Anti-ADAMTS13 Autoantibodies in Immune-Mediated Thrombotic Thrombocytopenic Purpura
Abstract
:1. Introduction
2. Formation and Structure of Anti-ADAMTS13 Antibodies
2.1. Molecular Mimicry and Antibody Pathogenesis
2.2. Role of T and B Lymphocytes in Autoimmune Response
2.3. Haplotype and Genetic Susceptibility to Autoimmunity
2.4. Structure of Anti-ADAMTS13 Antibodies
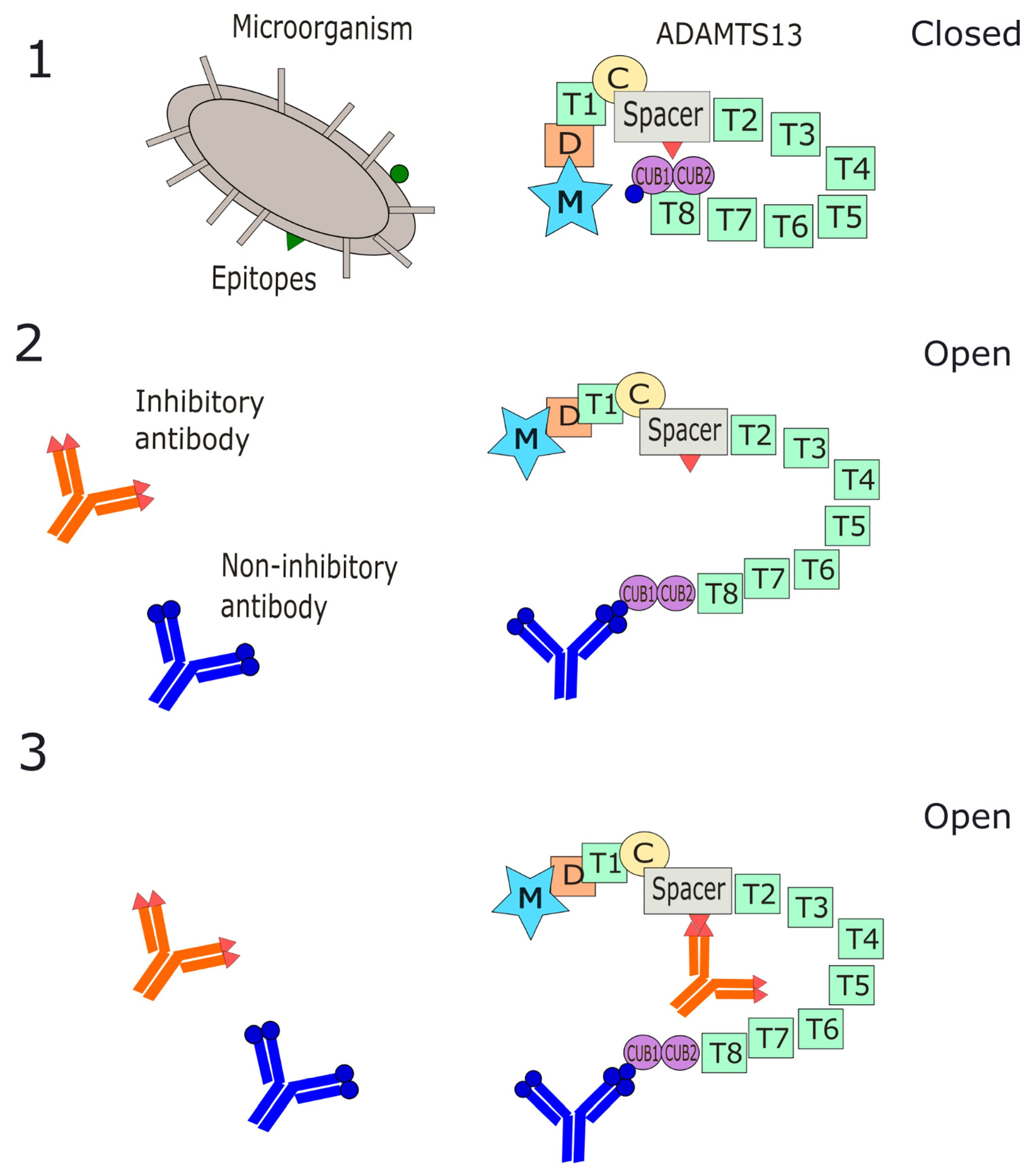
3. Mechanisms of Antibody Interaction with ADAMTS13
4. Function and Effects of Anti-ADAMTS13 Antibodies
5. Anti-ADAMTS 13 Antibodies and Clinical Correlates
6. Laboratory Detection of Anti-ADAMTS 13 Antibodies
7. Potential Role of Antibodies in Bone Marrow Suppression
8. Working Antibody-Mediated Disease Model and Therapeutic Options
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joly, B.S.; Coppo, P.; Veyradier, A. Thrombotic thrombocytopenic purpura. Blood 2017, 129, 2836–2846. [Google Scholar] [CrossRef]
- Zheng, X.L.; Vesely, S.K.; Cataland, S.R.; Coppo, P.; Geldziler, B.; Iorio, A.; Matsumoto, M.; Mustafa, R.A.; Pai, M.; Rock, G.; et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2020, 18, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Dolin, H.H.; Maitta, R.W. Pathological Mechanisms and Novel Testing Methods in Thrombotic Thrombocytopenic Purpura. Biomedicines 2024, 12, 621. [Google Scholar] [CrossRef]
- Kremer Hovinga, J.A.; Heeb, S.R.; Skowronska, M.; Schaller, M. Pathophysiology of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. J. Thromb. Haemost. 2018, 16, 618–629. [Google Scholar] [CrossRef]
- Furlan, M.; Robles, R.; Solenthaler, M.; Wassmer, M.; Sandoz, P.; Lammle, B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood 1997, 89, 3097–3103. [Google Scholar] [CrossRef]
- Tsai, H.M.; Lian, E.C. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N. Engl. J. Med. 1998, 339, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Setarehaseman, A.; Mohammadi, A.; Maitta, R.W. Thrombocytopenia in Sepsis. Life 2025, 15, 274. [Google Scholar] [CrossRef]
- Singh, K.; Kwong, A.C.; Madarati, H.; Kunasekaran, S.; Sparring, T.; Fox-Robichaud, A.E.; Liaw, P.C.; Kretz, C.A. Characterization of ADAMTS13 and von Willebrand factor levels in septic and non-septic ICU patients. PLoS ONE 2021, 16, e0247017. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.B.; Patel, N.; Hedges, M.A.; Benson, A.E.; Tomer, A.; Lo, J.O.; Shatzel, J.J. Hematologic Complications of Pregnancy. Eur. J. Haematol. 2025, 114, 596–614. [Google Scholar] [CrossRef]
- Moatti-Cohen, M.; Garrec, C.; Wolf, M.; Boisseau, P.; Galicier, L.; Azoulay, E.; Stepanian, A.; Delmas, Y.; Rondeau, E.; Bezieau, S.; et al. Unexpected frequency of Upshaw-Schulman syndrome in pregnancy-onset thrombotic thrombocytopenic purpura. Blood 2012, 119, 5888–5897. [Google Scholar] [CrossRef]
- Pos, W.; Luken, B.M.; Sorvillo, N.; Kremer Hovinga, J.A.; Voorberg, J. Humoral immune response to ADAMTS13 in acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2011, 9, 1285–1291. [Google Scholar] [CrossRef]
- Chaudhary, H.; Nasir, U.; Syed, K.; Labra, M.; Reggio, C.; Aziz, A.; Shah, P.; Reddy, R.; Sangha, N. COVID-19-Associated Thrombotic Thrombocytopenic Purpura: A Case Report and Systematic Review. Hematol. Rep. 2022, 14, 253–260. [Google Scholar] [CrossRef]
- Melissa, N.; Adit, S.; Junaid, H.; Sean, D. The perfect storm: Thrombotic thrombocytopenic purpura (TTP) associated with COVID-19, a clinical case series and review. EJHaem 2022, 3, 1358–1364. [Google Scholar] [CrossRef]
