A Novel Tetravalent Bispecific Immune Cell Engager Activates Natural Killer Cells to Kill Cancer Cells without Mediating Fratricide
Abstract
:1. Introduction
2. Experimental Methods
2.1. Cell Culture and Handling
2.2. Expression and Purification of BiKEs
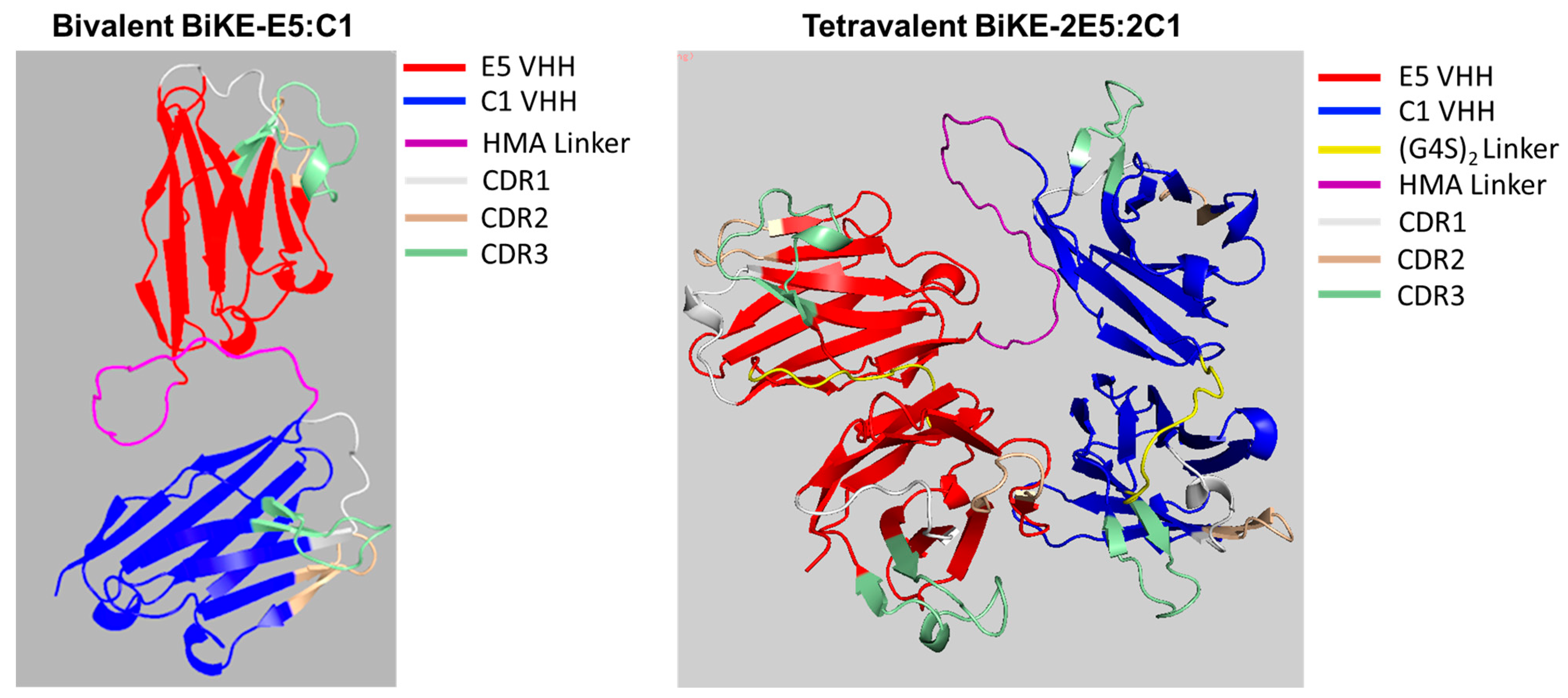
2.3. In Silico Modeling of Bivalent BiKE-E5:C1 and Tetravalent BiKE-2E5:2C1
2.4. Evaluation of the Ability of BiKE to Bind to CD16a and HER2 Antigens Using ELISA
2.5. Evaluation of the Ability of BiKE to Recognize HER2 and CD16a Antigens on Cells Using Flow Cytometry
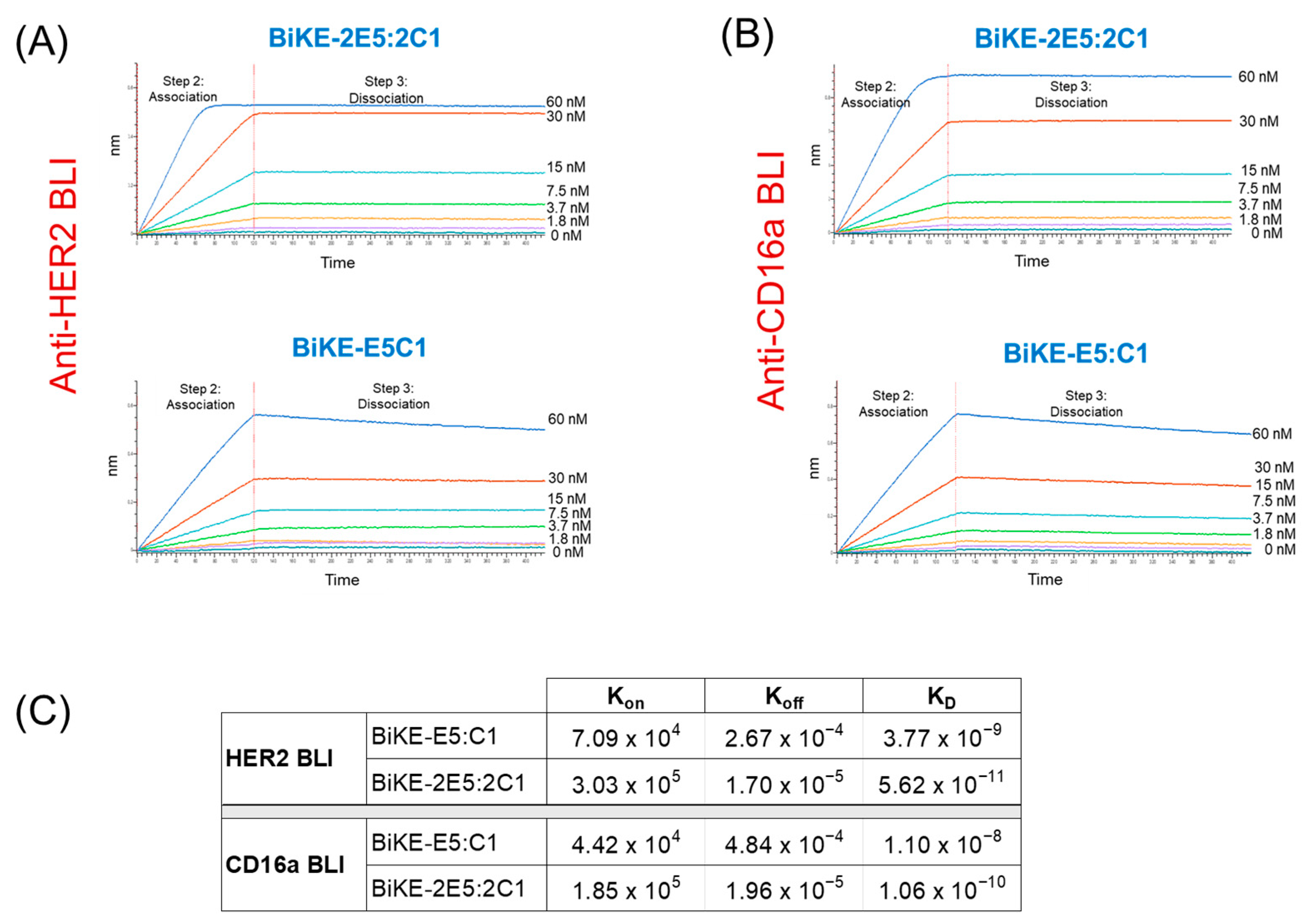
2.6. Measurement of the Binding Affinities of BiKEs Using Biolayer Interferometer
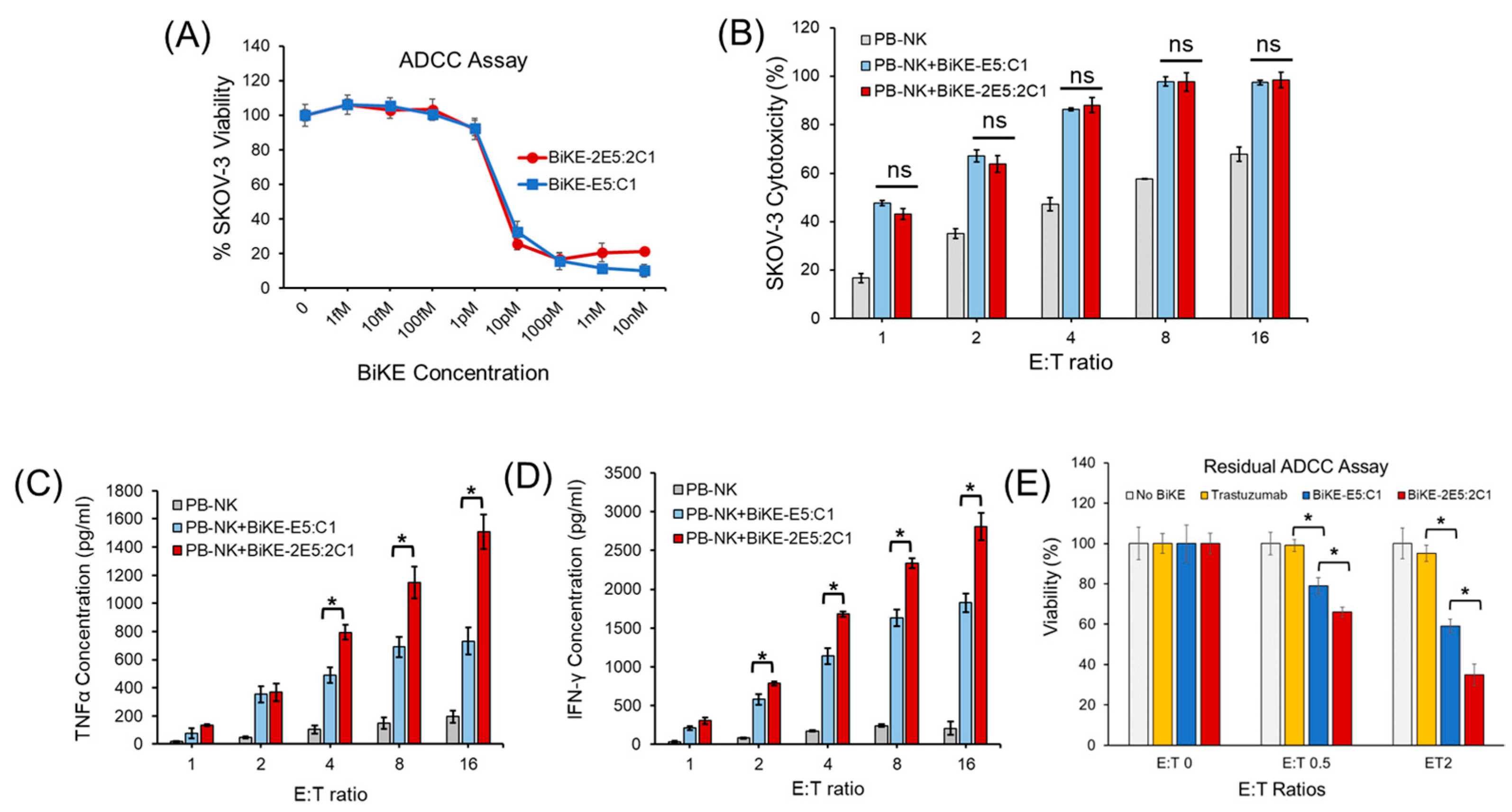
2.7. Measurement of Antibody-Dependent Cellular Cytotoxicity
2.8. Residual Antibody-Dependent Cellular Cytotoxicity Assay
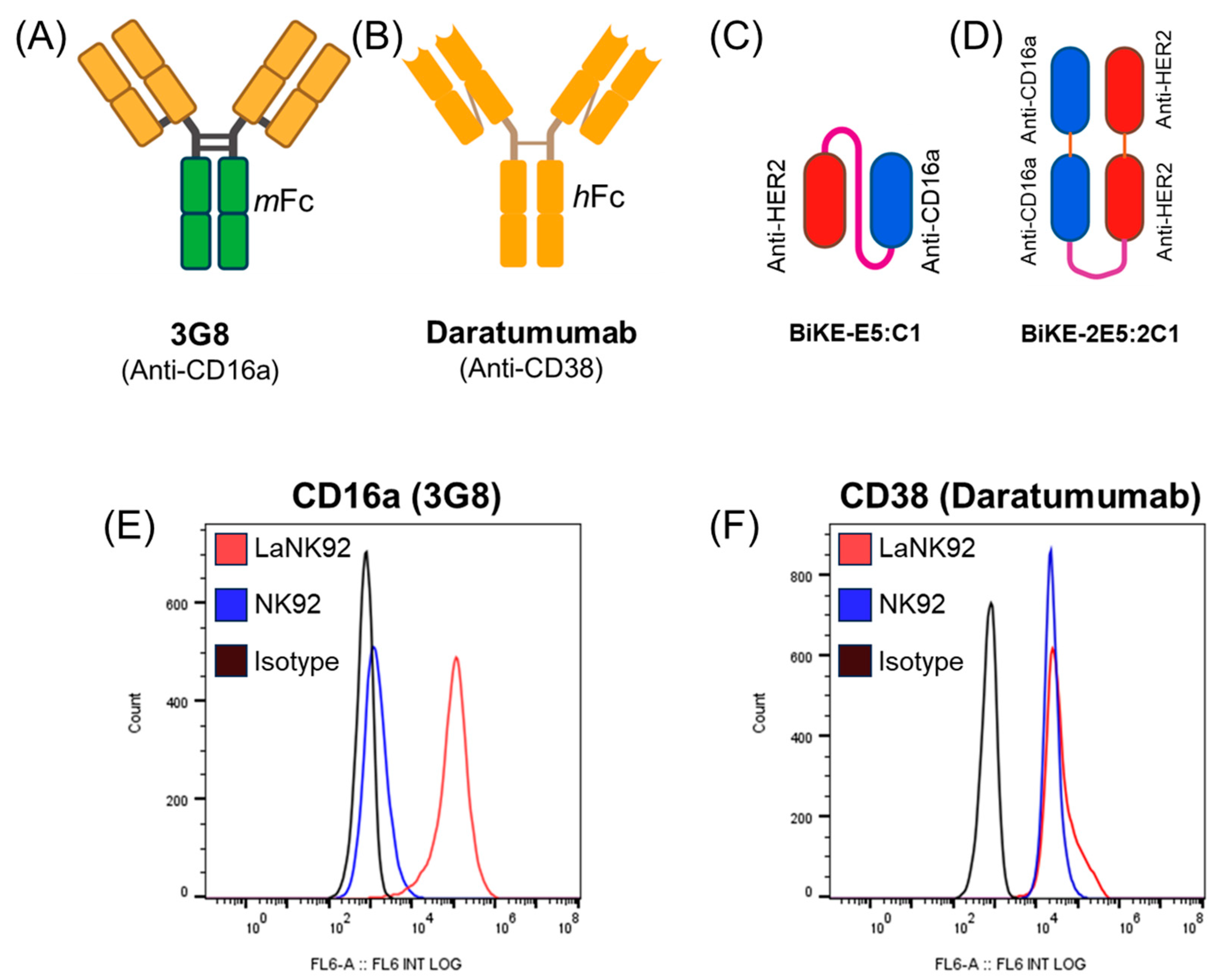
2.9. Measurement of Fratricide
