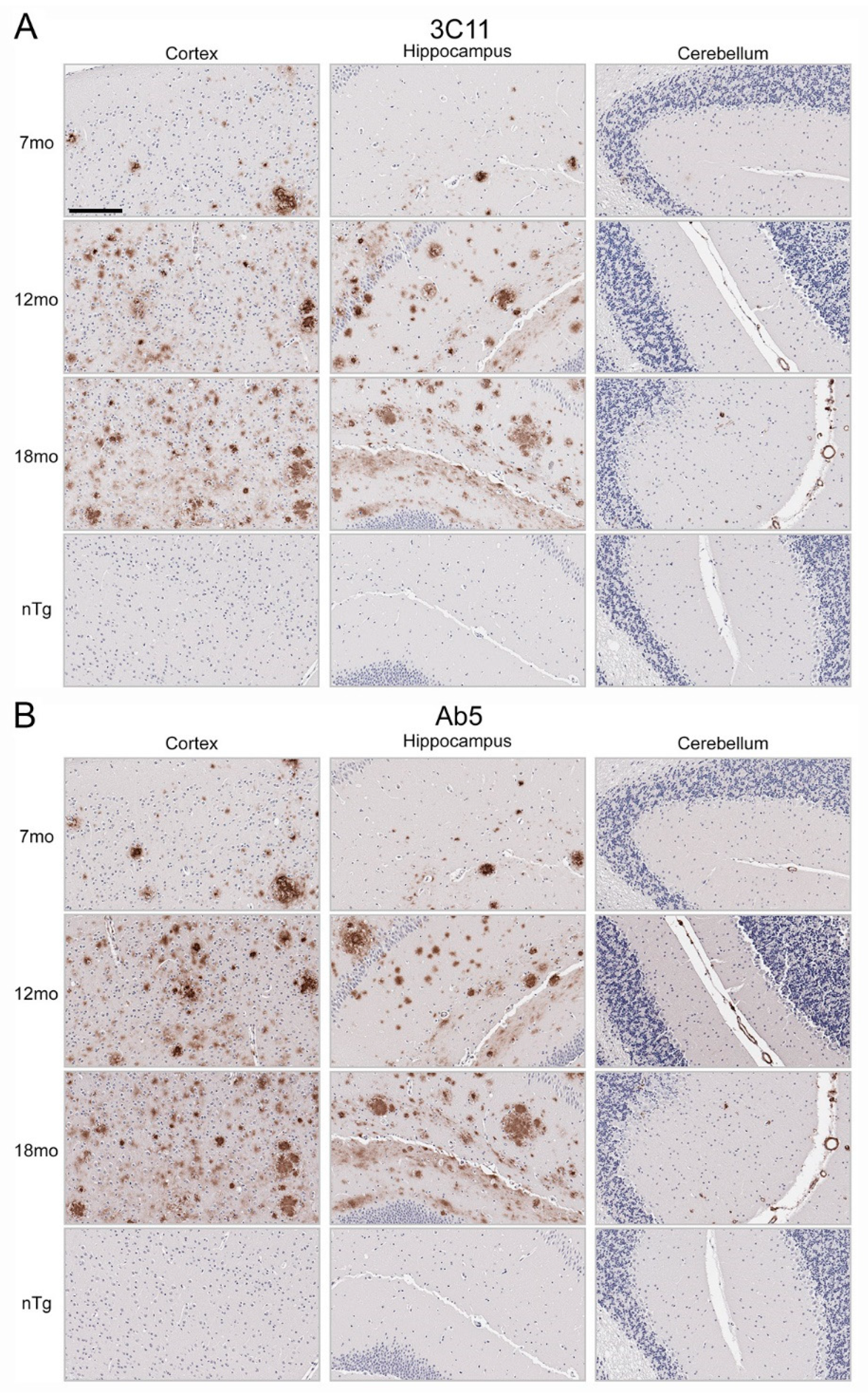
2.3. Tissue Processing and Immunohistochemistry
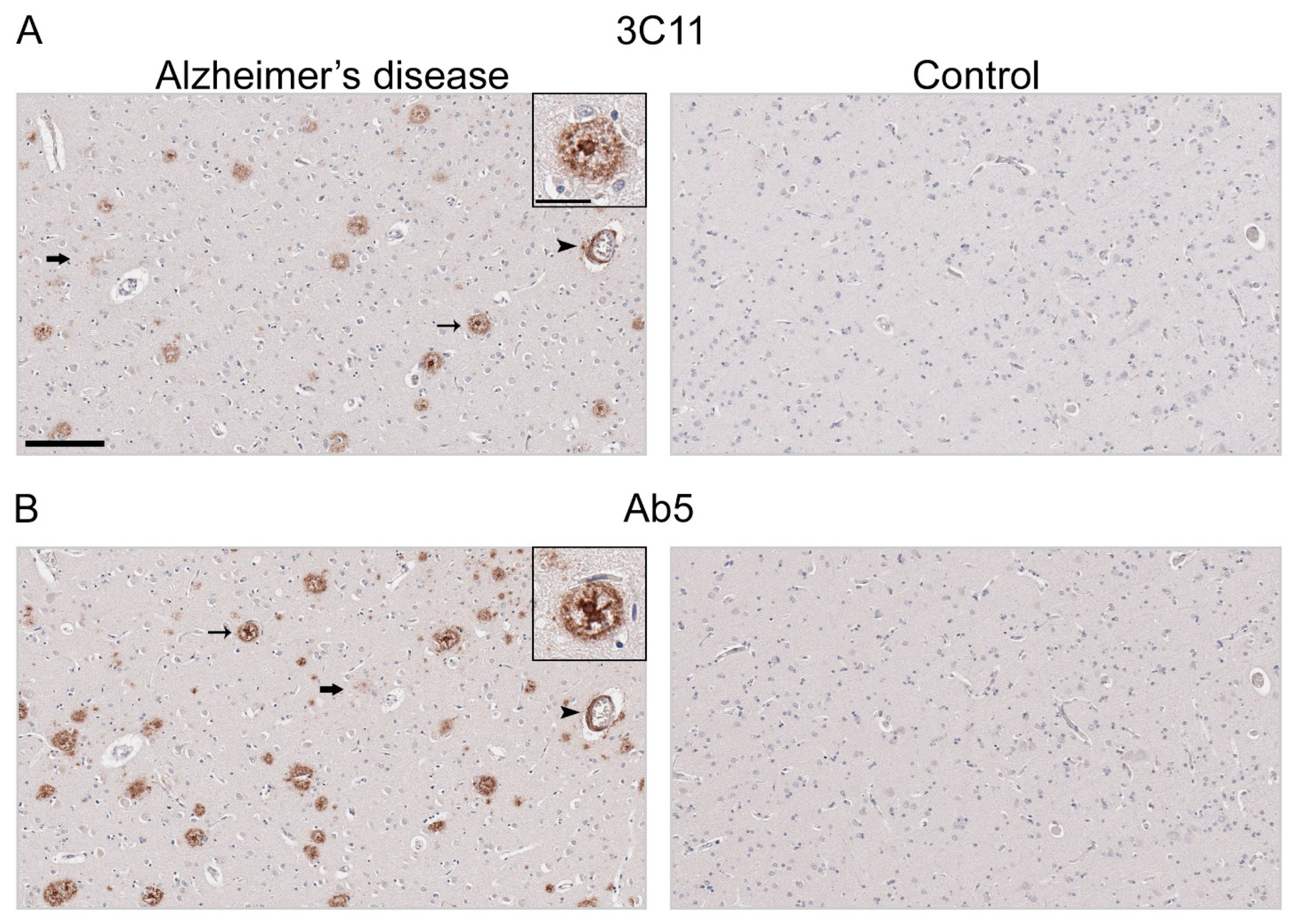
2.4. Quantification of Aβ Plaques in Human Brain Tissue
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Calcium Phosphate Transfection and Cell Harvest
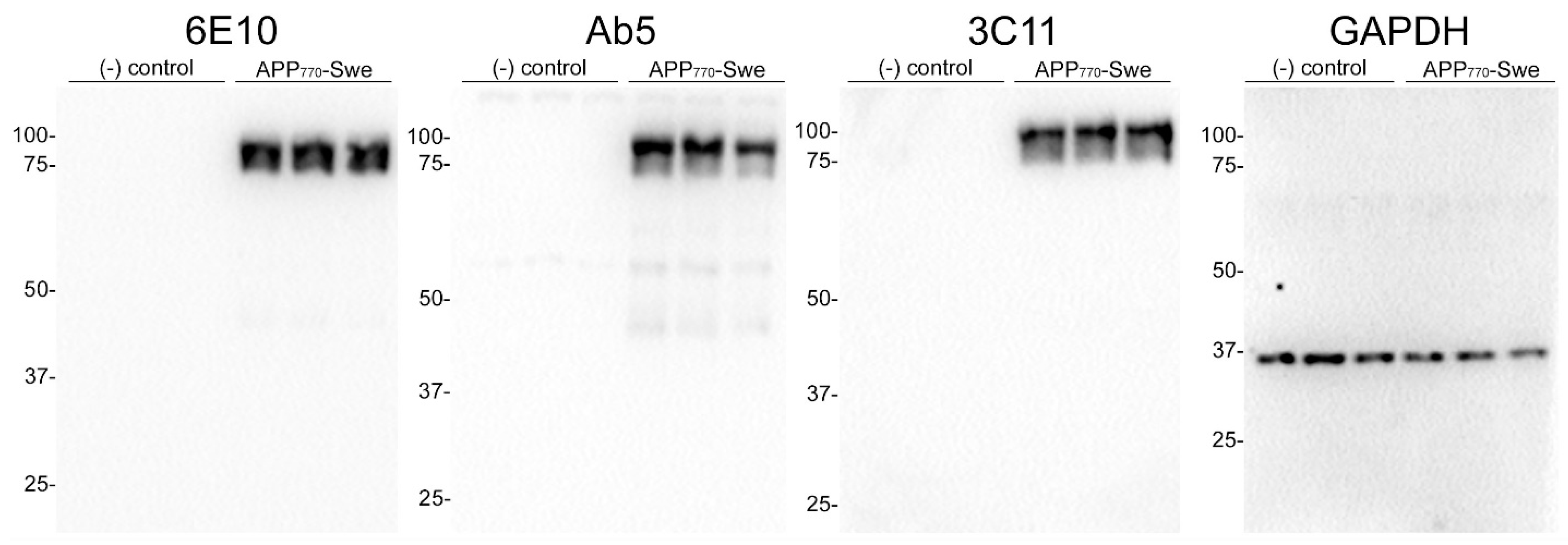
2.7. Western Blotting
3. Results
3.1. Epitope Characterization of Novel Monoclonal Antibody 3C11
3.2. Immunohistochemical Staining of Aβ Pathology Using Novel Monoclonal Antibody 3C11
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of A Beta-Deposition in the Human Brain and Its Relevance for the Development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K.; Alafuzov, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer Disease-Associated Neurofibrillary Pathology Using Paraffin Sections and Immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Attems, J. Sporadic Cerebral Amyloid Angiopathy: Pathology, Clinical Implications, and Possible Pathomechanisms. Acta Neuropathol. 2005, 110, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.C. Aβ Plaques. Free Neuropathol. 2020, 1, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s Disease: Initial Report of the Purification and Characterization of a Novel Cerebrovascular Amyloid Protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid Plaque Core Protein in Alzheimer Disease and Down Syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef]
- Joachim, C.L.; Duffy, L.K.; Morris, J.H.; Selkoe, D.J. Protein Chemical and Immunocytochemical Studies of Meningovascular β-Amyloid Protein in Alzheimer’s Disease and Normal Aging. Brain Res. 1988, 474, 100–111. [Google Scholar] [CrossRef]
- Kang, J.; Lemaire, H.G.; Unterbeck, A.; Salbaum, J.M.; Masters, C.L.; Grzeschik, K.H.; Multhaup, G.; Beyreuther, K.; Müller-Hill, B. The Precursor of Alzheimer’s Disease Amyloid A4 Protein Resembles a Cell-Surface Receptor. Nature 1987, 325, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, J.T.; Berger, E.P.; Lansbury, P.T. The Carboxy Terminus of the Beta Amyloid Protein Is Critical for the Seeding of Amyloid Formation: Implications for the Pathogenesis of Alzheimer’s Disease. Biochemistry 1993, 32, 4693–4697. [Google Scholar] [CrossRef] [PubMed]
- Burdick, D.; Soreghan, B.; Kwon, M.; Kosmoski, J.; Knauer, M.; Henschen, A.; Yates, J.; Cotman, C.; Glabe, C. Assembly and Aggregation Properties of Synthetic Alzheimer’s A4/Beta Amyloid Peptide Analogs. J. Biol. Chem. 1992, 267, 546–554. [Google Scholar] [CrossRef]