- Maitta, R.W.; Datta, K.; Lees, A.; Belouski, S.S.; Pirofski, L.A. Immunogenicity and efficacy of Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan peptide mimotope-protein conjugates in human immunoglobulin transgenic mice. Infect. Immun. 2004, 72, 196–208. [Google Scholar] [CrossRef]
- Laghmouchi, A.; Graca, N.A.G.; Voorberg, J. Emerging Concepts in Immune Thrombotic Thrombocytopenic Purpura. Front. Immunol. 2021, 12, 757192. [Google Scholar] [CrossRef]
- Saeki, Y.; Ishihara, K. Infection-immunity liaison: Pathogen-driven autoimmune-mimicry (PDAIM). Autoimmun. Rev. 2014, 13, 1064–1069. [Google Scholar] [CrossRef]
- Verbij, F.C.; Turksma, A.W.; de Heij, F.; Kaijen, P.; Lardy, N.; Fijnheer, R.; Sorvillo, N.; ten Brinke, A.; Voorberg, J. CD4+ T cells from patients with acquired thrombotic thrombocytopenic purpura recognize CUB2 domain-derived peptides. Blood 2016, 127, 1606–1609. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jimenez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramirez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Maoz-Segal, R.; Andrade, P. Chapter 3-Molecular Mimicry and Autoimmunity. In Infection and Autoimmunity, 2nd ed.; Shoenfeld, Y., Agmon-Levin, N., Rose, N.R., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 27–44. [Google Scholar]
- Sfriso, P.; Ghirardello, A.; Botsios, C.; Tonon, M.; Zen, M.; Bassi, N.; Bassetto, F.; Doria, A. Infections and autoimmunity: The multifaceted relationship. J. Leukoc. Biol. 2010, 87, 385–395. [Google Scholar] [CrossRef]
- Poole, B.D.; Scofield, R.H.; Harley, J.B.; James, J.A. Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity 2006, 39, 63–70. [Google Scholar] [CrossRef]
- Bennett, C.L.; Davidson, C.J.; Raisch, D.W.; Weinberg, P.D.; Bennett, R.H.; Feldman, M.D. Thrombotic thrombocytopenic purpura associated with ticlopidine in the setting of coronary artery stents and stroke prevention. Arch. Intern. Med. 1999, 159, 2524–2528. [Google Scholar] [CrossRef]
- Azarm, T.; Sohrabi, A.; Mohajer, H.; Azarm, A. Thrombotic Thrombocytopenic Purpura associated with Clopidogrel: A case report and review of the literature. J. Res. Med. Sci. 2011, 16, 353–357. [Google Scholar] [PubMed]
- Blank, M.; Barzilai, O.; Shoenfeld, Y. Molecular mimicry and auto-immunity. Clin. Rev. Allergy Immunol. 2007, 32, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, K.W.; Call, M.J.; Deng, L.; Mariuzza, R. Structural alterations in peptide-MHC recognition by self-reactive T cell receptors. Curr. Opin. Immunol. 2009, 21, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Subhan, M.O.; Cambridge, G.; Guo, Y.; de Groot, R.; Scully, M.; Thomas, M. Alterations in B- and circulating T-follicular helper cell subsets in immune thrombotic thrombocytopenic purpura. Blood Adv. 2022, 6, 3792–3802. [Google Scholar] [CrossRef]
- Wong, C.K.; Lit, L.C.; Tam, L.S.; Li, E.K.; Lam, C.W. Aberrant production of soluble costimulatory molecules CTLA-4, CD28, CD80 and CD86 in patients with systemic lupus erythematosus. Rheumatology 2005, 44, 989–994. [Google Scholar] [CrossRef]
- Coppo, P.; Busson, M.; Veyradier, A.; Wynckel, A.; Poullin, P.; Azoulay, E.; Galicier, L.; Loiseau, P.; French Reference Center for Thrombotic Microangiopathies. HLA-DRB1*11: A strong risk factor for acquired severe ADAMTS13 deficiency-related idiopathic thrombotic thrombocytopenic purpura in Caucasians. J. Thromb. Haemost. 2010, 8, 856–859. [Google Scholar] [CrossRef]
- John, M.L.; Hitzler, W.; Scharrer, I. The role of human leukocyte antigens as predisposing and/or protective factors in patients with idiopathic thrombotic thrombocytopenic purpura. Ann. Hematol. 2012, 91, 507–510. [Google Scholar] [CrossRef]