2.10. Measurement of the NK Cell Degranulation and Release of Cytokines and Effector Proteins
3. Results and Discussion
3.1. Prediction of 3D Molecular Structure of BiKE-2E5:2C1
3.2. Tetravalent BiKE-2E5:2C1 Binds to Its Target Antigens Similar to Bivalent BiKE-E5:C1
3.3. Tetravalent BiKE-2E5:2C1 Exhibits Picomolar Affinity toward Its Target Antigens
3.4. Tetravalent BiKE-2E5:2C1 Has Higher Anticancer Activity than Bivalent BiKE-E5:C1
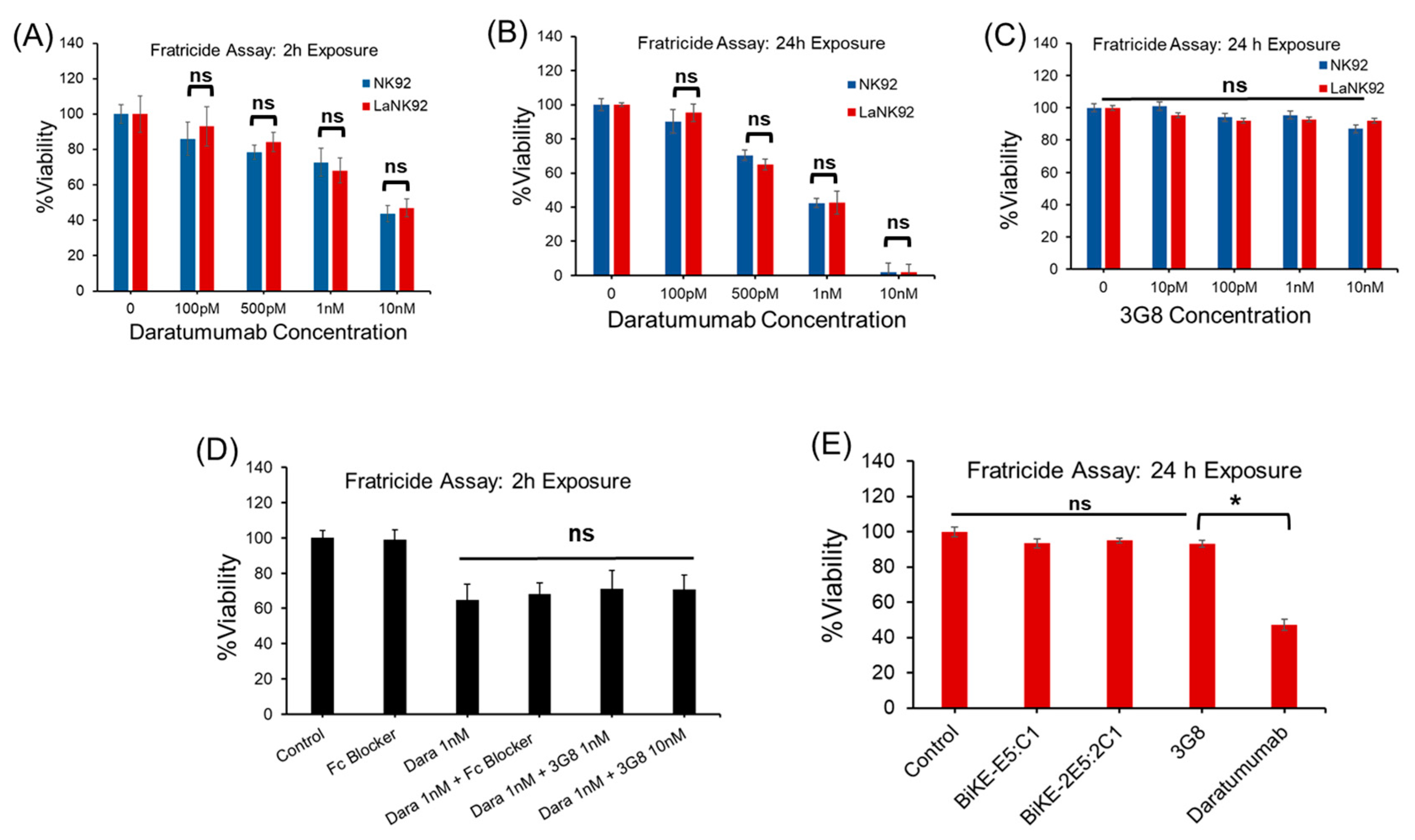
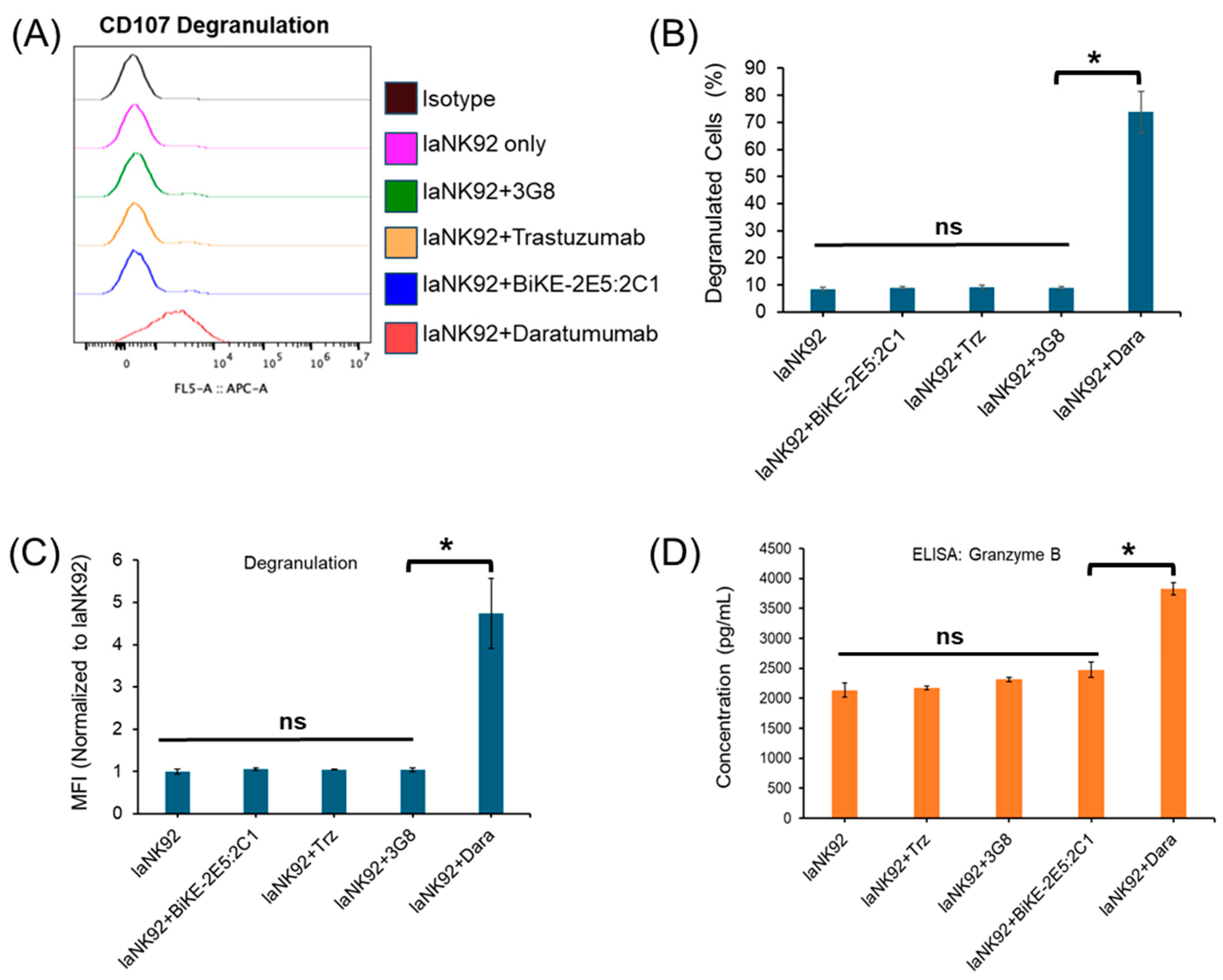
3.5. Tetravalent BiKE-2E5:2C1 Targeting CD16a Receptors Does Not Mediate NK Fratricide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steeland, S.; Vandenbroucke, R.E.; Libert, C. Nanobodies as therapeutics: Big opportunities for small antibodies. Drug Discov. Today 2016, 21, 1076–1113. [Google Scholar] [CrossRef] [PubMed]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Huet, H.A.; Growney, J.D.; Johnson, J.A.; Li, J.; Bilic, S.; Ostrom, L.; Zafari, M.; Kowal, C.; Yang, G.; Royo, A.; et