- Kirschner, D.A.; Abraham, C.; Selkoe, D.J. X-Ray Diffraction from Intraneuronal Paired Helical Filaments and Extraneuronal Amyloid Fibers in Alzheimer Disease Indicates Cross-Beta Conformation. Proc. Natl. Acad. Sci. USA 1986, 83, 503–507. [Google Scholar] [CrossRef]
- Giasson, B.I.; Lee, V.M.-Y.; Trojanowski, J.Q. Interactions of Amyloidogenic Proteins. Neuromol. Med. 2003, 4, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M. Alzheimer’s Disease—An Electron Microscopical Study. Brain 1964, 87, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, H.M.; Terry, R.D. Reexamination of the Pathogenesis of the Senile Plaque. In Progress in Neuropathology; Zimmerman, H.M., Ed.; Grune and Stratton: New York, NY, USA, 1973; pp. 1–26. [Google Scholar]
- Yang, Y.; Murzin, A.G.; Peak-Chew, S.; Franco, C.; Garringer, H.J.; Newell, K.L.; Ghetti, B.; Goedert, M.; Scheres, S.H.W. Cryo-EM Structures of Aβ40 Filaments from the Leptomeninges of Individuals with Alzheimer’s Disease and Cerebral Amyloid Angiopathy. Acta Neuropathol. Commun. 2023, 11, 191. [Google Scholar] [CrossRef]
- Yang, Y.; Arseni, D.; Zhang, W.; Huang, M.; Lövestam, S.; Schweighauser, M.; Kotecha, A.; Murzin, A.G.; Peak-Chew, S.Y.; Macdonald, J.; et al. Cryo-EM Structures of Amyloid-β 42 Filaments from Human Brains. Science 2022, 375, 167–172. [Google Scholar] [CrossRef]
- Wisniewski, H.M.; Wen, G.Y.; Kim, K.S. Comparison of Four Staining Methods on the Detection of Neuritic Plaques. Acta Neuropathol. 1989, 78, 22–27. [Google Scholar] [CrossRef]
- Kelényi, G. Thioflavin S Fluorescent and Congo Red Anisotropic Stainings in the Histologic Demonstration of Amyloid. Acta Neuropathol. 1967, 7, 336–348. [Google Scholar] [CrossRef]
- Beach, T.G. A History of Senile Plaques: From Alzheimer to Amyloid Imaging. J. Neuropathol. Exp. Neurol. 2022, 81, 387–413. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Quaranta, V.; Glenner, G.G. Neuritic Plaques and Cerebrovascular Amyloid in Alzheimer Disease Are Antigenically Related. Proc. Natl. Acad. Sci. USA 1985, 82, 8729–8732. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Wen, G.Y.; Bancher, C.M.; Chen, J.; Sapienza, V.J.; Hong, H.; Wisniewski, H.M. Detection and Quantitation of Amyloid B-Peptide with 2 Monoclonal Antibodies. Neurosci. Res. Commun. 1990, 7, 113–122. [Google Scholar]