- Sorvillo, N.; van Haren, S.D.; Kaijen, P.H.; ten Brinke, A.; Fijnheer, R.; Meijer, A.B.; Voorberg, J. Preferential HLA-DRB1*11-dependent presentation of CUB2-derived peptides by ADAMTS13-pulsed dendritic cells. Blood 2013, 121, 3502–3510. [Google Scholar] [CrossRef]
- Scully, M.; Brown, J.; Patel, R.; McDonald, V.; Brown, C.J.; Machin, S. Human leukocyte antigen association in idiopathic thrombotic thrombocytopenic purpura: Evidence for an immunogenetic link. J. Thromb. Haemost. 2010, 8, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, C.E.; Ha, J.P.; Maitta, R.W. Re-examination of 30-day survival and relapse rates in patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. PLoS ONE 2015, 10, e0127744. [Google Scholar] [CrossRef]
- Matsuyama, T.; Kuwana, M.; Matsumoto, M.; Isonishi, A.; Inokuma, S.; Fujimura, Y. Heterogeneous pathogenic processes of thrombotic microangiopathies in patients with connective tissue diseases. Thromb. Haemost. 2009, 102, 371–378. [Google Scholar] [CrossRef]
- Coppo, P.; Bengoufa, D.; Veyradier, A.; Wolf, M.; Bussel, A.; Millot, G.A.; Malot, S.; Heshmati, F.; Mira, J.P.; Boulanger, E.; et al. Severe ADAMTS13 deficiency in adult idiopathic thrombotic microangiopathies defines a subset of patients characterized by various autoimmune manifestations, lower platelet count, and mild renal involvement. Medicine 2004, 83, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Godel, P.; Fischer, J.; Scheid, C.; Gathof, B.S.; Wolf, J.; Rybniker, J. Familial acquired thrombotic thrombocytopenic purpura in siblings-no immunogenetic link with associated human leucocyte antigens. Eur. J. Haematol. 2017, 98, 311–313. [Google Scholar] [CrossRef]
- Studt, J.D.; Kremer Hovinga, J.A.; Radonic, R.; Gasparovic, V.; Ivanovic, D.; Merkler, M.; Wirthmueller, U.; Dahinden, C.; Furlan, M.; Lammle, B. Familial acquired thrombotic thrombocytopenic purpura: ADAMTS13 inhibitory autoantibodies in identical twins. Blood 2004, 103, 4195–4197. [Google Scholar] [CrossRef]
- Ferrari, S.; Mudde, G.C.; Rieger, M.; Veyradier, A.; Kremer Hovinga, J.A.; Scheiflinger, F. IgG subclass distribution of anti-ADAMTS13 antibodies in patients with acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2009, 7, 1703–1710. [Google Scholar] [CrossRef]
- Sinkovits, G.; Szilagyi, A.; Farkas, P.; Inotai, D.; Szilvasi, A.; Tordai, A.; Razso, K.; Reti, M.; Prohaszka, Z. Concentration and Subclass Distribution of Anti-ADAMTS13 IgG Autoantibodies in Different Stages of Acquired Idiopathic Thrombotic Thrombocytopenic Purpura. Front. Immunol. 2018, 9, 1646. [Google Scholar] [CrossRef]
- Rieger, M.; Mannucci, P.M.; Kremer Hovinga, J.A.; Herzog, A.; Gerstenbauer, G.; Konetschny, C.; Zimmermann, K.; Scharrer, I.; Peyvandi, F.; Galbusera, M.; et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood 2005, 106, 1262–1267. [Google Scholar] [CrossRef]
- Pos, W.; Luken, B.M.; Kremer Hovinga, J.A.; Turenhout, E.A.; Scheiflinger, F.; Dong, J.F.; Fijnheer, R.; Voorberg, J. VH1-69 germline encoded antibodies directed towards ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2009, 7, 421–428. [Google Scholar] [CrossRef]
- Zheng, X.; Chung, D.; Takayama, T.K.; Majerus, E.M.; Sadler, J.E.; Fujikawa, K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J. Biol. Chem. 2001, 276, 41059–41063. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.M. Pathophysiology of thrombotic thrombocytopenic purpura. Int. J. Hematol. 2010, 91, 1–19. [Google Scholar] [CrossRef]
- Luken, B.M.; Turenhout, E.A.; Hulstein, J.J.; Van Mourik, J.A.; Fijnheer, R.; Voorberg, J. The spacer domain of ADAMTS13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb. Haemost. 2005, 93, 267–274. [Google Scholar] [CrossRef]