al. Multivalent nanobodies targeting death receptor 5 elicit superior tumor cell killing through efficient caspase induction. mAbs 2014, 6, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Nikkhoi, S.K.; Li, G.; Eleya, S.; Yang, G.; Vandavasi, V.G.; Hatefi, A. Bispecific killer cell engager with high affinity and specificity toward CD16a on NK cells for cancer immunotherapy. Front. Immunol. 2022, 13, 1039969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gopal, S.; Kwon, S.J.; Ku, B.; Lee, D.W.; Kim, J.; Dordick, J.S. 3D tumor spheroid microarray for high-throughput, high-content natural killer cell-mediated cytotoxicity. Commun. Biol. 2021, 4, 893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nikkhoi, S.K.; Heydarzadeh, H.; Vandavasi, V.G.; Yang, G.; Louro, P.; Polunas, M.; Owji, H.; Hatefi, A. A high affinity and specificity anti-HER2 single-domain antibody (VHH) that targets trastuzumab’s epitope with versatile biochemical, biological, and medical applications. Immunol. Res. 2024, 72, 103–118. [Google Scholar] [CrossRef]
- Khoshtinat Nikkhoi, S.; Yang, G.; Owji, H.; Grizotte-Lake, M.; Cohen, R.I.; Gil Gonzalez, L.; Massumi, M.; Hatefi, A. Bispecific immune cell engager enhances the anticancer activity of CD16+ NK cells and macrophages in vitro, and eliminates cancer metastasis in NK humanized NOG mice. J. Immunother. Cancer 2024, 12, e008295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reusch, U.; Burkhardt, C.; Fucek, I.; Le Gall, F.; Le Gall, M.; Hoffmann, K.; Knackmuss, S.H.; Kiprijanov, S.; Little, M.; Zhukovsky, E.A. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. mAbs 2014, 6, 728–739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Bluemel, C.; Hausmann, S.; Fluhr, P.; Sriskandarajah, M.; Stallcup, W.B.; Baeuerle, P.A.; Kufer, P. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol. Immunother. 2010, 59, 1197–1209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Musolino, A.; Naldi, N.; Bortesi, B.; Pezzuolo, D.; Capelletti, M.; Missale, G.; Laccabue, D.; Zerbini, A.; Camisa, R.; Bisagni, G.; et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 2008, 26, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.K.; Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003, 21, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Hughes, T.; Zhang, J.; Caligiuri, M.A.; Benson, D.M.; Yu, J. Fratricide of NK Cells in Daratumumab Therapy for Multiple Myeloma Overcome by Ex Vivo-Expanded Autologous NK Cells. Clin. Cancer Res. 2018, 24, 4006–4017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lejeune, M.; Duray, E.; Peipp, M.; Clemenceau, B.; Baron, F.; Beguin, Y.; Caers, J. Balancing the CD38 Expression on Effector and Target Cells in Daratumumab-Mediated NK Cell ADCC against Multiple Myeloma. Cancers 2021, 13, 3072. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casneuf, T.; Xu, X.S.; Adams, H.C., 3rd; Axel, A.E.; Chiu, C.; Khan, I.; Ahmadi, T.; Yan, X.; Lonial, S.; Plesner, T.; et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017, 1, 2105–2114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nijhof, I.S.; Casneuf, T.; van Velzen, J.; van Kessel, B.; Axel, A.E.; Syed, K.; Groen, R.W.; van Duin, M.; Sonneveld, P.; Minnema, M.C.; et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016, 128, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.; Stikvoort, A.; Nolan, E.; Kirkham-McCarthy, L.; Khoruzhenko, S.; Shivakumar, R.; Zweegman, S.; Van de Donk, N.; Mutis, T.; Szegezdi, E.; et al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica 2022, 107, 437–445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural Killer Cells for Immunotherapy—Advantages of the NK-92 Cell Line over Blood NK Cells. Front. Immunol. 