- Dickson, D.W.; Crystal, H.A.; Mattiace, L.A.; Masur, D.M.; Blau, A.D.; Davies, P.; Yen, S.H.; Aronson, M.K. Identification of Normal and Pathological Aging in Prospectively Studied Nondemented Elderly Humans. Neurobiol. Aging 1992, 13, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, H.M.; Bancher, C.; Barcikowska, M.; Wen, G.Y.; Currie, J. Spectrum of Morphological Appearance of Amyloid Deposits in Alzheimer’s Disease. Acta Neuropathol. 1989, 78, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Iwatsubo, T.; Odaka, A.; Suzuki, N.; Mizusawa, H.; Nukina, N.; Ihara, Y. Visualization of A Beta 42(43) and A Beta 40 in Senile Plaques with End-Specific A Beta Monoclonals: Evidence That an Initially Deposited Species Is A Beta 42(43). Neuron 1994, 13, 45–53. [Google Scholar] [CrossRef]
- Dickson, D.W.; Farlo, J.; Davies, P.; Crystal, H.; Fuld, P.; Yen, S.H.C. Alzheimer’s Disease. A Double-Labeling Immunohistochemical Study of Senile Plaques. Am. J. Pathol. 1988, 132, 86–101. [Google Scholar] [PubMed]
- Dickson, D.W.; Crystal, H.; Mattiace, L.A.; Kress, Y.; Schwagerl, A.; Ksiezak-Reding, H.; Davies, P.; Yen, S.H. Diffuse Lewy Body Disease: Light and Electron Microscopic Immunocytochemistry of Senile Plaques. Acta Neuropathol. 1989, 78, 572–584. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hirai, S.; Morimatsu, M.; Shoji, M.; Ihara, Y. A Variety of Cerebral Amyloid Deposits in the Brains of the Alzheimer-Type Dementia Demonstrated by Beta Protein Immunostaining. Acta Neuropathol. 1988, 76, 541–549. [Google Scholar] [CrossRef]
- Allsop, D.; Landon, M.; Kidd, M.; Lowe, J.S.; Reynolds, G.P.; Gardner, A. Monoclonal Antibodies Raised against a Subsequence of Senile Plaque Core Protein React with Plaque Cores, Plaque Periphery and Cerebrovascular Amyloid in Alzheimer’s Disease. Neurosci. Lett. 1986, 68, 252–256. [Google Scholar] [CrossRef]
- Kim, K.S.; Miller, D.L.; Sapienza, V.J.; Chen, C.M.J.; Bai, C.; Grundke-Iqbal, I.; Currie, J.R.; Wisniewski, H.M. Production and Characterization of Monoclonal Antibodies Reactive to Synthetic Cerebrovascular Amyloid Peptide. Neurosci. Res. Commun. 