- Papakonstantinou, A.; Kalmoukos, P.; Mpalaska, A.; Koravou, E.E.; Gavriilaki, E. ADAMTS13 in the New Era of TTP. Int. J. Mol. Sci. 2024, 25, 8137. [Google Scholar] [CrossRef]
- Klaus, C.; Plaimauer, B.; Studt, J.D.; Dorner, F.; Lammle, B.; Mannucci, P.M.; Scheiflinger, F. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood 2004, 103, 4514–4519. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Wu, H.M.; Shang, D.; Falls, E.; Skipwith, C.G.; Cataland, S.R.; Bennett, C.L.; Kwaan, H.C. Multiple domains of ADAMTS13 are targeted by autoantibodies against ADAMTS13 in patients with acquired idiopathic thrombotic thrombocytopenic purpura. Haematologica 2010, 95, 1555–1562. [Google Scholar] [CrossRef]
- Thomas, M.R.; de Groot, R.; Scully, M.A.; Crawley, J.T. Pathogenicity of Anti-ADAMTS13 Autoantibodies in Acquired Thrombotic Thrombocytopenic Purpura. EBioMedicine 2015, 2, 942–952. [Google Scholar] [CrossRef]
- Kangro, K.; Roose, E.; Joly, B.S.; Sinkovits, G.; Falter, T.; von Auer, C.; Rossmann, H.; Reti, M.; Voorberg, J.; Prohaszka, Z.; et al. Anti-ADAMTS13 autoantibody profiling in patients with immune-mediated thrombotic thrombocytopenic purpura. Blood Adv. 2021, 5, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Soejima, K.; Matsumoto, M.; Kokame, K.; Yagi, H.; Ishizashi, H.; Maeda, H.; Nozaki, C.; Miyata, T.; Fujimura, Y.; Nakagaki, T. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood 2003, 102, 3232–3237. [Google Scholar] [CrossRef]
- Velasquez Pereira, L.C.; Roose, E.; Graca, N.A.G.; Sinkovits, G.; Kangro, K.; Joly, B.S.; Tellier, E.; Kaplanski, G.; Falter, T.; Von Auer, C.; et al. Immunogenic hotspots in the spacer domain of ADAMTS13 in immune-mediated thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2021, 19, 478–488. [Google Scholar] [CrossRef]
- Pos, W.; Sorvillo, N.; Fijnheer, R.; Feys, H.B.; Kaijen, P.H.; Vidarsson, G.; Voorberg, J. Residues Arg568 and Phe592 contribute to an antigenic surface for anti-ADAMTS13 antibodies in the spacer domain. Haematologica 2011, 96, 1670–1677. [Google Scholar] [CrossRef]
- Pos, W.; Crawley, J.T.; Fijnheer, R.; Voorberg, J.; Lane, D.A.; Luken, B.M. An autoantibody epitope comprising residues R660, Y661, and Y665 in the ADAMTS13 spacer domain identifies a binding site for the A2 domain of VWF. Blood 2010, 115, 1640–1649. [Google Scholar] [CrossRef]
- Roose, E.; Schelpe, A.S.; Joly, B.S.; Peetermans, M.; Verhamme, P.; Voorberg, J.; Greinacher, A.; Deckmyn, H.; De Meyer, S.F.; Coppo, P.; et al. An open conformation of ADAMTS-13 is a hallmark of acute acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2018, 16, 378–388. [Google Scholar] [CrossRef]
- South, K.; Luken, B.M.; Crawley, J.T.; Phillips, R.; Thomas, M.; Collins, R.F.; Deforche, L.; Vanhoorelbeke, K.; Lane, D.A. Conformational activation of ADAMTS13. Proc. Natl. Acad. Sci. USA 2014, 111, 18578–18583. [Google Scholar] [CrossRef]
- Jestin, M.; Benhamou, Y.; Schelpe, A.S.; Roose, E.; Provot, F.; Galicier, L.; Hie, M.; Presne, C.; Poullin, P.; Wynckel, A.; et al. Preemptive rituximab prevents long-term relapses in immune-mediated thrombotic thrombocytopenic purpura. Blood 2018, 132, 2143–2153. [Google Scholar] [CrossRef]
- Ercig, B.; Arfman, T.; Hrdinova, J.; Wichapong, K.; Reutelingsperger, C.P.M.; Vanhoorelbeke, K.; Nicolaes, G.A.F.; Voorberg, J. Conformational plasticity of ADAMTS13 in hemostasis and autoimmunity. J. Biol. Chem. 2021, 297, 101132. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, S.; Lammle, B.; Cataland, S.R. Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management. J. Clin. Med. 2021, 10, 536. [Google Scholar] [CrossRef]