2016, 7, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sconocchia, G.; Titus, J.A.; Mazzoni, A.; Visintin, A.; Pericle, F.; Hicks, S.W.; Malavasi, F.; Segal, D.M. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood 1999, 94, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mallone, R.; Funaro, A.; Zubiaur, M.; Baj, G.; Ausiello, C.M.; Tacchetti, C.; Sancho, J.; Grossi, C.; Malavasi, F. Signaling through CD38 induces NK cell activation. Int. Immunol. 2001, 13, 397–409. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Nikkhoi, S.K.; Owji, H.; Li, G.; Massumi, M.; Cervelli, J.; Vandavasi, V.G.; Hatefi, A. A Novel Tetravalent Bispecific Immune Cell Engager Activates Natural Killer Cells to Kill Cancer Cells without Mediating Fratricide. Antibodies 2024, 13, 75. https://doi.org/10.3390/antib13030075
Yang G, Nikkhoi SK, Owji H, Li G, Massumi M, Cervelli J, Vandavasi VG, Hatefi A. A Novel Tetravalent Bispecific Immune Cell Engager Activates Natural Killer Cells to Kill Cancer Cells without Mediating Fratricide. Antibodies. 2024; 13(3):75. https://doi.org/10.3390/antib13030075
Chicago/Turabian StyleYang, Ge, Shahryar Khoshtinat Nikkhoi, Hajar Owji, Geng Li, Mohammad Massumi, Jessica Cervelli, Venu Gopal Vandavasi, and Arash Hatefi. 2024. "A Novel Tetravalent Bispecific Immune Cell Engager Activates Natural Killer Cells to Kill Cancer Cells without Mediating Fratricide" Antibodies 13, no. 3: 75. https://doi.org/10.3390/antib13030075
APA StyleYang, G., Nikkhoi, S. K., Owji, H., Li, G., Massumi, M., Cervelli, J., Vandavasi, V. G., & Hatefi, A. (2024). A Novel Tetravalent Bispecific Immune Cell Engager Activates Natural Killer Cells to Kill Cancer Cells without Mediating Fratricide. Antibodies, 13(3), 75. https://doi.org/10.3390/antib13030075