1988, 2, 121–130. [Google Scholar]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer Disease Neuropathologic Changes with Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Jicha, G.A.; Schmitt, F.A.; Liu, H.; Davis, D.G.; Mendiondo, M.S.; Abner, E.L.; Markesbery, W.R. Clinicopathologic Correlations in a Large Alzheimer Disease Center Autopsy Cohort: Neuritic Plaques and Neurofibrillary Tangles “Do Count” When Staging Disease Severity. J. Neuropathol. Exp. Neurol. 2007, 66, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Boon, B.D.C.; Bulk, M.; Jonker, A.J.; Morrema, T.H.J.; van den Berg, E.; Popovic, M.; Walter, J.; Kumar, S.; van der Lee, S.J.; Holstege, H.; et al. The Coarse-Grained Plaque: A Divergent Aβ Plaque-Type in Early-Onset Alzheimer’s Disease. Acta Neuropathol. 2020, 140, 811–830. [Google Scholar] [CrossRef] [PubMed]
- Tsering, W.; Hery, G.P.; Phillips, J.L.; Lolo, K.; Bathe, T.; Villareal, J.A.; Ruan, I.Y.; Prokop, S. Transformation of Non-Neuritic into Neuritic Plaques during AD Progression Drives Cortical Spread of Tau Pathology via Regenerative Failure. Acta Neuropathol. Commun. 2023, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, H.M.; Sadowski, M.; Jakubowska-Sadowska, K.; Tarnawski, M.; Wegiel, J. Diffuse, Lake-like Amyloid-Beta Deposits in the Parvopyramidal Layer of the Presubiculum in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 1998, 57, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Allsop, D. Amyloid Deposition as the Central Event in the Aetiology of Alzheimer’s Disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.S.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef]
- Jia, J.; Ning, Y.; Chen, M.; Wang, S.; Yang, H.; Li, F.; Ding, J.; Li, Y.; Zhao, B.; Lyu, J.; et al. Biomarker Changes during 20 Years Preceding Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 712–722. [Google Scholar] [CrossRef]
- Li, Y.; Yen, D.; Hendrix, R.D.; Gordon, B.A.; Dlamini, S.; Barthélemy, N.R.; Aschenbrenner, A.J.; Henson, R.L.; Herries, E.M.; Volluz, K.; et al. Timing of Biomarker Changes in Sporadic Alzheimer’s Disease in Estimated Years from Symptom Onset. Ann. Neurol. 2024, 95, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Therriault, J.; Schindler, S.E.; Salvadó, G.; Pascoal, T.A.; Benedet, A.L.; Ashton, N.J.; Karikari, T.K.; Apostolova, L.; Murray, M.E.; Verberk, I.; et al. Biomarker-Based Staging of Alzheimer Disease: Rationale and Clinical Applications. Nat. Rev. Neurol. 