- Lancellotti, S.; Sacco, M.; Tardugno, M.; Ferretti, A.; De Cristofaro, R. Immune and Hereditary Thrombotic Thrombocytopenic Purpura: Can ADAMTS13 Deficiency Alone Explain the Different Clinical Phenotypes? J. Clin. Med. 2023, 12, 3111. [Google Scholar] [CrossRef]
- Sakai, K.; Matsumoto, M.; De Waele, L.; Dekimpe, C.; Hamada, E.; Kubo, M.; Tersteeg, C.; De Meyer, S.F.; Vanhoorelbeke, K. ADAMTS13 conformation and immunoprofiles in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura. Blood Adv. 2023, 7, 131–140. [Google Scholar] [CrossRef]
- Ostertag, E.M.; Kacir, S.; Thiboutot, M.; Gulendran, G.; Zheng, X.L.; Cines, D.B.; Siegel, D.L. ADAMTS13 autoantibodies cloned from patients with acquired thrombotic thrombocytopenic purpura: 1. Structural and functional characterization in vitro. Transfusion 2016, 56, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Scheiflinger, F.; Knobl, P.; Trattner, B.; Plaimauer, B.; Mohr, G.; Dockal, M.; Dorner, F.; Rieger, M. Nonneutralizing IgM and IgG antibodies to von Willebrand factor-cleaving protease (ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura. Blood 2003, 102, 3241–3243. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.I.; Alwan, F.; Thomas, M.R.; Scully, M.A.; Crawley, J.T.B. Autoantibodies enhance ADAMTS-13 clearance in patients with immune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2023, 21, 1544–1552. [Google Scholar] [CrossRef]
- Ferrari, S.; Palavra, K.; Gruber, B.; Kremer Hovinga, J.A.; Knobl, P.; Caron, C.; Cromwell, C.; Aledort, L.; Plaimauer, B.; Turecek, P.L.; et al. Persistence of circulating ADAMTS13-specific immune complexes in patients with acquired thrombotic thrombocytopenic purpura. Haematologica 2014, 99, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A.; Valsecchi, C.; Pontiggia, S.; Mancini, I.; Cannavo, A.; Artoni, A.; Mikovic, D.; Meloni, G.; Peyvandi, F. Measurement and prevalence of circulating ADAMTS13-specific immune complexes in autoimmune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2014, 12, 329–336. [Google Scholar] [CrossRef]
- Underwood, M.I.; Thomas, M.R.; Scully, M.A.; Crawley, J.T.B. ADAMTS-13 conformation influences autoimmune recognition in immune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2024, 22, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Roose, E.; Vidarsson, G.; Kangro, K.; Verhagen, O.; Mancini, I.; Desender, L.; Pareyn, I.; Vandeputte, N.; Vandenbulcke, A.; Vendramin, C.; et al. Anti-ADAMTS13 Autoantibodies against Cryptic Epitopes in Immune-Mediated Thrombotic Thrombocytopenic Purpura. Thromb. Haemost. 2018, 118, 1729–1742. [Google Scholar] [CrossRef]
- Roose, E.; Schelpe, A.S.; Tellier, E.; Sinkovits, G.; Joly, B.S.; Dekimpe, C.; Kaplanski, G.; Le Besnerais, M.; Mancini, I.; Falter, T.; et al. Open ADAMTS13, induced by antibodies, is a biomarker for subclinical immune-mediated thrombotic thrombocytopenic purpura. Blood 2020, 136, 353–361. [Google Scholar] [CrossRef]
- De Waele, L.; Curie, A.; Kangro, K.; Tellier, E.; Kaplanski, G.; Mannik, A.; Tersteeg, C.; Joly, B.S.; Coppo, P.; Veyradier, A.; et al. Anti-cysteine/spacer antibodies that open ADAMTS13 are a common feature in iTTP. Blood Adv. 2021, 5, 4480–4484. [Google Scholar] [CrossRef]
- Halkidis, K.; Zheng, X.L. ADAMTS13 conformations and mechanism of inhibition in immune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2022, 20, 2197–2203. [Google Scholar] [CrossRef]