2024, 20, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Möller, C.; Lannfelt, L. Lecanemab, Aducanumab, and Gantenerumab—Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics 2023, 20, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E.; Levey, A.I. Immunotherapies for Alzheimer’s Disease. Science 2023, 382, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.J.; Smith, J.; Donohue, M.C.; Delmar, P.; Abbas, R.; Salloway, S.; Wojtowicz, J.; Blennow, K.; Bittner, T.; Black, S.E.; et al. Two Phase 3 Trials of Gantenerumab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 389, 1862–1876. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Farlow, M.; McDade, E.; Clifford, D.B.; Wang, G.; Llibre-Guerra, J.J.; Hitchcock, J.M.; Mills, S.L.; Santacruz, A.M.; Aschenbrenner, A.J.; et al. A Trial of Gantenerumab or Solanezumab in Dominantly Inherited Alzheimer’s Disease. Nat. Med. 2021, 27, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The Antibody Aducanumab Reduces Aβ Plaques in Alzheimer’s Disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 Trials of Solanezumab for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.K.S.; Riffe, C.; Moore, B.D.; Ran, Y.; Chakrabarty, P.; Golde, T.E.; Giasson, B.I. A Novel Panel of α-Synuclein Antibodies Reveal Distinctive Staining Profiles in Synucleinopathies. PLoS ONE 2017, 12, e0184731. [Google Scholar] [CrossRef] [PubMed]
- Croft, C.L.; Moore, B.D.; Ran, Y.; Chakrabarty, P.; Levites, Y.; Golde, T.E.; Giasson, B.I. Novel Monoclonal Antibodies Targeting the Microtubule-Binding Domain of Human Tau. PLoS ONE 2018, 13, e0195211. [Google Scholar] [CrossRef] [PubMed]
- Levites, Y.; Das, P.; Price, R.W.; Rochette, M.J.; Kostura, L.A.; McGowan, E.M.; Murphy, M.P.; Golde, T.E. Anti-Abeta42- and Anti-Abeta40-Specific MAbs Attenuate Amyloid Deposition in an Alzheimer Disease Mouse Model. J. Clin. Investig. 2006, 116, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Waxman, E.A.; Giasson, B.I. A Novel, High-Efficiency Cellular Model of Fibrillar Alpha-Synuclein Inclusions and the Examination of Mutations That Inhibit Amyloid Formation. J. Neurochem. 2010, 113, 374–388. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Chishti, M.A.; Yang, D.S.; Janus, C.; Phinney, A.L.; Horne, P.; Pearson, J.; Strome, R.; Zuker, N.; Loukides, J.; French, J.; et al. Early-Onset Amyloid Deposition and Cognitive Deficits in Transgenic Mice Expressing a Double Mutant Form of Amyloid Precursor Protein 695. J. Biol. Chem. 2001, 276, 21562–21570. [Google Scholar] [CrossRef] [PubMed]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association Guidelines for the Neuropathologic Assessment of Alzheimer’s Disease: A Practical Approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; et al. National Institute on Aging-Alzheimer’s Association Guidelines for the Neuropathologic Assessment of Alzheimer’s Disease. Alzheimers Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef]
- Haass, C.; Schlossmacher, M.G.; Hung, A.Y.; Vigo-Pelfrey, C.; Mellon, A.; Ostaszewski, B.L.; Lieberburg, I.; Koo, E.H.; Schenk, D.; Teplow, D.B.; et al. Amyloid β-Peptide Is Produced by Cultured Cells during Normal Metabolism. Nature 1992, 359, 322–325. [Google Scholar] [CrossRef]
- Esch, F.S.; Keim, P.S.; Beattie, E.C.; Blacher, R.W.; Culwell, A.R.; Oltersdorf, T.; McClure, D.; Ward, P.J. Cleavage of Amyloid Beta Peptide during Constitutive Processing of Its Precursor. Science 1990, 248, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Golde, T.E.; Ghiso, J.; Cheung, T.T.; Estus, S.; Shaffer, L.M.; Cai, X.D.; McKay, D.M.; Tintner, R.; Frangione, B. Production of the Alzheimer Amyloid Beta Protein by Normal Proteolytic Processing. Science 1992, 258, 126–129. [Google Scholar] [CrossRef]
- Haass, C.; Kaether, C.; Thinakaran, G.; Sisodia, S. Trafficking and Proteolytic Processing of APP. Cold Spring Harb. Perspect. Med. 2012, 2, a006270. [Google Scholar] [CrossRef] [PubMed]
- Kummer, M.P.; Heneka, M.T. Truncated and Modified Amyloid-Beta Species. Alzheimers Res. Ther. 2014, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Vandersteen, A.; Hubin, E.; Sarroukh, R.; De Baets, G.; Schymkowitz, J.; Rousseau, F.; Subramaniam, V.; Raussens, V.; Wenschuh, H.; Wildemann, D.; et al. A Comparative Analysis of the Aggregation Behavior of Amyloid-β Peptide Variants. FEBS Lett. 2012, 586, 4088–4093. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.J.; Overman, M.J.; Cotman, C.W. Amino-Terminal Deletions Enhance Aggregation of Beta-Amyloid Peptides in Vitro. J. Biol. Chem. 1995, 270, 23895–23898. [Google Scholar] [CrossRef]
- Hatami, A.; Monjazeb, S.; Milton, S.; Glabe, C.G. Familial Alzheimer’s Disease Mutations within the Amyloid Precursor Protein Alter the Aggregation and Conformation of the Amyloid-β Peptide. J. Biol. Chem. 2017, 292, 3172–3185. [Google Scholar] [CrossRef] [PubMed]
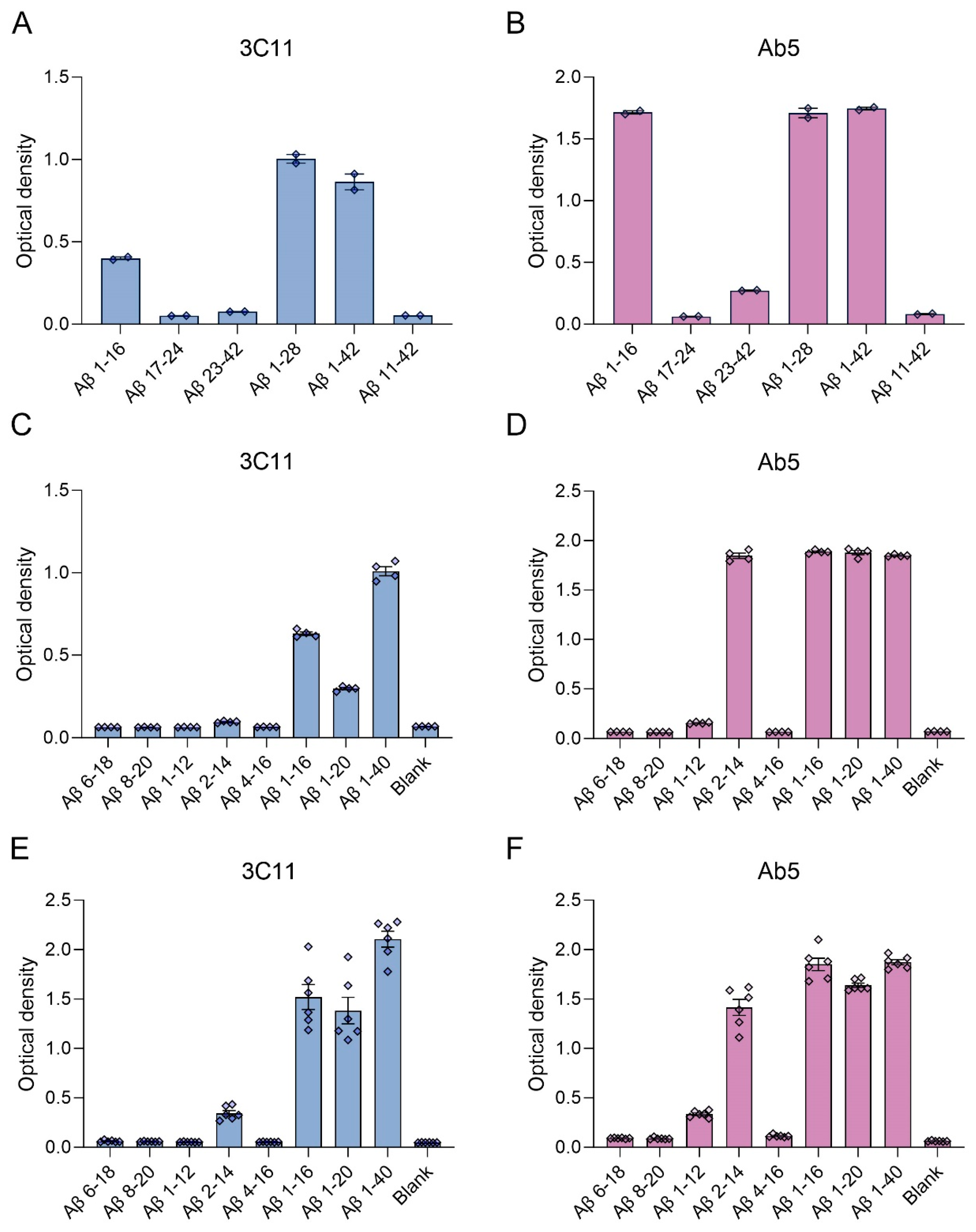

| Peptide | Primary Amino Acid Sequence |