- Bettoni, G.; Palla, R.; Valsecchi, C.; Consonni, D.; Lotta, L.A.; Trisolini, S.M.; Mancini, I.; Musallam, K.M.; Rosendaal, F.R.; Peyvandi, F. ADAMTS-13 activity and autoantibodies classes and subclasses as prognostic predictors in acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2012, 10, 1556–1565. [Google Scholar] [CrossRef]
- Alwan, F.; Vendramin, C.; Vanhoorelbeke, K.; Langley, K.; McDonald, V.; Austin, S.; Clark, A.; Lester, W.; Gooding, R.; Biss, T.; et al. Presenting ADAMTS13 antibody and antigen levels predict prognosis in immune-mediated thrombotic thrombocytopenic purpura. Blood 2017, 130, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Dainese, C.; Valeri, F.; Pizzo, E.; Valpreda, A.; Sivera, P.; Montaruli, B.; Porreca, A.; Massaia, M.; Bruno, B.; Borchiellini, A. ADAMTS13 Autoantibodies and Burden of Care in Immune Thrombotic Thrombocytopenic purpura: New Evidence and Future Implications. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221125785. [Google Scholar] [CrossRef] [PubMed]
- Coppo, P.; Wolf, M.; Veyradier, A.; Bussel, A.; Malot, S.; Millot, G.A.; Daubin, C.; Bordessoule, D.; Pene, F.; Mira, J.P.; et al. Prognostic value of inhibitory anti-ADAMTS13 antibodies in adult-acquired thrombotic thrombocytopenic purpura. Br. J. Haematol. 2006, 132, 66–74. [Google Scholar] [CrossRef]
- Peyvandi, F.; Lavoretano, S.; Palla, R.; Feys, H.B.; Vanhoorelbeke, K.; Battaglioli, T.; Valsecchi, C.; Canciani, M.T.; Fabris, F.; Zver, S.; et al. ADAMTS13 and anti-ADAMTS13 antibodies as markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica 2008, 93, 232–239. [Google Scholar] [CrossRef]
- Schieppati, F.; Russo, L.; Marchetti, M.; Barcella, L.; Cefis, M.; Gomez-Rosas, P.; Caldara, G.; Carpenedo, M.; D’Adda, M.; Rambaldi, A.; et al. Low levels of ADAMTS-13 with high anti-ADAMTS-13 antibodies during remission of immune-mediated thrombotic thrombocytopenic purpura highly predict for disease relapse: A multi-institutional study. Am. J. Hematol. 2020, 95, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Casper, T.C.; Cataland, S.R.; Kennedy, M.S.; Lin, S.; Li, Y.J.; Wu, H.M. Relationship between ADAMTS13 activity in clinical remission and the risk of TTP relapse. Br. J. Haematol. 2008, 141, 651–658. [Google Scholar] [CrossRef]
- Mancini, I.; Ferrari, B.; Valsecchi, C.; Pontiggia, S.; Fornili, M.; Biganzoli, E.; Peyvandi, F.; Italian Group of TTP Investigators. ADAMTS13-specific circulating immune complexes as potential predictors of relapse in patients with acquired thrombotic thrombocytopenic purpura. Eur. J. Intern. Med. 2017, 39, 79–83. [Google Scholar] [CrossRef]
- Smock, K.J. ADAMTS13 testing update: Focus on laboratory aspects of difficult thrombotic thrombocytopenic purpura diagnoses and effects of new therapies. Int. J. Lab. Hematol. 2021, 43 (Suppl. S1), 103–108. [Google Scholar] [CrossRef]
- Kokame, K.; Nobe, Y.; Kokubo, Y.; Okayama, A.; Miyata, T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br. J. Haematol. 2005, 129, 93–100. [Google Scholar] [CrossRef]
- Gomez-Segui, I.; Pascual Izquierdo, C.; de la Rubia Comos, J. Best practices and recommendations for drug regimens and plasma exchange for immune thrombotic thrombocytopenic purpura. Expert Rev. Hematol. 2021, 14, 707–719. [Google Scholar] [CrossRef]
- Masias, C.; Cataland, S.R. The role of ADAMTS13 testing in the diagnosis and management of thrombotic microangiopathies and thrombosis. Blood 2018, 132, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Simmons, S.C.; Williams, L.A., III; Staley, E.M.; Zheng, X.L.; Pham, H.P. ADAMTS13 test and/or PLASMIC clinical score in management of acquired thrombotic thrombocytopenic purpura: A cost-effective analysis. Transfusion 2017, 57, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Nixon, C.P.; Cheves, T.A.; Sweeney, J.D. Availability of an ADAMTS13 assay with rapid turnaround time may avoid interhospital transfer in patients with thrombotic microangiopathy. Transfusion 2018, 58, 1328–1329. [Google Scholar] [CrossRef]
- Nakashima, M.O.; Zhang, X.; Rogers, H.J.; Vengal, L.; Gibson, B., Jr.; Daly, T.M.; Kottke-Marchant, K. Validation of a panel of ADAMTS13 assays for diagnosis of thrombotic thrombocytopenic purpura: Activity, functional inhibitor, and autoantibody test. Int. J. Lab. Hematol. 2016, 38, 550–559. [Google Scholar] [CrossRef]
- Peyvandi, F.; Palla, R.; Lotta, L.A.; Mackie, I.; Scully, M.A.; Machin, S.J. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2010, 8, 631–640. [Google Scholar] [CrossRef]
- Vendramin, C.; Thomas, M.; Westwood, J.P.; Scully, M. Bethesda Assay for Detecting Inhibitory Anti-ADAMTS13 Antibodies in Immune-Mediated Thrombotic Thrombocytopenic Purpura. TH Open 2018, 2, e329–e333. [Google Scholar] [CrossRef]
- Dekimpe, C.; Roose, E.; Kangro, K.; Bonnez, Q.; Vandenbulcke, A.; Tellier, E.; Kaplanski, G.; Feys, H.B.; Tersteeg, C.; Mannik, A.; et al. Determination of anti-ADAMTS-13 autoantibody titers in ELISA: Influence of ADAMTS-13 presentation and autoantibody detection. J. Thromb. Haemost. 2021, 19, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Pasalic, L.; Henry, B.; Lippi, G. Laboratory testing for ADAMTS13: Utility for TTP diagnosis/exclusion and beyond. Am. J. Hematol. 2021, 96, 1049–1055. [Google Scholar] [CrossRef]
- Moore, G.W.; Vetr, H.; Binder, N.B. ADAMTS13 Antibody and Inhibitor Assays. Methods Mol. Biol. 2023, 2663, 549–565. [Google Scholar] [CrossRef]
- Zhu, M.L.; Reeves, H.M.; Maitta, R.W. Immature platelet dynamics correlate with ADAMTS13 deficiency and predict therapy response in immune-mediated thrombotic thrombocytopenic purpura. Thromb. Res. 2021, 198, 72–78. [Google Scholar] [CrossRef]
- Valsecchi, C.; Mirabet, M.; Mancini, I.; Biganzoli, M.; Schiavone, L.; Faraudo, S.; Mane-Padros, D.; Giles, D.; Serra-Domenech, J.; Blanch, S.; et al. Evaluation of a New, Rapid, Fully Automated Assay for the Measurement of ADAMTS13 Activity. Thromb. Haemost. 2019, 119, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Bonnez, Q.; Dekimpe, C.; Tellier, E.; Kaplanski, G.; Verhamme, P.; Tersteeg, C.; De Meyer, S.F.; Lammertyn, J.; Joly, B.; Coppo, P.; et al. Measuring ADAMTS-13 activity to diagnose thrombotic thrombocytopenic purpura: A novel, fast fiber-optic surface plasmon resonance immunoassay. Res. Pract. Thromb. Haemost. 2023, 7, 102171. [Google Scholar] [CrossRef] [PubMed]
- Bonnez, Q.; Dekimpe, C.; Bekaert, T.; Tellier, E.; Kaplanski, G.; Joly, B.S.; Veyradier, A.; Coppo, P.; Lammertyn, J.; Tersteeg, C.; et al. Diagnosis of thrombotic thrombocytopenic purpura: Easy-to-use fiber optic surface plasmon resonance immunoassays for automated ADAMTS-13 antigen and conformation evaluation. J. Thromb. Haemost. 2024, 22, 1936–1946. [Google Scholar] [CrossRef]
- Horta, S.; Qu, J.H.; Dekimpe, C.; Bonnez, Q.; Vandenbulcke, A.; Tellier, E.; Kaplanski, G.; Delport, F.; Geukens, N.; Lammertyn, J.; et al. Co(III)-NTA Mediated Antigen Immobilization on a Fiber Optic-SPR Biosensor for Detection of Autoantibodies in Autoimmune Diseases: Application in Immune-Mediated Thrombotic Thrombocytopenic Purpura. Anal. Chem. 2020, 92, 13880–13887. [Google Scholar] [CrossRef]
- Reeves, H.M.; Zhu, M.L.; Maitta, R.W. Immature platelet count responses of pediatric patients with immune-mediated thrombotic thrombocytopenic purpura. Thromb. Res. 2024, 241, 109085. [Google Scholar] [CrossRef]