|---|---|
| Aβ 1–12 | DAEFRHDSGYEV |
| Aβ 2–14 | AEFRHDSGYEVHH |
| Aβ 4–16 | FRHDSGYEVHHQK |
| Aβ 1–16 | DAEFRHDSGYEVHHQK |
| Aβ 6–18 | HDSGYEVHHQKLV |
| Aβ 8–20 | SGYEVHHQKLVFF |
| Aβ 1–20 | DAEFRHDSGYEVHHQKLVFF |
| Aβ 1–28 | DAEFRHDSGYEVHHQKLVFFAEDVGSNK |
| Aβ 17–24 | LVFFAEDV |
| Aβ 23–42 | DVGSNKGAIIGLMVGGVVIA |
| Aβ 11-42 | EVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA |
| Aβ 1–40 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV |
| Aβ 1–42 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA |
| Cases | Primary Neuropathological Diagnosis | Secondary Neuropathological Diagnosis | Tertiary Neuropathological Diagnosis | Thal Phase | Braak Stage | CERAD | APOE | Sex | Age |
|---|---|---|---|---|---|---|---|---|---|
| AD-1 | AD high | CAA widespread, mild to moderate | LATE stage 2 | 4 | VI | Frequent | 3/4 | f | 82 |
| AD-2 | AD high | CAA widespread, moderate | 5 | VI | Frequent | 4/4 | m | 70 | |
| AD-3 | AD high | CAA widespread, moderate | LBD amygdala predominant | 5 | V | Frequent | 3/3 | f | 82 |
| AD-4 | AD high | CAA widespread, moderate | 5 | VI | Frequent | 4/4 | m | 72 | |
| AD-5 | AD high | CAA, moderate, widespread | 5 | V | Frequent | 3/4 | f | 86 | |
| AD-6 | AD high | CAA widespread, moderate | LATE NC stage 2, hippocampal sclerosis | 4 | V | Frequent | 3/4 | f | 87 |
| AD-7 | AD high | CAA widespread, moderate | 5 | V | Frequent | 3/3 | f | 78 | |
| AD-8 | AD high | CAA widespread, moderate | LBD limbic-transitional | 5 | VI | Frequent | 3/3 | f | 81 |
| AD-9 | AD high | CAA widespread, moderate | LATE stage 1 | 5 | V | Frequent | 4/4 | f | 71 |
| AD-10 | AD high | CAA widespread, moderate | 5 | VI | Frequent | 3/4 | m | 63 | |
| AD-11 | AD high | CAA focal, mild | 5 | V | Frequent | 3/4 | f | 85 | |
| AD-12 | AD high | CAA widespread, moderate to severe | 3 | V | Frequent | 4/4 | f | 82 | |
| AD-13 | AD high | LBD diffuse neocortical | 5 | VI | Frequent | 3/4 | f | 84 | |
| AD-14 | AD high | CAA widespread, moderate to severe | cortical microinfarcts | 5 | V | Frequent | 3/4 | f | 85 |
| AD-15 | AD high | CAA widespread, moderate to severe | 5 | V | Frequent | 3/4 | m | 80 | |
| AD-16 | AD high | CAA widespread, mild to moderate | 5 | V | Frequent | 3/3 | f | 83 | |
| AD-17 | AD high | 5 | VI | Frequent | 3/3 | f | 76 | ||
| AD-18 | AD high | CAA widespread, moderate | LATE NC stage 2, hippocampal sclerosis | 5 | VI | Frequent | 3/4 | f | 99 |