- Reeves, H.M.; Maitta, R.W. Immature Platelet Dynamics in Immune-Mediated Thrombocytopenic States. Front. Med. 2020, 7, 597734. [Google Scholar] [CrossRef]
- Reeves, H.M.; Maitta, R.W. Comparison of absolute immature platelet count to the PLASMIC score at presentation in predicting ADAMTS13 deficiency in suspected thrombotic thrombocytopenic purpura. Thromb. Res. 2022, 215, 30–36. [Google Scholar] [CrossRef]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knobl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Scully, M.; Kremer Hovinga, J.A.; Cataland, S.; Knobl, P.; Wu, H.; Artoni, A.; Westwood, J.P.; Mansouri Taleghani, M.; Jilma, B.; et al. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2016, 374, 511–522. [Google Scholar] [CrossRef]
- Kubo, M.; Matsumoto, M. Frontiers in pathophysiology and management of thrombotic thrombocytopenic purpura. Int. J. Hematol. 2023, 117, 331–340. [Google Scholar] [CrossRef]
- Doyle, A.J.; Stubbs, M.J.; Lester, W.; Thomas, W.; Westwood, J.P.; Thomas, M.; Percy, C.; Prasannan, N.; Scully, M. The use of obinutuzumab and ofatumumab in the treatment of immune thrombotic thrombocytopenic purpura. Br. J. Haematol. 2022, 198, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Maitta, R.W. Anti-CD20 therapeutic options in immune-mediated thrombotic thrombocytopenic purpura. Br. J. Haematol. 2022, 198, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Antun, A.; Cataland, S.R.; Coppo, P.; Dossier, C.; Biebuyck, N.; Hassenpflug, W.A.; Kentouche, K.; Knobl, P.; Kremer Hovinga, J.A.; et al. Recombinant ADAMTS13 in Congenital Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2024, 390, 1584–1596. [Google Scholar] [CrossRef]
- Heo, Y.A. Apadamtase Alfa: First Approval. Drugs 2024, 84, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Plaimauer, B.; Kremer Hovinga, J.A.; Juno, C.; Wolfsegger, M.J.; Skalicky, S.; Schmidt, M.; Grillberger, L.; Hasslacher, M.; Knobl, P.; Ehrlich, H.; et al. Recombinant ADAMTS13 normalizes von Willebrand factor-cleaving activity in plasma of acquired TTP patients by overriding inhibitory antibodies. J. Thromb. Haemost. 2011, 9, 936–944. [Google Scholar] [CrossRef]
- Graca, N.A.G.; Ercig, B.; Carolina Velasquez Pereira, L.; Kangro, K.; Kaijen, P.; Nicolaes, G.A.F.; Veyradier, A.; Coppo, P.; Vanhoorelbeke, K.; Mannik, A.; et al. Modifying ADAMTS13 to modulate binding of pathogenic autoantibodies of patients with acquired thrombotic thrombocytopenic purpura. Haematologica 2020, 105, 2619–2630. [Google Scholar] [CrossRef]
- Halkidis, K.; Siegel, D.L.; Zheng, X.L. A human monoclonal antibody against the distal carboxyl terminus of ADAMTS-13 modulates its susceptibility to an inhibitor in thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2021, 19, 1888–1895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snyder, M.R.; Maitta, R.W. Anti-ADAMTS13 Autoantibodies in Immune-Mediated Thrombotic Thrombocytopenic Purpura. Antibodies 2025, 14, 24. https://doi.org/10.3390/antib14010024
Snyder MR, Maitta RW. Anti-ADAMTS13 Autoantibodies in Immune-Mediated Thrombotic Thrombocytopenic Purpura. Antibodies. 2025; 14(1):24. https://doi.org/10.3390/antib14010024
Chicago/Turabian StyleSnyder, Michael R., and Robert W. Maitta. 2025. "Anti-ADAMTS13 Autoantibodies in Immune-Mediated Thrombotic Thrombocytopenic Purpura" Antibodies 14, no. 1: 24. https://doi.org/10.3390/antib14010024
APA StyleSnyder, M. R., & Maitta, R. W. (2025). Anti-ADAMTS13 Autoantibodies in Immune-Mediated Thrombotic Thrombocytopenic Purpura. Antibodies, 14(1), 24. https://doi.org/10.3390/antib14010024