| AD-19 | AD high | CAA (moderate) | LATE NC 1 | 5 | VI | Frequent | 3/4 | m | 81 |
| AD-20 | AD high | CAA widespread, mild | LBD limbic-transitional | 5 | VI | Frequent | 4/4 | f | 76 |
| AD-21 | AD high | LBD amygdala predominant | CAA focal, mild | 5 | VI | Frequent | 4/4 | f | 75 |
| AD-22 | AD high | LBD amygdala predominant | CAA focal, mild | 5 | VI | Frequent | 3/3 | m | 70 |
| AD-23 | AD high | CAA widespread, mild to moderate | LBD amygdala predominant | 5 | VI | Frequent | 3/3 | m | 78 |
| AD-24 | AD high | LBD amygdala predominant | 5 | V | Frequent | 3/3 | f | 88 | |
| Control-1 | PART, definite, Braak II | 0 | II | None | 3/3 | f | 72 | ||
| Control-2 | No significant pathological findings | 0 | 0 | None | 3/4 | f | 55 | ||
| Control-3 | PART, definite, Braak I | 0 | I | None | 2/3 | f | 73 |
| Case | 3C11/Ab5 Immunoreactive Positivity of Dense Aβ Plaques |
|---|---|
| AD-1 | 0.55 |
| AD-2 | 0.92 |
| AD-4 | 0.52 |
| AD-7 | 0.90 |
| AD-8 | 0.86 |
| AD-10 | 0.85 |
| AD-11 | 0.98 |
| AD-20 | 0.60 |
| AD-21 | 1.32 |
| AD-22 | 1.10 |
| AD-23 | 0.60 |
| AD-24 | 0.51 |
| N | 12 |
| Mean of all cases (Standard deviation) | 0.81 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterno, G.; Moore, B.D.; Bell, B.M.; Gorion, K.-M.M.; Ran, Y.; Prokop, S.; Golde, T.E.; Giasson, B.I. Novel Monoclonal Antibody Specific toward Amyloid-β Binds to a Unique Epitope within the N-Terminal Region. Antibodies 2024, 13, 68. https://doi.org/10.3390/antib13030068
Paterno G, Moore BD, Bell BM, Gorion K-MM, Ran Y, Prokop S, Golde TE, Giasson BI. Novel Monoclonal Antibody Specific toward Amyloid-β Binds to a Unique Epitope within the N-Terminal Region. Antibodies. 2024; 13(3):68. https://doi.org/10.3390/antib13030068
Chicago/Turabian StylePaterno, Giavanna, Brenda D. Moore, Brach M. Bell, Kimberly-Marie M. Gorion, Yong Ran, Stefan Prokop, Todd E. Golde, and Benoit I. Giasson. 2024. "Novel Monoclonal Antibody Specific toward Amyloid-β Binds to a Unique Epitope within the N-Terminal Region" Antibodies 13, no. 3: 68. https://doi.org/10.3390/antib13030068
APA StylePaterno, G., Moore, B. D., Bell, B. M., Gorion, K.-M. M., Ran, Y., Prokop, S., Golde, T. E., & Giasson, B. I. (2024). Novel Monoclonal Antibody Specific toward Amyloid-β Binds to a Unique Epitope within the N-Terminal Region. Antibodies, 13(3), 68. https://doi.org/10.3390/antib13030068






